Abstract
General information is presented on ten agpaitic occurrences located in southern Brazil and at the border between Brazil and Paraguay. All the Brazilian agpaitic rocks are Late Cretaceous in age, whereas the Paraguayan ones are older than Early Triassic. The most significant occurrence is Poços de Caldas, the largest alkaline massif in South America. In general, these agpaitic rocks contain mineral assemblages that indicate presence of typical halogen-bearing Na–Ca–HFSE phases, eudialyte-, rinkite- and wöhlerite-group minerals being the most frequent ones. However, these associations are indeed more complex in terms of composition, with accessory phases in some cases consisting of various minerals, including U–Th oxides/silicates, Nb oxides, REE–Sr–Ba bearing carbonates–fluorocarbonates–phosphates–silicates and Zr–Na rich silicates. They usually form late magmatic stage to hydrothermal/deuteric assemblages linked with coarse and fine-grained, mainly silica-undersaturated evolved rocks. Data also indicate significant differences in type, amount and composition of agpaitic minerals in all investigated occurrences.
1. Introduction
Agpaitic rocks are defined by the IUGS Subcommission on the Systematics of Igneous Rocks as peralkaline nepheline syenites (and phonolites) characterized by complex Zr and Ti minerals, such as eudialyte and rinkite, rather than simple minerals such as zircon and ilmenite [1,2]. This term was first proposed by [3] to describe rocks from Ilímaussaq complex with a peralkaline index greater than 1.2 and its definition has undergone considerable change since its first introduction [4,5,6]. Recently, [7] proposed that the term “agpaitic rocks” should be used as descriptive terms to distinguish igneous rocks according to their primary magmatic HFSE mineralogy, irrespective of their whole-rock composition.
Agpaitic and miaskitic types can be identified via mineralogy which changes from one type to another [1,7,8]. On this basis, according to [7], the miaskitic types characteristically contain zircon, baddeleyite and titanite, whereas the agpaitic types contain aenigmatite, astrophyllite, eudialyte, lamprophyllite, F-disilicates, and wadeite. In addition, a detailed distinction between “normal” miaskitic variants and low, medium and highly agpaitic up to hyperagpaitic types, depending on the presence of diagnostic minerals, was previously proposed by [9].
This peculiar mineralogy is related to large presences of specific elements, such as Large Ion Lithophile Elements (LILE, such as Li, Na, K and Rb), halogens (F, and Cl), Rare Earth Elements (REE, the lanthanides) and High Field Strength Elements (HFSE, such as Zr, Ti, Nb and U–Th) [8]. Examined in more mineralogical detail, these mineral assemblages correspond to different types of silicates, mainly eudialyte and F-disilicates (rinkite and wöhlerite groups), but they can also involve a wide variety of other species, such as HFSE oxides, REE–Sr-rich phosphates, carbonates, and REE–F carbonates [8,10,11]. Mineral assemblages may also be characterized by the enrichment of major mafic constituents in alkali and iron and by the presence of accessory minerals with high concentrations of Na and Ca in their composition, as suggested by some F-disilicates (e.g., rosenbuschite, wöhlerite). These mineral associations, which are occasionally distinguished by a high content of volatile components (e.g., CO2, F, Cl and H2O), also include sodic and ferric varieties of clinopyroxene and amphibole. Late minerals are not only associated with deuteric stages of crystallization resulting from interactions among water-rich solutions during the cooling of the same magmatic body, but also with mineralizing fluids that percolate through the crystallizing body, leading to the formation of accessory fluorocarbonates and hydrate carbonates.
Agpaitic mineral assemblages are thought to originate from crystallization of residual magmatic liquids rich in very distinctive elements, such as REE, HFSE, LILE, and volatiles (F, Cl). These assemblages may preferentially form in the late-magmatic and hydrothermal/deuteric stages, as indicated by textural evidence (Table 1).

Table 1.
General information on agpaitic occurrences.
Agpaitic assemblages commonly derive from miaskitic peralkaline igneous rocks of foid syenite composition (mainly nepheline syenites and their fine-grained varieties) formed during final magma differentiation stages [7,8]. The petrological evolution of agpaitic rocks, and their associated eudialyte-group minerals, F-disilicates, astrophyllite-group minerals or aenigmatite crystallization, depends upon peralkalinity, silica, chlorine, fluorine, water activities and fO2 [7,10,35]. The processes by which agpaitic mineral assemblages are formed can be also responsible for unusual and exotic suites of interstitial minerals of different classes, mostly of highly complex chemical composition. Thus, some agpaitic occurrences are marked by an unusually large number of rare minerals, such as Lovozero and Khibina with over 500 identified minerals [9] and Mont Saint-Hilaire with approximately 250 minerals [8].
One of the most remarkable characteristics of the Mesozoic–Cenozoic alkaline magmatism that took place in the southern portion of the Brazilian platform is the abundance of more evolved rock-types [14,15,19,20,36,37,38,39,40,41]. Silicate alkaline rocks, the most abundant petrographic type present, include evolved nepheline syenites and syenites and their corresponding fine-grained varieties, forming large isolated, unique or composite intrusions or even multiple plutons. Main examples of these massifs are Poços de Caldas [14,42] and Itatiaia [15,43]. A small fraction of these evolved lithologies consists of agpaitic types, such as lujavrites (melanocratic—in this case, M > 30 vol.%—agpaitic variety of nepheline syenite rich in eudialyte, arfvedsonite and/or aegirine with a laminated structure), khibinites (a variety of eudialyte–nepheline syenite with aegirine, alkali amphibole and many accessory minerals, particularly those containing Ti and Zr) and eudialyte/aenigmatite phonolites [12,14,20,26]. However, the amount of information available on the exotic accessory assemblage found in these agpaitic rocks is still limited. In this paper, we present a general overview of the main agpaitic alkaline rocks of the southern Brazilian platform and the current knowledge of their exotic accessory mineralogy.
2. Alkaline Magmatism in Southern Brazilian Platform: Background Information
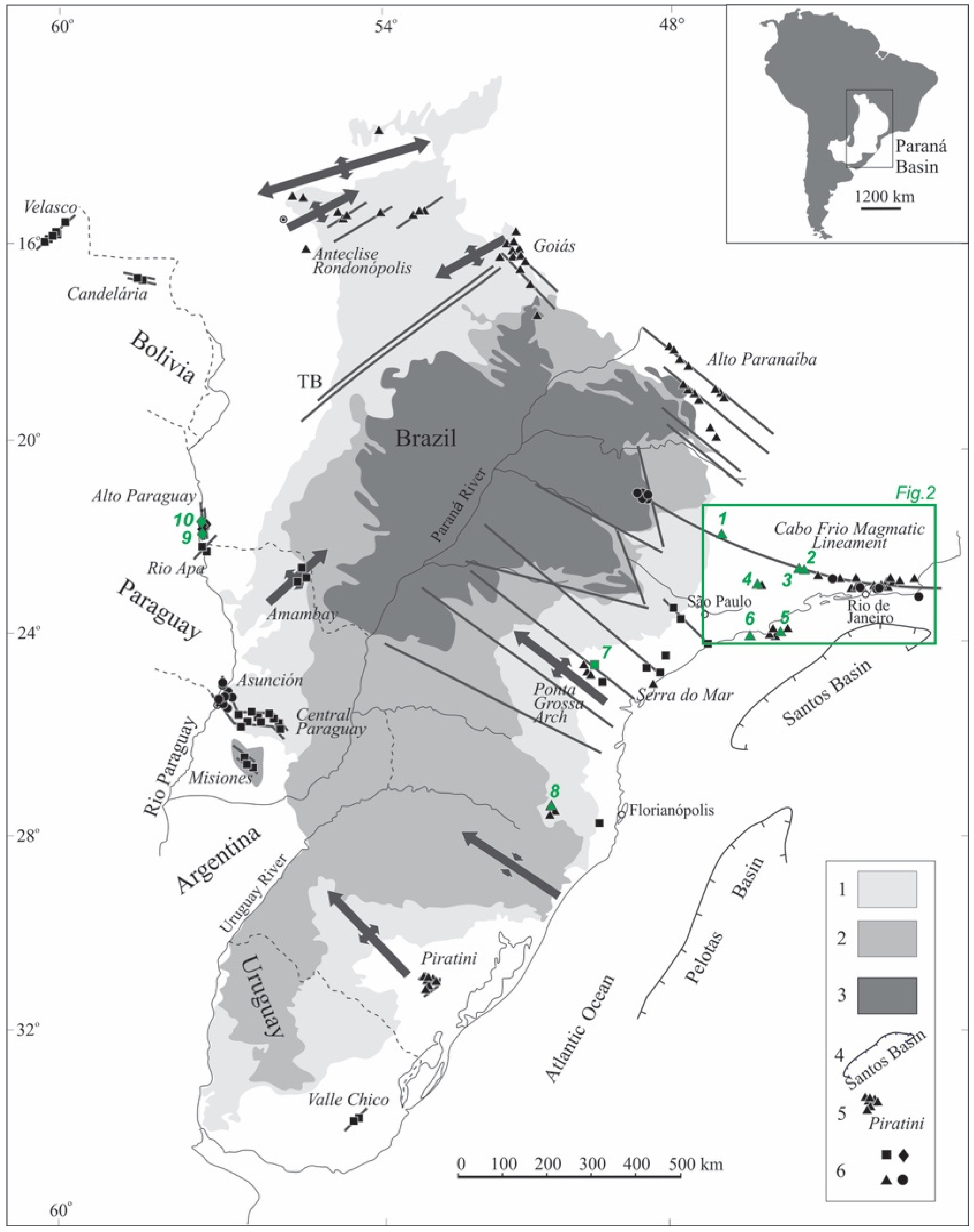
The abundance of alkaline rocks in Brazilian and Paraguayan territories is attested by over two hundred occurrences [44,45]. Subordinately, alkaline types are also present in Bolivia and Uruguay. They are found mainly in and around sedimentary formations of the Paraná and Bauru basins, with their emplacement controlled by regional tectonic factors [46,47] (Figure 1). In Brazil, alkaline centers occur predominantly dispersed over the eastern flanks of the country, but also as several individual bodies along two very distinct NE and NW trends that extend for many kilometers [47,48]. The first trend, which corresponds to the Serra do Mar province, consists of inland and island intrusions parallel to the coasts of São Paulo and Rio de Janeiro (Figure 2). The second trend includes occurrences located in inner portions of the country, forming two distinct groups in the states of Minas Gerais (Alto Paranaíba province) and Goiás (Goiás province), respectively. Some Brazilian carbonatite complexes of the first group, such as Araxá and Tapira, are of economic importance, having been exploited for niobium and phosphate in carbonatites. Poços de Caldas, the largest alkaline massif in South America, is tectonically related to the Cabo Frio Magmatic Lineament, a curved, 1150 km long WNW-ESE-trending feature [47]. Other Brazilian alkaline occurrences are distributed along a prominent NW-trending alignment, the Ponta Grossa arch [49]. Only two alkaline carbonatite complexes occur in southern Brazil, to the west of Florianópolis, being tectonically related to the Anitápolis-Lages lineament in the state of Santa Catarina [50] and the phonolitic volcanism of Piratini, which is associated with the Rio Grande structural feature [51]. In Paraguay, alkaline magmatism is concentrated mainly in the central-eastern part of the country, where a complex NW- to EW-oriented structure, the Asunción rift, controls several such intrusions [52]. This magmatism is also represented by occurrences within domains of the NE-trending Ponta Porã arch in the northeastern part of the country, the Amambay region [53,54], and, less frequently, in the southernmost areas in close relation to the NW-trending Santa Rosa lineament [55]. At the border between Brazil and Paraguay, alkaline bodies follow a narrow NS-trending belt along both margins of the Paraguay River, forming the Alto Paraguay province [32].
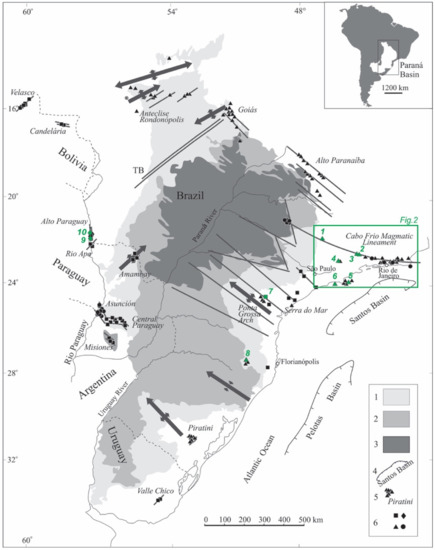
Figure 1.
