Selection of an Appropriate Depressant in Flotation Separation of Molybdenum Oxide from Fluorapatite
Abstract
:1. Introduction
2. Experimental
2.1. Samples and Reagents
2.2. Methods
2.2.1. Flotation Experiments
2.2.2. Adsorption Studies
2.2.3. Infrared Spectroscopic Analysis
2.2.4. Determination of Zeta Potentials
3. Results and Discussion
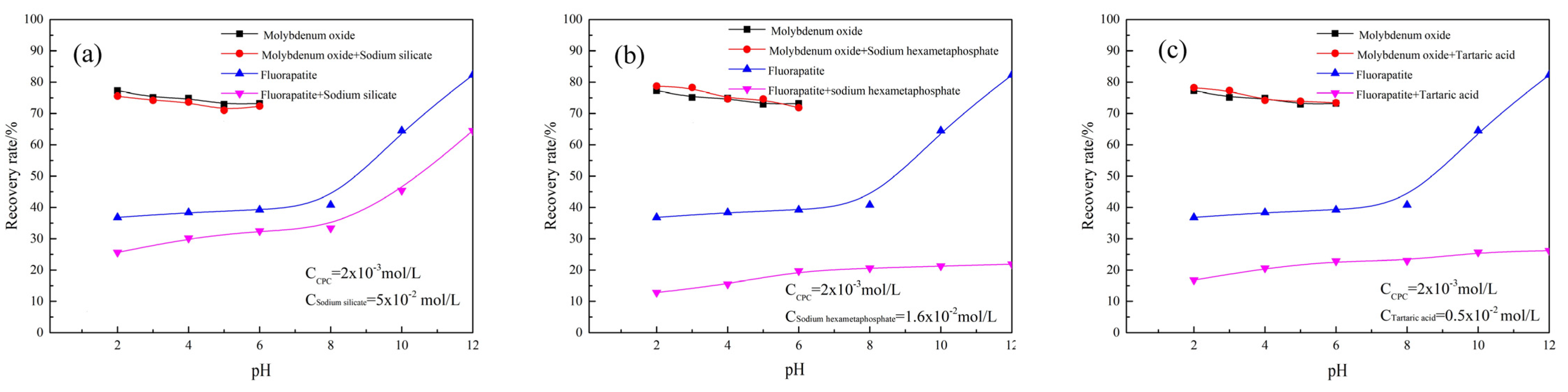
3.1. Floatability Tests of Minerals
3.2. Solubility Equilibrium Analysis of Fluorapatite
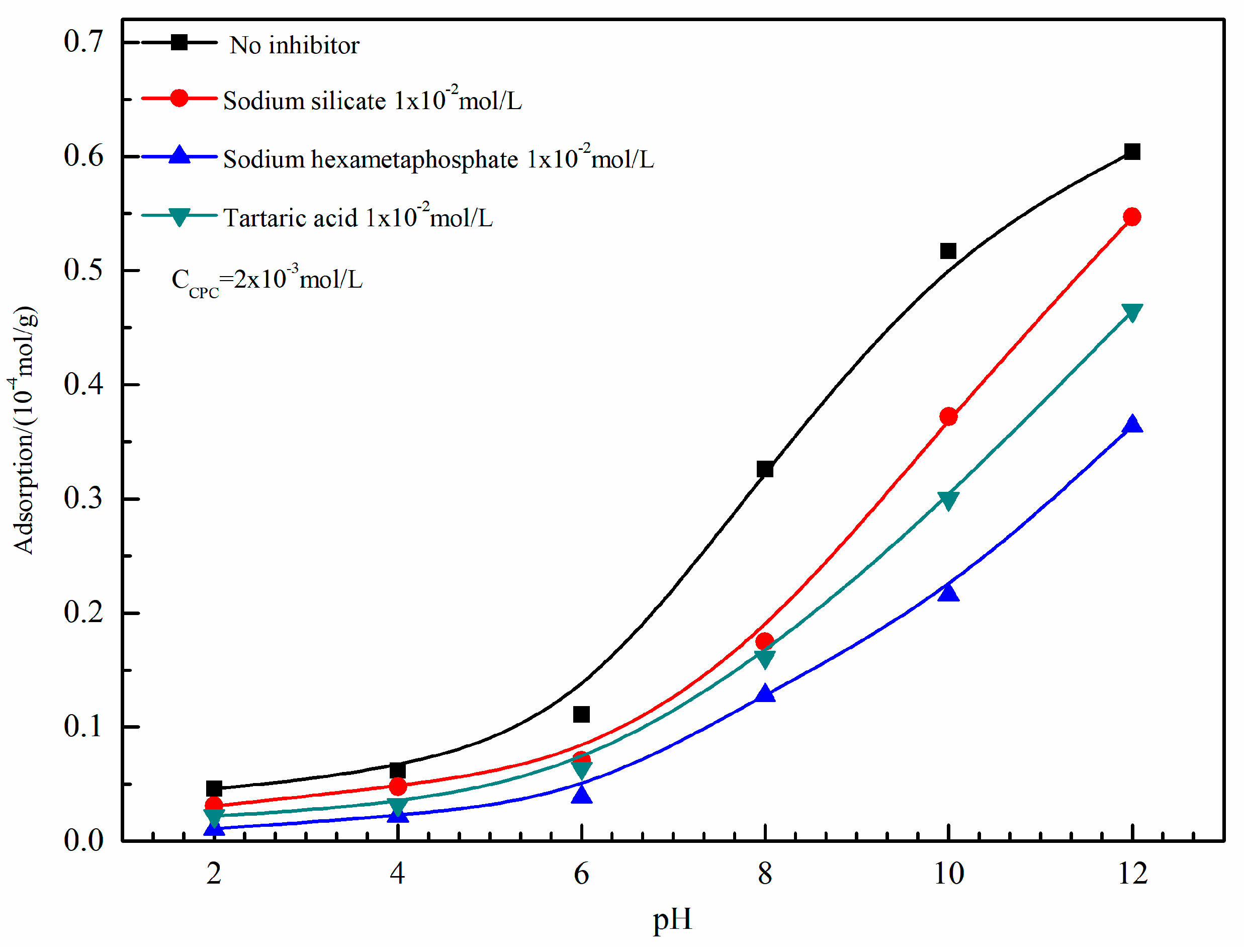
3.3. Adsorption Studies
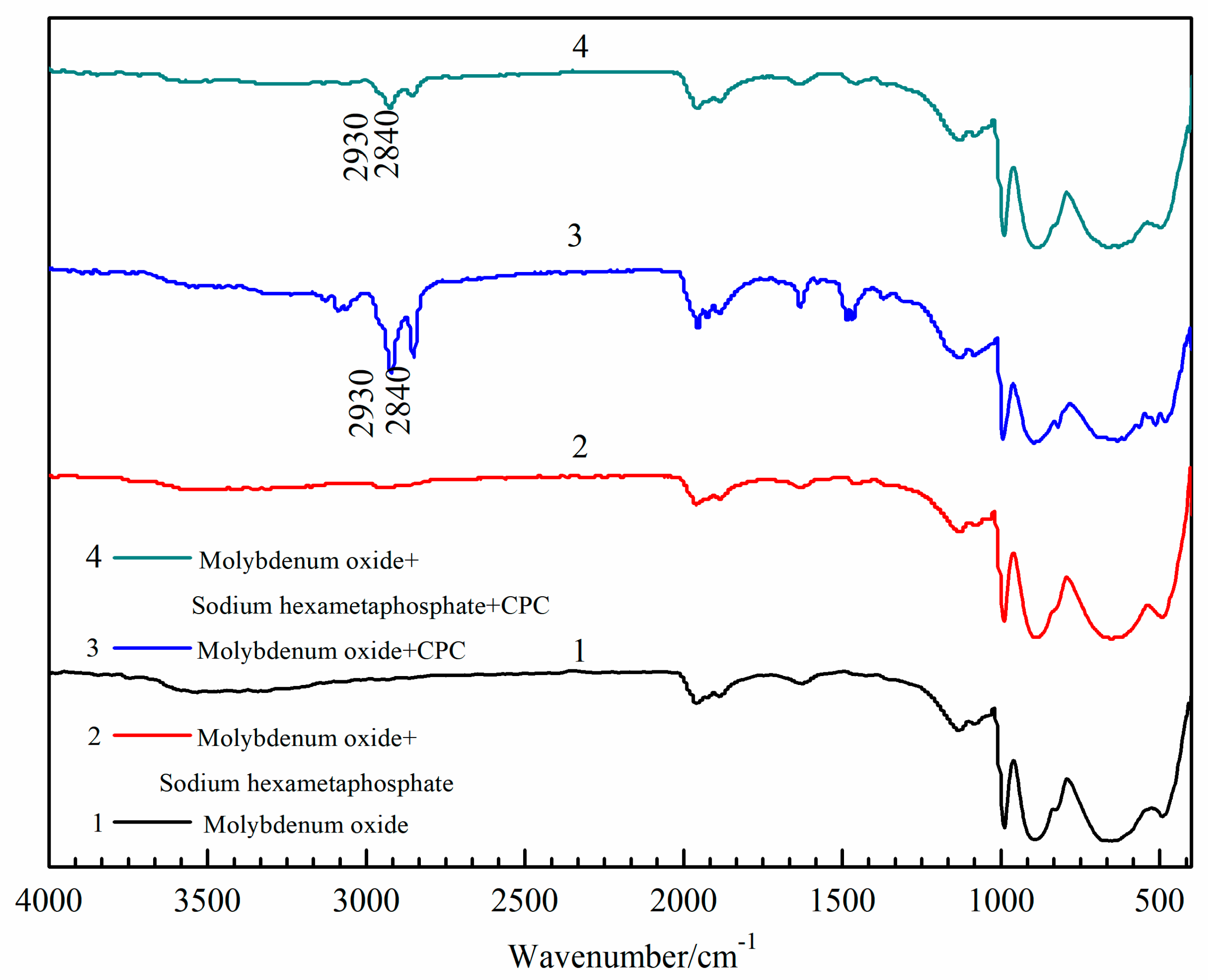
3.4. Infrared Spectroscopic Measurement
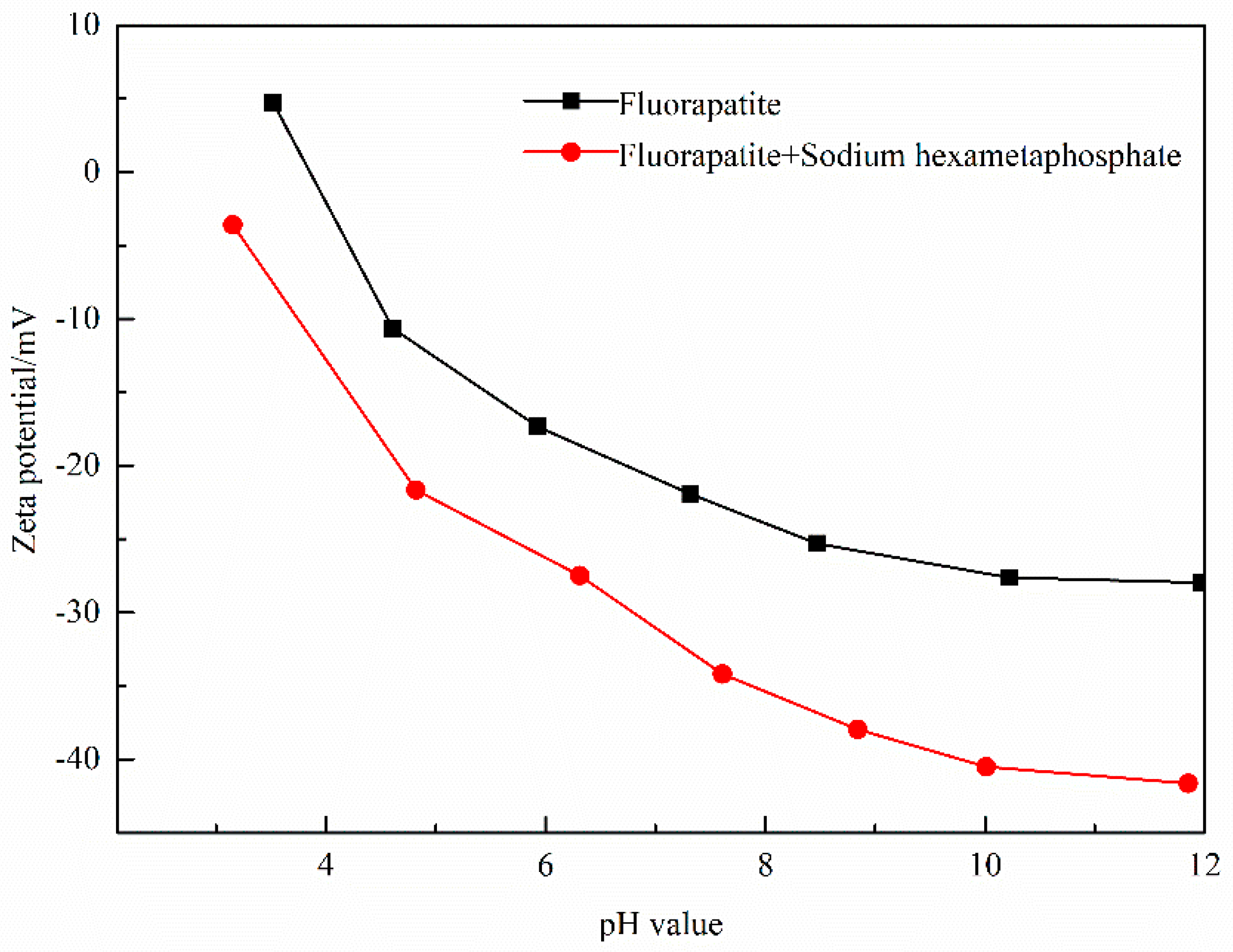
3.5. Zeta Potential Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, Z.; Li, J.; Cao, C.; Huo, G.; Zhang, G.; Li, H. Recovery and purification of molybdenum from Ni-Mo ore by direct air oxidation in alkaline solution. Hydrometallurgy 2010, 103, 68–73. [Google Scholar] [CrossRef]
- Fan, D.; Zhang, T.; Ye, J.; Pašava, J.; Kribek, B.; Dobes, P.; Varrin, I.; Zak, K. Geochemistry and origin of tin–polymetallic sulfide deposits hosted by the Devonian black shale series near Dachang, Guangxi, China. Ore Geol. Rev. 2004, 24, 103–120. [Google Scholar] [CrossRef]
- Wang, S.-F.; Wei, C.; Deng, Z.-G.; Li, C.-X.; Li, X.-B.; Wu, J.; Wang, M.-S.; Zhang, F. Extraction of molybdenum and nickel from Ni–Mo ore by pressure acid leaching. Trans. Nonferr. Metal. Soc. 2013, 23, 3083–3088. [Google Scholar] [CrossRef]
