Sequestration of Oxyanions of V(V), Mo(VI), and W(VI) Enhanced through Enzymatic Formation of Fungal Manganese Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sequestration of Oxyanions during the Cultivation of A. strictum KR21-2
2.2. Sequestration of Oxyanions by Newly Formed BMOs with and without Exogenous Mn2+
2.3. XRD Measurements of BMOs after the Sequestration Experiments
3. Results and Discussion
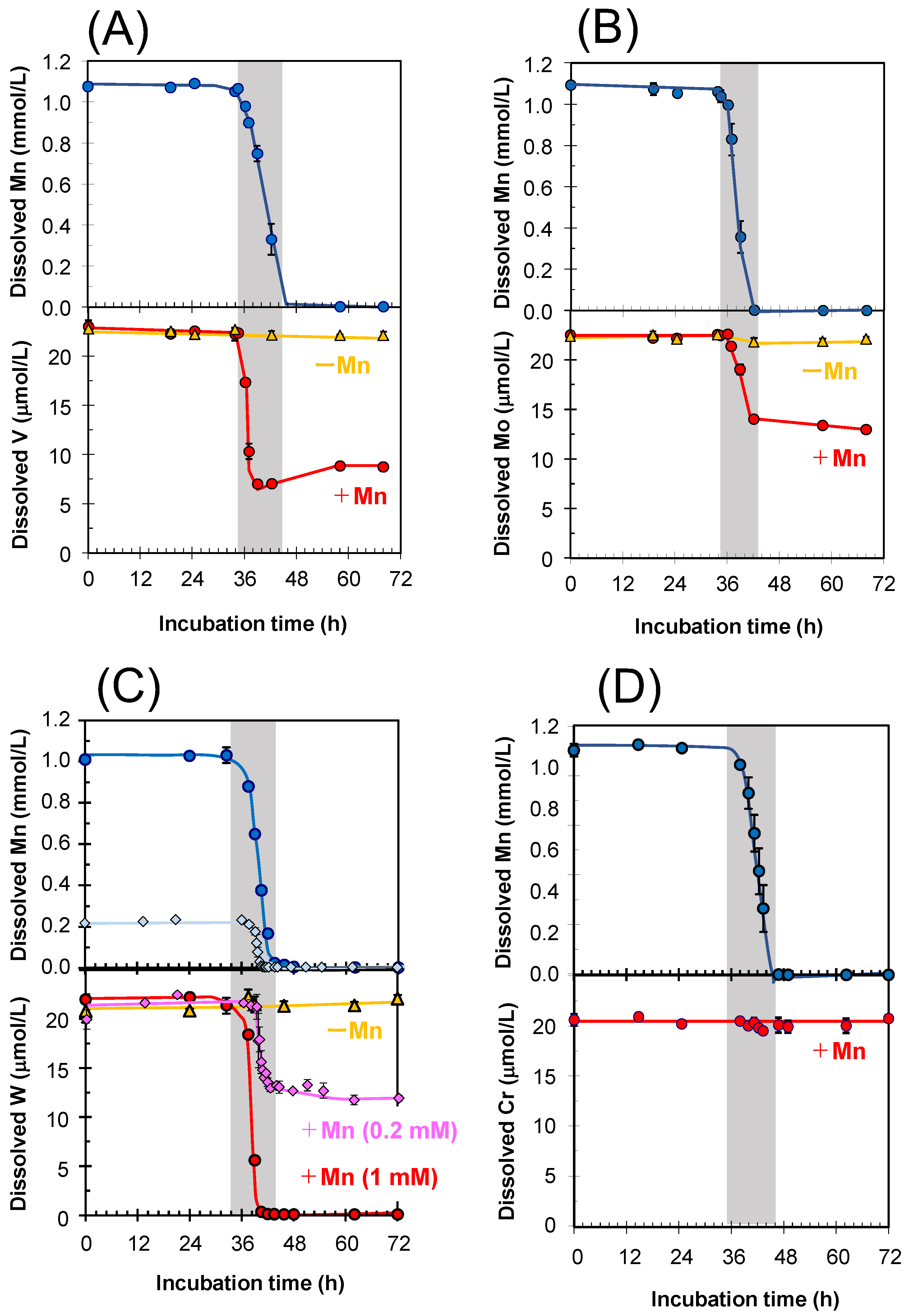
3.1. Sequestration of Oxyanions Concurrent with BMO Formation during the Incubation of A. strictum KR21-2
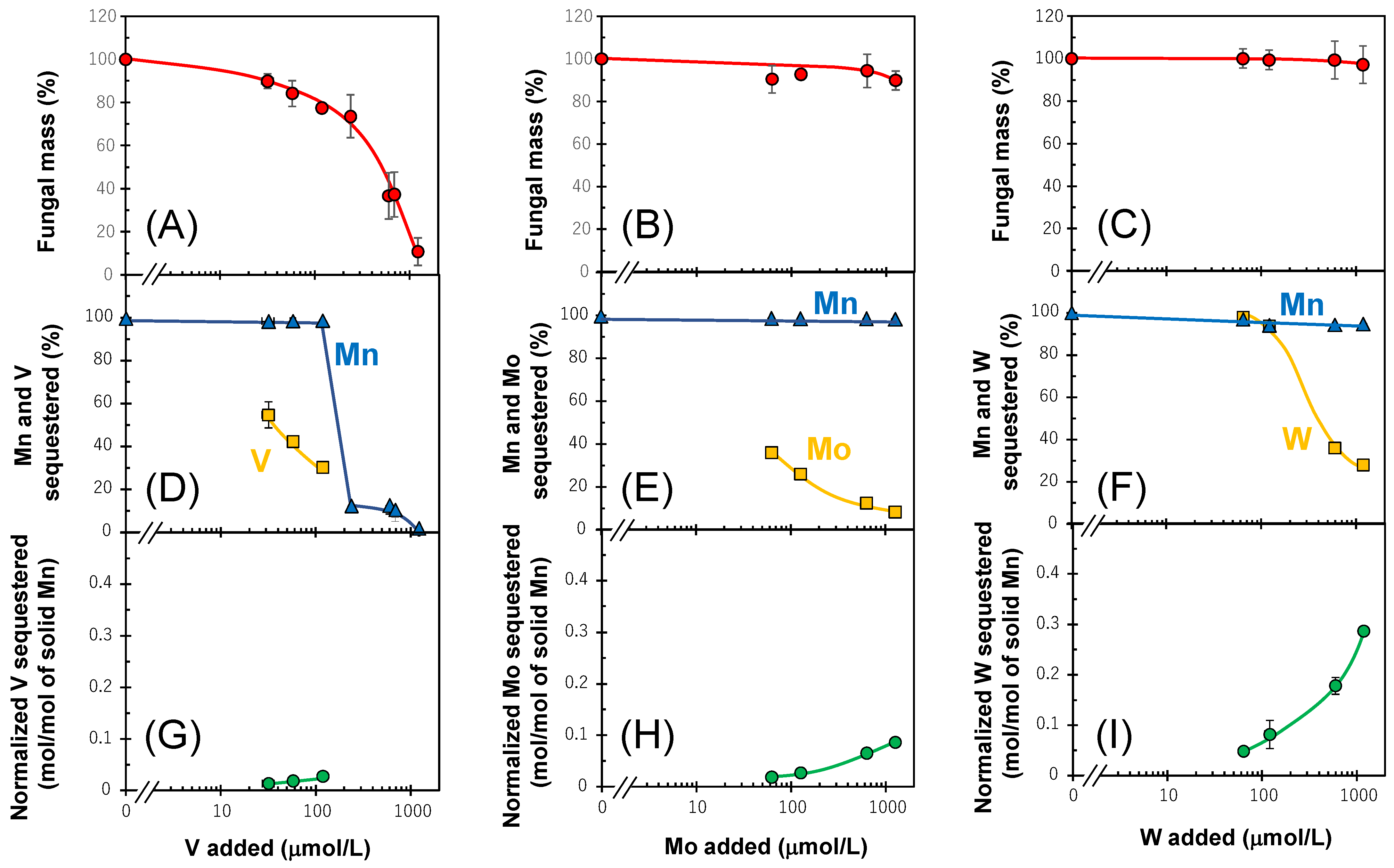
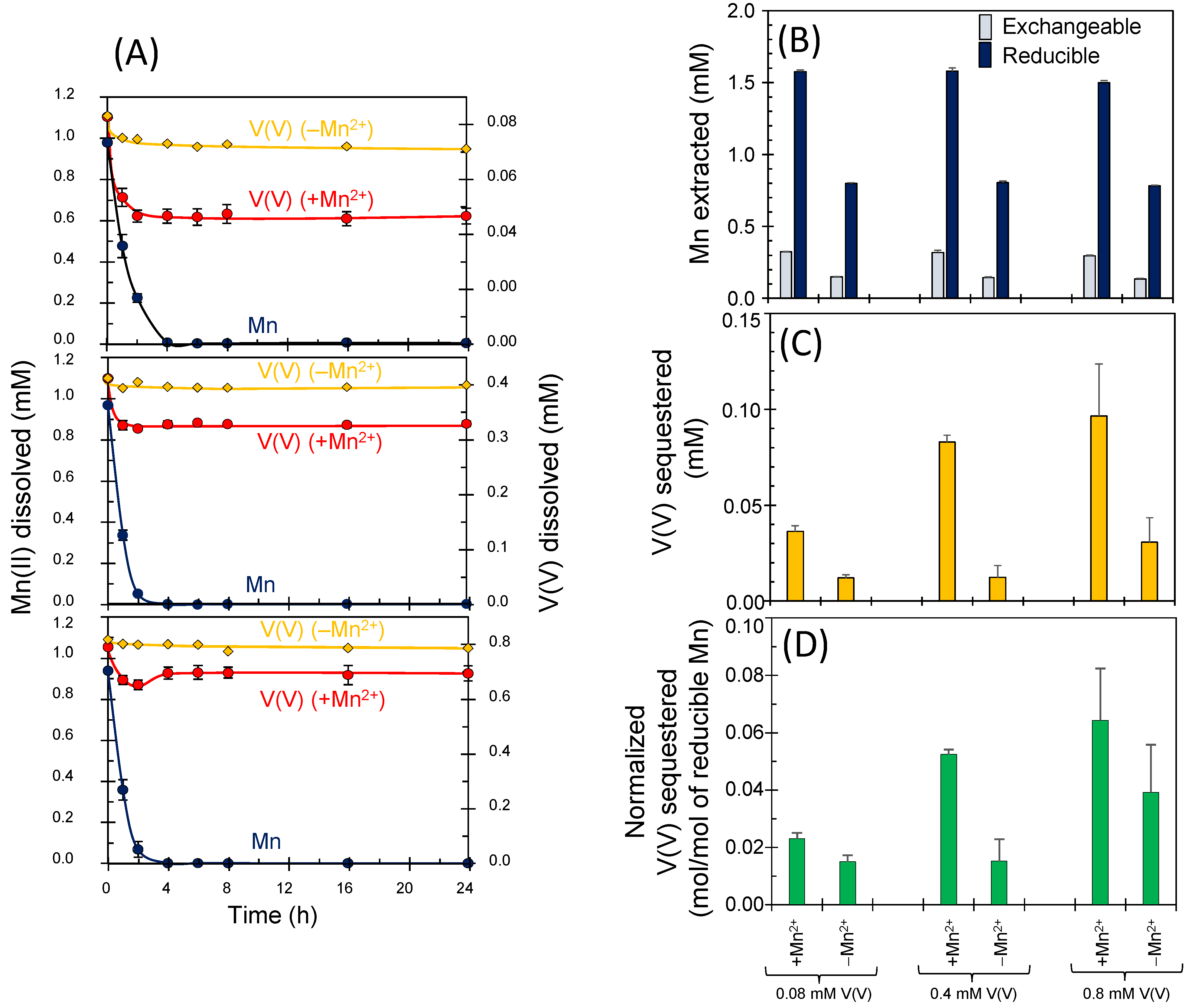
3.2. Sequestrations of Oxyanions by Newly Formed BMOs with an Mn(II) Oxidizing Enzymatic Activity
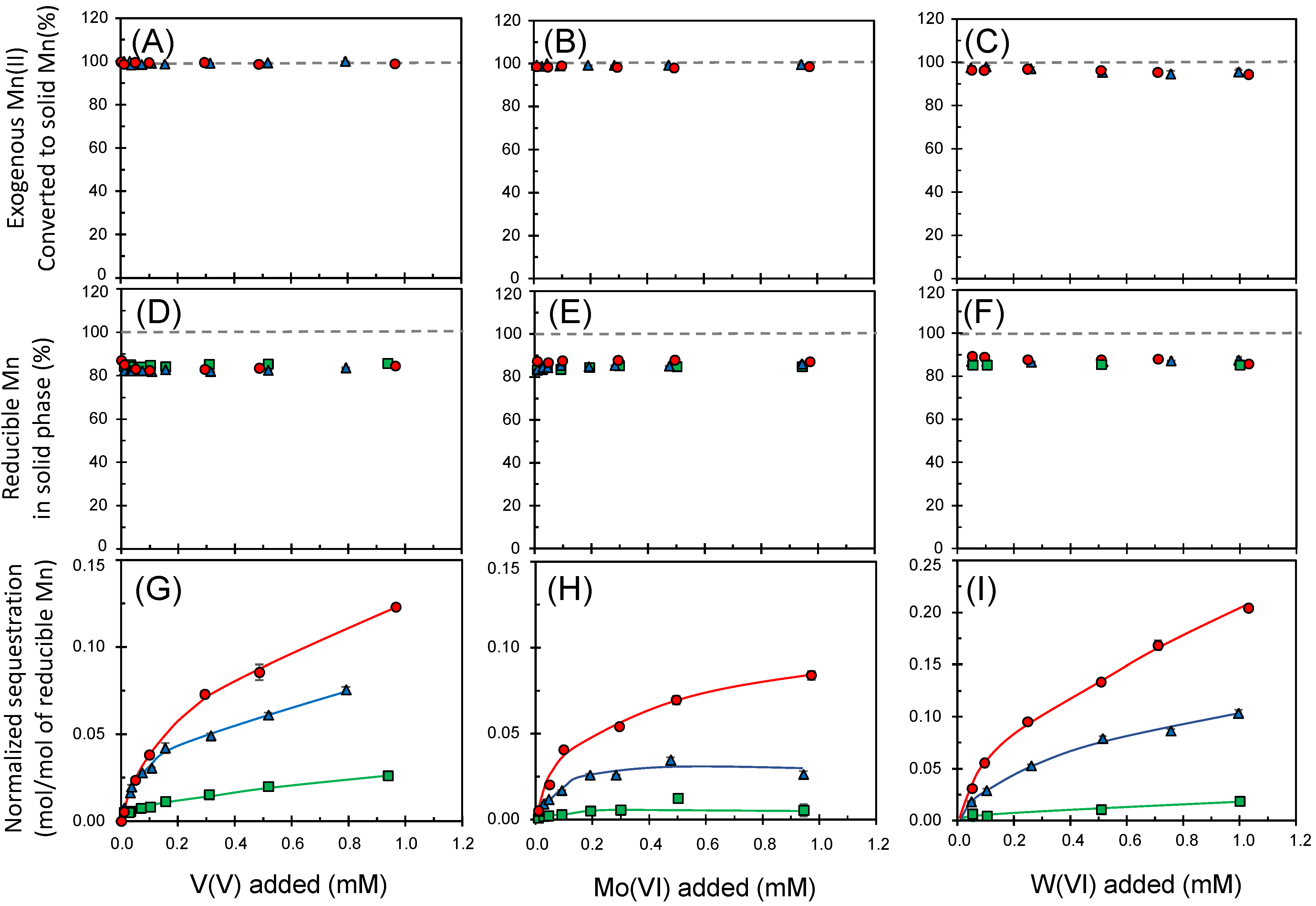
3.3. Sequestrations of Oxyanions through Enzymatic Mn(II) Oxidation
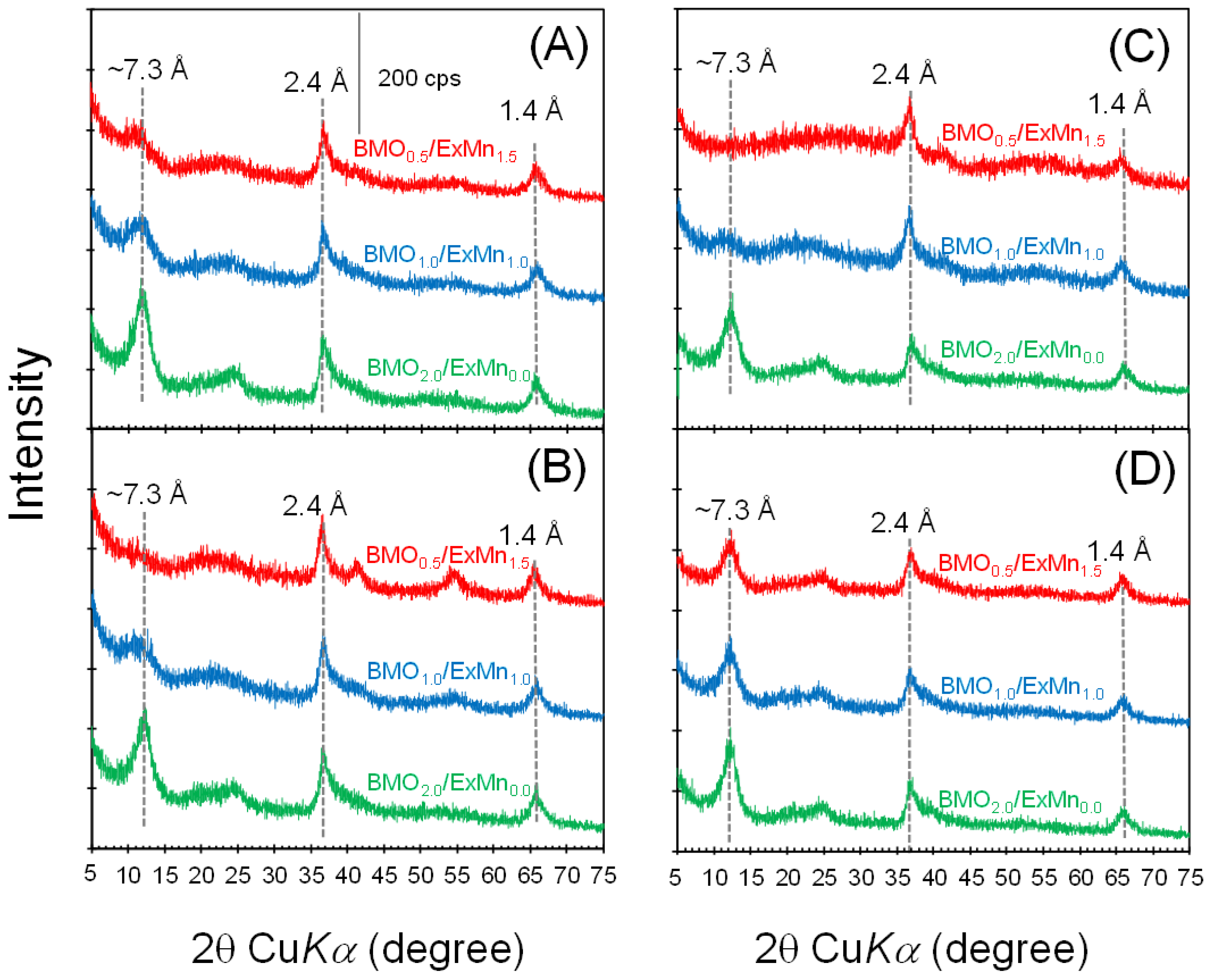
3.4. Mineralogical Characteristics of BMOs Formed with Coexisting V(V), Mo(VI), and W(VI)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global biogeochemical cycle of vanadium. Proc. Natl. Acad. Sci. USA 2017, 11, E11092–E11100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smedley, P.L.; Kinniburgh, D.G. Molybdenum in natural waters: A review of occurrence, distributions and controls. Appl. Geochem. 2017, 84, 387–432. [Google Scholar] [CrossRef] [Green Version]
