Zoisite-(Pb), a New Orthorhombic Epidote-Related Mineral from the Jakobsberg Mine, Värmland, Sweden, and Its Relationships with Hancockite
Abstract
:1. Introduction
2. Occurrence and Physical Properties
2.1. Chemical Data
2.2. Micro-Raman Spectroscopy
2.3. X-ray Crystallography
3. Discussion
3.1. Chemical Data
3.2. Crystal Structure Description of Zoisite-(Pb) and Hancockite
4. Relationships with Other Natural and Synthetic Phases
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prunier, A.R.; Hewitt, D.A. Experimental observations on coexisting clinozoisite and zoisite. Am. Mineral. 1985, 70, 375–378. [Google Scholar]
- Brunsmann, A.; Franz, G.; Heinrich, W. Experimental investigation of zoisite–clinozoisite phase equilibria in the system CaO–Fe2O3–Al2O3–SiO2–H2O. Contrib. Mineral. Petrol. 2002, 143, 115–130. [Google Scholar] [CrossRef]
- Armbruster, T.; Bonazzi, P.; Akasaka, M.; Bermanec, V.; Chopin, C.; Gieré, R.; Heuss-Assbichler, S.; Liebscher, A.; Menchetti, S.; Pan, Y.; et al. Recommended nomenclature of epidote-group minerals. Eur. J. Mineral. 2006, 18, 551–567. [Google Scholar] [CrossRef] [Green Version]
- Mills, S.J.; Hatert, F.; Nickel, E.H.; Ferraris, G. The standardisation of mineral group hierarchies: Application to recent nomenclature proposals. Eur. J. Mineral. 2009, 21, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Dörsam, G.; Liebscher, A.; Wunder, B.; Franz, G.; Gottschalk, M. Crystal chemistry of synthetic Ca2Al3Si3O12OH–Sr2Al3Si3O12OH solid-solution series of zoisite and clinozoisite. Am. Mineral. 2007, 92, 1133–1147. [Google Scholar] [CrossRef]
- Dörsam, G.; Liebscher, A.; Wunder, B.; Franz, G.; Gottschalk, M. Synthesis of Pb-zoisite and Pb-lawsonite. N. Jb. Miner. Abh. 2011, 188, 99–110. [Google Scholar] [CrossRef]
- Frei, D.; Liebscher, A.; Franz, G.; Dulski, P. Trace element geochemistry of epidote minerals. Rev. Mineral. 2004, 56, 553–605. [Google Scholar] [CrossRef]
- Penfield, S.L.; Warren, C.H. Some new minerals from the zinc mines at Franklin, N.J., and note concerning the chemical composition of ganomalite. Am. J. Sci. 1899, 8, 339–353. [Google Scholar] [CrossRef] [Green Version]
- Chukanov, N.V.; Varlamov, D.A.; Nestola, F.; Belakovskiy, D.I.; Goettlicher, J.; Britvin, S.N.; Lanza, A.; Jancev, S. Piemontite-(Pb), CaPbAl2Mn3+[Si2O7][SiO4]O(OH), a new mineral species of the epidote supergroup. N. Jb. Miner. Abh. 2012, 189, 275–286. [Google Scholar] [CrossRef]
- Moore, P.B. Mineralogy and chemistry of Långban-type deposits in Bergslagen, Sweden. Mineral. Rec. 1970, 1, 154–172. [Google Scholar]
- Magnusson, N.H. Långbans malmtrakt. Sverigs Geol. Undersökning 1930, 23, 111. [Google Scholar]
- Nysten, P.; Holtstam, D.; Jonsson, E. The Långban minerals. In Långban, the Mines, Their Minerals, History and Explorers; Holtstam, D., Langhof, J., Eds.; Raster Förlag: Stockholm, Sweden, 1999; 215p. [Google Scholar]
- Holtstam, D.; Mansfeld, J. Origin of a carbonate-hosted Fe–Mn–(Ba–As–Pb–Sb–W) deposit of Långban-type in Central Sweden. Miner. Deposita 2001, 36, 641–657. [Google Scholar] [CrossRef]
- Nordenskiõld, A.E. Nya mineralier från Långban. Geol. Foren. Stock. For. 1877, 3, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Igelström, L.J. Plumboferrit, ett nytt mineral från Jakobsbergs manganmalmsgrufva vid Nordmarken i Wermland. Kongl. Veten. Akad. För. 1881, 38, 27–31. [Google Scholar]
- Sjögren, H. Svabit, ett mineral af apatitgruppen från Harstigsgrufvan. Geol. Foren. Stock. For. 1891, 13, 789–796. [Google Scholar]
- Sjögren, H. Celsian, en anorthiten motsvarande bariumfältspat från Jakobsberg. Preliminärt meddelande. Geol. Foren. Stock. For. 1895, 17, 578–582. [Google Scholar] [CrossRef] [Green Version]
- Johansson, K. Mineralogische Mitteilungen. Z. Kristallogr. 1928, 68, 87–118. [Google Scholar] [CrossRef]
- Dunn, P.J.; Rouse, R.C. Morelandite, a new barium arsenate chloride member of the apatite group. Can. Mineral. 1978, 16, 601–604. [Google Scholar]
- Holstram, D.; Norrestam, R. Lindqvistite, Pb2MeFe16O27, a novel hexagonal ferrite mineral from Jakobsberg, Filipstad, Sweden. Am. Mineral. 1993, 78, 1304–1312. [Google Scholar]
- Holtstam, D.; Larsson, A. Tegengrenite, a new, rhombohedral spinel-related Sb mineral from the Jakobsberg Fe-Mn deposit, Värmland, Sweden. Am. Mineral. 2000, 85, 1315–1320. [Google Scholar] [CrossRef]
- Holtstam, D.; Cámara, F.; Karlsson, A.; Skogby, H.; Zack, T. Ferri-taramite, IMA 2021-046. CNMNC Newsletter 63. Mineral. Mag. 2021, 85, 910–915. [Google Scholar] [CrossRef]
- Mandarino, J.A. The Gladstone-Dale relationship. Part III. Some general applications. Can. Mineral. 1979, 17, 71–76. [Google Scholar]
- Mandarino, J.A. The Gladstone-Dale relationship. Part IV. The compatibility concept and its application. Can. Mineral. 1981, 19, 441–450. [Google Scholar]
- Warr, L.N. IMA-CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Holtstam, D.; Langhof, J. Hancockite from Jakobsberg, Filipstad: A second world occurrence. Mineral. Mag. 1994, 58, 172–174. [Google Scholar] [CrossRef]
- Christy, A.; Gatedal, K. Extremely Pb-rich rock-forming silicates including a beryllian scapolite and associated minerals in a skarn from Långban, Värmland, Sweden. Mineral. Mag. 2005, 69, 995–1018. [Google Scholar] [CrossRef]
- Hålenius, U.; Bosi, F.; Gatedal, K. Crystal structure and chemistry of skarn-associated bismuthian vesuvianite. Am. Mineral. 2013, 98, 566–573. [Google Scholar] [CrossRef]
- Holtstam, D.; Cámara, F.; Karlsson, A. Instalment of the margarosanite group, and data on walstromite–margarosanite solid solutions from the Jakobsberg Mn–Fe deposit, Värmland, Sweden. Mineral. Mag. 2021, 85, 224–232. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Makreski, P.; Jovanovski, G.; Kaitner, B.; Gajović, A.; Biljan, T. Minerals from Macedonia. XVIII. Vibrational spectra of some sorosilicates. Vibr. Spectr. 2007, 44, 162–170. [Google Scholar] [CrossRef]
- Liebscher, A. Spectroscopy of epidote minerals. Rev. Mineral. Geochem. 2004, 56, 125–170. [Google Scholar] [CrossRef]
- Della Ventura, G.; Mottana, A.; Parodi, G.C.; Griffin, W.L. FTIR spectroscopy in the OH-stretching region of monoclinic epidotes from Praborna (St. Marcel, Aosta valley, Italy). Eur. J. Mineral. 1996, 8, 655–665. [Google Scholar] [CrossRef]
- Bruker AXS Inc. Apex3; Bruker Advanced X-ray Solutions: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Comodi, P.; Zanazzi, P.F. The pressure behavior of clinozoisite and zoisite: An X-ray diffraction study. Am. Mineral. 1997, 82, 61–68. [Google Scholar] [CrossRef]
- Wilson, A.J.C. (Ed.) International Tables for Crystallography. Volume C: Mathematical, Physical and Chemical Tables; Kluwer Academic: Dordrecth, The Netherlands, 1992. [Google Scholar]
- Dollase, W.A. Refinement of the crystal structures of epidote, allanite and hancockite. Am. Mineral. 1971, 56, 447–464. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Brown, I.D. Bond Valence Parameters. 2016. Available online: http://www.iucr.org/data/assets/file/0007/126574/bvparm2016.cif (accessed on 22 December 2021).
