Abstract
Axial segregation of polydisperse granular mixtures in rotating drums have been observed in several experimental and discrete particle simulation studies reported in the literature. A common thread to both experimental and numerical studies is the formation of (alternating) bands which eventually coarsen in the long-time limit due to logarithmic merging. Models to explain the experimental observations are generally limited to bidisperse mixtures, and often unable to reproduce band coarsening. One such mechanism for bidisperse mixtures argues that the grains eventually diffuse into axial bands as a consequence of concentration fluctuations in the free surface layer caused by friction-limited mobility. We generalise this model to multi-species mixtures and show that the solution produces banding that evolves more complexly than binary mixtures, with sinusoidal-like variations of the band structure that evolves non-linearly in time. In addition, we successfully recover band coarsening with time—an observation that is generally difficult to reproduce, even experimentally. Contrary to literature findings, the configurations herein did not produce bands within bands for ternary and quaternary mixtures.
1. Introduction
Segregation, and the dual phenomena of mixing, play a vital role in several industrial processes. A multitude of factors are known to influence the segregation process [1], particularly at the industrial scale. Some of the segregation mechanisms reported in the literature include gravity, stress gradients, granular temperature gradients and porosity gradients. Particle properties such as size, density, inertia, surface roughness (friction) and shape often determines the extent to which a given segregation mechanism plays out. Other factors such as advection, diffusion, boundary conditions and interstitial fluid act to either reinforce or mitigate the effects of segregation. Real granular flow systems often involve combinations of these factors, which is arguably why a complete theory of segregation still remains elusive.
In the pharmaceutical industry, the effectiveness of the drug is directly related to the mixture of active ingredient and excipient. Central to the low efficiency (<5%) reported in mineral grinding systems like tumbling mills [2] is the inability to control the mixture of grinding balls and smaller rocks where energy is arguably dissipated [3] through shear dissipation. Ref. [4] point out the negative impact of radial segregation to the gas-particle contact required in the core of rotary kilns, while the undesirable case of complete segregation in drum mixers is discussed by [5].
The canonical rotating drum system encountered in mineral processing, food and pharmaceutical industries is well known to exhibit axial [6,7,8] and radial [9,10,11] mixing; however, their interplay for optimal performance is not well understand. The simplest rotating drum configuration—a binary mixture operated in batch mode—segregates into alternating bands along the length of the drum after several drum rotations. With sufficient further rotations of the drum, the bands merge into a coarser, more stable configuration. Since the experimentally observed segregation in equal-volume binary (by size) mixtures [12,13], theoretical models [4,14,15,16,17,18] and experiments [14,18,19,20,21] have emerged to better understand band formation and its evolution with time. Besides the development of theoretical models and experimental studies, computational simulation tools like the Discrete Element Method (DEM) and Monte Carlo techniques [22,23,24,25,26,27] have also grown rapidly with improvements in computing power (The above mentioned references contain high quality visualisations of banding and coarsening which may facilitate the previous and ensuing discussions).
Axial segregation investigations involving mixtures with species are limited. Ref. [26] experimentally investigated axial segregation of polydisperse mixtures in rotating drums. They used mixtures ranging from three to six different particle sizes with all other particle properties being identical. The ternary and quaternary mixtures resulted in the smaller-size particles forming bands within bands of the larger-size particles. They found no clear banding structure for mixtures of more than four particle sizes. Axial segregation was found to be a secondary instability following radial segregation. Discrete Monte Carlo simulations based solely on differing surface flow properties—which is essentially equivalent to differences in frictional properties—was successfully used to predict the experimental observations. Ref. [27] investigated axial segregation of a ternary mixture (by size) using discrete particle simulations. The observed band formation was such that the medium-sized particles tended to be located between alternating bands of big and small particles. In the long-time limit, the bands merged to form the coarsened banding structure found in experimental literature. Unlike the relatively few parameters found in their previous work, the large set of parameters spanned by the ternary mixture precluded a parametric study. The findings of [27] are consistent with experimental segregation studies in axially rotating drum flows. Notwithstanding the plethora of confounding factors influencing segregation, the literature does suggest that friction plays a major role in axial segregation within rotating drum flows. Finally, we recognise that axial segregation can be caused by fluctuations in the inner core of the bed due to radial segregation [28]. Future work will explore this idea in more detail.
