Mineralogy and Magnetic Behavior of Yellow to Red Xuanhua-Type Agate and Its Indication to the Forming Condition
Abstract
:1. Introduction
2. Materials and Methods
3. Results
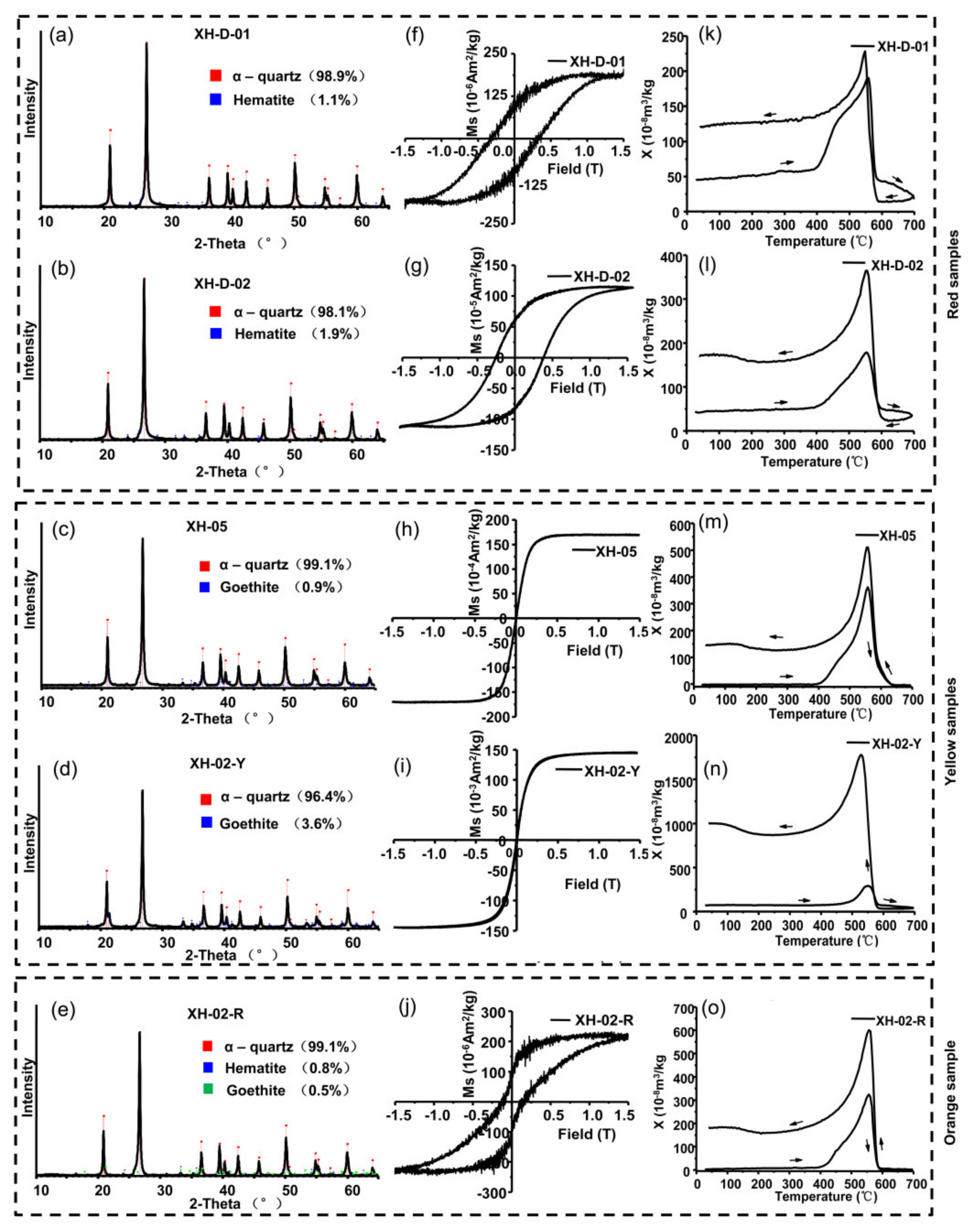
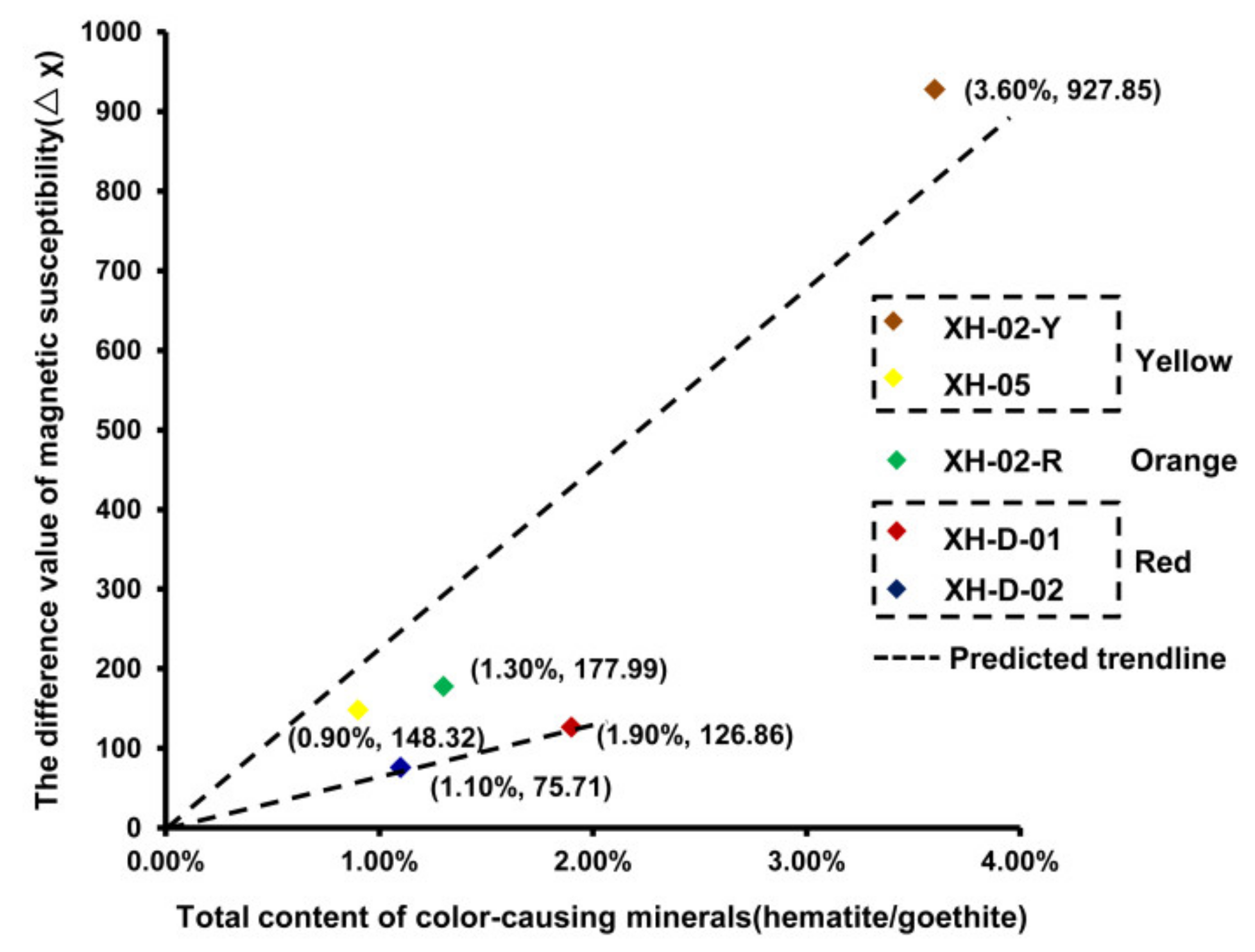
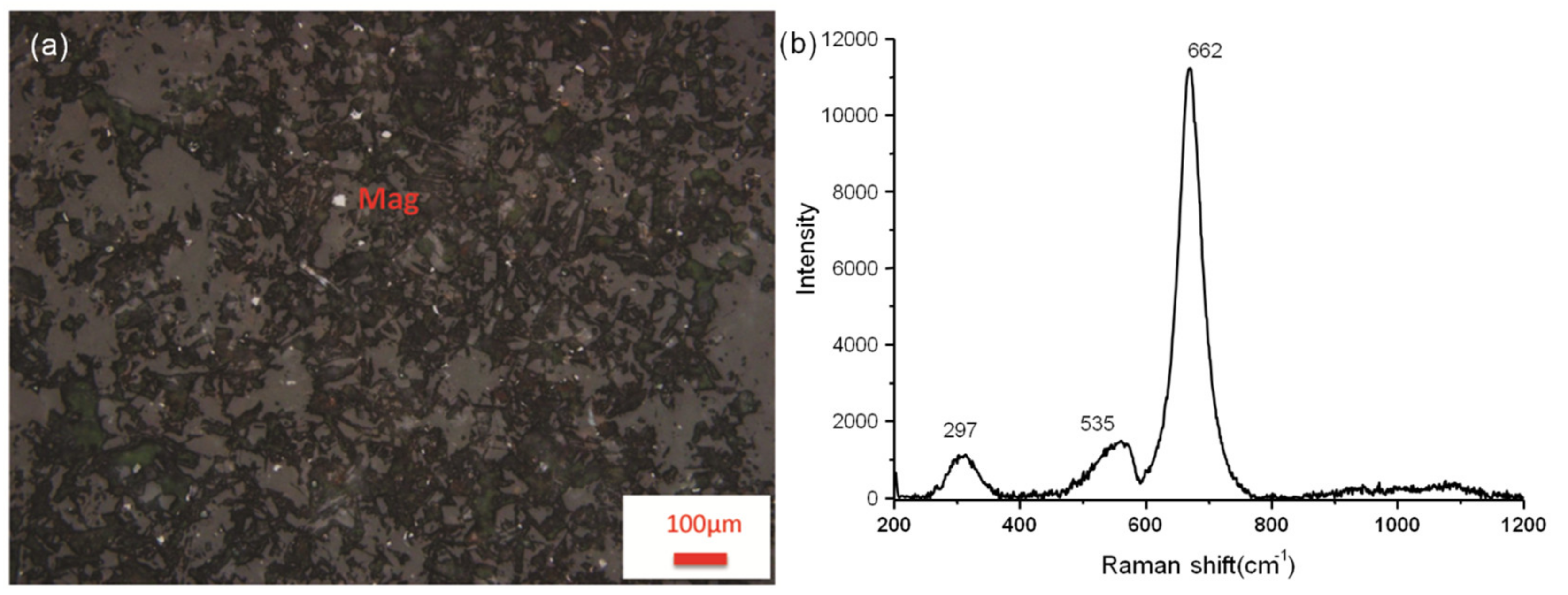
3.1. Major Mineral Components
3.2. Hysteresis Loop
3.3. Temperature Dependence of Magnetic Susceptibility (χ-T Curves)
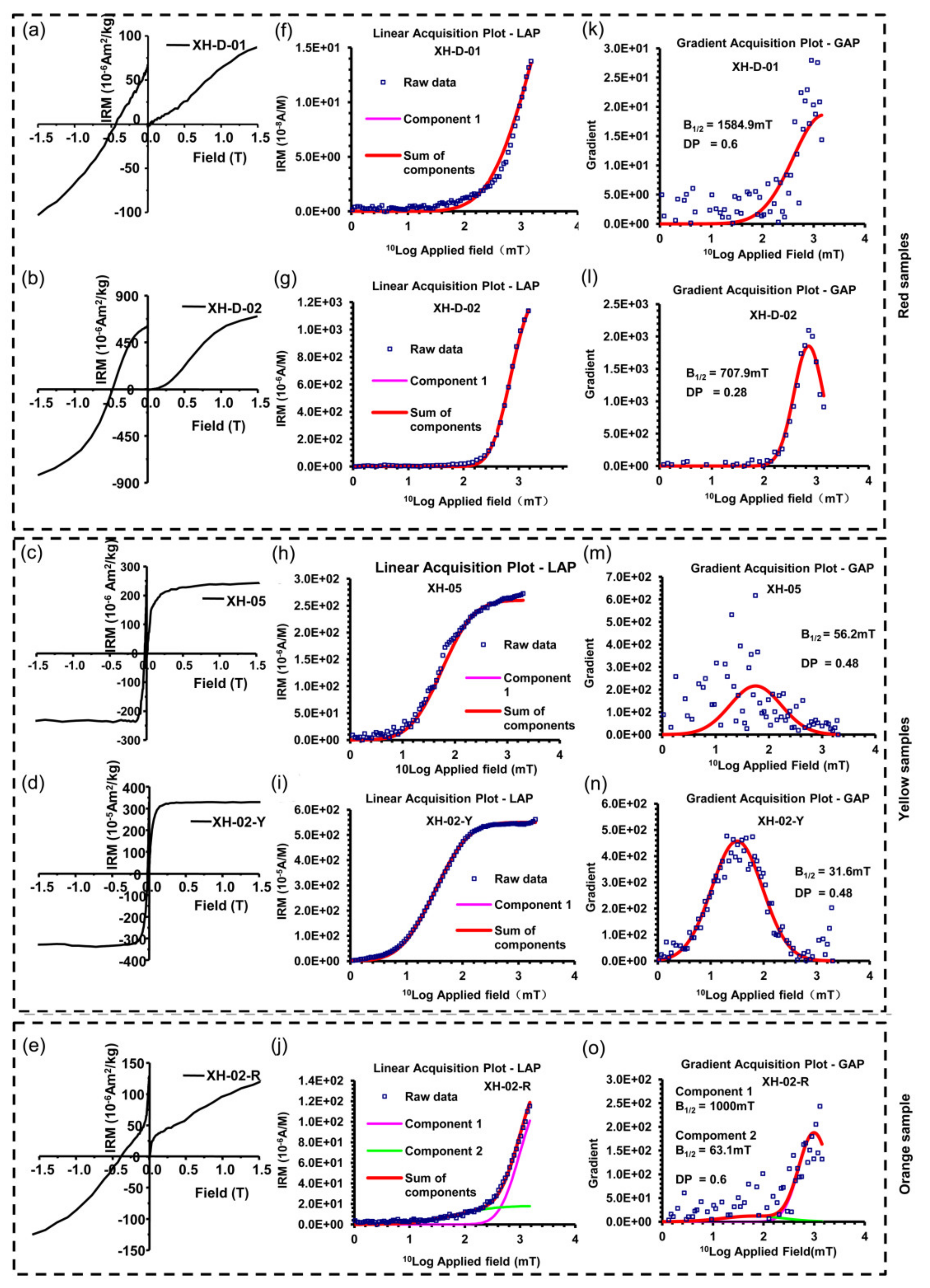
3.4. Isothermal Remnant Magnetization and Remnant Coercivity Components
3.5. Formation Temperature of Xuanhua-Type Agate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Götze, J.; Möckel, J.; Kempe, U.; Kapitonov, I. Characteristics and origin of agates in sedimentary rocks from the Dryhead area, Montana, USA. Mineral. Mag. 2009, 73, 673–690. [Google Scholar] [CrossRef]
- Meng, G.Q.; Chen, M.H.; Jiang, J.L.; Chen, S. Structural characteristic and cause of colour of “Zhanguohong” agate from Xuanhua, Hebei province. J. Gems Gemol. 2016, 18, 28–34. (In Chinese) [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses; Wiley: Weinheim, Germany, 2003; p. 664. [Google Scholar]
- Ao, H.; Deng, C.L. Review in the identification of magnetic minerals. Prog. Geophys. 2007, 22, 432–442. [Google Scholar]