- Koutsospyros, A.; Braida, W.; Christodoulatos, C.; Dermatas, D.; Strigul, N. A review of tungsten: From environmental obscurity to scrutiny. J. Hazard. Mater. 2006, 136, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Assem, F.L.; Levy, L.S. A review of current toxicological concerns on vanadium pentoxide and other vanadium compounds: Gaps in knowledge and directions for future research. J. Toxicol. Environ. Health B 2009, 12, 289–306. [Google Scholar] [CrossRef]
- Rojas-Lemus, M.; López-Valdez, N.; Bizarro-Nevares, P.; González-Villalva, A.; Ustarroz-Cano, M.; Zepeda-Rodríguez, A.; Pasos-Nájera, F.; García-Peláez, I.; Rivera-Fernández, N.; Fortoul, T.I. Toxic effects of inhaled vanadium attached to particulate matter: A literature review. Int. J. Environ. Res. Public Health 2021, 18, 8457. [Google Scholar] [CrossRef]
- Vyskocil, A.; Viau, C. Assessment of molybdenum toxicity in humans. J. Appl. Toxicol. 1999, 19, 185–192. [Google Scholar] [CrossRef]
- Strigul, N. Does speciation matter for tungsten ecotoxicology? Ecotoxicol. Environ. Saf. 2010, 73, 1099–1113. [Google Scholar] [CrossRef]
- Weidner, E.; Ciesielczyk, F. Removal of hazardous oxyanions from the environment using metal-oxide-based materials. Materials 2019, 12, 927. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Huang, Y.; Li, H.; Duan, H. Recent advances in removal techniques of vanadium from water: A comprehensive review. Chemosphere 2022, 287, 132021. [Google Scholar] [CrossRef]