In the present work we extended the axial diffusion model of [18] to describe ternary and quaternary mixtures. We also present a general formula to obtain the set of partial differential equations (PDEs) that describe axial segregation of any polydisperse mixture. The time evolution of the resulting axial concentration profile is then used to illustrate band formation and its eventual coarsening for binary, ternary and quaternary mixtures.
2. Model Derivation
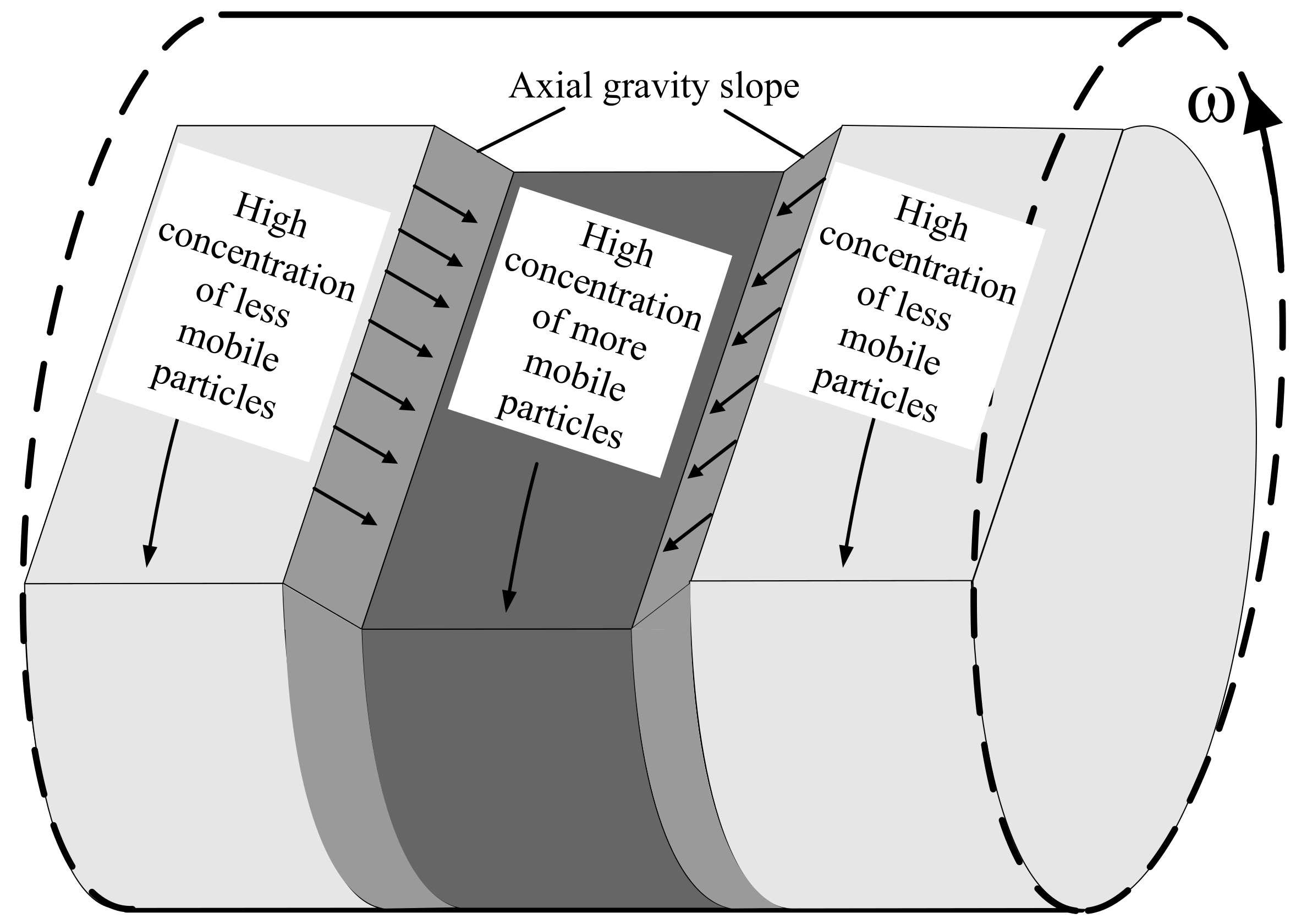
The axial diffusion model of [18] argues that spontaneous localised fluctuations in concentration seed alternating bands along the drum axis, as illustrated in Figure 1 for three such adjacent bands. The relatively higher slices—lighter grey bands in Figure 1—are rich in high friction (less mobile) particles while the central lower slice is rich in low friction (more mobile) particles. The height difference between the adjacent bands sets up a gravity slope that drives particles down the resulting local axial gradients—from higher slice (with higher repose angle) to adjacent lower slice (with lower repose angle)—while friction dictates the mobility of the particles. Accordingly, more mobile particles (with low friction) move preferentially down the axial gravity slope while the less mobile particles (with high friction) are impeded. With the passage of time, more mobile particles are depleted from the higher band while they accumulate in the lower band. The slope region also becomes richer in more mobile particles. Consequently, friction-limited mobility acts like a positive feedback mechanism that preferentially depletes the higher reposed slice of low friction species while preferentially feeding the lower reposed slice with low friction species. The alternating bands are thus reinforced. Ref. [18] successfully predicted alternating banding for drums more than half full of a binary mixture. Unfortunately, the model failed to predict band coarsening and was never extended to multiple species. We have shown previously [29] that:
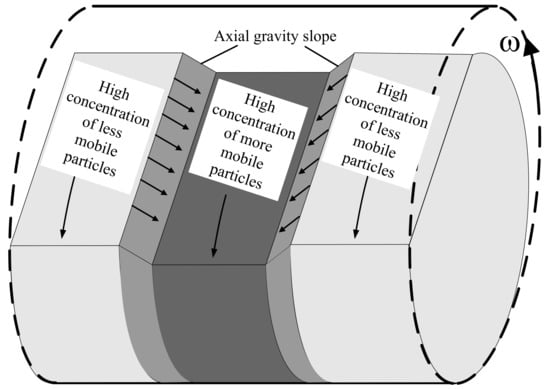
Figure 1.
Illustration of three alternating bands along axis of rotating drum. The central band is rich in low friction (more mobile) species while the other two are rich in high friction (less mobile) species. The high friction species exhibit a steeper angle of repose than the low friction species and consequently a gravity slope develops between the bands. More mobile particles are driven preferentially down the gravity slope, resulting in a positive feedback loop that re-enforces the banding pattern.
- (a)
- band coarsening can be achieved by introducing a second order binomial expansion to the axial gradient operator; and
- (b)
- to extend the theory to less than half full drums, a Bagnoldian shear stress assumption was required.
The present work extends the theory to multiple species with explicit solutions presented for three-, four- and n-species, where n represents any positive integer.
2.1. Three Species Mixture
To derive the set of diffusion equations that represent axial segregation of a three-species mixture using the surface flow mechanism, we follow the same argument that was applied for binary mixtures [18]. First, application of the continuity equation that exists for each species results in:
where is the concentration of the ith species with corresponding coefficient of friction and indexes the three species. z is the rotation axis of the drum with y denoting the vertical. is the total number density of the particles in the mixture. denotes the respective axial fluxes (per unit length) in the free surface layer and is given by Equation (4). The terms are the back flow fluxes where h is the depth of the flowing layer and is the flowing layer velocity in the axial direction.
At any given axial slice:
Addition of the three expressions in Equation (1) produces:
The details of axial fluxes in the free surface layer can be expressed as:
The constant where is the lithostatic pressure, is the viscosity, is density and g is the acceleration due to gravity. is the axial gradient operator and is the unit vector in the opposite direction of steepest descent, at a point on the surface.
Given that the total concentration is equal to 1, the concentration of the third species () can be eliminated using . The terms can then be rearranged such that:
The following equations were obtained after Equations (5) and (6) were substituted into Equations (2) and (3) respectively:
Application of the first order approximation to the axial gradient operator in a binomial expansion yields the axial component as: and Then Equation (7) becomes:
Following [19,22], the derivative is written through as:
Eventually, we get a coupled system of non-linear partial differential equations (PDEs) given by
2.2. Four Species Mixture
Following the same argument of segregation instability that was applied in Section 2.1, the set of PDEs that represents axial segregation of a four species mixture can be expressed as:
3. Generalization to n-Species Mixture
For a mixture of multi-species (n), the corresponding set of PDEs of axial segregation are obtained as follows:
- The number of the equations is given by where n is the number of the species of different types used in the mixture. The reduction to () equations results from:
- The corresponding set of differential equations is given by:
For example, a five species mixture, i.e., and , yields the following four equations:
4. Results and Discussion
The following two equations represent the initial conditions:
where n is the number of different-frictional species used in the mixture. At the initial state each different-frictional species in the mixture has the same (equal) value of normalised concentration. Periodic boundary conditions were applied along the drum axis.
