Effect of Fe2O3 Content and Acid on the Leaching Behavior of Phosphorus from Dephosphorization Slag
Abstract
:1. Introduction
2. Experimental
3. Results
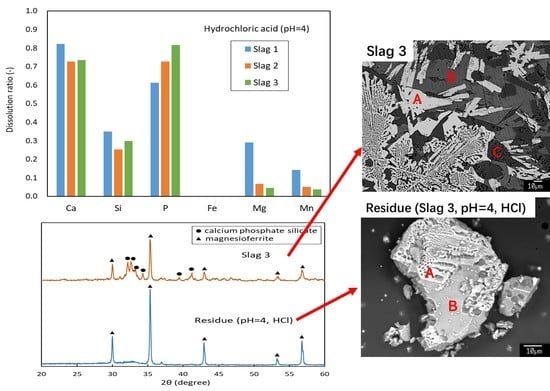
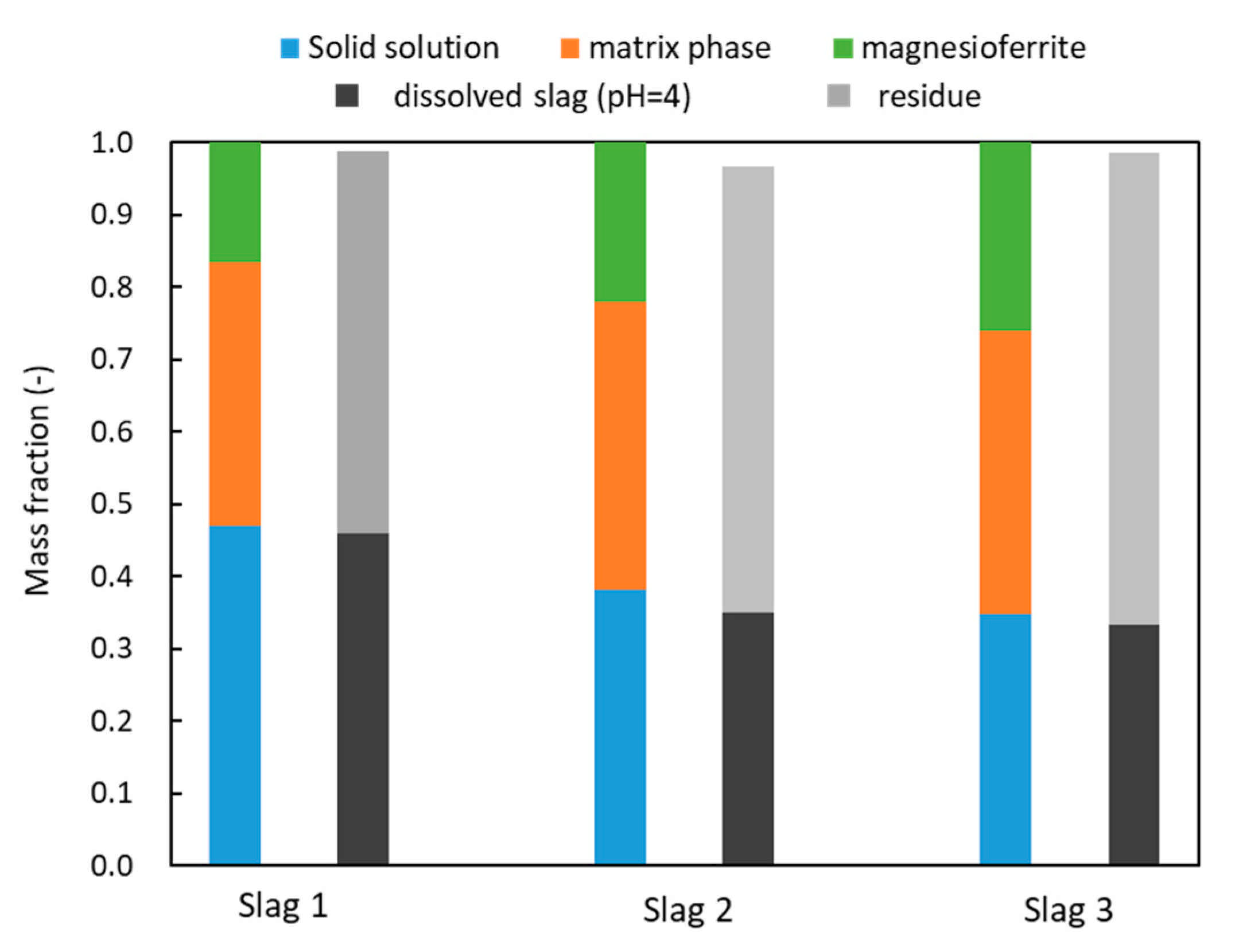
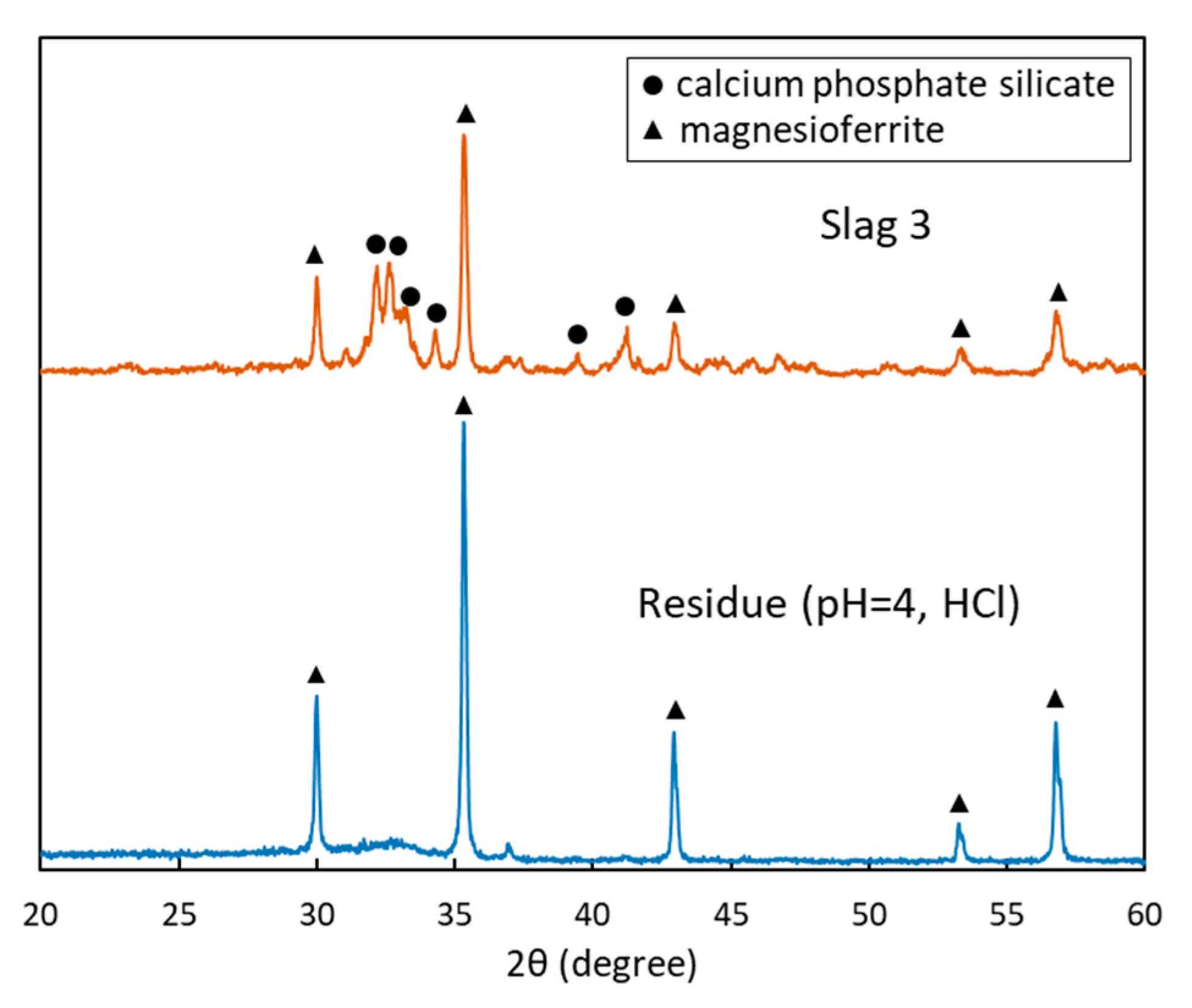
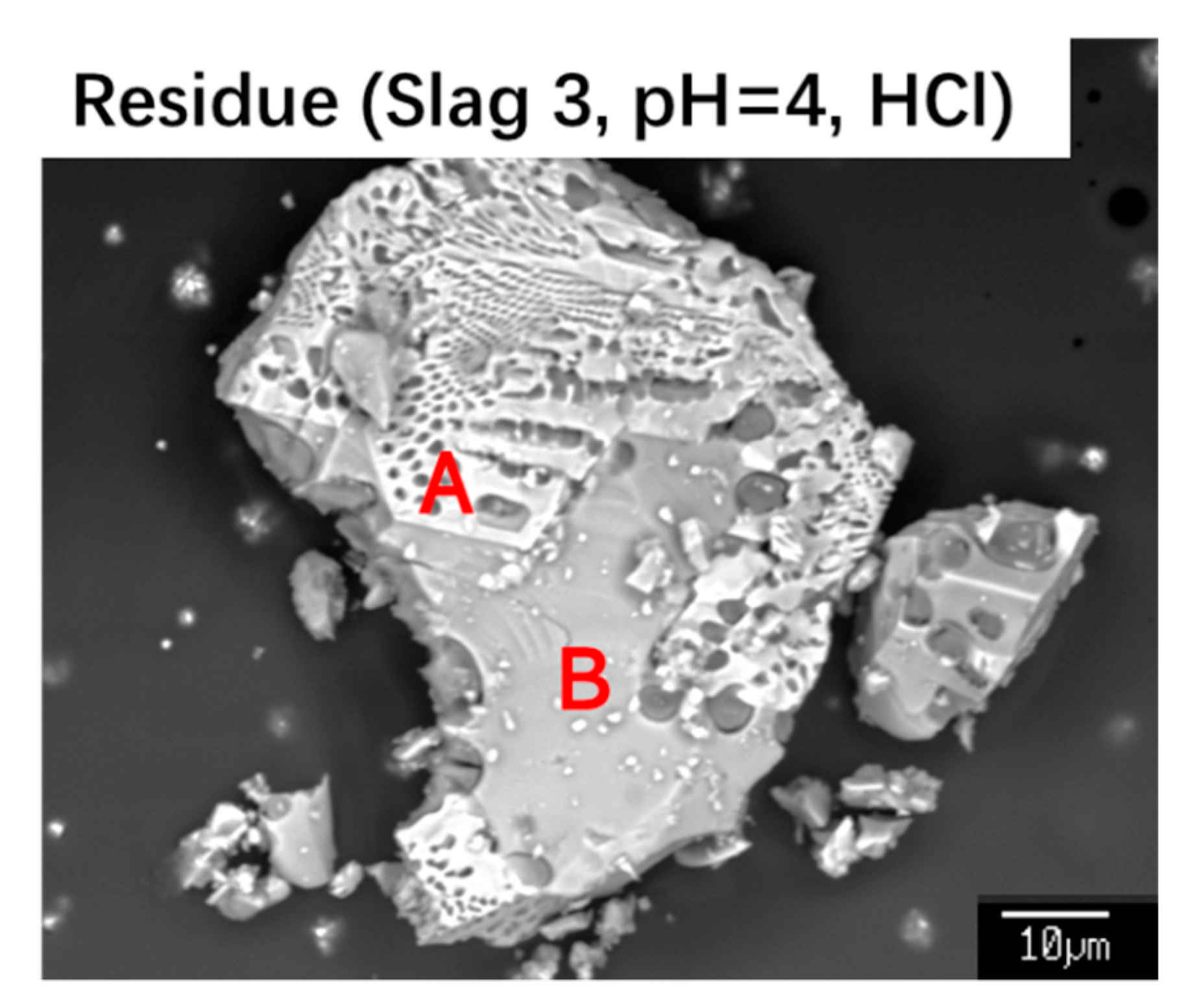
3.1. Mineralogical Composition
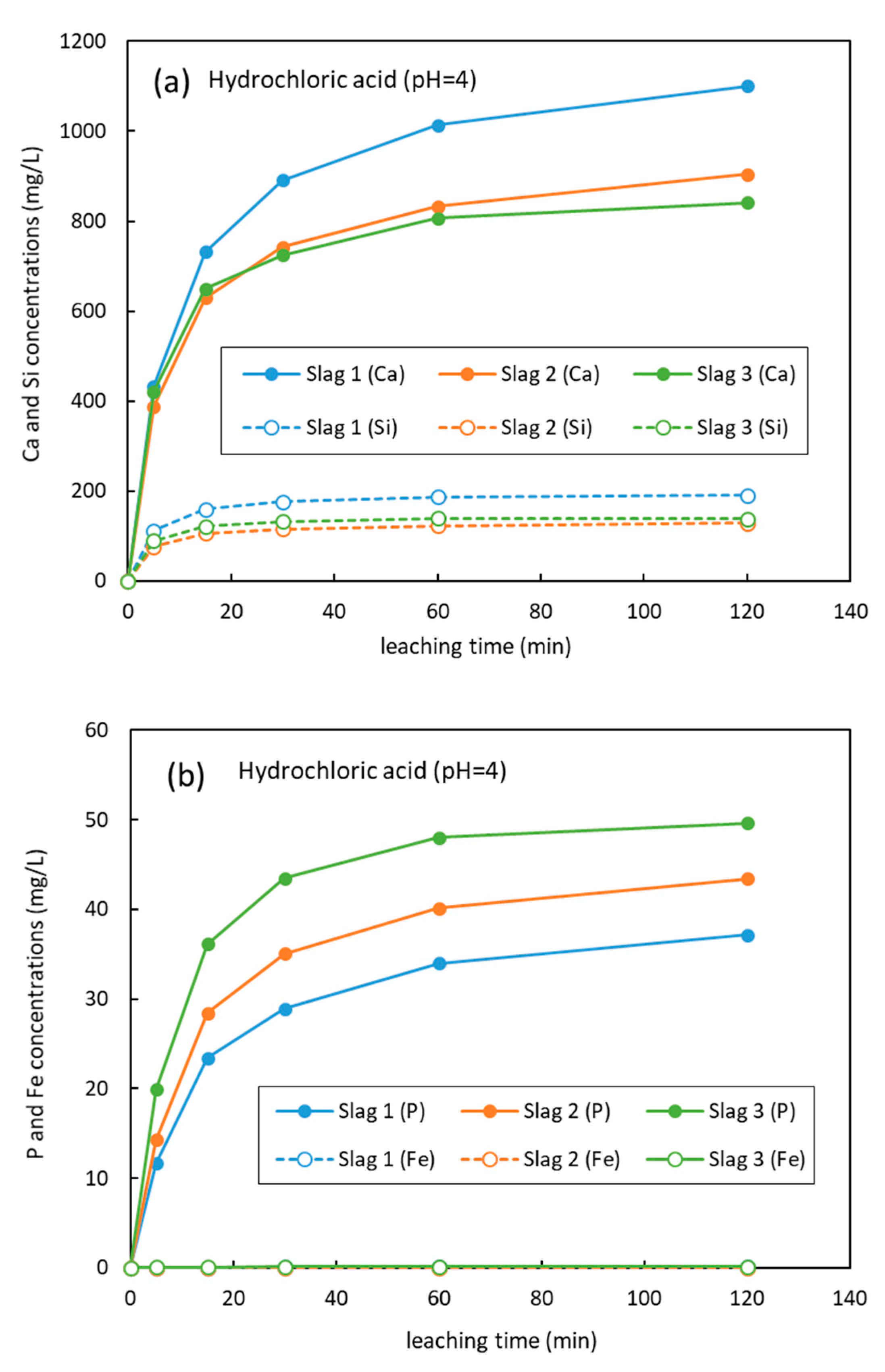
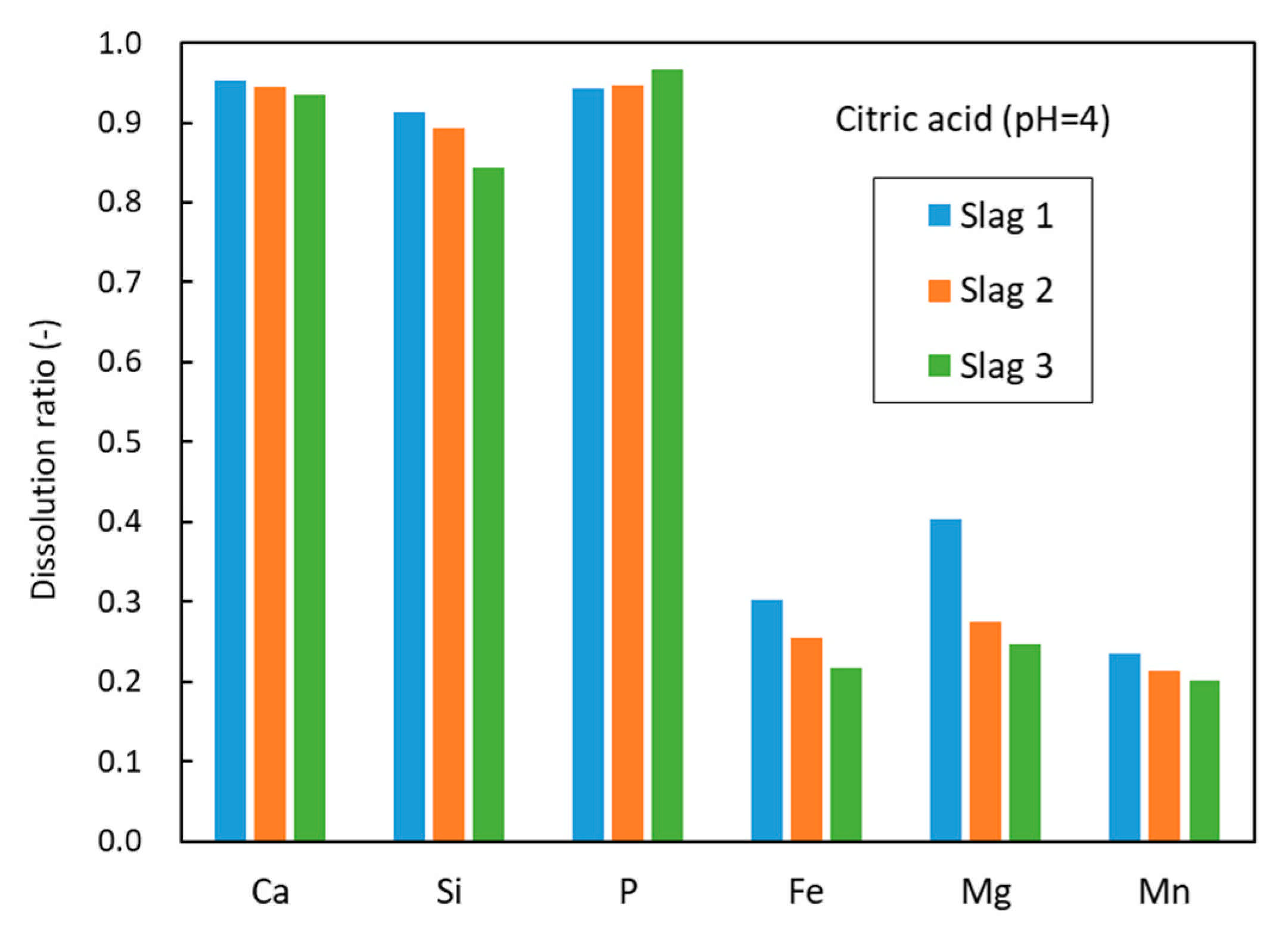
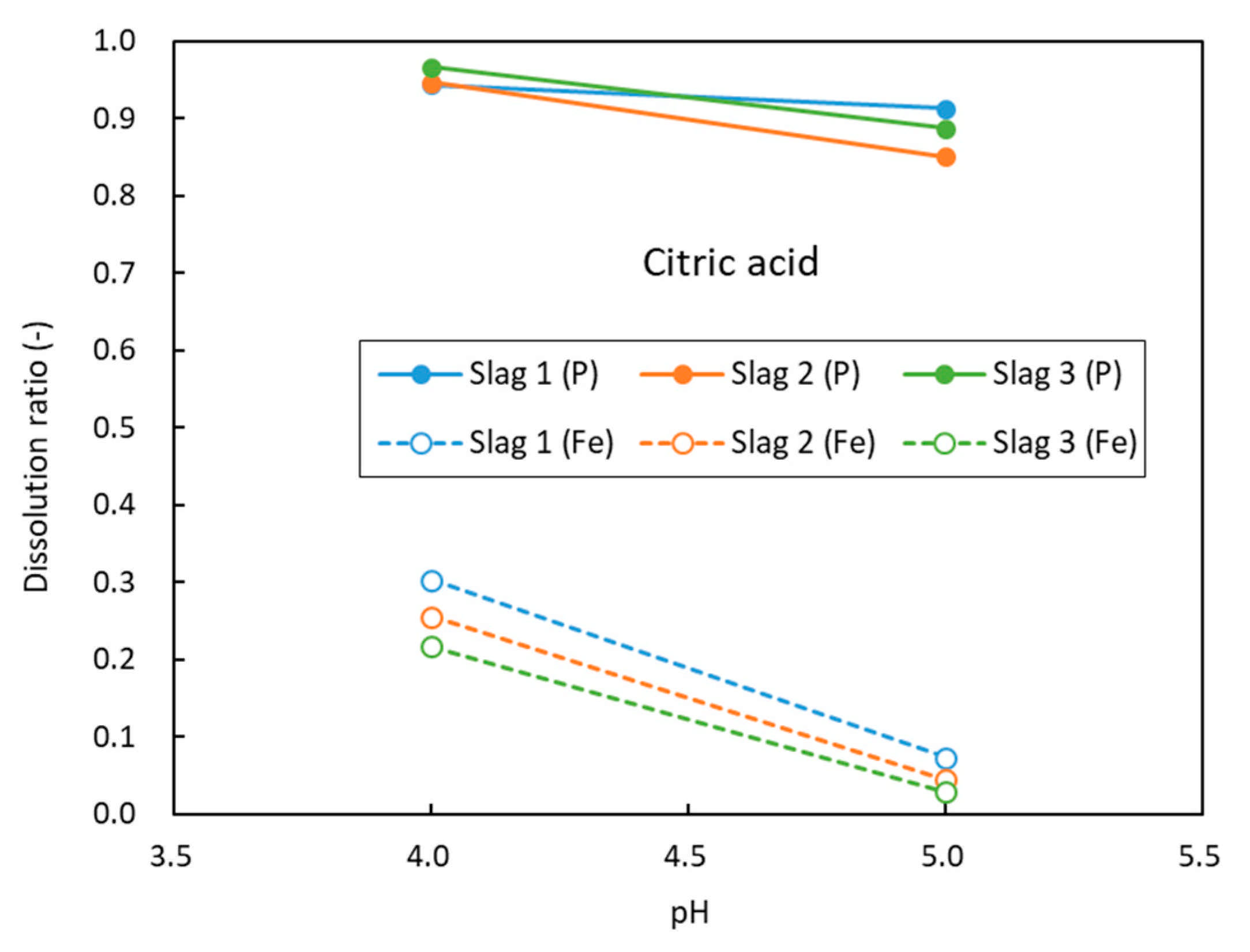
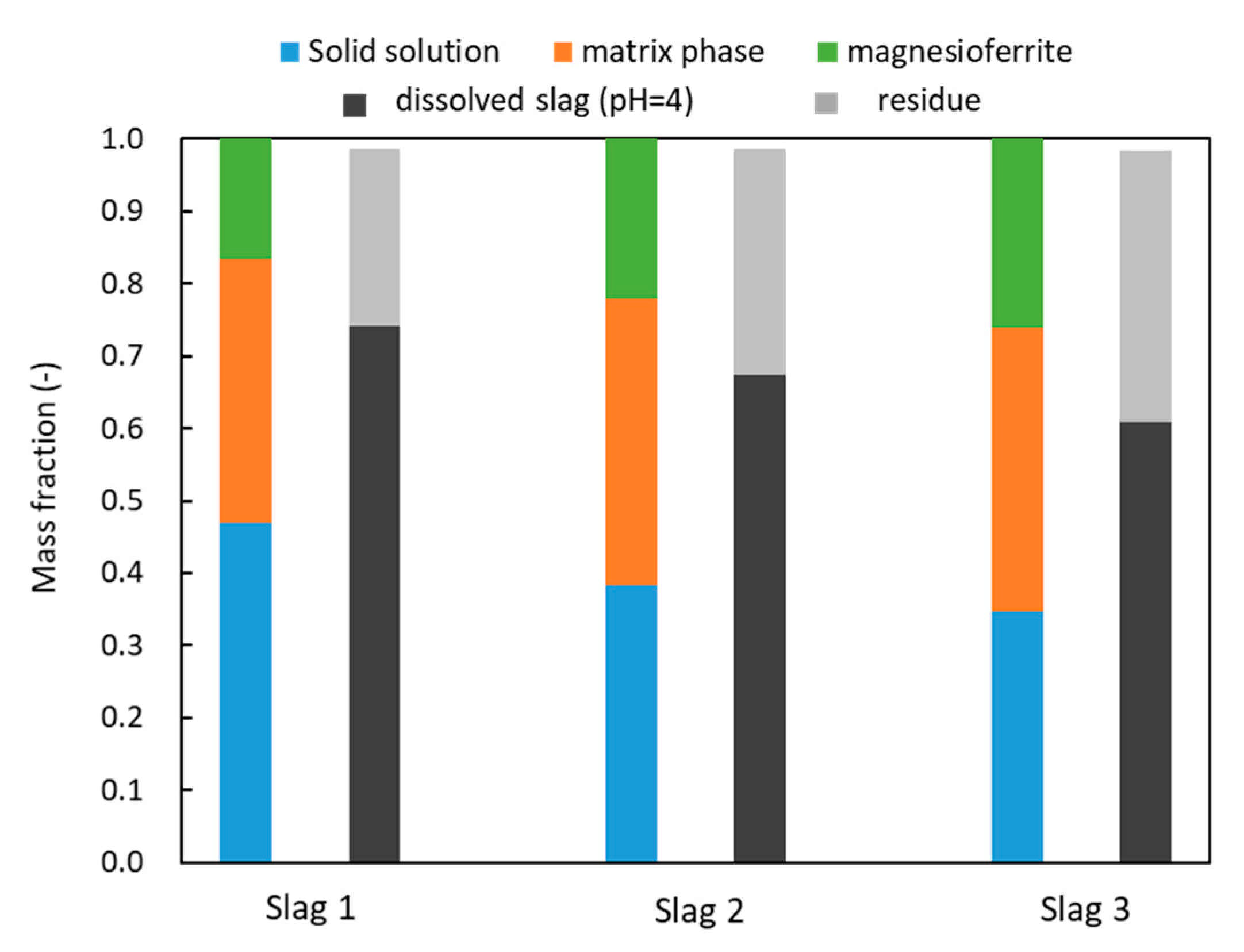
3.2. Leaching Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, C.; Gao, X.; Kitamura, S. Measures to decrease and utilize steelmaking slag. J. Sustain. Metall. 2019, 5, 141–153. [Google Scholar] [CrossRef]
- Lv, N.; Du, C.; Kong, H.; Yu, H. Leaching of phosphorus from quenched steelmaking slags with different composition. Metals 2021, 11, 1026. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Sun, H.; Yang, J.; Lu, X.; Liu, W.; Ye, G.; Zhang, R.; Yang, W. Dephosphorization in double slag converter steelmaking process at different temperatures by industrial experiments. Metals 2021, 11, 1030. [Google Scholar] [CrossRef]
- Aponte, D.; Soto Martín, O.; Valls del Barrio, S.; Barra Bizinotto, M. Ladle steel slag in activated systems for construction use. Minerals 2020, 10, 687. [Google Scholar] [CrossRef]
- Singh, S.K.; Rekha, P.; Surya, M. Utilization of Linz–Donawitz slag from steel industry for waste minimization. J. Mater. Cycles Waste Manag. 2020, 22, 611–627. [Google Scholar] [CrossRef]
- Diao, J.; Zhou, W.; Ke, Z.; Qiao, Y.; Zhang, T.; Liu, X.; Xie, B. System assessment of recycling of steel slag in converter steelmaking. J. Clean. Prod. 2016, 125, 159–167. [Google Scholar] [CrossRef]
- Matsubae, K.; Yamasue, E.; Inazumi, T.; Webeck, E.; Miki, T.; Nagasaka, T. Innovations in steelmaking technology and hidden phosphorus flows. Sci. Total Environ. 2016, 542, 1162–1168. [Google Scholar] [CrossRef] [Green Version]
