Crystal Structure Evolution of CaSiO3 Polymorphs at Earth’s Mantle Pressures
Abstract
:1. Introduction
Polymorphism and Polytypism in CaSiO3 System up to ca. 8 GPa
2. Materials and Methods
3. Results
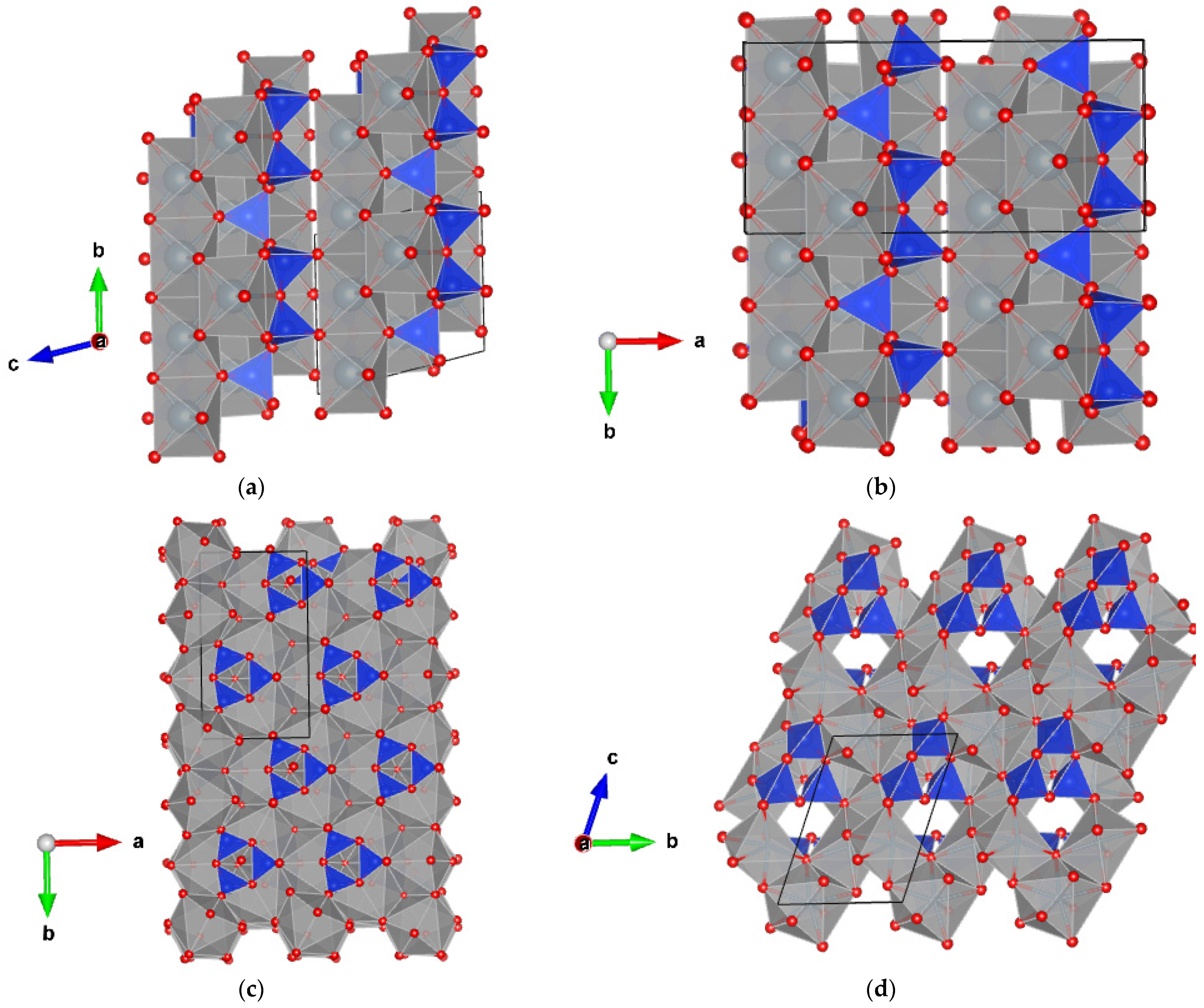
3.1. High-Pressure Polytypes of Wollastonite
3.2. High-Pressure Polytypes of CaSiO3-Walstromite
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palme, H.; Lodders, K.; Jones, A. Solar System Abundances of the Elements. In Planets, Asteroids, Comets and the Solar System, Treatise on Geochemistry, 2nd ed.; Davis, A.M., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 1, pp. 15–36. [Google Scholar]
- McDonough, W.F. Compositional Model for the Earth’s Core. In The Mantle and Core, Treatise on Geochemistry; Carlson, R.W., Ed.; Elsevier-Pergamon: Oxford, UK, 2003; Volume 2, pp. 547–568. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In The Crust, Treatise on Geochemistry; Rudnick, R.L., Ed.; Elsevier-Pergamon: Oxford, UK, 2003; Volume 3, pp. 1–64. [Google Scholar]
- Palme, H.; O’Neill, H.S.C. Cosmochemical estimates of mantle composition. In The Mantle and Core, Treatise on Geochemistry; Carlson, R.W., Ed.; Elsevier-Pergamon: Oxford, UK, 2003; Volume 2, pp. 1–38. [Google Scholar]
- Gasparik, T.; Wolf, K.; Smith, C.M. Experimental determination of phase relations in the CaSiO3 system from 8 to 15 GPa. Am. Mineral. 1994, 79, 1219–1222. [Google Scholar]
- Pearson, D.G.; Brenker, F.E.; Nestola, F.; McNeill, J.; Nasdala, L.; Hutchison, M.T.; Matveev, S.; Mather, K.; Silversmit, G.; Schmitz, S.; et al. Hydrous mantle transition zone indicated by ringwoodite included within diamond. Nature 2014, 507, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Nestola, F.; Korolev, N.; Kopylova, M.; Rotiroti, N.; Pearson, D.G.; Pamato, M.G.; Alvaro, M.; Peruzzo, L.; Gurney, J.J.; Moore, A.E.; et al. CaSiO3 perovskite in diamond indicates the recycling of oceanic crust into the lower mantle. Nature 2018, 555, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtstam, D.; Cámara, F.; Karlsson, A. Instalment of the margarosanite group, and data on walstromite-margarosanite solid solutions from the Jakobsberg Mn-Fe deposit, Värmland, Sweden. Mineral. Mag. 2021, 85, 224–232. [Google Scholar] [CrossRef]
- Ringwood, A.E.; Major, A. Some high-pressure transformations of geophysical significance. Earth Planet. Sci. Lett. 1967, 2, 106–110. [Google Scholar] [CrossRef]
- Oganov, A.R.; Hemley, R.J.; Hazen, R.M.; Jones, A.P. Structure, Bonding, and Mineralogy of Carbon at Extreme Conditions. In Carbon in Earth, Reviews in Mineralogy and Geochemistry; Hazen, R.M., Jones, A.P., Baross, J.A., Eds.; The Mineralogical Society of America: Chantilly, VA, USA, 2013; Volume 75, pp. 47–77. [Google Scholar]