Alkaline provinces of southeastern Brazil and their relationships with important structural features (axes of main arcs; TS syncline; major fracture zones; simplified after [47]). 1. Late Ordovician to Early Cretaceous Paraná Basin; 2. Early Cretaceous tholeiitic lava flows; 3. Late Cretaceous Bauru Basin; 4. Offshore marginal basins; 5. Alkaline provinces; 6. Age of alkaline rocks (diamonds, Permian-Triassic; squares, Early Cretaceous; triangles, Late Cretaceous; circles, Paleogene). Agpaitic occurrences as follows: 1. Poços de Caldas; 2. Itatiaia; 3. Passa Quatro; 4. Bom Repouso; 5. Búzios Island; 6. Monte de Trigo Island; 7. Itapirapuã; 8. Lages; 9. Cerro Siete Cabezas; 10. Cerro Boggiani.
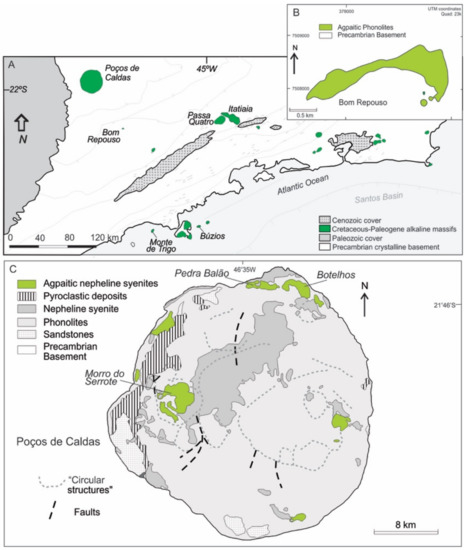
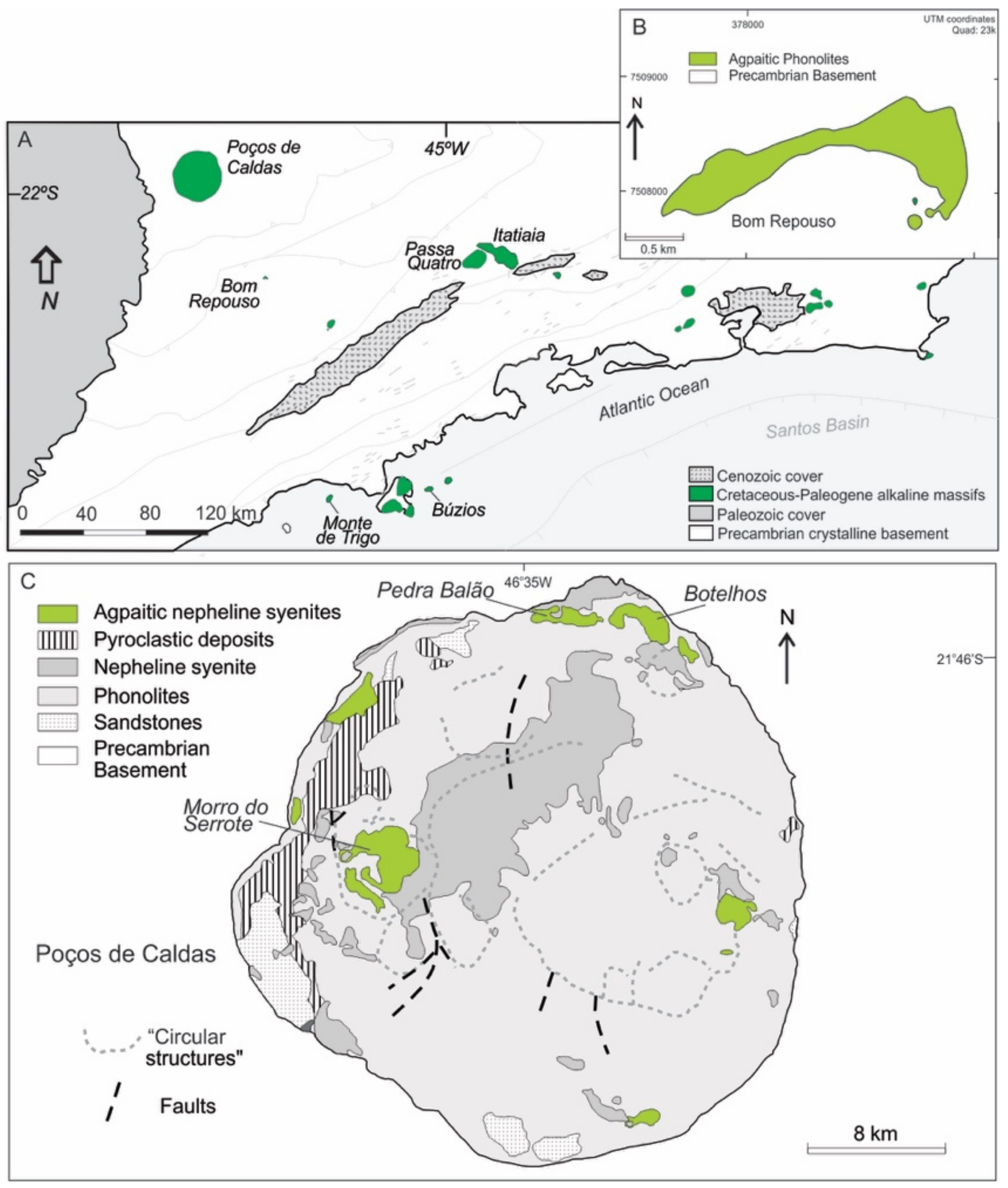
Figure 2.
(A) Simplified geological map of the Serra do Mar alkaline province and surrounding areas in southeastern Brazil [46], indicating the location of studied intrusions. (B) Geological map of the Bom Repouso occurrence, formed solely by agpaitic rocks [21]. (C) Geological map of the Poços de Caldas massif, indicating the mapped units of agpaitic bodies [42].
The alkaline rocks of the southern portions of the Brazilian platform correspond in age to a wide, approximately 200 Ma interval of magmatic activity pulses recorded from the Mesozoic to the Cenozoic [45]. The oldest events (241 Ma) are related to the intrusions that form the Alto Paraguay province [32], among which are agpaitic rocks of the Cerro Boggiani and Cerro Siete Cabezas stocks (Figure 1; Table 1). Some alkaline occurrences are contemporary to tholeiitic lava flows of the Paraná Large Igneous Province [56,57], with an estimated age of about 130 Ma. However, no typical agpaitic rocks are associated with this age interval. Itapirapuã, located in the Ponta Grossa Arch province, is a small occurrence whose recent radiometric Ar/Ar results confirm its age as falling within a 100–110 Ma interval [28] (Table 1). The magmatic events dated ~70–90 Ma [38,45] are the most abundant and widespread ones, assembling all the remaining Brazilian alkaline centers. They include rock bodies of agpaitic affinity, of which the Poços de Caldas [14,58,59,60,61], Itatiaia [15,40,43], Passa Quatro [19,20], Bom Repouso [21], Monte de Trigo Island [25,62], Lages [63] and Búzios Island [64,65] suites are examples.
In general, alkaline magmatism is characterized by the large number of occurrences in a large variety of igneous forms, yet intrusive forms clearly prevail [38,48]. The more abundant silicate alkaline rocks with agpaitic varieties consist of evolved nepheline syenites and syenites and their fine-grained equivalent types, forming large isolated, unique or composite intrusions, or even multiple plutons. On the other hand, less evolved types are present in minor amounts, mainly as small dikes in several occurrences.
Individual alkaline evolved suites with agpaitic rocks range in area from a few m2 to hundreds of km2. In addition to Poços de Caldas, which extends over approximately 800 km2, other occurrences occupying large areas are the Itatiaia (215 km2) and Passa Quatro (148 km2) massifs. Minor intrusions are the Búzios Island (7.5 km2), Itapirapuã (4 km2), Cerro Siete Cabezas (4 km2), Cerro Boggiani (4 km2), Monte de Trigo Island (1.3 km2) and Bom Repouso (1 km2) suites. In all these intrusions, the occurrences of agpaitic rocks are very subordinated or completely minor relative to other alkaline types.
3. Main Alkaline Massifs with Agpaitic Rocks
Except for the Poços de Caldas massif [42], where extensively outcropping agpaitic rocks form shallow subsurface lopolith bodies that are easily distinguished during fieldwork (e.g., lujavrite-khibinite outcrop of Pedra do Balão, khibinite Botelhos area, Morro do Serrote structure) (Figure 2C), and the irregular phonolitic intrusion of Bom Repouso [21] (Figure 2B), all remaining occurrences are present as minor components, especially compared to miaskitic types, being represented mainly by small bodies (stocks, dikes, sills and plugs) of fine-grained silica-undersaturated microsyenites and phonolites (Table 1). A general description of the main alkaline massifs containing agpaitic rocks is presented below.
3.1. Poços de Caldas
One of the largest alkaline igneous bodies in the world [42], Poços de Caldas intrudes Precambrian rocks, forming an isolated circular complex detached from numerous Late Cretaceous alkaline occurrences of the Alto Paranaíba (to the NE) and the Serra do Mar (to the SE) provinces (Figure 2C). The size of this intrusion (>800 km2) is smaller than Khibina massif (Kola Peninsula, ~1350 km2; [66]) but comparable to that of Lovozero (Kola Peninsula, 600 km2; [66]) and Pilanesberg (South Africa, ~625 km2; [67]). The massif includes dominant phonolites and their subvolcanic and plutonic counterparts (tinguaites and nepheline syenites, respectively), with a presence of strongly agpaitic types (lujavrites and khibinites) that are especially abundant in the northern ring area (Pedra Balão).
Along the western border of the Poços de Caldas complex, there is a depressed area, Vale do Quartel, which consists of pyroclastic products (agglomerates, lapilli, tuffs) and lava flow remnants, mostly of mafic–ultramafic composition. Phlogopite lamprophyric dikes occur in these and other sites, such as in the Osamu Utsumi uranium mine and amidst basement mangerites close to the massif’s border (Minas Pedras quarry), associated with a wide variety of basic–ultrabasic types and scarce carbonatite dikes. Considerable inner portions of the complex have been subject to hydrothermal alteration and brecciation to some extent due to subvolcanic magmatic activity, which led to the formation of different types of mineralization, mainly U, Th, Mo and Zr.
The bulk-rock major and trace element geochemistry of nepheline syenites and phonolites, with the marked presence of eudialyte, F-disilicates, aenigmatite and lamprophyllite, indicates the agpaitic affinity of these highly evolved melts. Unlike other agpaitic intrusions, a most peculiar feature in the Poços de Caldas is the absence of pure miaskitic rocks, because the prevailing nepheline syenites and phonolites are characterized by an assemblage showing the contemporaneous presence of minerals typical of miaskitic (e.g., zircon, baddeleyite and titanite) and agpaitic rocks (such as F-disilicates, eudialyte, aenigmatite and astrophyllite), [14] which are defined as a “transitional agpaitic” type, as proposed by [7]. The Poços de Caldas evolved melts are qualitatively derived from mafic–ultramafic melts via clinopyroxene removal in a prolonged fractional crystallization process. It is observable that the highly evolved melts are formed by removal of potassic alkali feldspar and nepheline associated with very small amounts of “incompatible” element-rich accessory phases [14].
3.2. Itatiaia
Itatiaia alkaline massif, the second largest alkaline occurrence in Brazil, intrudes the Precambrian basement, being overlain by Tertiary sedimentary rocks in some areas. The massif has a roughly ellipsoidal shape, its major, NW-trending axis lying parallel to regional tectonic lineaments of the Ribeira belt. The massif is located in the Serra da Mantiqueira ridge domains near the Passa Quatro and Morro Redondo massifs to the southeast of the Poços de Caldas complex, its terrain reaching altitudes as high as 2792 m at the Agulhas Negras peak, the fifth-highest peak in Brazil. The occurrence has been the subject of several studies [15,36,39,40]. A new geological map of Itatiaia published recently [43] suggests for the massif an evolution from a migratory magmatic center that manifested as ring structures and successive moon-shaped intrusions from SE to NW. Three different sectors are recognized in the Itatiaia alkaline massif based on lithological and geomorphological characteristics: a southeastern, a central, and a northern one. The southeastern sector consists of miaskitic to agpaitic nepheline syenites that are cut by aphyric to porphyritic dikes of phonolite and trachyte (some of them with pseudoleucites), and nephelinites. The central sector, which forms a ring-like structure, consists of most of the same lithologies plus silica-oversaturated rock types (pulaskites, nordmarkites and quartz-alkali feldspar syenites) in its inner portions. A small intrusive body of alaskite occurs near the center of the ring. The northern sector includes nepheline syenites and nordmarkites, and locally cumulatic melagabbro and biotite monzonite. The mineralogy, including peralkaline syenites and silica-oversaturated rock types, is characterized by the presence of variable F-disilicates and numerous accessory phases bearing HFSE and REE [40].