- Ferraris, G.; Ivaldi, G. Bond valence vs. bond length in O⋯O hydrogen bonds. Acta Crystallogr. 1988, 44, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation: A quantitative measure of distortion in coordination polyhedra. Science 1971, 172, 567–570. [Google Scholar] [CrossRef]
- Dunn, P.J. The lead silicates from Franklin, New Jersey: Occurrence and composition. Mineral. Mag. 1985, 49, 721–727. [Google Scholar] [CrossRef]
- Jancev, S.; Bermanec, V. Solid solution between epidote and hancockite from Nežilovo, Macedonia. Geol. Croat. 1998, 51, 23–26. [Google Scholar]
- Fesenko, E.G.; Rumanova, I.M.; Belov, N.V. The crystal structure of zoisite. Dokl. Acad. Sci. USSR 1955, 102, 275–278. [Google Scholar]
- Dollase, W.A. Refinement and comparison of the structure of zoisite and clinozoisite. Am. Mineral. 1968, 53, 1882–1888. [Google Scholar]
- Franz, G.; Liebscher, A. Physical and chemical properties of the epidote minerals—An introduction. Rev. Mineral. Geochem. 2004, 56, 1–82. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic study of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Pushkin, D.V.; Marukhnov, A.V.; Serezhkin, V.N. Coordination polyhedra PbOn in crystal structures. Russ. J. Inorg. Chem. 2006, 51, 99–107. [Google Scholar] [CrossRef]

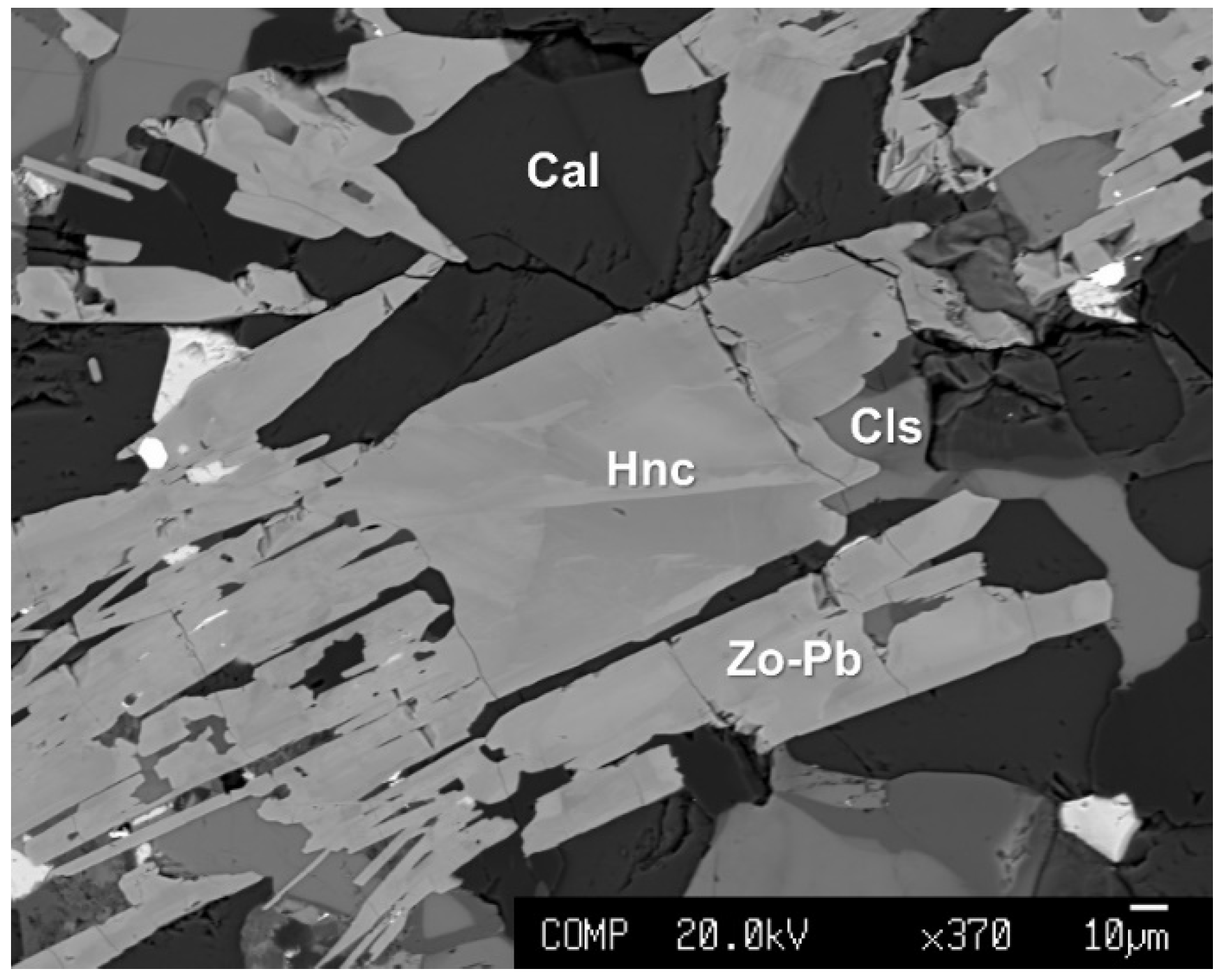
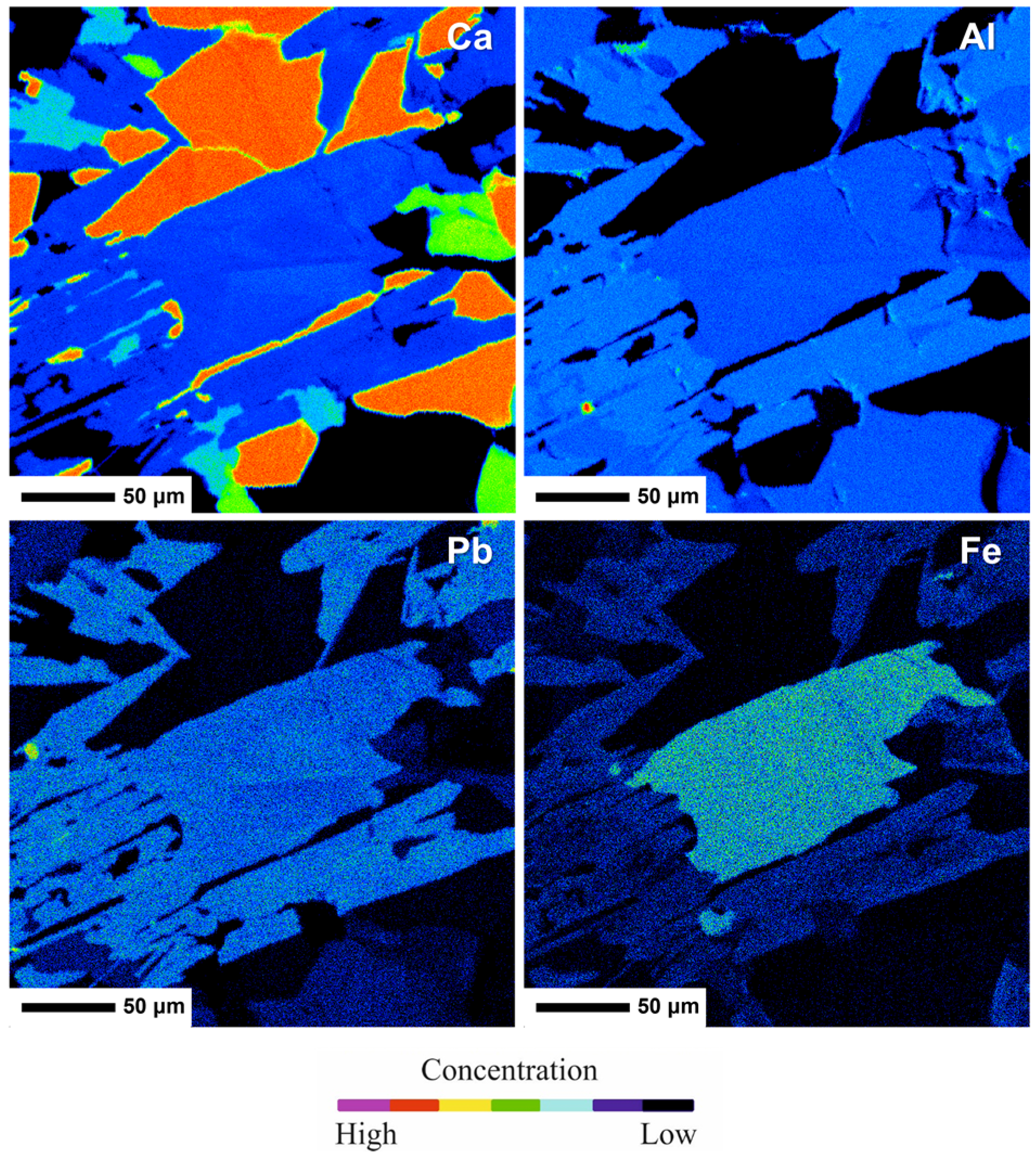
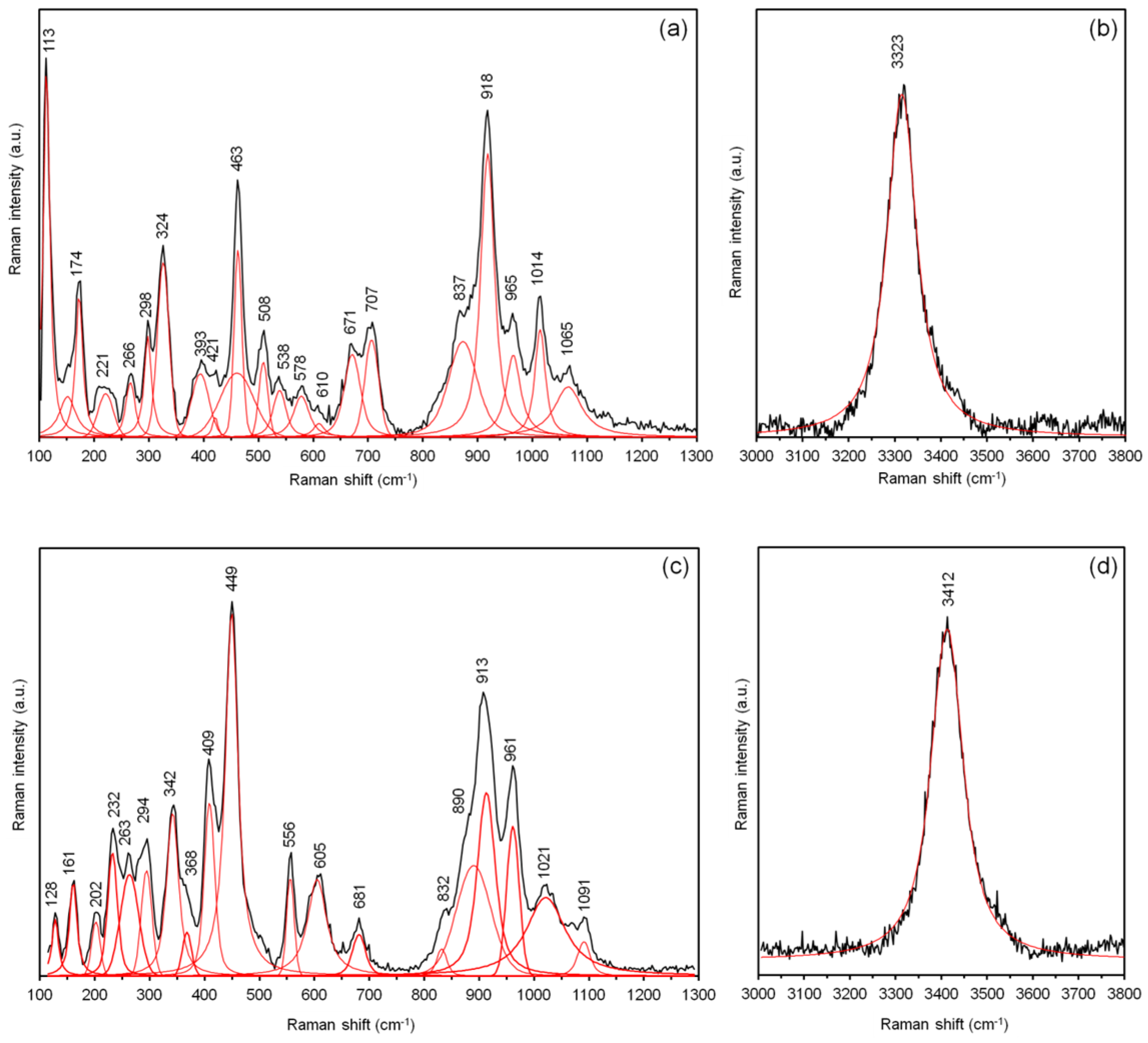
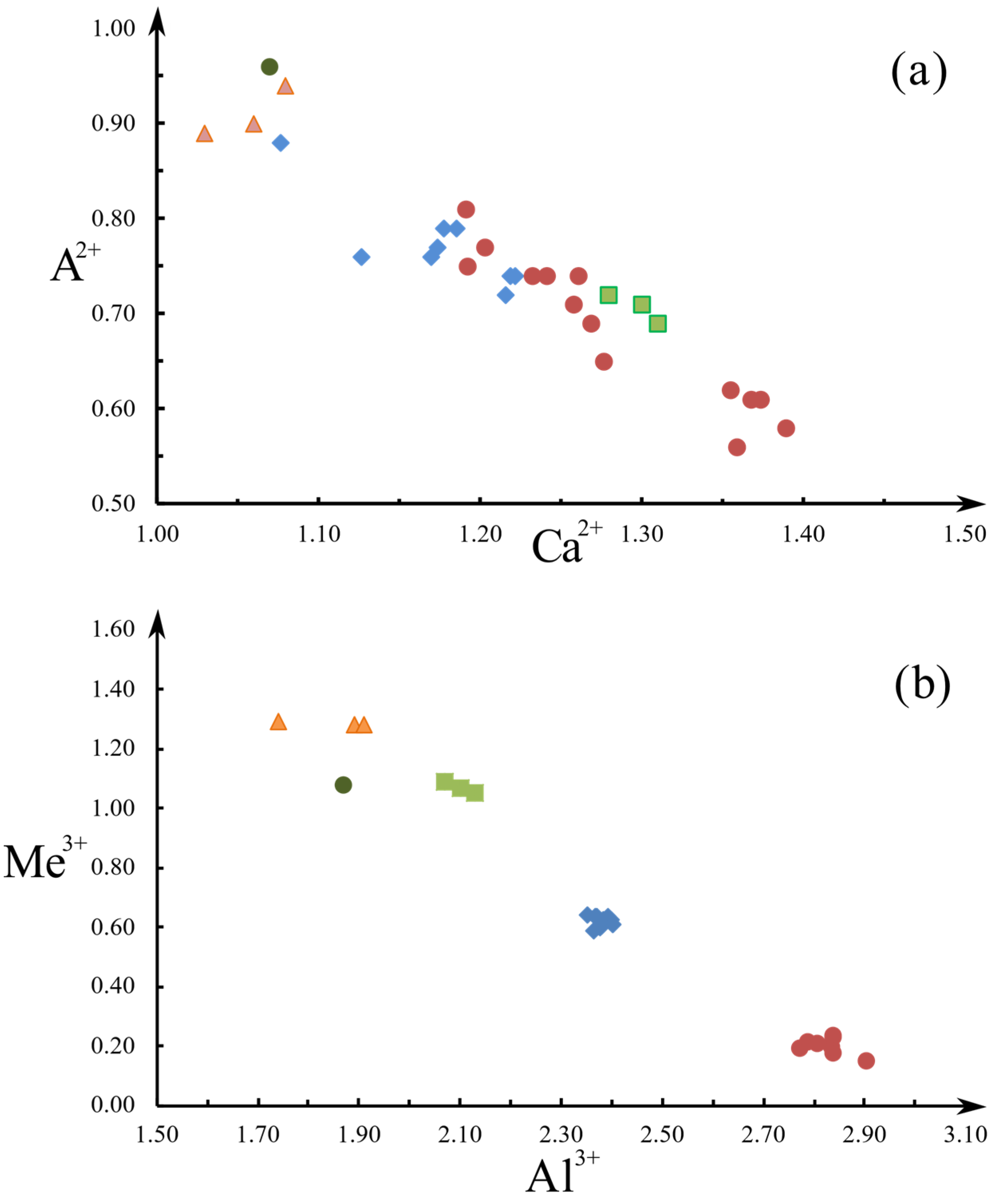
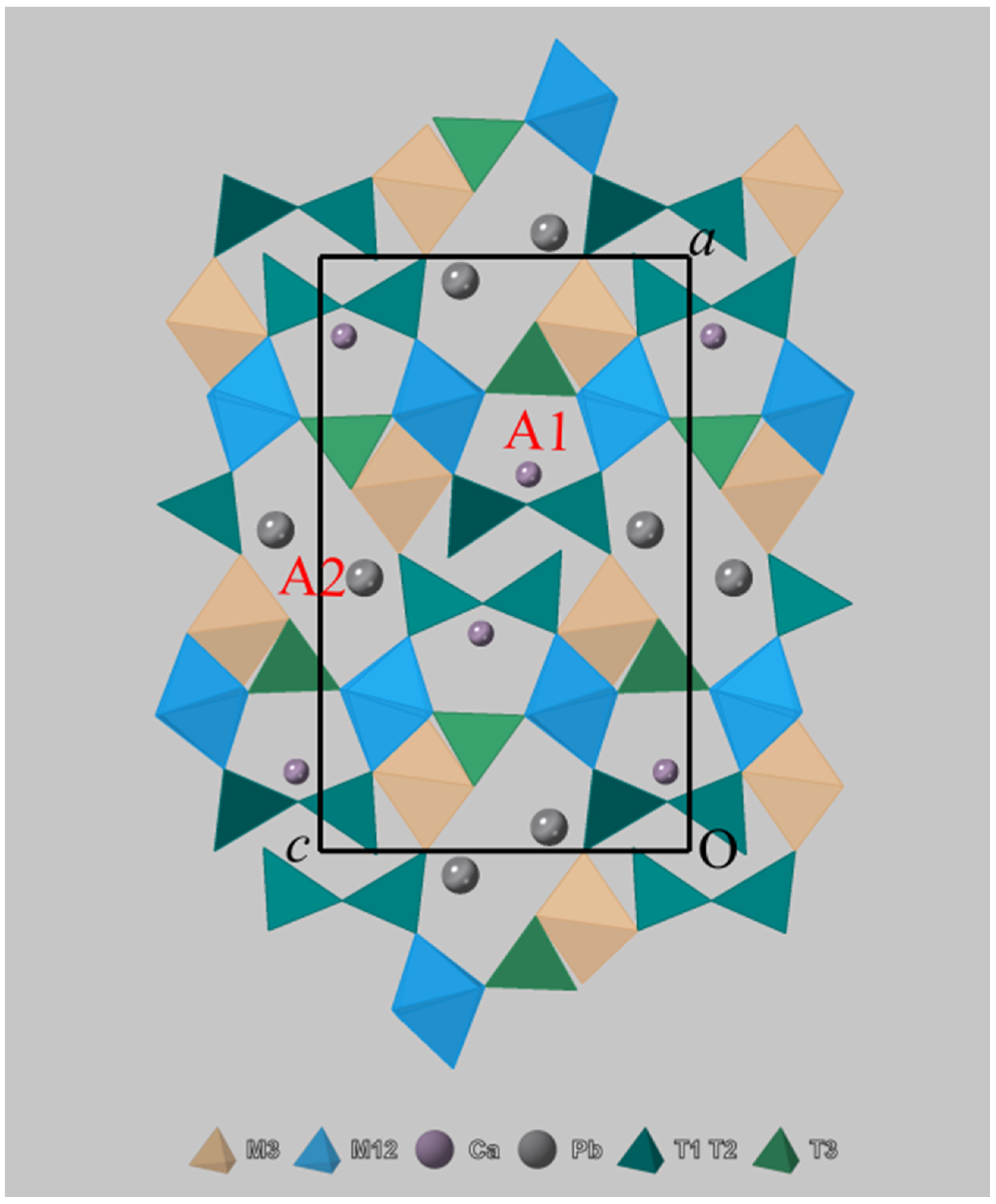

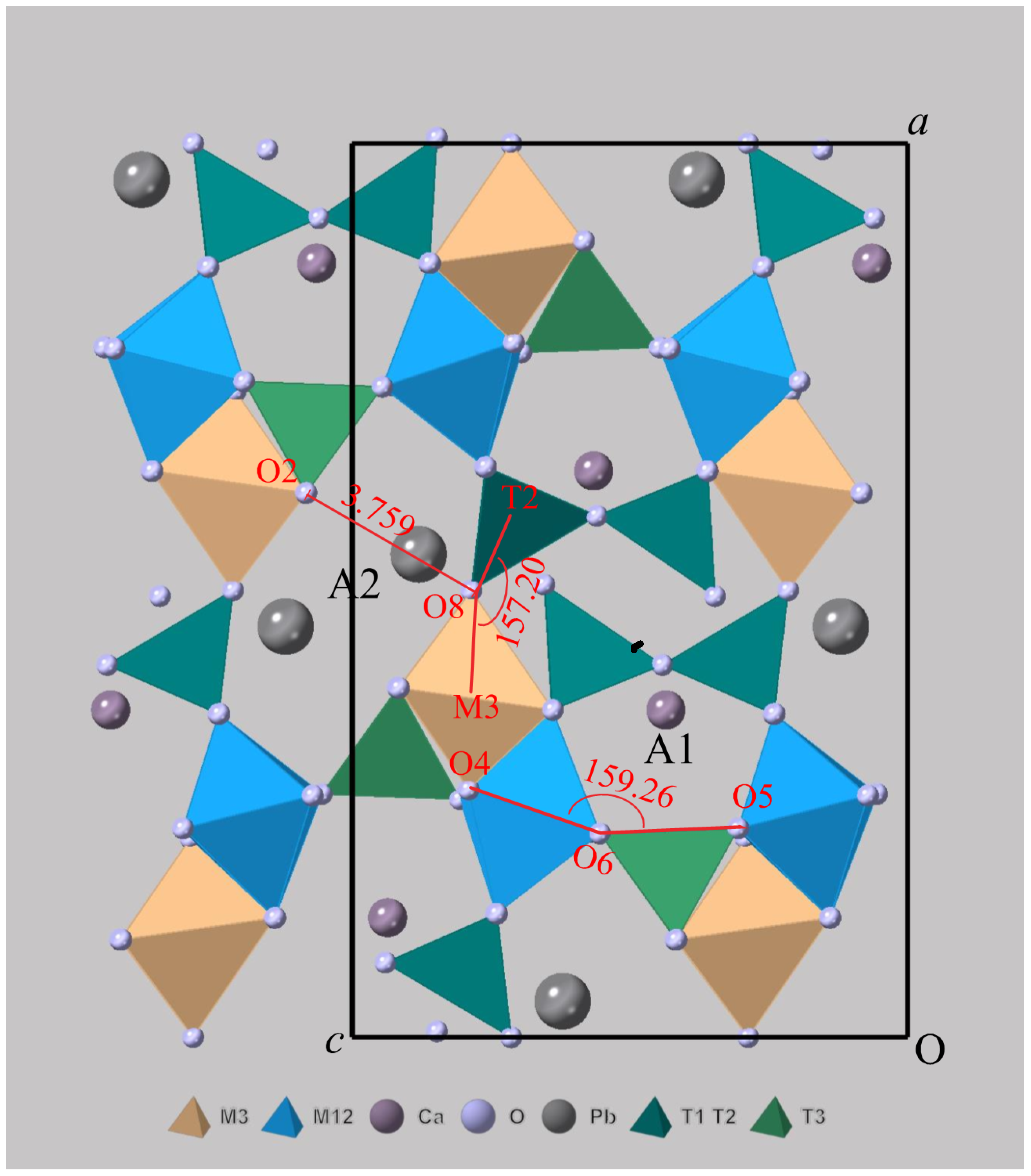

| Constituent | Zoisite-(Pb) | Hancockite | |||||
|---|---|---|---|---|---|---|---|
| Mean (n = 10) | Range | e.s.d. 1 | Mean (n = 5) | Range | e.s.d. 1 | Standard | |
| SiO2 | 30.11 | 29.45–31.56 | 0.64 | 30.19 | 29.93–30.45 | 0.20 | Albite |
| Al2O3 | 24.57 | 23.90–24.90 | 0.30 | 19.78 | 19.45–20.02 | 0.23 | Albite |
| Fe2O3(tot) | 1.32 | 1.23–1.45 | 0.07 | 9.34 | 9.11–9.46 | 0.14 | Magnetite |
| Fe2O3(calc) * | 1.32 | 8.74 | |||||
| FeO(calc) * | - | 0.54 | |||||
| CaO | 10.25 | 9.83–10.69 | 0.26 | 11.02 | 10.79–11.29 | 0.24 | Wollastonite |