- Ohtake, H.; Tsuneda, S. Phosphorus Recovery and Recycling; Springer: Singapor, 2019. [Google Scholar]
- Kikuchi, N.; Matsui, A.; Uchida, Y. Effect of lime dissolution rate in slag on hot metal dephosphorization. ISIJ Int. 2020, 60, 922–929. [Google Scholar] [CrossRef]
- Yan, Z.; Deng, Z.; Zhu, M. Effect of CaCl2 addition on phosphorus distribution between CaO–SiO2–FeOx–P2O5 slag and carbon-saturated iron at steelmaking temperature. Metall. Mater. Trans. B 2021, 52, 2806–2815. [Google Scholar] [CrossRef]
- Ye, G.F.; Yang, J.; Zhang, R.H.; Yang, W.K.; Sun, H. Behavior of phosphorus enrichment in dephosphorization slag at low temperature and low basicity. Int. J. Miner. Metall. 2021, 28, 66–75. [Google Scholar] [CrossRef]
- Wang, Z.; Sohn, I. A review on reclamation and reutilization of ironmaking and steelmaking slags. J. Sustain. Metall. 2019, 5, 127–140. [Google Scholar] [CrossRef]
- Ono, H.; Inagaki, A.; Masui, T.; Narita, H.; Mitsuo, T.; Nosaka, S.; Gonda, S. Removal of phosphorus from LD converter slag by floating of dicalcium silicate during solidification. Tetsu-to-Hagané 1980, 66, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Miki, T.; Kaneko, S. Separation of FeO and P2O5 from steelmaking slag utilizing capillary action. ISIJ Int. 2015, 55, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Gao, J.; Guo, Z. Isothermal enrichment of P-concentrating phase from CaO–SiO2–FeO–MgO–P2O5 melt with super gravity. ISIJ Int. 2016, 56, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Teratoko, T.; Maruoka, N.; Shibata, H.; Kitamura, S. Dissolution behavior of dicalcium silicate and tricalcium phosphate solid solution and other phases of steelmaking slag in an aqueous solution. High. Temp. Mater. Processes. 2012, 31, 329–338. [Google Scholar] [CrossRef]