3.3. Passa Quatro
Similarly to the Poços de Caldas and Itatiaia occurrences, the Passa Quatro massif, which reaches altitudes as high as 2797 m [68], is conditioned by the WNW-ESE-trending Cabo Frio alignment, a prominent structural feature that affects several alkaline intrusions in southern Brazil [39]. The massif also takes in part of the Serra do Mar province, which assembles the two abovementioned Late Cretaceous occurrences, lying mostly across the central portions of the lineament area. In plan view, it appears as a subcircular unit intruded into the Precambrian basement. Field relationships with host rocks are only observed at a few sites due to the presence of a large detrital talus and polymictic breccia bodies [19]. The massif is mainly composed of strongly undersaturated felsic intrusive (nepheline syenites and alkali syenites) and subvolcanic rocks (phonolites) forming small, dominantly NE-oriented dikes. Basic and intermediate lithologies are completely lacking. A noticeable characteristic of the Passa Quatro nepheline syenites and alkali syenites, with minor phonolitic dikes, is the marked presence of accessory minerals of complex and variable composition [20].
3.4. Bom Repouso
Bom Repouso corresponds to a crescent-moon shaped intrusion that forms an irregular, 2.6 km long EW-trending body and two small satellite plugs consisting primarily of silica-undersaturated agpaitic rocks (Figure 2B). Lying 62 km NNW of the Poços de Caldas complex intruding Precambrian granitoid rocks, Bom Repouso has been geologically investigated [21]. Phonolites and porphyritic phonolites are the dominant lithologies. The alkaline rocks are all of sodic composition and agpaitic affinity, with some exceedingly rare accessory minerals including calciohilairite and niobophyllite.
3.5. Búzios Island
In the state of São Paulo, Brazil, alkaline coastal islands include São Sebastião, the largest island encompassing three isolated syenitic stocks (São Sebastião, Serraria and Mirante), Búzios and Vitória to the northeast, and Monte do Trigo massif to the west. All these islands follow a NE trend that is tectonically related to the Southeastern Brazilian Continental Rifting [47]. Similarly to the previously mentioned alkaline centers, these coastal islands also take in part of the Serra do Mar province. Búzios Island corresponds to an alkaline body of irregular shape lying approximately 30 km from the continent [64,65]. In its NW and SW sectors, the stock is emplaced into Precambrian basement rocks, namely charnockites and, subordinately, gneisses and amphibolites. The stock consists principally of syenitic rocks cut by numerous dikes, most of them NE-trending ones. The dikes consist of two distinct suites. The first, felsic, of variable composition, ranges from silica-undersaturated (nepheline/sodalite microsyenites and sodalite phonolites) to silica-saturated (microsyenites and trachytes), the most widespread rocks across the island, and silica-oversaturated (rhyolites) types. The second suite is mafic–ultramafic in composition, consisting of different alkaline petrographic types (alkali basalts, basanites, tephrites, trachybasalts and lamprophyres). A primary assemblage of mostly silicate minerals bearing Zr, Ti, Nb and REE, and rare, chemically complex post-magmatic phases is identified in association with nepheline (sodalite) microsyenite and phonolite dikes of agpaitic affinity [65].
3.6. Monte de Trigo Island
Monte de Trigo is a small, ellipse-shaped alkaline stock located approximately 10 km from the coastline (Barra do Una beach). The stock intrudes Precambrian gneissic and migmatitic rocks of the Ribeira belt [25], being morphologically conditioned by N40W and N50E lineaments. Upon systematic investigation of the stock [25] it has been proposed that the development of this occurrence involved four distinct episodes of magmatic activity. A cumulate mafic–ultramafic association is thought to correspond to the oldest event, predominantly showing nepheline-bearing olivine melagabbros and melatheralites and their fine-grained varieties together with clinopyroxenites and olivine clinopyroxenites. An intrusive breccia pipe of about 50–60 m in diameter showing fragments derived from the mafic–ultramafic body and from the Precambrian country-rocks corresponds to the second magmatic event. The third magmatic event is represented by a miaskitic nepheline to nepheline-bearing alkali feldspar syenite stock in genetical association with nepheline microsyenite dikes of miaskitic and agpaitic affinity cutting all the previously described rock types. The last magmatic event corresponds to the intrusion of a series of porphyritic dikes of aphanitic groundmass whose composition varies from lamprophyre to phonolite. Agpaitic nepheline microsyenite dikes, the less frequent types, are thought to have crystallized from a more evolved magma fraction than the miaskitic dikes. These types are characterized by the presence of several exotic phases, such as eudialyte- and wöhlerite-group minerals [26].
3.7. Itapirapuã
Itapirapuã is a small alkaline stock of irregular NW-trending shape composed of silica-undersaturated rocks, melanite-bearing and melanite-free nepheline syenites, and subordinate mafic types (biotite melteigites, malignites) [27]. Decimetric, mainly NW-oriented phonolite (tinguaite) dikes cut both the intrusive body and the granitic country-rocks. Centimetric carbonatite veins penetrate the nepheline syenites. Magmatic breccia extending over a small area is also present at the southern part of the intrusion. Iron ore, chiefly consisting of idiomorphic magnetite, had been exploited in the area several years ago. Eudialyte-group minerals are described in association with peralkaline syenitic dikes [27].
3.8. Lages
Extending across 2100 km2 and emplaced into sedimentary Gondwanic sequences, the alkaline-carbonatitic district of Lages, which is limited to the west by the Paraná flood basalts, is related to the uplift of a large crustal block, the Lages Dome [29]. The alkaline rocks display hypabyssal forms and the volcanic intrusions follow a ring-shaped distribution dispersed as relatively small outcrops over a vast region. They are assembled into four main groups: carbonatites and associated rocks, pipe breccias and kimberlites, ultrabasic rocks, and leucocratic rocks [30,50]. Carbonatites occupy the northern sector of the dome, with Fazenda Varela, a 20 m wide tabular body, as their main exposure area. Kimberlites are found at a variety of sites. Diatremes and pipe breccias (tuffisitic breccias) are also known to exist. Ultrabasic rocks occur as dikes of variable dimensions and compositions (olivine melilitite, olivine nephelinite, basanite). Minor dikes of phonotephrite and trachyphonolite are also present. Leucocratic terms prevail over other rock types, corresponding to approximately 50 km2 of the total outcropping area, represented by peralkaline and porphyritic peralkaline phonolites and nepheline syenites. Agpaitic minerals are associated with peralkaline phonolites at Fazenda Nova do Tributo, an area in the central portion of the alkaline district [30].
3.9. Cerro Siete Cabezas
Cerro Siete Cabezas represents the southernmost alkaline occurrence of the Alto Paraguay province. The complex is circular in shape, with a central depression evidencing cauldron subsidence, being related to two minor satellite bodies [32]. Cerro Siete Cabezas rises up to 158 m above the alluvial plain of the Paraguay River, consisting mainly of medium to coarse-grained silica-undersaturated syenitic rocks. The satellites are characterized by a more variable composition, with prevalence of quartz-bearing varieties in stock I and the presence of both silica-undersaturated and silica-oversaturated syenitic types in stock II. The authors of [32] report eudialyte as a common accessory mineral in nepheline syenites within the main body of the complex.
3.10. Cerro Boggiani
The northernmost alkaline center along the Paraguay River, the rounded-shape intrusion of Cerro Boggiani is part of a series of major complexes and some minor stocks, lava flows and dikes that constitute the Early Triassic Alto Paraguay province, the oldest magmatic activity around the Paraná basin [32]. The intrusion consists of monadnocks that crop out above alluvial sediments of the Paraguay River, the largest intrusion forming a small, 0.5 km diameter hill lower than 150 m [34]. The complex mainly consists of sodalite syenites and nepheline syenites, with modal nepheline and sodalite contents of 16–28% and 8–17%, respectively. The extrusive rocks (lava flows and dikes) are phonolitic in composition. The Cerro Boggiani rocks show a late-stage magmatic to hydrothermal/deuteric mineralogy with several unusual accessory phases [34,69,70,71,72].
4. Main Features of Agpaitic Rocks
4.1. Rock-Forming Minerals
Data on rock-forming minerals were compiled from previous works [14,20,21,33,40,62,65]. The dominant mineral assemblage of the agpaitic rock types includes alkali feldspar and nepheline for the silica-undersaturated varieties and alkali feldspar and quartz for the silica-oversaturated varieties. These rocks are mostly leucocratic, with clinopyroxene and amphibole as the main mafic phases. Alkali feldspar with perthitic to mesoperthitic exsolution lamellae is the most abundant mineral. Plagioclase is very scarce, usually with a low anorthite content. Feldspathoids consist mainly of nepheline and, subordinately, sodalite, reaching high modal amounts in some occurrences (e.g., 42% in Cerro Boggiani). Clinopyroxene varies in composition, mainly from aegirine–augite to aegirine (predominant). Zoning is a common feature of the clinopyroxenes, as indicated by chemical and optical evidence. The normal zoning points to enrichment in Fe and Na content towards the crystal rims, frequently accompanied by corresponding variations in pleochroism, from light green to dark green. Amphiboles mainly present sodic compositions, including riebeckite, arfvedsonite and Mg-arfvedsonite, sometimes occurring as late-stage poikilitic crystals, partially or totally associated with clinopyroxenes.
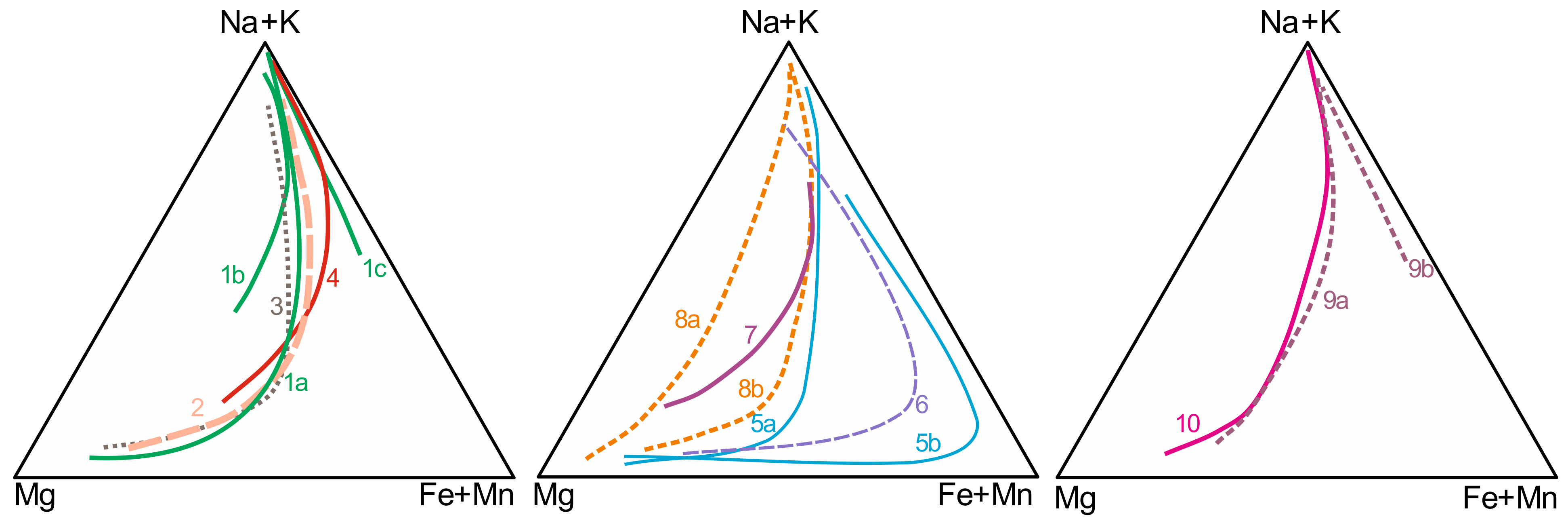
Clinopyroxene, present not just in agpaitic varieties but also in the main rocks of the studied alkaline massifs, is considered an important monitor of the degree of magmatic evolution [14,20,27,39]. The main trends, considering the differentiation of the entire massifs, indicate compositions varying from diopside to hedenbergite to aegirine–augite and finally to aegirine (Figure 3), characterizing progressive enrichments in Fe3+ and Na for the formation of agpaitic rocks. The main trends for Poços de Caldas, Itatitaia, Passa Quatro, Cierro Siete Cabezas, Cerro Boggiani, Itapirapuã, amphibole-free rocks from Búzios, and part of the peralkaline phonolites from Lages are very similar. The clinopyroxene of Monte de Trigo and part of Búzios Island matches present compositional variations partially controlled by amphibole crystallization, with initial extensive evolution in the diopside–hedenbergite series; on the other hand, some peralkaline dikes from Lages do not present significant Fe2+ enrichment prior to Na+ increase. Poços de Caldas, Bom Repouso and Passa Quatro aegirines present high amounts of Zr (up to 3 wt% and 1.73 of ZrO2 in Passa Quatro and Bom Repouso, respectively), especially for agpaitic varieties [20,21,73], whereas in Itatiaia clinopyroxenes Zr is very low [40]. The incorporation of Zr contents in clinopyroxene could have an important influence in the assemblage of rare accessory minerals [11].