- Dekkers, M.J. Environmental magnetism: An introduction. Geol. Mijnb. 1997, 76, 163–182. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Liu, Q.S. Quantification of hematite and its climatic significances. Quat. Sci. 2016, 36, 674–689. (In Chinses) [Google Scholar]
- King, J.W.; Channell, J.E.T. Sedimentary magnetism, environmental magnetism, and magnetostratigraphy. Rev. Geophys. 1991, 29, 358–370. [Google Scholar] [CrossRef]
- Liu, S.Z.; Deng, C.L.; Xiao, J.L.; Li, J.H.; Paterson, G.A.; Chang, L.; Yi, L.; Qin, H.F.; Pan, Y.X.; Zhu, R.X. Insolation driven biomagnetic response to the Holocene Warm Period in semi-arid East Asia. Sci. Rep. 2014, 5, 8001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.Z.; Krijgsman, W.; Dekkers, M.J.; Palcu, D.V. Early diagenetic greigite as an indicator of paleosalinity changes in the middle Miocene Paratethys Sea of central Europe. Geochem. Geophys. Geosyst. 2017, 18, 2634–2645. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.S.; Deng, C.L. Magnetic susceptibility and its environmental significances. Chin. J. Geophys. 2009, 52, 1041–1048. (In Chinese) [Google Scholar]
- Zhu, R.X.; Hoffman, K.A.; Nomade, S.; Renne, P.R.; Shi, R.; Pan, Y. Geomagnetic paleointensity and direct age determination of the ISEA (M0r?) chron. Earth Planet. Sci. Lett. 2004, 217, 285–295. [Google Scholar] [CrossRef]
- Zhu, R.X.; Lo, C.H.; Shi, R.P.; Shi, G.H.; Pan, Y.X.; Shao, J. Palaeointensities determined from the middle Cretaceous basalt in Liaoning Province, northeastern China. Phys. Earth Planet. Inter. 2004, 142, 49–59. [Google Scholar] [CrossRef]
