Effects of Alkanolamine Absorbents in Integrated Absorption–Mineralization
Abstract
:1. Introduction
2. Materials and Methods
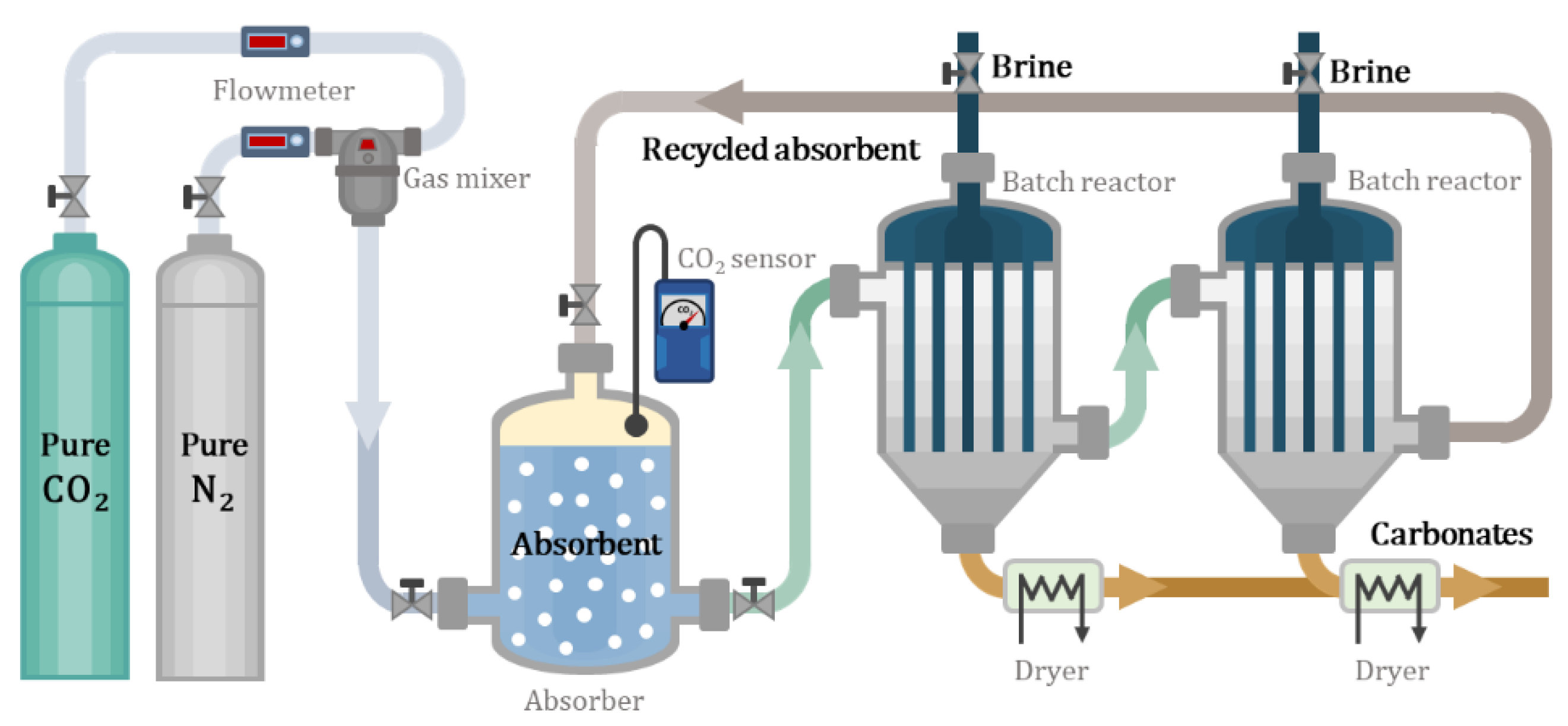
2.1. Absorption
| Alkanolamine | Classification | Chemical Structure | Advantage | Disadvantage |
|---|---|---|---|---|
| Monoethanolamine (MEA) | Primary amine | OH–C2H4–NH2 |
|
|
| Diethanolamine (DEA) | Secondary amine | HN(CH2CH2OH)2 |
|
|
| Triethanolamine (TEA) | Tertiary amine | N(CH2CH2OH)3 |
|
|
| Aminomethyl propanol (AMP) | Steric hindrance amine | H2NC(CH3)2CH2OH |
|
|
2.2. Precipitation
2.3. Regeneration
3. Results
3.1. Effects of the Developed Model of CO2 Absorption Capacity
3.2. Effects of the Increased Number of Precipitation Steps
3.3. Comparison of Absorption in Alkanolamine Absorbents
3.3.1. CO2 Absorption Capacity
3.3.2. CO2 Absorption Rate
3.4. Comparison of Conversion in Alkanolamine Absorbents
4. Discussion
4.1. Degradation of Alkanolamine Absorbents
4.2. CO2 Conversion of Alkanolamine Absorbents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, G.; O’keefe, P.; Rose, J.; Wisner, B. Climate change and disaster management. Disasters 2006, 30, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Carrington, D. Guardian. Climate Crisis: 11,000 Scientists Warn of ‘Untold Suffering’, London, 2019. Available online: https://www.theguardian.com/environment/2019/nov/05/climate-crisis-11000-scientists-warn-of-untold-suffering (accessed on 10 September 2022).
- Bataille, C.; Åhman, M.; Neuhoff, K.; Nilsson, L.J.; Fischedick, M.; Lechtenböhmer, S.; Solano-Rodriquez, B.; Denis-Ryang, A.; Stieberth, S.; Waismana, H.; et al. A review of technology and policy deep decarbonization pathway options for making energy-intensive industry production consistent with the Paris Agreement. J. Clean. Prod. 2018, 187, 960–973. