Hydrothermal Alteration and Its Superimposed Enrichment for Qianjiadian Tabular-Type Uranium Deposit in Southwestern Songliao Basin
Abstract
:1. Introduction
2. Geological Setting
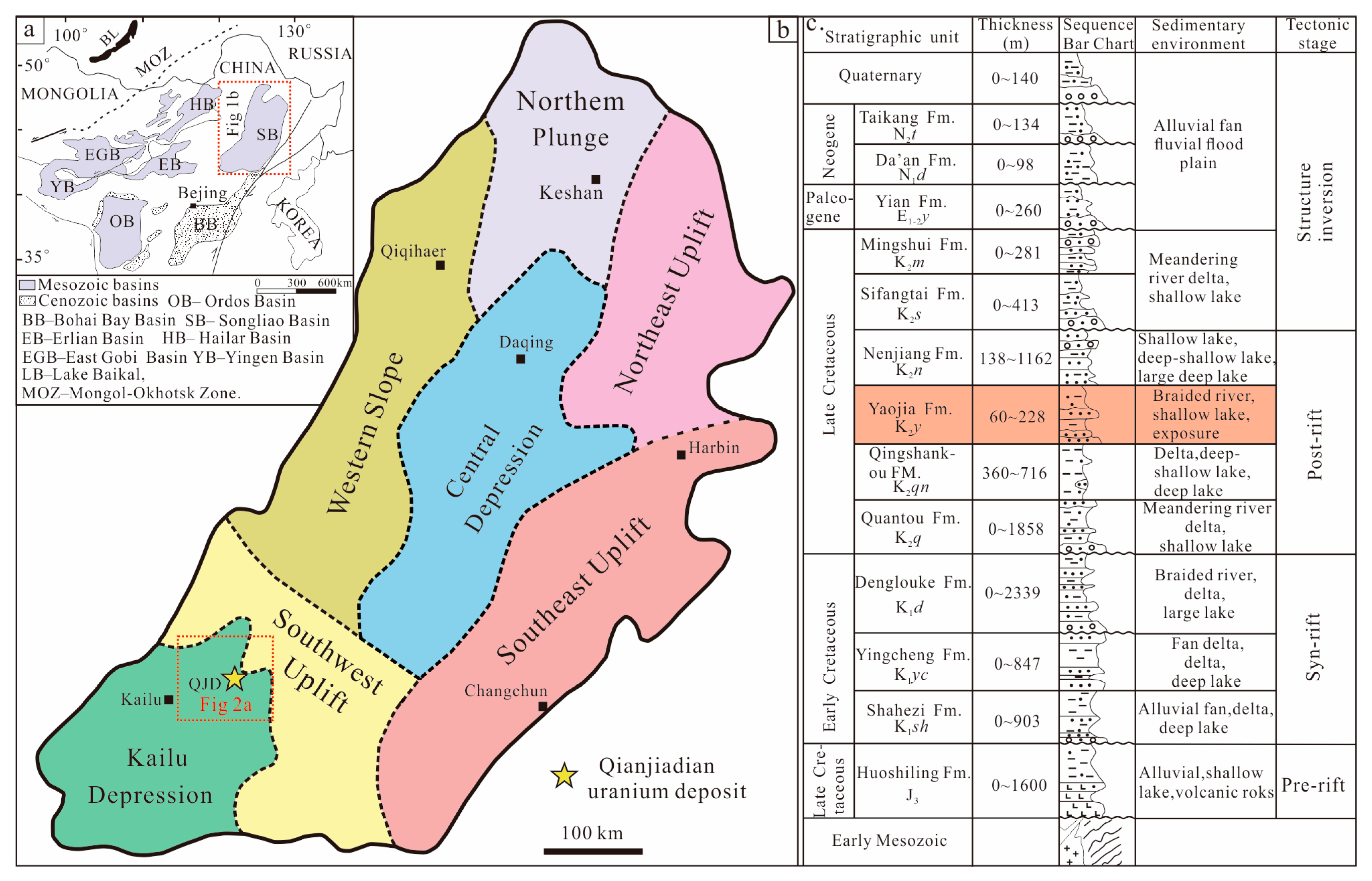
2.1. The Songliao Basin
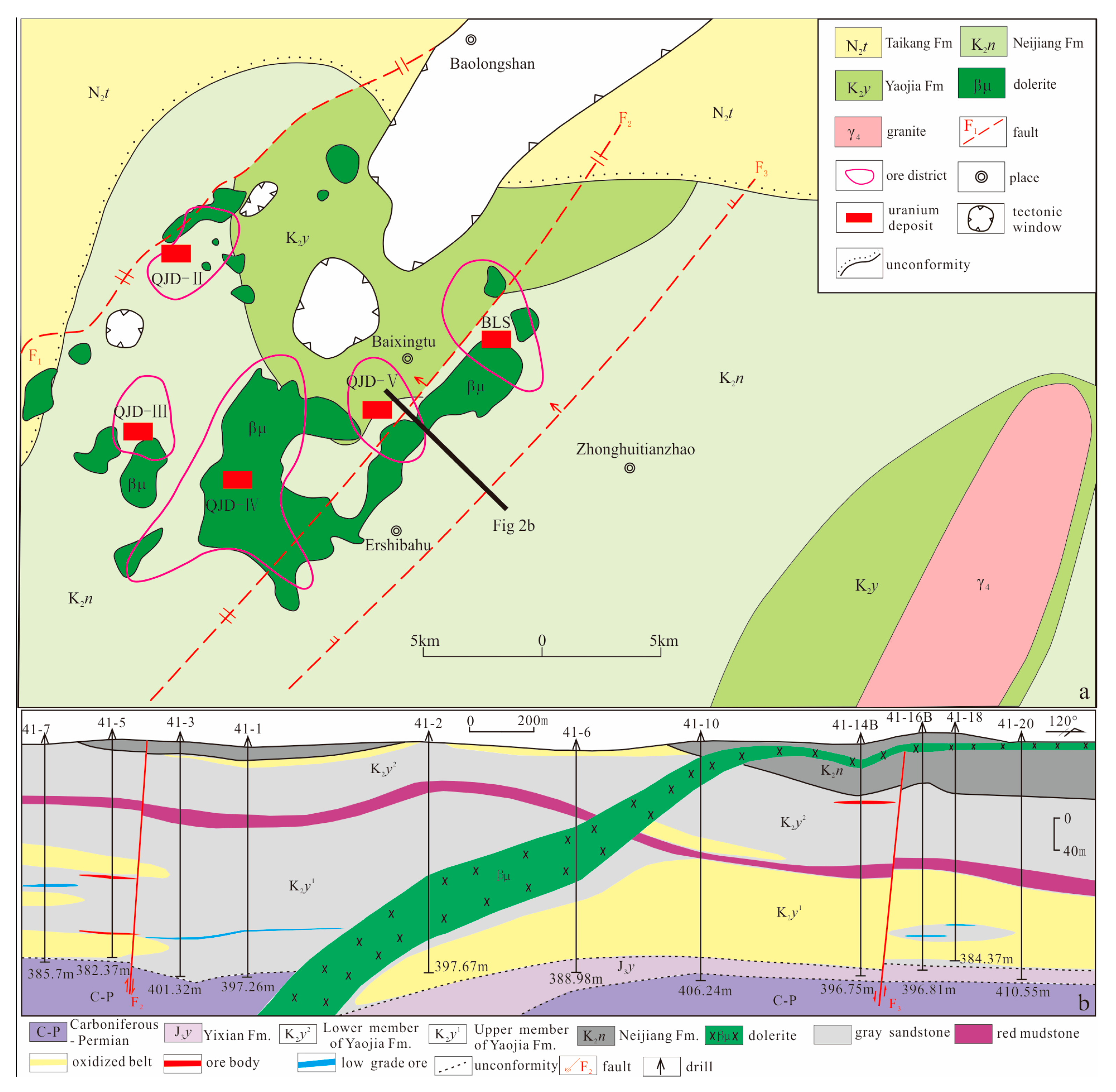
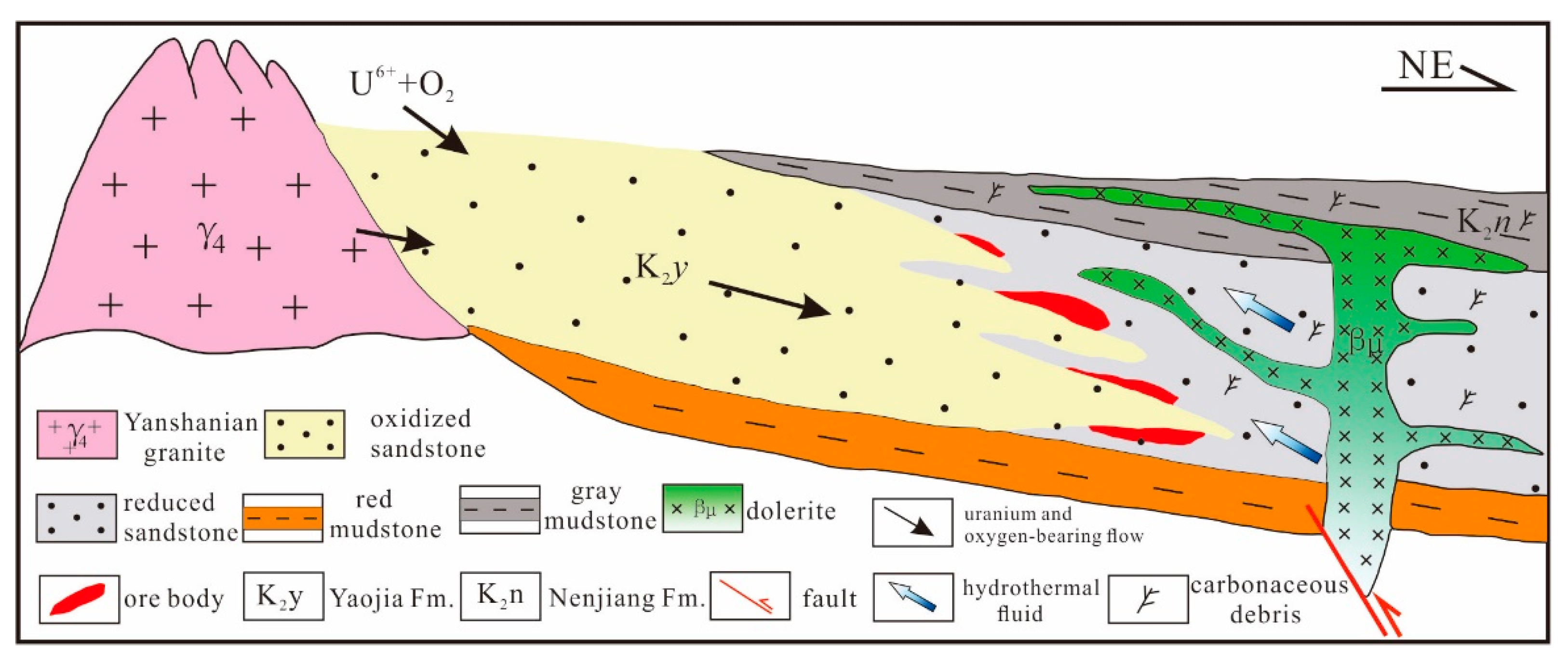
2.2. The QJD Uranium Ore Field
3. Sample Collection and Analysis
3.1. Electron Microprobe Analysis
3.2. Carbon and Oxygen Isotope Analyses
3.3. Sulfur Isotopic Analysis
3.4. Fluid Inclusion Analysis
4. Results
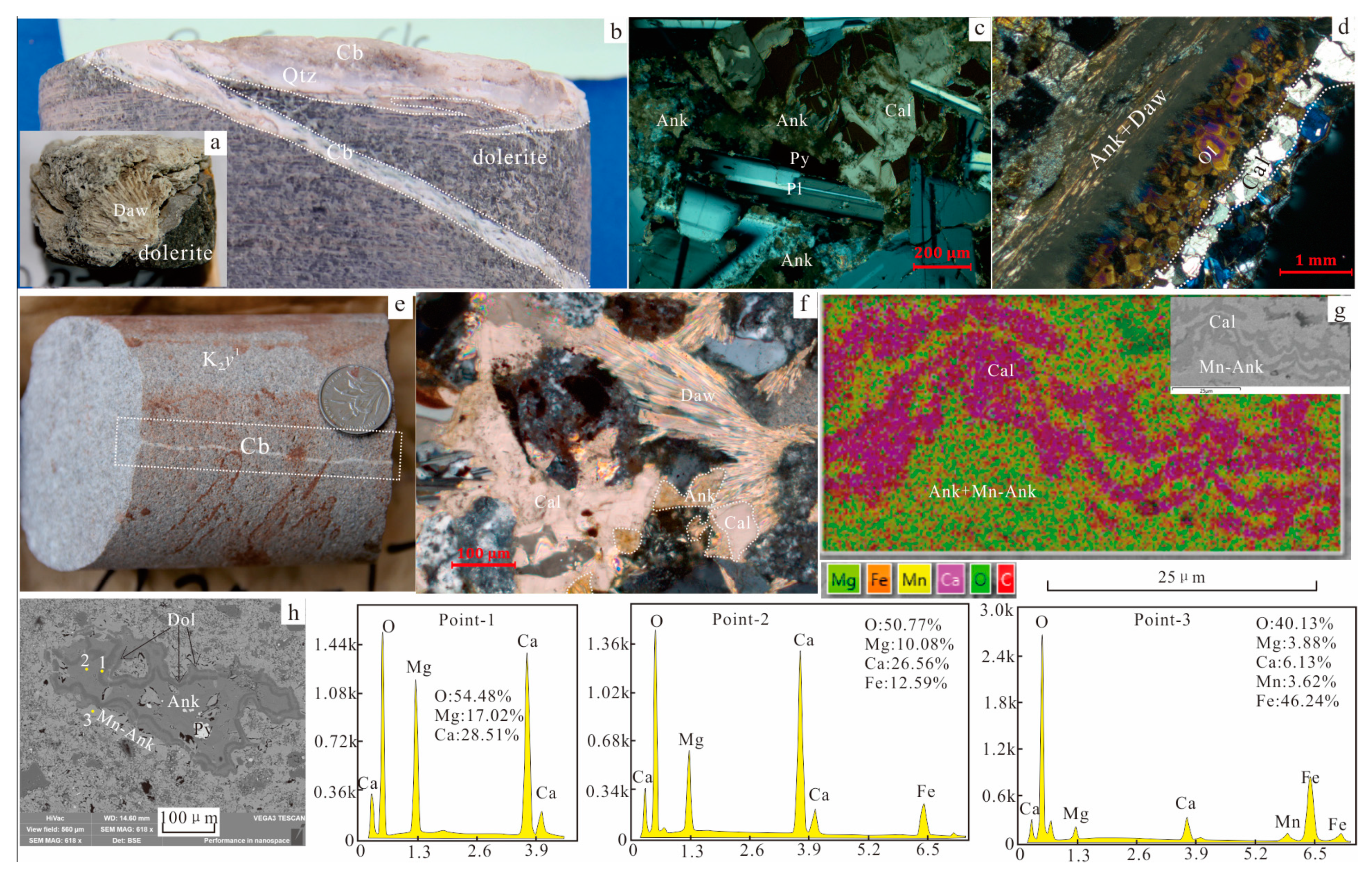
4.1. Hydrothermal Altered Minerals
4.1.1. Carbonatization
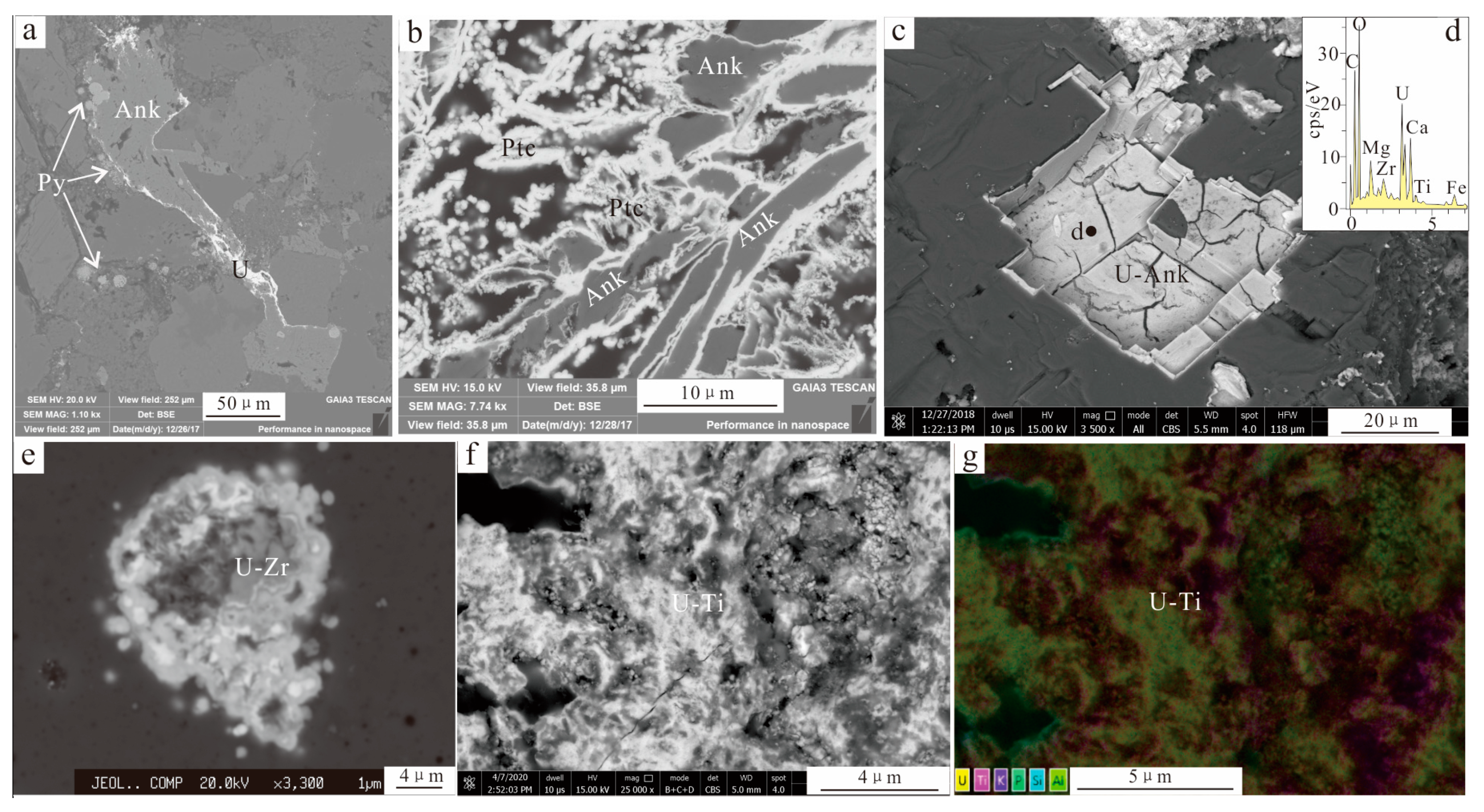
4.1.2. Metal Mineralization
4.1.3. Silicification and Biotitization
4.2. Hydrothermal Uranium Mineralization
4.3. Stable Isotopes
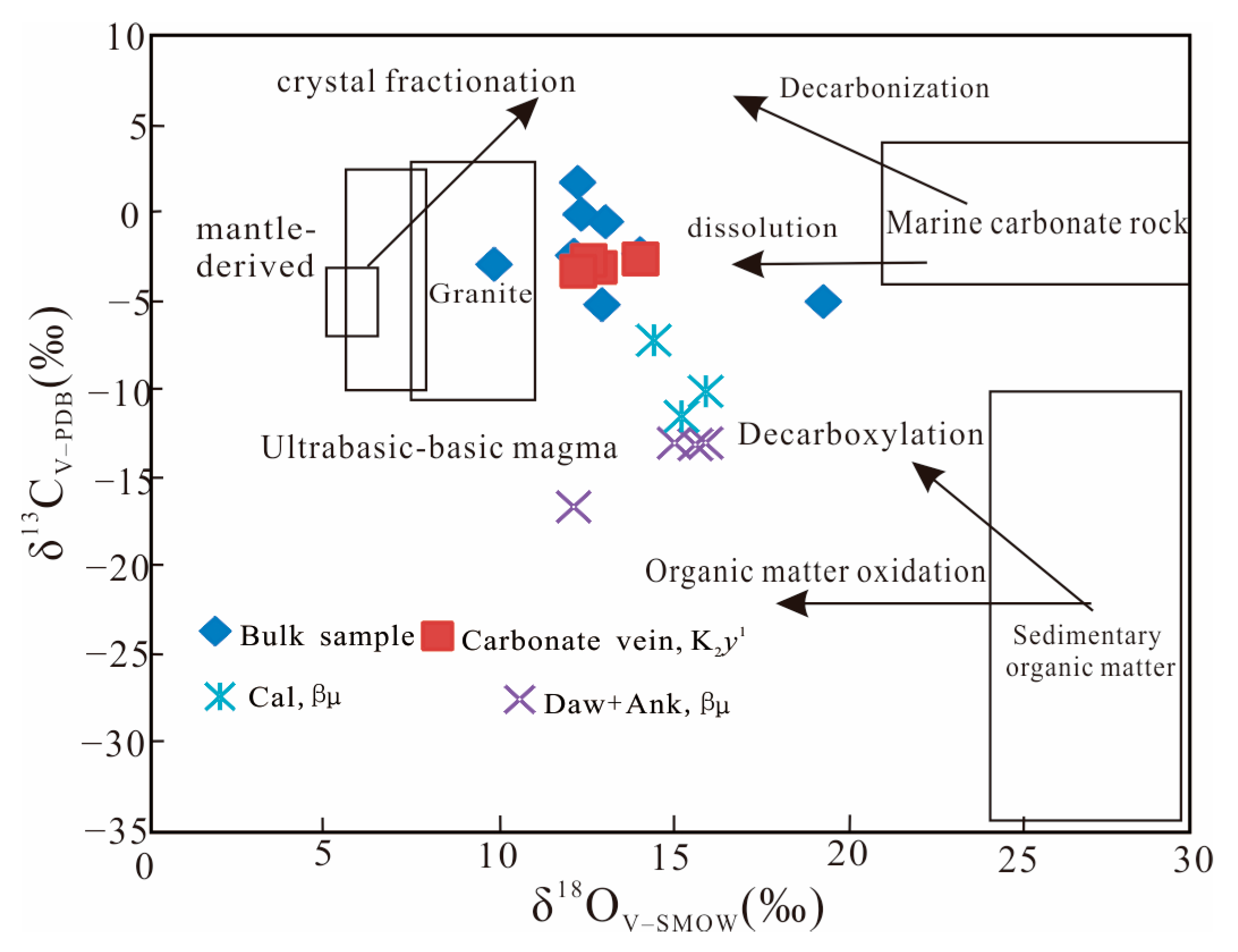
4.3.1. C-O Isotope
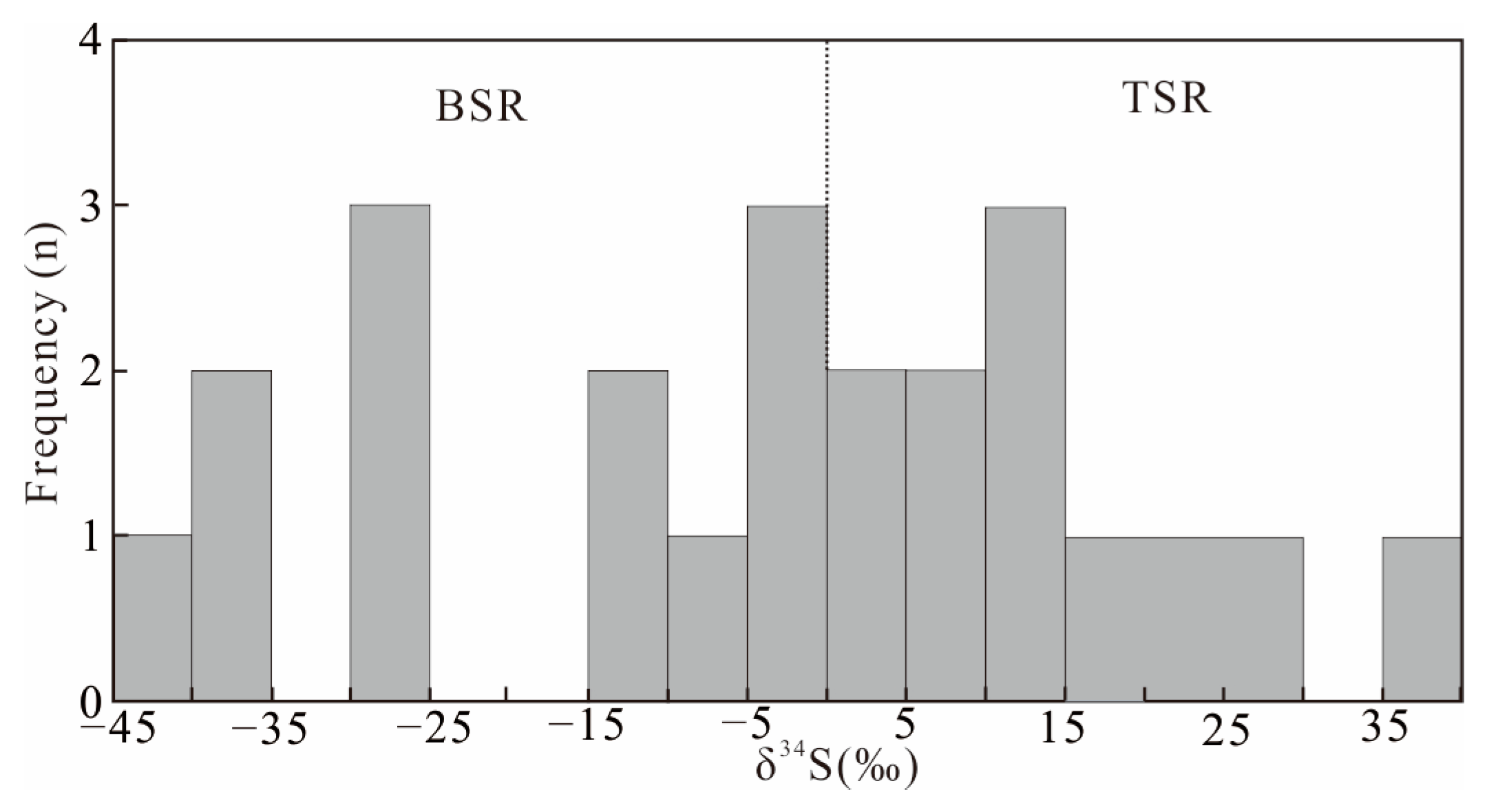
4.3.2. Sulfur Isotope
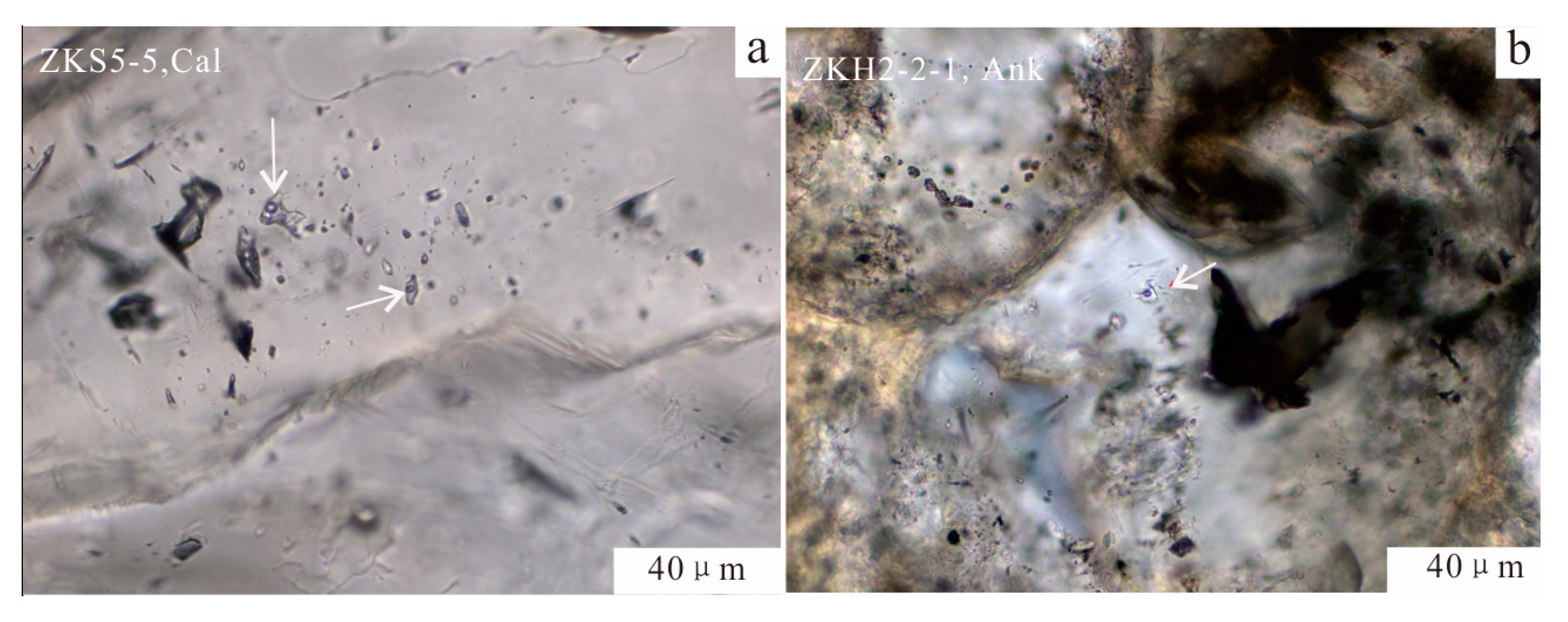
4.4. Fluid Inclusions in the Calcite Cement
4.4.1. Petrographic Characteristics
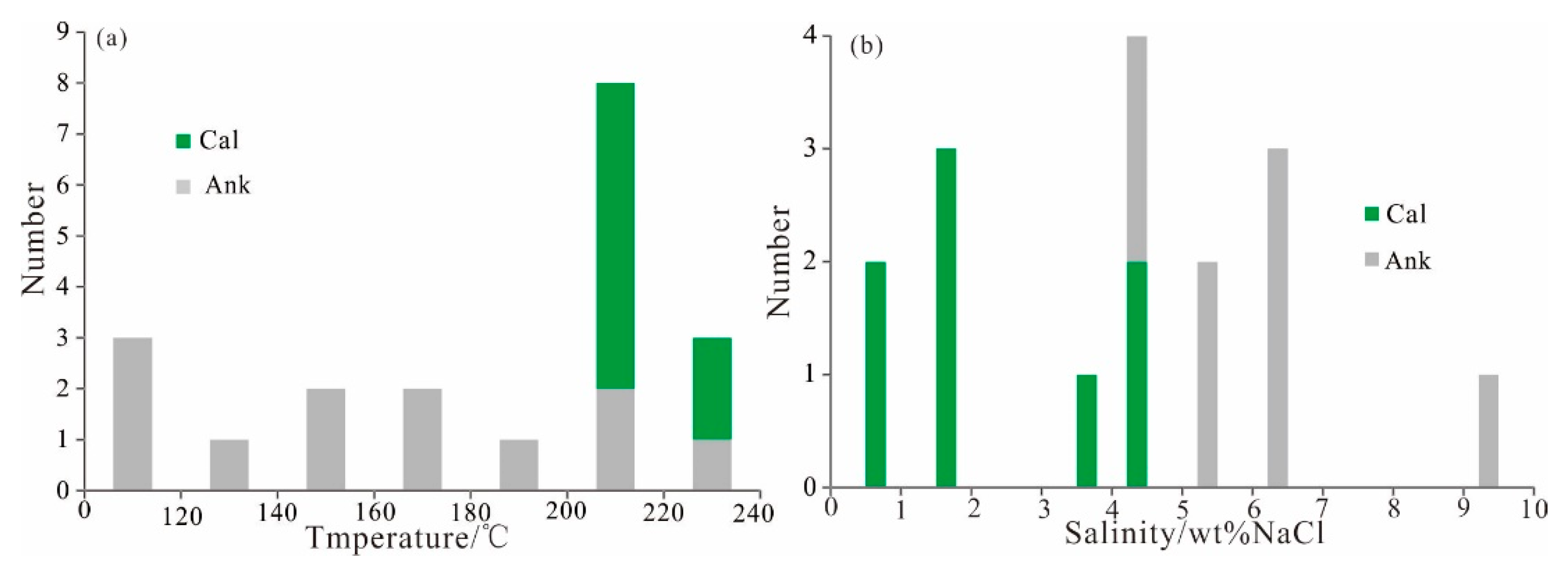
4.4.2. Homogeneous Temperature and Salinity
5. Discussion
5.1. Hydrothermal Fluid and Superimposed Mineralization
5.2. Effects of the Hydrothermal Fluids on Uranium Mineralization
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Z.L.; Zhang, S.; Gao, S.L.; Cui, J.P.; Liu, X.S. Relationship between thermal history and various energy mineral deposits in Dongsheng area, Yimeng uplift. Oil Gas Geol. 2006, 27, 187–193. [Google Scholar]
- Cuney, M. Evolution of uranium fractionation processes through time; driving the secular variation of uranium deposit types. Econ. Geol. 2010, 105, 553–569. [Google Scholar] [CrossRef]
- Cai, C.F.; Dong, H.L.; Li, H.T. Mineralogical and geochemical evidence for coupled bacterial uranium mineralization and hydrocarbon oxidation in the Shashagetai Deposit, NW China. Chem. Geol. 2007, 236, 167–179. [Google Scholar] [CrossRef]
- Lan, Q.; Hu, R.Z.; Bi, X.W.; Liu, H.; Xiao, J.F.; Fu, S.L.; Santosh, M.; Tang, Y.Y. The source of organic matter and its role in producing reduced sulfur for the giant sediment-hosted Jinding zinc-lead deposit, Lanping Basin, Yunnan, southwest China. Econ. Geol. 2021, 116, 1537–1560. [Google Scholar] [CrossRef]
- Sillitoe, R.H.; Brogi, A. Geothermal systems in the Northern Apennines, Italy; modern analogues of carlin-style gold deposits. Econ. Geol. 2021, 116, 1491–1501. [Google Scholar] [CrossRef]
- Chi, G.X.; Xue, C.J. Hydrodynamic regime as a major control on localization of uranium mineralization in sedimentary basins. Science China. Earth Sci. 2014, 57, 2928–2933. [Google Scholar]