- Dekkers, M.J. Magnetic behaviour of natural goethite during thermal demagnetization. Geophys. Res. Lett. 1988, 15, 538–541. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Dunlop, D.J. Hysteresis and coercivity of hematite. J. Geophys. Res. Solid Earth 2014, 119, 2582–2594. [Google Scholar] [CrossRef]
- Hu, J.M. Gemological Characteristic of Zhanguo Red Agate from Xuanhua District, Hebei Province. Master’s Thesis, China University of Geosciences Beijing, Beijing, China, 2015. [Google Scholar]
- Gou, Z.N.; He, X.M. Study on the Coloration of Red-Yellow Zhanguohong Agate from Xuanhua, Hebei Province, China. In Proceedings of the China Gems & Jewelry Academic Conference, Beijing, China, 30 November 2015. [Google Scholar]
- Xu, W.H.; Xu, X.C.; Yang, L.L.; Wu, H.Y. Ore-geology and metallogenesis of ‘Zhanguohong’ agate from Xuanhua, Hebei Province. J. Gems Gemol. 2017, 19, 1–11. [Google Scholar]
- Xu, C.; Liang, R.; Yang, X.H.; Zhao, J. Prospecting of agate deposit in Zhangjiakou, Hebei province. J. Gems Gemol. 2017, 19, 17–24. [Google Scholar]
- Zhu, R.X.; Xu, Y.G.; Zhu, G.; Zhang, H.F.; Xia, Q.K.; Zheng, T.Y. Destruction of the North China Craton. Sci. China Earth Sci. 2012, 42, 1135–1159. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, G.; Zhang, S.H.; Liu, J.M.; Hu, J.M.; Liu, J.; Pei, J.L. Pre-Yanshanian geological events in the northern margin of the North China Craton and its adjacent areas. Geol. China 2010, 37, 900–915. [Google Scholar]