- Brenker, F.E.; Vollmer, C.; Vincze, L.; Vekemans, B.; Szymanski, A.; Janssens, K.; Szaloki, I.; Nasdala, L.; Joswig, W.; Kaminsky, F. Carbonates from the lower part of transition zone or even the lower mantle. Earth Planet. Sci. Lett. 2007, 260, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hazen, R.M.; Hemley, R.J.; Mangum, A.J. Carbon in Earth’s Interior: Storage, Cycling, and Life. Eos 2012, 93, 17–18. [Google Scholar] [CrossRef]
- Merlini, M.; Cerantola, V.; Gatta, G.D.; Gemmi, M.; Hanfland, M.; Kupenko, I.; Lotti, P.; Müller, H.; Zhang, L. Dolomite-IV: Candidate structure for a carbonate in the Earth’s lower mantle. Am. Mineral. 2017, 102, 1763–1766. [Google Scholar] [CrossRef]
- Pickard, C.J.; Needs, R.J. Structures and stability of calcium and magnesium carbonates at mantle pressures. Phys. Rev. B 2015, 91, 104101. [Google Scholar] [CrossRef] [Green Version]
- Holland, T.J.B.; Powell, R. An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J. Metamorph. Geol. 2011, 29, 333–383. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals, 2nd ed.; Singlechain Silicates; Geological Society Publishing House: London, UK, 1997; Volume 2. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Hesse, K.-F. Refinement of the crystal structure of wollastonite-2M (parawollastonite). Z. Krist. 1984, 168, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Prewitt, C.T. On the crystal structure of pseudowollastonite (CaSiO3). Am. Mineral. 1999, 84, 929–932. [Google Scholar] [CrossRef]
- Schiavinato, G. Il Giacimento a wollastonite ed altri minerali di contatto presso Alpe Bazena (Adamello meridionale). Mem. Ist. Geol. Padova 1947, 15, 1–63. [Google Scholar]
- Allen, E.T.; White, W.P.; Wright, F.E. On wollastonite and pseudo-wollastonite-polymorphic forms of calcium metasilicate. Am. Jour. Sci. 1906, 21, 89–108. [Google Scholar] [CrossRef]
- Prewitt, C.T. Structure and Crystal Chemistry of Wollastonite and Pectolite. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1962. [Google Scholar]
- Peacock, M.A. On wollastonite and parawollastonite. Am. J. Sci. 1935, 30, 495–529. [Google Scholar] [CrossRef]
- Ito, T. X-ray Studies on Polymorphism; Maruzen: Tokyo, Japan, 1951; pp. 93–110. [Google Scholar]
- Wenk, H.-R. Polymorphism of wollastonite. Contrib. Mineral. Petrol. 1969, 22, 238–247. [Google Scholar] [CrossRef]
- Hutchison, J.L.; McLaren, A.C. Two-dimensional lattice images of stacking disorder in wollastonite. Contrib. Mineral. Petrol. 1976, 55, 303–309. [Google Scholar] [CrossRef]
- Henmi, C.; Kawahara, A.; Henmi, K.; Kusachi, I.; Takeuchi, Y. The 3 T, 4 T and 5 T polytypes of wollastonite from Kushiro, Hiroshima Prefecture, Japan. Am. Mineral. 1983, 68, 156–163. [Google Scholar]
- Seryotkin, Y.V.; Sokol, E.V.; Kokh, S.N. Natural pseudowollastonite: Crystal structure, associated minerals, and geological context. Lithos 2012, 134–135, 75–90. [Google Scholar] [CrossRef]