| MnO(tot) | 0.59 | 0.52–0.64 | 0.03 | 0.65 | 0.62–0.71 | 0.04 | Rhodonite |
| Mn2O3(calc) * | 0.50 | ||||||
| MnO(calc) * | 0.14 | ||||||
| BaO | 0.05 | 0.01–0.10 | 0.02 | 0.07 | 0.02–0.11 | 0.03 | Baryte |
| PbO | 32.23 | 31.14–33.73 | 0.74 | 27.20 | 26.84–27.96 | 0.47 | Crocoite |
| Na2O | 0.07 | 0.01–0.14 | 0.04 | - | - | - | Albite |
| H2O(calc) ** | 1.50 | 1.50 | |||||
| Total | 100.75 | 99.69 | |||||
| Celsian-1 | Celsian-2 | Vesuvianite | Grossular | |
|---|---|---|---|---|
| SiO2 (wt%) | 35.86 | 44.44 | 34.75 | 38.17 |
| Al2O3 | 26.13 | 23.12 | 13.93 | 13.75 |
| Fe2O3 | 0.00 | 0.00 | 0.00 | 12.10 |
| FeO | 0.02 | 0.03 | 3.20 | 0.00 |
| MnO | 0.00 | 0.00 | 0.32 | 1.01 |
| CaO | 0.03 | 0.01 | 32.28 | 34.84 |
| MgO | 0.00 | 0.00 | 1.98 | 0.11 |
| BaO | 30.64 | 18.03 | 0.00 | 0.00 |
| SrO | 0.11 | 0.00 | 0.00 | 0.00 |
| PbO | 6.94 | 8.20 | 1.83 | 0.00 |
| CuO | 0.11 | 0.09 | 1.36 | 0.00 |
| Na2O | 0.32 | 1.46 | 0.29 | 0.00 |
| K2O | 1.28 | 4.67 | 0.00 | 0.00 |
| F | 0.00 | 0.00 | 0.23 | 0.00 |
| Sum | 101.44 | 100.05 | 90.17 | 99.98 |
| 0.00 | 0.00 | −0.10 | 0.00 | |
| Total | 101.44 | 100.05 | 90.07 | 99.98 |
| * | * | ** | *** | |
| Si (a.p.f.u.) | 2.16 | 2.48 | 18.54 | 3.00 |
| Al | 1.85 | 1.52 | 8.76 | 1.27 |
| Fe3+ | 0.00 | 0.00 | 0.00 | 0.72 |
| Fe2+ | 0.00 | 0.00 | 1.52 | 0.00 |
| Mn | 0.00 | 0.00 | 0.15 | 0.07 |
| Ca | 0.00 | 0.00 | 18.45 | 2.94 |
| Mg | 0.00 | 0.00 | 1.57 | 0.01 |
| Ba | 0.72 | 0.39 | 0.00 | 0.00 |
| Sr | 0.00 | 0.00 | 0.00 | 0.00 |
| Pb | 0.11 | 0.12 | 0.26 | 0.00 |
| Cu | 0.01 | 0.00 | 0.55 | 0.00 |
| Na | 0.04 | 0.16 | 0.30 | 0.00 |
| K | 0.1 | 0.33 | 0.00 | 0.00 |
| F | 0.00 | 0.00 | 0.38 | 0.00 |
| Iobs | dobs | Icalc | dcalc | h k l | Iobs | dobs | Icalc | dcalc | h k l |
|---|---|---|---|---|---|---|---|---|---|
| s | 8.63 | 100 | 8.658 | 1 0 1 | w | 2.136 | 16 | 2.131 | 6 0 3 |
| mw | 8.11 | 38 | 8.199 | 2 0 0 | vw | 2.128 | 9 | 2.129 | 7 0 2 |
| vw | 6.45 | 10 | 6.389 | 2 0 1 | mw | 2.085 | 19 | 2.090 | 2 2 3 |
| vw | 5.124 | 9 | 5.098 | 0 0 2 | 13 | 2.083 | 5 2 1 | ||