- Kuhn, T.; Bostick, B.C.; Koschinsky, A.; Halbach, P.; Fendorf, S. Enrichment of Mo in hydrothermal Mn precipitates: Possible Mo sources, formation process and phase associations. Chem. Geol. 2003, 199, 29–43. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Takahashi, Y.; Marcus, M.A.; Uruga, T.; Tanida, H.; Terada, Y.; Usui, A. Tungsten species in natural ferromanganese oxides related to its different behavior from molybdenum in oxic ocean. Geochim. Cosmochim. Acta 2013, 106, 364–378. [Google Scholar] [CrossRef]
- Tanaka, M.; Ariga, D.; Kashiwabara, T.; Takahashi, Y. Adsorption mechanism of molybdenum (VI) on manganese oxides causing a large isotope fractionation. ACS Earth Space Chem. 2018, 2, 1187–1195. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Khan, Z.M.; Naz, I.; Sultan, M.; Ali, M.; Abbasi, I.A.; Islam, T.; Ye, T. Overview of microbes based fabricated biogenic nanoparticles for water and wastewater treatment. J. Environ. Manag. 2019, 230, 128–150. [Google Scholar] [CrossRef] [PubMed]
- Hennebel, T.; De Gusseme, B.; Boon, N.; Verstraete, W. Biogenic metals in advanced water treatment. Trends Biotechnol. 2009, 27, 90–98. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef] [Green Version]
- Villalobos, M.; Bargar, J.; Sposito, G. Trace metal retention on biogenic manganese oxide nanoparticles. Elements 2005, 1, 223–226. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Sakata, M.; Iwahori, K. Microbial manganese oxide formation and interaction with toxic metal ions. J. Biosci. Bioeng. 2007, 104, 1–8. [Google Scholar] [CrossRef]