- Numata, M.; Maruoka, N.; Kim, S.; Kitamura, S. Fundamental experiment to extract phosphorous selectively from steelmaking slag by leaching. ISIJ Int. 2014, 54, 1983–1990. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Diao, J.; Liu, X.; Li, X.; Zhang, T.; Xie, B. Dephosphorization of steelmaking slag by leaching with acidic aqueous solution. JOM 2016, 68, 2511–2519. [Google Scholar] [CrossRef]
- Du, C.; Gao, X.; Ueda, S.; Kitamura, S. A kinetic study on selective leaching of phosphorus from dephosphorization slag. J. Sustain. Metall. 2020, 6, 724–738. [Google Scholar] [CrossRef]
- Du, C.; Gao, X.; Ueda, S.; Kitamura, S. Effect of Na2O addition on phosphorus dissolution from steelmaking slag with high P2O5 content. J. Sustain. Metall. 2017, 3, 671–682. [Google Scholar] [CrossRef]
- Du, C.; Gao, X.; Ueda, S.; Kitamura, S. Effect of Fe2+/T.Fe ratio on the dissolution behavior of P from steelmaking slag with high P2O5 content. J. Sustain. Metall. 2018, 4, 443–454. [Google Scholar] [CrossRef]
- Du, C.; Gao, X.; Ueda, S.; Kitamura, S. Recovery of phosphorus from modified steelmaking slag with high P2O5 content via leaching and precipitation. Tetsu-to-Hagané 2021, 107, 103–111. [Google Scholar] [CrossRef]
- Futatsuka, T.; Shitogiden, K.; Miki, T.; Nagasaka, T.; Hino, M. Dissolution behavior of nutrition elements from steelmaking slag into seawater. ISIJ Int. 2004, 44, 753–761. [Google Scholar] [CrossRef]
- Bassi, R.; Prasher, S.O.; Simpson, B.K. Extraction of metals from a contaminated sandy soil using citric acid. Environ. Prog. 2000, 19, 275–282. [Google Scholar] [CrossRef]
- Astuti, W.; Hirajima, T.; Sasaki, K.; Okibe, N. Comparison of effectiveness of citric acid and other acids in leaching of low-grade Indonesian saprolitic ores. Miner. Eng. 2016, 85, 1–16. [Google Scholar] [CrossRef]




| CaO | SiO2 | Fe2O3 | P2O5 | MgO | MnO | |
|---|---|---|---|---|---|---|
| Slag 1 | 40.7 | 25.3 | 24.0 | 3.0 | 4.0 | 3.0 |
| Slag 2 | 36.7 | 22.8 | 30.5 | 3.0 | 4.0 | 3.0 |