- Liu, J.D.; Sun, W. Flotation technology and adsorption mechanism of collector CSU-M to molybdenum oxide in Ni-Mo ore. J. Cent. South Univ. 2014, 45, 4105–4110. [Google Scholar]
- Peng, J.; Wang, X.; Jiang, C.; Wang, M.; Ma, Y.; Xiang, X. Separation of Mo(VI) and Fe(III) from the acid leaching solution of carbonaceous Ni-Mo ore by ion exchange. Hydrometallurgy 2014, 142, 116–120. [Google Scholar] [CrossRef]
- Xian, P.-F.; Zhou, S.-F.; Wang, M.-Y.; Wang, X.-W.; Chen, B.-F. Extraction of molybdenum and nickel from roasted Ni–Mo ore by hydrochloric acid leaching, sulphation roasting and water leaching. Trans. Nonferr. Metal. Soc. 2017, 27, 220–226. [Google Scholar] [CrossRef]
- Wang, M.-S.; Wei, C.; Fan, G.; Deng, Z.-G.; Wang, S.-F.; Wu, J. Molybdenum recovery from oxygen pressure water leaching residue of Ni–Mo ore. Rare Met. 2013, 32, 208–212. [Google Scholar] [CrossRef]
- Deng, Z.G.; Bai, J.Y.; Wei, C.; Fan, G.; Li, X.; Li, M.; Li, C. Pressure-Leaching Behavior of Nickel from Ni-Mo Ore in Aqueous Oxygenated Media. Int. J. Chem. React. Eng. 2018, 16, 20170209. [Google Scholar]
- Gadd, M.G.; Peter, J.; Jackson, S.E.; Yang, Z.; Petts, D. Platinum, Pd, Mo, Au and Re deportment in hyper-enriched black shale Ni-Zn-Mo-PGE mineralization, Peel River, Yukon, Canada. Ore Geol. Rev. 2019, 107, 600–614. [Google Scholar] [CrossRef]