- Stoppa, F.; Sharygin, V.V. Melilitolite intrusion and pelite digestion by high temperature kamafugitic magma at Colle Fabbri, Spoleto, Italy. Lithos 2009, 112, 306–320. [Google Scholar] [CrossRef]
- Ingrin, J. TEM imaging of polytypism in pseudowollastonite. Phys. Chem. Miner. 1993, 20, 56–62. [Google Scholar] [CrossRef]
- Brenker, F.; Nestola, F.; Brenker, L.; Peruzzo, L.; Secco, L.; Harris, J.W. Breyite, IMA 2018-062, CNMNC Newsletter. Mineral. Mag. 2018, 82, 1225–1232. [Google Scholar]
- Brenker, F.E.; Vincze, L.; Vekemans, B.; Nasdala, L.; Stachel, T.; Vollmer, C.; Kersten, M.; Somogyi, A.; Adams, F.; Joswig, W. Detection of a Ca-rich lithology in the Earth’s deep (>300 km) convecting mantle. Earth Planet. Sci. Lett. 2005, 236, 579–587. [Google Scholar] [CrossRef]
- Anzolini, C.; Angel, R.J.; Merlini, M.; Derzsi, M.; Tokar, K.; Milani, S.; Krebs, M.Y.; Brenker, F.E.; Nestola, F.; Harris, J.W. Depth of formation of CaSiO3 -walstromite included in super-deep diamonds. Lithos 2016, 265, 138–147. [Google Scholar] [CrossRef]
- Brenker, F.E.; Nestola, F.; Brenker, L.; Peruzzo, L.; Harris, J.W. Origin, properties, and structure of breyite: The second most abundant mineral inclusion in super-deep diamonds. Am. Mineral. 2021, 106, 38–43. [Google Scholar] [CrossRef]
- Woodland, A.B.; Girnis, A.V.; Bulatov, V.K.; Brey, G.P.; Hofer, H.E. Breyite inclusions in diamond: Experimental evidence for possible dual origin. Eur. J. Mineral. 2020, 32, 171–185. [Google Scholar] [CrossRef] [Green Version]
- Joswig, W.; Paulus, E.F.; Winkler, B.; Milman, V. The crystal structure of CaSiO3 -walstromite, a special isomorph of wollastonite-II. Z. Krist. 2003, 218, 811–818. [Google Scholar] [CrossRef]
- Bulanova, G.P.; Smith, C.B.; Blundy, J.; Walter, M.J.; Kohn, S.C.; Gobbo, L.; Armstrong, L.S. Mineral inclusions in sublithospheric diamonds from Collier 4 kimberlite pipe, Juina, Brazil: Subducted protoliths, carbonated melts and primary kimberlite magmatism. Contrib. Mineral. Petrol. 2010, 160, 489–510. [Google Scholar] [CrossRef]
- Trojer, F.J. The crystal structure of a high-pressure polymorph of CaSiO3. Z. Krist. 1969, 130, 185–206. [Google Scholar] [CrossRef]
- Barkley, M.C.; Downs, R.T.; Yang, H. Structure of walstromite, BaCa2Si3O9, and its relationship to CaSiO3-walstromite and wollastonite-II. Am. Mineral. 2011, 96, 797–801. [Google Scholar] [CrossRef]
- Hamilton, D.L.; Henderson, C.M.B. The preparation of silicate composition by gelling method. Mineral. Mag. 1968, 36, 832–838. [Google Scholar] [CrossRef]
- Fumagalli, P.; Poli, S. Experimentally determined phase relations in hydrous peridotites to 6.5 GPa and their consequences on the dynamics of subduction zones. J. Petrol. 2005, 46, 555–578. [Google Scholar] [CrossRef]