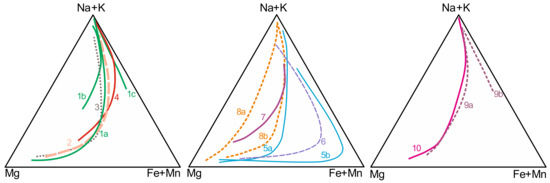
Figure 3.
Main clinopyroxene trends of alkaline massifs with agpaitic rocks in southeastern Brazil. 1. Poços de Caldas [14,73]; a. main trend of both nepheline syenites and phonolites, b. lujavrite and khibinite types, c. eudialyte-bearing phonolites and nepheline syenites; 2. Itatiaia [15,40]; 3. Passa Quatro [19,20]; 4. Bom Repouso [21]; 5. Búzios Island [65]; 6. Monte de Trigo Island [62]; 7. Itapirapuã [27]; 8. Lages [30]; 9. Cerro Siete Cabezas [32]; 10. Cerro Boggiani [32].
4.2. Geochemical Characteristics
The peralkaline varieties of the main massifs of this study share important geochemical fingerprints, summarized in Table 2, mainly presenting a typical sodic affinity and high levels of Zr (up to 7847 ppm in Cerro Boggiani), Nb (up to 1211 ppm in Búzios Island), and variable REE contents, with the light ones displaying higher concentrations (La and Ce, predominantly). They also present very low MgO, Cr, Ni and V values. Poços de Caldas is the best-known agpaitic occurrence in the Brazilian platform, with higher values in the peralkaline index (PI up to 1.37; [14,42]). No significant Eu anomaly (Eu/Eu* = EuN/(SmNxGdN)0.5 = 1.0–1.1) is recorded in Poços de Caldas nepheline syenites and phonolites [14]. The peralkaline Itatiaia rocks correspond predominantly to varieties of strongly undersaturated nepheline syenites (ne-normative > 20%; [15,40]. The Passa Quatro peralkaline suite includes silica-undersaturated lithotypes, represented mainly by nepheline syenites and alkali syenites, with minor phonolitic dikes with a high degree of incompatible element enrichment, especially Zr and Nb [20]. The Bom Repouso occurrence is represented by felsic silica-undersaturated rocks of phonolitic composition forming the main body and the two small satellites, with high ne-normative contents (7.5–28.8%) being the highest values related to the satellites [21]. In the Búzios island suite, peralkaline phonolitic dikes are the exception in a dominant potassic association of miaskitic affinity [65]. The peralkaline rocks of Monte de Trigo occur as agpaitic nepheline microsyenite dikes of highly variable mineralogy, found only at a few sites within the massif, mainly intrusives in melatheralites, and present high ne- and ac-normative contents [26]. For Itapirapuã massif [27,74], agpaitic compositions are found for some nepheline syenites and a phonolite dike with higher peralkaline index values. Itatiaia, Passa Quatro, Bom Repouso and Monte de Trigo peralkaline rocks present strong light to heavy REE fractionation and significant negative spikes for Eu. The normative composition of Lages peralkaline phonolites are characterized by the abundance of nepheline and a great amount of acmite; almost all samples of this occurrence lack a negative Eu anomaly [75]. The peralkaline varieties from the Cerro Siete Cabezas (associated with the main intrusion and formed by silica-undersaturated syenitic rocks) and from the Cerro Boggiani (formed by fine- and coarse-grained silica-undersaturated varieties) are between trachytic and phonolitic minima in the petrogenetic residual diagram; the Eu negative anomaly is considered a peculiar feature in all complexes of the Alto Paraguay Province, including Cerro Siete Cabezas and Cerro Boggiani [32].

Table 2.
Whole rock geochemical characteristics of peralkaline varieties from the studied massifs. Souces: 1. [14], 2. [15,40]; 3. [19]; 4. [21]; 5. [65]; 6. [26]; 7. [27,74]. * just one sample for La and Ce values; 8. [75]; 9. and 10. [32].
4.3. Mineral Resources
Poços de Caldas is the most important agpaitic occurrence in the Brazilian platform in terms of mineral deposits associated with fine-grained nepheline syenitic rocks (tinguaites and phonolites). These intensely fractured rocks were modified by hydrothermal and weathering processes, especially in the central-eastern area of the massif. U–Mo–Zr (Usamu Utsumi or Cercado mine, decommissioned in 1995), Mo–U–Zr (Agostinho deposit) and Th–REE–Nb (Morro do Ferro deposit) deposits have been intensively exploited from Poços de Caldas rocks [76]. Most of the active exploration sites associated with hydrothermal altered rocks were decommissioned in the 1980s, including the Zr mine and “garimpo” [42]. Bauxite (laterite with 80–90 wt% Al2O3, in anhydrous basis) mines and deposits, mainly related to the tinguaite ring-dike, have been explored in many localities in inner parts of the plateau, particularly around the city of Poços de Caldas [76]. Bauxite mines and deposits are also present in Bom Repouso [21]. Small local economically unimportant deposits of bauxite are also known to be present in inner parts of the Passa Quatro massif [77].
5. Rare Accessory Minerals of Agpaitic Rocks
Several rare and exotic mineral phases occur in agpaitic rocks as a result of their crystallization from highly evolved magmas that are enriched in HFSEs and other incompatible elements and halogens. In agpaitic rocks, defining the mineral assemblages is not too easy: they can be subdivided into the early magmatic (±liquidus), late-stage, and hydrothermal/deuteric assemblages. Generally, in the compared intrusions, the early magmatic assemblages are made up of alkali feldspar, nepheline and pyroxene followed by opaque oxides ± fluorite ± sodalite. The late-stages are mainly related to the crystallization of F-disilicates (rinkite- and wöhlerite-groups) ± eudialyte ± aenigmatite ± lamprophyllite; the replacement (hydrothermal/deuteric) assemblages are pectolite, catapleiite, and so on until reaching alteration assemblages (Table 1 and Table 3). Among these mineral species, aenigmatite and eudialyte-, rinkite- and wöhlerite-groups are the most abundant and characteristic ones, with Table 4, Table 5, Table 6 and Table 7 showing the results of some representative analyses.

Table 3.
HFSE accessory mineralogy of agpaitic rocks.

Table 4.
Disilicate compositions.

Table 5.
Cyclosilicate compositions.

Table 6.
Inosilicate compositions.

Table 7.
Phyllosilicate compositions.
The rinkite mineral group includes several Ti-rich disilicates, such as rinkite-(Ce), and end-members, such as hainite, mosandrite-(Ce) and rosenbuschite [88], which are commonly found in several occurrences. Chemical analyses of hainite from two localities within the Poços de Caldas massif (Bortolan and Prefeitura quarries, in nepheline syenites and phonolites) reveal a calcium silicate mineral of quite homogeneous composition bearing high amounts of TiO2 (8.80–9.83 wt%), Na2O (6.17–8.40 wt%) and F (5.13–7.06 wt%) and some LREE (Table 4). Mosandrite-(Ce), a rare Ca-Ti silicate, has only been identified in the Poços de Caldas massif, Búzios Island and Cerro Boggiani rocks. Data from the Poços de Caldas massif and Búzios Island rocks indicate that the mineral contains significant amounts of Na2O (4.27–7.35 and 8.57 wt%, respectively) and F (3.93–5.40 and 7.02 wt%, respectively), having been strongly enriched in LREE, with Ce2O3 concentrations reaching up to 8.88 and 10.44 wt%, respectively. Compositions of rosenbuschite exhibit small variations in major components TiO2 (6.79–8.56 wt%), ZrO2 (12.1–14.8 wt%), CaO (24.09–25.91 wt%), Na2O (9.34–10.9 wt%) and F (5.04–8.16 wt%) and allow the Passa Quatro specimen to be distinguished as having the lowest Nb2O5 (0.58–0.97 wt%) and highest MnO (3.75–4.56 wt%) contents. Rinkite-(Ce)ss is present in the Passa Quatro, Itatiaia and Poços de Caldas assemblages. When compared to other minerals of the same group, especially rosenbuschite, data indicate, for the first specimen, a low concentration of ZrO2 and considerable amounts of LREE, with Ce2O3 clearly prevailing over La2O3 (Table 4). An important Ti host mineral is lamprophyllite, described in the Poços de Caldas massif [14,78,82] (Table 4). The Poços de Caldas lamprophyllites have high average concentrations of TiO2 (26.74–29.06 wt%), MnO (4.31–6.97 wt%), Na2O (10.99–11.51 wt%) and SrO (13.06–16.96 wt%) along with some REE content (REE up to 2.03 wt%) and F (0–2.05 wt%). Representative mineral analysis of the Morro do Serrote rocks [78] does not differ greatly from the previously available mean composition, with high contents of TiO2 (27.82 wt%), MnO (6.97 wt%), Na2O (11.51 wt%) and SrO (13.06 wt%; a second analysis showing 16 wt%).
Wöhlerite, hiortdahlite, låvenite and normandite, Na-, Ca- and Zr-rich disilicates of the cuspidine–wöhlerite group [89], are known to be present in most of the compiled occurrences (Table 3). Hiortdahlite, a rich calcium-silicate phase (27.48–31.24 wt%), had its composition determined at four occurrences, where it was characterized by high proportions of Zr2O (16.0–17.76 wt%), Na2O (6.80–9.41 wt%) and F (6.33–8.36 wt%). A sample collected from the Itatiaia massif is noteworthy for its unusual concentration of TiO2 (6.98 wt%). Låvenite is a common constituent of the group, analyses having been performed for five occurrences indicating heterogeneous composition and large differences regarding TiO2 (2.94–8.43 wt%), ZrO2 (16.5–27.76 wt%), MnO (4.23–9.66 wt%), CaO (8.09–14.89 wt%) and F (2.97–5.76 wt%). SiO2 and Na2O variations lie within a narrow interval (Table 4). Analyses for normandite derive from Poços de Caldas and Passa Quatro syenitic rocks, showing significant differences in the average values. Compared to those of Passa Quatro, Poços de Caldas samples are higher in TiO2 and poor in ZrO2 and tend to be slightly enriched in FeO and CaO. Na2O and F remain constant, whereas MnO is higher in Passa Quatro than in Poços de Caldas analyses (Table 4). Wöhlerite is a Ca–Zr–Nb silicate whose composition remains, to a great extent, constant regarding major elements of different occurrences: ZrO2 (14.0–15.91 wt%), Nb2O5 (9.25–13.43 wt%), CaO (25.7–28.98 wt%), Na2O (7.40–8.23 wt%) and F (3.29–4.67 wt%). MnO concentrations are higher in Passa Quatro mineral specimens (Table 4).
Eudialyte-group minerals are one of the major Zr-hosts in agpaitic rocks and include several Na- and Ca-rich cyclosilicates with variable amounts of Fe, Mn, Nb, and REE, among other cations [7,90,91]. Minerals of this group occur in various localities (Table 3) and eudialytess have been analyzed in most of the investigated occurrences. They are compositionally variable in SiO2 (47.60–51.80 wt%), CaO (8.17–12.51 wt%), MnO (4.97–5.38 wt%), and Na2O (8.11–13.05 wt%). ZrO2 and FeO concentrations are practically constant (11.94–13.35 wt% and 3.33–5.09 wt%, respectively). A Mn-rich variety, the manganoeudialyte, is found at the locality of Pedra Balão in the Poços de Caldas massif, and also as single crystal in the Poços de Caldas nepheline syenites. The manganoeudialytes show lower SiO2 and ZrO2 (42.76–44.01 wt% and 10.32–10.99 wt%, respectively) and higher MnO (6.04–9.56 wt%) and SrO (4.68–6.54 wt%) contents. Nb2O5 reaches up to 3.97 wt% (Table 5). Kentbrooksite is restricted to the Passa Quatro massif with the mineral presenting high proportions of ZrO2 (10.8 wt%) and MnO (6.29 wt%) and significant amounts of LREE. Other analyzed minerals include catapleiite and Ca-catapleiite, Zr-rich silicates represented by two chemically distinct varieties. The first is from Poços de Caldas and is rich in Na2O (9.09 wt%); the second is from Cerro Boggiani and contains high CaO (13.82 wt%) (Table 5).
