Environmental Impact of Potentially Toxic Elements on Tropical Soils Used for Large-Scale Crop Commodities in the Eastern Amazon, Brazil
Abstract
:1. Introduction
2. Material and Methods
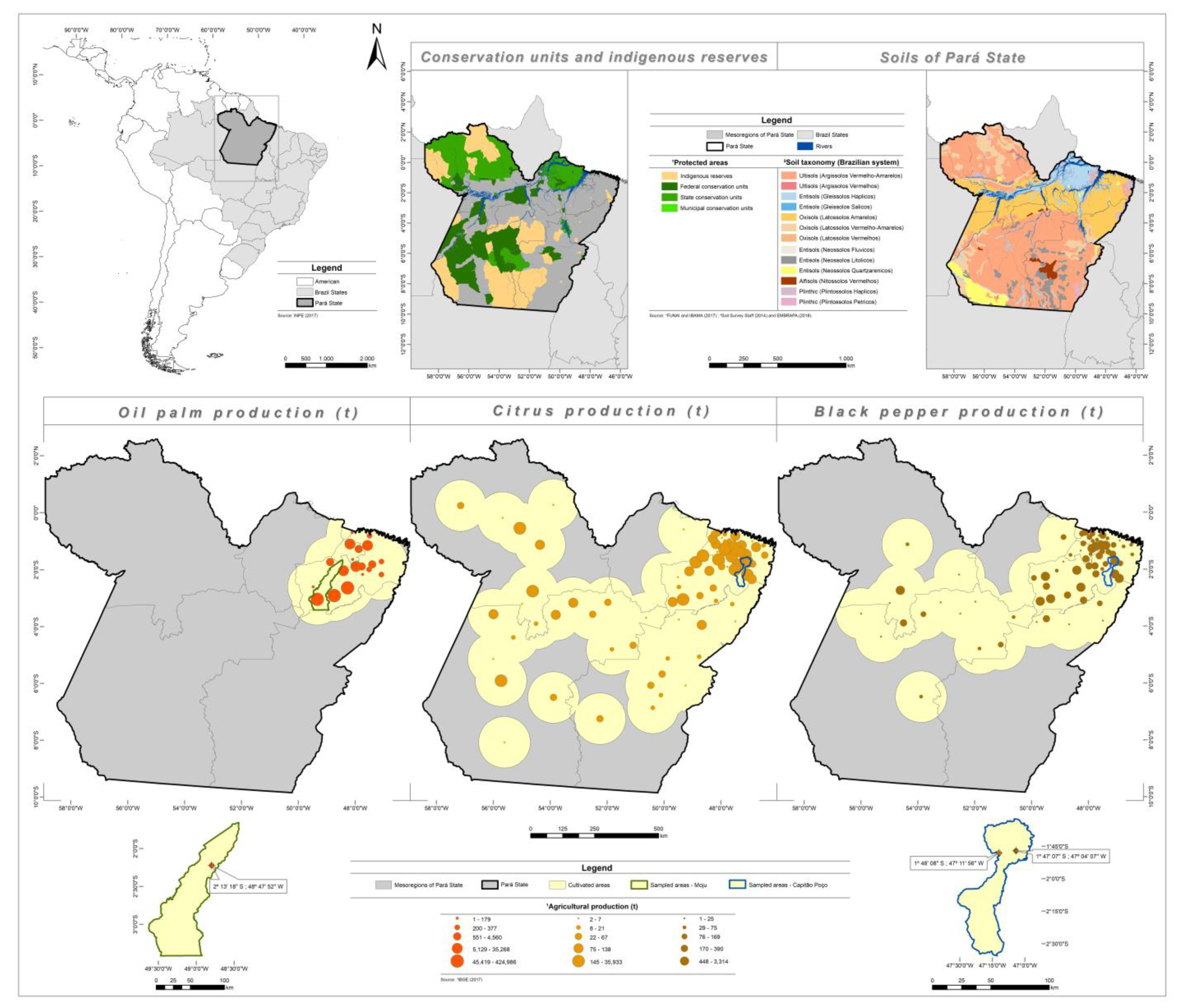
2.1. Study Site
2.2. Sampling and Analyzes of Soils and Plants
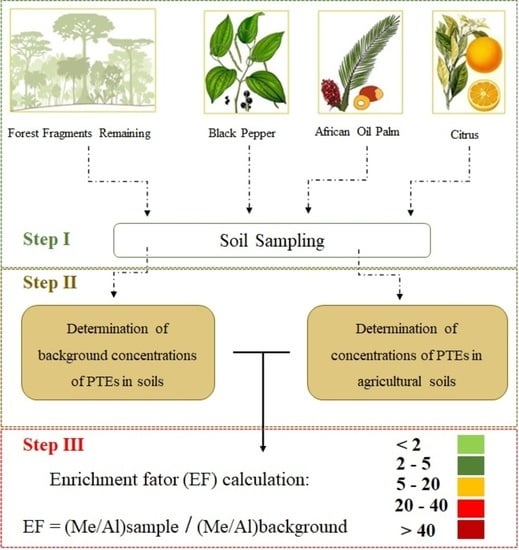
2.3. Enrichment and Bioaccumulation Factors
2.4. Statistical Analyses
3. Results
3.1. Soil Attributes
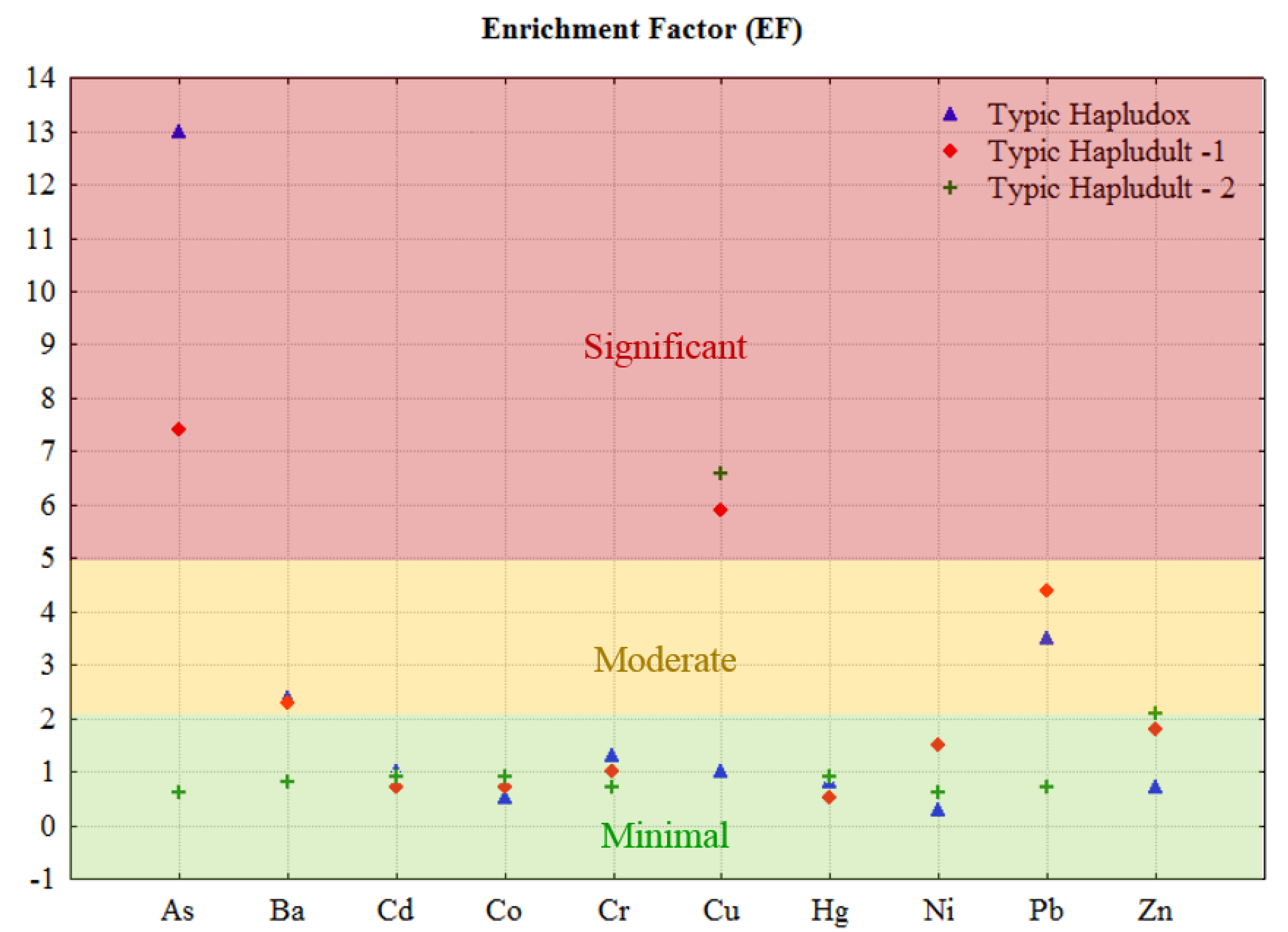
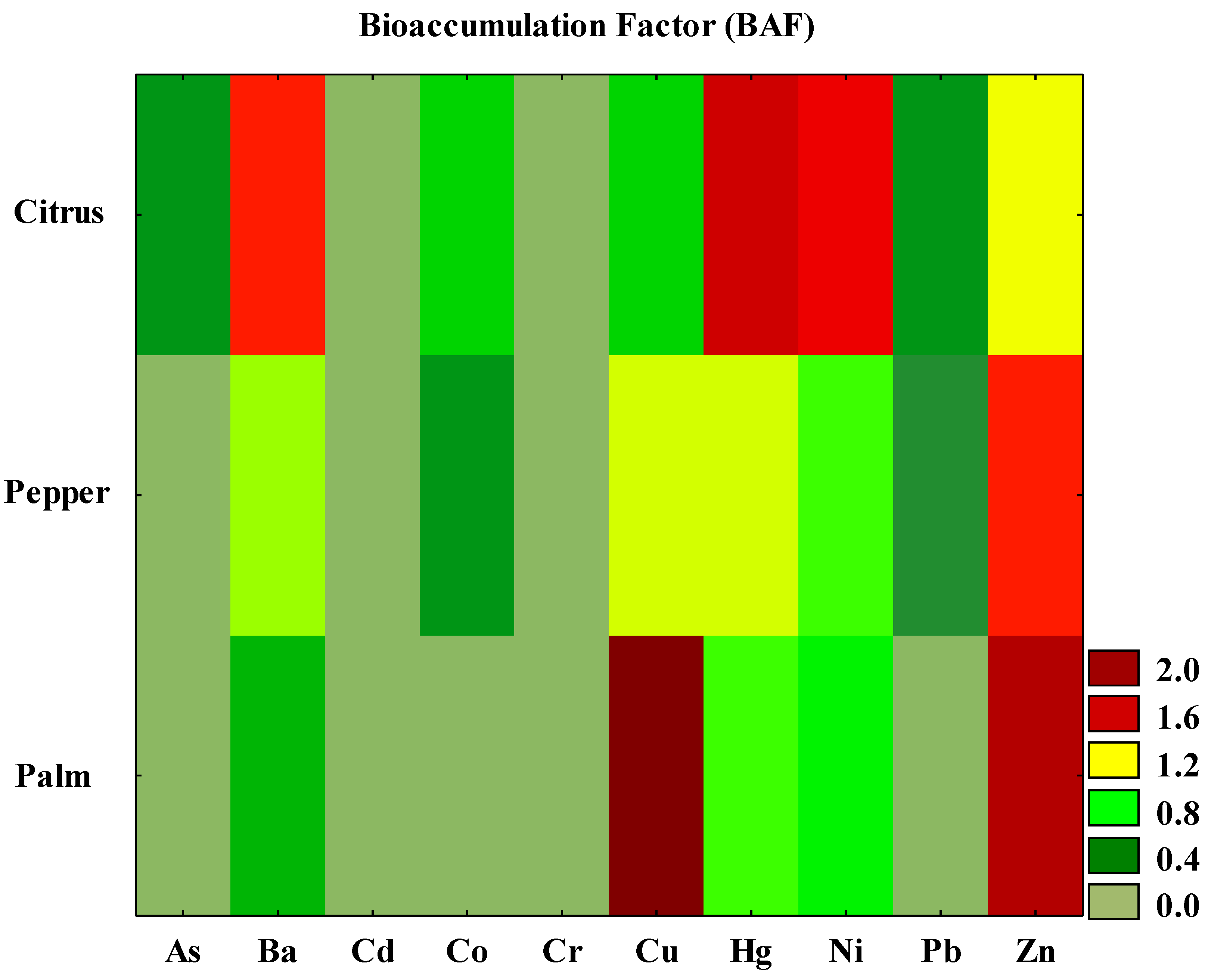
3.2. Concentrations, Enrichment and Bioaccumulation of PTEs
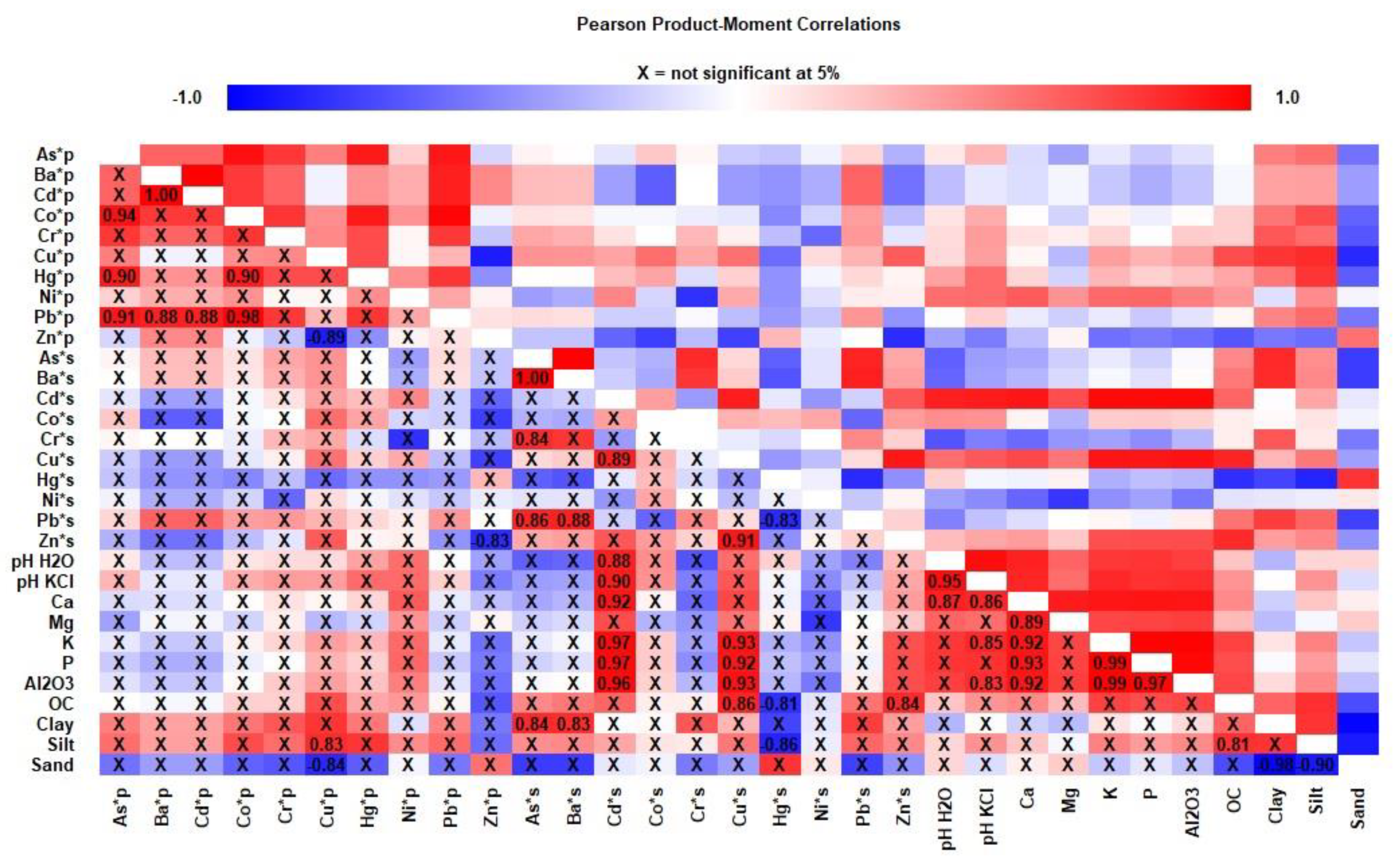
3.3. Correlation Study
4. Discussion
4.1. Soil Attributes
