Abstract
Microbial community changes in response to acid stress in microbial fuel cells (MFCs) were studied. Acid mine drainage (AMD) wastewater is usually difficult to treat because of the high concentration of sulfate and heavy metals. MFCs, which have multiple functions based on the principle of synergistically treating organic and heavy metal wastewater while generating electrical energy, represent a promising direction for the development of new heavy metal wastewater treatment technologies. Maintaining a neutral or slightly alkaline wastewater pH in MFCs facilitates the growth of electricity-producing microorganisms in the anode chamber. Studies on the response of anode electroactive biofilms to acidic pH stress and its correlation with changes in AMD treatment capacity have not been reported. Results showed that the anolyte pH of 4.0 and 5.0 affected the electron output capacity of the electrogenic microbial community in the MFCs. In contrast, MFCs working at an anolyte pH of 6.0 exhibited a high efficiency of chemical energy conversion to electrical energy. The microbial abundance and microbial diversity of the electroactive biofilm were significantly affected by the H+ concentration in the medium when the ambient acidity was continuously reduced. The classic exoelectrogen Geobacter decreased gradually with the increase of H+ concentration in the medium. In addition, Cu2+ was recovered from the simulated AMD in the MFCs cathodic chambers at low anode pH, but the removal rate of Cu2+ decreased as the pH of the anode environment decreased. At 48 h, 86.2% of Cu2+ was removed from the MFCs cathode solution at pH 5.0, while the removal rate of Cu2+ from the MFCs cathode solution at pH 4.0 was 84.2%. Trace amounts of Cu2O and Cu3(OH)2[CO3]2 were present on the cathode, which reduced the amount of Cu2+ that precipitated on the cathode carbon cloth. Conversely, the concentration of Cu2+ in the catholyte of MFCs with electroactive biofilm at pH 6.0 decreased rapidly, and by 36 h, no detectable Cu2+ was present in the cathodic solution. This study will provide researchers with valuable information regarding the optimal pH for resource recovery with MFCs.
1. Introduction
Acid mine drainage (AMD) is extremely acidic and highly contaminated leachate [1], typically generated from underground operations in abandoned mines and from the accumulation of tailings or waste rock [2], and one of the most serious water pollution problems worldwide [3]. This AMD typically contains high concentrations of sulfate (350–13,000 SO24− mg/L) and heavy metals (10−3–104 mg/L) [3]. Direct discharge of untreated AMD may have toxic effects on organisms in rivers. Acidic wastewater also erodes the soil and causes plant decay [4]. Therefore, finding effective treatment methods to remove heavy metals and neutralize AMD will help in wastewater treatment and recycling.
Conventional techniques for AMD remediation include various physicochemical methods, but these methods suffer from excessive use of chemicals and high costs [5]. Therefore, biological treatment has emerged as an effective, economical, and environmentally friendly alternative for the remediation of AMD. Bioremediation of AMD mostly relies on microorganisms, such as bacteria and fungi [6], to help remediate acidic mine drainage by designing bioreactors with microbial attachment to promote the reduction of metals, sulfates, and produce alkalinity [7]. AMD treatment has been studied in recent years with various methods such as constructed wetlands, microbial, neutralization, and adsorption. The constructed wetlands method is less costly, but its remediation efficiency and sustainability depend largely on climate and vegetation [8]. Microorganisms can remove metals differentially without secondary contamination, but the remediation efficiency of microorganisms is mainly controlled by heavy metal ions, and the heavy metal precipitation produced during remediation wraps around the bacterial surface, further hindering material exchange [9]. Neutralization is a simple process but requires large amounts of neutralizer and generates a large amount of sludge after the remediation of AMD [10]. Adsorption is an important technique for the removal of heavy metals from wastewater due to its strong affinity and high loading [11]. However, all these techniques suffer from low remediation efficiency or high costs, and in addition, some of them usually generate new wastes that require further treatment.
Microbial fuel cells (MFCs) are currently one of the most promising technologies for the treatment of acid mine drainage [12]. The minimal external energy required and the low cost of using MFCs for wastewater treatment make it an economically advantageous treatment method [13]. The classical MFCs consist of an anaerobic anode chamber, an aerobic cathode chamber, a proton exchange membrane (PEM), and an external circuit [14]. In the anode chamber, microorganisms oxidize organic matter or biomass and produce electrons and protons. The electrons are transferred to the cathode via an external circuit, while protons are transferred via the internal membrane. In the cathode chamber, electrons and protons are oxidized by oxidants (usually oxygen or, in the absence of oxygen, nitrate, sulfate, iron, manganese, selenate, and arsenate) [15,16], and it is the series of processes described above that allows the anode chamber to maintain a stable pH [17,18]. The microbial community in the anode chamber oxidizes organic matter in the wastewater under limited oxygen conditions, resulting in acidic conditions in the anode chamber, which affects the growth of the electroactive microbial community and its ability to produce electricity [19,20]. The kinetics of redox reactions are generally faster in an alkaline environment than in an acidic environment [21], so maintaining a neutral or slightly alkaline wastewater pH is favorable for the growth of electrogenic microorganisms in the anode chamber. However, there are relatively few applications of MFCs for the treatment of acidic wastewater [22]. For studies comparing the electrochemical activity of anodic electroactive biofilms, the response of microbial communities to acidic pH stress and their effects on the treatment of AMD has not been reported.
Previous studies have demonstrated that acidic pH can affect the performance of anodic electroactive biofilms in MFCs [23,24]. Margaria [23], for example, reported that at a pH lower than 5.5, there was a significant drop in MFCs performance as well as a decrease in viable population. In another study, it was also reported that dual-chamber MFCs operating at pH 5.0 had the least power generation [24]. However, the impacts of the various acidic pH on the microbial communities in the MFCs were barely studied. In addition, the effect of different acidic pH on the ability of electroactive biofilms to act as biocatalysts in the treatment of AMD has not been reported. Therefore, the aim of this study was to investigate (i) the effects of varied acidic pH (i.e., pH 4.0, 5.0, and 6.0) on the electrochemical performance of MFCs, (ii) the impact of these pH on electrogenic microbial communities in MFCs, and (iii) the impact of the different acidic pH on the ability of electroactive biofilms to act as biocatalysts in the treatment of AMD. This work will provide researchers with valuable information regarding the optimal pH for resource recovery with MFCs.
2. Materials and Methods
2.1. Configuration of Microbial Fuel Cell Reactor
In this paper, single-chamber MFCs with a cubic shape and a cylindrical chamber (3 cm in diameter and 4 cm in length) were constructed, and the main material for these MFCs was polymethyl methacrylate [25]. The core components of each single-chamber MFCs reactor were an anode carbon brush (1.5 cm radius and 3 cm length) and a cathode carbon cloth (7.07 cm2 surface area). The working volume of each reactor in this experiment was 28 mL, and its anode and cathode were connected by titanium wire with an external resistance of 1000 Ω. Dual-chamber MFCs were used to treat the simulated AMD. The dual-chamber MFCs consisted of two main components: an anode chamber with a working volume of 28 mL and a cathode chamber with a working volume of 15 mL. The anode enriched in the single-chamber MFCs was used in the dual-chamber MFCs, and the cathode was a rectangular carbon cloth (2.5 cm long × 0.9 cm wide). The two chambers were separated by an anion exchange membrane (Hangzhou Glion Environmental Technology Co., Ltd., Hangzhou, China). The cathode and the electroactive anode of this dual-chamber MFCs were connected with a 10 Ω external resistor. The pretreatment of the carbon brushes and carbon cloth was performed as described by Ai [26].
2.2. Startup and Operation of MFCs
In this experiment, abiotic single-chamber MFCs without inoculation of anaerobic sludge and single-chamber MFCs inoculated with anaerobic sludge from the Changsha municipal wastewater treatment plant were established, and duplicate single-chamber MFCs were constructed as described in previous work [25]. The anolyte medium used for the enrichment of the electroactive biofilm was made up of 50 mM phosphate buffer (4.56 g/L, Na2HPO4; 2.45 g/L, NaH2PO4; 0.31 g/L, NH4Cl; 0.13 g/L, KCL; 0.02 g/L, CaCl2), 20 mM lactate, trace element solution (100 μL/L), and Wolfe’s vitamins (0.5 mL/L), as reformed from a previous study [27]. The MFCs’ bioanodes were initially acclimated for approximately three weeks, and the process was stopped when power generation was stable. To investigate the effects of different acidic pHs on electrochemical activity, the pH of the anolyte was adjusted to 4.0, 5.0, and 6.0 using NaOH or HCl. The experimental MFCs were operated in batch replenishment mode in an incubator at 30 °C. When the output voltage of the MFCs dropped below 50 mV, the medium was replaced. Dual-chamber MFCs containing electroactive anodes were constructed in order to examine the impact of the various acidic pH (4.0, 5.0, and 6.0) on the ability of the electroactive biofilms to treat simulated AMD. The medium used to maintain the electroactive biofilm growth in the anode chamber of the dual-chamber MFCs was the same as that used in the single-chamber MFCs. The cathode compartments of the dual-chamber MFCs were filled with simulated AMD, which contained mainly 150 mg/L Cu2+ at pH 3.0, while abiotic dual-chamber MFCs without enriched biofilm were established as control groups. At regular intervals, 1.5 mL of the cathode solution was taken to examine the changes in pH with time, and 0.4 mL of the cathode solution was subsequently retained to determine the Cu2+ concentration during the experiment.
