Swelling Pressure and Permeability of Compacted Bentonite from 10th Khutor Deposit (Russia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geological Site and Composition of 10X
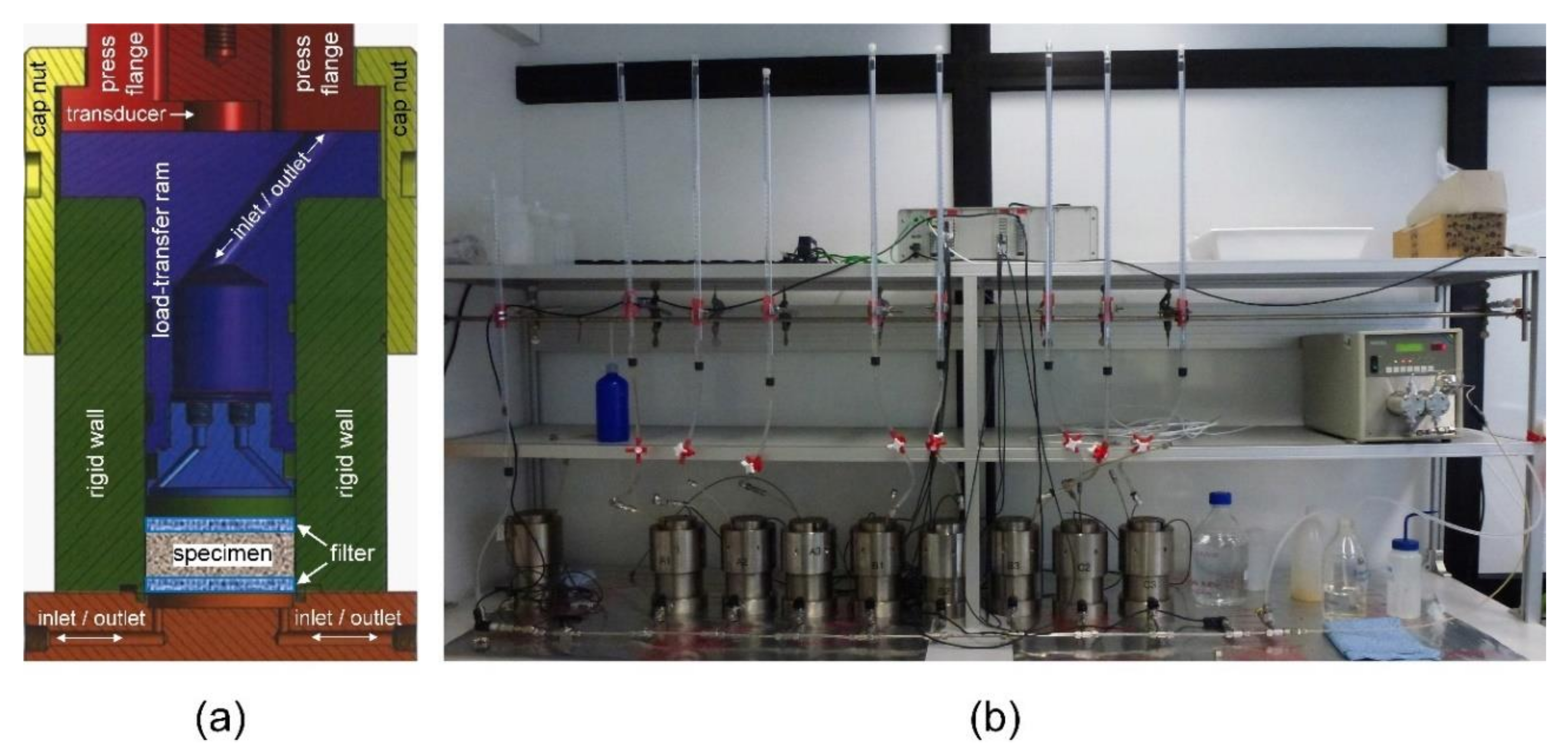
2.2. Experiments and Conditions
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abramov, A.A.; Bolshov, L.A.; Dorofeeev, A.N.; Igin, I.M.; Kazakov, K.S.; Krasilnikov, V.Y.; Linge, I.I.; Trokhov, N.N.; Utkin, S.S. Underground research laboratory in the Nizhnekanskiy massif: Evolutionary design study. Radioact. Waste 2020, 1, 9–21. (In Russian) [Google Scholar] [CrossRef]
- Dorofeev, A.N.; Bolshov, L.A.; Linge, I.I.; Utkin, S.S.; Saveleva, E.A. Strategic master-plan for research demonstrating the safety of construction, operation and closure of a deep geological repository for radioactive waste. Radioact. Waste 2017, 1, 19–26. [Google Scholar]
- Krupskaya, V.V.; Biryukov, D.V.; Belousov, P.E.; Lekhov, V.A.; Romanchuk, A.Y.; Kalmykov, S.N. The use of natural clay materials to increase the nuclear and radiation safety level of nuclear legacy facilities. Radioact. Waste 2018, 2, 30–43. [Google Scholar]
- Krupskaya, V.V.; Zakusin, S.V.; Lekhov, V.A.; Dorzhieva, O.V.; Belousov, P.E.; Tyupina, E.A. Buffer properties of bentonite barrier systems for radioactive waste isolation in geological repository in the Nizhnekanskiy Massif. Radioact. Waste 2020, 1, 35–55. [Google Scholar] [CrossRef]
- Birgersson, M.; Hedström, M.; Karnland, O.; Sjöland, A. Bentonite buffer: Macroscopic performance from nanoscale properties. In Geological Repository Systems for Safe Disposal of Spent Nuclear Fuels and Radioactive Waste, 2nd ed.; Michael, J.A., Joonhong, A., Eds.; Woodhead Publishing: Duxford, UK, 2017; Volume 12, pp. 319–364. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R. Distinguishing between more and less suitable bentonites for storage of high-level radioactive waste. Clay Miner. 2016, 51, 289–302. [Google Scholar] [CrossRef] [Green Version]
- Sellin, P.; Leupin, O.X. The use of clay as an engineered barrier in radioactive-waste management—A review. Clays Clay Miner. 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Belousov, P.E.; Krupskaya, V.V. Bentonite clays of Russia and neighboring countries. Georesursy 2019, 21, 79–90. [Google Scholar] [CrossRef]