4.2. Concentrations, Enrichment and Bioaccumulation
4.3. Comprehensive Correlation of PTEs in Soil–Crop Systems
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vale, P.; Gibbs, H.; Vale, R.; Christie, M.; Florence, E.; Munger, J.; Sabaini, D. The expansion of intensive beef farming to the Brazilian Amazon. Glob. Environ. Chang. 2019, 57, 101922. [Google Scholar] [CrossRef]
- Cortner, O.; Garrett, R.D.; Valentim, J.F.; Ferreira, J.; Niles, M.T.; Reis, J.; Gil, J. Perceptions of integrated crop-livestock systems for sustainable intensification in the Brazilian Amazon. Land Use Policy 2019, 82, 841–853. [Google Scholar] [CrossRef]
- Bowman, M.S.; Soares-Filho, B.S.; Merry, F.D.; Nepstad, D.C.; Rodrigues, H.; Almeida, O.T. Persistence of cattle ranching in the Brazilian Amazon: A spatial analysis of the rationale for beef production. Land Use Policy 2012, 29, 558–568. [Google Scholar] [CrossRef]
- de Souza, R.A.; Miziara, F.; De Marco, P.J. Spatial variation of deforestation rates in the Brazilian Amazon: A complex theater for agrarian technology, agrarian structure and governance by surveillance. Land Use Policy 2013, 30, 915–924. [Google Scholar] [CrossRef]
- IBGE—Brazilian Institute of Geography and Statistics. Municipal Agricultural Research. 2017. Available online: https://cidades.ibge.gov.br/brasil/pa/pesquisa/15/11863 (accessed on 4 January 2019).