2.3. Calculation and Analysis of Power Production Efficiency
In this study, the voltage across the external resistance (1000 Ω) of the single-chamber MFCs was recorded every 50 s by a data acquisition unit (ADAM-4017 analog input model, Advantech Technology Co., Ltd., Shenzhen, China) connected to a computer. The polarization curve of the MFCs, as well as the power density, were obtained by gradually varying the external resistance from 10 Ω to 51,000 KΩ [28]. The power density was normalized to the geometric surface area of the anode (7 cm2) [29], and the calculation of the Coulombic efficiency (CE) of the single-chamber MFCs was based on a previous study [30]. The electrochemical behavior of the electroactive biofilms in the MFCs was ascertained with electrochemical impedance spectroscopy (EIS) using a potentiostat (Gamry reference 600+ workstation, Philadelphia, PA, USA). For the EIS experiments, a three-electrode configuration was utilized, with the anode functioning as the working electrode and the cathode serving as the counter electrode, as well as a saturated Ag/AgCl reference electrode. The EIS test was carried out in the frequency range of 1000 kHz to 0.01 Hz, with a signal amplitude of 5 mV, and was interpreted with the software Zview. The Cu2+ concentration in the leachate of the cathode chamber was determined by ICP after dilution (SPECTROBLUE FMS386, AMETEK Materials Analysis Division, Kleve, Germany). The pH value of the cathodic solution was determined using a pH meter (SJ-4A, Lei Chi, Shanghai, China). Scanning electron microscopy was used to examine the morphologies of the electroactive biofilms on the anode (SEM, JSM-6490LV, JEOL, Tokyo, Japan). The samples were prepared as previously described prior to SEM analysis [26]. Energy-dispersive X-ray spectroscopy (EDXS; Elect super, EDAX AMETEK, Kleve, Germany) coupled with scanning electron microscopy (SEM) and X-ray powder diffraction (XRD; D8 Advance, Bruker Corporation, Germany) were used to obtain detailed information about the morphologies and elemental compositions of the cathodes after the treatment of the simulated AMD.
2.4. Genomic DNA Extraction and MiSeq Sequencing of Bioelectrically Active Biofilms
The MFCs with stable output voltage were disassembled, and a small amount of anode carbon fiber containing electroactive biofilms was carefully cut with sterile scissors. Total DNA was subsequently extracted from the electroactive biofilms of the carbon fiber using a bacterial soil DNA extraction kit (DNeasy PowerSoil DNA Isolation Kit (QIAGEN, Chatsworth, CA, USA). The purity and approximate concentration of the extracted samples were determined by NanoDrop1000, and the integrity of the DNA was initially determined using a 1% (w/v) agarose gel. The V4 region of bacterial and archaeal 16S rDNA genes was amplified using Illumina splice sequence plus universal primer pairs 515FmodF (5′-GTGYCAGCMGCCGCGGTAA-3′) and 806RmodR (5′-GGACTACNVTCTAAT-3′) [31]. PCR amplification was performed using Applied Biosystems GeneAmp®9700 thermal cycler (ABI Inc., Los Angeles, CA, USA). The PCR system (25 μL) consisted of 1 μL of template DNA, 2 μL of primers, 9.5 μL of sterile deionized water, and 12.5 μL of 2× Taq PCR DNA polymerase (TransGen, Beijing, China). Each genomic DNA sample was mixed after 3 separate amplifications to minimize potential amplification bias; the amplified target fragments were separated by 2% agarose gel electrophoresis and recovered using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). The concentration of the recovered PCR products was accurately determined using a QuantiFluor TM_ST fluorometer (Promega Corporation, Fitchburg, WI, USA). Sequencing libraries were prepared and sequenced on the Illumina MiSeq platform with a PE250 sequencing strategy (Shanghai Meiji Biomedical Technology Co., Ltd., Shanghai, China). The raw data of 16S rDNA gene sequences obtained from the MiSeq sequencer were in FASTQ format, and Illumina sequencing junctions and other specific sequences were removed before proceeding. Double-ended sequenced sequences that overlapped by at least 10 bp and had a mismatch rate of less than 5% were then merged using FLASH software [32]. Sequences shorter than 240 bp, chimeric sequences, and low-quality sequences were filtered, cropped, and removed using correlation software [33]. Using the UPARSE method, the operable classification units (OTUs) of each sample were obtained using a 97% similarity threshold [34]. Based on the Bayesian algorithm, the representative sequences of OTU were phylogenetically classified using an RDP classifier at a threshold of 70% confidence [35]. Community richness, Ace and Shannon indices, and Chao1 richness estimates were obtained for each sample by MOTHUR software (Version 1.35.1) [36].
3. Results and Discussion
3.1. Comparison of Electricity Production Capacity of Electroactive Biofilm at Different Low pH
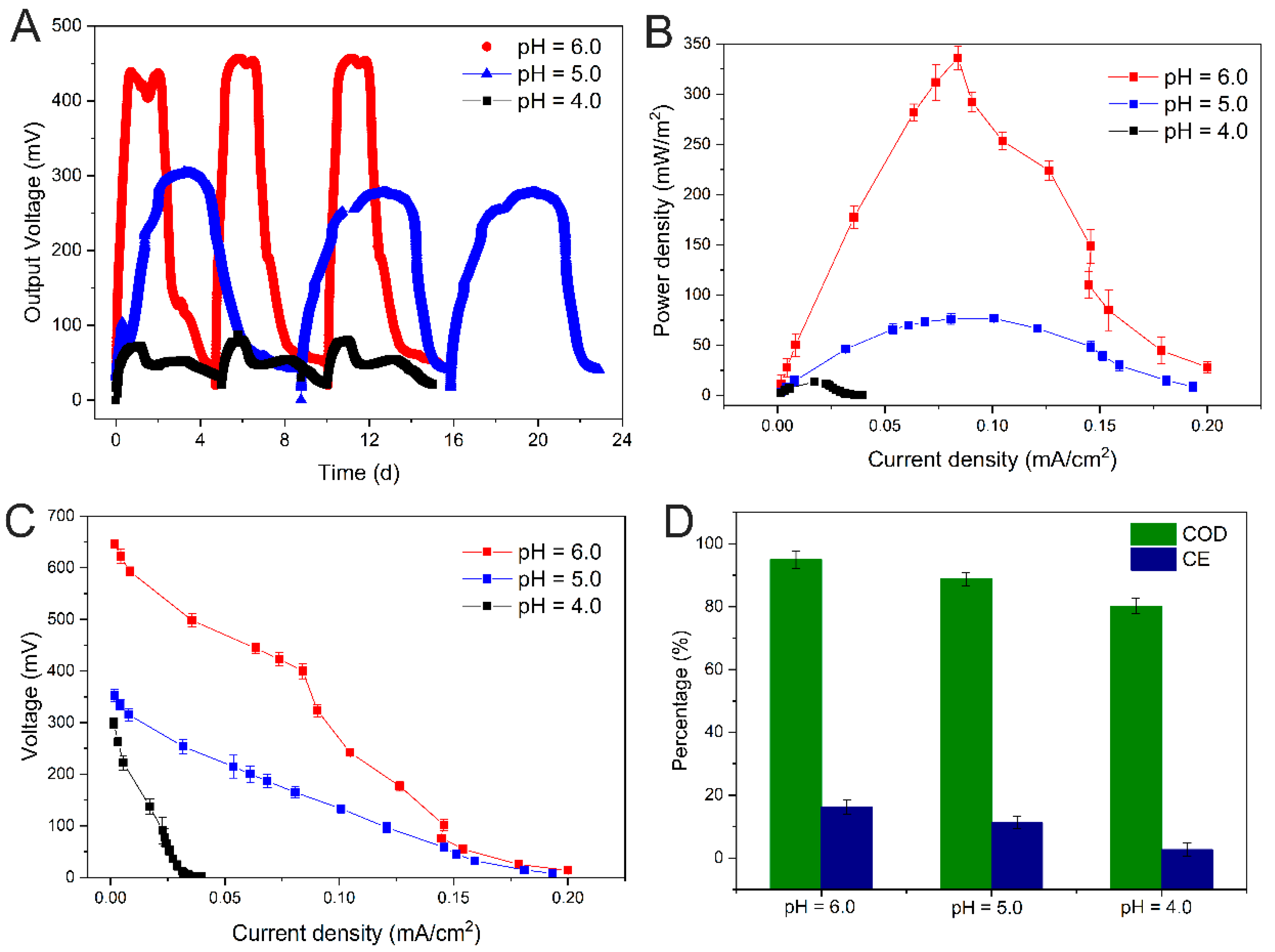
Different low pH phosphate media have different degrees of influence on the electrochemical characteristics of electroactive biofilms, such as maximum output voltage, power density, and CE (Figure 1). The electroactive biofilm stimulated with a pH 6.0 medium had a stable power production cycle of five days with a maximum output voltage of 456 mV and a maximum power density of 336.16 mW/m2, while the electroactive biofilm stimulated with a pH 5.0 medium had an extended power production cycle of eight days and a lower output voltage and power density of 305.29 mV and 76.66 mW/m2, respectively. Meanwhile, the electroactive biofilm stimulated with a pH 4.0 medium had the least voltage output and power density of 80.11 mV and 13.5 mW/m2, respectively. The electroactive biofilm produced in a pH 4.0 medium had a similar cycle time to the electroactive biofilm produced in a pH 6.0 medium, which was approximately five days, but its maximum output voltage was 82.43% lower than that of the latter and only 73.76% lower than that of the electroactive biofilm produced in pH 5.0 medium (Figure 1A,B). The polarization curves showed that the output voltage of the electrogenic MFCs at pH 6.0 was much higher than that of the MFCs at other conditions under different external resistance (Figure 1C), and on the contrary, the output voltage of the electrogenic MFCs at pH 4.0 was the lowest. This indicates that the higher the H+ content in the anode medium under acidic conditions, the greater the damage to the electroactive biofilm and the lower the ability to produce electricity. The chemical oxygen demand (COD) removal efficiencies of the MFCs at pH 6.0, 5.0, and 4.0 were 95.0 ± 2.8%, 88.8 ± 2.1%, and 80.2 ± 2.4%, respectively (Figure 1D). The results suggest that the increase in H+ content impeded electroactive biofilm activities, which subsequently decreased the COD removal efficiency. The CE of electroactive biofilms at pH 6.0, 5.0, and 4.0 were 16.19 ± 2.4%, 11.26 ± 1.9%, and 2.72 ± 2.1%, respectively (Figure 1D). The difference in CE between the pH 6.0 and 5.0 gradients was not significant, likely because as pH decreased, the metabolism of microorganisms was inhibited, and their growth rate slowed, resulting in a longer power production cycle; however, the consumption of organic matter did not decrease. All of the above indicates that the highest discharge efficiency of electroactive biofilms was achieved at pH 6.0 for electricity production in the medium.