- Merlini, M.; Hanfland, M. Single crystal diffraction at megabar conditions by synchrotron radiation. High Press. Res. 2013, 33, 511–522. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Chervin, J.C.; Canny, B.; Mancinelli, M. Ruby-spheres as pressure gauge for optically transparent high pressure cells. High Press. Res. 2001, 21, 305–314. [Google Scholar] [CrossRef]
- Klotz, S.; Chervin, J.-C.; Munsch, P.; Le Marchand, G. Hydrostatic limits of 11 pressure transmitting media. J. Phys. D. 2009, 42, 075413. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System; Version 1.171.40.67a (2019); Rigaku Corporation: Wroclaw, Poland, 2019. [Google Scholar]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist. 2014, 229, 345–352. [Google Scholar]
- Angel, R.J.; Gonzales-Platas, J.; Alvaro, M. EoSFit7c and a Fortran module (library) for equation of state calculations. Z. Krist. 2014, 229, 405–419. [Google Scholar] [CrossRef]
- Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Angel, R.J. Equations of state. In High-Temperature and High-Pressure Crystal Chemistry, Reviews in Mineralogy and Geochemistry; Hazen, R.M., Downs, R.T., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 2000; Volume 41, pp. 35–59. [Google Scholar]
- Skoko, Ž.; Zamir, S.; Naumov, P.; Bernstein, J. The Thermosalient Phenomenon. “Jumping Crystals” and Crystal Chemistry of the Anticholinergic Agent Oxitropium Bromide. J. Am. Chem. Soc. 2010, 132, 14191–14202. [Google Scholar] [CrossRef] [PubMed]
- Vaiday, S.N.; Bailey, S.; Pasternack, T.; Kennedy, G.C. Compressibility of fifteen minerals to 45 kilobars. J. Geophys. Res. 1973, 78, 6893–6898. [Google Scholar] [CrossRef]
- Dera, P. All Different Flavors of Synchrotron Single Crystal X-ray Diffraction Experiments. In High-Pressure Crystallography; NATO Science for Peace and Security Series B: Physics and Biophysics; Boldyreva, E., Dera, P., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 11–22. [Google Scholar]
- Rothkirch, A.; Gatta, G.D.; Meyer, M.; Merkel, S.; Merlini, M.; Liermann, H.-P. Single-crystal diffraction at the Extreme Conditions beamline P02.2: Procedure for collecting and analyzing high-pressure single-crystal data. J. Synchrotron Radiat. 2013, 20, 711–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotti, P.; Milani, S.; Merlini, M.; Joseph, B.; Alabarse, F.; Lausi, A. Single-crystal diffraction at the high-pressure Indo-Italian beamline Xpress at Elettra, Trieste. J. Synchrotron Radiat. 2020, 27, 222–229. [Google Scholar] [CrossRef]
- Oganov, A.R.; Glass, C.W.; Ono, S. High-pressure phases of CaCO3: Crystal structure prediction and experiment. Earth Planet. Sci. Lett. 2006, 241, 95–103. [Google Scholar] [CrossRef]