- Belousov, P.E.; Krupskaya, V.V.; Zakusin, S.V.; Zhigarev, V.V. Bentonite clays from 10th Khutor deposite: Features of genesis, composition and adsorption properties. RUDN J. Eng. Res. 2017, 18, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Belousov, P.; Chupalenkov, N.; Zakusin, S.; Morozov, I.; Dorzhieva, O.; Chernov, M.; Krupskaya, V. 10th Khutor bentonite deposit (Russia): Geology, mineralogy and industrial application. Appl. Clay Sci. 2021, in press. [Google Scholar]
- Belousov, P.E.; Pokidko, B.V.; Zakusin, S.V.; Krupskaya, V.V. Quantitative methods for quantification of montmorillonite content in bentonite clays. Georesursy 2020, 22, 38–47. [Google Scholar] [CrossRef]
- Post, J.E.; Bish, D.L. Rietveld refinement of crystal structures using powder X-ray diffraction data. Rev. Miner. Geochem. 1989, 20, 277–308. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GOST 5180-2015 Soils. Laboratory Methods for Determination of Physical Characteristics; Standartinform: Moscow, Russia, 2019. [Google Scholar]
- ASTM D854-14. Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Lorenz, P.; Meier, L.; Kahr, G. Determination of the cation exchange capacity (CEC) of clays minerals using the complexes of copper (II) ion with triethylenetetramine and tetraethylenepentamine. Clays Clay Miner. 1999, 47, 386–388. [Google Scholar]
- Dohrmann, R.; Genske, D.; Karnland, O.; Kaufhold, S.; Kiviranta, L.; Olsson, S.; Plötze, M.; Sandén, T.; Sellin, P.; Svensson, D.; et al. Interlaboratory CEC and exchangeable cation study of bentonite buffer materials: I. Cu(II)-triethylenetetramine method. Clays Clay Miner. 2012, 60, 162–175. [Google Scholar] [CrossRef]
- Stanjek, H.; Künkel, D. CEC determination with Cu-triethylenetetramine: Recommendations for improving reproducibility and accuracy. Clay Miner. 2016, 51, 1–17. [Google Scholar] [CrossRef]
- Rozov, K.B.; Rumynin, V.G.; Nikulenkov, A.M.; Leskova, P.G. Sorption of 137Cs, 90Sr, Se, 99Tc, 152(154)Eu, 239(240)Pu on fractured rocks of the Yeniseysky site (Nizhne-Kansky massif, Krasnoyarsk region, Russia). J. Environ. Radioact. 2018, 192, 513–523. [Google Scholar] [CrossRef]
- Meleshyn, A.Y. Swelling behaviour and permeability of compacted bentonites at high salinity as influenced by fluid pressure changes. Radioact. Waste 2020, 2, 109–119, (In Russian; the English version is in press). [Google Scholar] [CrossRef]
- Meleshyn, A. Mechanisms of Transformation of Bentonite Barriers (UMB); Report GRS-390 BMWi-FKZ 02 E 11344A.; GRS: Braunschweig, Germany, 2019; Available online: https://www.grs.de/publikationen/grs-390 (accessed on 30 June 2021).
