Contribution of the Paragenetic Sequence of Clay Minerals to Re-Examination of the Alteration Zoning in the Krafla Geothermal System
Abstract
:1. Introduction
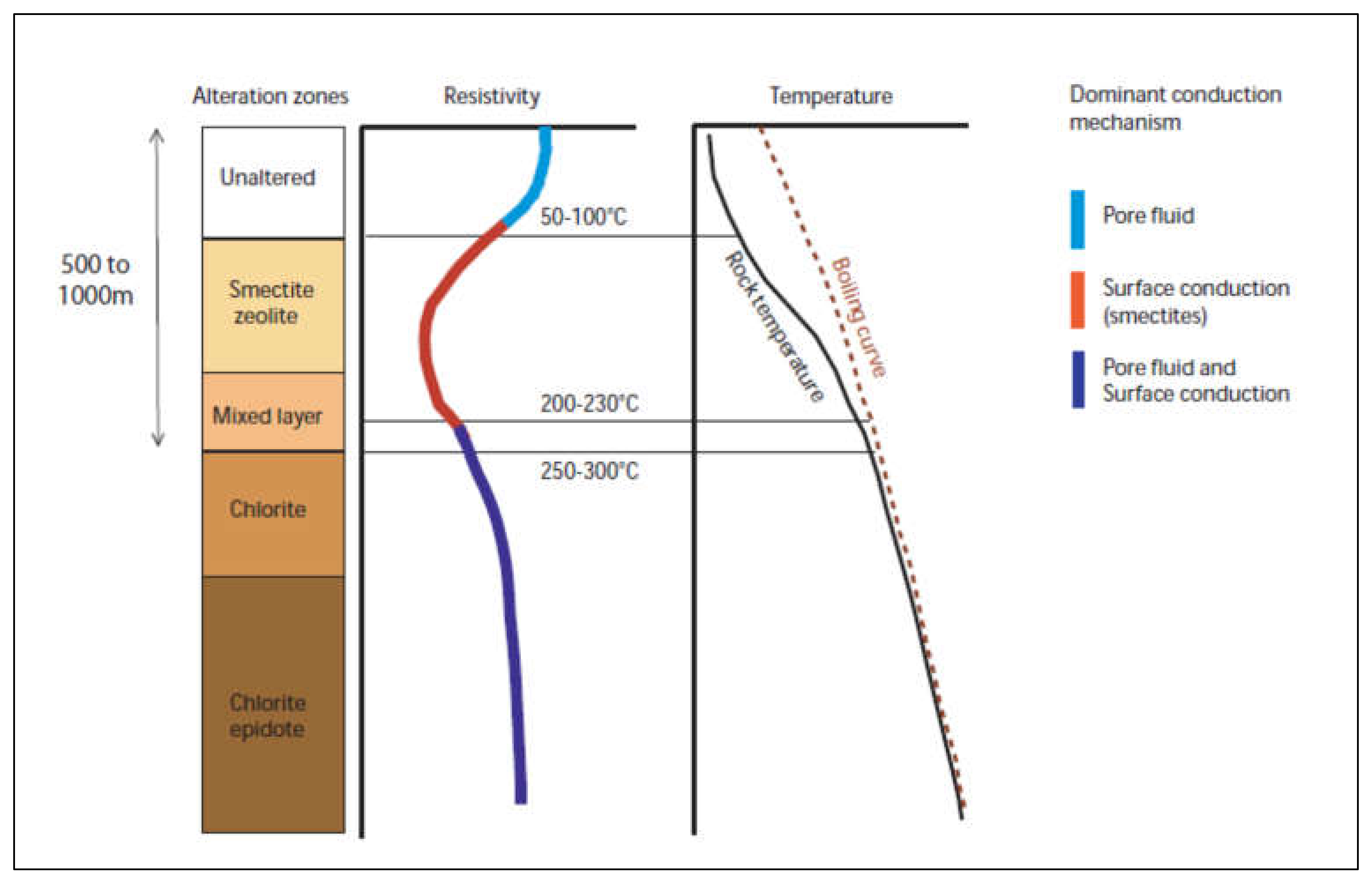
2. Geological Background
3. Materials and Methods
3.1. X-ray Diffraction
3.2. CEC Analysis
3.3. Petrographic Observations and Punctual Chemical Analyses
3.4. Porosity
4. Results
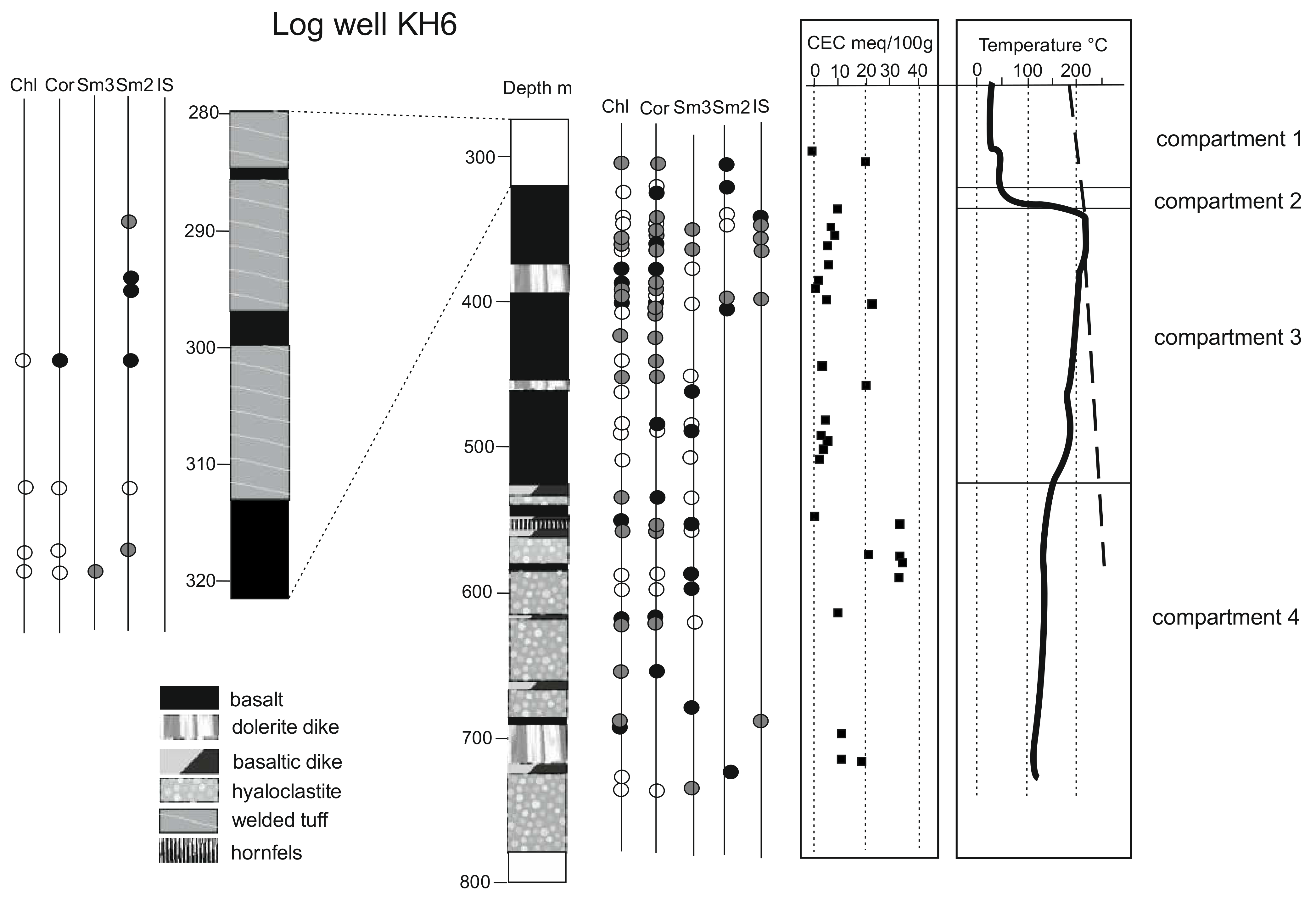
4.1. Lithology and Temperature of the KH6 Drill Hole
- -
- The first compartment (compartment 1, as indicated in Figure 4) is located from the surface down to a 300–320 m depth, where the temperature was stable at approximately 20–40 °C. This level constitutes the upper part of the drill hole where thermal transfers are mainly convective (low vertical thermal gradient). In this interval, the lithology is dominated by altered to strongly altered tuffs with some intercalations of poorly altered basalts. Outside the basaltic intercalations, the porosity reaches 35% and is mainly linked to the microporous clay matrixes. The bottom of this interval corresponds to a major change in lithology.
- -
- The second compartment, which is ten to twenty meters thick, corresponds to dominant basaltic lavas with the occurrence of an intercalated doleritic dike at a 324 m depth. Along this interval, the temperature abruptly increased to reach a maximum temperature of 215 °C, which exceeds that of the boiling curve of geothermal fluids near 350 m depths. Associated thermal transfers are mainly conductive, and this compartment seems to act as a cap for the deeper high-temperature geothermal system.
- -
- Below 350 m and above approximately a 530 m depth (compartment 3), the tempe-rature slightly and progressively decreased to 180 °C to a 475 m depth and increased again up to 190 °C near a 490 m depth before sharply decreasing to a 550 m depth. The shape of the thermal gradient in this zone suggests that this level is affected by fracture-controlled circulation of hot fluids close to boiling conditions. This compartment corresponds to a fractured reservoir with a lateral recharge of hot fluids. The lithology consists of basaltic lavas intercalated by two doleritic dikes. This compartment includes (in its upper part) the main fractured zones identified in the well. Due to fracture-controlled alteration, the porosity of the rocks in this compartment is variable, with values ranging from 5% to 35%.
- -
- Below a 550 m depth (compartment 4), the temperature is nearly constant (125–130 °C) until the well bottom. This shape of the thermal gradient is associated with convective thermal transfers. In this compartment, the lithology changes drastically with the predominance of hyaloclastic breccias originating from subglacial eruptions. Fractures are scarce, but the still high porosity (25–30%) and permeability can be explained by the textural properties of the rocks.