- Boulard, E.; Pan, D.; Galli, G.; Liu, Z.; Mao, W.L. Tetrahedrally coordinated carbonates in Earth’s lower mantle. Nat. Comm. 2015, 6, 6311. [Google Scholar] [CrossRef]
- Cerantola, V.; Bykova, E.; Kupenko, I.; Merlini, M.; Ismailova, L.; McCammon, C.; Bykov, M.; Chumakov, A.I.; Petitgirard, S.; Kantor, I.; et al. Stability of iron-bearing carbonates in the deep Earth’s interior. Nat. Comm. 2017, 8, 15960. [Google Scholar] [CrossRef]





| Phase | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | Space Group | Reference |
|---|---|---|---|---|---|---|---|---|
| wollastonite | 7.079(2) | 7.3309(4) | 7.938(2) | 103.41(2) | 95.30(2) | 90.07 (2) | P | This study |
| wollastonite-2M | 15.409(3) | 7.322(1) | 7.063(1) | 90 | 95.30(2) | 90 | P21/a | [18] |
| pseudowollastonite | 6.8394(5) | 11.8704(9) | 19.6313(7) | 90 | 90.667(6) | 90 | C2/c | [19] |
| CaSiO3-walstromite | 6.6442(4) | 6.6886(4) | 9.282(8) | 69.71(3) | 83.68(2) | 76.21(1) | P | This study |
| P (GPa) | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | V (Å3) |
|---|---|---|---|---|---|---|---|
| wollastonite-I | |||||||
| 0.0001 | 7.079(2) | 7.3309(4) | 7.938(2) | 103.41(2) | 95.30(2) | 90.07(2) | 398.9(2) |
| 1.08 | 7.049(3) | 7.3105(4) | 7.901(3) | 103.44(2) | 95.12(3) | 90.04(2) | 393.3(2) |
| 1.44 | 7.030(3) | 7.3054(4) | 7.882(3) | 103.45(2) | 94.96(3) | 90.04(2) | 392.1(2) |
| 1.78 | 7.022(3) | 7.2990(4) | 7.871(3) | 103.45(2) | 94.92(3) | 90.04(2) | 390.8(2) |
| 2.29 | 7.015(3) | 7.2905(5) | 7.858(3) | 103.46(2) | 94.91(3) | 90.03(2) | 389.3(2) |
| 2.72 | 7.007(3) | 7.2833(4) | 7.850(3) | 103.46(2) | 94.88(3) | 90.01(2) | 388.1(2) |
| 3.45 | 6.991(3) | 7.2717(3) | 7.828(2) | 103.47(2) | 94.77(3) | 90.00(2) | 385.6(2) |
| 3.90 | 6.983(2) | 7.2645(4) | 7.814(2) | 103.48(2) | 94.70(3) | 90.00(2) | 384.1(2) |
| 4.43 | 6.969(2) | 7.2556(4) | 7.797(2) | 103.49(2) | 94.59(3) | 89.99(2) | 382.1(2) |
| 4.86 | 6.958(2) | 7.2478(3) | 7.783(2) | 103.50(2) | 94.50(3) | 89.99(2) | 380.4(2) |
| 5.38 | 6.947(2) | 7.2390(3) | 7.766(2) | 103.52(2) | 94.39(3) | 89.98(2) | 378.6(2) |
| 5.97 | 6.934(2) | 7.2300(3) | 7.750(2) | 103.53(1) | 94.27(2) | 89.97(1) | 376.6(2) |
| 6.62 | 6.922(2) | 7.2202(4) | 7.731(2) | 103.55(2) | 94.11(2) | 89.95(2) | 374.7(2) |
| 7.16 | 6.914(2) | 7.2127(4) | 7.716(2) | 103.58(2) | 94.00(2) | 89.93(2) | 373.1(2) |
| 7.75 | 6.904(2) | 7.2033(3) | 7.699(2) | 103.59(1) | 93.83(2) | 89.94(1) | 371.3(2) |
| 8.29 | 6.895(2) | 7.1945(3) | 7.684(2) | 103.62(1) | 93.66(2) | 89.92(1) | 369.6(3) |
| wollastonite-II | |||||||
| 9.27 | 6.693(2) | 7.2561(3) | 14.927(9) | 76.048(9) | 86.95(2) | 89.951(9) | 702.5(3) |
| 9.76 | 6.696(2) | 7.2461(3) | 14.896(4) | 76.06(1) | 86.94(2) | 89.99(2) | 700.3(3) |
| 9.91 | 6.679(2) | 7.2509(5) | 14.872(2) | 76.012(9) | 87.11(2) | 89.966(9) | 697.9(2) |
| 10.13 | 6.647(2) | 7.2183(4) | 14.786(4) | 76.00(2) | 87.26(2) | 89.98(2) | 687.6(3) |