Compositions for aenigmatite, a Ca–Ti–Na mineral bearing high amounts of FeO (35.01–39.80 wt%), are available from Búzios Island, Cerro Boggiani and Poços de Caldas rocks, whereas analysis for lorenzenite, a silicate with abundant TiO2 (43.46 wt%) and Na2O (17.4 wt%), is available for Poços de Caldas samples (Table 6). Pectolite and serandite are described in Lages and Poços de Caldas occurrences, with chemical analyses given in the last table. Pectolite is richer in CaO (20.41–30.63 wt%) while serandite is higher in MnO (19.63–21.70 wt%). Analysis of pectolite from Poços de Caldas samples [82] also shows high MnO content (14.08 wt%). Na2O remains practically constant in both minerals (pectolite: 8.92–9.77 wt%; serandite: 7.89–8.40 wt%). Chemical compositions of astrophyllite are determined from three occurrences, indicating a FeO-rich (20.66–26.79 wt%) silicate mineral with some variation in TiO2 (8.87–10.90 wt%) and MnO (8.28–13.3 wt%). A specimen from Passa Quatro is enriched in ZrO2 (3.75 wt%). Kupletskite, a mineral present in Passa Quatro rocks, is abundant in MnO (25.8 wt%) and contains significant amounts of TiO2 (8.71 wt%), ZrO2 (5.00 wt%), FeO (10.3 wt%) and K2O (6.05 wt%).
The hydrate mineral neptunite (5.38 wt% H2O) is characterized by high TiO2 (17.39 wt%), FeO (14.66 wt%) and K2O (6.67 wt%) concentrations. It is described in the Bortolan quarry within the Poços de Caldas massif [78]. Another hydrate mineral (12.63 wt% H2O), tuperssuatsite, is a rare accessory identified at the same quarry [79], being characterized by rich Fe2O3 (25.45 wt%) content and the significant presence of Na2O (4.26 wt%) (Table 7).
In addition to the above constituents, Itatiaia, Passa Quatro and Cerro Boggiani rocks carry a very extensive list of highly diversified minerals formed under varied conditions, mostly late-stage magmatic and hydrothermal/deuteric. Some even derived from metasomatic fluids. The Poços de Caldas massif is especially marked by the outstanding amount of poorly known accessory phases, mostly found in outcrops of the decommissioned Bortolan quarry. In total, 28 different species are preliminarily discussed upon investigating major and accessory minerals of local lithotypes [78]. Some minerals, such as belovite-(Ce) NaCeSr3(PO4)3F, chlobartonite K6Fe24S26(Cl), götzenite Ca4NaCa2Ti(Si2O7)2(OF)F2, polezhaevaite-(Ce), polezhaevaite-(La) NaSr(Ce,La)F6, stronadelphite Sr5(PO4)3F, strontiofluorite SrF2, and vishnevite Na8(Al6Si6)O24(SO4)·2H2O, mostly analyzed through EDS, have been described and reported for the first time in Brazilian rocks [78]. Recently, the presence of fluorcaphite SrCaCa3(PO4)3F, strontium apatite (Ca,Sr)5(PO4)3F, and georgechaoite NaKZr(Si3O9)·2H2O, together with stronadelphite Sr5(PO4)3F and strontiofluorite SrF2, both already identified, were also reported for the first time in this massif [14].
A great number of rare accessory minerals occur in association with agpaitic and miaskitic rocks (Table 8). Pyrochlore is the most significant Nb-bearing oxide, being represented by three different species: plumbopyrochlore, uranopyrochlore and yttropyrochlore. Uraninite and thorianite correspond to the main U and Th oxides, respectively, whereas thorite, a rare Th-rich silicate, is also present. Other oxide phases include baddeleyite, perovskite and zirconolite. REE-rich minerals are represented by numerous crystalline phases consisting of phosphates, silicates and fluorocarbonates. Monazite-(Ce) and -(La) is one of the most common phosphate minerals. In some occurrences britholite-(Ce), a basic phosphate/silicate phase containing high amounts of SiO2, La2O3, Ce2O3 and CaO and low amounts of P2O5, is found coexisting or inter-grown with apatite. Rare LREE–HFSE-bearing silicates have chevkinite-(Ce) and perrierite-(Ce) as their principal members, showing a significant compositional range that indicates inverse correlation of FeO and CaO, as observed in Itatiaia rocks [40]. Carbonates and fluorocarbonates, mostly REE-bearing phases, are frequent and include various constituents that are largely present in e.g., Cerro Boggiani peralkaline nepheline syenites and phonolites [34,69]. In such occurrences, the minerals present are burbankite and remondite-(Ce), rare Sr and Ce carbonates; fluorocarbonates of the bastnäsite–parisite–synchysite series forming fine-scale complex intergrowths fibroradial to plumose aggregates; cordylite-(Ce), a Ba- fluorocarbonate; and hydrate carbonates containing ancylite-(Ce) and galgenbergite-(La) as main phases.

Table 8.
Rare accessory minerals in agpaitic and miaskitic rocks.
6. Petrological Considerations
A few studies addressing petrogenetic aspects of the agpaitic occurrences discussed are available in the literature, the most recent and complete ones being the contributions on Itatiaia [40] and Passa Quatro [20] massifs and, most recently, a paper on the mineralogy and geochemistry of Poços de Caldas agpaitic rocks [14]. In general, however, these and other alkaline occurrences in southern Brazil and eastern Paraguay have been interpreted by various authors [20,32,42,62,65] as formed by extensive long-term fractional crystallization processes operating at shallow surface levels (approximately <5 km) from alkaline mafic to ultramafic parental magmas. The occurrences are also believed to have originated from low-degree partial melting of geochemically enriched mantle lithologies. Thus, an episode of mantellic metasomatic pre-enrichment is commonly suggested in order to explain particularities such as the enrichment in halogens, HFSEs, REEs, and incompatible elements [7,8]. A basanitic (plagioclase-bearing) and/or nephelinitic (feldspar-free) composition is preferentially proposed as the primary magma. Small dikes of basanite rocks are commonly known in many places, either directly associated with evolved (miaskitic and agpaitic) peralkaline rocks in the field (e.g., the Búzios and Monte de Trigo coastal islands [25,65]) or emplaced close to alkaline intrusions over distinct areas along the Serra da Mantiqueira [36,41,59].
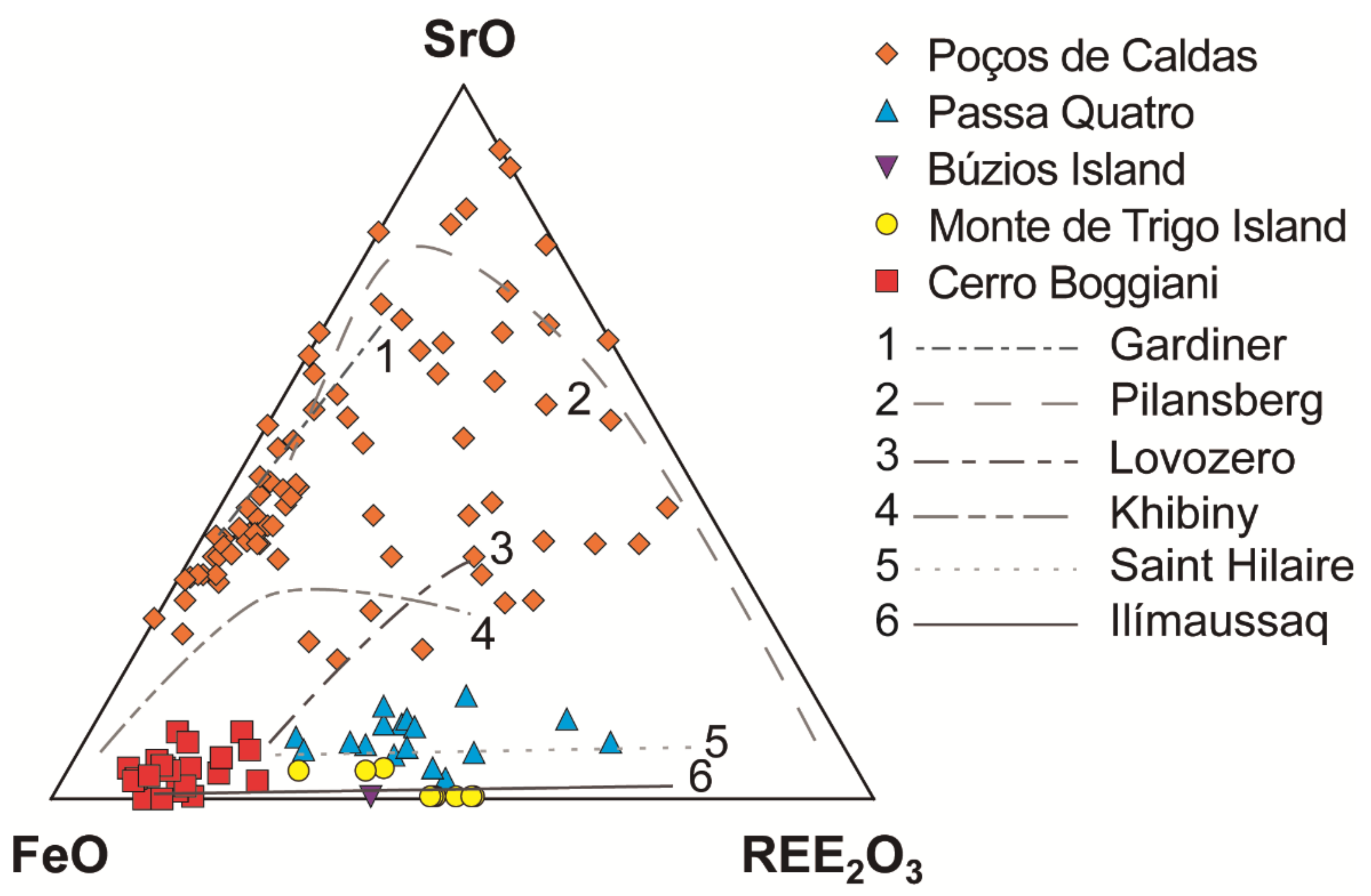
Numerous studies suggest that both compositions are likely to represent the most important types of parental melts for agpaitic rocks [7]. The distinction of the parental melts of each locality can also be suggested based on the distribution of Sr and REE in eudialyte-group minerals [26,91]. Agpaitic rocks derived from alkali basaltic or basanite magmas are normally low in Sr and have pronounced negative Eu anomalies, due to the extensive plagioclase fractionation that occurred during magmatic evolution. The composition of the eudialyte-group minerals reflects this geochemical signature. Figure 4 exhibits the low Sr content of eudialyte from the Monte de Trigo, Passa Quatro and Búzios massifs, supporting the interpretation of a basanite/alkali basalt parental magma for the whole suite of rocks [20,26,65]. Moreover, no Sr-bearing mineral occurs in these localities. In contrast, when derived from more SiO2-undersaturated magmas, the Sr content is high, and no marked spikes are noticed for Eu. The parental mafic magma of the agpaitic rocks of the Poços de Caldas massif is interpreted as a more SiO2-undersaturated parental magma that evolved without plagioclase fractionation [14,42], and where the eudialyte-group minerals have very high Sr content (Figure 4), together with the occurrence of lamprophyllite, a Sr-rich disilicate, among several other Sr-rich minerals (Table 3 and Table 8).
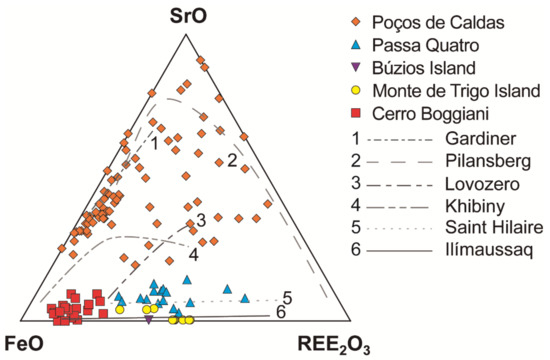
Figure 4.
Ternary plot of FeO-SrO-REE2O3 for the eudialyte-group minerals. Data sources: [14,20,26,34,65,72,81,82,92]. Trend lines of the worldwide EGM compositions according to [26] and references therein.
In the model proposed for the evolution of the Passa Quatro massif [20], differentiation starts from mafic to ultramafic parental magmas, with generation of phonolites in the first stages being followed by generation of peralkaline phonolites in subsequent stages and, finally, late and post-magmatic phases. Removal of diopside-alkali feldspar–plagioclase would promote generation of alkali syenites, whereas removal of nepheline–aegirine–titanite would lead to generation of nepheline syenites. Removal of many different accessories, e.g., zircon, fluorite, F-disilicates, eudialyte, etc., would originate eudialyte-bearing nepheline syenites and phonolites.
Suppression of such phases is responsible for the enrichment of the residual magmatic liquids with incompatible elements, which onsets the crystallization of HFSE- and REE-F-rich minerals. At the end of this crystallization path, phases containing CO2 and H2O in their composition form as REE-carbonates and REE-fluorocarbonates. Therefore, according to the abovementioned authors, the magma that originated the Passa Quatro agpaitic rocks is characterized by geochemical traits such as enrichment in incompatible elements (HFSEs, REEs), high F, and minor CO2 and H2O under low oxygen fugacity and water activity conditions.