- Smidt, G.A.; Koschinsky, A.; de Carvalho, L.M.; Monserrat, J.; Schnug, E. Heavy metal concentrations in soils in the vicinity of a fertilizer factory in Southern Brazil. Agric. For. Res. 2011, 61, 353–364. [Google Scholar]
- Ramos, S.J.; Dinali, G.S.; de Carvalho, T.S.; Chaves, L.C.; Siqueira, J.O.; Guilherme, L.R.G. Rare earth elements in raw materials and products of the phosphate fertilizer industry in South America: Content, signature, and crystalline phases. J. Geochem. Explor. 2016, 168, 177–186. [Google Scholar] [CrossRef]
- Bosco-Santos, A.; Luiz-Silva, W.; da Silva-Filho, E.V.; de Souza, M.D.C.; Dantas, E.L.; Navarro, M.S. Fractionation of rare earth and other trace elements in crabs, Ucides cordatus, from a subtropical mangrove affected by fertilizer industry. J. Environ. Sci. 2017, 54, 69–76. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.B.V.; do Nascimento, C.W.A.; Muniz Araújo, P.R. Environmental risk of trace elements in P-containing fertilizers marketed in Brazil. J. Soil Sci. Plant Nutr. 2017, 17, 635–647. [Google Scholar] [CrossRef] [Green Version]
- Michalak, I.; Saeid, A.; Chojnacka, K.; Gramza, M. Trace elements as fertilizer micronutrients. In Recent Advances in Trace Elements; Chojnacka, K., Saeid, A., Eds.; Wiley-Blackwell: Oxford, UK, 2018; pp. 299–318. ISBN 9781119133773. [Google Scholar]
- Dutra, P.H.; Feliciano, V.M.D.; Carvalho Filho, C.A.d. Distribution of major and trace elements in bottom sediments of the Taquari River Basin, Caldas municipality (Brazil). Ambient. Agua—Interdiscip. J. Appl. Sci. 2019, 14. [Google Scholar] [CrossRef]
- Júnior, E.P.S.; da Conceição, F.T.; Menegário, A.A.; Fernandes, A.M.; Sardinha, D.d.S. The fertilizer-effect on Al, Ba, Fe, Mn and Ni released in a watershed with influence of sugar cane crops in the São Paulo state, Brazil. J. Water Resour. Prot. 2019, 11, 638–650. [Google Scholar] [CrossRef] [Green Version]
- Parameswari, E.; Davamani, V.; Premalatha, R.; Muthumanickam, D. Assessment of trace elements concentration of soils in vineyard plantation under intensive system. J. Pharmacogn. Phytochem. 2019, 8, 2456–2459. [Google Scholar]
- Qian, L.; Zhang, C.; Zuo, F.; Zheng, L.; Li, D.; Zhang, A.; Zhang, D. Effects of fertilizers and pesticides on the mineral elements used for the geographical origin traceability of rice. J. Food Compos. Anal. 2019, 83, 103276. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Gong, Y.; Liu, Q.; Yang, S.; Ma, J.; Zhao, L.; Hou, H. Status of copper accumulation in agricultural soils across China (1985–2016). Chemosphere 2020, 244, 125516. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, D.G.; Lopes, A.R.; Martins, I.K.; Leandro, L.P.; Nunes, M.E.M.; de Carvalho, N.R.; Rodrigues, N.R.; Macedo, G.E.; Saidelles, A.P.; Aguiar, C.; et al. Mancozeb exposure results in manganese accumulation and Nrf2-related antioxidant responses in the brain of common carp Cyprinus carpio. Environ. Sci. Pollut. Res. 2018, 25, 15529–15540. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Gong, Q.; Chen, P.; Shi, R.; Gao, Y.; Zheng, S.-A.; Xu, Y.; Shao, C.; Zheng, X. Health assessment of trace metal concentrations in organic fertilizer in Northern China. Int. J. Environ. Res. Public Health 2019, 16, 1031. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, R.; Xia, S.; Wang, L.; Liu, C.; Zhang, R.; Fan, Z.; Chen, F.; Liu, Y. Interactions between N, P and K fertilizers affect the environment and the yield and quality of satsumas. Glob. Ecol. Conserv. 2019, 19, e00663. [Google Scholar] [CrossRef]
- CETESB—Environmental Agency of the State of Sao Paulo. Report Establishment of Guiding Values for Soils and Groundwater of the State of Sao Paulo; CETESB: São Paulo, Brazil, 2005; p. 247.
- Martinez-lladó, X.; Vilà, M.; Martí, V.; Rovira, M.; Domènech, J.A.; Pablo, J. Trace element distribution in topsoils in Catalonia: Background and reference values and relationship with regional geology. Environ. Eng. Sci. 2008, 25, 863–878. [Google Scholar] [CrossRef]
- Lado, L.R.; Hengl, T.; Reuter, H.I. Heavy metals in European soils: A geostatistical analysis of the FOREGS geochemical database. Geoderma 2008, 148, 189–199. [Google Scholar] [CrossRef]
- CONAMA—National Council for the Environment. Brazil. Resolution No. 420/2009. 2009. Available online: http://www.mma.gov.br/port/conama/legiabre.cfm?codlegi=620 (accessed on 16 January 2019).
- Nael, M.; Khademi, H.; Jalalian, A.; Schulin, R.; Kalbasi, M.; Sotohian, F. Effect of geo-pedological conditions on the distribution and chemical speciation of selected trace elements in forest soils of western Alborz, Iran. Geoderma 2009, 152, 157–170. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Souza, E.S.d.; de Souza Braz, A.M.; Birani, S.M.; Alleoni, L.R.F. Quality reference values and background concentrations of potentially toxic elements in soils from the Eastern Amazon, Brazil. J. Geochem. Explor. 2018, 190, 453–463. [Google Scholar] [CrossRef]