| m | 4.895 | 40 | 4.905 | 0 1 1 | w | 2.015 | 17 | 2.019 | 4 1 4 |
| mw | 4.825 | 20 | 4.817 | 3 0 1 | 3 | 2.013 | 5 0 4 | ||
| w | 4.620 | 13 | 4.621 | 2 1 0 | w | 1.963 | 28 | 1.965 | 5 2 2 |
| m | 4.210 | 35 | 4.209 | 2 1 1 | vw | 1.921 | 12 | 1.924 | 8 1 0 |
| mw | 3.793 | 26 | 3.803 | 4 0 1 | vw | 1.910 | 4 | 1.911 | 4 2 3 |
| s | 3.660 | 57 | 3.672 | 1 1 2 | 4 | 1.910 | 3 0 5 | ||
| 52 | 3.651 | 3 1 1 | vw | 1.867 | 8 | 1.871 | 1 2 4 | ||
| w | 3.336 | 15 | 3.332 | 1 0 3 | 10 | 1.868 | 2 1 5 | ||
| w | 3.302 | 19 | 3.307 | 4 1 0 | vw | 1.835 | 14 | 1.836 | 2 2 4 |
| w | 3.140 | 17 | 3.146 | 4 1 1 | vw | 1.765 | 16 | 1.770 | 6 1 4 |
| mw | 3.097 | 32 | 3.102 | 3 1 2 | vw | 1.741 | 7 | 1.742 | 1 3 2 |
| s | 2.900 | 89 | 2.904 | 0 1 3 | 5 | 1.737 | 4 1 5 | ||
| mw | 2.750 | 53 | 2.758 | 5 0 2 | vw | 1.693 | 16 | 1.694 | 7 2 2 |
| m | 2.725 | 67 | 2.726 | 5 1 1 | vw | 1.652 | 14 | 1.655 | 5 1 5 |
| w | 2.642 | 33 | 2.648 | 2 2 0 | 11 | 1.640 | 9 1 2 | ||
| 14 | 2.640 | 6 0 1 | vw | 1.635 | 12 | 1.636 | 0 3 3 | ||
| w | 2.558 | 23 | 2.565 | 3 1 3 | vw | 1.619 | 10 | 1.622 | 3 0 6 |
| 13 | 2.549 | 0 0 4 | 11 | 1.618 | 1 1 6 | ||||
| w | 2.431 | 17 | 2.434 | 2 0 4 | vw | 1.573 | 8 | 1.573 | 10 1 0 |
| vw | 2.350 | 6 | 2.350 | 2 2 2 | vw | 1.554 | 8 | 1.555 | 8 1 4 |
| w | 2.250 | 20 | 2.254 | 4 2 1 | vw | 1.485 | 3 | 1.487 | 8 2 3 |
| w | 2.235 | 18 | 2.238 | 3 2 2 | 6 | 1.483 | 7 1 5 | ||
| vw | 2.175 | 7 | 2.176 | 5 1 3 |
| Crystal Data | Zoisite-(Pb) | Hancockite |
|---|---|---|
| Crystal size (mm) | 0.062 × 0.033 × 0.023 | 0.075 × 0.041 × 0.027 |
| Cell setting, space group | Orthorhombic, Pnma | Monoclinic, P21/m |
| a (Å) | 16.3978 (6) | 8.9496 (3) |
| b (Å) | 5.5953 (2) | 5.6474 (2) |
| c (Å) | 10.1953 (3) | 10.2724 (3) |
| β = 114.362 (1)° | ||
| V (Å3) | 936.43 (1) | 472.96 (1) |
| Z | 4 | 2 |
| Data collection and refinement | ||
| Radiation, wavelength (Å) MoKα, | 0.71073 | 0.71073 |
| Temperature (K) | 293 | 293 |
| 2θmax (°) | 70.60 | 71.25 |
| Measured reflections | 17129 | 9184 |
| Unique reflections | 2109 | 2139 |
| Reflections with Fo > 4σ (Fo) | 2013 | 2041 |
