Study of the Effect of Absorbed Cu Species on the Surface of Specularite (0 0 1) by the DFT Calculations
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. The Effect of Cu Species Adsorbed on the Structure of the Specularite (0 0 1) Surface
3.2. The Effect of Cu Species Adsorption on the Electronic Properties of Specularite
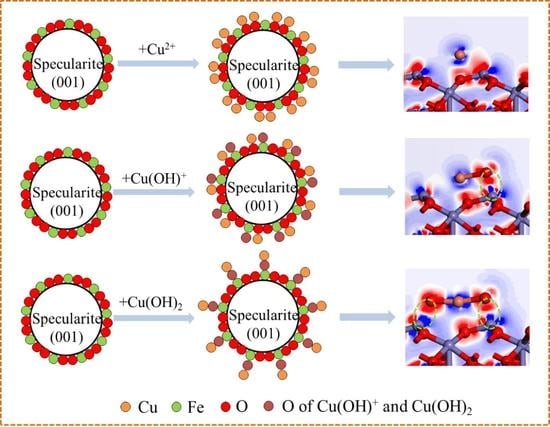
3.3. Adsorption of Cu Species on the Surface of Specularite
3.3.1. The Role of Cu Species Absorbed on the Surface of Specularite
3.3.2. Density of States Analysis of Interaction between Cu Species and Specularite (0 0 1) Surface
3.3.3. Interatomic Charge Transfer Analysis of Interaction between Cu Species and Specularite (0 0 1) Surface
3.3.4. Analysis of Bond Properties of Cu Species Adsorbed on the Specularite Surface
4. Conclusions
- (1)
- The results of crystalline structure and electronic properties of a specularite (0 0 1) suface show that the geometric structure and electronic properties of the surface of specularite (0 0 1) are significantly changed after absorbing the Cu species, and the structure of O3–Fe1–O1 near the absorbate is more easily affected.
- (2)
- The results of adsorption energy analysis show that Cu2+, Cu(OH)+, and Cu(OH)2 can be spontaneously adsorbed on the surface of specularite (0 0 1), and the adsorption stability of Cu components that are on the surface of specularite increase in the order of Cu2+ < Cu(OH)+ < Cu(OH)2.
- (3)
- The results of the adsorption module and PDOS analysis show that the adsorptions of Cu species on the surface of specularite are different. Cu2+ may be adsorbed on the surface of specularite through a Cu atom directly interacting with O atoms to form Cu–O complexes, while Cu(OH)+ and Cu(OH)2 may be adsorbed on the surface of specularite through the interaction between OI (OII) atoms in –OH and Fe atoms on the surface of specularite to form Cu–O–Fe complexes.
- (4)
- The results of charge transfer analysis show that the charges in the Cu–O bond formed after the adsorption of absorbates were mainly transferred from the Cu 4s orbital to the O 2p orbital and that the charges in the Fe–O bond formed were mainly transferred from the 4s and 2p orbitals of the Fe atom to the 2p orbital of the O atom.
- (5)
- By comparing Cu–O and Fe–O of the three Cu components on the surface of specularite (0 0 1), it can be seen that the bond populations of Cu–O are smaller than those of Fe–O, indicating that Cu–O is more ionic and Fe–O is more covalent.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef]
- Chen, Z.; Yoon, R.-H. Electrochemistry of copper activation of sphalerite at pH 9.2. Int. J. Miner. Process. 2000, 58, 57–66. [Google Scholar] [CrossRef]
- Deng, J.; Wen, S.-M.; Liu, J.; Wu, D.-D.; Feng, Q. Adsorption and activation of copper ions on chalcopyrite surfaces: A new viewpoint of self-activation. Trans. Nonferr. Met. Soc. China 2014, 24, 3955–3963. [Google Scholar]
- Cao, Z.; Zhang, Y.H.; Sun, C.Y.; Cao, Y.D. Activation mechanism of serpentine by Cu (II) and Ni (II) ions in copper-nickel sulfide ore flotation. Zhongguo Youse Jinshu Xuebao/Chin. J. Nonferr. Met. 2014, 24, 506–510. [Google Scholar]
- Qiu, T.; Qiu, X.; Yang, X. Density functional theory and experimental studies of Cu2+ activation on a cyanide-leached sphalerite surface. J. Ind. Eng. Chem. 2016, 45, 307–315. [Google Scholar] [CrossRef]