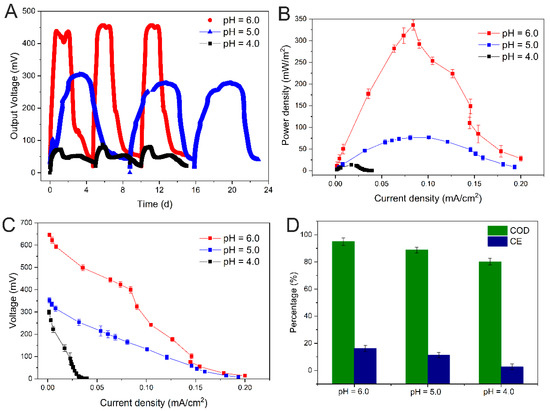
Figure 1.
Output voltage (A), power density (B), polarization curves (C), and COD removal efficiency and CE (D) of single-chamber MFCs under different pH stresses.
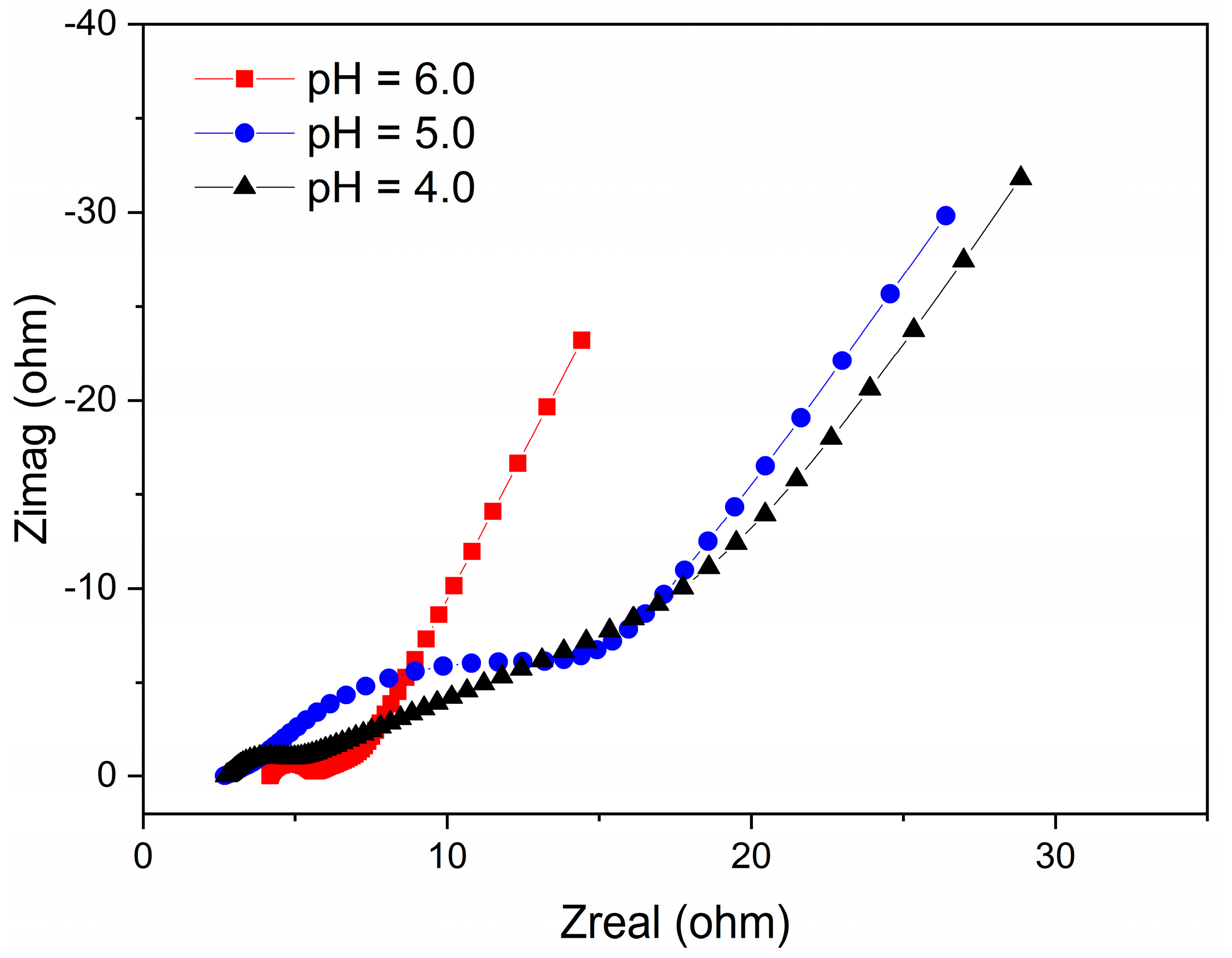
The total internal resistance of the electroactive biofilms was evaluated using EIS to better understand the influence of the different pH conditions on the biofilms. Of note, the total internal resistance is comprised of both the charge transfer resistance and ohmic resistance, which can be estimated from the semicircle and the intercept at high frequency, as stated previously [37]. From the Nyquist plots, the total internal resistance of the electroactive biofilms fed with pH 6.0, 5.0, and 4.0 media were 17 Ω, 24 Ω, and 21 Ω, respectively (Figure 2). This result shows that at high H+ content (pH 5.0 and 4.0), the total internal resistance of the MFCs increased, revealing that these pH conditions had harmful effects on the anodic biofilms, lowering microbial activity and hindering electron transport, consistent with the results in Figure 1D. Therefore, the differences in maximum voltage output, power density, and CE of the single-chamber MFCs operating at different low pH levels indicate that acidic pH has a significant effect on the microbial community structure of the electroactive biofilm enriched on the anode surface and affects the electron output capacity of the electricity-producing bacteria.
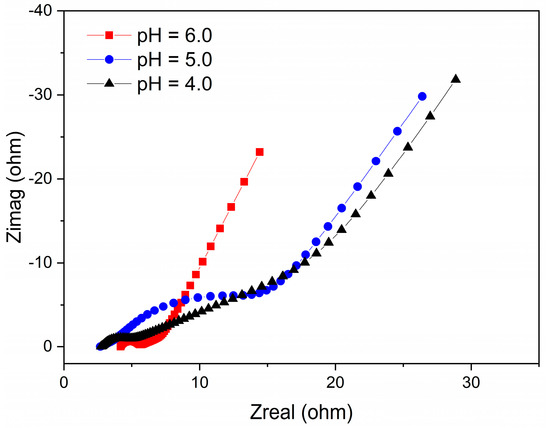
Figure 2.
EIS of MFCs under different pH conditions.
3.2. Observation of Electroactive Biofilm Morphology at Different Low pH
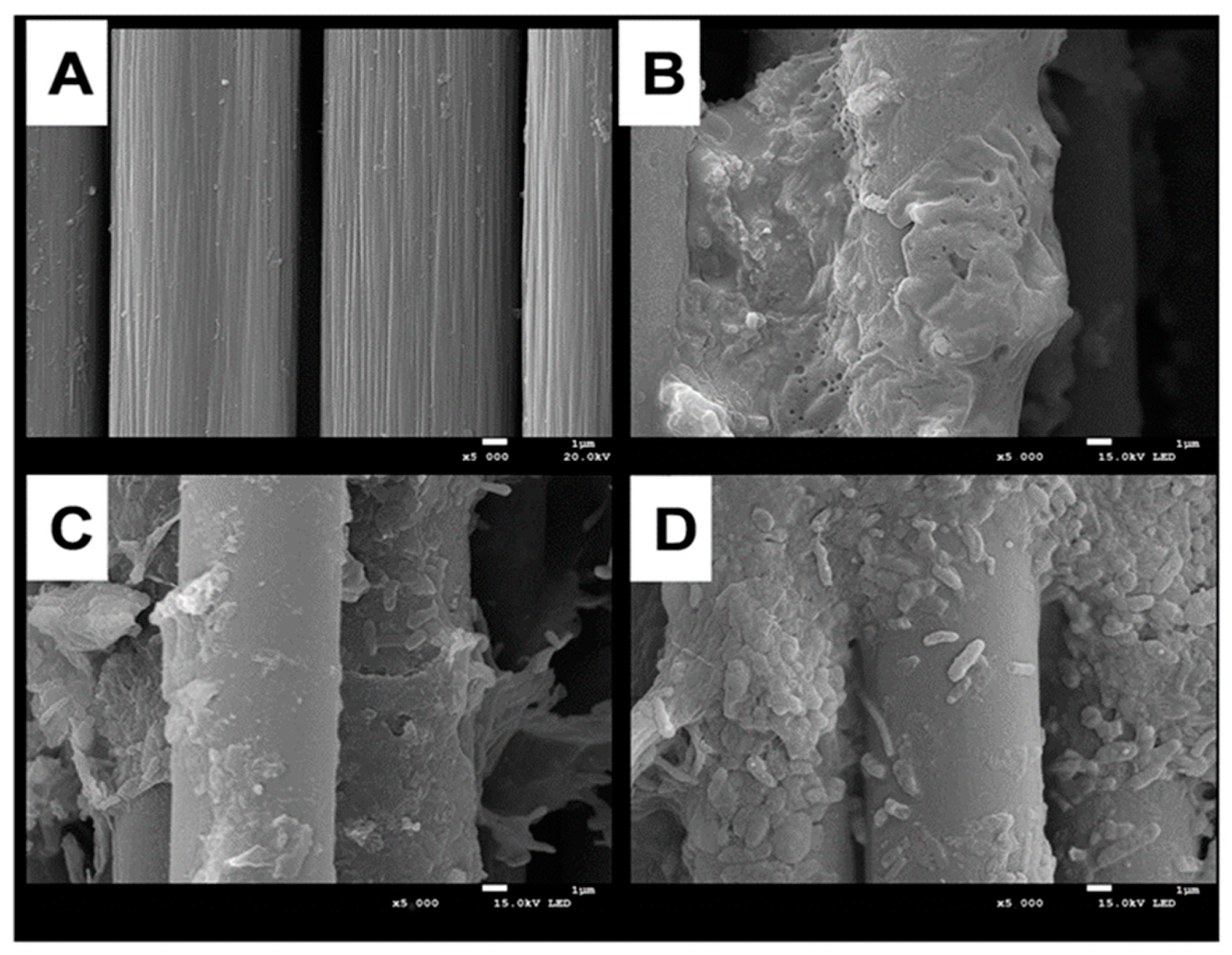
Previous studies on MFCs have shown that the bacterial species, abundance, and activity in the anode biofilm mainly affect the power generation performance of the MFCs [38]. Figure 3 shows SEM images of anode carbon brushes before system start-up and after stable operation of MFCs at different pH conditions. Before system operation, the anode carbon fibers were arranged in an orderly manner, and all surfaces were relatively clean and smooth without microorganisms attached (Figure 3A). The carbon fibers of the anode electrode grown at pH 6.0 were covered with electroactive biofilm, and microorganisms were closely attached to the electrode surface (Figure 3B), with a predominance of rod-shaped bacteria. It has been reported in the literature that some rod-shaped bacteria have hair-like structures (microbial nanowires), which can easily transfer the generated electrons from the internal cell membrane to the anode electrode [39]. The colonization of biofilms grown at pH 5.0 medium became loose and sparse, with a significant decrease in bacterial species and abundance (Figure 3C). The same phenomenon was more pronounced in the anodic electroactive biofilm at pH 4.0 (Figure 3D), which indicates that a high concentration of H+ decreases the bacterial species and abundance of the anodic electroactive biofilm microbial community, thus reducing its ability to produce electricity and affecting the power generation capacity of MFCs.