- Granger, H.C.; Santos, E.S.; Dean, B.G.; Moore, F.B. Sandstone-type uranium deposits at Ambrosia Lake, New Mexico; an interim report. Econ. Geol. 1961, 56, 1179–1210. [Google Scholar] [CrossRef]
- Qin, M.K.; He, Z.B.; Liu, Z.Y.; Guo, Q.; Song, J.Y.; Xu, Q. Study on Metallogenic Environments and Prospective Direction of Sandstone Type Uranium Deposits in Junggar Basin. Geol. Rev. 2017, 63, 1255–1269. [Google Scholar]
- Huang, S.H.; Qin, M.K.; Xu, Q.; He, Z.B.; Guo, Q. Hydrocarbon Fluid Geological Characteristics of the Xishanyao Formation and Its Uranium Metallogenic Significance, Northwest Junggar Basin. Earth Sci. 2019, 44, 3060–3073. [Google Scholar]
- Lin, S.X.; Gong, X.F.; Zhang, T.L. Deep Geofluid and Uranium Metallogenesis in Meso-Cenozoic Basin. Uranium Geol. 2017, 33, 321–328. [Google Scholar]
- Fuchs, S.; Schumann, D.; Williams-Jones, A.E.; Vali, H. The growth and concentration of uranium and titanium minerals in hydrocarbons of the Carbon Leader Reef, Witwatersrand Supergroup, South Africa. Chem. Geol. 2015, 393–394, 55–66. [Google Scholar] [CrossRef]
- Goswami, S.; Bhagat, S.; Zakaulla, S.; Kumar, S.; Rai, A.K. Role of organic matter in uranium mineralisation in Vempalle dolostone; Cuddapah basin, India. J. Geol. Soc. India 2017, 89, 145–154. [Google Scholar] [CrossRef]
- Xu, Q.; Qin, M.K.; Fan, H.H.; Gu, D.Z.; Wang, S.Y. Preliminary discussion on ore-controlling factors of Azelik sandstone type uranium deposits in Niger. World Nucl. Geosci. 2012, 29, 149–155. [Google Scholar]
- Liu, Z.Y.; Peng, S.P.; Qin, M.K.; He, Z.B.; Guo, Q.; Xu, Q. Multistage Enrichment of the Sawafuqi Uranium Deposit: New Insights into Sandstone-hosted Uranium Deposits in the Intramontane Basins of Tian Shan, China. Acta Geol. Sin. (Engl. Ed.) 2017, 6, 2138–2152. [Google Scholar] [CrossRef]
- Xiao, X.J.; Li, Z.Y.; Fang, X.H.; Ou, G.X.; Sun, Y.; Chen, A.P. The Evidences and Significances of Epithermal Mineralization Fluid in the Dongsheng Sandstone Type Uranium Deposit. Bull. Mineral. Petrol. Geochem. 2004, 23, 301–304. [Google Scholar]
- Zhu, Q.; Yu, R.A.; Feng, X.X.; Li, J.G.; Sima, X.Z.; Tang, C.; Xu, Z.L.; Liu, X.X.; Si, Q.H.; Li, G.Y.; et al. Mineralogy, geochemistry, and fluid action process of uranium deposits in the Zhiluo Formation, Ordos Basin, China. Ore Geol. Rev. 2019, 111, 102984. [Google Scholar] [CrossRef]
- Wang, F.G.; Hou, S.R.; Zhang, L.; Men, H.; Wang, J.L. Study on the Characteristics of Water-Rock Interaction and Its Relation to Uranium Mineralization in Tamusu Uranium Deposit, Southern Bayin Gobi Basin. Geol. Rev. 2018, 64, 633–646. [Google Scholar]