Based on textural relationships and overall accessory mineral chemistry, the Cerro Boggiani nepheline syenites and phonolites are characterized as a sequence of different minerals that usually crystallize under magmatic, late-stage magmatic and hydrothermal conditions [32,34,69,70,71]. Among the hydrothermal fluids affecting those rocks, it is suggested that a distinction be made between deuteric (H2O-rich) and metasomatic of carbonatitic origin (CO2-rich) stage ones. Included in the magmatic stages are U–Th minerals (uraninite–thorianite and thorite), thorianite being more frequently generated under hydrothermal/deuteric conditions. Nb-bearing minerals with pyrochlore as their main phase (oxide) originate in the magmatic field, advancing progressively into the hydrothermal field. Zr-rich silicate phases (eudialyte and lorite), ks, ions, and minor compatible elements, however data for the first mineral is more consistent. Rosenbuschite is restricted to the hydrothermal stage. It is inferred that carbonatitic fluids rich in F, Na and REE percolated through the subvolcanic system and metasomatically interacted with the peralkaline and agpaitic silicate melts during and after their late-stage magmatic crystallization and hydrothermal/deuteric alteration. As a result, a highly diverse mineral assemblage consisting of silicates (Ca-catapleiite), phosphates (monazite), phosphates/silicates (britholite) and especially of different types of carbonates (hydrate carbonates: ancylite, galgenbergite; REE-carbonates: burbankite; REE-fluorocarbonates: cordylite, bastnäsite–parisite–synchisite) was generated from REE–Ca–Sr–Ba-rich carbonatitic fluids of dominantly metasomatic origin under hydrothermal conditions, at relatively low temperatures.
The agpaitic occurrence of Monte de Trigo Island is associated with small nepheline microsyenite dikes that are genetically related to a miaskitic nepheline syenite stock, which occupies mainly the central parts of the intrusion. Compositionally, these dikes consist of alkali feldspar, nepheline, mafic minerals (clinopyroxene, amphibole, biotite) and varied accessories including magnetite, titanite, apatite, zircon, perovskite, pyrochlore, baddeleyite, and zirconolite, which impart a miaskitic character to the rocks. The evolution of the nepheline-bearing alkali feldspar syenite from fractional crystallization led to the formation, as final differentiates, of agpaitic synplutonic nepheline microsyenite dikes, with a high concentration of volatiles and pronounced enrichment in incompatible elements [62]. The rock may be classified as transitional agpaitic [7,8]. The major characteristic of the assemblage is the abundance of late-crystallized calcic minerals. Britholite-(Ce) and Zr–Nb-silicates of the cuspidine–wöhlerite series showing high Ca and Na contents are relatively common. The eudialyte-group minerals are marked by a significant enrichment in Ca, a moderate enrichment in Nb and REE, and a somewhat low Na content when compared to their counterparts in other Brazilian alkaline occurrences [26]. REE and Y concentrate preferentially in britholite-(Ce) (30.9–58.5 wt%) and perovskite (33 wt%), but they are also found in significant amounts in zirconolite (7.7–14.2 wt%), pyrochlore (3.2–13.3 wt%), eudialyte (2.4–4.1 wt%), and in minerals of the cuspidine–wöhlerite group (<3 wt%) [62]. Instead of contributing to the origin of Zr–Nb-bearing silicates, Ti tends to form titanite. At the borders of the dike, accessory minerals are represented exclusively by låvenite and eudialyte-group forms.
Itatiaia rocks are defined as forming a continuous spectrum from nepheline to quartz syenite and granite fields across the SiO2-saturation boundary [15,40]. In the petrogenetic residual diagram, the nepheline syenites clearly trend towards the phonolitic minimum, while the silica-oversaturated syenites trend towards the rhyolitic minimum without reaching it. The dikes that cut the intrusions are mainly phonolitic in composition. The accessory minerals vary widely in composition, being identified, in addition to the widespread phases (e.g., titanite, Fe–Ti oxides, apatite, zircon), as other, less common ones of complex composition (e.g., REE-HFSE silicates, Zr and Nb oxides, phosphates). Secondary phases comprise Fe-carbonates, fluorocarbonates and hydrate phases (catapleiite). Additionally, variations in major- and trace-element compositions of Itatiaia rocks are directly related to the amount of accessory phases present, as suggested by the enriched chondrite-normalized REE distribution patterns of least- and most-evolved nepheline syenites [40]. The presence of chevknite/perrierite (±allanite) and the abundance of pseudobrookite in quartz syenites and the presence of Ca–F-disilicates, britholite, and pyrophanite-rich compositions in nepheline syenites are among the most striking differences in the accessory phase assemblages. Fluorapatite, an early crystallizing phosphate phase, is typical of silica-oversaturated rocks in contrast to britholite, which frequently joins the apatite in the nepheline syenite. The britholite present is characterized by a remarkable concentration of REE that reaches up to 62 wt%, with La2O3 prevailing over Ce2O3. The latest crystallized phases in Itatiaia rocks are found as interstitial material, consisting of pectolite, hydrate catapleiite and REE-F carbonates. Two main mineral reactions, both recognized on thin sections, are considered very characteristic of the Itatiaia rocks [40]: (1) the corrosion of early formed titanite crystals and (2) the instability of zircon + fluorite, which react to form F-disilicates due to the remaining concentration of fluorine and other elements in interstitial liquids after the predominant crystallization of felsic phases.
Despite being the occurrence that carries the highest diversity of accessory and exotic phases in the entire agpaitic suite, the mineralogy of Poços de Caldas has not been yet investigated systematically in detail. The only such study available [78] identified, using various analytical techniques, 28 different mineral species, all collected at the Bortolan quarry. These minerals were described and classified mainly based on chemical (WDS and EDS analyses) and crystallographic data; no petrological information on them is presented in the study mentioned, nor is there any other descriptive contribution focusing specifically on tuperssuatsiaite [79], hainite [80], eudialyte [81] and lamprophyllite–normandite [82]. New major oxide, trace element and Sr–Nd isotope analyses coupled with detailed petrographic and mineralogical descriptions, supported by microprobe analyses and LA–ICP–MS mineral chemical study, of the main accessory phases for the Poços de Caldas were carried out by [14]. The eudialyte-group minerals are associated with main to late-stage crystallization assemblages and have colorless to slightly pinkish large anhedral/poikilitic or anhedral aspects. They are especially abundant in lujavrites and khibinites, totaling between 5% and 15% of the modal composition. Lamprophyllite is a phase of late stage of crystallization, commonly forming aggregates, sometimes radiated, and intergrowths with aegirine crystals. Normandite and rinkite occur mainly as single crystals, sometimes as aggregates.
The agpaitic massifs of the Brazilian platform also present distinctive Ti- and Zr-bearing complex silicate assemblages (Table 3 and references therein). Zr- and F-rich disilicates, such as hiortdahlite, wöhlerite and låvenite, prevail in agpaitic rocks from Monte de Trigo Island, in addition to eudialyte. There are no complex Ti-rich silicates, as this element preferentially appears in titanite. The agpaitic rocks from Búzios Island, Passa Quatro, Cerro Boggiani and Itatiaia massifs have somewhat similar paragenesis, with the presence of complex Ti- and Zr-rich silicates. They contain the same Zr- and F-rich disilicates that occur in the Monte de Trigo Island, in addition to rosenbuschite and Ti- and F-rich disilicates, such as hainite, normadite, rinkite-(Ce), and mosandrite-(Ce). In these localities, Ti also occurs in aenigmatite and astrophyllite-group minerals. Eudialyte occurs in all of them except Itatiaia. Thus, the increasing fluorine trend identified by [10] in agpaitic rocks from the Oslo Rift is the main trend of magmatic evolution that led to the formation of the agpaitic rocks from Monte de Trigo Island, Búzios Island, Passa Quatro, Cerro Boggiani and Itatiaia, although locally some samples also show trends of increasing alkali and water.
On the other hand, the Poços de Caldas agpaitic rocks have practically no Zr- and F-rich disilicates, but have several complex Ti-rich silicates, such as hainite, normandite, rinkite-(Ce), lamprophyllite, aenigmatite, and astrophyllite-group minerals. The eudialyte-group minerals, which are quite abundant in this massif, are the major carrier of Zr, along with hydrated zirconosilicates and some Zr that is incorporated in aegirine. It seems that in this case the increasing alkali trend [10] is the main trend that led to the formation of the agpaitic rocks from Poços de Caldas. Moreover, Poços de Caldas has villiaumite, an almost pure NaF mineral that occurs in rocks with extreme alkali enrichment, often associated with hyperagpaitic rocks [7,8].
7. Final Remarks
- ▪
- Agpaitic rocks occur as minor components, being usually subordinate and younger compared to associated miaskitic units in Mesozoic to Cenozoic occurrences in the Brazilian platform.
- ▪
- The Poços de Caldas massif displays the largest exposure of agpaitic rocks, including lujavrite and khibinite bodies, carrying mineral assemblages that are exclusive to and typical of agpaitic conditions. The massif contains the most significant number of exotic minerals among the occurrences.
- ▪
- Eudialyte-group, rinkite-group and cuspidine–wöhlerite-group minerals are the main typical constituents of the studied agpaitic rocks, but other exotic phases, such as aenigmatite, astrophyllite, lamprophyllite, etc., are also described in agpaitic rocks.
- ▪
- The studied agpaitic rocks were generated from extensive long-term fractional crystallization processes operating at shallow surface levels (<about 5 km), from highly alkaline mafic to ultramafic parental magmas and deriving from a metasomatized mantle source.
- ▪
- After the early stages of differentiation, fractional crystallization continues after the generation of miaskitic phonolites, reaching peralkaline phonolite compositions in subsequent stages; late and post-magmatic processes also play important roles in the formation of the exotic mineralogy.
- ▪
- After formation of exotic mineralogylites, the enrichment of residual magmatic liquids in incompatible elements may occur. Subsequently, mineral phases such as REE-carbonates and REE-fluorocarbonates form, containing CO2 and H2O in their composition.
- ▪
- Geochemical evidence suggests enrichment in REE and incompatible elements and slight to strong LREE/HREE fractionation. Normalized diagrams for incompatible elements are marked by strong negative anomalies in Ba, Sr, P and Ti, whereas the distribution of chondrite-normalized REE normally exhibits a concave upward pattern.
- ▪
- Some occurrences (e.g., Poços de Caldas massif and Monte de Trigo Island) would be more appropriately described as transitional agpaitic, as they contain HFSE minerals such as titanite and eudialyte, which are characteristic of both miaskitic and agpaitic suites.
- ▪
- Villiaumite, a mineral marking the transition from agpaitic to strongly agpaitic stages and characterized by extreme alkali enrichment, is described in fine-grained nepheline syenitic rocks of the Poços de Caldas massif.
Author Contributions
Conceptualization, C.d.B.G.; investigation, C.d.B.G., R.G.A., G.E.E.R., V.G. and E.R.; data curation, all; writing—original draft preparation, all; writing—review and editing, all; visualization, G.E.E.R., R.G.A. and V.G.; project administration, C.d.B.G.; funding acquisition, C.d.B.G. and E.R. All authors have read and agreed to the published version of the manuscript.
Funding
São Paulo Research Foundation, FAPESP (Processes 2019/13174-3 and 2019/22084-8).