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, C.J. The 2015 Paris climate change conference: COP21. Sci. Progress 2016, 9, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Haug, N.; Geyrhofer, L.; Londei, A.; Dervic, E.; Desvars-Larrive, A.; Loreto, V.; Pinior, B.; Thurner, S.; Klimek, P. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat. Hum. Behav. 2022, 4, 1303–1312. [Google Scholar] [CrossRef]
- Tiseo, I. Annual production of plastics worldwide from 1950 to 2020 (in million metric tons). Stat. Stat. Portal 2021, 10, 2021. [Google Scholar]
- Legg, S. IPCC, 2021: Climate Change 2021-the Physical Science basis. Interaction 2021, 49, 44–45. [Google Scholar]
- IEA. CCUS in Clean Energy Transitions, Paris, 2020. Available online: https://www.iea.org/reports/ccus-in-clean-energy-transitions (accessed on 10 September 2022).
- Baylin-Stern, A.; Berghout, N. Is Carbon Capture too Expensive? Paris, 2021. Available online: https://www.iea.org/commentaries/is-carbon-capture-too-expensive (accessed on 10 September 2022).
- Bachu, S. Identification of oil reservoirs suitable for CO2-EOR and CO2 storage (CCUS) using reserves databases, with application to Alberta, Canada. Int. J. Greenh. Gas Control. 2016, 44, 152–165. [Google Scholar] [CrossRef]
- Yu, S.; Horing, J.; Liu, Q.; Dahowski, R.; Davidson, C.; Edmonds, J.; Liu, B.; Mcjeona, H.; McLeod, J.; Patel, P.; et al. CCUS in China’s mitigation strategy: Insights from integrated assessment modeling. Int. J. Greenh. Gas Control. 2019, 84, 204–218. [Google Scholar] [CrossRef]
- Yu, C.; Huang, C.; Tan, C. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef] [Green Version]
- IEA. Putting CO2 to Use, Paris, 2019. Available online: https://www.iea.org/reports/putting-co2-to-use (accessed on 10 September 2022).