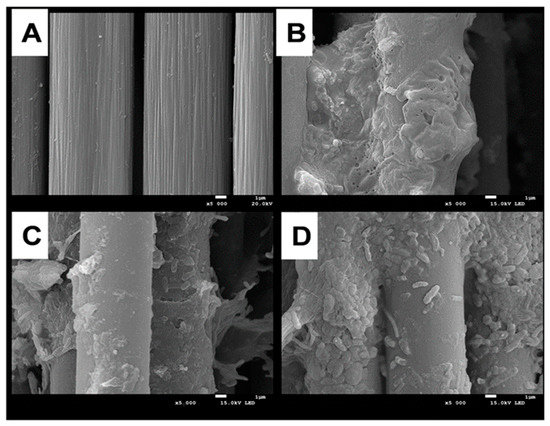
Figure 3.
Comparative SEM morphological analysis of the differences in the anodic electroactive biofilms of MFCs at different pH conditions. (A): sterile control; (B): pH = 6.0; (C): pH = 5.0; (D): pH = 4.0.
3.3. Comparative Analysis of Electroactive Biofilm Microbial Communities at Different Low pH
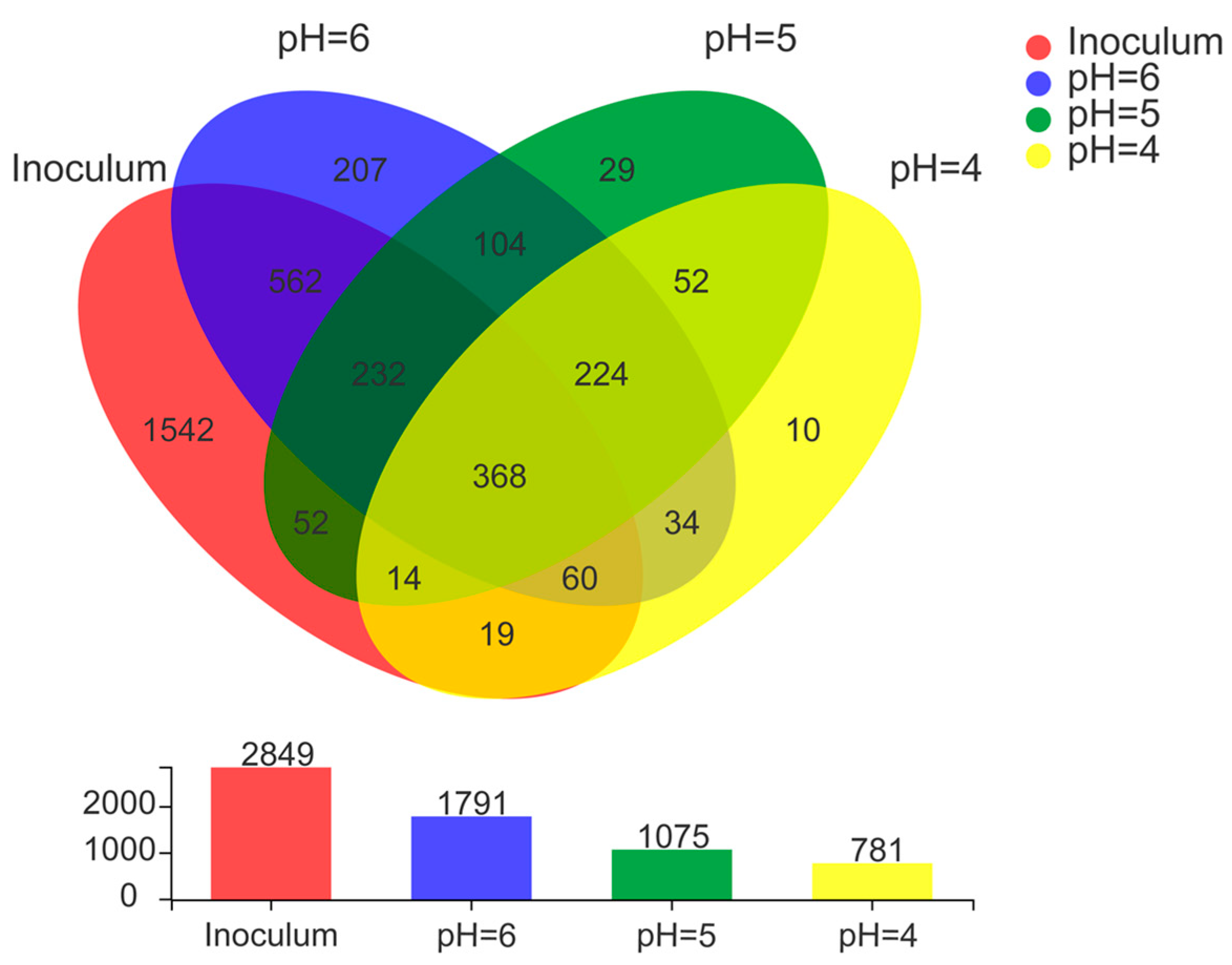
Microbial communities of anode electroactive biofilms directly affect the power generation performance of MFCs [40]. In this study, the microbial community structure of biofilm samples collected from the surface of anodic carbon brushes was analyzed by high-throughput sequencing technology to reveal the differences in microbial community diversity of electroactive biofilms grown under different low pH media. 2849 OTUs were detected in the inoculated anaerobic activated sludge (Figure 4). During the process of electroactive biofilm enrichment, microbial succession occurred at the OTU level, and when the MFCs reached a stable maximum output voltage, there were 1791 OTUs in the electroactive biofilm enriched in the lactic acid medium at pH 6.0, but the number of OTUs contained in the electroactive biofilm decreased as the pH of the medium decreased, and the electroactive biofilm working at pH 5.0 contained only 1075 OTUs. Based on the level of OTUs in each MFC, Venn diagrams were used to assess the variability and similarity of electroactive biofilms at different pH, comparing the number of shared OTUs and unique OTUs in the three MFCs systems, as shown in Figure 4. Hence, 207, 29, and 10 unique OTUs were identified in MFCs with pH 6.0, 5.0, and 4.0, respectively. Previous studies have shown that differences in the presence of specific OTUs lead to changes in the composition of bacterial communities with different treatment performances [41], and the present study, in agreement with previous studies, verified that different fractions of unique OTUs led to different electrical production capacities of the MFCs systems. A total of 592 unique OTUs were shared by these three MFCs systems. It is possible that the similarity stems from the adoption of similar environmental and growth conditions during domestication. As a result of shared OTUs, different MFCs had comparable microbial communities as well as the capacity to oxidize lactate and generate power. Thus, the combined effect of unique OTUs and shared OTUs composition resulted in different bacterial community diversity with the ability to oxidize organic matter and export electrons.
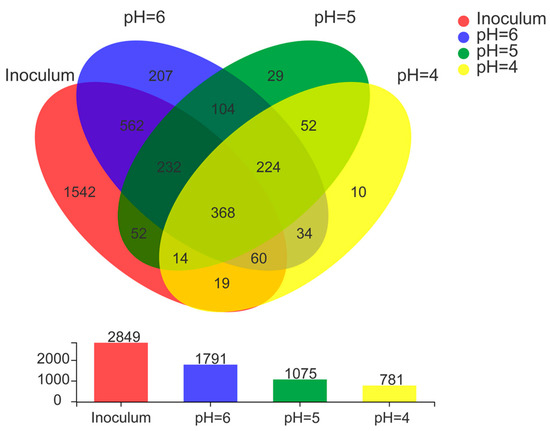
Figure 4.
Venn diagram analysis of OTU of electroactive biofilm at different pH.
Table 1 shows the results of assessing species richness and bacterial community diversity using an α-diversity index. The microbial abundance and diversity of the electroactive biofilm fed with a pH 4.0 medium were smaller than those with pH 5.0 and 6.0 media. These results show that the H+ concentration in the medium had a substantial effect on the microbial abundance and diversity of the electroactive biofilm. The microbial abundance and diversity of the electroactive biofilm gradually declined as the H+ concentration in the medium increased.

Table 1.
α-diversity analysis of electroactive biofilms enriched on carbon brushes.