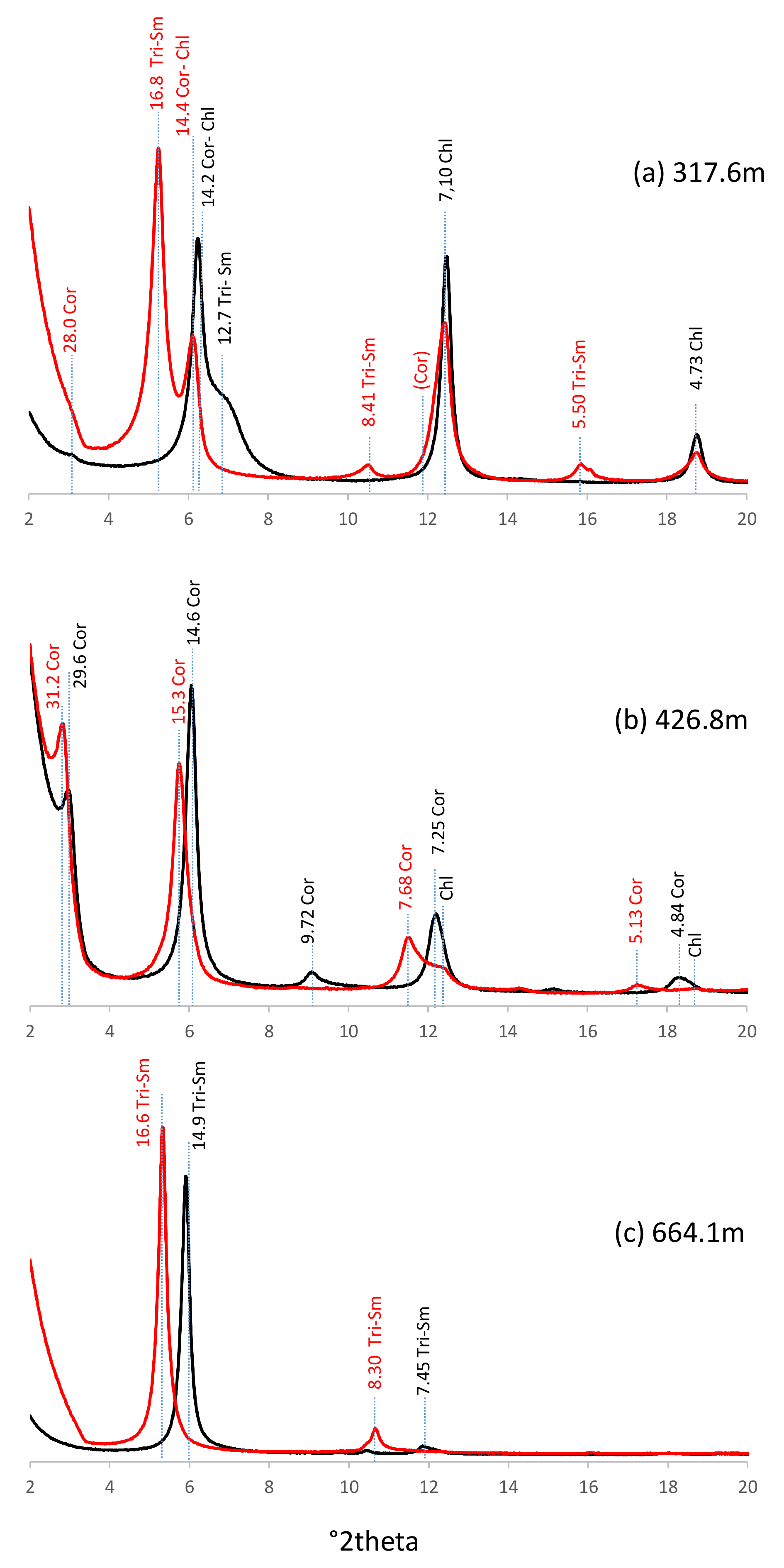
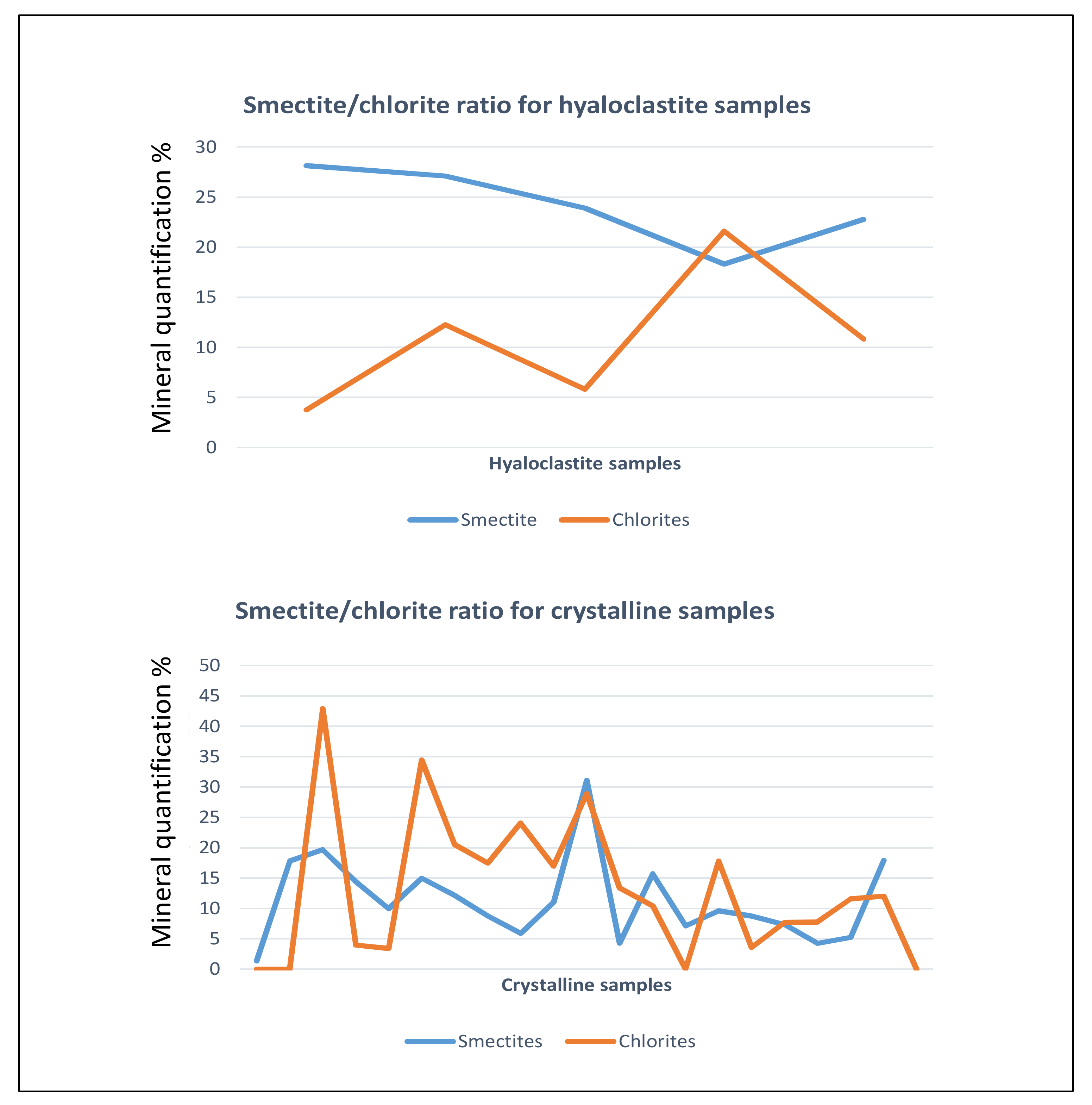
4.2. X-ray Identification of Clay Species
4.3. Vertical Distribution of Clay Species
4.4. Alteration Parageneses
- (1)
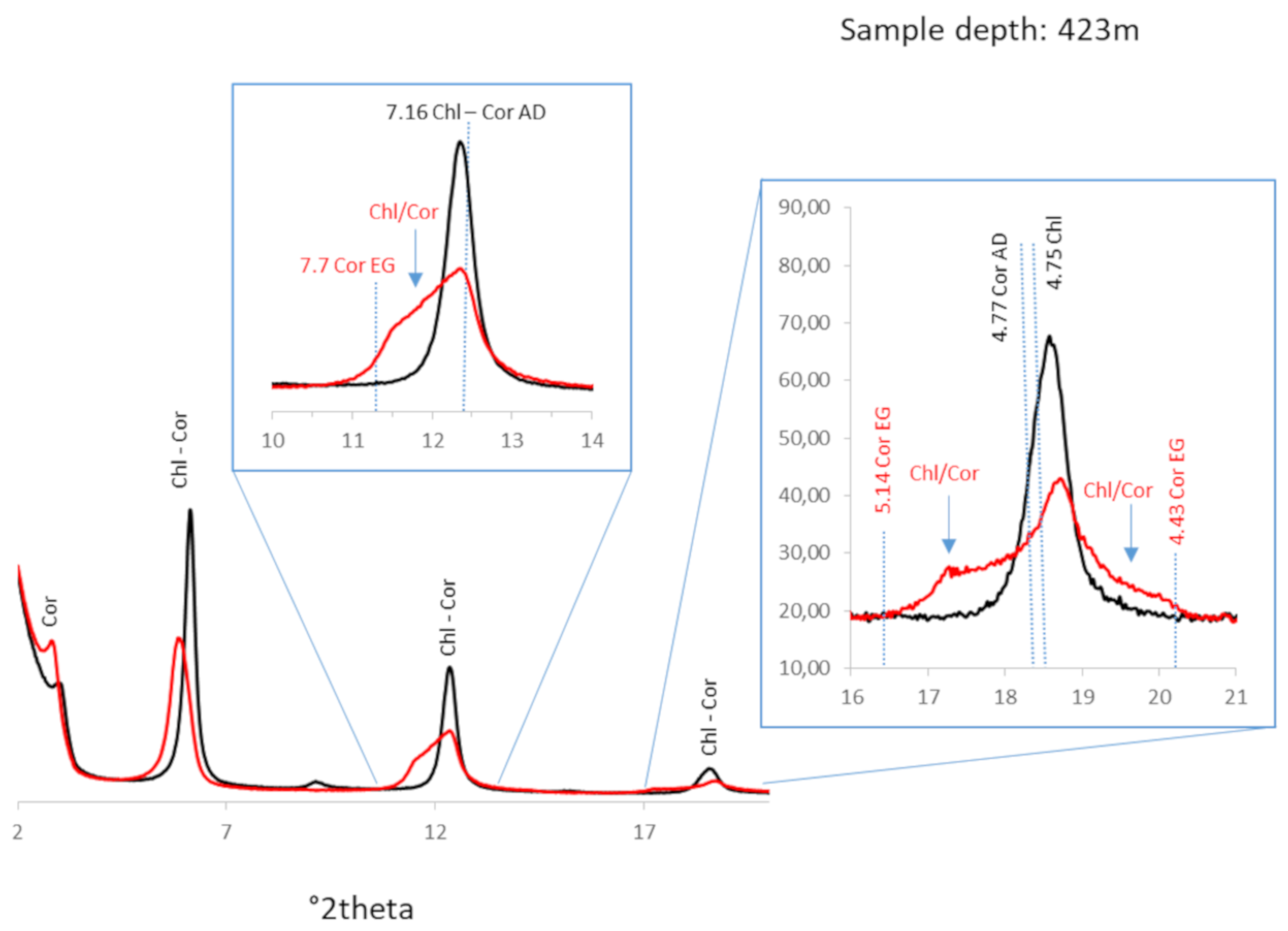
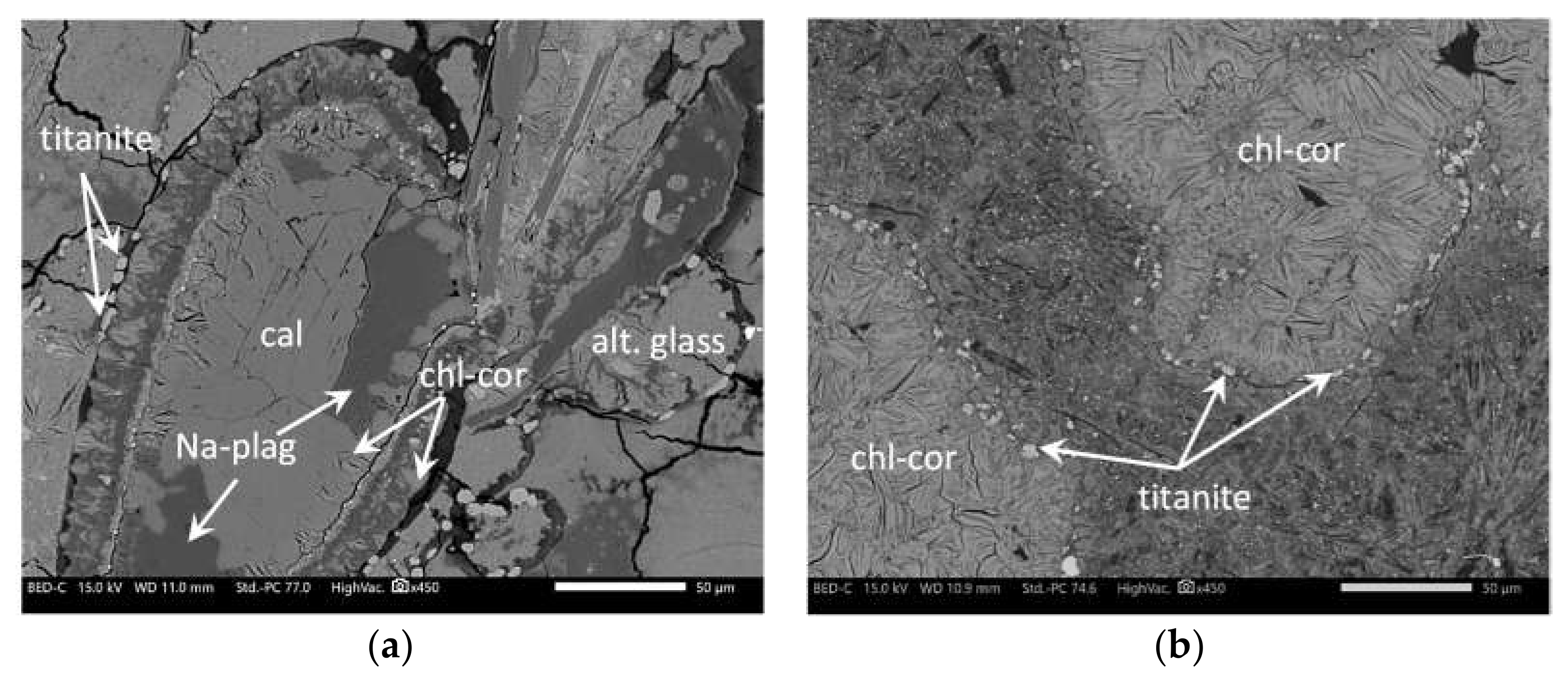
- A first stage of argillization linked to fracture-controlled fluid circulation (vein infillings) is expressed by coprecipitation of chlorite (+ Chl/Cor), corrensite, platy calcite and quartz with some “feathery” habits (Figure 10). It is particularly developed in the third compartment at a depth of approximately 425 m. Chlorite and corrensite (+ Chl/Cor) are very abundant alteration products of the wall rock in which they have replaced all mesostases and most coarser-grained primary (or secondary) minerals.
- (2)
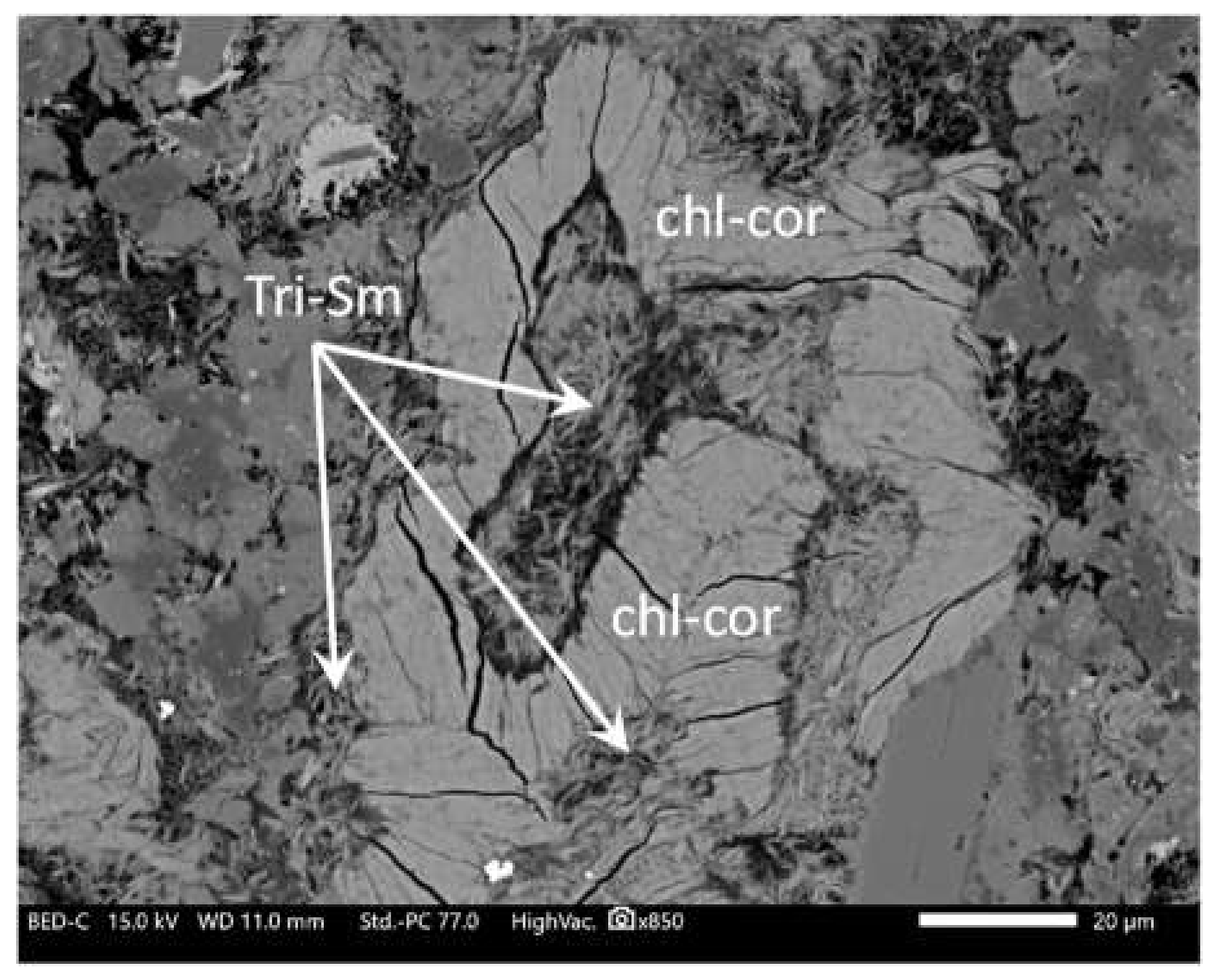
- An alteration stage with trioctahedral smectite + calcite + quartz as vein infillings is also observed locally. Its relationships with chlorite-bearing veins are complex, suggesting that both types of veins are contemporaneous. Sometimes saponite veins postdate chlorite veins, while chlorite (±Chl/Cor) veins seem to postdate saponite veins in other places (Figure 11).