- Teir, S.; Eloneva, S.; Fogelholm, C.J.; Zevenhoven, R. Dissolution of steelmaking slags in acetic acid for precipitated calcium carbonate production. Energy 2007, 32, 528–539. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, J.S.; Kawatra, S.K.; Eisele, T.C.; Sutter, L.L. Carbon dioxide sequestration in cement kiln dust through mineral carbonation. Environ. Sci. Technol. 2009, 43, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Eloneva, S.; Said, A.; Fogelholm, C.J.; Zevenhoven, R. Preliminary assessment of a method utilizing carbon dioxide and steelmaking slags to produce precipitated calcium carbonate. Appl. Energy 2012, 90, 329–334. [Google Scholar] [CrossRef]
- Kang, D.; Jo, H.; Lee, M.G.; Park, J. Carbon dioxide utilization using a pretreated brine solution at normal temperature and pressure. Chem. Eng. J. 2016, 284, 1270–1278. [Google Scholar] [CrossRef]
- Solis, B.H.; Cui, Y.; Weng, X.; Seifert, J.; Schauermann, S.; Sauer, J.; Shaikhutdinov, S.; Freund, H.J. Initial stages of CO2 adsorption on CaO: A combined experimental and computational study. Phys. Chem. Chem. Phys. 2017, 19, 4231–4242. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Eldrup, N.H.; Normann, F.; Skagestad, R.; Øi, L.E. Cost estimation of CO2 absorption plants for CO2 mitigation–method and assumptions. Int. J. Greenh. Gas Control. 2019, 88, 10–23. [Google Scholar] [CrossRef]
- Raksajati, A.; Ho, M.T.; Wiley, D.E. Reducing the cost of CO2 capture from flue gases using aqueous chemical absorption. Ind. Eng. Chem. Res. 2013, 52, 16887–16901. [Google Scholar] [CrossRef]
- IEA. Direct Air Capture, Paris, 2022. Available online: https://www.iea.org/reports/direct-air-capture (accessed on 15 September 2022).
- Wang, Y.; Song, L.; Ma, K.; Liu, C.; Tang, S.; Yan, Z.; Yue, H.; Liang, B. An integrated absorption–mineralization process for CO2 capture and sequestration: Reaction mechanism, recycling stability, and energy evaluation. ACS Sustain. Chem. Eng. 2021, 9, 16577–16587. [Google Scholar] [CrossRef]
- Rochelle, G.T. Conventional amine scrubbing for CO2 capture. Absorption-based post-combustion capture of carbon dioxide. Woodhead Publ. 2016, 2016, 35–67. [Google Scholar] [CrossRef]
- Thamsiriprideeporn, C.; Suekane, T. Investigation and development of the multicycle of CO2 mineralization with wastewater under standard conditions. Greenh. Gases Sci. Technol. 2021, 12, 67–84. [Google Scholar] [CrossRef]
- Soliman, M.N.; Guen, F.Z.; Ahmed, S.A.; Saleem, H.; Khalil, M.J.; Zaidi, S.J. Energy consumption and environmental impact assessment of desalination plants and brine disposal strategies. Process Saf. Environ. Prot. 2021, 147, 589–608. [Google Scholar] [CrossRef]
- Samco Technologies, Inc. How Much Does It Cost to Treat Brine for Reuse and/or Disposal? 2018. Available online: https://samcotech.com/cost-treat-brine-reuse-disposal/ (accessed on 20 September 2022).