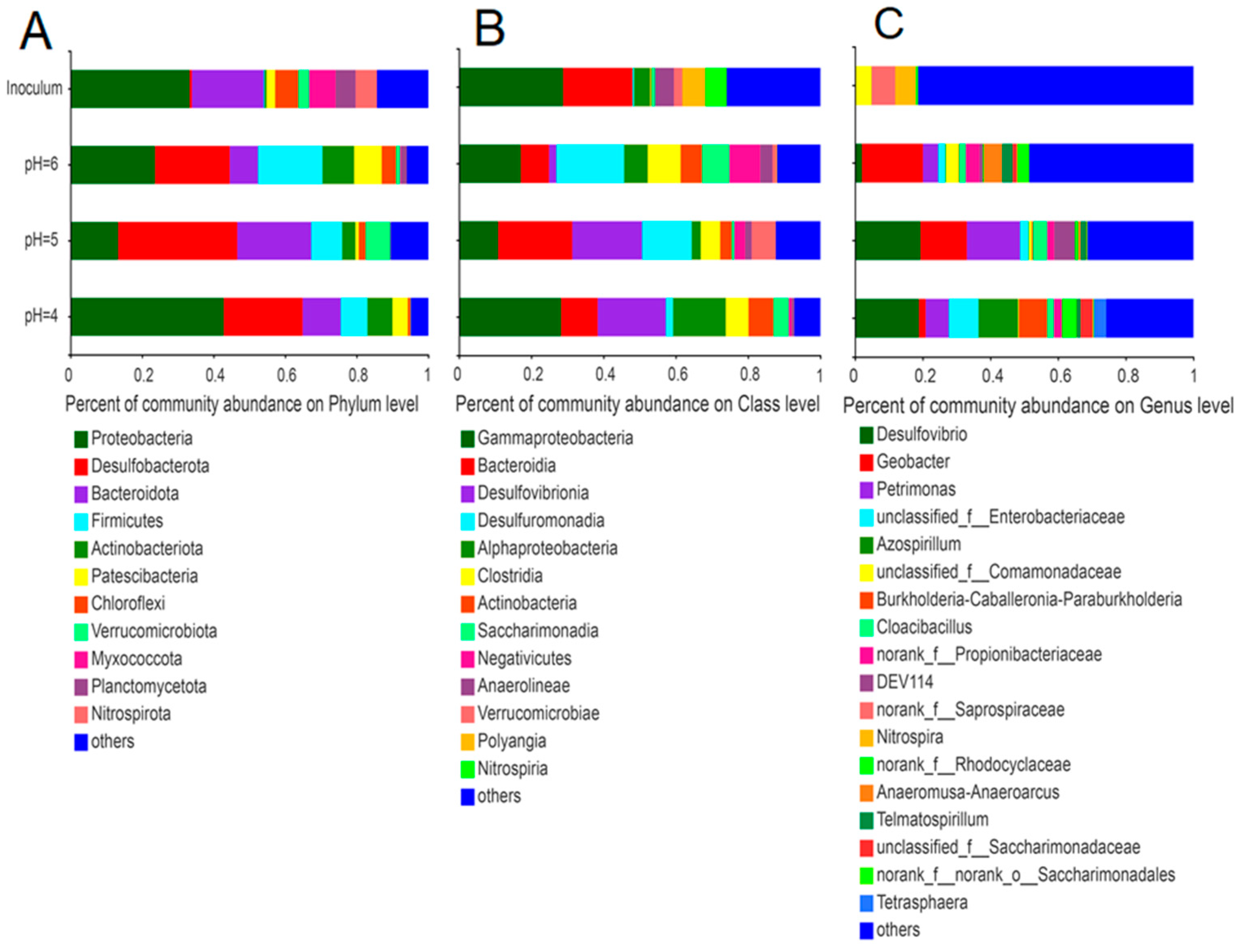
Similar to our previous study [42], the most prominent phyla in the initial inoculum (anaerobic sludge) were Proteobacteria, Bacteroidetes, and Myxococcota (Figure 5); the phyla Proteobacteria and Bacteroidetes remained dominant in the electroactive biofilm. Among these electroactive biofilms, Desulfobacterota and Firmicutes were enriched as one of the dominant phyla. At the same time, the total proportions of the phyla Proteobacteria, Bacteroidetes, Desulfobacterota, and Firmicutes in the electroactive biofilms increased as the pH of the medium decreased (Figure 5A).
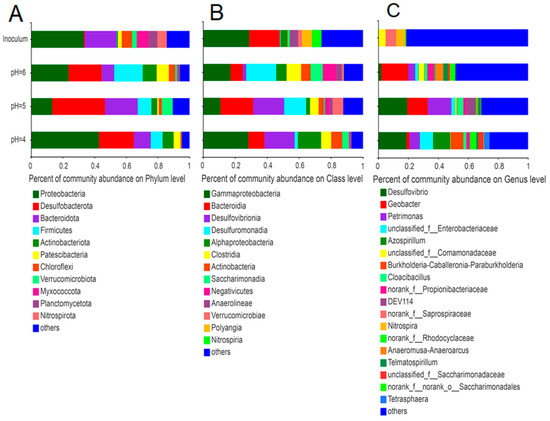
Figure 5.
Differences in microbial community structure at the phylum (A), order (B), and genus (C) levels for electroactive biofilms at different pH.
The microbial communities in each electroactive biofilm also differed significantly at the class level (Figure 5B). The dominant phyla in the anaerobic sludge were γ-proteobacteria (28.8%) and Bacteroidia (19.1%). Among the electroactive biofilms grown at pH 6.0, the percentages of both γ-proteobacteria and Bacteroidia decreased to 17.1% and 7.8%, respectively, while the percentages of Desulfuromonadia, Clostridia, and Negativicutes increased significantly to 18.8%, 9.1%, and 8.5%, respectively. Compared with the electroactive biofilms grown at pH 6.0, the percentage of γ-proteobacteria in the electroactive biofilms grown at pH 5.0 decreased to 10.8%, while the percentage of Desulfuromonadia increased to 20.4%. However, the most significant change was in Desulfovibrionia, which increased from 2.0% to 13.7% and became the subdominant class of this biofilm. The electroactive biofilms grown at pH 4.0 differed from the preceding two in that the proportion of γ-proteobacteria increased to 28.2%, Desulfuromonadia decreased to 10%, Desulfovibrionia remained at 18.9%, and α-proteobacteria increased to 14.6%, establishing a new subdominant class.
The microbial communities of the anodic electroactive biofilms at different pH differed significantly at the genus level (Figure 5C). The genus Geobacter (18.0%) was dominant in the electroactive biofilm grown at pH 6.0. Desulfovibrio (19.3%), Petrimonas (15.7%), and Geobacter (13.6%) were the dominant genera in the electroactive biofilms grown at pH 5.0. In contrast, the proportion of Petrimonas and Geobacter in electroactive biofilms at pH 4.0 decreased, while unclassified_f_Enterobacteriaceae (8.8%) and Azospirillum (11.7%) became the dominant genera. The abundance of the classic exoelectrogen Geobacter in the MFCs fed with pH 6.0 medium corresponds to the highest output voltage observed in these MFCs. Geobacter, a member of the Proteobacteria, is a well-known electrochemically active bacteria capable of transferring electrons from organic matter to extracellular electron acceptors [43]. Furthermore, Geobacter has several c-type cytochrome genes, some of which are needed for extracellular electron transfer and biofilm electroactivity [44]. They also participate in direct interspecies electron transfer and provide electrons to other species through their electrically conductive pili [45]. The decline in Geobacter in the electroactive biofilms formed at pH 4.0 and 5.0 correlated with the decrease in voltage output in these MFCs. In previous studies, a decrease in the relative abundance of Geobacter was associated with a reduction in the voltage output of MFCs [37,46]. According to a related study, Geobacter can use small organic molecules (e.g., acetate, malate, and oxalate) and hydrogen to provide electrons, while it has DNA repair genes that can rapidly repair DNA damage in a low pH anodic environment [47], which explains why the electro conversion efficiency of electroactive biofilms operating at pH 5.0 was not significantly affected.
3.4. Effect of Different Low pH on the Ability of Electroactive Biofilm to Treat AMD
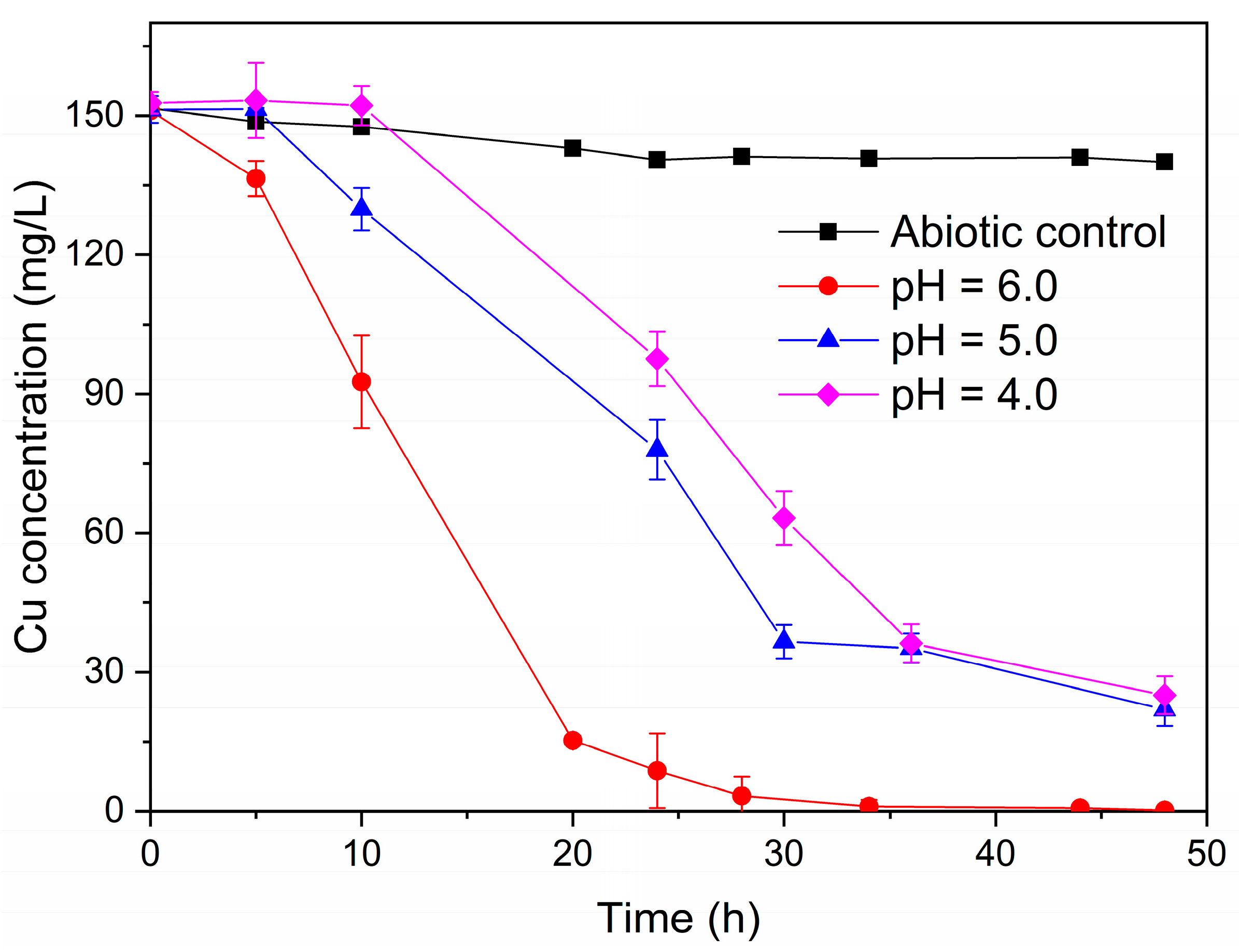
The single-chamber MFCs were dismantled to construct dual-chamber MFCs in order to investigate the effect of the various acidic pH (4.0, 5.0, and 6.0) on the ability of the electroactive biofilms to treat simulated AMD. Herein, anodes with electroactive biofilm enriched in the single-chamber MFCs with varying acidic pH were used as anodes in dual-chamber MFCs. The dual-chamber MFCs with electroactive biofilm in different low pH environments in the anode chamber were able to recover copper metal from acid mine wastewater. As shown in Figure 6, the concentration of Cu2+ in the cathode solution of the MFCs with electroactive biofilm at pH 6.0 decreased rapidly at the beginning of the reaction, and no detectable Cu2+ was found in the cathode solution at 36 h. However, there was a delay in the reduction of Cu2+ by the MFCs of the electroactive biofilm at pH 5.0 and 4.0, with Cu2+ beginning to gain electrons and being reduced at 5 h and 10 h, respectively. This may be because the electroactive biofilm is less capable of producing electricity at low pH, and it takes some time to reach the standard redox potential of Cu2+. Meanwhile, at 48 h, 86.2% of Cu2+ was removed from the cathode solution of the MFCs with an anode pH of 5.0, whereas the removal rate of Cu2+ from the cathode solution of the MFCs with an anode pH of 4.0 was even lower (84.2%).