- (3)
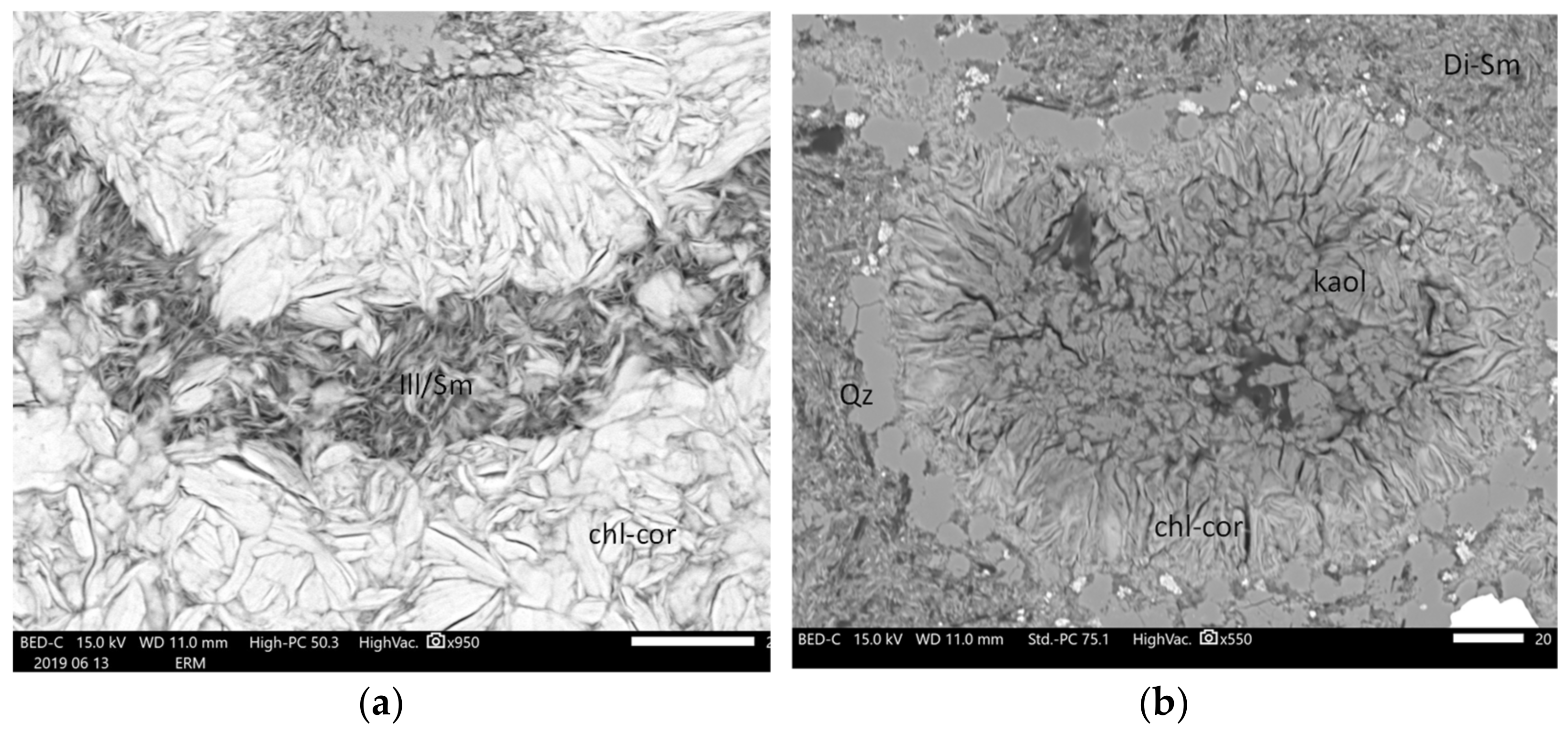
- Another paragenetic assemblage, located at the top of the studied section (first and third compartments), is expressed by the crystallization of aluminous clay minerals, including dioctahedral smectite, Ill/Sm and scarce kaolinite. Ill/Sm are observed at the top of the third compartment from 340 to 400 m depth, mainly in the replacement of plagioclase microlites or as veinlets crosscutting earlier aggregates of chlorite-corrensite minerals (Figure 12). Aluminous smectites are observed in the first compartment and on the top and base of Ill/Sm zones. They replace feldspar microlites in basaltic lavas. These zones are highly altered with almost complete replacement of primary minerals. Kaolinite is observed in one sample in the first compartment (Figure 12). This event is associated with intense leaching of the basaltic rocks and tuffs, which resulted in strongly argillized rock (compared with deeper compartments) and is not related to felsic rocks. Aluminous clay minerals are associated with quartz and calcite ± pyrite.