- Zhang, C.Y.; Nie, F.J.; Jiao, Y.Q.; Deng, W.; Peng, Y.B.; Hou, S.R.; Dai, M.J.; Ye, T.F. Characterization of ore-forming fluids in the Tamusu sandstone-type uranium deposit, Bayingobi Basin, China: Constraints from trace elements, fluid inclusions and C–O–S isotopes. Ore Geol. Rev. 2019, 111, 102999. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Cai, Y.Q.; Cai, J.F.; Liu, Z.Y. Discussion on the relationship between diabase intrusion and sandstone-type uranium mineralization in the southwestern Songliao Basin: C-O isotope evidence from carbonate cement. Geol. Rev. 2019, 65, 113–114. [Google Scholar]
- Li, Z.Y.; Fang, X.H.; Chen, A.P.; Ou, G.X.; Sun, Y.; Zhang, K.; Xia, Y.L.; Zhou, W.B.; Chen, F.Z.; Li, M.G. Superposition metallogenic model of sandstone-type uranium deposit in the Northeastern Ordos basin. Uranium Geol. 2009, 25, 65–70. [Google Scholar]
- Liu, C.; Xie, Q.B.; Wang, G.W.; Song, Y.F.; Wang, Y.H.; Tang, Y. Diagenetic and metamorphic characteristics and implications for hydrocarbon reservoirs in the country rocks influenced by magmatic emplacement: A case study from an outcrop of diabase intrusion in the southern Songliao Basin. Chin. J. Geol. 2017, 52, 453–469. [Google Scholar]
- Gao, Y.Q.; Liu, L.; Zhang, S.H.; Yue, K.; Sun, X.M. Alteration to semi-concreted sandstone by diabase intrusion –taking an example from the cretaceous in the southeast of Songliao Basin. Xinjiang Geol. 2003, 21, 474–478. [Google Scholar]
- Liu, H.B.; Jin, G.S.; Han, J.; Zhang, J.F.; Li, J.J.; Zhang, J.; Shi, X. Element and isotope geochemical characteristics of diabase in Qianjiadian uranium deposit. World Nucl. Geosci. 2021, 38, 135–143. [Google Scholar]
- Rong, H.; Jiao, Y.Q.; Wu, L.Q.; Zhao, X.F.; Gao, M.Q.; Liu, W.H. Effects of igneous intrusions on diagenesis and reservoir quality of sandstone in the Songliao Basin, China. Mar. Pet. Geol. 2021, 127, 104980. [Google Scholar] [CrossRef]
- Xia, Y.L.; Zheng, J.W.; Li, Z.Y.; Li, L.Q.; Tian, S.F. Metallogenic characteristics and metallogenic model of Qianjiadian uranium deposit in Songliao Basin. Miner. Depos. 2010, 29, 154–155. [Google Scholar]
- Wu, R.G.; Xu, Z.; Gong, W.J.; Cai, J.F.; Ning, J. Discussion on the Genesis of Baixintu Uranium Deposit in Sonliao Basin. Uranium Geol. 2012, 28, 142–147. [Google Scholar]
- Ding, B.; Liu, H.X.; Liu, Z.Y.; Qiu, L.F.; Jia, L.C.; Zhang, Z.M. Discovery of swartzite in Baolongshan uranium deposit in Songliao Basin and new way of sandstone-type uranium mineralization. Geol. China 2020, 48, 966–967. [Google Scholar]