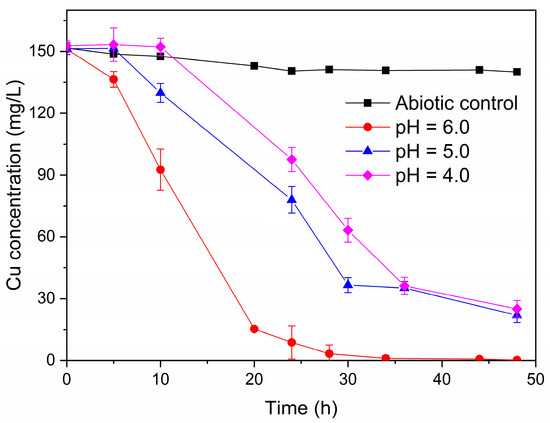
Figure 6.
Cu2+ concentration variation curve in the cathode chamber of dual-chamber MFCs under different anolyte pH conditions.
The color of the cathode carbon cloth was significantly different after MFCs treatment of the AMD with electroactive biofilms in different low pH environments (Figure 7). After treating the simulated AMD with dual-chamber MFCs operating at anodic pH 6.0, the cathodic carbon cloth changed from black to brown, indicating that Cu elements were likely heavily deposited on the cathode surface. Meanwhile, as the pH of the anode environment decreased, the color of the cathodic carbon cloth lightened, and the deposition of Cu elements decreased gradually. The cathodic surface of the MFCs with an anolyte pH of 4.0 was partially covered with a layer of a light green substance.

Figure 7.
Cathode color after simulated AMD treatment with dual-chamber MFCs with electroactive biofilm at different pH (1: abiotic, 2: pH = 6.0, 3: pH = 5.0, and 4: pH = 4.0).
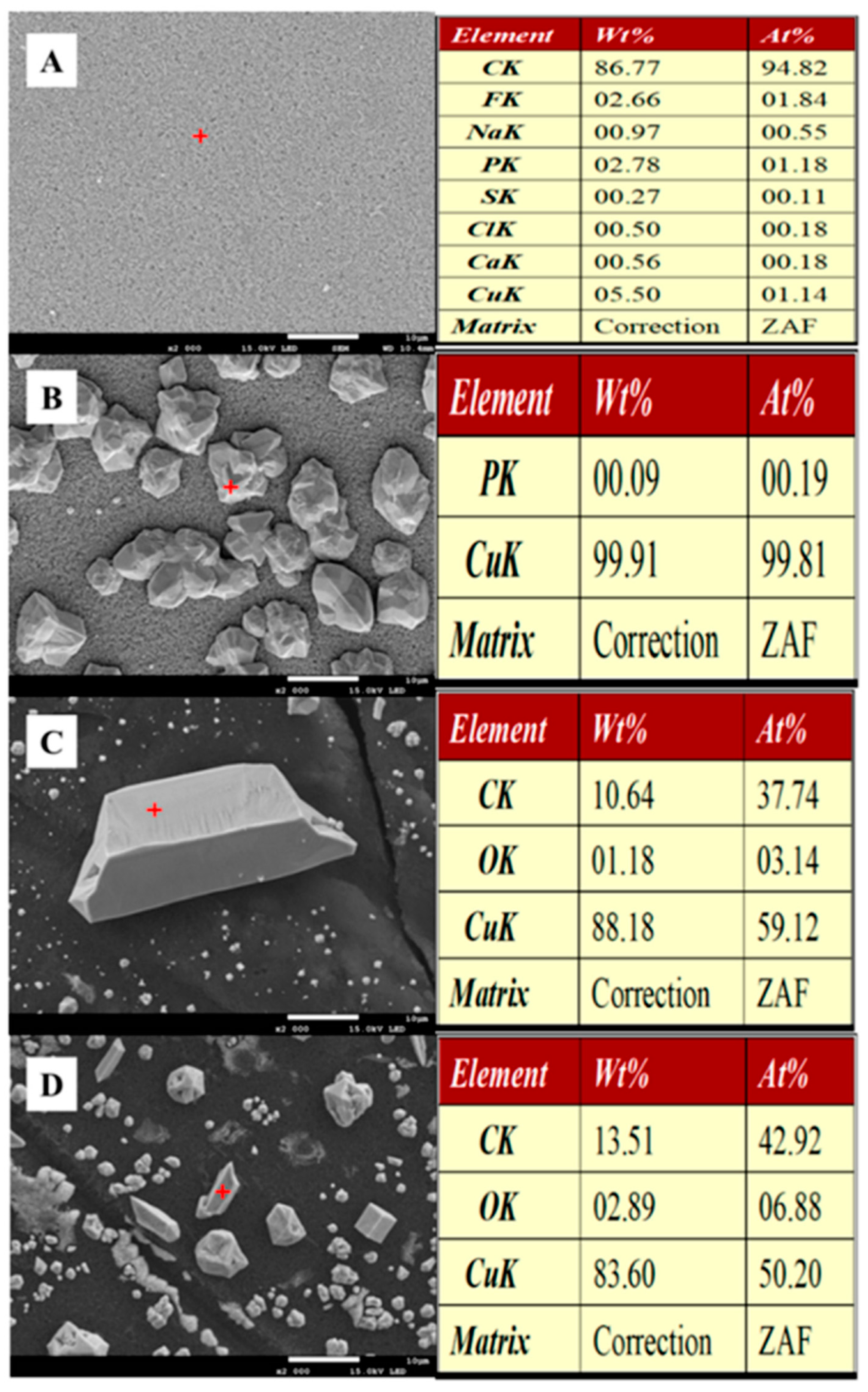
To better understand the recovery quality of copper, the surface material of the carbon cloth of the dual-chambered MFCs cathodes after 48 h of AMD treatment was analyzed using SEM and XRD. SEM micrographs of the cathode surfaces of these MFCs were similar in structure and morphology (Figure 8). Compared to the cathodic carbon cloth surface of the non-biological MFCs (Figure 8A), the cathodic carbon cloth surface of the MFCs with an anode ambient pH of 6.0 was covered with irregular crystal particles (Figure 8B), and the elemental analysis showed that the crystals were aggregates of copper elements, presumably copper monomers. The same irregular crystals were also found on the surface of the MFCs cathode carbon cloth at pH 5.0 in the anode environment (Figure 8C), but the number of crystals was significantly reduced, and the elemental analysis showed not only the aggregation of copper but also the aggregation of carbon and oxygen elements. Presumably, the crystals contained not only copper monomers but also copper oxides. With the anode environment of pH 4.0, fewer crystals were deposited on the carbon cloth surface of the MFCs cathode, and the content of carbon and oxygen elements was higher, presumably with a higher percentage of copper oxides (Figure 8D).
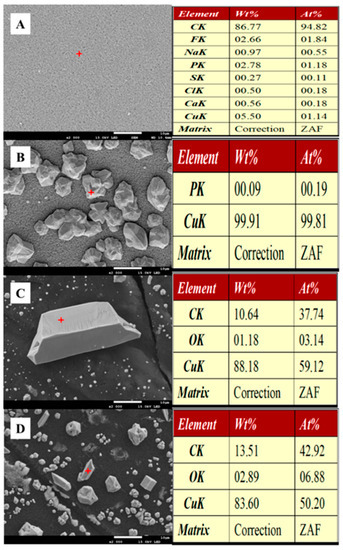
Figure 8.
SEM morphology and constituent element analysis of the cathode of MFCs after simulated AMD treatment. (A): abiotic; (B): pH = 6.0; (C): pH = 5.0; (D): pH = 4.0.