| 10.42 | 6.671(2) | 7.2430(4) | 14.835(4) | 76.00(2) | 87.21(2) | 89.98(2) | 694.6(3) |
| 10.94 | 6.668(2) | 7.2371(3) | 14.799(2) | 75.977(7) | 87.23(2) | 89.984(7) | 692.1(2) |
| 11.54 | 6.659(2) | 7.2314(3) | 14.762(2) | 75.956(7) | 87.33(2) | 89.990(8) | 688.8(2) |
| 12.06 | 6.653(2) | 7.2245(4) | 14.721(4) | 75.96(1) | 87.42(2) | 90.01(1) | 685.6(3) |
| 12.34 | 6.670(2) | 7.2239(5) | 14.724(4) | 75.94(2) | 87.30(3) | 90.02(2) | 687.4(3) |
| 12.75 | 6.644(2) | 7.2163(2) | 14.673(2) | 75.927(6) | 87.49(2) | 90.001(7) | 681.7(2) |
| 13.21 | 6.638(2) | 7.2108(2) | 14.640(2) | 75.907(6) | 87.56(2) | 90.005(7) | 679.0(2) |
| 13.71 | 6.631(2) | 7.2046(2) | 14.604(2) | 75.887(6) | 87.65(2) | 90.007(7) | 676.0(2) |
| 14.37 | 6.626(2) | 7.1975(3) | 14.568(2) | 75.870(6) | 87.70(2) | 90.015(7) | 673.1(2) |
| 15.11 | 6.620(2) | 7.1899(3) | 14.525(2) | 75.849(7) | 87.79(2) | 90.027(7) | 669.8(2) |
| 15.78 | 6.615(2) | 7.1835(3) | 14.486(2) | 75.831(6) | 87.87(2) | 90.037(8) | 666.9(2) |
| 16.38 | 6.609(2) | 7.1783(3) | 14.449(2) | 75.802(6) | 87.93(2) | 90.038(7) | 664.0(2) |
| 16.98 | 6.604(2) | 7.1733(2) | 14.411(2) | 75.768(6) | 87.99(2) | 90.046(6) | 661.3(2) |
| 17.67 | 6.594(2) | 7.1688(3) | 14.366(2) | 75.729(6) | 88.07(2) | 90.061(7) | 657.7(2) |
| 18.50 | 6.587(2) | 7.1632(3) | 14.313(2) | 75.670(6) | 88.23(2) | 90.038(7) | 654.0(2) |
| 19.20 | 6.578(2) | 7.1587(3) | 14.259(2) | 75.627(6) | 88.44(2) | 90.023(7) | 650.2(2) |
| 19.85 | 6.575(2) | 7.1563(3) | 14.200(2) | 75.556(6) | 88.60(2) | 90.032(7) | 646.8(2) |
| wollastonite-III | |||||||
| 20.39 | 6.571(2) | 14.142(2) | 14.3107(5) | 75.488(6) | 89.967(7) | 91.22(2) | 1287.1(3) |
| 20.98 | 6.568(2) | 14.012(2) | 14.3262(5) | 75.324(6) | 89.834(7) | 90.81(2) | 1275.3(3) |
| 22.11 | 6.561(2) | 13.925(2) | 14.3264(5) | 75.237(6) | 89.789(7) | 90.61(2) | 1265.5(3) |
| 22.29 | 6.564(2) | 13.910(3) | 14.3224(6) | 75.222(7) | 89.850(7) | 90.62(2) | 1264.3(3) |
| Phase | Wollastonite-I | Wollastonite-II | Wollastonite-III |
|---|---|---|---|
| a (Å) | 7.079(2) | 6.693(2) | 6.571(2) |
| b (Å) | 7.3309(4) | 7.2561(3) | 14.142(2) |
| c (Å) | 7.938(2) | 14.9270(3) | 14.3107(5) |
| α (°) | 103.41(2) | 76.048(9) | 75.488(6) |
| β (°) | 95.29(2) | 86.95(2) | 89.967(7) |
| γ (°) | 90.07(2) | 89.951(9) | 91.22(2) |
| V (Å3) | 398.9(2) | 702.5(3) | 1287.1(3) |
| P (GPa) | 0.0001(1) | 9.27(5) | 20.39(5) |
| Space group | P | P | P |
| λ (Å) | 0.41105 | 0.41105 | 0.41105 |
| θ max (°) | 18.51 | 18.43 | 18.42 |
| No. measured reflections | 1216 | 2206 | 12838 |
| No. unique reflections | 766 | 1426 | 3184 |
| No. refined parameters | 61 | 121 | 241 |
| Rint | 0.028 | 0.0488 | 0.1711 |
| R1 (F) | 0.0493 | 0.0657 | 0.0816 |
| wR2 (F2) | 0.0604 | 0.0790 | 0.0882 |
| GooF | 3.74 | 3.92 | 3.47 |