To visualise the solutions for binary (Figure 2), ternary (Figure 3, Figure 4 and Figure 5) and quaternary (Figure 6) mixtures, we plot concentration profiles along the axial length of the drum at different snapshots in times. To facilitate the ensuing analysis, we fix the diffusion coefficients, thereby obviating the need to physically account for the pressure, viscosity, density and spatial gradients (Future work will systematically study the influence of these parameters on the segregation pattern.). Consequently, the only mechanical property used to differentiate the individual species was the friction coefficient () which satisfied the following mixture combinations:
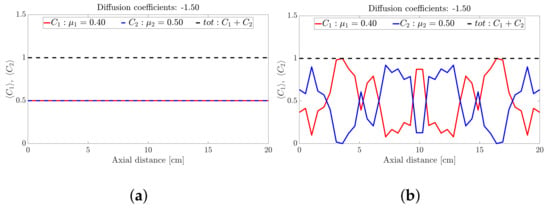
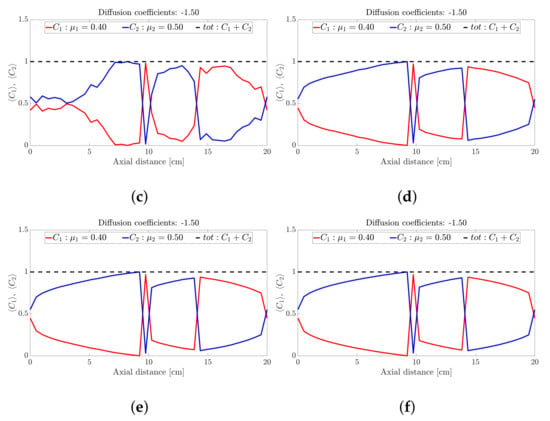
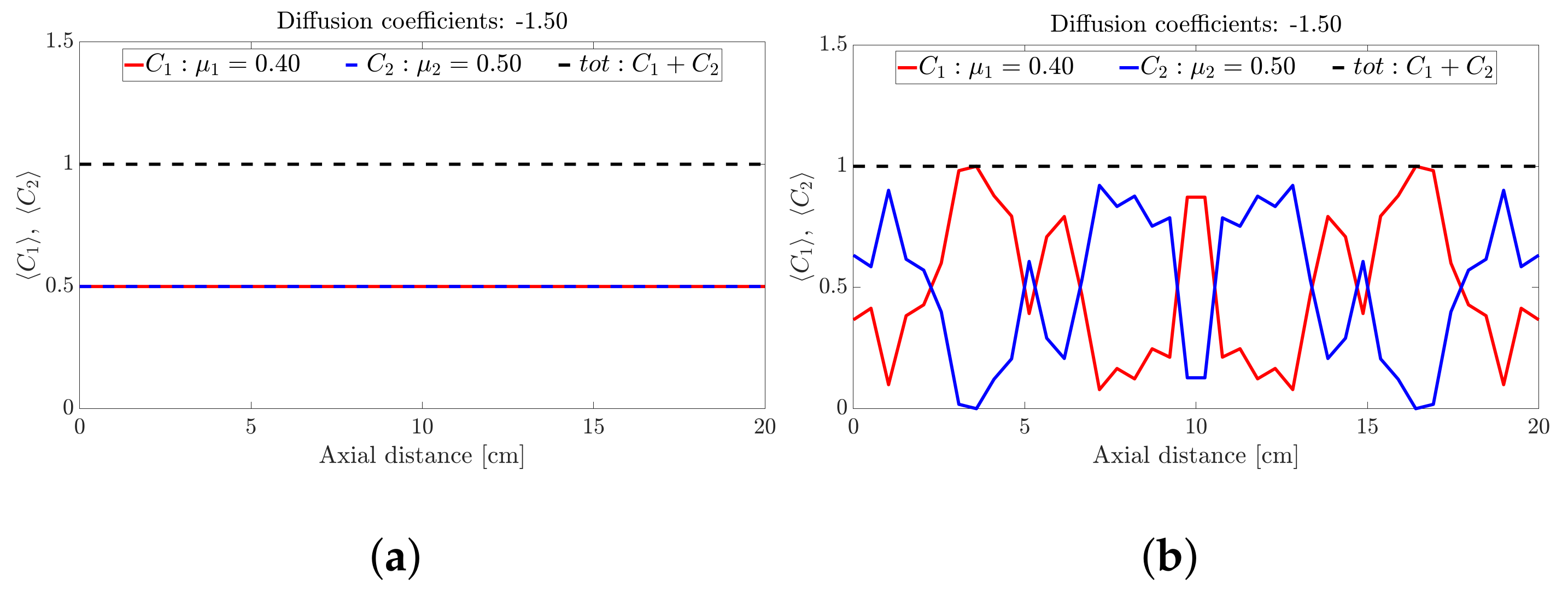
Figure 2.
Snapshots of binary mixture at: (a) 0 s, (b) 17 s, (c) 50 s, (d) 500 s, (e) 1000 s and (f) 5000 s with a fixed diffusion coefficient of −1.5.