- Yin, W.Z.; Wang, J.Z.; Wang, N.L.; Luo, X.M.; Wang, Y.L. Effect of flotation reagents on direct reverse flotation of carbonate-containing iron ores. Beijing Keji Daxue Xuebao/J. Univ. Sci. Technol. Beijing 2014, 36, 153–160. [Google Scholar]
- Lin, L.; Jiong-tian, L.; Yong-tian, W.; Yi-jun, C.; Hai-jun, Z.; He-sheng, Y. Experimental research on anionic reverse flotation of hematite with a flotation column. Procedia Earth Planet. Sci. 2009, 1, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Mowla, D.; Karimi, G.; Ostadnezhad, K. Removal of hematite from silica sand ore by reverse flotation technique. Sep. Purif. Technol. 2008, 58, 419–423. [Google Scholar] [CrossRef]
- Ng, W.S.; Sonsie, R.; Forbes, E.; Franks, G.V. Flocculation/flotation of hematite fines with anionic temperature-responsive polymer acting as a selective flocculant and collector. Miner. Eng. 2015, 77, 64–71. [Google Scholar] [CrossRef]
- Araujo, A.; Viana, P.; Peres, A.E.C. Reagents in iron ores flotation. Miner. Eng. 2005, 18, 219–224. [Google Scholar]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. A study of flotation characteristics of monazite, hematite, and quartz using anionic collectors. Int. J. Miner. Process. 2017, 158, 55–62. [Google Scholar] [CrossRef]
- Ze, C.; Mingyang, L.; Yiming, H.; Xiangpeng, G.; Jun, L. Activation Effect and Mechanism of Cu(Ⅱ)/Ni(Ⅱ) lons on Specularite and Chlorite Flotation. Bull. Chin. Ceram. Soc. 2020, 39, 182–186. [Google Scholar]
- Kaka, A. Study the Electronic Structure ofIn 1 x Al x P with variable concentration of Aluminum using Density Functional Theory. Int. J. Therm. Technol. 2015, 55, 2277–4114. [Google Scholar]
- Zaky, R.; Fekri, A.; Gaber, Y.; Moustafa, H.; Abdulrahman, Y. Structural, spectral, dft, ion-flotation and biological studies on transition metal complexes of 2-aminothiazole derivatives. Int. J. Adv. Res. 2016, 4, 1705–1717. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.H.; Chen, Y.; Yu-Qiong, L.I. DFT calculation of amine cation collectors for zinc oxide flotation. J. Guangxi Univ. (Nat. Sci. Ed.) 2009, 34, 67–72. [Google Scholar]
- Jiang, D.; Deng, W.; Lan, Y. A DFT Study of the Effect of Natural Impurities on the Electronic Structure of Sphalerite. Adv. Mater. Res. 2013, 669, 39–45. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Khoso, S.; Sun, W.; Hu, Y. Understanding the activation mechanism of Pb2+ ion in benzohydroxamic acid flotation of spodumene: Experimental findings and DFT simulations. Miner. Eng. 2019, 143, 106006. [Google Scholar] [CrossRef]
- Zhao, G.; Zhong, H.; Qiu, X.; Wang, S.; Gao, Y.; Dai, Z.; Huang, J.; Liu, G. The DFT study of cyclohexyl hydroxamic acid as a collector in scheelite flotation. Miner. Eng. 2013, 49, 54–60. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, J.; Zhao, C. Structure-activity of chelating collectors for flotation: A DFT study. Miner. Eng. 2020, 146, 106133. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, D.; Feng, Q.; Wen, S.; Chang, W. DFT insights into the electronic properties and adsorption mechanism of HS− on smithsonite (1 0 1) surface. Miner. Eng. 2019, 141, 105846. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.; Wang, Y.; Deng, J.; Chen, X. Transition state search study on the migration of Cu absorbed on the S sites of sphalerite (110) surface. Int. J. Miner. Process. 2016, 147, 28–30. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.; Chen, X.; Bai, S.; Liu, D.; Cao, Q. DFT computation of Cu adsorption on the S atoms of sphalerite (110) surface. Miner. Eng. 2013, 46–47, 1–5. [Google Scholar] [CrossRef]