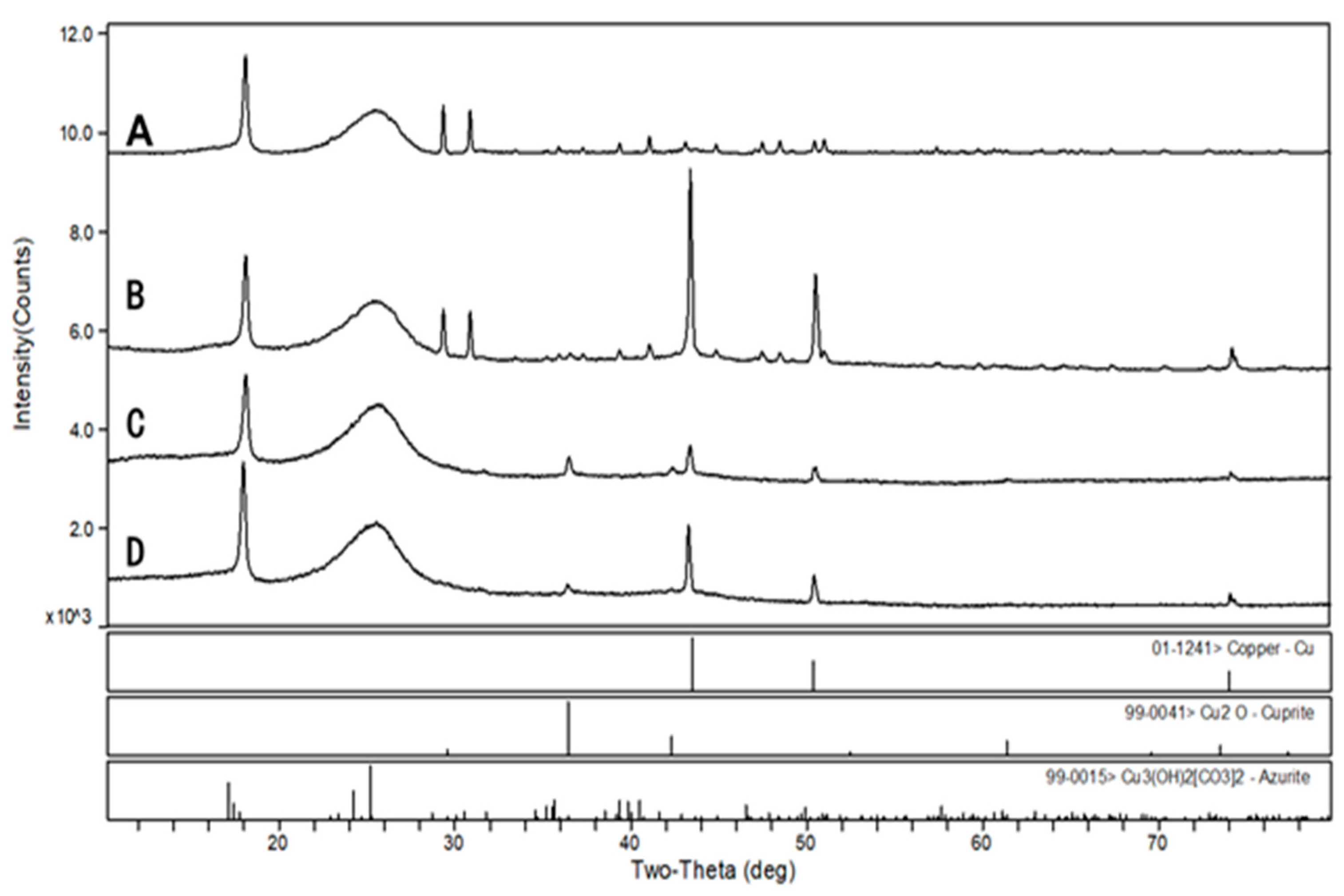
XRD analysis showed that the metallic copper (Cu0) on the cathodic surface of the three MFCs at a low anodic pH environment had characteristic peaks at 2θ with 43.3, 50.4, and 74.1 degrees (Figure 9), and the largest peak area was observed at 6.0 anodic pH environment. The characteristic peaks of Cu2O [48] and trace amounts of Cu3(OH)2[CO3]2 [49] were also detected in the MFCs with an anode pH of 5.0 and 4.0, which explains the green layer attached to the cathode carbon cloth. Therefore, when treating organic wastewater and AMD simultaneously with the MFCs, the pH of the electroactive biofilm working environment should be controlled to ensure the treatment efficiency of this system.
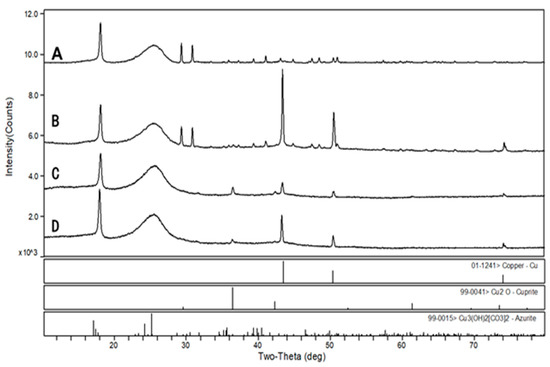
Figure 9.
Physical phase analysis of MFCs cathode surface deposits after treatment of simulated AMD. (A): abiotic, (B): pH = 6.0, (C): pH = 5.0, and (D): pH = 4.0.
3.5. Comparison of This Work with Previous Research
The pH of the anodic chamber is a significant factor that can affect both optimal microbial growth and substrate metabolic activity, thereby influencing electron and proton generation mechanisms. Consequently, few studies have examined the effects of different anodic pH on the electrochemical performance of MFCs. In comparing our study with these previous studies, we found the following differences: (i) The microbial community structure of the anodic electroactive biofilms enriched with different pH conditions was barely studied in most previous research. (ii) The effects of the varied pH were investigated on pure culture in other studies. (iii) The impact of alkaline pH conditions on electroactive biofilms was only investigated in some studies. (iv) Other studies analyzed the anodic electroactive biofilms with traditional culture-dependent techniques or denaturing gradient gel electrophoresis (DGGE); (v). Furthermore, to our knowledge, no research has been published on the effect of different acidic pH on the ability of electroactive biofilms to act as biocatalysts in the treatment of AMD.
MFCs have been employed in heavy metal recovery from wastewater. To be consistent with the target heavy metal in our study, we selected and compared research works that focused on the treatment of Cu2+-contaminated wastewater (Table 2). In contrast to our research, these earlier studies only utilized a single anolyte pH condition for the treatment of heavy metal-contaminated wastewater. Taken altogether, the results of this study will provide researchers with valuable information regarding the optimal pH for resource recovery with MFCs.

Table 2.
Comparison of this work with other related studies.
4. Conclusions and Future Perspectives
The results of this study revealed that electroactive biofilms of the genus Geobacter, a typical electro-producing microorganism enriched with sodium lactate as an energy substrate, showed significant differences in maximum output voltage, power density, CE, and total internal resistance when operating at different low pH environments. The bacterial species and abundance of the microbial community of the electroactive biofilm decreased when the environmental acidity was continuously reduced. Although the genus Geobacter has the function of rapid repair of DNA damage in low pH anodic environment, which can buffer the stress of low pH on electroactive biofilms for a period of time, electroactive biofilms still cannot be exposed to a low pH environment for a long time. In addition, the effect of different acidic pH on the ability of electroactive biofilms to act as biocatalysts in the treatment of AMD was investigated. The results showed that the dual-chamber MFCs could recover Cu from simulated AMD at low anode pH, but the removal rate of Cu2+ decreased with decreasing anode pH, and the quality of Cu deposited on the cathode carbon cloth decreased with the presence of trace amounts of Cu2O and Cu3(OH)2[CO3]2. For the practical application of MFCs, future research should focus on the treatment of various types of real wastewater, taking anolyte pH control into consideration. Furthermore, large-scale MFCs for onsite use should be developed, and a wider range of actual wastewater pH should be investigated to determine the best pH condition for real-world application.