4.5. Crystal Chemical Properties of Clay Minerals
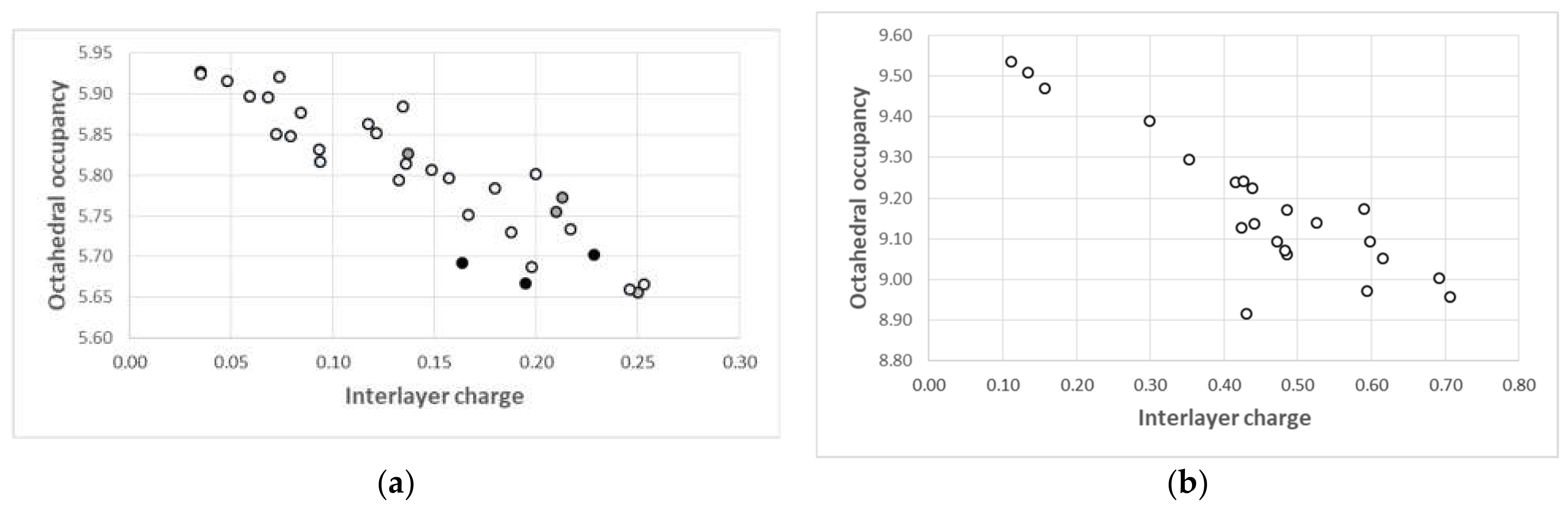
4.5.1. Chloritic Phases
4.5.2. Trioctahedral Smectites
4.5.3. Dioctahedral Smectites
4.5.4. Illite/Smectite
5. Discussion
5.1. Chronology of Hydrothermal Events and Associated (Paleo) Conditions
5.1.1. Earlier Chlorite-Corrensite Assemblage
5.1.2. Pervasive Replacement of the Groundmass by Trioctahedral Clay Minerals (Chlorite, Corrensite, and Smectite)
5.1.3. Clay Minerals Related to Fracture-Controlled Fluid Circulation
Clay Minerals Resulting from Direct Precipitation from Geothermal Fluids
Clay Minerals Associated with Intense Leaching of the Host Rocks
5.2. Relationships between Hydrothermal Alteration and Hydrodynamics
5.3. Implications for Geothermal Exploration
- -
- First, both di- and trioctahedral smectites and/or expandable clay minerals are observed frequently and not strictly linked to cap rock formations. Indeed, smectites have been identified everywhere both close to the surface and at the base of the fracturated zone affected by current fluid flows (dioctahedral smectites) or as replacements of basaltic glass and vein infillings (trioctahedral smectites).
- -
- Second, chlorite and corrensite are also present everywhere and result from different hydrothermal processes, some of which are not representative of fluid circulation.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hersir, G.; Flóvenz, Ó. Resistivity surveying and electromagnetic methods. In IGA Academy Report 0110–2013, Geothermal Exploration Best Practices, Geology, Exploration Drilling, Geochemistry, Geophysics; Bracke, R., Harvey, C., Rueter, H., Eds.; IGA: Bochum, Germany, 2013; Volume 1, p. 10. [Google Scholar]
- Einarnason, P. Plate boundaries, rifts and transforms in Iceland. Jökull 2008, 58, 35–58. [Google Scholar]
- Flóvenz, Ó.G.; Hersir, G.P.; Saemundsson, K.; Ármannsson, H.; Fridriksson, T.H. Geothermal energy exploration techniques. In Comprehensive Renewable Energy; Sayigh, A., Ed.; Elsevier: Oxford, UK, 2012; Volume 7, pp. 51–95. [Google Scholar]
- Lévy, L.; Gibert, B.; Sigmundsson, F.; Flóvenz, Ó.G.; Hersir, G.P.; Briole, P.; Pezard, P.A. The role of smectites in the electrical conductivity of active hydrothermal systems: Electrical properties of core samples from Krafla volcano, Iceland. Geophys. J. Intern. 2018, 215, 1558–1582. [Google Scholar] [CrossRef]
- Flóvenz, Ó.G.; Spangenberg, E.; Kulenkampff, J.; Árnason, K.; Karlsdóttir, R.; Huenges, E. The role of electrical conduction in geothermal exploration. In Proceedings of the World Geothermal Congress, Antalya, Turkey, 24–29 April 2005. [Google Scholar]
- Arnason, K. New conceptual model for the magma-hydrothermal-tectonic system of Krafla, NE Iceland. Geosciences 2020, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Beaufort, D.; Patrier, P.; Meunier, A.; Ottaviani, M.M. Chemical variations in assemblages including epidote and/or chlorite in the fossil hydrothermal system of Saint Martin (Lesser Antilles). J. Volcanol. Geotherm. Res. 1992, 51, 95–114. [Google Scholar] [CrossRef]
- Inoue, A.; Kitagawa, R. Morphological characteristics of illitic clay minerals from a hydrothermal system. Am. Mineral. 1994, 79, 700–711. [Google Scholar]
- Essene, E.J.; Peacor, D.R. Clay mineral thermometry. A critical perspective. Clays Clay Miner. 1995, 43, 540–553. [Google Scholar] [CrossRef]
- Berger, G.; Beaufort, D.; Antoine, R. Clay minerals related to the late magmatic activity of the Piton des Neiges (Réunion Island): Consequence for the primitive crusts. Clay Miner. 2018, 53, 675–690. [Google Scholar] [CrossRef]
- Yoneda, T.; Mokko, H.; Matsumoto, A.; Sato, T. Comparison of smectite–corrensite–chlorite series minerals in the todoroki and hishikari Au–Ag deposits: Applicability of mineralogical properties as exploration index for epithermal systems. Nat. Resour. Res. 2020, 1–20. [Google Scholar] [CrossRef]
- Lonker, S.W.; Fitz Gerald, J.D.; Hedenquist, J.W.; Walshe, J.L. Mineral-fluid interactions in the Broadlands-Ohaaki geothermal system, New Zealand. Am. J. Sci. 1990, 290, 995–1068. [Google Scholar] [CrossRef]
- De Caritat, P.; Hutcheon, I.; Walshe, J.L. Chlorite geothermometry: A review. Clays Clay Miner. 1993, 41, 219–239. [Google Scholar] [CrossRef]
- Beaufort, D.; Papapanagiotou, P.; Fujimoto, K.; Patrier, P.; Kasai, K. High temperature smectites in active geothermal systems. In Water–Rock Interaction; Kharaka, Y.K., Chudaev, O.V., Eds.; Taylor & Francis: Abingdon, UK, 1995; pp. 493–496. [Google Scholar]
- Patrier, P.; Papapanagiotou, P.; Beaufort, D.; Traineau, H.; Bril, H.; Rojas, J. Role of permeability versus temperature in the distribution of the fine (<0.2 µm) clay fraction in the Chipilapa geothermal system (El Salvador, Central America). J. Volcanol. Geotherm. Res. 1996, 72, 101–120. [Google Scholar] [CrossRef]
- Martinez-Serrano, R.G.; Dubois, M. Chemical variations in chlorite at the Los Humeros geothermal system, Mexico. Clays Clay Miner. 1998, 46, 615–628. [Google Scholar] [CrossRef]
- Robinson, D.; Schmidt, S.T.; De Zamora, A.S. Reaction pathways and reaction progress for the smectite-to-chlorite transformation: Evidence from hydrothermally altered metabasites. J. Metamorph. Geol. 2002, 20, 167–174. [Google Scholar] [CrossRef]
- Staudigel, H.; Hart, S.R. Alteration of basaltic glass: Mechanisms and significance for the oceanic crust-sea water budget. Geochim. Cosmochim. Acta 1983, 47, 337–350. [Google Scholar] [CrossRef]
- Lévy, L.E.; Gibert, B.; Escobedo, D.; Patrier, P.; Lanson, B.; Beaufort, D.; Loggia, D.; Pezard, P.A.; Marino, N. Relationships between lithology, permeability, clay mineralogy and electrical conductivity in Icelandic altered volcanic rocks. In Proceedings of the World Geothermal Congress, Reykjavik, Iceland, 21–26 April 2020. [Google Scholar]
- Kristmandottir, H. Alteration of basaltic rock by hydrothermal activity at 100–300 °C. In International Clay Conference; Mortland, M.M., Farmer, V.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1979; pp. 359–367. [Google Scholar]
- Bakht, M.S. Drill hole geology and hydrothermal alteration of well KJ- 28 Krafla high temperature area, NE-Iceland. In Proceedings of the 26th Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, CA, USA, 29–31 January 2001. [Google Scholar]
- Franzson, H.; Zierenberg, R.; Schiffman, P. Chemical transport in geothermal systems in Iceland evidence from hydrothermal alteration. J. Volcanol. Geotherm. Res. 2008, 173, 217–229. [Google Scholar] [CrossRef]
- Franzson, H.; Gunnlaugsson, E. Formation of clays and chlorites in the upper Icelandic crust. In Proceedings of the World Geothermal Congress 2020, Reykjavik, Iceland, 21–26 April 2020. [Google Scholar]
- Gudmundsson, A. Dynamics of volcanic systems in Iceland: Example of tectonism and volcanism at juxtaposed hot spot and mid-ocean ridge systems. Annu. Rev. Earth Planet. Sci. 2000, 28, 107–140. [Google Scholar] [CrossRef]
- Einarsson, P. Earthquakes and present-day tectonism in Iceland. Tectonophysics 1991, 189, 261–279. [Google Scholar] [CrossRef]
- Einarsson, P. Structure and evolution of the Iceland hotspot. Deutsch. Geophys. Ges. Mitt. 2001, 1, 11–14. [Google Scholar]
- Saemundsson, K. Outline of the geology of Iceland. Jokull 1979, 29, 7–28. [Google Scholar]