- Deng, J.; Lei, Y.-H.; Wen, S.; Chen, Z. Modeling interactions between ethyl xanthate and Cu/Fe ions using DFT/B3LYP approach. Int. J. Miner. Process. 2015, 140, 1–37. [Google Scholar] [CrossRef]
- Lin, S.; He, J.; Liu, R.; Hu, Y.; Sun, W. Depression behavior and mechanism of pyrogallol on bismuthinite flotation. J. Clean. Prod. 2021, 281, 125322. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.; Chen, J. DFT simulation of the adsorption of sodium silicate species on kaolinite surfaces. Appl. Surf. Sci. 2016, 370, 403–409. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liu, J.; Gao, X.; Hu, Y.; Yuan, Q. Surface Properties and Floatability Comparison of Aegirite and Specularite by Density Functional Theory Study and Experiment. Minerals 2019, 9, 782. [Google Scholar] [CrossRef] [Green Version]
- Sarvaramini, A.; Larachi, F.; Hart, B. Collector attachment to lead-activated sphalerite—Experiments and DFT study on pH and solvent effects. Appl. Surf. Sci. 2016, 367, 459–472. [Google Scholar] [CrossRef]
- Zhang, H.L.; Xu, Z.J.; Chen, D.X.; Hu, B.; Zhou, Q.Q.; Chen, S.D.; Li, S.; Sun, W.; Zhang, C.Y. Adsorption mechanism of water molecules on hematite (104) surface and the hydration microstructure. Appl. Surf. Sci. 2021, 550, 149328. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Pan, M.; Wen, S. Interaction of salicylhydroxamic acid with the surface of MgTi2O5: A study combined DFT and experiment. J. Alloys Compd. 2018, 774, 222–228. [Google Scholar] [CrossRef]
- Yin, X.; Wang, H.; Han, E.-H. Effects of solvation and applied potential on the adsorption behaviors of H, O, OH and H2O on Fe(110) surface. Surf. Sci. 2019, 691, 121504. [Google Scholar] [CrossRef]
- Zhong, W.; Yin, W.; Wang, Y.; Yao, J. Selective flotation of magnesite from dolomite using α-chloro-oleate acid as collector. Powder Technol. 2020, 373, 147–151. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, S.; Deng, J.; Zhao, W. DFT study on the interaction between hydrogen sulfide ions and cerussite (110) surface. Appl. Surf. Sci. 2017, 396, 920–925. [Google Scholar] [CrossRef]
- Rabanal-León, W.; Arratia-Perez, R. Relativistic-DFT study of the electronic structure, bonding and energetic of the [ReF8]− and [UF8]2− ions. J. Chil. Chem. Soc. 2013, 58, 2020–2024. [Google Scholar] [CrossRef] [Green Version]
- Jian-Hua, C.; Xian-Hao, L.; Cui-Hua, Z.; Duan, K.; Jin, G. DFT calculation on relaxation and electronic structure of sulfide minerals surfaces in presence of H2O molecule. J. Cent. South Univ. Technol. 2014, 21, 3945–3954. [Google Scholar]
- Gao, Z.; Li, C.; Sun, W.; Hu, Y. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Nourmohamadi, H.; Aghazadeh, V.; Esrafili, M. A comparative DFT study of Fe3+ and Fe2+ ions adsorption on (100) and (110) surfaces of pyrite: Electrochemical point of view. Surf. Interface Anal. 2019, 52, 1–9. [Google Scholar]
- Wu, D.; Mao, Y.; Deng, J.; Wen, S. Activation mechanism of ammonium ions on sulfidation of malachite (–201) surface by DFT study. Appl. Surf. Sci. 2017, 410, 126–133. [Google Scholar] [CrossRef]
- Wang, Y.; Xian, Y.; Wen, S.; Deng, J.; Wu, D. The electronic structures of magnesium-bearing anosovite (MgnTi3-nO5 0 ≤ n ≤ 1) and its response to flotation. J. Alloys Compd. 2017, 708, 982–988. [Google Scholar] [CrossRef]







| Adsorbates | Bond Length (Å) | |||
|---|---|---|---|---|