- Grangeon, S.; Bataillard, P.; Coussy, S. The nature of manganese oxides in soils and their role as scavengers of trace elements: Implication for soil remediation. In Environmental Soil Remediation and Rehabilitation; van Hullebusch, E.D., Huguenot, D., Pechaud, Y., Simonnot, M.-O., Colombano, S., Eds.; Springer Nature: Cham, Switzerland, 2020; Chapter 7; pp. 399–429. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Iwahori, K.; Soma, M. Enzymatic formation of manganese oxides by an Acremonium-like hyphomycete fungus, strain KR21-2. FEMS Microbiol. Ecol. 2004, 47, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Miyata, N.; Tani, Y.; Maruo, K.; Tsuno, H.; Sakata, M.; Iwahori, K. Manganese(IV) oxide production by Acremonium sp. strain KR21-2 and extracellular Mn(II) oxidase activity. Appl. Environ. Microbiol. 2006, 72, 6467–6473. [Google Scholar] [CrossRef]
- Tojo, F.; Kitayama, A.; Miyata, N.; Okano, K.; Fukushima, J.; Suzuki, R.; Tani, Y. Molecular cloning and heterologous expression of manganese(II)-oxidizing enzyme from Acremonium strictum strain KR21-2. Catalysts 2020, 10, 686. [Google Scholar] [CrossRef]
- Geszvain, K.; Butterfield, C.; Davis, R.E.; Madison, A.S.; Lee, S.-W.; Parker, D.L.; Soldatova, A.; Spiro, T.G.; Luther, G.W., III; Tebo, B.M. The molecular biogeochemistry of manganese(II) oxidation. Biochem. Soc. Trans. 2012, 40, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Tani, Y.; Miyata, N.; Iwahori, K.; Soma, M.; Tokuda, S.; Seyama, H.; Theng, B.K.G. Biogeochemistry of manganese oxide coatings on pebble surfaces in the Kikukawa River System, Shizuoka, Japan. Appl. Geochem. 2003, 18, 1541–1554. [Google Scholar] [CrossRef]