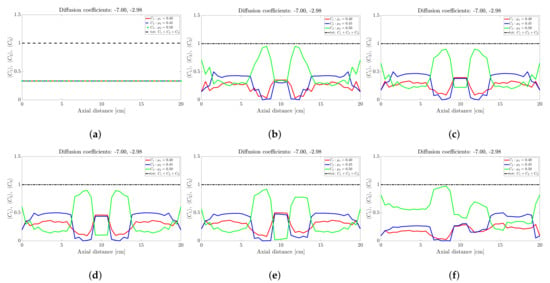
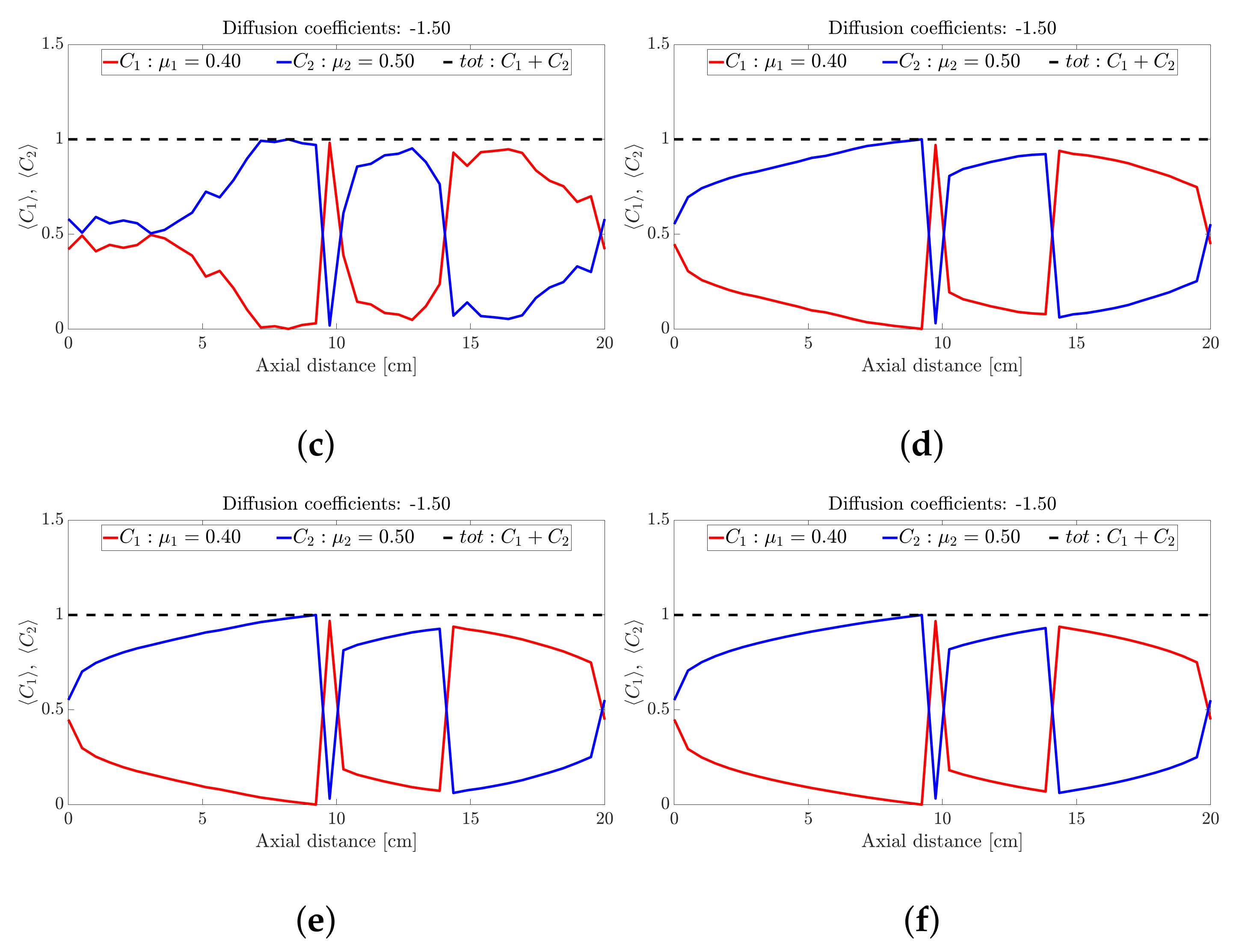
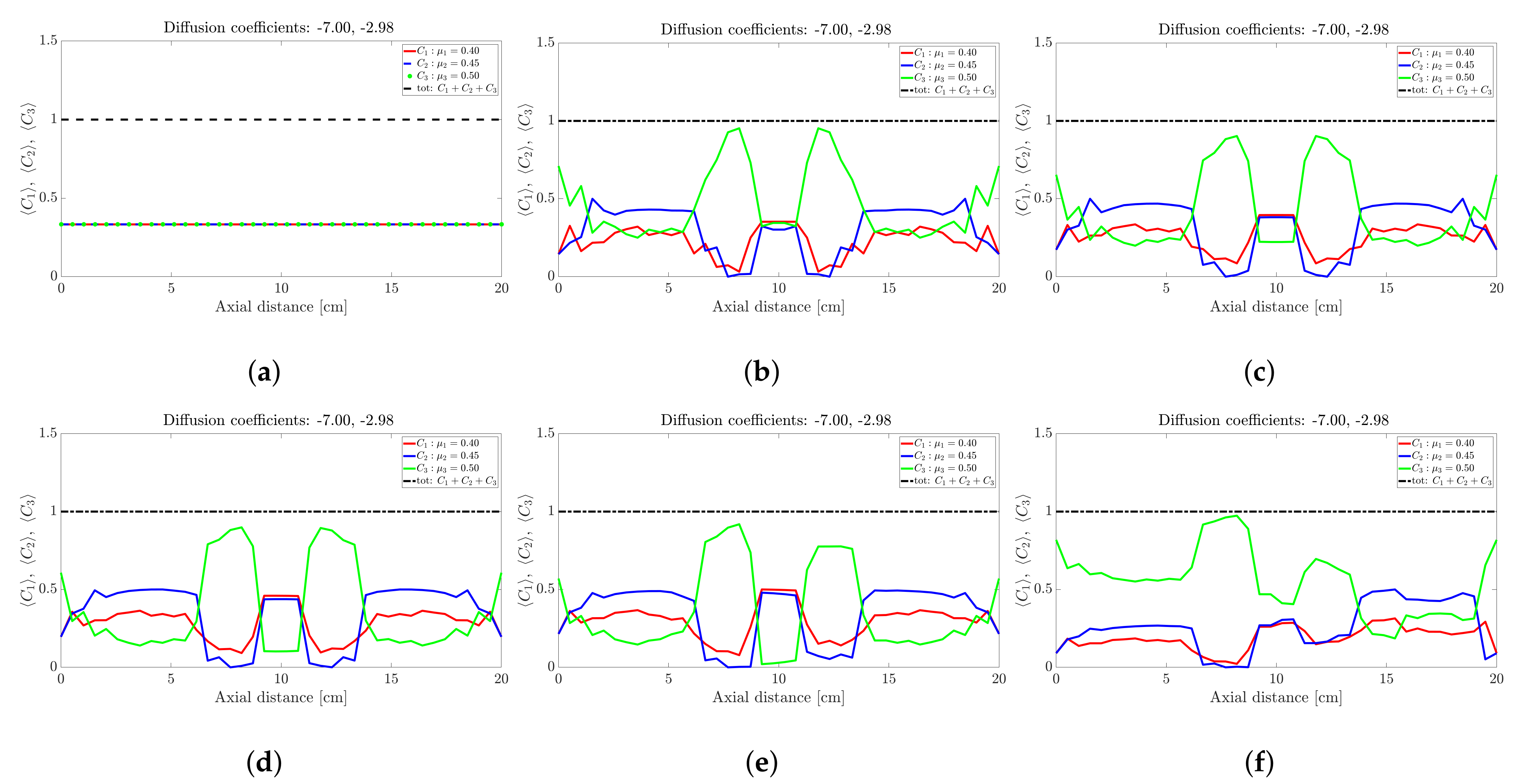
Figure 3.