- Orberger, B.; Vymazalova, A.; Wagner, C.; Fialin, M.; Gallien, J.P.; Wirth, R.; Pasava, J.; Montagnac, G. Biogenic origin of intergrown Mo-sulphide and carbonaceous matter in Lower Cambrian black shales (Zunji Formation, S China). Chem. Geol. 2007, 238, 213–231. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.M.; Peng, Y. The effect of sodium hydrosulfide on molybdenite flotation in seawater and diluted seawater. Miner. Eng. 2020, 158, 106589. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, L.S.; Xiao, C.L.; Gong, B.F. Study on leaching of molybdenum and nickel from Ni–Mo ore using sodium chlorate. Can. Metall. Quart. 2013, 52, 335–341. [Google Scholar] [CrossRef]
- Wang, M.; Chen, B.; Huang, S.; Yang, H.; Hu, B.; Zhang, C.; Wang, X.; Xu, Y. Extraction of molybdenum and nickel from Ni-Mo ore by acid leaching combined with chlorate oxidation and phosphate complexation. Miner. Eng. 2018, 124, 63–67. [Google Scholar] [CrossRef]
- Xu, L.; Lehmann, B.; Mao, J. Seawater contribution to polymetallic Ni–Mo–PGE–Au mineralization in Early Cambrian black shales of South China: Evidence from Mo isotope, PGE, trace element, and REE geochemistry. Ore Geol. Rev. 2013, 52, 66–84. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Wei, Q. Utilization of iron ions to improve the depressive efficiency of tartaric acid on the flotation separation of scheelite from calcite. Miner. Eng. 2021, 168, 106925. [Google Scholar] [CrossRef]
- Abdel-Khalek, N.A. Evaluation of flotation strategies for sedimentary phosphates with siliceous and carbonates gangues. Miner. Eng. 2000, 13, 793. [Google Scholar] [CrossRef]
- Chander, H.S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar]
- Yepsen, R.; Roa, J.; Toledo, P.; Gutiérrez, L. Chalcopyrite and Molybdenite Flotation in Seawater: The Use of Inorganic Dispersants to Reduce the Depressing Effects of Micas. Minerals 2021, 11, 539. [Google Scholar] [CrossRef]
- Rammal, M.B.; Omanovic, S. Synthesis and characterization of NiO, MoO3, and NiMoO4 nanostructures through a green, facile method and their potential use as electrocatalysts for water splitting. Mater. Chem. Phys. 2020, 255, 123570. [Google Scholar] [CrossRef]
- Hu, H.; Deng, C.; Xu, J.; Zhang, K.; Sun, M. Metastableh-MoO3 and stableα-MoO3 microstructures: Controllable synthesis, growth mechanism and their enhanced photocatalytic activity. J. Exp. Nanosci. 2015, 10, 1336–1346. [Google Scholar] [CrossRef]