- Rong, H.; Jiao, Y.Q.; Wu, L.Q.; Ji, D.M.; Li, H.L.; Zhu, Q.; Cao, M.Q.; Wang, X.M.; Li, Q.C.; Xie, H.L. Epigenetic Alteration and Its Constraints on Uranium Mineralization from the Qianjiadian Uranium Deposit, Southern Songliao Basin. Earth Sci. 2016, 41, 153–166. [Google Scholar]
- Jia, J.M.; Rong, H.; Jiao, Y.Q.; Wu, L.Q.; Guo, X.J.; Cao, M.Q.; Cui, Z.J.; Tao, Z.P. Occurrence of Carbonate Cements and Relationship between Carbonate Cementation and Uranium Mineralization of Qianjiadian Uranium Deposit, Songliao Basin. Earth Sci. 2018, 43, 149–161. [Google Scholar]
- Yan, X.L. Characteristics of upper Cretaceous diabase and uranium mineralization in Qianjiadian area, Songliao Basin. J. Northeast. Pet. Univ. 2018, 42, 40–48. [Google Scholar]
- Nie, F.J.; Yan, Z.B.; Xia, F.; Li, M.G.; Lu, Y.Y.; Cai, J.F.; Guo, F.N.; Ning, J. Hot fluid flows in the sandstone-type uranium deposit in the Kailu basin, Northeast China. Geol. Bull. China 2017, 36, 1850–1866. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Li, Q.C.; Liu, M.Y.; Song, B.; Cao, M.; Wang, M. Uranium mineralization formed through multi-stage superposition: Case of the Qianjiadian deposit in Songliao Basin, China. Energy Geosci. 2021, 2, 32–40. [Google Scholar] [CrossRef]
- Bonnetti, C.; Zhou, L.L.; Riegler, T.; Brugger, J.; Fairclough, M. Large S isotope and trace element fractionations in pyrite of uranium roll front systems result from internally-driven biogeochemical cycle. Geochim. Et Cosmochim. Acta 2020, 282, 113–132. [Google Scholar] [CrossRef]
- Liu, J.L.; Qin, M.K.; Cai, Y.Q.; Liu, Z.Y.; Zhang, Z.M.; Yao, L. Late Mesozoic tectonic evolution of the southern Great Xing’an Range, northeastern China: Constraints from detrital zircon U–Pb and Hf isotopes of Late Cretaceous sandstones in the southwestern Songliao Basin. Geol. J. 2020, 55, 4415–4425. [Google Scholar] [CrossRef]
- Jia, J.M.; Rong, H.; Jiao, Y.Q.; Wu, L.Q.; Wang, Y.; Li, H.L.; Cao, M.Q. Mineralogy and geochemistry of carbonate cement in sandstone and implications for mineralization of the Qianjiadian sandstone-hosted uranium deposit, southern Songliao Basin, China. Ore Geol. Rev. 2020, 123, 103590. [Google Scholar] [CrossRef]
- Cai, J.F.; Yan, Z.B.; Zhang, L.L.; Feng, Z.B.; Huang, X.; Nie, F.J.; Xia, F. Relationship Between Grey Sandstone and Uranium Mineralization in Yaojia Formation of Upper Cretaceous in Tongliao, Inner Mongolia. J. East China Univ. Technol. 2018, 41, 328–335. [Google Scholar]
- Lin, J.R.; Tian, H.; Dong, W.M.; Xia, Y.L.; Zheng, J.W.; Qi, D.N.; Yao, S.C. Original geochemical types and epigenetic alteration of rocks in prospecting target stratum for uranium deposit in the southeast of Songliao basin. Uranium Geol. 2009, 25, 202–207. [Google Scholar]