| Rint | 0.0340 | 0.0364 |
| Rσ | 0.0206 | 0.0306 |
| Range of h, k, l | −26 ≤ h ≤ 26, −8 ≤ k ≤ 8, −15 ≤ l ≤ 16 | −14 ≤ h ≤ 14, −9 ≤ k ≤ 8, −16 ≤ l ≤ 15 |
| R [Fo > 4σ (Fo)] | 0.0213 | 0.0254 |
| R (all data) | 0.0235 | 0.0283 |
| wR (on Fo2) | 0.0409 | 0.0780 |
| Goof | 1.194 | 1.244 |
| Number of least-squares parameters | 120 | 124 |
| Maximum and minimum residual peak (e Å−3) | 1.03 (at 1.85 Å from O9) −1.96 (at 0.55 Å from Si3) | 1.18 (at 0.51 Å from A2) −1.18 (at 1.12 Å from Si2) |
| Site | Occupancy | x | y | z | Ueq |
|---|---|---|---|---|---|
| A1 | Ca1 | 0.36622 (4) | ¼ | 0.43549 (6) | 0.00817 (13) |
| A2 | Pb0.86Ca0.14 | 0.45965 (2) | ¼ | 0.12028 (2) | 0.01013 (4) |
| M12 | Al1.00 | 0.25250 (4) | 0.99680 (12) | 0.18358 (7) | 0.00487 (13) |
| M3 | Al0.86Fe0.14 | 0.10965 (5) | ¾ | 0.29542 (9) | 0.00503 (25) |
| Si1 | Si1.00 | 0.08471 (5) | ¼ | 0.10020 (9) | 0.00474 (16) |
| Si2 | Si1.00 | 0.41239 (5) | ¾ | 0.28124 (9) | 0.00489 (16) |
| Si3 | Si1.00 | 0.16555 (5) | ¼ | 0.42498 (8) | 0.00457 (16) |
| O1 | O1.00 | 0.13368 (10) | 0.00207 (30) | 0.13943 (17) | 0.00752 (29) |
| O2 | O1.00 | 0.10909 (10) | 0.01413 (31) | 0.41682 (17) | 0.00841 (30) |
| O3 | O1.00 | 0.36182 (9) | 0.98858 (30) | 0.24009 (17) | 0.00734 (29) |
| O4 | O1.00 | 0.22261 (14) | ¾ | 0.29060 (23) | 0.00569 (38) |
| O5 | O1.00 | 0.23370 (13) | ¼ | 0.30518 (23) | 0.00496 (38) |
| O6 | O1.00 | 0.27210 (13) | ¾ | 0.05404 (22) | 0.00471 (37) |
| O7 | O1.00 | 0.99353 (15) | ¼ | 0.15323 (25) | 0.00935 (43) |
| O8 | O1.00 | 1.00020 (15) | ¾ | 0.28603 (25) | 0.01052 (45) |
| O9 | O1.00 | 0.41653 (18) | ¾ | 0.44046 (26) | 0.01829 (57) |
| O10 | O1.00 | 0.27052 (15) | ¼ | 0.07163 (24) | 0.00829 (42) |
| H10 | H1.00 | 0.2650 (38) | ¼ | 0.9836 (60) | 0.033 (17) * |
| A1 | −O7 | 2.275 (3) | A2 | –O7 | 2.375 (2) | M12 | –O4 | 1.827 (2) | M3 | –O8 | 1.797 (3) |
| –O3 | 2.473 (2) × 2 | –O3 | 2.491 (2) × 2 | –O10 | 1.843 (2) | –O4 | 1.853 (2) | ||||
| –O1 | 2.513 (2) × 2 | –O2 | 2.785 (2) × 2 | –O3 | 1.884 (2) | –O2 | 1.928(2) × 2 | ||||