| Fe1–O1 | O1–Fe2 | Fe2–O2 | O3–Fe1 | |
| Original | 1.749 | 1.918 | 1.910 | 1.749 |
| Cu2+ | 1.809 | 1.943 | 1.925 | 1.764 |
| Cu(OH)+ | 1.887 | 1.912 | 1.926 | 1.775 |
| Cu(OH)2 | 1.844 | 1.908 | 1.930 | 1.722 |
| Adsorbates | Bond Angles (°) | ||||
|---|---|---|---|---|---|
| Fe1–O1–Fe2 | O1–Fe2–O2 | O3–Fe1–O1 | O1–Fe2–O4 | O4–Fe2–O2 | |
| Original | 118.461 | 101.402 | 115.903 | 86.237 | 82.717 |
| Cu2+ | 119.474 | 100.695 | 108.848 | 87.559 | 83.609 |
| Cu(OH)+ | 119.829 | 99.038 | 111.672 | 86.879 | 83.956 |
| Cu(OH)2 | 118.235 | 100.270 | 112.594 | 87.574 | 83.873 |
| Adsorbates | DCu–Fe1 (Å) | DCu–O1 (Å) | DFe1–OI (Å) | DFe2–OII (Å) | Energy (eV) | |
|---|---|---|---|---|---|---|
| Cu2+ | Before | 1.614 | 1.613 | – | – | – |
| After | 2.387 | 1.987 | – | – | −0.76 | |
| CuOH+ | Before | 1.665 | 1.661 | 1.538 | – | – |
| After | 2.808 | 1.985 | 1.854 | – | −0.85 | |
| Cu(OH)2 | Before | 1.708 | 1.713 | 1.721 | 2.296 | – |
| After | 2.750 | 2.146 | 1.887 | 1.909 | −1.78 |
| Adsorbates | Atom | s | p | d | f | Total | Charge/e | |
|---|---|---|---|---|---|---|---|---|
| Cu2+ | Cu | Before | 1.00 | 0.00 | 10.00 | 0.00 | 11.00 | 0.00 |
| After | 0.78 | 0.12 | 9.81 | 0.00 | 10.71 | 0.29 | ||
| Fe1 | Before | 0.40 | 0.24 | 6.49 | 0.00 | 7.14 | 0.86 | |
| After | 0.41 | 0.38 | 6.54 | 0.00 | 7.32 | 0.68 | ||
| O1 | Before | 1.88 | 4.71 | 0.00 | 0.00 | 6.59 | −0.59 | |
| After | 1.86 | 4.76 | 0.00 | 0.00 | 6.62 | −0.62 | ||
| CuOH+ | Cu | Before | 0.65 | 0.11 | 9.76 | 0.00 | 10.52 | 0.48 |
| After | 0.53 | 0.17 | 9.79 | 0.00 | 10.50 | 0.50 | ||
| OI | Before | 1.90 | 5.03 | 0.00 | 0.00 | 6.93 | −0.93 | |
| After | 1.87 | 4.96 | 0.00 | 0.00 | 6.83 | −0.83 | ||
| Fe1 | Before | 0.40 | 0.24 | 6.49 | 0.00 | 7.14 | 0.86 | |
| After | 0.27 | 0.32 | 6.45 | 0.00 | 7.05 | 0.95 | ||
| O1 | Before | 1.88 | 4.71 | 0.00 | 0.00 | 6.59 | −0.59 | |
| After | 1.86 | 4.78 | 0.00 | 0.00 | 6.64 | −0.64 | ||
| Cu(OH)2 | Cu | Before | 0.62 | 0.09 | 9.44 | 0.00 | 10.16 | 0.84 |
| After | 0.58 | 0.08 | 9.67 | 0.00 | 10.33 | 0.67 | ||
| OI | Before | 1.90 | 4.98 | 0.00 | 0.00 | 6.87 | −0.87 | |
| After | 1.87 | 4.94 | 0.00 | 0.00 | 6.81 | −0.81 | ||
| OII | Before | 1.90 | 4.99 | 0.00 | 0.00 | 6.88 | −0.88 | |
| After | 1.86 | 4.96 | 0.00 | 0.00 | 6.83 | −0.83 | ||
| Fe1 | Before | 0.40 | 0.24 | 6.49 | 0.00 | 7.14 | 0.86 | |
| After | 0.27 | 0.35 | 6.43 | 0.00 | 7.05 | 0.95 | ||
| O1 | Before | 1.88 | 4.71 | 0.00 | 0.00 | 6.59 | −0.59 | |
| After | 1.87 | 4.76 | 0.00 | 0.00 | 6.62 | −0.62 |
| Adsorbates | Bond | Population |
|---|---|---|
| Cu2+ | O1–Cu | 0.14 |
| Cu(OH)+ | O1–Cu | 0.17 |
| OI–Fe1 | 0.33 | |
| Cu(OH)2 | OI–Fe1 | 0.30 |
| OII –Fe5 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huangfu, M.; Li, J.; Zhang, X.; Hu, Y.; Deng, J.; Wang, Y.; Wei, P. Study of the Effect of Absorbed Cu Species on the Surface of Specularite (0 0 1) by the DFT Calculations. Minerals 2021, 11, 930. https://doi.org/10.3390/min11090930
Huangfu M, Li J, Zhang X, Hu Y, Deng J, Wang Y, Wei P. Study of the Effect of Absorbed Cu Species on the Surface of Specularite (0 0 1) by the DFT Calculations. Minerals. 2021; 11(9):930. https://doi.org/10.3390/min11090930
Chicago/Turabian StyleHuangfu, Mingzhu, Jiaxin Li, Xi Zhang, Yiming Hu, Jiushuai Deng, Yu Wang, and Pingping Wei. 2021. "Study of the Effect of Absorbed Cu Species on the Surface of Specularite (0 0 1) by the DFT Calculations" Minerals 11, no. 9: 930. https://doi.org/10.3390/min11090930