- Zhao, Y.; Zhai, M.G.; Chen, H.; Zhang, S.H. Paleozoic-early Jurassic tectonic evolution of North China Craton and its adjacent orogenic belts. Geol. China 2017, 44, 44–60. [Google Scholar]
- Tauxe, L.; Bertram, H.N.; Seberino, C. Physical interpretation of hysteresis loops: Micromagnetic modeling of fine particle magnetite. Geochem. Geophys. Geosyst. 2013, 3, 1–22. [Google Scholar] [CrossRef]
- Roberts, A.P.; Cui, Y.; Verosub, K.L. Wasp-waisted hysteresis loops: Mineral magnetic characteristics and discrimination of components in mixed magnetic systems. J. Geophys. Res. Solid Earth 1995, 100, 17909–17924. [Google Scholar] [CrossRef]
- Dekkers, M.J. Magnetic properties of natural goethite-I. grain-size dependence of some low- and high-field related rockmagnetic parameters measured at room temperature. Geophys. J. Int. 1989, 97, 323–340. [Google Scholar] [CrossRef] [Green Version]
- Dekkers, M.J. Magnetic properties of natural goethite-III. Magnetic behaviour and properties of minerals originating from goethite dehydration during thermal demagnetization. Geophys. J. Int. 1990, 103, 233–250. [Google Scholar] [CrossRef] [Green Version]