- Salomão, G.N.; Dall’Agnol, R.; Angélica, R.S.; Figueiredo, M.A.; Sahoo, P.K.; de Medeiros Filho, C.A.; da Costa, M.F. Geochemical mapping and estimation of background concentrations in soils of Carajás mineral province, eastern Amazonian Craton, Brazil. Geochem. Explor. Environ. Anal. 2019, 19, 431–447. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Dall’Agnol, R.; Salomão, G.N.; da Silva Ferreira, J.J.; da Silva, M.S.; Martins, G.C.; e Souza Filho, P.W.M.; Powell, M.A.; Maurity, C.W.; Angelica, R.S.; et al. Source and background threshold values of potentially toxic elements in soils by multivariate statistics and GIS-based mapping: A high density sampling survey in the Parauapebas basin, Brazilian Amazon. Environ. Geochem. Health 2020, 42, 255–282. [Google Scholar] [CrossRef] [PubMed]
- Loska, K.; Cebula, J.; Pelczar, J.; Wiechuła, D.; Kwapuliński, J. Use of enrichment, and contamination factors together with geoaccumulation indexes to evaluate the content of Cd, Cu, and Ni in the Rybnik water reservoir in Poland. Water Air Soil Pollut. 1997, 93, 347–365. [Google Scholar] [CrossRef]
- Loska, K.; Wiechuła, D.; Korus, I. Metal contamination of farming soils affected by industry. Environ. Int. 2004, 30, 159–165. [Google Scholar] [CrossRef]
- Blaser, P.; Zimmermann, S.; Luster, J.; Shotyk, W. Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb, and Zn in Swiss forest soils. Sci. Total Environ. 2000, 249, 257–280. [Google Scholar] [CrossRef]
- Martínez Cortizas, A. Distribution of some selected major and trace elements in four Italian soils developed from the deposits of the Gauro and Vico volcanoes. Geoderma 2003, 117, 215–224. [Google Scholar] [CrossRef]
- Tijani, M.N.; Okunlola, O.A.; Abimbola, A.F. Lithogenic concentrations of trace metals in soils and saprolites over crystalline basement rocks: A case study from SW Nigeria. J. Afr. Earth Sci. 2006, 46, 427–438. [Google Scholar] [CrossRef]
- Dragović, S.; Mihailović, N.; Gajić, B. Heavy metals in soils: Distribution, relationship with soil characteristics and radionuclides and multivariate assessment of contamination sources. Chemosphere 2008, 72, 491–495. [Google Scholar] [CrossRef]
- Muñoz-Barbosa, A.; Gutiérrez-Galindo, E.A.; Daesslé, L.W.; Orozco-Borbón, M.V.; Segovia-Zavala, J.A. Relationship between metal enrichments and a biological adverse effects index in sediments from Todos Santos Bay, northwest coast of Baja California, México. Mar. Pollut. Bull. 2012, 64, 405–409. [Google Scholar] [CrossRef]
- Sello Likuku, A.; Mmolawa, K.; Kabelo Gaboutloeloe, G. Assessment of heavy metal enrichment and degree of contamination around the copper-nickel mine in the Selebi Phikwe region, Eastern Botswana. Environ. Ecol. Res. 2013, 1, 32–40. [Google Scholar] [CrossRef]
- Alagarsamy, R.; Zhang, J. Geochemical characterisation of major and trace elements in the coastal sediments of India. Environ. Monit. Assess. 2010, 161, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Rubio, B.; Nombela, M.; Vilas, F. Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): An assessment of metal pollution. Mar. Pollut. Bull. 2000, 40, 968–980. [Google Scholar] [CrossRef]
- Facchinelli, A.; Sacchi, E.; Mallen, L. Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ. Pollut. 2001, 114, 313–324. [Google Scholar] [CrossRef]
- Sipter, E.; Auerbach, R.; Gruiz, K.; Mathe-Gaspar, G. Change of bioaccumulation of toxic metals in vegetables. Commun. Soil Sci. Plant Anal. 2009, 40, 285–293. [Google Scholar] [CrossRef]
- Oti, W.O. bioaccumulation factors and pollution indices of heavy metals in selected fruits and vegetables from a derelict mine and their associated health implications. Int. J. Environ. Sustain. 2015, 4, 2015. [Google Scholar] [CrossRef]
- USDA. Keys to Soil Taxonomy, 12th ed.; USDA–Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Souza, E.S.d.; Fernandes, A.R.; Souza Braz, A.M.d.; Oliveira, F.J.d.; Alleoni, L.R.F.; Campos, M.C.C. Physical, chemical, and mineralogical attributes of a representative group of soils from the eastern Amazon region in Brazil. Soil 2018, 4, 195–212. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.F.; Cruz, E.S. Manual of Sample Collection of Black Pepper Leaves for Nutritional Diagnosis; Embrapa Amazônia Oriental: Belém, Brazil, 1999. [Google Scholar]
- Dias, J.R.M.; Tucci, C.A.F.; Wadt, P.G.S.; da Silva, A.M.; Santos, J.Z.L. Níveis críticos e faixas de suficiência nutricional em laranjeira-pêra na Amazônia Central obtidas pelo método DRIS. Acta Amaz. 2013, 43, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.R.L.; Amblard, P.; Barcelos, E.; Macedo, J.L.V.; Cunha, R.N.V.; Tavares, A.M. Evaluation of the nutritional status of oil palm: Leaf analysis. Reformulated. In Circular técnica; Embrapa Amazônia Ocidental: Manaus, Brazil, 2006; Volume 26. [Google Scholar]
- Silva, C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- McGrath, S.P.; Cunliffe, C.H. A simplified method for the extraction of the metals Fe, Zn, Cu, Ni, Cd, Pb, Cr, Co and Mn from soils and sewage sludges. J. Sci. Food Agric. 1985, 36, 794–798. [Google Scholar] [CrossRef]
- Araújo, G.C.L.; Gonzalez, M.H.; Ferreira, A.G.; Nogueira, A.R.A.; Nóbrega, J.A. Effect of acid concentration on closed-vessel microwave-assisted digestion of plant materials. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 2121–2132. [Google Scholar] [CrossRef]
- Wu, J.; Teng, Y.; Lu, S.; Wang, Y.; Jiao, X. Evaluation of soil contamination indices in a mining area of Jiangxi, China. PLoS ONE 2014, 9, e112917. [Google Scholar] [CrossRef]