| Residuals (e−/Å3) | −5.10/+10.36 | −1.40/+1.42 | −2.41/+7.54 |
| P (GPa) | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | V (Å3) |
|---|---|---|---|---|---|---|---|
| CaSiO3-walstromite-I | |||||||
| 0.0001 | 6.6442(3) | 6.6886(4) | 9.282(8) | 69.71(3) | 83.68(2) | 76.205(5) | 375.6(3) |
| 1.39 d | 6.605(3) | 6.6457(6) | 9.230(4) | 69.99(3) | 83.45(4) | 76.41(3) | 369.8(2) |
| 1.71 d | 6.591(2) | 6.6347(5) | 9.213(3) | 70.03(2) | 83.45(3) | 76.45(2) | 367.9(2) |
| 2.60 d | 6.573(2) | 6.6109(6) | 9.186(3) | 70.14(2) | 83.28(3) | 76.55(2) | 364.8(2) |
| 3.45 d | 6.550(2) | 6.5911(7) | 9.156(3) | 70.21(2) | 83.25(2) | 76.63(2) | 361.5(2) |
| 4.76 d | 6.514(2) | 6.5592(8) | 9.114(3) | 70.28(2) | 83.16(2) | 76.73(2) | 356.4(2) |
| 6.02 | 6.4908(3) | 6.5385(3) | 9.087(5) | 70.28(2) | 83.03(2) | 76.804(5) | 353.1(2) |
| 6.60 d | 6.468(2) | 6.5156(7) | 9.061(3) | 70.36(2) | 82.97(3) | 76.87(2) | 349.8(2) |
| 6.97 | 6.4602(3) | 6.5084(3) | 9.018(7) | 70.34(3) | 82.89(2) | 76.874(5) | 347.3(3) |
| 7.68 | 6.4445(4) | 6.4935(3) | 9.009(6) | 70.38(2) | 82.82(2) | 76.919(5) | 345.4(3) |
| 8.48 | 6.4273(3) | 6.4777(3) | 9.007(5) | 70.41(2) | 82.77(2) | 76.970(4) | 343.7(2) |
| CaSiO3-walstromite-II | |||||||
| 7.84 d | 10.2206(9) | 7.9369(9) | 8.6213(7) | 90 | 105.217(9) | 90 | 674.8(4) |
| 8.77 d | 10.1897(2) | 7.9217(2) | 8.5966(2) | 90 | 105.137(2) | 90 | 669.8(4) |
| 9.37 | 10.1649(2) | 7.9143(3) | 8.5806(2) | 90 | 105.098(2) | 90 | 666.5(5) |
| 9.37 d | 10.1707(4) | 7.9119(5) | 8.5811(3) | 90 | 105.087(4) | 90 | 666.7(4) |
| 9.75 d | 10.1613(2) | 7.9078(2) | 8.5745(2) | 90 | 105.057(2) | 90 | 665.3(3) |
| 10.23 | 10.1432(3) | 7.9020(3) | 8.5629(2) | 90 | 104.9817(2) | 90 | 663.0(4) |
| 11.10 d | 10.1208(4) | 7.8892(4) | 8.5429(3) | 90 | 104.926(4) | 90 | 659.1(3) |
| 11.13 | 10.1155(4) | 7.8877(5) | 8.5405(2) | 90 | 104.907(2) | 90 | 658.5(4) |
| 12.05 d | 10.0924(2) | 7.8725(2) | 8.5205(2) | 90 | 104.856(2) | 90 | 654.3(4) |
| 12.08 | 10.0892(2) | 7.8729(3) | 8.51979(9) | 90 | 104.832(2) | 90 | 654.2(4) |
| CaSiO3-walstromite-III | |||||||
| 12.54 d | 8.5111(2) | 7.8542(3) | 10.0964(3) | 90 | 104.886(3) | 90 | 652.3(4) |
| 13.05 | 8.4985(2) | 7.8294(2) | 10.0985(2) | 90 | 104.907(2) | 90 | 649.3(4) |
| 13.88 d | 8.4813(2) | 7.8018(3) | 10.0916(3) | 90 | 104.926(2) | 90 | 645.2(4) |
| 13.98 | 8.4769(3) | 7.7959(4) | 10.0874(4) | 90 | 104.915(4) | 90 | 644.2(4) |
| 14.59 d | 8.4636(3) | 7.7752(4) | 10.0787(3) | 90 | 104.925(3) | 90 | 640.9(4) |
| 15.26 | 8.4534(2) | 7.7631(3) | 10.0734(3) | 90 | 104.904(3) | 90 | 638.8(4) |
| 15.90 | 8.4396(2) | 7.7443(2) | 10.0622(2) | 90 | 104.895(2) | 90 | 635.6(3) |
| 15.93 d | 8.4419(2) | 7.7435(2) | 10.0682(2) | 90 | 104.909(2) | 90 | 636.0(3) |
| 16.52 d | 8.4306(2) | 7.7280(3) | 10.0587(3) | 90 | 104.892(3) | 90 | 633.3(3) |