- Opheim, J.A.; Guðmundsson, A. Formation and geometry of fractures, and related volcanism, of the Krafla fissure swarm, northeast Iceland. Geol. Soc. Am. Bull. 1989, 101, 1608–1622. [Google Scholar] [CrossRef]
- Saemundsson, K. Geology of the Krafla system. In The Natural History of Lake Myvatn; Gardarsson, A., Einarsson, P., Eds.; The Icelandic Natural History Society: Reykjavık Iceland, 1991; pp. 24–95. (In Icelandic) [Google Scholar]
- Einarsson, P. S-wave shadows in the Krafla caldera in NE-Iceland, evidence for a magma chamber in the crust. Bull. Volcanol. 1978, 41, 187–195. [Google Scholar] [CrossRef]
- Brandsdottir, B.; Menke, W.; Einarsson, P.; White, R.S.; Staples, R.K. Färoe-Iceland ridge experiment 2. Crustal structure of the Krafla central volcano. J. Geophys. Res. 1997, 102, 7867–7886. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, A.K.; Egilson, T.; Gautason, B.; Árnadóttir, S.; Gudmundsson, Á. Stratigraphy, alteration mineralogy, permeability and temperature conditions of well IDDP-1, Krafla, NE-Iceland. Geothermics 2014, 49, 31–41. [Google Scholar] [CrossRef]
- Thordarson, T.; Larsen, G. Volcanism in Iceland in historical time: Volcano types, eruption styles and eruptive history. J. Geodyn. 2007, 43, 118–152. [Google Scholar] [CrossRef]
- Armannsson, H.; Gudmundsson, A.; Steingrimsson, B. Exploration and development of the Krafla geothermal area. Jokull 1987, 37, 13–30. [Google Scholar]
- Langella, G.; Paoletti, V.; Dipippoc, R.; Amoresano, A.; Steinunnardóttir, K.; Milano, M.; Langella, G.; Paoletti, V.; Dipippoc, R.; Amoresano, A.; et al. Krafla geothermal system, northeastern Iceland: Performance assessment of alternative plant configurations. Geothermics 2017, 69, 74–92. [Google Scholar] [CrossRef]
- Fridleifsson, G.Ó.; Ármannsson, H.; Mortensen, A.K. Geothermal Conditions in the Krafla Caldera with Focus on Well KG-26; Iceland Geosurvey: Reykjavík, Iceland, 2006; p. 37. [Google Scholar]
- Arnorsson, S. Geothermal systems in Iceland. Structure and conceptual models-1. High temperature areas. Geothermics 1995, 24, 561–601. [Google Scholar] [CrossRef]
- Pope, E.C.; Bird, D.; Arnosson, S.; Giroud, N. Hydrogeology of the Krafla geothermal system, northeast Iceland. Geofluids 2016, 16, 175–197. [Google Scholar] [CrossRef]
- Gudmundsson, Á.; Mortensen, A.K. Well locations consideration of purpose, objectives and achievement with emphasis on recent drilling in the Krafla geothermal area. In Proceedings of the World Geothermal Congress 2015, Melbourne, Australia, 19–24 April 2015. [Google Scholar]
- Lévy, L.; Fridriksson, T.; Findling, N.; Lanson, B.; Fraisse, B.; Marino, N.; Gibert, B. Smectite quantification in hydrothermally altered volcanic rocks. Geothermics 2020, 85, 101748. [Google Scholar] [CrossRef]
- Meunier, A. Les argiles par la pratique: Cristallochimie, minéralogie, géologie. In Sciences & Techniques; Vuibert: Paris, France, 2013. [Google Scholar]
- Ammann, L.; Bergaya, F.; Lagaly, G. Determination of the cation exchange capacity of clays with copper complexes revisited. Clay Miner. 2005, 40, 441–453. [Google Scholar] [CrossRef]
- Stanjek, H.; Kunkel, D. CEC determination with Cu triethylene tetramine: Recommendations for improving reproducibility and accuracy. Clay Miner. 2016, 51, 1–17. [Google Scholar] [CrossRef]
- Meier, L.; Kahr, G. Determination of the cation exchange capacity (CEC) of clay miner. Using the complexes of copper(II) ion with triethylenetetramine and tetraethylenepentamine. Clays Clay Miner. 1999, 47, 386–388. [Google Scholar] [CrossRef]
- Gautason, B.; Egilson, T.; Blishke, A.; Danielsen, P.E. Krafla: Borun Tveggja Kjarnahola, KH5 og KH6, Veturinn 2006–2007; ISOR: Reykjavik, Iceland, 2007. [Google Scholar]
- Franzson, H.; Gudfinnsson, G.H.; Frolova, J.; Helgadóttir, H.M.; Pauly, B.; Mortensen, A.K.; Jakobsson, S.P. Icelandic Hyaloclastite Tuffs, Petrophysical Properties, Alteration and Geochemical Mobility; ISOR: Reykjavik, Iceland, 2011. [Google Scholar]
- Beaufort, D.; Rigault, C.; Billon, S.; Billault, V.; Inoue, A.; Inoue, S.; Patrier, P. Chlorite and chloritization processes through mixed-layer mineral series in low-temperature geological systems—A review. Clay Miner. 2015, 50, 497–523. [Google Scholar] [CrossRef]
- Brindley, G.W.; Brown, G. X-ray diffraction procedures for clay mineral identification. In Crystal Structures of Clay Mineral and Their X-ray Identification; Brindley, G.W., Brown, G., Eds.; Mineralogical Society: Chantilly, VA, USA, 1980; pp. 305–356. [Google Scholar]
- Beaufort, D.; Baronnet, A.; Lanson, B.; Meunier, A. Corrensite: A single phase or a mixed-layer phyllosilicate in the saponite-to-chlorite conversion series? A case study of Sancerre-Couy deep drill hole (France). Am. Mineral. 1997, 82, 109–124. [Google Scholar] [CrossRef]
- Vidal, O.; Baldeyrou, A.; Beaufort, D.; Fritz, B.; Geoffroy, N.; Lanson, B. Experimental study of the stability and phase relations of clays at high temperature in a thermal gradient. Clays Clay Miner. 2012, 60, 200–225. [Google Scholar] [CrossRef]
- Gudmundsson, B.T.; Arnosson, S. Secondary mineral–fluid equilibria in the Krafla and Namafjall geothermal systems, Iceland. Appl. Geochem. 2005, 20, 1607–1625. [Google Scholar] [CrossRef]
- Thien, B.M.J.; Kosakowski, G.; Kulik, D.A. Differential alteration of basaltic lava flows and hyaloclastites in Icelandic hydrothermal systems. Geotherm. Energy 2015, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Merle, R.; Caroff, M.; Girardeau, J.; Cotten, J.; Guivel, C. Segregation vesicles, cylinders, and sheets in vapor-differentiated pillow lavas: Examples from Tore-Madeira Rise and Chile triple junction. J. Volcanol. Geotherm. Res. 2005, 141, 109–122. [Google Scholar] [CrossRef]
- Meunier, A.; Petit, S.; Ehlmann, B.L.; Dudoignon, P.; Westall, F.; Mas, A.; El Albani, A.; Ferrage, E. Magmatic precipitation as a possible origin of Noachian clays on Mars. Nat. Geosci. 2012, 5, 739–743. [Google Scholar] [CrossRef]
- De Andrade, V.; Vidal, O.; Lewin, E.; O’Brien, P.; Agard, P. Quantification of electron microprobe compositional maps of rock thin sections: An optimized method and examples. J. Metamorph. Geol. 2006, 24, 655–658. [Google Scholar] [CrossRef]
- Muñoz, M.; De Andrade, V.; Vidal, O.; Lewin, É.; Pascarelli, S.; Susini, J. Redox and speciation micromapping using dispersive X-ray absorption spectroscopy: Application to iron in chlorite mineral of a metamorphic rock thin section. Geochem. Geophys. Geosyst. 2006, 7, 11. [Google Scholar] [CrossRef]
- Vidal, O.; Lanari, P.; Munoz, M.; Bourdelle, F.; De Andrade, V. Deciphering temperature, pressure and oxygen-activity conditions of chlorite formation. Clays Miner. 2016, 51, 615–633. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, P.; Fridleifsson, G.O. The smectite chlorite transition in drillhole NJ-15, Nesjavellir geothermal field, Iceland—XRD, BSE and electron-microscope investigations. J. Metamorph. Geol. 1991, 9, 679–696. [Google Scholar] [CrossRef]
- Shau, Y.H.; Peacor, D.R. Phyllosilicates in hydrothermally altered basalts from DSDP Hole 504B, Leg 83—A TEM and AEM study. Contrib. Miner. Pet. 1992, 112, 119–133. [Google Scholar] [CrossRef]
- Roberson, H.E.; Reynolds, R.C., Jr.; Jenkins, D.M. Hydrothermal synthesis of corrensite: A study of the transformation of saponite to corrensite. Clays Clay Miner. 1999, 47, 212–218. [Google Scholar] [CrossRef]