- Luo, Y.; Ma, H.F.; Xia, Y.L.; Zhang, Z.G. Geologic characteristics and metal logenic model of Qianjiadian uranium deposit in Songliao basin. Uranium Geol. 2007, 23, 193–200. [Google Scholar]
- Xu, Z.L.; Li, J.G.; Zhu, Q.; Wei, J.L.; Li, H.L.; Zhang, B. Late Cretaceous paleoclimate change and its impact on uranium mineralization in the Kailu Depression, southwest Songliao Basin. Ore Geol. Rev. 2019, 104, 403–421. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zheng, J.W.; Tian, S.F.; Xia, Y.L.; Liu, H.B. Research on existing state of uranium and uranium ore-formation age at Qianjiadian uranium deposit in Kailu depression. Uranium Geol. 2005, 21, 213–218. [Google Scholar]
- Wang, B.; Tian, W.; Fu, B.; Fang, J.Q. Channelized CO2-Rich Fluid Activity along a Subduction Interface in the Paleoproterozoic Wutai Complex, North China Craton. Minerals 2021, 11, 748. [Google Scholar] [CrossRef]
- Lin, S.R.; Hu, K.; Cao, J.; Bai, T.; Liu, Y.; Han, S.C. An in situ sulfur isotopic investigation of the origin of Carlin-type gold deposits in Youjiang Basin, southwest China. Ore Geol. Rev. 2021, 134, 104187. [Google Scholar] [CrossRef]
- Nikiforov, A.V.; Dubinina, E.O.; Polyakov, N.A.; Sugorakova, A.M.; Khertek, A.K. Influence of Host Marble Rocks on the Formation of Intrusive Alkaline Rocks and Carbonatites of Sangilen (E. Siberia, Russia). Minerals 2021, 11, 666. [Google Scholar] [CrossRef]
- Troshin, Y.P.; Lomonosov, I.S.; Bryukhanova, N.N. Conditions of formation of ore-geochemical specialization of modern hydrotherms in the Baikal rift zone. Russ. Geol. Geophys. 2008, 49, 169–175. [Google Scholar] [CrossRef]
- Ming, X.R.; Liu, L.; Yu, M.; Bai, H.G.; Yu, L.; Peng, X.L.; Yang, T.H. Bleached mudstone, iron concretions, and calcite veins: A natural analogue for the effects of reducing CO2-bearing fluids on migration and mineralization of iron, sealing properties, and composition of mudstone cap rocks. Geofluids 2017, 16, 1017–1042. [Google Scholar] [CrossRef]
- Rong, H.; Jiao, Y.Q.; Wu, L.Q.; Wan, D.; Cui, Z.J.; Guo, X.J.; Jia, J.M. Origin of the carbonaceous debris and its implication for mineralization within the Qianjiadian uranium deposit, southern Songliao Basin. Ore Geol. Rev. 2019, 107, 336–352. [Google Scholar] [CrossRef]
- Pointer, C.; Achworth, J.R.; Simpson, P.R. Genesis of coffinite and the U-Ti association in lower Old Red Sandstone sediments, Ousdale, Caithness, Scotland. Miner. Depos. 1989, 24, 117–123. [Google Scholar] [CrossRef]