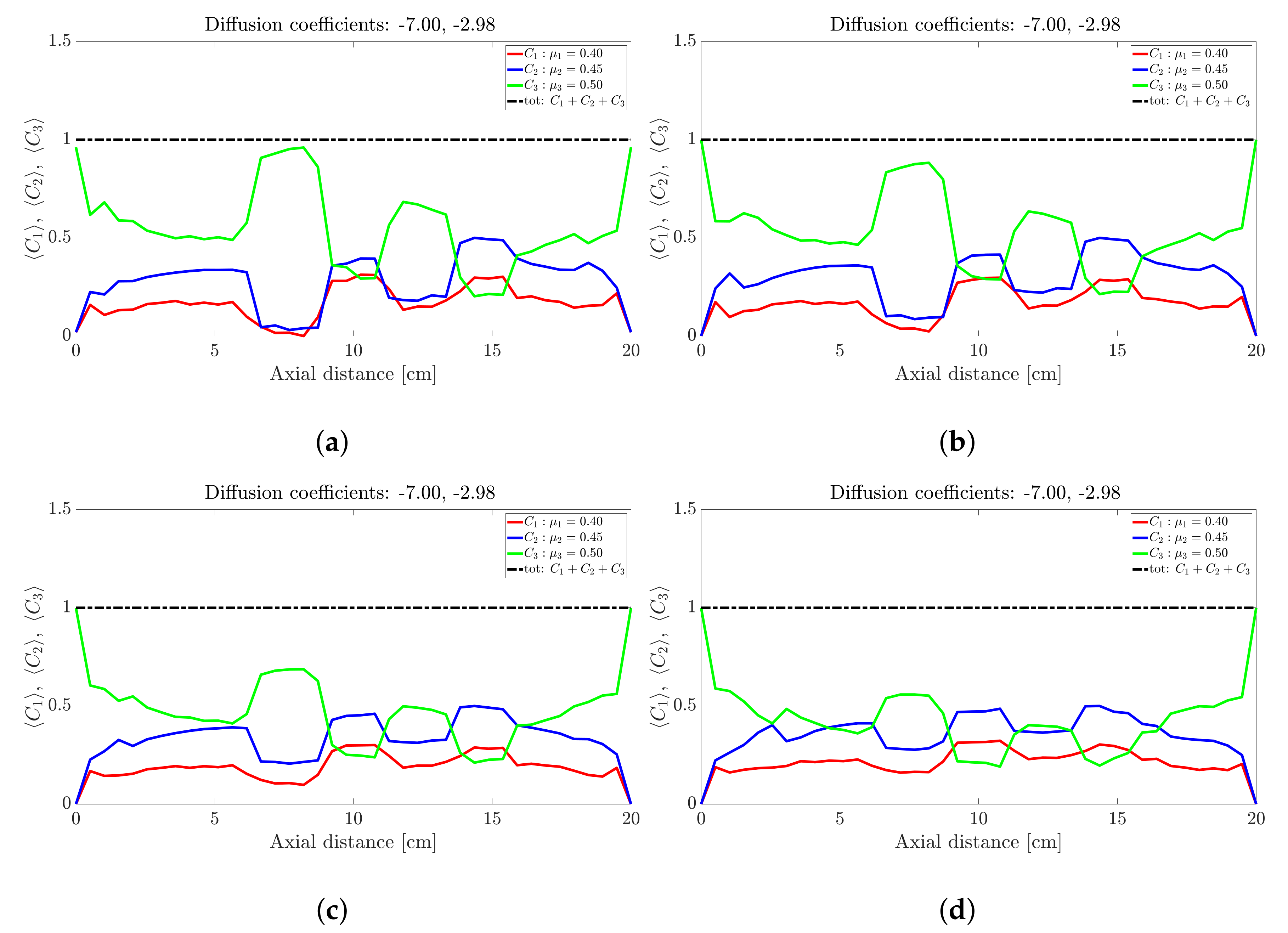
Snapshots of ternary mixture at: (a) 0 s (b) 20 s, (c) 25 s, (d) 30 s, (e) 32 s and (f) 40 s. The corresponding diffusion coefficients are −7 and −2.98.
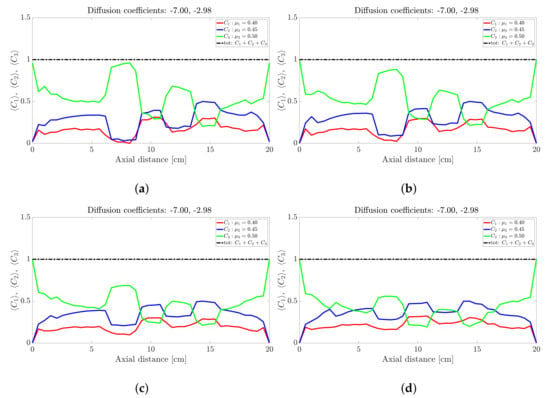
Figure 4.
Snapshots of ternary mixture at: (a) 45 s, (b) 50 s, (c) 70 s, (d) 100 s. The corresponding diffusion coefficients are −7 and −2.98.