- Reyes, A.G. Petrology of Philippine geothermal systems and the application of alteration mineralogy to their assessment. J. Volcanol. Geotherm. Res. 1990, 43, 279–309. [Google Scholar] [CrossRef]
- Inoue, A. Formation of clay minerals in hydrothermal environments. In Origin and Mineralogy of Clays; Velde, B., Ed.; Springer: Berlin, Germany, 1995; pp. 268–329. [Google Scholar]
- Meunier, A.; Mas, A.; Beaufort, D.; Patrier, P.; Dudoignon, P. Clay minerals in basalt-hawaiite rocks from Mururoa atoll (French) Polynesia. II. Petrography and geochemistry. Clays Clay Miner. 2008, 56, 730–750. [Google Scholar] [CrossRef]
- Deer, W.A. An Introduction to the Rock Forming Minerals; Deer, W.A., Howie, R.A., Zussman, J., Eds.; Mineralogical Society: Chantilly, VA, USA, 2013. [Google Scholar]
- Le Gal, X.; Crovisier, J.L.; Gauthier Lafaye, F.; Honnorez, J.; Grambow, B. Altération météorique de verres volcaniques d’Islande: Changement du mécanisme à long terme: Meteoric alteration of Icelandic volcanic glass: Long-term changes in the mechanism. Earth Planet. Sci. 1999, 329, 175–181. [Google Scholar]
- Stroncik, N.A.; Schmincke, H.-U. Palagonite—A review. Int. J. Earth Sci. 2002, 91, 680–697. [Google Scholar] [CrossRef]
- Simmons, S.F.; Christenson, S.W. Origins of calcite in a boiling geothermal system. Am. J. Sci. 1994, 294, 361–400. [Google Scholar] [CrossRef]
- Marosvölgyi, K.; Kristmannsdóttir, H.; Lacasse, C. Retrograde alteration of basaltic rocks in the Theistareykir high-temperature geothermal field, North-Iceland. In Proceedings of the World Geothermal Congress 2010, Bali, Indonesia, 25–30 April 2010; p. 9. [Google Scholar]
- Mingez, H.A.; Ortega, L.; Lunar, R.; Frias, J.M.; Ruben, P. Mineralogy of the hydrothermal alteration in the námafjall geothermal field (Iceland). Rev. Soc. Esp. Mineral. 2011, 15, 25–26. [Google Scholar]
- Markusson, S.H.; Stefansson, A. Geothermal surface alteration of basalts, Krysuvik Iceland-alteration mineralogy, water chemistry and the effects of acid supply on the alteration process. J. Volcanol. Geotherm. Res. 2011, 206, 46–59. [Google Scholar] [CrossRef]
- Beaufort, D.; Westercamp, D.; Legendre, O.; Meunier, A. The fossil hydrothermal system of Saint Martin, Lesser Antilles: Geology and lateral distribution of alterations. J. Volcanol. Geotherm. Res. 1990, 40, 219–243. [Google Scholar] [CrossRef]
- Pirajno, F. Hydrothermal Mineral Deposits, Principles and Fundamental Concepts for the Exploration Geologist; Springer: Berlin, Germany, 1992; p. 709. [Google Scholar]
- Warmer, N.H.; Farmer, J.D. Subglacial hydrothermal alteration minerals in Jökulhlaup deposits of southern Iceland, with implications for detecting past or present habitable environments on Mars. Astrobiology 2010, 10, 5. [Google Scholar] [CrossRef]
- Papapanagiotou, P. Evolution des Minéraux Argileux en Relation avec la Dynamique des Champs Géothermiques Haute Enthalpie: L’exemple du Champ de Chipilapa (Salvador). Ph.D. Thesis, University of Poitiers, Poitiers, France, 1994. [Google Scholar]
- Yang, K.; Browne, P.R.L.; Huntington, J.F.; Walshe, J.L. Characterising the hydrothermal alteration of the Broadlands Ohaaki geothermal system, New Zealand, using short-wave infrared spectroscopy. J. Volcanol. Geotherm. Res. 2001, 106, 53–65. [Google Scholar] [CrossRef]
- Inoue, A.; Meunier, A.; Beaufort, D. Illite-smectite mixed-layer minerals in felsic volcaniclastic rocks from drill cores, Kakkonda, Japan. Clays Clay Miner. 2004, 52, 66–84. [Google Scholar] [CrossRef]
- Guisseau, D.; Patrier, P.; Beaufort, D.; Girard, J.P.; Inoue, A.; Sanjuan, B.; Genter, A.; Lens, A. Significance of the depth-related transition montmorillonite-beidellite in the geothermal field of Bouillante (Guadeloupe, Lesser Antilles). Am. Mineral. 2007, 92, 1800–1813. [Google Scholar] [CrossRef]
- Hemley, J.J.; Jones, W.R. Chemical aspects of hydrothermal alteration with emphasis on hydrogen metasomatism. Econ. Geol. 1964, 59, 538–569. [Google Scholar] [CrossRef]
- Dekov, V.M.; Scholten, J.; Botz, R.; Garbe-Schönberg, D.; Thiry, M.; Stoffers, P.; Schmidt, M. Occurrence of kaolinite and mixed-layer kaolinite/smectite in hydrothermal sediments of Grimsey Graben, Tjörnes Fracture Zone (north of Iceland). Mar. Geol. 2005, 215, 159–170. [Google Scholar] [CrossRef]
- Beane, R.E. Hydrothermal alteration in silicate rocks: South-Western North America. In Advances in Geology of the Porphyry COPPER Deposits, South-Western North America; Titley, S.R., Ed.; University of Arizona Press: Tucson, AZ, USA, 1982; pp. 117–138. [Google Scholar]
- Velde, B. Clay Minerals a Physico-Chemical Explanation of Their Occurrence; Elsevier: Amsterdam, The Netherlands, 1985; p. 427. [Google Scholar]
- Carson, G.L. Hydrothermal Acid-sulfate Alteration at Krafla and Námafjall, Ne Iceland: Implications for Gusev Crater and Meridiani Planum, Mars. Master’s Thesis, University of Wisconsin Milwaukee, Milwaukee, WI, USA, 2015. [Google Scholar]
- Escobedo, D. Study of Hydrothermal Alteration and Petrophysical Properties of Well KH6 Krafla Geothermal Field, NE Iceland. Master’s Thesis, Geosciences Montpellier, Montpellier, France, 2018. [Google Scholar]
- Lonker, S.W.; Franzson, H.; Kristmannsdottir, H. Mineral-fluid interactions in the Reykjanes and Svartsengi geothermal systems, Iceland. Am. J. Sci. 1993, 293, 605–670. [Google Scholar] [CrossRef]
- Franzson, H.; Thordarson, S.; Bjornsson, G.; Gudlaugsson, S.T. Reykjanes high-temperature field, SW-Iceland. In Geology and hydrothermal alteration of well RN-10. In Proceedings of the twenty-seventh Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, CA, USA, 28–30 January 2002. [Google Scholar]
- Friðleifsson, G.Ó.; Elders, W.A. The Iceland deep drilling project: A search for deep unconventional geothermal resources. Geothermics 2005, 34, 269–285. [Google Scholar] [CrossRef]
- Komori, S.; Kagiyama, T.; Hoshizumi, H.; Takakura, S.; Mimura, M. Vertical mapping of hydrothermal fluids and alteration from bulk conductivity: Simple interpretation on the USDP-1 site, Unzen Volcano, SW Japan. J. Volcanol. Geotherm. Res. 2010, 198, 339–347. [Google Scholar] [CrossRef]
- Gonzales, K.; Finizola, A.; Lénat, J.F.; Macedo, O.; Ramos, D.; Thouret, J.C.; Fournier, N.; Cruz, V.; Pistre, K. Asymmetrical structure, hydrothermal system and edifice stability: The case of Ubinas volcano, Peru, revealed by geophysical surveys. J. Volcanol. Geotherm. Res. 2014, 276, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, G. Exploring for geothermal resources with electromagnetic methods. Surv. Geophys. 2014, 35, 101–122. [Google Scholar] [CrossRef]
- Gasperikova, E.; Rosenkjaer, G.; Arnason, K.; Newman, G.; Lindsey, N. Resistivity characterization of the Krafla and Hengill geothermal fields through 3D MT inverse modeling. Geothermics 2015, 57, 246–257. [Google Scholar] [CrossRef] [Green Version]
- Hersir, G.P.; Árnason, K.; Vilhjálmsson, A.M.; Saemundsson, K.; Ágústsdóttir, Þ.; Fridleifsson, G.Ó. Krýsuvík high temperature geothermal area in SW Iceland: Geological setting and 3D inversion of magnetotelluric (MT) resistivity data. J. Volcanol. Geotherm. Res. 2020, 391, 106500. [Google Scholar] [CrossRef]