- Islam, S.; Ahmed, K.; Habibullah-Al-Mamun; Masunaga, S. Potential ecological risk of hazardous elements in different land-use urban soils of Bangladesh. Sci. Total Environ. 2015, 512–513, 94–102. [Google Scholar] [CrossRef]
- Sutherland, R.A. Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ. Geol. 2000, 39, 611–627. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, J.; Chopra, A.K. Assessment of plant growth attributes, bioaccumulation, enrichment, and translocation of heavy metals in water lettuce (Pistia stratiotes L.) grown in sugar mill effluent. Int. J. Phytoremediation 2018, 20, 507–521. [Google Scholar] [CrossRef]
- Khaokaew, S.; Landrot, G. A field-scale study of cadmium phytoremediation in a contaminated agricultural soil at Mae Sot District, Tak Province, Thailand: (1) Determination of Cd-hyperaccumulating plants. Chemosphere 2015, 138, 883–887. [Google Scholar] [CrossRef]
- Covre, W.P.; Pereira, W.V.d.S.; Gonçalves, D.A.M.; Teixeira, O.M.M.; Amarante, C.B.d.; Fernandes, A.R. Phytoremediation potential of Khaya ivorensis and Cedrela fissilis in copper contaminated soil. J. Environ. Manag. 2020, 268, 110733. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Venegas, V.H.A.; Novais, R.F.; Barros, N.F.; Cantarutti, R.B.; Lopes, A.S. Interpretation of soil analysis results. In Recommendations for the Use of Correctives and Fertilizers in Minas Gerais; Comissão de Fertilidade do Solo do Estado de Minas Gerais: Viçosa, Brazil, 1999; pp. 25–32. [Google Scholar]
- Birani, S.M.; Fernandes, A.R.; de Souza Braz, A.M.; Pedroso, A.J.S.; Alleoni, L.R.F. Available contents of potentially toxic elements in soils from the Eastern Amazon. Geochemistry 2015, 75, 143–151. [Google Scholar] [CrossRef]
- Moreira, A.; Fageria, N.K. Soil chemical attributes of Amazonas State, Brazil. Commun. Soil Sci. Plant Anal. 2009, 40, 2912–2925. [Google Scholar] [CrossRef]
- Frazão, L.A.; Paustian, K.; Pellegrino Cerri, C.E.; Cerri, C.C. Soil carbon stocks and changes after oil palm introduction in the Brazilian Amazon. GCB Bioenergy 2013, 5, 384–390. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Rodrigues, M.; Soltangheisi, A.; de Carvalho, T.S.; Guilherme, L.R.G.; Benites, V.d.M.; Gatiboni, L.C.; de Sousa, D.M.G.; Nunes, R.d.S.; Rosolem, C.A.; et al. Transitions to sustainable management of phosphorus in Brazilian agriculture. Sci. Rep. 2018, 8, 2537. [Google Scholar] [CrossRef] [PubMed]
- Bayer, C.; Mielniczuk, J. Características químicas do solo afetadas por métodos de preparo e sistemas de cultura. Soil Sci. 1997, 21, 105–112. [Google Scholar]
- Bowman, R.A.; Reeder, J.D.; Lober, R.W. Changes in soil properties in a central plains rangeland soil after 3, 20, and 60 years of cultivation. Soil Sci. 1990, 150, 851–857. [Google Scholar] [CrossRef]
- de Souza Braz, A.M.; Fernandes, A.R.; Alleoni, L.R.F. Soil attributes after the conversion from forest to pasture in the Amazon. Land Degrad. Dev. 2013, 24, 33–38. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Ramalho, J.F.G.P.; Amaral Sobrinho, N.M.B.; Velloso, A.C.X. Acúmulo de metais pesados em solos cultivados com cana-de-açúcar pelo uso contínuo de adubação fosfatada e água de irrigação. Rev. Bras. Ciência Solo 1999, 23, 971–979. [Google Scholar] [CrossRef]
- Aini Azura, A.; Fauziah, C.I.; Samsuri, A.W. Cadmium and Zinc concentrations in soils and oil palm tissues as affected by long-term application of phosphate rock fertilizers. Soil Sediment Contam. Int. J. 2012, 21, 586–603. [Google Scholar] [CrossRef]
- Lokesh, M.S.; Gangadharappa, P.M. Management of Phytophthora foot rot and nematode diseases in black pepper (Piper nigrum L.). J. Spices Aromat. Crop. 1995, 4, 61–63. [Google Scholar]
- Fan, J.; He, Z.; Ma, L.Q.; Stoffella, P.J. Accumulation and availability of copper in citrus grove soils as affected by fungicide application. J. Soils Sediments 2011, 11, 639–648. [Google Scholar] [CrossRef]
- Behlau, F.; Scandelai, L.H.M.; da Silva Junior, G.J.; Lanza, F.E. Soluble and insoluble copper formulations and metallic copper rate for control of citrus canker on sweet orange trees. Crop. Prot. 2017, 94, 185–191. [Google Scholar] [CrossRef]
- Mirlean, N.; Roisenberg, A.; Chies, J.O. Metal contamination of vineyard soils in wet subtropics (southern Brazil). Environ. Pollut. 2007, 149, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.-C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Preston, W.; da Silva, Y.J.A.B.; do Nascimento, C.W.A.; da Cunha, K.P.V.; Silva, D.J.; Ferreira, H.A. Soil contamination by heavy metals in vineyard of a semiarid region: An approach using multivariate analysis. Geoderma Reg. 2016, 7, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Alloway, B.J. Heavy Metals in Soils; Blackie Academic & Professional: London, UK, 1995. [Google Scholar]
- Campos, M.L.; Pierangeli, M.A.P.; Guilherme, L.R.G.; Marques, J.J.; Curi, N. Baseline concentration of heavy metals in Brazilian Latosols. Commun. Soil Sci. Plant Anal. 2003, 34, 547–557. [Google Scholar] [CrossRef]
- Campos, M.L.; Guilherme, L.R.G.; Lopes, R.S.; Antunes, A.S.; Marques, J.J.G.S.M.; Curi, N. Teor e capacidade máxima de adsorção de arsênio em Latossolos brasileiros. Rev. Bras. Ciência Solo 2007, 31, 1311–1318. [Google Scholar] [CrossRef]