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the São Paulo Research Foundation, FAPESP, for the financial support (Processes 2019/13174-3 and 2019/22084-8). The authors appreciated and acknowledge the helpful comments and suggestions of two anonymous reviewers, and the editorial support of Aleksandra Milićev, Assistant Editor of Minerals journal.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sørensen, H. On the agpaitic rocks. In Proceedings of the Report of the International Geological Congress 21st Session Norden, Copenhagen, Denmark, 15–25 August 1960; Volume 13, pp. 319–327. [Google Scholar]
- Le Maitre, R.W. Igneous Rocks. A Classification and Glossary of Terms. In Reccomendations of the International Union of Geological Sciences, Subcommission on the Systematics of Igneous Rocks, 2nd ed.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Ussing, N.V. Geology of country around Julianchaab, Greenland. Medd. Om Groland 1911, 38, 376. [Google Scholar]
- Gerasimovskii, V.I. Geochemistry and mineralogy of bepheline syenite intrusions. Geochemistry 1956, 5, 494–510. [Google Scholar]
- Sørensen, H. The Alkaline Rocks; Jonh Wiley & Sons: Hoboken, NJ, USA, 1974; p. 622. [Google Scholar]
- Edgar, A.D. On the use of the term ‘Agpaitic’. Mineral. Mag. 1974, 39, 729–730. [Google Scholar] [CrossRef]
- Marks, A.W.M.; Markl, G. A global review on agpaitic rocks. Earth-Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Sørensen, H. The agpaitic rocks: An overview. Miner. Mag. 1997, 61, 485–498. [Google Scholar] [CrossRef]
- Khomyakov, A.P. Mineralogy of Hiperagpaitic Rocks; Claredon Press: Oxford, UK, 1995; p. 223. [Google Scholar]
- Andersen, T.; Erambert, M.; Larsen, A.O.; Selbekk, R.S. Petrology of nepheline syenite pegmatites in the Oslo Rift, Norway: Zirconium silicate mineral assemblages as indicators of alkalinity and volatile fugacity in mildly agpaitic magma. J. Pet. 2010, 51, 2303–2325. [Google Scholar] [CrossRef]
- Andersen, T.; Elburg, M.; Erambert, M. Contrasting trends of agpaitic crystallization in nepheline syenite in the Pilanesberg Alkaline Complex, South Africa. Lithos 2018, 312–313, 375–388. [Google Scholar] [CrossRef]
- Ulbrich, H.H.G.J.; Ulbrich, M.N.C. The lujavrite and khibinite bodies in the Poços de Caldas alkaline massif, southeastern Brazil: A structural and petrographic study. Rev. Bras. Geoc. 2000, 30, 615–622. [Google Scholar] [CrossRef]
- Vlach, S.R.F.; Ulbrich, H.H.G.J.; Ulbrich, M.N.C.; Vasconcelos, P.M. Melanite-bearing nepheline syenite fragments and 40Ar/39Ar age of phlogopite megacrysts in conduit breccia from the Poços de Caldas Alkaline Massif (MG/SP), and implications. Braz. J. Geol. 2018, 48, 391–402. [Google Scholar] [CrossRef]
- Guarino, V.; Lustrino, M.; Zanetti, A.; Tassinari, C.C.G.; Ruberti, E.; De Gennaro, R.; Melluso, L. Mineralogy and geochemistry of a giant magma reservoir: The Late Cretaceous Poços de Caldas potassic alkaline complex (SE Brazil). Lithos 2021, 398–399, 106330. [Google Scholar] [CrossRef]
- Brotzu, P.; Gomes, C.B.; Melluso, L.; Morbidelli, L.; Morra, V.; Ruberti, E. Petrogenesis of coexisting SiO2-undersaturated to SiO2-oversaturated felsic igneous rocks: The alkaline complex of Itatiaia, Southeastern Brazil. Lithos 1997, 40, 133–156. [Google Scholar] [CrossRef]
- Rosa, P.A.S.; Ruberti, E. Nepheline syenites to granitic rocks from the Itatiaia Alkaline Massif, southern Brazil: New geological and petrological insights. In Proceedings of the 8th Hutton Symposium on Granites and Related Rocks, Book of Abstracts, Florianópolis, Brazil, 20–25 September 2015; Volume 1, pp. 40–200. [Google Scholar]
- Rosa, P.A.S. Geologia e Evolução Petrogenética do Maciço Alcalino de Itatiaia. Ph.D. Thesis, University of São Paulo, Sao Paolo, Brazil, 2017; p. 137. [Google Scholar]
- Sonoki, L.K.; Garda, G.M. Idades K-Ar de rochas alcalinas do Brasil Meridional e Paraguai Oriental: Compilação e adaptação às novas constantes de decaimento. Bol. Ig-Usp Série Científica 1988, 19, 63–85. [Google Scholar] [CrossRef]
- Brotzu, P.; Barbieri, M.; Beccaluva, L.; Garbarino, C.; Gomes, C.B.; Macciotta, G.; Melluso, L.; Morbidelli, L.; Ruberti, E.; Sigolo, J.B.; et al. Petrology and geochemistry of the Passa Quatro alkaline complex, Southeastern Brazil. J. S. Am. Earth Sci. 1992, 6, 237–252. [Google Scholar] [CrossRef]
- Guarino, V.; Gennaro, R.; Melluso, L.; Ruberti, E.; Azzone, R.G. The transition from miaskitic to agpaitic rocks, as highlighted by the accessory phase assemblages in the Passa Quatro alkaline complex (Southeastern Brazil). Can. Miner. 2019, 57, 1–23. [Google Scholar] [CrossRef]
- Rosa, P.A.S. Geologia e Petrologia da Suíte Alcalina do Bom Repouso, MG. Master’s Thesis, University of São Paulo, Sao Paolo, Brazil, 2012; p. 182. [Google Scholar]
- Rosa, P.A.S.; Ruberti, E.; Vasconcelos, P.M.; Thiede, D.S. 40Ar/39Ar Ages of Bom Repouso Alkaline Suite, MG State. In Proceedings of theProgram Abstract, 9th South American Symposium on Isotope Geology, São Paulo, Brazil, 6–9 April 2014; Volume 1, p. 184. [Google Scholar]
- Enrich, G.E.R.; Ruberti, E.; Gomes, C.B.; Alves, F.R. Caracterização faciológica dos diques de nefelina microssienitos e fonólitos agpaíticos da Ilha dos Búzios (SP). In Proceedings of the X Simpósio de Geologia do Sudeste, Diamantina, Brazil, 1–4 November 2007; p. 24. [Google Scholar]
- Enrich, G.E.R.; Ruberti, E.; Azzone, R.G.; Gomes, C.B. Chemical composition of eudialyte from Búzios Island massif. In Proceedings of the 34st International. Geology Congres Brisbane, Brisbane, Australia, 5–10 August 2012. (cd-rom). [Google Scholar]
- Enrich, G.E.R.; Ruberti, E.; Gomes, C.B. Geology and geochronology of Monte de Trigo Island alkaline suite, southeastern Brazil. Rev. Bras. Geoc. 2009, 39, 67–80. [Google Scholar] [CrossRef]
- Enrich, G.E.R.; Ruberti, E.; Azzone, R.G.; Gomes, C.B. Eudialyte-group minerals from the Monte de Trigo alkaline suite, Brazil: Composition and petrological implications. Braz. J. Geol. 2016, 46, 411–426. [Google Scholar]
- Gomes, C.B. Petrologia do maciço alcalino de Itapirapuã, SP. Bol. Iga-Usp 1970, 1, 77–197. [Google Scholar] [CrossRef][Green Version]
- Gomes, C.B.; Azzone, R.G.; Ruberti, E.; Vasconcelos, P.M.; Sato, K.; Enrich, G.E.R. New age determinations for the Banhadão and Itapirapuã complexes in the Ribeira Valley, Southern Brazil. Braz. J. Geol. 2018, 48, 403–414. [Google Scholar] [CrossRef]
- Scheibe, L.F. Geologia e Petrologia do Distrito Alcalino de Lages, SC, Brasil. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 1986; p. 224. [Google Scholar]
- Traversa, G.; Scheibe, L.F.; Barbieri, M.; Beccaluva, L.; Coltorti, M.; Conte, A.M.; Garbarino, C.; Gomes, C.B.; Macciota, G.; Morbidelli, L.; et al. Petrology and mineral chemistry of the alkaline district of Lages, SC, Brazil. Geochim. Bras. 1994, 8, 179–214. [Google Scholar]
- Roldan, L.F.; Machado, R.; Steiner, S.S.; Warren, L.V. Análise de lineamentos estruturais no Domo de Lages (SC) com uso de Imagens de satélite e mapas de relevo sombreado. Geologia USP Série Científica 2010, 10, 57–72. [Google Scholar] [CrossRef]
- Comin-Chiaramonti, P.; Gomes, C.B.; Censi, P.; Gasparon, M.; Velázquez, V.F. Alkaline complexes from the Alto Paraguay Province at the border of Brazil (Mato Grosso do Sul State) and Paraguay. In Mesozoic Cenozoic Alkaline Magmat Brazil Platform; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 71–148. [Google Scholar]
- Comin-Chiaramonti, P.; Gomes, C.B.; DeMin, A.; Ernesto, M.; Gasparon, M. Magmatism along the high Paraguay River at the border of Brazil and Paraguay: A review and new constrains on emplacement ages. J. S. Am. Earth Sci. 2015, 58, 72–81. [Google Scholar] [CrossRef]
- Comin-Chiaramonti, P.; Renzulli, A.; Ridolfi, F.; Enrich, G.E.R.; Gomes, C.B.; De Min, A.; Azzone, R.G.; Ruberti, E. Late-stage to deuteric/metasomatic accessory phases from the Cerro Boggiani agpaitic complex (Alto Paraguay Alkaline Province). J. Am. Earth Sci. 2016, 71, 248–261. [Google Scholar] [CrossRef]
- Markl, G.; Marks, M.A.W.; Frost, B.R. On the controls of oxygen fugacity in the generation and crystallization of peralkaline rocks. J. Petrol. 2010, 51, 1831–1847. [Google Scholar] [CrossRef]
- Brotzu, P.; Melluso, L.; D’Amelio, F.; Lustrino, M. Potassic Dykes and Intrusions of the Serra do Mar Igneous Province (SE Brazil); Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 443–472. [Google Scholar]
- Brotzu, P.; Melluso, L.; Bennio, L.; Gomes, C.B.; Lustrino, M.; Morbidelli, L.; Morra, V.; Ruberti, E.; Tassinari, C.C.G.; D’Antonio, M. Petrogenesis of the Early Cenozoic potassic alkaline complex of Morro de São João, southeastern Brazil. J. Am. Earth Sci. 2007, 24, 93–115. [Google Scholar] [CrossRef]
- Morbidelli, L.; Gomes, C.B.; Beccaluva, L.; Brotzu, P.; Conte, A.M.; Ruberti, E.; Traversa, G. Mineralogical, petrological and geochemical aspects of alkaline and alkaline-carbonatite associations from Brazil. Earth-Sci. Rev. 1995, 39, 135–168. [Google Scholar] [CrossRef]
- Enrich, G.E.R.; Azzone, R.G.; Ruberti, E.; Gomes, C.B.; Comin-Chiaramonti, P. Itatiaia, Passa Quatro and São Sebastião Island, the Major Alkaline Syenitic Complexes from the Serra do Mar Region; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 419–441. [Google Scholar]
- Melluso, L.; Guarino, V.; Lustrino, M.; Morra, V.; Gennaro, R. The REE-and HFSE-bearing phases in the Itatiaia alkaline complex (Brazil) and geochemical evolution of feldspar-rich felsic melts. Miner. Mag. 2017, 81, 217–250. [Google Scholar] [CrossRef]
- Azzone, R.G.; Ruberti, E.; Silva, J.C.L.; Gomes, C.B.; Enrich, G.E.R.; Hollanda, M.H.B.M.; Tassinari, C.C.G. Upper Cretaceous weakly to strongly silica-undersaturated alkaline dike series of the Mantiqueira Range, Serra do Mar alkaline province: Crustal assimilation processes and mantle source signatures. Braz. J. Geol. 2018, 48, 373–390. [Google Scholar] [CrossRef]
- Ulbrich, H.H.; Vlach, S.R.F.; Demaiffe, D.; Ulbrich, M.N.C. Structure and Origin of the Poços de Caldas Alkalinesi; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 367–418. [Google Scholar]
- Rosa, P.A.S.; Ruberti, E. Nepheline syenites to syenites and granitic rocks of the Itatiaia alkaline massif, Southeastern Brazil: New geological insights into a migratory ring complex. Braz. J. Geol. 2018, 48, 347–372. [Google Scholar] [CrossRef]
- Gomes, C.B.; Comin-Chiaramonti, P. An Introduction to the Alkaline anda Alkaline-Carbonatitic Magmatism in and around the Paraná Basin; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Platf. Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 21–29. [Google Scholar]
- Gomes, C.B.; Comin-Chiaramonti, P. Magmat. Alcalino Cont. da Região Merid. Da Plataforma Bras; Edusp/Fapesp: São Paulo, Brazil, 2017; 608p. [Google Scholar]
- Almeida, F.F.M. Relações tectônicas das rochas alcalinas mesozoicas da região meridional da Plataforma Sul-Americana. Rev. Bras. Geoc. 1983, 13, 139–158. [Google Scholar] [CrossRef]