- France, D.E.; Oldfield, F. Identifying goethite and hematite from rock magnetic measurements of soils and sediments. J. Geophys. Res. Solid Earth 2000, 105, 2781–2795. [Google Scholar] [CrossRef]
- Dekkers, M.J. Magnetic properties of natural goethite-II. TRM behaviour during thermal and alternating field demagnetization and low-temperature treatment. Geophys. J. R. Astron. Soc. 1989, 97, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, Ö.; Dunlop, D.J. Thermoremanence and Neel temperature of goethite. Geophys. Res. Lett. 1996, 23, 921–924. [Google Scholar] [CrossRef]
- Shive, P.N.; Diehl, J.F. Thermomagnetic analysis of natural and synthetic hematite. Geophys. Res. Lett. 1977, 4, 159–162. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.X.; Lü, B.; Ma, M.M.; Zhao, G.Y.; Chen, J.S. Study of rock magnetic properties and its variation mechanism of loess in Nileke, Xinjiang. Quat. Sci. 2014, 34, 491–503. [Google Scholar]
- Pisutha-Arnond, V.; Rochd, C.; Atichat, W.; Wathanakul, P.; Narudeesombat, N. Role of BE in Reduction and Oxidation Heating of Sapphire. In Proceedings of the 34th International Gemmological Conference, Vilnius, Lithuania, 27–30 August 2015. [Google Scholar]
- Kruiver, P.P.; Dekkers, M.J.; Heslop, D. Quantification of magnetic coercivity components by the analysis of acquisition curves of isothermal remnant magnetization. Earth Planet. Sci. Lett. 2001, 189, 269–276. [Google Scholar] [CrossRef]