- Marques, J.J.G.S.M. Trace Element Distributions in Brazilian Cerrado at the Landscape and Micrometer Scales. Ph.D. Thesis, Purdue University, Lafayette, IN, USA, 2000. [Google Scholar]
- Fitz, W.J.; Wenzel, W.W. Arsenic transformations in the soil–rhizosphere–plant system: Fundamentals and potential application to phytoremediation. J. Biotechnol. 2002, 99, 259–278. [Google Scholar] [CrossRef]
- Smedley, P.; Kinniburgh, D. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- WHO—World Health Organization. Arsenic and Arsenic Compounds, 2nd ed.; WHO: Geneva, Switzerland, 2001; p. 187. [Google Scholar]
- dos Santos, S.N.; Alleoni, L.R.F. Reference values for heavy metals in soils of the Brazilian agricultural frontier in Southwestern Amazônia. Environ. Monit. Assess. 2013, 185, 5737–5748. [Google Scholar] [CrossRef]
- Biondi, C.M.; Nascimento, C.W.A.d.; Fabricio Neta, A.d.B.; Ribeiro, M.R. Teores de Fe, Mn, Zn, Cu, Ni E Co em solos de referência de Pernambuco. Rev. Bras. Ciência Solo 2011, 35, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- do Nascimento, C.W.A.; Lima, L.H.V.; da Silva, F.L.; Biondi, C.M.; Campos, M.C.C. Natural concentrations and reference values of heavy metals in sedimentary soils in the Brazilian Amazon. Environ. Monit. Assess. 2018, 190, 606. [Google Scholar] [CrossRef]
- de Matos, A.T.; Fontes, M.P.F.; da Costa, L.M.; Martinez, M.A. Mobility of heavy metals as related to soil chemical and mineralogical characteristics of Brazilian soils. Environ. Pollut. 2001, 111, 429–435. [Google Scholar] [CrossRef]
- Kellman, M. Nutrient retention by tropical ecosystems: Soil adsorption and plant absorption as synergistic processes. J. Trop. Ecol. 2002, 18, 877–895. [Google Scholar] [CrossRef]
- Ordóñez Fernandez, R.; Giráldez Cervera, J.V.; Vanderlinden, K.; Carbonell Bojollo, R.; González Fernández, P. Temporal and spatial monitoring of the pH and heavy metals in a soil polluted by mine spill. Post cleaning effects. Water Air Soil Pollut. 2007, 178, 229–243. [Google Scholar] [CrossRef]
- Tume, P.; Bech, J.; Reverter, F.; Bech, J.; Longan, L.; Tume, L.; Sepúlveda, B. Concentration and distribution of twelve metals in Central Catalonia surface soils. J. Geochem. Explor. 2011, 109, 92–103. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Luan, Y. Meta-analysis of soil mercury accumulation by vegetables. Sci. Rep. 2018, 8, 1261. [Google Scholar] [CrossRef] [Green Version]
- Duressa, T.F.; Leta, S. Determination of levels of As, Cd, Cr, Hg and Pb in soils and some vegetables taken from river mojo water irrigated farmland at Koka Village, Oromia state, East Ethiopia. Int. J. Sci. Basic Appl. Res. 2015, 21, 352–372. [Google Scholar]
- Sebastian, U.N.; Godwin, O.O. Heavy metal concentrations of citrus species (Citrus reticulata and Citrus sinensis) cultivated on road sides in Uyo metropolis in Akwa Ibom state, Nigeria. Int. J. Ecol. Sci. Environ. Eng. 2017, 4, 86–92. [Google Scholar]
- Mirzaei, M.; Marofi, S.; Solgi, E.; Abbasi, M.; Karimi, R.; Riyahi Bakhtyari, H.R. Ecological and health risks of soil and grape heavy metals in long-term fertilized vineyards (Chaharmahal and Bakhtiari province of Iran). Environ. Geochem. Health 2020, 42, 27–43. [Google Scholar] [CrossRef]
- Rehman, I.; Ishaq, M.; Ali, L.; Muhammad, S.; Din, I.U.; Yaseen, M.; Ullah, H. Potentially toxic elements’ occurrence and risk assessment through water and soil of Chitral urban environment, Pakistan: A case study. Environ. Geochem. Health 2020, 42, 4355–4368. [Google Scholar] [CrossRef]
- Hu, W.; Wang, H.; Dong, L.; Huang, B.; Borggaard, O.K.; Bruun Hansen, H.C.; He, Y.; Holm, P.E. Source identification of heavy metals in peri-urban agricultural soils of southeast China: An integrated approach. Environ. Pollut. 2018, 237, 650–661. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Di, L.; Wu, X.; You, W.; Huang, B. Occurrence, speciation, and risks of trace metals in soils of greenhouse vegetable production from the vicinity of industrial areas in the Yangtze River Delta, China. Environ. Sci. Pollut. Res. 2019, 26, 8696–8708. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Huang, B.; Borggaard, O.K.; Ye, M.; Tian, K.; Zhang, H.; Holm, P.E. Soil threshold values for cadmium based on paired soil-vegetable content analyses of greenhouse vegetable production systems in China: Implications for safe food production. Environ. Pollut. 2018, 241, 922–929. [Google Scholar] [CrossRef]
- Sawut, R.; Kasim, N.; Maihemuti, B.; Hu, L.; Abliz, A.; Abdujappar, A.; Kurban, M. Pollution characteristics and health risk assessment of heavy metals in the vegetable bases of northwest China. Sci. Total Environ. 2018, 642, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.S.D.; Moura, N.B.A.; Mazur, N.; Oliveira, C.D. Heavy metal contamination in soil and plants by intensive use of pesticides and fertilizers. In Proceedings of the 29th Symposium, 17th World Congress of Soil Science, Bangkok, Thailand, 14–21 August 2002; pp. 1441–1446. [Google Scholar]