| 16.57 | 8.4288(2) | 7.7282(2) | 10.0544(2) | 90 | 104.892(2) | 90 | 632.9(3) |
| 17.19 | 8.4187(2) | 7.7133(2) | 10.0479(2) | 90 | 104.882(2) | 90 | 630.6(3) |
| 17.32 d | 8.4196(2) | 7.7112(3) | 10.0512(3) | 90 | 104.886(3) | 90 | 630.7(3) |
| 17.86 | 8.4078(2) | 7.6983(2) | 10.0405(2) | 90 | 104.873(2) | 90 | 628.1(3) |
| 18.02 d | 8.4014(3) | 7.6884(4) | 10.0342(4) | 90 | 104.885(4) | 90 | 626.4(3) |
| 18.83 | 8.3921(3) | 7.6763(4) | 10.0280(4) | 90 | 104.867(4) | 90 | 624.4(3) |
| 19.07 d | 8.3860(3) | 7.6658(5) | 10.0214(5) | 90 | 104.859(4) | 90 | 622.7(3) |
| 19.59 | 8.3815(3) | 7.6598(4) | 10.0196(4) | 90 | 104.857(4) | 90 | 621.8(3) |
| 19.61 d | 8.3786(3) | 7.6551(4) | 10.0163(4) | 90 | 104.851(4) | 90 | 621.0(3) |
| 20.15 | 8.3756(3) | 7.6512(4) | 10.0147(4) | 90 | 104.851(4) | 90 | 620.3(3) |
| Phase | CaSiO3-Walstromite-I | CaSiO3-Walstromite-II | CaSiO3-Walstromite-III |
|---|---|---|---|
| a (Å) | 6.6442(3) | 10.2206(9) | 8.5111(2) |
| b (Å) | 6.6886(4) | 7.9369(9) | 7.8542(3) |
| c (Å) | 9.282(8) | 8.6213(7) | 10.0964(3) |
| α (°) | 69.71(3) | 90 | 90 |
| β (°) | 83.68(2) | 105.217(9) | 104.886(3) |
| γ (°) | 76.205(5) | 90 | 90 |
| V (Å3) | 375.6(3) | 674.8(4) | 652.3(4) |
| P (GPa) | 0.0001(1) | 9.37(5) | 12.54(5) |
| Space group | P | C2/m | P21/c |
| λ (Å) | 0.41105 | 0.41105 | 0.41105 |
| θ max (°) | 19.18 | 19.76 | 19.11 |
| No. measured reflections | 890 | 699 | 1219 |
| No. unique reflections | 593 | 460 | 732 |
| No. refined parameters | 61 | 37 | 61 |
| Rint | 0.0301 | 0.083 | 0.047 |
| R1 (F) | 0.0582 | 0.0498 | 0.0415 |
| wR2 (F2) | 0.0632 | 0.0594 | 0.0489 |
| GooF | 3.69 | 4.05 | 2.70 |
| Residuals (e−/Å3) | −2.23/+5.77 | −4.17/+5.29 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milani, S.; Comboni, D.; Lotti, P.; Fumagalli, P.; Ziberna, L.; Maurice, J.; Hanfland, M.; Merlini, M. Crystal Structure Evolution of CaSiO3 Polymorphs at Earth’s Mantle Pressures. Minerals 2021, 11, 652. https://doi.org/10.3390/min11060652
Milani S, Comboni D, Lotti P, Fumagalli P, Ziberna L, Maurice J, Hanfland M, Merlini M. Crystal Structure Evolution of CaSiO3 Polymorphs at Earth’s Mantle Pressures. Minerals. 2021; 11(6):652. https://doi.org/10.3390/min11060652
Chicago/Turabian StyleMilani, Sula, Davide Comboni, Paolo Lotti, Patrizia Fumagalli, Luca Ziberna, Juliette Maurice, Michael Hanfland, and Marco Merlini. 2021. "Crystal Structure Evolution of CaSiO3 Polymorphs at Earth’s Mantle Pressures" Minerals 11, no. 6: 652. https://doi.org/10.3390/min11060652
APA StyleMilani, S., Comboni, D., Lotti, P., Fumagalli, P., Ziberna, L., Maurice, J., Hanfland, M., & Merlini, M. (2021). Crystal Structure Evolution of CaSiO3 Polymorphs at Earth’s Mantle Pressures. Minerals, 11(6), 652. https://doi.org/10.3390/min11060652