| –O5 | 2.547 (2) | –O2 | 2.809 (2) × 2 | −O5 | 1.908 (2) | –O1 | 2.162 (2) × 2 | ||||
| –O6 | 2.570 (2) | –O8 | 3.030 (1) × 2 | −O6 | 1.938 (2) | <M3-O> | 1.970 | ||||
| -O9 | 2.917 (1) × 2 | −O1 | 2.000 (2) | QE | 1.0235 | ||||||
| <A1[7]> | 2.482 | <A2[7]> | 2.651 | <M12-O> | 1.901 | ||||||
| QE | 1.0081 | ||||||||||
| Si1 | –O7 | 1.590 (2) | Si2 | –O8 | 1.595 (2) | Si3 | –O2 | 1.614 (2) × 2 | |||
| –O9 | 1.629 (3) | –O9 | 1.625 (3) | –O5 | 1.656 (2) | ||||||
| –O1 | 1.652 (2) × 2 | –O3 | 1.627 (2) × 2 | –O6 | 1.666 (2) | ||||||
| <Si1-O> | 1.631 | <Si2-O> | 1.619 | <Si3-O> | 1.639 | ||||||
| A1 | A2 | M12 | M3 | Si1 | Si2 | Si3 | Σav | |
|---|---|---|---|---|---|---|---|---|
| O1 | 0.25↓ × 2 | 0.34 | 2 x → 0.18↓ × 2 | 0.92↓ × 2 | 1.87 | |||
| O2 | 0.15↓ × 2 0.16↓ × 2 | 0.55↓ × 2 | 1.08↓ × 2 | 1.94 | ||||
| O3 | 0.28↓ × 2 | 0.38↓ × 2 | 0.52 | 0.97↓ × 2 | 2.15 | |||
| O4 | 2x → 0.62 | 0.71 | 1.95 * | |||||
| O5 | 0.22 | 2x → 0.48 | 0.93 | 2.11 | ||||
| O6 | 0.21 | 2x → 0.44 | 0.89 | 1.98 | ||||
| O7 | 0.42 | 0.49 | 1.15 | 2.06 | ||||
| O8 | 2x → 0.06↓× 2 | 0.82 | 1.09 | 2.03 | ||||
| O9 | 2x → 0.05↓ × 2 | 1.00 | 0.97 | 2.07 | ||||
| O10 | 2x → 0.59 | 1.18 * | ||||||
| Σcv | 2.01 | 1.99 | 2.99 | 2.99 | 3.99 | 4.00 | 3.98 |
| Site | Occupancy | x | y | z | Ueq |
|---|---|---|---|---|---|
| A1 | Ca1 | 0.7647 (1) | ¾ | 0.1562 (1) | 0.01021 (20) |
| A2 | Pb0.76Ca0.24 | 0.5881 (3) | ¾ | 0.4097 (3) | 0.01285 (8) |
| M1 | Al1.00 | 0 | 0 | 0 | 0.00572 (26) |
| M2 | Al1.00 | 0 | 0 | ½ | 0.00503 (25) |
| M3 | Fe0.67Al0.33 | 0.2876 (1) | ¼ | 0.2199 (1) | 0.00605 (25) |
| Si1 | Si1.00 | 0.3345 (2) | ¾ | 0.0412 (1) | 0.00630 (24) |
| Si2 | Si1.00 | 0.6845 (2) | ¼ | 0.2785 (1) | 0.00662 (25) |
| Si3 | Si1.00 | 0.1733 (2) | ¾ | 0.3099 (1) | 0.00655 (24) |
| O1 | O1.00 | 0.2330 (3) | 0.9930 (5) | 0.0409 (3) | 0.00967(45) |
| O2 | O1.00 | 0.2892 (3) | 0.9814 (5) | 0.3416 (3) | 0.01118 (47) |
| O3 | O1.00 | 0.7907 (3) | 0.0122 (5) | 0.3466 (3) | 0.01033 (46) |
| O4 | O1.00 | 0.0485 (4) | ¼ | 0.1254 (4) | 0.00769 (59) |
| O5 | O1.00 | 0.0333 (4) | ¾ | 0.1413 (4) | 0.00823 (61) |