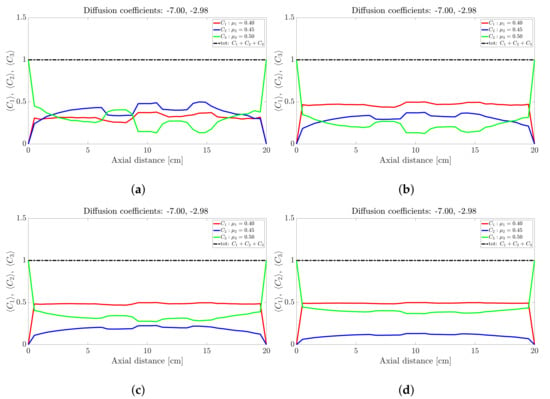
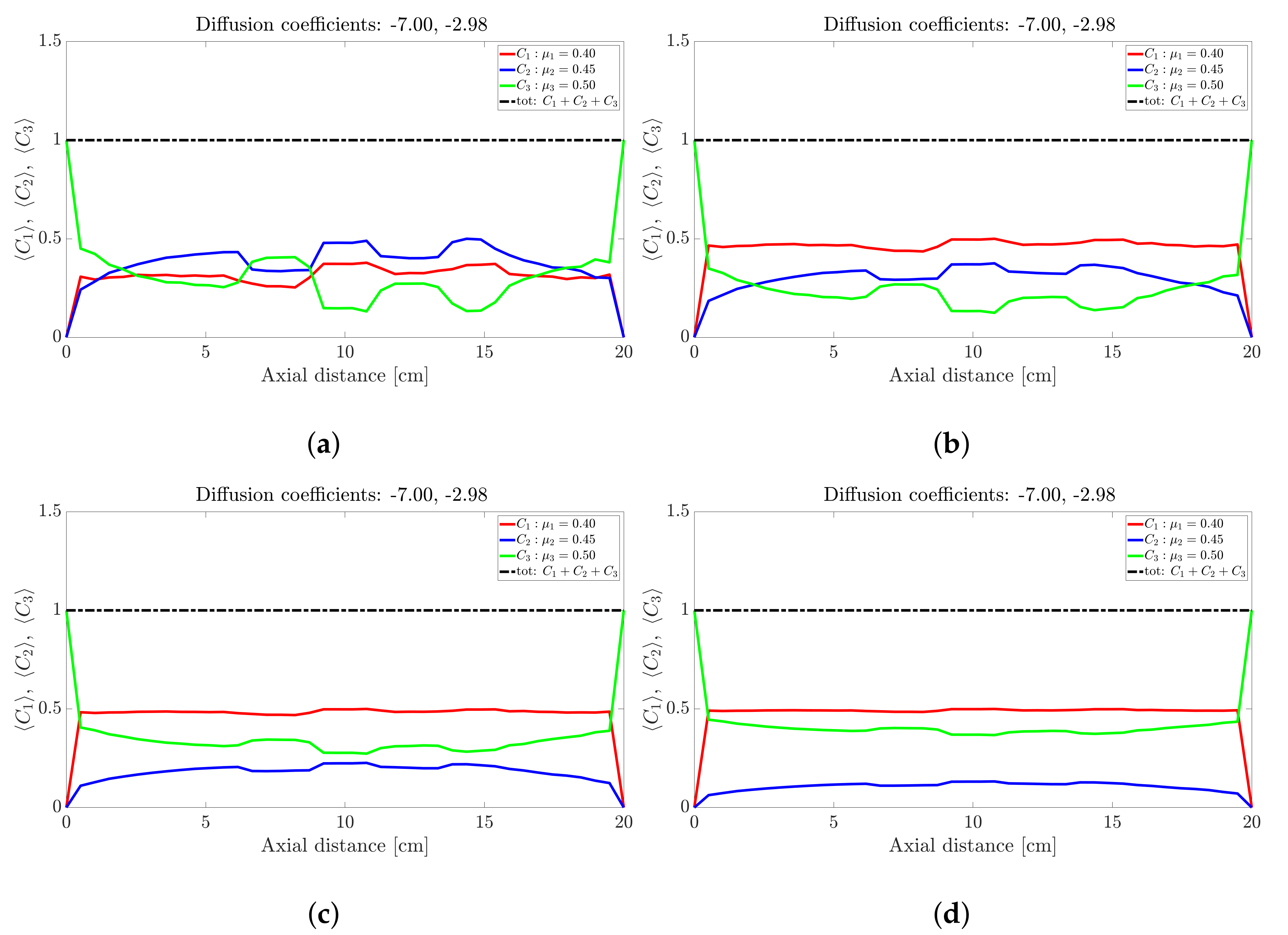
Figure 5.
Snapshots of ternary mixture at: (a) 200 s, (b) 500 s, (c) 1000 s, (d) 2000 s. The corresponding diffusion coefficients are −7 and −2.98.
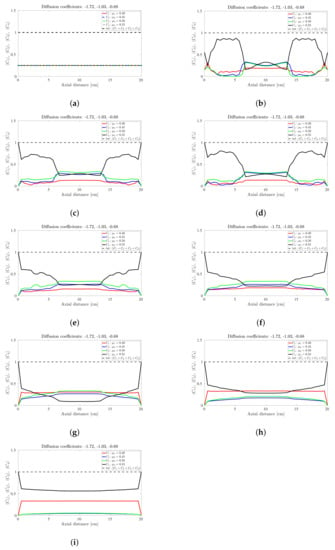
Figure 6.
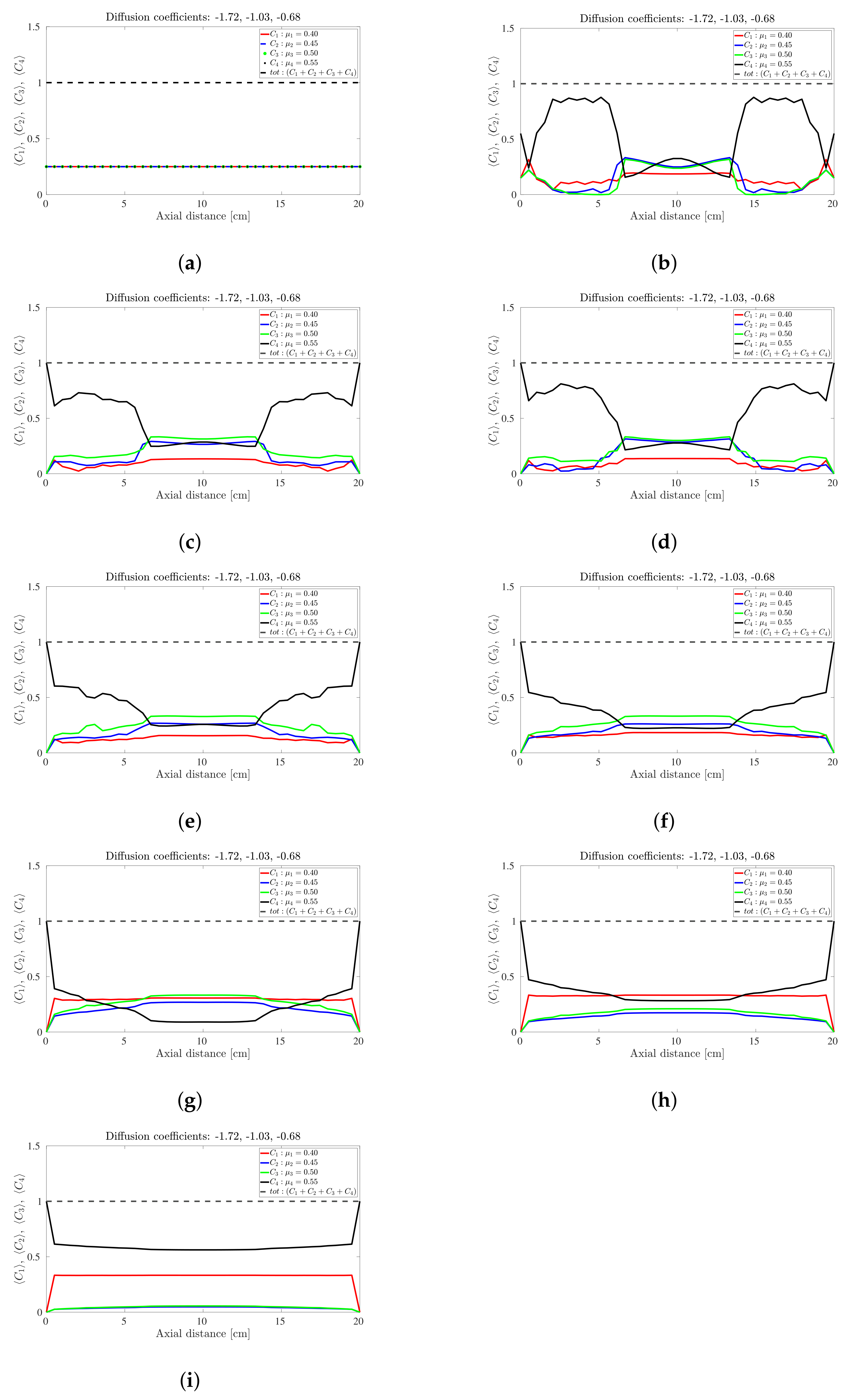
Snapshots of quaternary mixture at: (a) 0 s, (b) 24 s, (c) 30, s (d) 40 s, (e) 100 s, (f) 200 s, (g) 500 s, (h) 1000 s and (i) 5000 s. The corresponding diffusion coefficients are −1.74, −1.02 and −0.71.