- Yu, Z.C.; Liu, L.; Qu, X.Y.; Yang, H.D.; Shao, M.L. Formation Temperature of Dawsonite from Qingshankou Formation in the Honggang Oilfield, Southern Songliao Basin. Acta Sedimentol. Sin. 2011, 29, 293–302. [Google Scholar]
- Qu, X.Y.; Zhu, W.H.; Liu, L.; Dong, F.X. Petrology characteristics and the origin of dawsonite-bearing sandstone reservoir in southern Songliao Basin. Sci. Technol. Rev. 2015, 33, 13–19. [Google Scholar]
- Ma, P.J.; Lin, C.Y.; Jens, J.R.; Jahren, J.; Dong, C.; Rena, L.H.; Hellevang, H. Cyclic zoning in authigenic saddle dolomite-ankerite; indications of a complex interplay between fault-rupturing and diagenetic alteration. Chem. Geol. 2021, 559, 119831. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.L.; Yang, J.H. Tracing water-rock interaction in carbonate replacement deposits; a SIMS pyrite S-Pb isotope perspective from the Chinese Xinqiao System. Ore Geol. Rev. 2019, 107, 248–257. [Google Scholar] [CrossRef]
- René, M. Anomalous rare earth element, yttrium and zirconium mobility associated with uranium mineralization. Terra Nova 2008, 20, 52–58. [Google Scholar] [CrossRef]
- You, X.Y.; Jia, W.Q.; Xu, F.; Liu, Y. Mineralogical Characteristics of Ankerite and Mechanisms of Primary and Secondary Origins. Earth Sci. 2018, 43, 4046–4055. [Google Scholar]
- Cheng, Y.H.; Wang, S.Y.; Zhang, T.F.; Teng, X.M.; Ao, C.; Jin, R.S.; Li, H.L. Regional sandstone-type uranium mineralization rooted in Oligo–Miocene tectonic inversion in the Songliao Basin, NE China. Gondwana Res. 2020, 88, 88–105. [Google Scholar] [CrossRef]
- Wigley, M.; Kampman, N.; Dubacq, B.; Bickle, M. Fluid-mineral reactions and trace metal mobilization in an exhumed natural CO2 reservoir, Green River, Utah. Geology 2012, 40, 555–558. [Google Scholar] [CrossRef]
- Zaccarini, F.; Stumpfl, E.; Garuti, G. Zirconolite and Zr-Th-U minerals in chromitites of the Finero Complex, Western Alps, Italy; evidence for carbonatite-type metasomatism in a subcontinental mantle plume. Can. Minerals. 2004, 42, 1825–1845. [Google Scholar] [CrossRef]
- Mikysek, P.; Zikmund, T.; Dosbaba, M.; Brinek, A.; Slobodnik, M.; Adamovic, J.; Meszarosova, N.; Trojek, T.; Kaiser, J. Multi-scale visualization of uranium-rich domains dispersed in U-Zr mineralization of sandstone-type (Břevniště, Czech Republic). Ore Geol. Rev. 2021, 138, 104358. [Google Scholar] [CrossRef]
- Qiu, L.F.; Li, X.D.; Liu, W.S.; Hu, B.Q.; Gao, L.; He, Z.B. Uranium Deposits of Erlian Basin (China): Role of Carbonaceous Debris Organic Matter and Hydrocarbon Fluids on Uranium Mineralization. Minerals 2021, 11, 532. [Google Scholar] [CrossRef]

| Samples | Results (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Point | Al2O3 | FeO | UO2 | Na2O | MgO | TiO2 | Y2O3 | SiO2 | PbO | |