| O6 | O1.00 | 0.0583 (4) | ¾ | 0.4015 (4) | 0.00751(60) |
| O7 | O1.00 | 0.5134 (5) | ¾ | 0.1644 (4) | 0.01114 (65) |
| O8 | O1.00 | 0.5173 (5) | ¼ | 0.2994 (5) | 0.01587 (8) |
| O9 | O1.00 | 0.6466 (6) | ¼ | 0.1096 (4) | 0.01840 (83) |
| O10 | O1.00 | 0.0723 (5) | ¼ | 0.4235 (4) | 0.00894 (62) |
| A1 | –O7 | 2.285 (5) | A2 | –O7 | 2.325 (5) | M1 | –O4 | 1.838 (3) × 2 | M2 | –O10 × 2 | 1.857 (4) × 2 |
| –O3 | 2.386 (4) × 2 | –O3 | 2.621 (4) × 2 | –O1 | 1.950 (3) × 2 | –O3 × 2 | 1.887 (3) × 2 | ||||
| –O5 | 2.471 (5) | –O2 | 2.778 (3) × 2 | –O5 | 1.958 (3) × 2 | –O6 × 2 | 1.931 (4) × 2 | ||||
| –O1 | 2.499 (4) × 2 | –O2 | 2.794 (3) × 2 | <M1-O> | 1.915 | <M2-O> | 1.892 | ||||
| –O6 | 2.790 (4) | –O10 | 2.807 (4) | QE | 1.0072 | QE | 1.0054 | ||||
| –O9 | 2.984 (2) × 2 | –O8 | 3.011 (2) × 2 | ||||||||
| <A1[7]-O> | 2.474 | <A2[8]-O> | 2.690 | M3 | –O8 | 1.873 (5) | |||||
| –O4 | 1.951 (4) | ||||||||||
| Si1 | –O7 | 1.579 (4) | Si2 | –O8 | 1.597 (6) | Si3 | –O2 | 1.616 (3) × 2 | –O2 | 1.962 (4) × 2 | |
| –O9 | 1.626 (6) | –O9 | 1.625 (5) | –O6 | 1.655 (5) | –O1 | 2.233 (4) × 2 | ||||
| –O1 | 1.645 (2) × 2 | –O3 | 1.627 (3) × 2 | –O5 | 1.667 (4) | <M3-O> | 2.036 | ||||
| <Si1-O> | 1.624 | <Si2-O> | 1.619 | <Si3-O> | 1.639 | QE | 1.0279 | ||||
| A1 | A2 | M1 | M2 | M3 | Si1 | Si2 | Si3 | Σav | |
|---|---|---|---|---|---|---|---|---|---|
| O1 | 0.25↓ × 2 | 0.44↓ × 2 | 0.18↓ × 2 | 0.92↓ × 2 | 1.79 | ||||
| O2 | 0.16↓ × 2 0.17↓ × 2 | 0.61↓ × 2 | 1.08↓ × 2 | 2.02 | |||||
| O3 | 0.34↓ × 2 | 0.28↓ × 2 | 0.50↓ × 2 | 0.97↓ × 2 | 2.09 | ||||
| O4 | 2x → 0.63↓ × 2 | 0.63 | 1.89 * | ||||||
| O5 | 0.27 | 2x → 0.43↓ × 2 | 0.89 | 2.02 | |||||
| O6 | 0.08 | 2x → 0.44↓ × 2 | 0.94 | 1.90 | |||||
| O7 | 0.42 | 0.50 | 1.17 | 2.09 | |||||
| O8 | 2x → 0.06↓ × 2 | 0.78 | 1.08 | 1.98 | |||||
| O9 | 2x → 0.02 | 0.99 | 0.98 | 2.02 | |||||
| O10 | 0.14 | 2x → 0.55↓ × 2 | 1.24 * | ||||||
| Σcv | 1.97 | 1.98 | 3.00 | 2.98 | 2.99 | 4.00 | 4.00 | 3.99 |
| (1) | (2) | (3) | (4) | |
|---|---|---|---|---|
| a | 16.212 | 16.3978 | 16.3548 | 16.4529 |
| b | 5.555 | 5.5953 | 5.5985 | 5.6432 |