- Yigzaw, Z.G.; Cuisinier, O.; Massat, L.; Masrouri, F. Role of different suction components on swelling behavior of compacted bentonites. Appl. Clay Sci. 2016, 120, 81–90. [Google Scholar] [CrossRef]
- Massat, L.; Cuisinier, O.; Bihannic, I.; Claret, F.; Pelletier, M.; Masrouri, F.; Gaboreau, S. Swelling pressure development and inter-aggregate porosity evolution upon hydration of a compacted swelling clay. Appl. Clay Sci. 2016, 124, 197–210. [Google Scholar] [CrossRef]
- Zhu, C.M.; Ye, W.M.; Chen, Y.G.; Chen, B.; Cui, Y.J. Influence of salt solutions on the swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite. Eng. Geol. 2013, 166, 74–80. [Google Scholar] [CrossRef]
- Ye, W.M.; Wan, M.; Chen, B.; Chen, Y.G.; Cui, Y.J.; Wang, J. Temperature effects on the swelling pressure and saturated hydraulic conductivity of the compacted GMZ01 bentonite. Environ. Earth Sci. 2013, 68, 281–288. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, W.M.; Wang, Q.; Chen, Y.G.; Chen, B. An insight into the swelling pressure of GMZ01 bentonite with consideration of salt solution effects. Eng. Geol. 2019, 251, 190–196. [Google Scholar] [CrossRef]
- Chapuis, R.P. Sand–bentonite liners: Predicting permeability from laboratory tests. Can. Geotech. J. 1990, 27, 47–57. [Google Scholar] [CrossRef]
- Pane, V.; Croce, P.; Znidarcic, D.; Ko, H.Y.; Olsen, H.W.; Schiffman, R.L. Effects of consolidation on permeability measurements for soft clay. Geotechnique 1983, 33, 67–72. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, A.M.; Cui, Y.J.; Delage, P.; Gatmiri, B. Experimental study on the swelling behaviour of bentonite/claystone mixture. Eng. Geol. 2012, 124, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Olsen, H.W.; Nichols, R.W.; Rice, T.L. Low gradient permeability measurements in a triaxial system. Geotechnique 1985, 35, 145–157. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, Y.J.; Tang, A.M.; Barnichon, J.D.; Saba, S.; Ye, W.M. Hydraulic conductivity and microstructure changes of compacted bentonite/sand mixture during hydration. Eng. Geol. 2013, 164, 67–76. [Google Scholar] [CrossRef]
- Osipov, V.I.; Sokolov, V.N. Clays and Its Properties. In Composition, Structure and Properties Formation; GEOS: Moscow, Russia, 2013; p. 576. ISBN 978-5-89118-617-0. [Google Scholar]
- Osipov, V.I.; Babak, V.G. The nature and mechanism of clay swelling. Eng. Geol. 1987, 5, 18–27. [Google Scholar]