- Lei, Z.; Cagnetta, G.; Li, X.; Qu, J.; Li, Z.; Zhang, Q.; Huang, J. Enhanced adsorption of potassium nitrate with potassium cation on H3PO4 modified kaolinite and nitrate anion into Mg-Al layered double hydroxide. Appl. Clay Sci. 2018, 154, 10–16. [Google Scholar] [CrossRef]



| Chemical Equation | Equilibrium Constant (log k) |
|---|---|
| Ca10(PO4)6(F)2 + 6H+ = 10 Ca2+ + 6PO43− + 2F− | 11.8 |
| Ca2+ + H2PO4− = CaH2PO4+ | 1.1 |
| Ca2+ + HPO42− = CaHPO4(aq) | 2.7 |
| CaHPO4(S) = Ca2+ + HPO42− | −7.0 |
| Ca2+ + 2OH− = Ca(OH)2(aq) | 2.77 |
| Ca2+ + OH− = CaOH+ | 1.4 |
| Ca2+ + PO43− = CaPO4− | 6.46 |
| Ca2+ + F− = CaF+ | 1.9 |
| CaF2(S) = Ca2+ + 2F− | −10.4 |
| H2PO4− + H+ = H3PO4 | 2.15 |
| H2PO4− = H+ + HPO42− | −7.20 |
| HPO42− = H+ + PO43− | −12.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Peng, B.; Zhao, L.; Bai, F.; Lei, Z. Selection of an Appropriate Depressant in Flotation Separation of Molybdenum Oxide from Fluorapatite. Minerals 2021, 11, 1110. https://doi.org/10.3390/min11101110
Liu J, Peng B, Zhao L, Bai F, Lei Z. Selection of an Appropriate Depressant in Flotation Separation of Molybdenum Oxide from Fluorapatite. Minerals. 2021; 11(10):1110. https://doi.org/10.3390/min11101110
Chicago/Turabian StyleLiu, Jiandong, Binbin Peng, Liping Zhao, Fengwei Bai, and Zhiwu Lei. 2021. "Selection of an Appropriate Depressant in Flotation Separation of Molybdenum Oxide from Fluorapatite" Minerals 11, no. 10: 1110. https://doi.org/10.3390/min11101110