- Lévy, L.; Maurya, P.K.; Byrdina, S.; Vandemeulebrouck, J.; Sigmundsson, F.; Arnason, K.; Ricci, T.; Deldicque, D.; Roger, M.; Gibert, B.; et al. Electrical resistivity tomography and time-domain induced polarization field investigations of geothermal areas at Krafla, Iceland: Comparison to borehole and laboratory frequency-domain electrical observations. Geophys. J. Int. 2019, 218, 1469–1489. [Google Scholar] [CrossRef] [Green Version]
- Hebert, B. Approche Quantitative par Spectrométrie Vis-NIR des Minéraux Argileux et Uranifères Dans les Sables du Gisement de Tortkuduk, Kazakhstan. Ph.D. Thesis, University of Poitiers, Poitiers, France, 2018. [Google Scholar]
- Glaas, C.; Vidal, J.; Patrier, P.; Girard, J.F.; Beaufort, D.; Petit, S.; Genter, A. How do secondary minerals in granite help distinguish paleo—From present-day permeable fracture zones? Joint interpretation of SWIR spectroscopy and geophysical logs in the geothermal wells of Northern Alsace. Geofluids 2019, 2019, 8231816. [Google Scholar] [CrossRef]





| Chlorite | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 486.5 m | 727.3 m | 356.3 m | 461.32 m | 387 m | 364.5 m | 405 m | |||||||||||||
| Na2O | 0.32 | 0.12 | 0.33 | 0.17 | 0.44 | 0.10 | 0.13 | 0.05 | 0.04 | 0.03 | 0.08 | 0.12 | 0.07 | 0.05 | 0.07 | 0.09 | 0.08 | 0.11 | 0.12 |
| MgO | 12.43 | 11.88 | 16.49 | 15.39 | 11.27 | 13.86 | 13.56 | 15.16 | 13.93 | 14.88 | 14.30 | 13.36 | 12.52 | 12.64 | 14.03 | 13.69 | 14.17 | 13.69 | 14.42 |
| Al2O3 | 15.92 | 12.58 | 18.03 | 16.57 | 14.03 | 16.66 | 16.99 | 16.49 | 17.22 | 16.34 | 16.56 | 17.00 | 16.35 | 15.10 | 15.93 | 15.65 | 17.26 | 16.52 | 17.22 |
| SiO2 | 26.33 | 24.29 | 30.30 | 29.36 | 29.89 | 28.14 | 27.48 | 28.66 | 27.84 | 28.46 | 28.08 | 27.81 | 26.65 | 28.00 | 29.86 | 29.84 | 26.38 | 26.27 | 27.72 |
| K2O | 0.03 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 |
| CaO | 0.24 | 0.35 | 0.31 | 0.39 | 0.19 | 0.25 | 0.18 | 0.11 | 0.28 | 0.12 | 0.14 | 0.26 | 0.41 | 0.20 | 0.26 | 0.31 | 0.19 | 0.22 | 0.21 |
| TiO2 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.04 | 0.01 | 0.00 | 0.00 | 0.04 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 |
| MnO | 0.21 | 0.22 | 0.26 | 0.38 | 0.17 | 0.16 | 0.20 | 0.34 | 0.30 | 0.27 | 0.33 | 0.23 | 0.21 | 0.14 | 0.15 | 0.21 | 0.27 | 0.15 | 0.27 |
| FeO | 27.95 | 22.62 | 24.51 | 26.46 | 32.94 | 26.74 | 27.83 | 27.46 | 28.81 | 27.58 | 27.77 | 28.23 | 27.48 | 29.77 | 29.08 | 29.05 | 22.12 | 23.53 | 23.42 |
| Si | 2.98 | 3.14 | 3.06 | 3.07 | 3.23 | 3.05 | 2.98 | 3.03 | 2.96 | 3.03 | 3.01 | 3.00 | 3.00 | 3.09 | 3.13 | 3.15 | 2.99 | 3.01 | 3.04 |
| AlIV | 1.02 | 0.86 | 0.94 | 0.93 | 0.77 | 0.95 | 1.02 | 0.97 | 1.04 | 0.97 | 0.99 | 1.00 | 1.00 | 0.91 | 0.87 | 0.85 | 1.01 | 0.99 | 0.96 |
| AlVI | 1.11 | 1.06 | 1.21 | 1.11 | 1.01 | 1.17 | 1.16 | 1.08 | 1.12 | 1.08 | 1.11 | 1.16 | 1.16 | 1.06 | 1.10 | 1.09 | 1.30 | 1.24 | 1.27 |
| Mg | 2.10 | 2.29 | 2.49 | 2.40 | 1.81 | 2.24 | 2.19 | 2.39 | 2.21 | 2.36 | 2.29 | 2.15 | 2.10 | 2.08 | 2.19 | 2.15 | 2.40 | 2.34 | 2.36 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mn | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.01 | 0.02 | 0.03 | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.03 | 0.01 | 0.03 |
| Fe2+ | 2.65 | 2.45 | 2.07 | 2.31 | 2.97 | 2.42 | 2.53 | 2.43 | 2.56 | 2.46 | 2.49 | 2.55 | 2.58 | 2.75 | 2.55 | 2.56 | 2.10 | 2.25 | 2.15 |
| oct | 5.88 | 5.83 | 5.79 | 5.85 | 5.81 | 5.85 | 5.90 | 5.93 | 5.92 | 5.92 | 5.92 | 5.88 | 5.86 | 5.90 | 5.85 | 5.83 | 5.82 | 5.84 | 5.81 |
| Na | 0.07 | 0.03 | 0.06 | 0.03 | 0.09 | 0.02 | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 |
| Ca | 0.03 | 0.05 | 0.03 | 0.04 | 0.02 | 0.03 | 0.02 | 0.01 | 0.03 | 0.01 | 0.02 | 0.03 | 0.05 | 0.02 | 0.03 | 0.03 | 0.02 | 0.03 | 0.02 |
| K | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Int Ch | 0.13 | 0.14 | 0.13 | 0.12 | 0.14 | 0.08 | 0.07 | 0.04 | 0.07 | 0.04 | 0.05 | 0.08 | 0.12 | 0.06 | 0.07 | 0.09 | 0.06 | 0.08 | 0.08 |
| Fe/(Fe + Mg) | 0.56 | 0.52 | 0.45 | 0.49 | 0.62 | 0.52 | 0.54 | 0.50 | 0.54 | 0.51 | 0.52 | 0.54 | 0.55 | 0.57 | 0.54 | 0.54 | 0.47 | 0.49 | 0.48 |
| Corrensite | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 364.5 m | 425 m | 582.1 | 461.32 | 305.1 m | 501 m | ||||||||||||||
| Na2O | 0.57 | 0.57 | 0.68 | 0.60 | 0.57 | 0.62 | 0.37 | 0.53 | 0.64 | 0.98 | 1.04 | 1.07 | 0.04 | 0.50 | 0.41 | 0.93 | 0.50 | 0.05 | 0.11 |
| MgO | 16.31 | 15.97 | 18.90 | 16.24 | 16.21 | 15.17 | 15.63 | 15.70 | 11.79 | 9.96 | 10.27 | 10.76 | 13.74 | 13.17 | 12.77 | 10.18 | 11.72 | 12.00 | 11.97 |
| Al2O3 | 15.73 | 15.30 | 17.54 | 15.54 | 13.73 | 15.98 | 16.92 | 16.88 | 12.11 | 12.62 | 13.10 | 13.60 | 15.08 | 14.63 | 14.73 | 14.15 | 13.99 | 15.14 | 15.93 |
| SiO2 | 33.29 | 32.64 | 36.80 | 33.14 | 33.38 | 31.53 | 31.44 | 32.11 | 29.28 | 31.62 | 32.39 | 32.30 | 30.01 | 29.53 | 29.43 | 30.76 | 31.15 | 31.37 | 30.82 |
| K2O | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.02 | 0.05 | 0.03 | 0.05 | 0.05 | 0.04 | 0.00 | 0.04 | 0.01 | 0.03 | 0.03 | 0.02 | 0.02 |
| CaO | 0.78 | 0.78 | 0.72 | 0.78 | 0.83 | 0.96 | 0.89 | 1.04 | 0.93 | 0.61 | 0.56 | 0.47 | 0.88 | 0.74 | 0.74 | 0.60 | 0.92 | 1.19 | 1.18 |
| TiO2 | 0.02 | 0.00 | 0.00 | 0.02 | 0.00 | 0.02 | 0.03 | 0.00 | 0.01 | 0.00 | 0.06 | 0.03 | 0.05 | 0.10 | 0.10 | 0.07 | 0.00 | 0.02 | 0.12 |
| MnO | 0.17 | 0.24 | 0.15 | 0.21 | 0.26 | 0.19 | 0.21 | 0.15 | 0.19 | 0.12 | 0.13 | 0.21 | 0.15 | 0.22 | 0.13 | 0.16 | 0.22 | 0.28 | 0.25 |
| FeO | 22.13 | 22.63 | 22.25 | 22.45 | 23.49 | 21.84 | 22.62 | 22.67 | 25.06 | 30.53 | 31.48 | 31.60 | 22.82 | 23.68 | 23.16 | 29.46 | 26.77 | 25.84 | 26.36 |
| Si | 6.00 | 5.97 | 6.01 | 5.99 | 6.11 | 5.88 | 5.76 | 5.81 | 6.08 | 6.19 | 6.16 | 6.07 | 5.89 | 5.86 | 5.90 | 6.00 | 6.06 | 6.00 | 5.86 |
| AlIV | 2.00 | 2.03 | 1.99 | 2.01 | 1.89 | 2.12 | 2.24 | 2.19 | 1.92 | 1.81 | 1.84 | 1.93 | 2.11 | 2.14 | 2.10 | 2.00 | 1.94 | 2.00 | 2.14 |
| AlVI | 1.34 | 1.27 | 1.39 | 1.30 | 1.08 | 1.39 | 1.41 | 1.41 | 1.05 | 1.10 | 1.10 | 1.09 | 1.37 | 1.28 | 1.38 | 1.26 | 1.26 | 1.42 | 1.43 |
| Mg | 4.38 | 4.36 | 4.60 | 4.37 | 4.43 | 4.22 | 4.26 | 4.23 | 3.65 | 2.91 | 2.91 | 3.02 | 4.02 | 3.90 | 3.82 | 2.96 | 3.40 | 3.42 | 3.40 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.02 |
| Mn | 0.03 | 0.04 | 0.02 | 0.03 | 0.04 | 0.03 | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.02 | 0.04 | 0.02 | 0.03 | 0.04 | 0.04 | 0.04 |
| Fe2+ | 3.33 | 3.46 | 3.04 | 3.39 | 3.60 | 3.41 | 3.46 | 3.43 | 4.35 | 5.00 | 5.01 | 4.97 | 3.74 | 3.93 | 3.88 | 4.81 | 4.35 | 4.14 | 4.19 |
| oct | 9.08 | 9.13 | 9.06 | 9.10 | 9.14 | 9.05 | 9.17 | 9.09 | 9.09 | 9.03 | 9.05 | 9.12 | 9.17 | 9.16 | 9.11 | 9.06 | 9.05 | 9.03 | 9.08 |
| Na | 0.20 | 0.20 | 0.22 | 0.21 | 0.20 | 0.22 | 0.13 | 0.18 | 0.26 | 0.37 | 0.38 | 0.39 | 0.02 | 0.19 | 0.16 | 0.35 | 0.19 | 0.02 | 0.04 |
| Ca | 0.15 | 0.15 | 0.13 | 0.15 | 0.16 | 0.19 | 0.17 | 0.20 | 0.21 | 0.13 | 0.11 | 0.10 | 0.19 | 0.16 | 0.16 | 0.13 | 0.19 | 0.24 | 0.24 |
| K | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 |
| Ch int | 0.50 | 0.51 | 0.47 | 0.51 | 0.53 | 0.62 | 0.49 | 0.60 | 0.68 | 0.64 | 0.62 | 0.59 | 0.39 | 0.52 | 0.48 | 0.61 | 0.58 | 0.51 | 0.52 |
| Fe/(Fe + Mg) | 0.43 | 0.44 | 0.40 | 0.44 | 0.45 | 0.45 | 0.45 | 0.45 | 0.54 | 0.63 | 0.63 | 0.62 | 0.48 | 0.50 | 0.50 | 0.62 | 0.56 | 0.55 | 0.55 |
| Trioctahedral Smectite | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 727.3 m | 461.32 m | 501 m | 582.1 m | ||||||||||||||