| ZKX97-5-1B | 1 | 0.69 | 0.73 | 72.50 | 1.81 | 0.08 | 1.72 | 0.08 | 3.17 | / |
| 2 | / | 0.32 | 70.87 | 0.58 | 0.04 | 1.98 | 0.13 | 0.83 | / | |
| 3 | 0.47 | 1.17 | 71.86 | 0.92 | 0.20 | 1.81 | 0.18 | 1.90 | 0.24 | |
| Point | Ce2O3 | Nd2O3 | CaO | MnO | P2O5 | SO3 | ZrO2 | Total amount | Mineral | |
| 1 | 0.14 | / | 2.54 | 0.07 | 1.65 | 0.10 | 5.06 | 90.34 | Pitchblende | |
| 2 | 0.17 | / | 2.81 | 0.08 | 2.15 | 0.24 | 5.55 | 85.75 | Pitchblende | |
| 3 | 0.10 | 0.17 | 3.67 | 0.25 | 1.77 | 0.22 | 6.36 | 91.29 | Pitchblende | |
| SL19-24 | Point | MgO | FeO | CaO | MnO | UO2 | CO2 | Total | Mineral | — |
| 1 | 13.37 | 6.45 | 34.07 | 0.47 | 0.05 | 45.59 | 100.00 | Ankerite | — | |
| 2 | 9.43 | 41.70 | 7.47 | 0.35 | 0.08 | 41.95 | 100.98 | Fe-Ankerite | — | |
| 3 | 20.19 | 0.14 | 32.04 | / | / | 47.28 | 99.65 | Dolomite | — | |
| 4 | 9.57 | 12.53 | 32.75 | 1.13 | / | 44.53 | 100.51 | Ankerite | — | |
| 5 | 9.93 | 9.63 | 29.99 | 1.35 | / | 41.12 | 92.02 | Ankerite | — | |
| SL19-35 | 1 | 7.23 | 11.82 | 30.86 | 6.12 | 0.05 | 43.17 | 99.25 | Mn-Ankerite | — |
| 2 | 11.05 | 11.99 | 31.59 | 0.46 | 0.13 | 44.53 | 99.75 | Ankerite | — | |
| SL19-37 | 1 | 0.17 | 0.07 | 54.73 | 1.19 | / | 43.92 | 100.08 | Calcite | — |
| SL19-47 | 1 | 0.50 | 10.63 | 11.77 | 37.01 | 0.07 | 39.27 | 99.25 | Mn-Ankerite | — |
| 2 | 9.83 | 13.38 | 31.31 | 1.10 | / | 44.18 | 99.80 | Ankerite | — | |
| 3 | 0.21 | 10.74 | 9.41 | 39.54 | / | 38.73 | 98.63 | Mn-Ankerite | — | |
| 4 | 10.61 | 12.95 | 31.25 | 0.66 | / | 44.45 | 99.92 | Ankerite | — | |
| Samples | Lithology and Stratigraphy | δ13CPDB | δ18OPDB | δ18OPDB |
|---|---|---|---|---|
| SL19-2 | Bulk sample, K2y1 | −0.5 | −17.3 | 13.1 |
| SL19-04 | 1.7 | −18 | 12.3 | |
| SL19-06 | −2.3 | −16.3 | 14.1 | |
| SL19-17 | −0.1 | −18 | 12.4 | |
| SL19-21 | −2.4 | −18.1 | 12.2 | |
| SL19-22 | −2.9 | −20.4 | 9.9 | |
| SL19-37 | −5.2 | −17.4 | 13 | |
| SL19-47 | −5 | −11.1 | 19.4 | |
| ZKS5-5-2 | Carbonate vein, K2y1 | −3.1 | −17.5 | 12.9 |
| ZKS25-61-1 | −2.7 | −17.8 | 12.6 | |
| ZKS5-5-1 | −3.3 | −18 | 12.3 | |
| ZKS25-61-3 | −2.6 | −16.3 | 14.1 | |
| SL20-39 | Dawsonite and ankerite, βμ | −13 | −14.5 | 16 |
| SL20-40-1 | −13 | −15.4 | 15.1 | |
| SL20-65-1 | −13.1 | −14.7 | 15.7 | |
| SL20-67 | −16.6 | −18.2 | 12.2 | |
| ZKS1-41 | Calcite, βμ | −7.2 | −15.9 | 14.5 |
| SL20-40 | −11.5 | −15.1 | 15.3 | |
| SL20-65 | −10.1 | −14.5 | 16 |
| Methods | Samples | Lithology and Stratigraphy | δ34S (‰) |
|---|---|---|---|
| TIMS, sample dissolving | SL19-06 | Bulk sample, K2y1 | 19.8 |
| SL19-37 | −27.8 | ||
| SL19-47 | −14.9 | ||