- Al-Taie, L.; Pusch, R.; Knutsson, S. Hydraulic properties of smectite rich clay controlled by hydraulic gradients and filter types. Appl. Clay Sci. 2014, 87, 73–80. [Google Scholar] [CrossRef]
- Mondol, N.H.; Bjørlykke, K.; Jahren, J. Experimental compaction of clays: Relationship between permeability and petrophysical properties in mudstones. Pet. Geosci. 2008, 14, 319–337. [Google Scholar] [CrossRef]








| a—Particle Size Distribution | ||||||||||||
| Mods, µm | Median, µm | Particle Size Distribution, % | ||||||||||
| 0.1–1 µm | 1–10 µm | 10–25 µm | ||||||||||
| 1.79 | 2.90 | 15.6 | 72.6 | 11.8 | ||||||||
| b—Mineral Composition (%) | ||||||||||||
| Smectite (Montmorillonite) | Chlorite | Quartz | Feldspars (Microcline and Albite) | Calcite | Anatase | Gypsum | ||||||
| 73.0 | 0.9 | 13.9 | 10.8 | 0.7 | 0.3 | 0.4 | ||||||
| c—Chemical Composition | ||||||||||||
| Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | Fe2O3 | FeO | MnO | LOI (105–1000 °C) | ||
| 0.107 | 2.67 | 18.84 | 51.87 | 0.48 | 6.58 | 0.70 | 3.37 | 0.33 | 0.01 | 7.64 | ||
| iH, m/m | σ, MPa | κ, 10−20 m2 | ||||
|---|---|---|---|---|---|---|
| 1.4 g/cm3 | 1.6 g/cm3 | 1.8 g/cm3 | 1.4 g/cm3 | 1.6 g/cm3 | 1.8 g/cm3 | |
| 180 | 0.7 ± 0.2 | 1.5 ± 0.6 | 5.3 ± 0.9 | - | - | - |
| 2000 | 0.8 ± 0.3 | 2.2 ± 0.6 | 6.3 ± 0.3 | 27 ± 15 | 3.4 ± 0.8 | 0.96 ± 0.26 |
| 5000 | 0.9 ± 0.3 | 2.3 ± 0.6 | 6.4 ± 0.3 | 25 ± 14 | 3.2 ± 0.4 | 0.54 ± 0.10 |
| 20,000 | 2.1 ± 0.2 | 3.1 ± 0.6 | 6.9 ± 0.3 | 11 ± 4 | 2.9 ± 0.4 | 0.68 ± 0.02 |
| 80,000 | 7.6 ± 0.1 | 8.3 ± 0.8 | 9.7 ± 0.3 | 5.4 ± 1.5 | 2.0 ± 0.2 | 0.67 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meleshyn, A.Y.; Zakusin, S.V.; Krupskaya, V.V. Swelling Pressure and Permeability of Compacted Bentonite from 10th Khutor Deposit (Russia). Minerals 2021, 11, 742. https://doi.org/10.3390/min11070742
Meleshyn AY, Zakusin SV, Krupskaya VV. Swelling Pressure and Permeability of Compacted Bentonite from 10th Khutor Deposit (Russia). Minerals. 2021; 11(7):742. https://doi.org/10.3390/min11070742
Chicago/Turabian StyleMeleshyn, Artur Yu., Sergey V. Zakusin, and Victoria V. Krupskaya. 2021. "Swelling Pressure and Permeability of Compacted Bentonite from 10th Khutor Deposit (Russia)" Minerals 11, no. 7: 742. https://doi.org/10.3390/min11070742
APA StyleMeleshyn, A. Y., Zakusin, S. V., & Krupskaya, V. V. (2021). Swelling Pressure and Permeability of Compacted Bentonite from 10th Khutor Deposit (Russia). Minerals, 11(7), 742. https://doi.org/10.3390/min11070742