- Nelson, Y.M.; Lion, L.W.; Shuler, M.L.; Ghiorse, W.C. Effect of oxide formation mechanisms on lead adsorption by biogenic manganese (hydr)oxides, iron (hydr)oxides, and their mixtures. Environ. Sci. Technol. 2002, 36, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, M.; Bargar, J.; Sposito, G. Mechanisms of Pb(II) sorption on a biogenic manganese oxide. Environ. Sci. Technol. 2005, 39, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Toner, B.; Manceau, A.; Webb, S.M.; Sposito, G. Zinc sorption to biogenic hexagonal-birnessite particles within a hydrated bacterial biofilm. Geochim. Cosmochim. Acta 2006, 70, 27–43. [Google Scholar] [CrossRef]
- Grangeon, S.; Lanson, B.; Miyata, N.; Tani, Y.; Manceau, A. Structure of nanocrystalline phyllomanganates produced by freshwater fungi. Am. Mineral. 2010, 95, 1608–1616. [Google Scholar] [CrossRef] [Green Version]
- Tani, Y.; Ohashi, M.; Miyata, N.; Seyama, H.; Iwahori, K.; Soma, M. Sorption of Co(II), Ni(II) and Zn(II) ions on biogenic manganese oxide produced by a Mn-oxidizing fungus, strain KR21-2. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2004, 39, 2641–2660. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Seyama, H.; Tanaka, K. Sequestration of Cd(II) and Ni(II) ions on fungal manganese oxides associated with Mn(II) oxidase activity. Appl. Geochem. 2014, 47, 198–208. [Google Scholar] [CrossRef]
- Zheng, H.; Tani, Y.; Naitou, H.; Miyata, N.; Tojo, F.; Seyama, H. Sequestration of La3+ by fungal manganese oxides and the effect of Mn(II) oxidase activity. J. Environ. Chem. Eng. 2017, 5, 735–743. [Google Scholar] [CrossRef]