- Li, Y.N.; He, L.N.; Diao, Z.F.; Yang, Z.Z. Carbon capture with simultaneous activation and its subsequent transformation. Adv. Inorg. Chem. 2014, 66, 289–345. [Google Scholar] [CrossRef]
- Vega, F.; Cano, M.; Camino, S.; Fernández, L.M.G.; Portillo, E.; Navarrete, B. Solvents for carbon dioxide capture. Carbon Dioxide Chem. Capture Oil Recovery 2018, 1, 142–163. [Google Scholar] [CrossRef]
- Vega, F.; Sanna, A.; Maroto-Valer, M.M.; Navarrete, B.; Abad-Correa, D. Study of the MEA degradation in a CO2 capture process based on partial oxy-combustion approach. Int. J. Greenh. Gas Control. 2016, 54, 160–167. [Google Scholar] [CrossRef]
- Einbu, A.; Pettersen, T.; Morud, J.; Tobiesen, A.; Jayarathna, C.K.; Skagestad, R.; Nysæther, G. Energy assessments of onboard CO2 capture from ship engines by MEA-based post combustion capture system with flue gas heat integration. Int. J. Greenh. Gas Control. 2022, 113, 103526. [Google Scholar] [CrossRef]
- Lv, B.; Guo, B.; Zhou, Z.; Jing, G. Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes. Environ. Sci. Technol. 2015, 49, 10728–10735. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bautista, A.; Palmero, E.M.; Moya, A.J.; Gómez-Díaz, D.; La Rubia, M.D. Characterization of alkanolamine blends for carbon dioxide absorption. Corrosion and regeneration studies. Sustainability 2021, 13, 4011. [Google Scholar] [CrossRef]
- Kim, Y.E.; Lim, J.A.; Jeong, S.K.; Yoon, Y.I.; Bae, S.T.; Nam, S.C. Comparison of carbon dioxide absorption in aqueous MEA, DEA, TEA, and AMP solutions. Bull. Korean Chem. Soc. 2013, 34, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Hadri, N.E.; Quang, D.V.; Rayer, A.V.; Abu-Zahra, M.R. Development of amine-blend systems for CO2 post-combustion capture. In Proceedings of the 4th international gas processing symposium; Elsevier: Amsterdam, The Netherlands, 2015; pp. 59–68. [Google Scholar] [CrossRef]
- Wincek, R.T.; Miller, B.G.; Johnson, D.K.; Decker, G.E.; Hart, P.J.; DE, W.P.U.G.N.; No, S. On-Site Treatment of Brine. The Energy Institute and Hart Resource Technologies, Inc., 2002. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.164.2188&rep=rep1&type=pdf (accessed on 13 August 2022).
- Smit, B.; Reimer, J.A.; Oldenburg, C.M.; Bourg, I.C. Introduction to carbon capture and sequestration; World Scientific: Singapore, 2014. [Google Scholar] [CrossRef]
- Steel, K.M.; Alizadehhesari, K.; Balucan, R.D.; Bašić, B. Conversion of CO2 into mineral carbonates using a regenerable buffer to control solution pH. Fuel 2013, 111, 40–47. [Google Scholar] [CrossRef]
- Yiin, B.S.; Margerum, D.W. Non-metal redox kinetics: Reactions of trichloramine with ammonia and with dichloramine. Inorg. Chem. 1990, 29, 2135–2141. [Google Scholar] [CrossRef]
- Eloneva, S.; Teir, S.; Salminen, J.; Fogelholm, C.J.; Zevenhoven, R. Fixation of CO2 by carbonating calcium derived from blast furnace slag. Energy 2008, 33, 1461–1467. [Google Scholar] [CrossRef]
- Yamasaki, H.; Kamei, S.; Kuroki, T.; Okubo, M. Adsorbed CO2 dissociation using argon and helium nonthermal plasma flows. IEEE Trans. Ind. Appl. 2020, 56, 6983–6989. [Google Scholar] [CrossRef]