| c | 10.034 | 10.1953 | 10.2600 | 10.3631 |
| V (Å3) | 903.6 | 935.43 | 939.43 | 962.18 |
| <A1VII-O> | 2.464 | 2.480 | 2.560 | 2.666 |
| O9 × 2 | 2.914 | 2.917 | 2.902 | 2.913 |
| <A2VII-O> | 2.548 | 2.649 | 2.650 | 2.664 |
| O8 × 2 | 3.007 | 3.030 | 3.099 | 3.130 |
| A2-O10 | 3.003 | 3.141 | 3.038 | 3.066 |
| A2+ | 0 | 0.86 | 2 | 2 |
| O2-O8 | O4-O6-O5 | T2-O8-M3 | |
|---|---|---|---|
| (1) | 3.428 | 160.83 | 148.44 |
| (2) | 3.759 | 159.26 | 157.20 |
| (3) | 3.791 | 157.68 | 160.71 |
| (4) | 4.020 | 153.46 | 165.41 |
| V | λ | σ2 | ||
|---|---|---|---|---|
| T1 | 1.624 | 2.186 | 1.0036 | 11.9616 |
| T2 | 1.619 | 2.174 | 1.0012 | 4.7143 |
| T3 | 1.639 | 2.246 | 1.0039 | 15.1322 |
| M1 | 1.915 | 9.281 | 1.0072 | 19.7561 |
| M2 | 1.892 | 8.954 | 1.0054 | 7.4723 |
| M3 | 2.036 | 10.87 | 1.0279 | 74.2204 |
| [9]A1 | 2.587 | 28.28 | ||
| [10]A2 | 2.754 | 41.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perchiazzi, N.; Mauro, D.; Vignola, P.; Zaccarini, F.; Eldjarn, K. Zoisite-(Pb), a New Orthorhombic Epidote-Related Mineral from the Jakobsberg Mine, Värmland, Sweden, and Its Relationships with Hancockite. Minerals 2022, 12, 51. https://doi.org/10.3390/min12010051
Perchiazzi N, Mauro D, Vignola P, Zaccarini F, Eldjarn K. Zoisite-(Pb), a New Orthorhombic Epidote-Related Mineral from the Jakobsberg Mine, Värmland, Sweden, and Its Relationships with Hancockite. Minerals. 2022; 12(1):51. https://doi.org/10.3390/min12010051
Chicago/Turabian StylePerchiazzi, Natale, Daniela Mauro, Pietro Vignola, Federica Zaccarini, and Knut Eldjarn. 2022. "Zoisite-(Pb), a New Orthorhombic Epidote-Related Mineral from the Jakobsberg Mine, Värmland, Sweden, and Its Relationships with Hancockite" Minerals 12, no. 1: 51. https://doi.org/10.3390/min12010051
APA StylePerchiazzi, N., Mauro, D., Vignola, P., Zaccarini, F., & Eldjarn, K. (2022). Zoisite-(Pb), a New Orthorhombic Epidote-Related Mineral from the Jakobsberg Mine, Värmland, Sweden, and Its Relationships with Hancockite. Minerals, 12(1), 51. https://doi.org/10.3390/min12010051