- Fallick, A.E.; Jocelyn, J.; Donnelly, T.; Guy, M.; Behan, C. Origin of agates in volcanic rocks from Scotland. Nature 1985, 313, 672–674. [Google Scholar] [CrossRef]
- Harris, C. Oxygen-isotope zonation of agates from Karoo volcanics of the Skeleton Coast, Namibia. Am. Mineral. 1989, 74, 476–481. [Google Scholar]
- Moxon, T.J. Agate: A study of ageing. Eur. J. Mineral. 2002, 14, 1109–1118. [Google Scholar] [CrossRef]
- Dekkers, M.J. Some Rockmagnetic Parameters for Natural Goethite, Pyrrhotite and Fine-Grained Hematite. Ph.D. Thesis, University of Utrecht, Utrecht, The Netherlands, 1988. [Google Scholar]
- Dunlop, D.J. Theory and application of the day plot (Mrs/Ms versus Hcr/Hc) 1. Theoretical curves and tests using titanomagnetite data. J. Geophys. Res. Solid Earth 2002, 107, 4–22. [Google Scholar]
- Dunlop, D.J. Theory and application of the day plot (Mrs/Ms versus Hcr/Hc) 2. Application to data for rocks, sediments, and soils. J. Geophys. Res. Solid Earth 2002, 107, 5–15. [Google Scholar]
- Regazzoni, A.E.; Urrutia, G.A.; Blesa, M.A.; Maroto, A.J.G. Some observations on the composition and morphology of synthetic magnetites obtained by different routes. J. Inorg. Nucl. Chem. 1981, 43, 1489–1493. [Google Scholar] [CrossRef]
- Tamaura, Y.; Buduan, P.V.; Katsura, T. Studies on the oxidation of iron (II) ions during the formation of Fe3O4 and α-FeO(OH) by air oxidation of Fe(OH)2 suspensions. JCS Dalton 1981, 1807–1811. [Google Scholar] [CrossRef]