- Inthorn, D.; Tani, Y.; Chang, J.; Naitou, H.; Miyata, N. Magnetically modified fungal Mn oxides with high sequestration efficiency for simultaneously removing multiple heavy metal ions from wastewater. J. Environ. Chem. Eng. 2014, 2, 1635–1641. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Tojo, F.; Seyama, H. Zn(II) sequestration by fungal biogenic manganese oxide through enzymatic and abiotic processes. Chem. Geol. 2014, 383, 155–163. [Google Scholar] [CrossRef]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Seyama, H. Fungal Mn oxides supporting Mn(II) oxidase activity as effective Mn(II) sequestering materials. Environ. Technol. 2013, 34, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Tani, Y.; Kakinuma, S.; Chang, J.; Tanaka, K.; Miyata, N. Preferential elimination of Ba2+ through irreversible biogenic manganese oxide sequestration. Minerals 2021, 11, 53. [Google Scholar] [CrossRef]
- Aoshima, M.; Tani, Y.; Fujita, R.; Tanaka, K.; Miyata, N.; Umezawa, K. Simultaneous sequestration of Co2+ and Mn2+ by fungal manganese oxide through asbolane formation. Minerals 2022, 12, 358. [Google Scholar] [CrossRef]
- Tani, Y.; Miyata, N.; Ohashi, M.; Ohnuki, T.; Seyama, H.; Iwahori, K.; Soma, M. Interaction of inorganic arsenic with biogenic manganese oxide produced by a Mn-oxidizing fungus, strain KR21-2. Environ. Sci. Technol. 2004, 38, 6618–6624. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Tani, Y.; Naitou, H.; Miyata, N.; Seyama, H.; Tanaka, K. Cobalt(II) sequestration on fungal biogenic manganese oxide enhanced by manganese(II) oxidase activity. Appl. Geochem. 2013, 37, 170–178. [Google Scholar] [CrossRef]