| Na2O | 0.93 | 0.29 | 1.61 | 3.26 | 2.95 | 2.60 | 3.23 | 3.00 | 2.85 | 1.75 | 1.51 | 1.44 | 1.60 | 1.59 | 1.61 | 2.52 | 0.54 |
| MgO | 12.46 | 13.20 | 13.89 | 8.98 | 9.09 | 10.15 | 9.67 | 9.21 | 8.81 | 9.62 | 9.10 | 8.80 | 9.19 | 9.25 | 8.36 | 9.70 | 10.10 |
| Al2O3 | 8.47 | 9.29 | 8.71 | 11.34 | 11.08 | 12.27 | 11.49 | 11.03 | 10.76 | 10.73 | 10.06 | 10.18 | 10.49 | 10.47 | 10.19 | 11.00 | 7.41 |
| SiO2 | 37.69 | 38.21 | 39.74 | 41.03 | 40.06 | 40.37 | 41.61 | 40.68 | 39.55 | 39.59 | 36.82 | 37.22 | 38.43 | 38.35 | 34.60 | 39.79 | 32.03 |
| K2O | 0.06 | 0.05 | 0.04 | 0.19 | 0.17 | 0.10 | 0.22 | 0.22 | 0.23 | 0.04 | 0.05 | 0.02 | 0.03 | 0.04 | 0.03 | 0.05 | 0.09 |
| CaO | 3.13 | 3.16 | 1.19 | 1.19 | 1.14 | 0.93 | 0.89 | 1.00 | 1.01 | 2.95 | 1.86 | 1.88 | 1.99 | 1.72 | 1.49 | 1.52 | 1.88 |
| TiO2 | 1.29 | 0.89 | 0.00 | 0.05 | 0.14 | 0.00 | 0.06 | 0.04 | 0.02 | 1.12 | 0.13 | 0.00 | 0.14 | 0.02 | 0.02 | 0.00 | 0.03 |
| MnO | 0.14 | 0.13 | 0.10 | 0.12 | 0.13 | 0.14 | 0.12 | 0.10 | 0.12 | 0.24 | 0.18 | 0.24 | 0.35 | 0.28 | 0.15 | 0.14 | 0.17 |
| FeO | 16.35 | 18.58 | 18.02 | 25.97 | 26.90 | 27.66 | 26.18 | 26.61 | 26.80 | 22.88 | 23.84 | 23.98 | 24.06 | 23.19 | 24.48 | 24.01 | 17.49 |
| Si | 3.21 | 3.15 | 3.27 | 3.18 | 3.14 | 3.08 | 3.17 | 3.17 | 3.16 | 3.15 | 3.15 | 3.17 | 3.17 | 3.19 | 3.08 | 3.18 | 3.21 |
| AlIV | 0.79 | 0.85 | 0.73 | 0.82 | 0.86 | 0.92 | 0.83 | 0.83 | 0.84 | 0.85 | 0.85 | 0.83 | 0.83 | 0.81 | 0.92 | 0.82 | 0.79 |
| AlVI | 0.07 | 0.06 | 0.12 | 0.22 | 0.17 | 0.18 | 0.21 | 0.18 | 0.17 | 0.15 | 0.16 | 0.19 | 0.19 | 0.22 | 0.15 | 0.21 | 0.09 |
| Mg | 1.58 | 1.63 | 1.70 | 1.04 | 1.06 | 1.15 | 1.10 | 1.07 | 1.05 | 1.14 | 1.16 | 1.12 | 1.13 | 1.15 | 1.11 | 1.15 | 1.51 |
| Ti | 0.08 | 0.06 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mn | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 |
| Fe2+ | 1.17 | 1.28 | 1.24 | 1.68 | 1.76 | 1.76 | 1.67 | 1.73 | 1.79 | 1.52 | 1.70 | 1.71 | 1.66 | 1.62 | 1.82 | 1.60 | 1.47 |
| oct | 2.91 | 3.03 | 3.07 | 2.95 | 3.01 | 3.10 | 2.98 | 3.00 | 3.02 | 2.90 | 3.04 | 3.03 | 3.01 | 3.01 | 3.10 | 2.98 | 3.09 |
| Na | 0.15 | 0.05 | 0.26 | 0.49 | 0.45 | 0.38 | 0.48 | 0.45 | 0.44 | 0.27 | 0.25 | 0.24 | 0.26 | 0.26 | 0.28 | 0.39 | 0.11 |
| Ca | 0.29 | 0.28 | 0.11 | 0.10 | 0.10 | 0.08 | 0.07 | 0.08 | 0.09 | 0.25 | 0.17 | 0.17 | 0.18 | 0.15 | 0.14 | 0.13 | 0.20 |
| K | 0.01 | 0.01 | 0.00 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| Int ch | 0.73 | 0.61 | 0.47 | 0.71 | 0.66 | 0.55 | 0.64 | 0.64 | 0.64 | 0.78 | 0.60 | 0.58 | 0.61 | 0.57 | 0.57 | 0.66 | 0.52 |
| Fe/(Fe + Mg) | 0.42 | 0.44 | 0.42 | 0.62 | 0.62 | 0.60 | 0.60 | 0.62 | 0.63 | 0.57 | 0.60 | 0.60 | 0.59 | 0.58 | 0.62 | 0.58 | 0.49 |
| Dioctahedral Smectites | Ill/Sm | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 305.1 m | 405 m | 356.3 m | ||||||||||||||||||||
| Na2O | 1.14 | 2.35 | 0.56 | 0.52 | 0.41 | 0.75 | 0.86 | 0.79 | 0.82 | 0.95 | 1.25 | 1.06 | 1.28 | 1.38 | 1.57 | 0.26 | 0.08 | 0.29 | 0.27 | 0.35 | 0.25 | 0.28 |
| MgO | 2.85 | 1.77 | 1.66 | 2.04 | 1.93 | 1.04 | 1.07 | 1.02 | 0.69 | 1.09 | 1.05 | 1.12 | 1.07 | 1.27 | 1.22 | 1.79 | 1.22 | 1.56 | 1.14 | 1.10 | 1.32 | 1.31 |
| Al2O3 | 18.78 | 18.87 | 23.63 | 22.54 | 20.98 | 28.26 | 28.90 | 27.98 | 27.96 | 27.69 | 28.00 | 26.23 | 26.93 | 27.04 | 29.27 | 34.50 | 33.07 | 34.51 | 34.56 | 34.99 | 34.28 | 34.66 |
| SiO2 | 43.66 | 46.41 | 48.66 | 49.19 | 45.04 | 49.48 | 49.43 | 47.46 | 48.93 | 49.52 | 49.38 | 47.32 | 48.36 | 48.71 | 52.11 | 51.70 | 49.14 | 52.51 | 51.26 | 51.81 | 51.54 | 51.96 |
| K2O | 0.15 | 0.18 | 0.17 | 0.19 | 0.18 | 1.55 | 1.44 | 1.90 | 0.08 | 0.37 | 0.95 | 0.41 | 0.79 | 0.80 | 0.68 | 7.84 | 7.87 | 8.29 | 7.86 | 7.71 | 6.88 | 8.10 |
| CaO | 1.50 | 1.48 | 1.54 | 1.90 | 1.63 | 1.20 | 1.68 | 1.18 | 1.79 | 1.31 | 1.03 | 1.21 | 0.90 | 0.88 | 1.09 | 0.50 | 0.42 | 0.46 | 0.58 | 0.78 | 0.63 | 0.62 |
| TiO2 | 0.03 | 0.42 | 0.07 | 0.23 | 0.18 | 0.07 | 0.03 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.03 | 0.05 | 0.04 | 0.13 | 0.00 | 0.03 | 0.00 | 0.07 | 0.05 | 0.00 |
| MnO | 0.00 | 0.00 | 0.00 | 0.02 | 0.05 | 0.02 | 0.00 | 0.02 | 0.00 | 0.04 | 0.02 | 0.00 | 0.00 | 0.07 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.03 | 0.00 | 0.01 |
| FeO | 2.59 | 1.60 | 1.24 | 1.07 | 1.91 | 0.25 | 0.20 | 0.27 | 0.23 | 0.30 | 0.44 | 0.42 | 0.44 | 0.68 | 0.33 | 1.21 | 0.66 | 0.95 | 0.50 | 0.44 | 0.57 | 0.56 |
| Si | 3.70 | 3.79 | 3.69 | 3.73 | 3.69 | 3.54 | 3.50 | 3.49 | 3.56 | 3.57 | 3.55 | 3.57 | 3.57 | 3.56 | 3.55 | 3.27 | 3.28 | 3.30 | 3.28 | 3.28 | 3.31 | 3.29 |
| AlIV | 0.30 | 0.21 | 0.31 | 0.27 | 0.31 | 0.46 | 0.50 | 0.51 | 0.44 | 0.43 | 0.45 | 0.43 | 0.43 | 0.44 | 0.45 | 0.73 | 0.72 | 0.70 | 0.72 | 0.72 | 0.69 | 0.71 |
| AlVI | 1.58 | 1.60 | 1.80 | 1.74 | 1.72 | 1.92 | 1.91 | 1.92 | 1.95 | 1.93 | 1.92 | 1.91 | 1.91 | 1.89 | 1.91 | 1.84 | 1.89 | 1.85 | 1.90 | 1.89 | 1.90 | 1.88 |
| Mg | 0.36 | 0.22 | 0.19 | 0.23 | 0.24 | 0.11 | 0.11 | 0.11 | 0.08 | 0.12 | 0.11 | 0.13 | 0.12 | 0.14 | 0.12 | 0.17 | 0.12 | 0.15 | 0.11 | 0.10 | 0.13 | 0.12 |
| Ti | 0.00 | 0.03 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fe2+ | 0.18 | 0.11 | 0.08 | 0.07 | 0.13 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.03 | 0.03 | 0.03 | 0.04 | 0.02 | 0.06 | 0.04 | 0.05 | 0.03 | 0.02 | 0.03 | 0.03 |
| oct | 2.12 | 1.95 | 2.07 | 2.05 | 2.10 | 2.05 | 2.04 | 2.05 | 2.04 | 2.07 | 2.06 | 2.06 | 2.06 | 2.07 | 2.05 | 2.08 | 2.04 | 2.05 | 2.03 | 2.02 | 2.06 | 2.03 |
| Na | 0.19 | 0.37 | 0.08 | 0.08 | 0.07 | 0.10 | 0.12 | 0.11 | 0.12 | 0.13 | 0.17 | 0.16 | 0.18 | 0.20 | 0.21 | 0.03 | 0.01 | 0.04 | 0.03 | 0.04 | 0.03 | 0.03 |
| Ca | 0.14 | 0.13 | 0.12 | 0.15 | 0.14 | 0.09 | 0.13 | 0.09 | 0.14 | 0.10 | 0.08 | 0.10 | 0.07 | 0.07 | 0.08 | 0.03 | 0.03 | 0.03 | 0.04 | 0.05 | 0.04 | 0.04 |
| K | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.14 | 0.13 | 0.18 | 0.01 | 0.03 | 0.09 | 0.04 | 0.07 | 0.07 | 0.06 | 0.63 | 0.67 | 0.66 | 0.64 | 0.62 | 0.56 | 0.65 |
| Int ch | 0.48 | 0.65 | 0.35 | 0.40 | 0.37 | 0.43 | 0.50 | 0.48 | 0.40 | 0.37 | 0.42 | 0.39 | 0.40 | 0.41 | 0.43 | 0.73 | 0.74 | 0.76 | 0.76 | 0.77 | 0.68 | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobedo, D.; Patrier, P.; Beaufort, D.; Gibert, B.; Levy, L.; Findling, N.; Mortensen, A. Contribution of the Paragenetic Sequence of Clay Minerals to Re-Examination of the Alteration Zoning in the Krafla Geothermal System. Minerals 2021, 11, 935. https://doi.org/10.3390/min11090935
Escobedo D, Patrier P, Beaufort D, Gibert B, Levy L, Findling N, Mortensen A. Contribution of the Paragenetic Sequence of Clay Minerals to Re-Examination of the Alteration Zoning in the Krafla Geothermal System. Minerals. 2021; 11(9):935. https://doi.org/10.3390/min11090935
Chicago/Turabian StyleEscobedo, David, Patricia Patrier, Daniel Beaufort, Benoit Gibert, Léa Levy, Nathaniel Findling, and Annette Mortensen. 2021. "Contribution of the Paragenetic Sequence of Clay Minerals to Re-Examination of the Alteration Zoning in the Krafla Geothermal System" Minerals 11, no. 9: 935. https://doi.org/10.3390/min11090935
APA StyleEscobedo, D., Patrier, P., Beaufort, D., Gibert, B., Levy, L., Findling, N., & Mortensen, A. (2021). Contribution of the Paragenetic Sequence of Clay Minerals to Re-Examination of the Alteration Zoning in the Krafla Geothermal System. Minerals, 11(9), 935. https://doi.org/10.3390/min11090935