| SL19-37 | Pyrite grain, K2y1 | −36.5 | |
| SL19-47 | −28.2 | ||
| LA-ICP-MS, in-situ | SL19-06-2 | Colloidal pyrite, K2y1 | 24.02 |
| SL19-06-3 | 3.27 | ||
| SL19-06-4 | −0.44 | ||
| SL19-06-5 | 36.31 | ||
| SL19-06-6 | 10.49 | ||
| SL19-06-7 | 12.43 | ||
| SL19-06-8 | 25.46 | ||
| SL19-06-9 | 12.64 | ||
| SL19-37-1 | −28.92 | ||
| SL19-37-2 | −43.91 | ||
| SL19-37-3 | −39.72 | ||
| SL19-37-4 | 3.77 | ||
| SL19-37-5 | −4.42 | ||
| SL19-37-6 | −12.45 | ||
| SL19-47-1 | −3.26 | ||
| SL19-47-2 | −5.48 | ||
| SL19-47-3 | 8.44 | ||
| SL19-47-4 | 7.45 |
| Samples | Mineral | Inclusion Type | Size (μm) | Homogenization Temperature (°C) | Salinity (wt% NaCl) |
|---|---|---|---|---|---|
| ZKH2-2-1 | Ankerite cement | two-phase (vapor-liquid) inclusions | 8 × 11 | 178 | 9.98 |
| 4 × 6 | 166 | / | |||
| 4 × 6 | 202 | 6.59 | |||
| ZKH0-2-4 | two-phase (aqueous-rich) inclusions | 4 × 8 | 213 | 4.34 | |
| 2 × 5 | 232 | 4.18 | |||
| KT14-25-1 | 3 × 5 | 156 | / | ||
| 3 × 25 | 100 | 5.56 | |||
| 2 × 14 | 107 | 5.26 | |||
| 5 × 9 | 111 | / | |||
| 8 × 5 | 140 | 6.88 | |||
| 3 × 6 | 198 | 6.72 | |||
| ZKS5-5 | Calcite cement | two-phase (aqueous-rich) inclusions | 2 × 5 | 214 | 4.18 |
| 2 × 3 | 223 | 4.34 | |||
| 4 × 6 | 203 | 1.91 | |||
| 2 × 6 | 215 | 1.91 | |||
| 4 × 16 | 215 | 0.71 | |||
| 3 × 14 | 213 | 0.71 | |||
| two-phase (vapor-liquid) inclusions | 13 × 18 | 234 | 1.91 | ||
| 3 × 6 | 213 | 3.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, M.-K.; Huang, S.-H.; Liu, J.-L.; Liu, Z.-Y.; Guo, Q.; Jia, L.-C.; Jiang, W.-J. Hydrothermal Alteration and Its Superimposed Enrichment for Qianjiadian Tabular-Type Uranium Deposit in Southwestern Songliao Basin. Minerals 2022, 12, 52. https://doi.org/10.3390/min12010052
Qin M-K, Huang S-H, Liu J-L, Liu Z-Y, Guo Q, Jia L-C, Jiang W-J. Hydrothermal Alteration and Its Superimposed Enrichment for Qianjiadian Tabular-Type Uranium Deposit in Southwestern Songliao Basin. Minerals. 2022; 12(1):52. https://doi.org/10.3390/min12010052
Chicago/Turabian StyleQin, Ming-Kuan, Shao-Hua Huang, Jia-Lin Liu, Zhang-Yue Liu, Qiang Guo, Li-Cheng Jia, and Wen-Jian Jiang. 2022. "Hydrothermal Alteration and Its Superimposed Enrichment for Qianjiadian Tabular-Type Uranium Deposit in Southwestern Songliao Basin" Minerals 12, no. 1: 52. https://doi.org/10.3390/min12010052
APA StyleQin, M.-K., Huang, S.-H., Liu, J.-L., Liu, Z.-Y., Guo, Q., Jia, L.-C., & Jiang, W.-J. (2022). Hydrothermal Alteration and Its Superimposed Enrichment for Qianjiadian Tabular-Type Uranium Deposit in Southwestern Songliao Basin. Minerals, 12(1), 52. https://doi.org/10.3390/min12010052