| Slag 3 | 33.3 | 20.7 | 36.0 | 3.0 | 4.0 | 3.0 |
| Mineral Phase | CaO | SiO2 | Fe2O3 | P2O5 | MgO | MnO | |
|---|---|---|---|---|---|---|---|
| Slag 1 | A | 1.4 | 0.2 | 76.5 | 0.0 | 12.8 | 9.1 |
| B | 32.6 | 31.1 | 33.1 | 0.6 | 1.2 | 1.4 | |
| C | 59.9 | 29.7 | 1.8 | 7.1 | 0.8 | 0.7 | |
| Slag 2 | A | 2.3 | 0.1 | 78.2 | 0.0 | 10.4 | 9.0 |
| B | 32.2 | 31.8 | 33.8 | 0.5 | 0.9 | 0.8 | |
| C | 59.7 | 28.4 | 1.9 | 9.2 | 0.4 | 0.4 | |
| Slag 3 | A | 2.1 | 0.1 | 79.1 | 0.0 | 9.9 | 8.8 |
| B | 31.5 | 33.4 | 33.2 | 0.4 | 0.8 | 0.7 | |
| C | 59.5 | 27.4 | 1.6 | 10.8 | 0.4 | 0.3 | |
| CaO | SiO2 | P2O5 | Fe2O3 | MgO | MnO | |
|---|---|---|---|---|---|---|
| Residue (slag 1) | 10.0 | 32.3 | 2.1 | 45.5 | 5.0 | 5.1 |
| Residue (slag 2) | 11.6 | 27.4 | 0.9 | 50.4 | 5.1 | 4.6 |
| Residue (slag 3) | 11.4 | 22.7 | 0.7 | 56.5 | 4.5 | 4.2 |
| Point | Ca | Si | Fe | P | Mg | Mn |
|---|---|---|---|---|---|---|
| A | 1.3 | 0 | 55.1 | 0 | 5.8 | 6.4 |
| B | 22.6 | 15.2 | 23.0 | 0.2 | 0.4 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-W.; Li, P.-P.; Du, C.-M.; Lv, N.-N. Effect of Fe2O3 Content and Acid on the Leaching Behavior of Phosphorus from Dephosphorization Slag. Minerals 2021, 11, 972. https://doi.org/10.3390/min11090972
Liu S-W, Li P-P, Du C-M, Lv N-N. Effect of Fe2O3 Content and Acid on the Leaching Behavior of Phosphorus from Dephosphorization Slag. Minerals. 2021; 11(9):972. https://doi.org/10.3390/min11090972
Chicago/Turabian StyleLiu, Shi-Wei, Ping-Ping Li, Chuan-Ming Du, and Ning-Ning Lv. 2021. "Effect of Fe2O3 Content and Acid on the Leaching Behavior of Phosphorus from Dephosphorization Slag" Minerals 11, no. 9: 972. https://doi.org/10.3390/min11090972
APA StyleLiu, S.-W., Li, P.-P., Du, C.-M., & Lv, N.-N. (2021). Effect of Fe2O3 Content and Acid on the Leaching Behavior of Phosphorus from Dephosphorization Slag. Minerals, 11(9), 972. https://doi.org/10.3390/min11090972