- Riccomini, C.; Velázquez, V.F.; Gomes, C.B. Tectonic controls of the Mesozoic and Cenozoic alkaline magmatism in the Central-Southeastern Brazilian Platform; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 31–56. [Google Scholar]
- Ulbrich, H.H.; Gomes, C.B. Alkaline rocks from continental Brazil. Earth-Sci. Rev. 1981, 17, 135–154. [Google Scholar] [CrossRef]
- Gomes, C.B.; Ruberti, E.; Comin-Chiaramonti, P.; Azzone, R.G. Alkaline magmatism in the Ponta Grossa Arch, SE Brazil: A review. J. Am. Earth Sci. 2011, 32, 152–168. [Google Scholar] [CrossRef]
- Scheibe, L.F.; Kawashita, K.; Gomes, C.B. Contribuição à geocronologia do complexo alcalino de Lages, SC. Ii Simpósio Sul-Bras. De Geol. Florianópolis An. 1985, 2, 299–307. [Google Scholar]
- Philipp, R.P.; Viero, A.P.; Comin-Chiaramonti, P.; Gomes, C.B. Mesozoic alkaline rocks of Rio Grande do Sul; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 573–590. [Google Scholar]
- Comin-Chiaramonti, P.; DeMin, A.; Gomes, C.B.; Appendix, I. Magmatic rock-types from the Asunción-Sapucai graben: Description of the Occurrences and Petrographical Notes; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 1996; pp. 275–330. [Google Scholar]
- Gomes, C.B.; Comin-Chiaramonti, P.; Velázquez, V.F. A synthesis on the alkaline magmatism of Eastern Paraguay. Braz. J. Geol. 2013, 43, 745–761. [Google Scholar] [CrossRef]
- Comin-Chiaramonti, P.; Cundari, A.; Degraff, J.M.; Gomes, C.B.; Piccirillo, E.M. Early Cretaceous-Tertiary magmatism in Eastern Paraguay (Western Paraná Basin): Geological, geophysical and geochemical relationships. J. Geod. 1999, 28, 375–391. [Google Scholar] [CrossRef]
- Velázquez, V.F.; Comin-Chiaramonti, P.; Cundari, A.; Gomes, C.B.; Riccomini, C. Cretaceous Na-alkaline magmatism from Misiones Province (Paraguay): Relationships with the Paleogene Na-alkaline analogue from Asunción and geodynamic significance. J. Geol. 2006, 114, 593–614. [Google Scholar] [CrossRef]
- Thiede, D.S.; Vasconcelos, P.M. Paraná flood basalts: Rapid extrusion hypothesis confirmed by new 40Ar/39Ar results. Geology 2010, 38, 747–750. [Google Scholar] [CrossRef]
- Janasi, V.A.; Freitas, V.A.; Heaman, L.H. The onset of flood basalt volcanism, Northern Paraná Basin, Brazil: A precise U-Pb baddeleyite/zircon age for a Chapecó-type dacite. Earth Plan. Sci. Lett. 2011, 302, 147–153. [Google Scholar] [CrossRef]
- Shea, M.E. Isotopic geochemical characteristics of selected nepheline syenites and phonolites from the Poços de Caldas alkaline complex, Minas Gerais, Brazil. J. Geochem. Explor. 1992, 54, 173–214. [Google Scholar] [CrossRef]
- Thompson, R.N.; Gibson, S.A.; Mitchell, J.G.; Dickin, A.P.; Leonardos, O.H.; Brod, J.A.; Greenwood, J.C. Migrating Cretaceous-Eocene Magmatism in the Serra do Mar Alkaline Province, SE Brazil: Melts from the deflected Trindade Mantle Plume? J. Pet. 1998, 39, 493–1526. [Google Scholar] [CrossRef]
- Ulbrich, H.H.; Vlach, S.R.F.; Ulbrich, M.N.C.; Kavashita, K. Penecontemporaneous syenitic-phonolitic and basic-ultrabasic-carbonatitic rocks at the Poços de Caldas alkaline massif, SE Brazil: Geologic and geochronologic evidence. Rev. Bras. Geoc. 2002, 32, 15–26. [Google Scholar] [CrossRef]
- Vlach, S.R.F.; Vilalva, F.C.J.; Ulbrich, M.N.C.; Ulbrich, H.H.; Vasconcelos, P.M. Phlogopite from carbonatitic veins associated with the Poços de Caldas alkaline massif, SE Brazil: Mineralogy and 40Ar/39Ar dating by the Laser Step Heating Method. In Proceedings of the IV South American Symposium on Isotope Geology, Salvador, Brazil, 24–27 August 2003; Volume 2, pp. 702–705. [Google Scholar]
- Enrich, G.E.R. Petrogênese Da Suite Alcalina Da Ilha Monte De TrigoSp. Ph.D. Thesis, University of São Paulo, Sao Paulo, Brazil, 2005; p. 229. [Google Scholar]
- Scheibe, L.F.; Furtado, S.M.; Comin-Chiaramonti, P.; Gomes, C.B. Cretaceous Alkaline Magmatism from Santa Catarina State, Southern Brazil; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 523–572. [Google Scholar]
- Alves, F.R.; Gomes, C.B. Idades das rochas alcalinas do litoral do Estado de São Paulo: A Ilha de Búzios. Xli Congr. Bras. Geol. João Pessoa An. 2002, 535. Available online: https://repositorio.usp.br/item/001275878 (accessed on 24 August 2021).
- Gomes, C.B.; Alves, F.R.; Azzone, R.G.; Enrich, G.E.R.; Ruberti, E. Geochemistry and petrology of the Búzios Island alkaline massif. Braz. J. Geol. 2017, 47, 127–145. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Lahaye, Y.; Brey, G.P. Plume-related mantle source of super-large rare metal deposits from the Lovozero and Khibiny massifs on the Kola Peninsula, Eastern part of Baltic Shield: Sr, Nd and Hf isotope systematics. Mineral. Petrol. 2010, 98, 197–208. [Google Scholar] [CrossRef]
- Cawthorn, R.G. The geometry and emplacement of the Pilanesberg Complex, South Africa. Geol. Mag. 2015, 152, 802–812. [Google Scholar] [CrossRef]
- Chiessi, C.M. Tectônica Cenozoica do Maciço Alcalino de Passa Quatro (SP-MG-RJ). Master’s Thesis, University of São Paulo, São Paulo, Brazil, 2004; p. 116. [Google Scholar]
- Enrich, G.E.R.; Gomes, C.B.; Ruberti, E. Química mineral de carbonatos de elementos terras raras em nefelina sienitos e fonólitos agpaíticos do maciço de Cerro Boggiani, Província Alto Paraguay, Paraguai. In Proceedings of the X Congresso de Geoquímica dos Países de Língua Portuguesa, Porto, portugal, 28 March–1 April 2010; pp. 223–227. [Google Scholar]
- Enrich, G.E.R.; Gomes, C.B.; Ruberti, E.; Azzone, R.G. Silicatos de Zircônio do Grupo da Cuspidina-Wöhlerita Nas Rochas Vulcânicas Agpaíticas do Maciço Alcalino de Cerro Boggiani, Paraguai. In Proceedings of the V Simpósio De Vucanismo E Ambientes Assoc. Abstr. Cidade de Goiás, Anais, v. Cd-Rom; Available online: https://repositorio.usp.br/item/002213598 (accessed on 24 August 2021).
- Enrich, G.E.R.; Gomes, C.B.; Ruberti, E.; Azzone, R.G. Eudialitas do Maciço Alcalino de Cerro Boggiani, Paraguai: Ocorrência e composição química. In Proceedings of the 46th Congres Brasil De Geology, San Paulo, Brazil, 20–24 August 2012; Volume 15. [Google Scholar]
- Carbonin, S.; Liziero, F.; Fuso, C. Mineral Chemistry of Accessory Minerals in Alkaline Complexes from the Alto Paraguay Province; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 149–158. [Google Scholar]
- Ulbrich, M.N.C. Aspectos mineralógicos e petrológicos de nefelina sienitos do maciço alcalino de Poços de Caldas, MG-SP. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 1983; 369p. [Google Scholar]
- Ruberti, E.; Gomes, C.B.; Comin-Chiaramonti, P. The Alkaline Magmatism from the Ponta Grossa Arch; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 473–522. [Google Scholar]
- Traversa, G.; Barbieri, M.; Beccaluva, L.; Coltorti, M.; Conte, A.M.; Garbarino, C.; Gomes, C.B.; Macciota, G.; Morbidelli, L.; Ronca, S. Mantle sources and differentiation of alkaline magmatic suite of Lages, Santa Catarina, Brazil. Eur. J. Miner. 1996, 8, 193–208. [Google Scholar] [CrossRef]
- Biondi, J.C. Brazilian Mineral Deposits Associated with Alkaline and Alkaline-Carbonatite Complexes; Comin-Chiaramonti, P., Gomes, C.B., Eds.; Edusp/Fapesp: São Paulo, Brazil, 2005; pp. 707–750. [Google Scholar]
- Sigolo, J.B. B. Formações Lateríticas Do Maciço Alcalino De Passa QuatroMg. Sua Evolução Micromorfológica Geoquímica E Implicações Do Relev. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 1988; p. 186. [Google Scholar]
- Azzi, A.A. Caracterização Cristaloquímica de Minerais do Complexo Alcalino de Poços de Caldas, MG-Internal Report; Research Project Fapesp no 2017/25426-1; University of São Paulo: São Paulo, Brazil, 2019; Unpublished work. [Google Scholar]
- Atencio, D.; Coutinho, J.M.V.; Vlach, S.R.F. Tuperssuatsiaite from the Bortolan quarry Poços de Caldas, Minas Gerais, Brazil. Miner. Rec. 2005, 36, 275–280. [Google Scholar]
- Atencio, D.; Coutinho, J.M.V.; Ulbrich, M.N.C.; Vlach, S.R.F.; Rastvetaeva, R.K.; Pushcharovky, D.Y. Hainite from Poços de Caldas, Minas Gerais, Brazil. Can. Miner. 1999, 37, 91–98. [Google Scholar]
- Gualda, G.A.R.; Vlach, S.R.F. Eudialitas-eucolitas do maciço alcalino de Poços de Caldas (MG-SP): Quimismo e correlações com comportamento óptico. In Proceedings of the XXXIX Congresso Brasileiro de Geologia, Salvador, Brazil; 1996; Volume 3, pp. 34–36. Available online: https://repositorio.usp.br/directbitstream/9f0f87bd-6c1b-4f3d-88a1-6ffe52c08904/0905871.pdf (accessed on 24 August 2021).
- Gualda, G.A.R. Variações Químicas em Minerais Máficos e a Evolução dos Magmas Agpaíticos do Corpo Lujaurítico-Chibinítico do Anel Norte—Maciço Alcalino de Poços de Caldas (Mg-Sp); Monografia de Trabalho de Formatura; Institute of Geosciences, University of São Paulo: Sao Paolo, Brazil, 1998; 91p. [Google Scholar]
- Matioli, P.A.; Atencio, D. Gaidonnayite from Poços de Caldas, Minas Gerais, Brazil. Acad. Bras. Ci. 1994, 66, 500–501. [Google Scholar]
- Matioli, P.A.; Atencio, D.; Coutinho, J.M.V. Barium-free burbankite from Poços de Caldas, Minerais Gerais, Brazil. In Proceedings of the 16th General Meeting International Mineralogical Association, Pisa, Italy, 4–9 September 1994; p. 268. [Google Scholar]
- Gualda, G.A.R.; Vlach, S.R.F. Pectolita-serandita do maciço alcalino de Poços de Caldas (MG-SP). V Simpósio De Iniciação Científica Do Igc-Usp 1997, 2, 420. [Google Scholar]
- Atencio, D.; Coutinho, J.M.V.; Matioli, P.A.; Giardullo, P.; Ulbrich, H.H. Quartzo, narsarsukita e tainiolita em rochas alcalinas de Poços de Caldas, Minas Gerais, Brasil. Acad. Bras. Ci. 1997, 69, 432. [Google Scholar]
- Atencio, D.; Coutinho, J.M.V.; Silva, J.F. Kentbrooksite, ferrokentbrooksite and eudialyte from Poços de Caldas, Minas Gerais. In Proceedings of 4th International Conference Mineralogy and Museums, Melbrone, Australia, 4–7 December 2000; p. 12. [Google Scholar]
- Sokolova, E.; Cámara, F. The seidozerite supergroup of TS-block minerals: Nomenclature and classification, with change of the following names: Rinkite to rinkite-(Ce), mosandrite to mosandrite-(Ce), hainite to hainite-(Y) and innelite-1 T to innelite-1 A. Miner. Mag. 2017, 81, 1457–1484. [Google Scholar] [CrossRef]
- Merlino, S.; Perchiazzi, N. Modular mineralogy in the cuspidine group of minerals. Can. Miner. 1988, 26, 933–943. [Google Scholar]
- Johnsen, O.; Ferraris, G.; Gault, R.A.; Grice, J.D.; Kampf, A.R.; Perov, I.V. The nomenclature of eudialyte-group minerals. Can. Miner. 2003, 41, 785–794. [Google Scholar] [CrossRef]
- Schilling, J.; Wu, F.-Y.; McCammon, C.; Wenzel, T.; Marks, M.A.W.; Pfaff, K.; Jacob, D.E.; Markl, G. The compositional variability of eudialyte-group minerals. Mineral. Mag. 2011, 75, 87–115. [Google Scholar] [CrossRef]
- Wu, F.-Y.; Yang, Y.-H.; Marks, M.A.W.; Liu, Z.-C.; Zhou, Q.; Ge, W.-C.; Yang, J.-S.; Zhao, Z.-S.; Mitchell, R.H.; Markl, G. In situ U-Pb, Nd and Hf isotopic analysis of eudialyte by LA-(MC)-ICP-MS. Chem. Geol. 2010, 273, 8–34. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).