- Rosson, R.A.; Nealson, K.H. Manganese binding and oxidation by spores of a marine bacillus. J. Bacteriol. 1982, 151, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- De Vrind, J.P.; de Vrind-de Jong, E.W.; de Voogt, J.W.; Westbroek, P.; Boogerd, F.C.; Rosson, R.A. Manganese oxidation by spores and spore coats of a marine Bacillus species. Appl. Environ. Microbiol. 1986, 52, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, J.; Tani, Y.; Miyata, N.; Seyama, H.; Mitsunobu, S.; Naitou, H. Concurrent sorption of As(V) and Mn(II) during biogenic manganese oxide formation. Chem. Geol. 2012, 306–307, 123–128. [Google Scholar] [CrossRef]
- Suzuki, R.; Tani, Y.; Naitou, H.; Miyata, N.; Tanaka, K. Sequestration and oxidation of Cr(III) by fungal Mn oxides with Mn(II) oxidizing activity. Catalysts 2020, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Feng, X.; Tan, W.; Koopal, L.K.; Hu, T.; Zhu, M.; Liu, F. Structure and properties of vanadium(V)-doped hexagonal turbostratic birnessite and its enhanced scavenging of Pb2+ from solutions. J. Hazard. Mater. 2015, 288, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Abernathy, M.J.; Schaefer, M.V.; Vessey, C.J.; Liu, H.; Ying, S.C. Oxidation of V(IV) by birnessite: Kinetics and surface complexation. Environ. Sci. Technol. 2021, 55, 11703–11712. [Google Scholar] [CrossRef] [PubMed]