- (i)
- Binary case: and
- (ii)
- Ternary case: and
- (iii)
- Quaternary case: , and
4.1. Inferring Band Height
As depicted in Figure 1, the geometric implication of the [18] hypothesis for species that differ only by their frictional properties—that differences in species concentration along the drum axis lead to alternating (in height) bands—is that:
- IV.A(1)
- Bands with higher concentrations of higher frictional species (species with higher values of ) are physically higher in height than bands with higher concentrations of lower frictional species (species with lower values of ).
- IV.A(2)
- A direct consequence of Section 4.1 is that the average friction coefficient is directly proportional to the concentration distribution of frictional species within a band.
- IV.A(3)
- Bands with equal (or similar) concentrations of frictional species have the same (or similar) physical band height.
- IV.A(4)
- If two adjacent bands, initially with different concentrations of frictional species, evolve over time to have equal (or similar) concentrations of frictional species, the bands are assumed to have evolved to equal (or similar) physical heights and are therefore assumed to have merged into a larger band. We will use this criteria as the basis for band coarsening.
4.2. Binary Mixture
Figure 2 depicts the axial concentration variation of a binary mixture at six snapshots in time. Each species follows a sinusoidal-like concentration variation along the drum axis that is out of phase with the other species. Noting that the height of the band is determined by the relative proportions of frictional species (the higher the proportion of high frictional species, the higher the band), we conclude that the binary mixture forms an alternating banding pattern (a repeating high-low pattern) along the drum axis. It is also clear from Figure 2 that the bands appear to coarsen with time up to s (Figure 2c,d) and maintains the band structure for s (Figure 2e,f).
4.3. Ternary Mixture
The ternary mixture exhibits a more complex time-evolution of the band structure. To illustrate the complexity, lets focus on the central band located about 10 cm along the drum axis; see Figure 3. At seconds all species are approximately at ; see Figure 3a. Over the next 30 s; see Figure 3b,c, the highest frictional species () drops to nearly zero while the other two species increase slightly. This corresponds to a steady drop in band height over the 10 s period for the centrally located band. Over the next 10 s—Figure 3e,f—the reverse process occurs: The highest frictional species () rapidly increases in concentration with a corresponding drop in concentration observed for the other two species, implying an increase in height of the centrally located band. Beyond 40 s—Figure 4b,d—the process reverses once more, but now over a longer period of approximately 50 s. Beyond 100 s; see Figure 5b,d, the concentration profile appears to stabilise and flatten out. A similar concentration variation is observed at most other axial locations. The overall flattening out of the concentration profile is consistent with band coarsening as defined in Section 4.1. Distinct from [26,27], no bands within bands were observed. Changing the difference between friction coefficients did not work. Even changes to the diffusion coefficient did not recover bands within bands. It must be noted that these changes were not systematic, so there might well be suitable choices of friction coefficients and/or diffusion coefficients that we haven’t tried. Barring a full parametric study, we defer further comment to future work.
4.4. Quaternary Mixture
The sinusoidal-like variation of the concentration profile at a given axial slice persists with the quaternary mixture, but over a much larger timescale; see Figure 6. To facilitate the discussion, let’s limit our attention once more to the centrally located band about the 10 cm axial position: At s the highest frictional species () has the highest concentration, the lowest frictional species () has the lowest concentration while the intermediate frictional species ( and ) are roughly midway between the highest and lowest concentrations. Over the next 500 s (Figure 6b–g) the -species (highest frictional species) drops steadily to approximately zero with the -species (lowest frictional species) now occupying the highest concentration. The increase (respectively, decrease) in concentration of the low (respectively, high) frictional species over this 500 s period corresponds to a steady decrease in the height of the centrally-located band. Beyond 500 s the reverse process occurs albeit over a much longer timescale (over 5000 s), with an additional flattening of the concentration profile along the drum length. We thus conclude that bands within the quaternary mixture follows a sinusoidal-like variation over time that eventually coarsens. Again, no bands within bands were observed for the quaternary mixtures investigated despite anecdotal changes to the friction coefficients and/or diffusion coefficients. Notwithstanding it is worth noting that [30] also found that bands within bands are not well pronounced in quaternary mixtures, suggesting that only very unique combinations of friction and diffusion coefficients can yield banding patterns similar to [26,27].
5. Conclusions
This paper presents a generalisation of the axial segregation model of [18] to include a general solution strategy for an n-species mixture, and explicit representations of the binary (), ternary () and quaternary () mixtures. Consistent with all experimental findings reported in the literature [14,18,19,20,21], the binary mixture was shown to follow the classical alternating band formation that coarsened with time. The ternary mixture produced bands that oscillated in height. Initially, each oscillation occurred at roughly the same frequency. The oscillations continued in the long-time limit, but over an order-of-magnitude-larger time frame. Notwithstanding the more complex band evolution, band coarsening was successfully recovered. Again, the findings are consistent with the experimental findings of [26,27]. Similar to the ternary mixture, the bands within the quaternary mixture oscillated with time and eventually coarsened. A notable difference between the ternary and quaternary mixtures is that the latter evolved at a significantly lower frequency.
There are still several aspects worthy of further investigation. The exact reasons for slower evolution of the band structure towards eventual coarsening needs further investigation. The use of fixed diffusion coefficients (a lumped parameter) greatly facilitated the numerical solution of the polydisperse mixtures; however, it prevented apriori a deeper probing of the root cause to complex time-evolution of the banding structure in ternary and quaternary mixtures. A detailed parametric study of the individual constituents making up the diffusion coefficient is currently underway. Finally, we are hopeful that a complete parametric study will also provide insights into the band-within-bands and when exactly they occur.