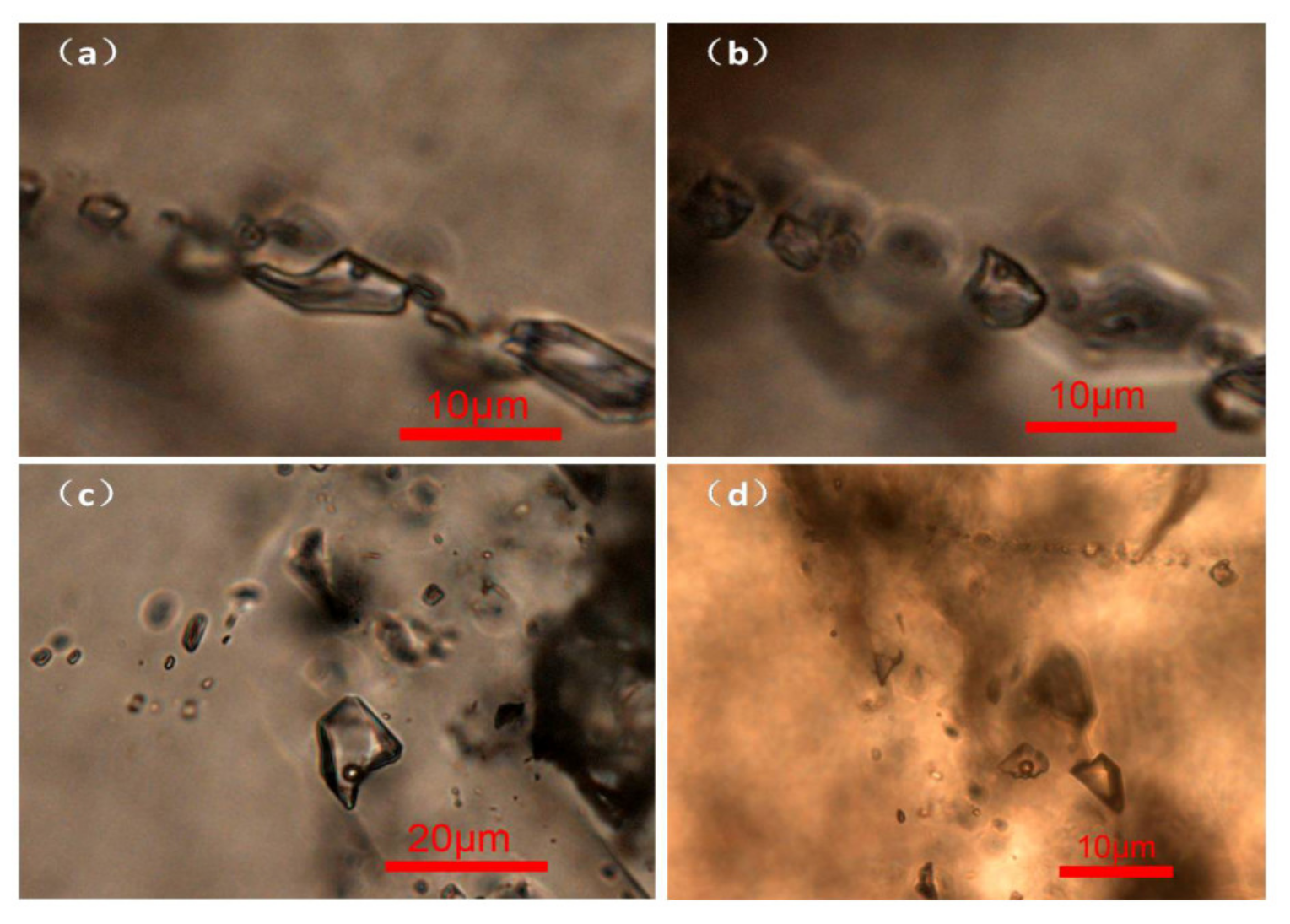

| Picture Number | Inclusion Type | Size (μm) | Phase Ratio of Gas/Fluid (%) | Homogenization Temperature (°C) |
|---|---|---|---|---|
| Figure 7a | gas-liquid | 5 × 10 | 5 | 168.8 |
| Figure 7b | gas-liquid | 6 × 7 | 10 | 178.8 |
| Figure 7c | gas-liquid | 12 × 17 | 10 | 216.3 |
| Figure 7d | gas-liquid | 3 × 4 | 20 | 264.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Shi, G.; Liu, S.; Wu, B. Mineralogy and Magnetic Behavior of Yellow to Red Xuanhua-Type Agate and Its Indication to the Forming Condition. Minerals 2021, 11, 877. https://doi.org/10.3390/min11080877
Zhou D, Shi G, Liu S, Wu B. Mineralogy and Magnetic Behavior of Yellow to Red Xuanhua-Type Agate and Its Indication to the Forming Condition. Minerals. 2021; 11(8):877. https://doi.org/10.3390/min11080877
Chicago/Turabian StyleZhou, Danyi, Guanghai Shi, Suzhen Liu, and Bailing Wu. 2021. "Mineralogy and Magnetic Behavior of Yellow to Red Xuanhua-Type Agate and Its Indication to the Forming Condition" Minerals 11, no. 8: 877. https://doi.org/10.3390/min11080877
APA StyleZhou, D., Shi, G., Liu, S., & Wu, B. (2021). Mineralogy and Magnetic Behavior of Yellow to Red Xuanhua-Type Agate and Its Indication to the Forming Condition. Minerals, 11(8), 877. https://doi.org/10.3390/min11080877