| Primary BMOs | Sequestration Process | |
|---|---|---|
| BMO2.0/ExMn0.0 1 | The newly formed BMO 2 (1 mM as Mn) was reacted with exogenous Mn2+ (1 mM) to form additional BMO phases in 20 mM HEPES at 7.0 for 24 h. This primary BMO contained 2 mM Mn. | V(V), Mo(VI), or W(VI) (up to ~1 mM) without exogenous Mn2+ in 20 mM HEPES (at pH 7.0) for 24 h. |
| BMO1.0/ExMn1.0 1 | Newly formed BMOs 2 (1 mM as Mn) | V(V), Mo(VI), or W(VI) (up to ~1 mM) with 1 mM exogenous Mn2+ in 20 mM HEPES (at pH 7.0) for 24 h. |
| BMO0.5/ExMn1.5 1 | Newly formed BMOs 2 (0.5 mM as Mn) | V(V), Mo(VI), or W(VI) (up to ~1 mM) with 1.5 mM exogenous Mn2+ in 20 mM HEPES (at pH 7.0) for 24 h. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tani, Y.; Wu, T.; Shirakura, T.; Umezawa, K.; Miyata, N. Sequestration of Oxyanions of V(V), Mo(VI), and W(VI) Enhanced through Enzymatic Formation of Fungal Manganese Oxides. Minerals 2022, 12, 1368. https://doi.org/10.3390/min12111368
Tani Y, Wu T, Shirakura T, Umezawa K, Miyata N. Sequestration of Oxyanions of V(V), Mo(VI), and W(VI) Enhanced through Enzymatic Formation of Fungal Manganese Oxides. Minerals. 2022; 12(11):1368. https://doi.org/10.3390/min12111368
Chicago/Turabian StyleTani, Yukinori, Tingting Wu, Takumi Shirakura, Kazuhiro Umezawa, and Naoyuki Miyata. 2022. "Sequestration of Oxyanions of V(V), Mo(VI), and W(VI) Enhanced through Enzymatic Formation of Fungal Manganese Oxides" Minerals 12, no. 11: 1368. https://doi.org/10.3390/min12111368