Author Contributions
E.M.E.A. refined the theoretical work, performed the numerical analysis, and contributed to writing the paper. I.G. conceptualized the project, developed the initial model, and wrote of the paper. A.N.M. contributed to writing the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work supported by the Centre for Minerals Research (CMR)-University of Cape Town, Physics Department-University of Cape Town and the Department of Chemical at the Engineering-University of KwaZulu-Natal.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hill, K. Handbook of Granular Materials, Chapter 10: Segregation in Dense Sheared Systems; Taylor and Francis: New York, NY, USA; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Wills, B.; Napier-Munn, T. Mineral Processing Technology, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2006; pp. 573–580. [Google Scholar]
- Nordell, L.; Potapov, A. Novel comminution machine may vastly improve crushing-grinding efficiency. In Proceedings of the International Conference on Autogeneous and Semiautogeneous Grinding Technology, Vancouver, BC, Canada, 24–28 September 2012; Flintoff, B., Ed.; CIM: Montreal, QC, Canada, 2012. [Google Scholar]
- Gupta, S.D.; Khakhar, D.; Bhatia, S. Axial segregation of particles in a horizontal rotating drum. Chem. Eng. Sci. 1991, 46, 1513–1517. [Google Scholar] [CrossRef]
- Donald, M.; Roseman, B. Mixing and demixing of solid particles, part i, mechanisms in horizontal drum mixer. Br. Chem. Eng. 1962, 7, 749–753. [Google Scholar]
- Hardin, M.T.; Howes, T.; Mitchell, D.A.; Whittaker, A.K. Axial mixing in rotating drums using magnetic resonance imaging using bran as a model for solid state fermentations. Biotechnol. Lett. 2002, 24, 521–525. [Google Scholar] [CrossRef]
- Sherritt, R.G.; Chaouki, J.; Mehrotra, A.K.; Behie, L.A. Axial dispersion in the three-dimensional mixing of particles in a rotating drum reactor. Chem. Eng. Sci. 2003, 58, 401–415. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, G.; Xu, J.; Ge, W. Enhanced axial mixing of rotating drums with alternately arranged baffles. Powder Technol. 2015, 286, 276–287. [Google Scholar] [CrossRef]
- Hajra, S.K.; Khakhar, D.V. Radial mixing of granular materials in a rotating cylinder: Experimental determination of particle self-diffusivity. Phys. Fluids 2005, 17, 013101. [Google Scholar] [CrossRef]
- Khakhar, D.V.; McCarthy, J.J.; Shinbrot, T.; Ottino, J.M. Transverse flow and mixing of granular materials in a rotating cylinder. Phys. Fluids 1997, 9, 1. [Google Scholar] [CrossRef]
- Lehmberg, J.; Hehl, M.; Schügerl, K. Transverse mixing and heat transfer in a horizontal drum mixer. Powder Technol. 1977, 18, 149. [Google Scholar] [CrossRef]
- Oyama, Y.; Ayaki, K. Studies on the mixing of particulate solids. Kaguku Kikai 1956, 20, 6–13. [Google Scholar]
- Weidenbaum, S. Mixing of solids. Adv. Chem. Eng. 1958, 2, 209–324. [Google Scholar]
- Hill, K.M.; Caprihan, A.; Kakalios, J. Bulk segregation in rotated granular material measured by magnetic resonance imaging. Phys. Rev. Lett. 1997, 78, 50–53. [Google Scholar] [CrossRef]
- Jain, N.; Khakhar, D.V.; Lueptow, R.M.; Ottino, J.M. Self-organization in granular slurries. Phys. Rev. Lett. 2001, 86, 3771–3774. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, M. Axial segregation of granular flows in a horizontal rotating cylinder. Chem. Eng. Sci. 1994, 49, 2540–2544. [Google Scholar] [CrossRef]
- Taberlet, N.; Newey, M.; Richard, P.; Losert, W. On axial segregation in a tumbler: An experimental and numerical study. Stat. Mech. Theory Exp. 2006, 2006, 041302. [Google Scholar] [CrossRef]
- Zik, O.; Levine, D.; Lipson, S.G.; Shtrikman, S.; Stavans, J. Rotationally induced segregation of granular materials. Phys. Rev. Lett. 1994, 73, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.; Molteno, T.C.A.; Morris, S.W. Traveling granular segregation patterns in a long drum mixer. Phys. Rev. Lett. 1997, 79, 2975–2978. [Google Scholar] [CrossRef] [Green Version]
- Frette, V.; Stavans, J. Avalanche-mediated transport in a rotated granular mixture. Phys. Rev. E 1997, 56, 6981–6990. [Google Scholar] [CrossRef]
- Khan, Z.S.; Tokaruk, W.A.; Morris, S.W. Oscillatory granular segregation in a long drum mixer. Europhys. Lett. 2004, 66, 212. [Google Scholar] [CrossRef]
- Bertr, F.; Leclaire, L.A.; Levecque, G. Dem-based models for the mixing of granular materials. Chem. Eng. Sci. 2005, 60, 2517–2531. [Google Scholar] [CrossRef]
- Ch, R.; Khaskheli, M.A.; Qadir, A.; Ge, B.; Shi, Q. Discrete particle simulation of radial segregation in horizontally rotating drum: Effects of drum-length and non-rotating end-plates. Physica A 2012, 391, 4590–4596. [Google Scholar]
- Cundall, P.; Strack, O. A discrete numerical model for granular assemblies. Geotechnique 1979, 29, 47–65. [Google Scholar] [CrossRef]
- Matuttis, H.G.; Luding, S.; Herrmann, H.J. Discrete element methods for the simulation of dense packings and heaps made of spherical and non-spherical particles. Powder Technol. 2000, 109, 278–292. [Google Scholar] [CrossRef]
- Newey, M.; Ozik, J.; van der Meer, S.M.; Ott, E.; Losert, W. Band-in-band segregation of multidisperse granular mixtures. Europhys. Lett. 2004, 66, 205. [Google Scholar] [CrossRef]
- Rapaport, D.C. Simulated three-component granular segregation in a rotating drum. Phys. Rev. E. 2007, 76, 041302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taberlet, N.; Losert, W.; Richard, P. Understanding the dynamics of segregation bands of simulated granular material in a rotating drum. Europhys. Lett. 2004, 68, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Govender, I.; Ahmed, E. A mechanistic model of axial charge segregation in rotating drums. In Proceedings of the XXVIII International Mineral Processing Congress (IMPC 2016), Quebec City, QC, Canada, 11–15 September 2016. [Google Scholar]
- Losert, W. Segregation Summer School: Granular Materials from Simulations to Astrophysical Applications; CSCAMM and the University of Maryland-Burgers Program in Fluid Dynamics: College Park, MD, USA, 2011. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).