- Huertas, J.I.; Gomez, M.D.; Giraldo, N.; Garzón, J. CO2 absorbing capacity of MEA. J. Chem. 2015, 2015, 965015. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.C.; Cho, H.H.; Jhuang, J.H.; Ku, C.H. Selection of Mixed Amines in the CO2 Capture Process. C 2021, 7, 25. [Google Scholar] [CrossRef]
- Smith, T.J. MolView: A program for analyzing and displaying atomic structures on the Macintosh personal computer. J. Mol. Graph. 1995, 13, 122–125. [Google Scholar] [CrossRef]
- Rinker, E.B.; Ashour, S.S.; Sandall, O.C. Kinetics and modeling of carbon dioxide absorption into aqueous solutions of diethanolamine. Ind. Eng. Chem. Res. 1996, 35, 1107–1114. [Google Scholar] [CrossRef]
- Liu, B.; Cui, Z.; Tian, W. The Kinetics Investigation of CO2 Absorption into TEA and DEEA Amine Solutions Containing Carbonic Anhydrase. Processes 2021, 9, 2140. [Google Scholar] [CrossRef]
- Park, S.W.; Choi, B.S.; Lee, J.W. Chemical absorption of carbon dioxide with triethanolamine in non-aqueous solutions. Korean J. Chem. Eng. 2006, 23, 138–143. [Google Scholar] [CrossRef]
- Yeh, A.C.; Bai, H. Comparison of ammonia and monoethanolamine solvents to reduce CO2 greenhouse gas emissions. Sci. Total Environ. 1999, 228, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Fang, M.; Zhong, L.; Zhang, C.; Luo, Z. Semi-batch experimental study on CO2 absorption characteristic of aqueous ammonia. Energy Procedia 2011, 4, 156–163. [Google Scholar] [CrossRef] [Green Version]
- OriginLab Corp. OriginPro2021 Manual, 2021. Available online: https://www.originlab.com/doc/Origin-Help/PD-Dialog-Line-Tab (accessed on 6 August 2022).
- Jensen, J.H.; Gordon, M.S. On the Number of Water Molecules Necessary to Stabilize the Glycine Zwitterion. J. Am. Chem. Soc. 1995, 117, 8159–8170. [Google Scholar] [CrossRef]
- Chi, S.; Rochelle, G.T. Oxidative degradation of monoethanolamine. Ind. Eng. Chem. Res. 2002, 41, 4178–4186. [Google Scholar] [CrossRef]
- McCann, N.; Phan, D.; Wang, X.; Conway, W.; Burns, R.; Attalla, M.; Graeme, P.; Maeder, M. Kinetics and mechanism of carbamate formation from CO2 (aq), carbonate species, and monoethanolamine in aqueous solution. J. Phys. Chem. A 2009, 113, 5022–5029. [Google Scholar] [CrossRef] [PubMed]
- Vevelstad, S.J.; Eide-Haugmo, I.; da Silva, E.F.; Svendsen, H.F. Degradation of MEA; a theoretical study. Energy Procedia 2011, 4, 1608–1615. [Google Scholar] [CrossRef]
- Benyahia, F.; Eljack, F.T. Overview of Conversion of Greenhouse Gas Carbon dioxide to Hydrocarbons. In Proceedings of the 2nd Annual Gas Processing Symposium, Doha, Qatar, 10–14 January 2010; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thamsiriprideeporn, C.; Tetsuya, S. Effects of Alkanolamine Absorbents in Integrated Absorption–Mineralization. Minerals 2022, 12, 1386. https://doi.org/10.3390/min12111386
Thamsiriprideeporn C, Tetsuya S. Effects of Alkanolamine Absorbents in Integrated Absorption–Mineralization. Minerals. 2022; 12(11):1386. https://doi.org/10.3390/min12111386
Chicago/Turabian StyleThamsiriprideeporn, Chanakarn, and Suekane Tetsuya. 2022. "Effects of Alkanolamine Absorbents in Integrated Absorption–Mineralization" Minerals 12, no. 11: 1386. https://doi.org/10.3390/min12111386
APA StyleThamsiriprideeporn, C., & Tetsuya, S. (2022). Effects of Alkanolamine Absorbents in Integrated Absorption–Mineralization. Minerals, 12(11), 1386. https://doi.org/10.3390/min12111386