- Hu, W.; Huang, B.; Shi, X.; Chen, W.; Zhao, Y.; Jiao, W. Accumulation and health risk of heavy metals in a plot-scale vegetable production system in a peri-urban vegetable farm near Nanjing, China. Ecotoxicol. Environ. Saf. 2013, 98, 303–309. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Wang, M.; Peng, C. Regional accumulation characteristics of cadmium in vegetables: Influencing factors, transfer model and indication of soil threshold content. Environ. Pollut. 2016, 219, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.; Boluda, R.; Ramos, J. Determination and evaluation of cadmium, lead and nickel in greenhouse soils of Almería (Spain). Chemosphere 2004, 55, 1027–1034. [Google Scholar] [CrossRef]
- Guo, X.; Sun, Q.; Zhao, Y.; Cai, H. Identification and characterisation of heavy metals in farmland soil of Hunchun basin. Environ. Earth Sci. 2019, 78, 310. [Google Scholar] [CrossRef]
- Fang, H.; Li, W.; Tu, S.; Ding, Y.; Wang, R.; Rensing, C.; Li, Y.; Feng, R. Differences in cadmium absorption by 71 leaf vegetable varieties from different families and genera and their health risk assessment. Ecotoxicol. Environ. Saf. 2019, 184, 109593. [Google Scholar] [CrossRef]
| Crops | Oil Palm | Black Pepper | Citrus | |||
|---|---|---|---|---|---|---|
| Areas | Cultivation | Reference | Cultivation | Reference | Cultivation | Reference |
| Soil Order | Typic Hapludox | Oxisol | Typic Hapludult | Ultisol | Typic Hapludult | Ultisol |
| pH (in H2O) | 4.6 ± 0.32 | 4 ± 0.26 | 5.2 ± 0.80 | 4.1 ± 0.06 | 4.7 ± 0.21 | 4.4 ± 0.25 |
| pH (in KCl) | 4.3 ± 0.29 | 3.7 ± 0.15 | 4.7 ± 0.62 | 3.7 ± 0.15 | 4.1 ± 0.49 | 3.8 ± 0.24 |
| Ca2+ (mmolc kg−1) | 9.6 ± 0.38 | 1.3 ± 3.01 | 34 ± 1.97 | 11.3 ± 0.42 | 13.7 ± 0.72 | 5.1 ± 1.0 |
| Mg2+ (mmolc kg−1) | 2.5 ± 0.1 | 1.7 ± 1.82 | 15 ± 0.57 | 10 ± 0.1 | 9 ± 0.17 | 1.2 ± 0.57 |
| K+ (mmolc kg−1) | 0.9 ± 0.09 | 0.6 ± 0.25 | 4.2 ± 0.36 | 0.5 ± 0.02 | 0.5 ± 0.05 | 0.1 ± 0.28 |
| P (mg kg−1) | 5 ± 4.8 | 2.6 ± 2.94 | 234 ± 194.32 | 1 ± 0.0 | 8 ± 7 | 1.5 ± 0.3 |
| Al2O3 (g kg−1) | 54.1 ± 9.11 | 54 ± 10.82 | 71.7 ± 31.55 | 52.9 ± 36.98 | 53.6 ± 12.82 | 48.8 ± 10.31 |
| Organic carbon (g kg−1) | 11.6 ± 3.5 | 13.5 ± 2.54 | 16 ± 7.46 | 11 ± 3.05 | 6.9 ± 3.16 | 9.7 ± 2.05 |
| Clay (g kg−1) | 277 ± 78.11 | 303 ± 102.39 | 171 ± 76.51 | 168 ± 50.86 | 60 ± 11.55 | 63 ± 49.65 |
| Sand (g kg−1) | 590 ± 115.36 | 599 ± 155.88 | 712 ± 58.51 | 758 ± 60.35 | 919 ± 16.65 | 879 ± 92.86 |
| Silt (g kg−1) | 133 ± 40.25 | 98 ± 40.41 | 117 ± 24.68 | 74 ± 9.87 | 21 ± 6.11 | 58 ± 43.89 |
| Area | As | Ba | Cd | Co | Cr | Cu | Hg | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | ||||||||||
| Oil palm | 1.3 ± 3.06 | 24.8 ± 4.04 | 0.4 ± 2.82 | 1.0 ± 0.56 | 50.0 ± 0.41 | 2.0 ± 0.82 | 0.05 ± 12.88 | 2.0 ± 0.34 | 7.0 ± 0.43 | 8.0 ± 0.98 |
| Reference | 0.1 ± 0.09 | 10.3 ± 4.97 | 0.4 ± 2.00 | 2.0 ± 0.06 | 40.0 ± 0.38 | 2.0 ± 0.80 | 0.06 ± 12.00 | 6.0 ± 0.86 | 2.0 ± 0.66 | 11.0 ± 1.48 |
| Black Pepper | 2.0 ± 1.53 | 32.3 ± 0.87 | 0.4 ± 3.41 | 2.0 ± 0.43 | 70.0 ± 6.02 | 16.0 ± 1.78 | 0.05 ± 12.01 | 4.0 ± 0.47 | 6.0 ± 0.18 | 19.0 ± 1.23 |
| Reference | 0.2 ± 1.06 | 10.3 ± 0.69 | 0.4 ± 2.89 | 2.0 ± 0.24 | 50.0 ± 8.00 | 2.0 ± 0.89 | 0.07 ± 11.98 | 2.0 ± 0.55 | 1.0 ± 0.07 | 8.0 ± 2.00 |
| Citrus | 0.6 ± 2.89 | 17.0 ± 0.25 | 0.4 ± 3.10 | 2.0 ± 0.60 | 40.0 ± 0.52 | 36.0 ± 1.70 | 0.05 ± 13.01 | 2.0 ± 1.83 | 4.0 ± 0.29 | 21.0 ± 2.65 |
| Reference | 0.9 ± 1.91 | 18.9 ± 0.91 | 0.4 ± 3.00 | 2.0 ± 0.10 | 50.0 ± 0.28 | 5.0 ± 1.49 | 0.05 ± 12.57 | 3.0 ± 1.38 | 5.0 ± 0.38 | 9.0 ± 2.12 |
| QRV- Pará 75th a | 1.4 | 14.3 | 0.4 | - | 24.1 | 9.9 | 0.26 | 1.4 | 4.8 | 7.2 |
| QRV- Pará 90th a | 2.6 | 33.4 | 0.9 | - | 35.5 | 18.2 | 0.45 | 6.1 | 7.5 | 21.0 |
| QRV- Brazil b | 3.5 | 75.0 | <0.5 | 13.0 | 40.0 | 35.0 | 0.05 | 13.0 | 17.0 | 60.0 |
| PV- Brazil b | 15.0 | 150.0 | 1.3 | 25.0 | 75.0 | 60.0 | 0.50 | 30.0 | 72.0 | 300.0 |
| RV- Brazil b | 35.0 | 300.0 | 3.0 | 35.0 | 150.0 | 200.0 | 12.0 | 70.0 | 180.0 | 450.0 |
| Crops | As | Ba | Cd | Co | Cr | Cu | Hg | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | ||||||||||
| Oil palm | 0.09 ± 0.00 | 9.00 ± 0.01 | 0.01 ± 0.00 | 0.10 ± 0.00 | 3.00 ± 1.73 | 4.10 ± 1.34 | 0.03 ± 0.01 | 1.03 ± 0.15 | 0.23 ± 0.06 | 13.33 ± 3.21 |
| Black pepper | 0.09 ± 0.00 | 23.33 ± 5.17 | 0.01 ± 0.01 | 0.57 ± 0.21 | 2.67 ± 0.57 | 13.50 ± 0.62 | 0.04 ± 0.01 | 2.70 ± 0.52 | 0.70 ± 0.35 | 25.00 ± 1.73 |
| Citrus | 0.16 ± 0.12 | 23.33 ± 5.77 | 0.01 ± 0.01 | 0.97 ± 0.64 | 3.33 ± 0.58 | 16.57 ± 0.40 | 0.08 ± 0.03 | 2.83 ± 0.93 | 1.03 ± 0.84 | 20.67 ± 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Braz, A.M.; da Costa, M.L.; Ramos, S.J.; Dall’Agnol, R.; Fernandes, A.R. Environmental Impact of Potentially Toxic Elements on Tropical Soils Used for Large-Scale Crop Commodities in the Eastern Amazon, Brazil. Minerals 2021, 11, 990. https://doi.org/10.3390/min11090990
de Souza Braz AM, da Costa ML, Ramos SJ, Dall’Agnol R, Fernandes AR. Environmental Impact of Potentially Toxic Elements on Tropical Soils Used for Large-Scale Crop Commodities in the Eastern Amazon, Brazil. Minerals. 2021; 11(9):990. https://doi.org/10.3390/min11090990
Chicago/Turabian Stylede Souza Braz, Anderson Martins, Marcondes Lima da Costa, Sílvio Junio Ramos, Roberto Dall’Agnol, and Antonio Rodrigues Fernandes. 2021. "Environmental Impact of Potentially Toxic Elements on Tropical Soils Used for Large-Scale Crop Commodities in the Eastern Amazon, Brazil" Minerals 11, no. 9: 990. https://doi.org/10.3390/min11090990