Author Contributions
Conceptualization, W.Z.; methodology, J.J. and C.A.; software, R.A.; validation, C.A., X.Z. and R.A.; formal analysis, J.J. and C.A.; investigation, J.J., C.A. and X.Z; resources, W.Z.; data curation, J.J. and C.A.; writing—original draft preparation, J.J.; writing—review and editing, C.A. and R.A.; visualization, X.Z.; supervision, W.Z.; project administration, G.Q. and W.Z; funding acquisition, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 51934009, No. 52074353), the National Key Research and Development Program of China (No. 2019YFC1803600), and the Natural Science Foundation of Hunan Province (No. 2021JJ30855).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Skousen, J.G.; Sexstone, A.; Ziemkiewicz, P.F. Acid mine drainage control and treatment. In Reclamation of Drastically Disturbed Lands American Society of Agronomy and American Society for Surface Mining and Reclamation; Agronomy No. 41; Barnhisel, R.I., Darmody, R.G., Daniels, W.L., Eds.; American Society of Agronomy: Madison, WI, USA, 2000. [Google Scholar]
- Zhang, X.; Tang, S.; Wang, M.; Sun, W.; Xie, Y.; Peng, H.; Zhong, A.; Liu, H.; Zhang, X.; Yu, H.; et al. Acid mine drainage affects the diversity and metal resistance gene profile of sediment bacterial community along a river. Chemosphere 2019, 217, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Bergerson, J.; Lave, L. Life Cycle Analysis of Power Generation Systems. In Encyclopedia of Energy; Cleveland, C.J., Ed.; Elsevier: New York, NY, USA, 2004; pp. 635–645. [Google Scholar]
- Yang, M.; Lu, C.; Quan, X.; Cao, D. Mechanism of Acid Mine Drainage Remediation with Steel Slag: A Review. ACS Omega 2021, 6, 30205–30213. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Galán, M.; Baena-Moreno, F.M.; Vázquez, S.; Arroyo-Torralvo, F.; Vilches, L.F.; Zhang, Z. Remediation of acid mine drainage. Environ. Chem. Lett. 2019, 17, 1529–1538. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- RoyChowdhury, A.; Sarkar, D.; Datta, R. Remediation of Acid Mine Drainage-Impacted Water. Curr. Pollut. Rep. 2015, 1, 131–141. [Google Scholar] [CrossRef]
- Jing, Q.; Zhang, M.; Liu, X.; Li, Y.; Wang, Z.; Wen, J. Bench-scale microbial remediation of the model acid mine drainage: Effects of nutrients and microbes on the source bioremediation. Int. Biodeterior. Biodegrad. 2018, 128, 117–121. [Google Scholar] [CrossRef]
- Moodley, I.; Sheridan, C.; Kappelmeyer, U.; Akcil, A. Environmentally sustainable acid mine drainage remediation: Research developments with a focus on waste/by-products. Miner. Eng. 2018, 126, 207–220. [Google Scholar] [CrossRef]
- El-Azim, H.A.; Seleman, M.M.E.-S.; Saad, E.M. Applicability of water-spray electric arc furnace steel slag for removal of Cd and Mn ions from aqueous solutions and industrial wastewaters. J. Environ. Chem. Eng. 2019, 7, 102915. [Google Scholar] [CrossRef]
- Xu, L.; Yu, W.; Graham, N.; Zhao, Y.; Qu, J. Application of Integrated Bioelectrochemical-Wetland Systems for Future Sustainable Wastewater Treatment. Environ. Sci. Technol. 2019, 53, 1741–1743. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Umar, K.; Parveen, T.; Ahmad, A.; Lokhat, D.; Setapar, S.H.M. A glimpse into the microbial fuel cells for wastewater treatment with energy generation. Desalination Water Treat. 2021, 214, 379–389. [Google Scholar] [CrossRef]
- Peng, X.; Tang, T.; Zhu, X.; Jia, G.; Ding, Y.; Chen, Y.; Yang, Y.; Tang, W. Remediation of acid mine drainage using microbial fuel cell based on sludge anaerobic fermentation. Environ. Technol. 2017, 38, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.N.M.; Ahmad, A.; Yaqoob, A.A.; Ibrahim, M.N.M. Application of rotten rice as a substrate for bacterial species to generate energy and the removal of toxic metals from wastewater through microbial fuel cells. Environ. Sci. Pollut. Res. 2021, 28, 62816–62827. [Google Scholar] [CrossRef]
- Lim, K.; Wong, C.; Wong, W.; Loh, K.; Selambakkannu, S.; Othman, N.; Yang, H. Radiation-Grafted Anion-Exchange Membrane for Fuel Cell and Electrolyzer Applications: A Mini Review. Membranes 2021, 11, 397. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Yaakop, A.S.; Ahmad, A. Application of microbial fuel cells energized by oil palm trunk sap (OPTS) to remove the toxic metal from synthetic wastewater with generation of electricity. Appl. Nanosci. 2021, 11, 1949–1961. [Google Scholar] [CrossRef]
- Bagchi, S.; Behera, M. Evaluation of the effect of anolyte recirculation and anolyte pH on the performance of a microbial fuel cell employing ceramic separator. Process Biochem. 2021, 102, 207–212. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Khatoon, A.; Mohd Setapar, S.H.; Umar, K.; Parveen, T.; Mohamad Ibrahim, M.N.; Ahmad, A.; Rafatullah, M. Outlook on the Role of Microbial Fuel Cells in Remediation of Environmental Pollutants with Electricity Generation. Catalysts 2020, 10, 819. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Di, J.; Jiang, Y.; Wang, M.; Dong, Y. Preparation of biologically activated lignite immobilized SRB particles and their AMD treatment characteristics. Sci. Rep. 2022, 12, 3964. [Google Scholar] [CrossRef]
- Margaria, V.; Tommasi, T.; Pentassuglia, S.; Agostino, V.; Sacco, A.; Armato, C.; Chiodoni, A.; Schilirò, T.; Quaglio, M. Effects of pH variations on anodic marine consortia in a dual chamber microbial fuel cell. Int. J. Hydrog. Energy 2017, 42, 1820–1829. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, B.; Zhou, S.; Zhong, S.; Zhuang, L. Electrocatalytic activity of anodic biofilm responses to pH changes in microbial fuel cells. Bioresour. Technol. 2011, 102, 6887–6891. [Google Scholar] [CrossRef] [PubMed]
- Amanze, C.; Zheng, X.; Man, M.; Yu, Z.; Ai, C.; Wu, X.; Xiao, S.; Xia, M.; Yu, R.; Wu, X.; et al. Recovery of heavy metals from industrial wastewater using bioelectrochemical system inoculated with novel Castellaniella species. Environ. Res. 2022, 205, 112467. [Google Scholar] [CrossRef]
- Ai, C.; Hou, S.; Yan, Z.; Zheng, X.; Amanze, C.; Chai, L.; Qiu, G.; Zeng, W. Recovery of Metals from Acid Mine Drainage by Bioelectrochemical System Inoculated with a Novel Exoelectrogen, Pseudomonas sp. E8. Microorganisms 2020, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Yan, Z.; Hou, S.; Huo, Q.; Chai, L.; Qiu, G.; Zeng, W. Sequentially recover heavy metals from smelting wastewater using bioelectrochemical system coupled with thermoelectric generators. Ecotoxicol. Environ. Saf. 2020, 205, 111174. [Google Scholar] [CrossRef] [PubMed]
- Niessen, J.; Schröder, U.; Scholz, F. Exploiting complex carbohydrates for microbial electricity generation—A bacterial fuel cell operating on starch. Electrochem. Commun. 2004, 6, 955–958. [Google Scholar] [CrossRef]
- Rabaey, K.; Boon, N.; Siciliano, S.D.; Verhaege, M.; Verstraete, W. Biofuel Cells Select for Microbial Consortia That Self-Mediate Electron Transfer. Appl. Environ. Microbiol. 2004, 70, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Devasahayam, M.; Masih, S.A. Microbial fuel cells demonstrate high coulombic efficiency applicable for water remediation. Indian J. Exp. Biol. 2012, 50, 430–438. [Google Scholar] [PubMed]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2016, 1, e00009-15. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Gevers, D.; Westcott, S.L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE 2011, 6, e27310. [Google Scholar] [CrossRef] [PubMed]
- Amanze, C.; Zheng, X.; Anaman, R.; Wu, X.; Fosua, B.A.; Xiao, S.; Xia, M.; Ai, C.; Yu, R.; Wu, X.; et al. Effect of nickel (II) on the performance of anodic electroactive biofilms in bioelectrochemical systems. Water Res. 2022, 222, 118889. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Aulenta, F.; Schröder, U.; Harnisch, F. 6.43—Microbial Electrochemical Technologies: Industrial and Environmental Biotechnologies Based on Interactions of Microorganisms With Electrodes. In Comprehensive Biotechnology, 3rd. ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2016; pp. 545–563. [Google Scholar]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous Electricity Generation at High Voltages and Currents Using Stacked Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Xie, B.; Deng, S.; Fan, Y.; Tang, X.; Liang, H. Enhancement of anaerobic digestion effluent treatment by microalgae immobilization: Characterized by fluorescence excitation-emission matrix coupled with parallel factor analysis in the photobioreactor. Sci. Total Environ. 2019, 678, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Yan, Z.; Hou, S.; Zheng, X.; Zeng, Z.; Amanze, C.; Dai, Z.; Chai, L.; Qiu, G.; Zeng, W. Effective Treatment of Acid Mine Drainage with Microbial Fuel Cells: An Emphasis on Typical Energy Substrates. Minerals 2020, 10, 443. [Google Scholar] [CrossRef]
- Kouzuma, A.; Ishii, S.I.; Watanabe, K. Metagenomic insights into the ecology and physiology of microbes in bioelectrochemical systems. Bioresour. Technol. 2018, 255, 302–307. [Google Scholar] [CrossRef]
- Steidl, R.J.; Lampa-Pastirk, S.; Reguera, G. Mechanistic stratification in electroactive biofilms of Geobacter sulfurreducens mediated by pilus nanowires. Nat. Commun. 2016, 7, 12217. [Google Scholar] [PubMed]
- Zheng, S.; Li, M.; Liu, Y.; Liu, F. Desulfovibrio feeding Methanobacterium with electrons in conductive methanogenic aggregates from coastal zones. Water Res. 2021, 202, 117490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, G.; Wen, J.; Xu, Y.; Sun, J.; Ning, X.-A.; Lu, X.; Wang, Y.; Yang, Z.; Yuan, Y. Electrochemical and microbial community responses of electrochemically active biofilms to copper ions in bioelectrochemical systems. Chemosphere 2018, 196, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wan, X.; Liu, W.; Xia, X.; Huang, F.; Wang, A.; Smith, J.A.; Dang, Y.; Holmes, D.E. Characterization of the genome from Geobacter anodireducens, a strain with enhanced current production in bioelectrochemical systems. RSC Adv. 2019, 9, 25890–25899. [Google Scholar]
- Zhu, H.; Zhang, J.; Li, C.; Pan, F.; Wang, T.; Huang, B. Cu2O thin films deposited by reactive direct current magnetron sputtering. Thin Solid Films 2009, 517, 5700–5704. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Feng, Q.; Wen, S.; Zhou, Y.; Nie, W.; Liu, J. Identification of sulfidization products formed on azurite surfaces and its correlations with xanthate adsorption and flotation. Appl. Surf. Sci. 2020, 511, 145594. [Google Scholar] [CrossRef]
- Lusk, B.G.; Parameswaran, P.; Popat, S.C.; Rittmann, B.E.; Torres, C.I. The effect of pH and buffer concentration on anode biofilms of Thermincola ferriacetica. Bioelectrochemistry 2016, 112, 47–52. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Luo, H.; Liu, G.; Torres, C.I.; Zhang, R. Effect of pH on bacterial distributions within cathodic biofilm of the microbial fuel cell with maltodextrin as the substrate. Chemosphere 2021, 265, 129088. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Ding, L.; Xu, K.; Ren, H. Influences of initial pH on performance and anodic microbes of fed-batch microbial fuel cells. J. Chem. Technol. Biotechnol. 2011, 86, 1226–1232. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhou, S.; Li, Y.; Yuan, Y. Enhanced performance of air-cathode two-chamber microbial fuel cells with high-pH anode and low-pH cathode. Bioresour. Technol. 2010, 101, 3514–3519. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, J.; Li, X.; Yang, N.; Wang, X. Enhanced bioelectricity generation of air-cathode buffer-free microbial fuel cells through short-term anolyte pH adjustment. Bioelectrochemistry 2018, 120, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Modin, O.; Wang, X.; Wu, X.; Rauch, S.; Fedje, K.K. Bioelectrochemical recovery of Cu, Pb, Cd, and Zn from dilute solutions. J. Hazard. Mater. 2012, 235–236, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Miran, W.; Jang, J.; Nawaz, M.; Shahzad, A.; Jeong, S.E.; Jeon, C.O.; Lee, D.S. Mixed sulfate-reducing bacteria-enriched microbial fuel cells for the treatment of wastewater containing copper. Chemosphere 2017, 189, 134–142. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).