Hydrochemical and Isotope (18O, 2H and 3H) Characteristics of Karst Water in Central Shandong Province: A Case Study of the Pingyi-Feixian Region
Abstract
:1. Introduction
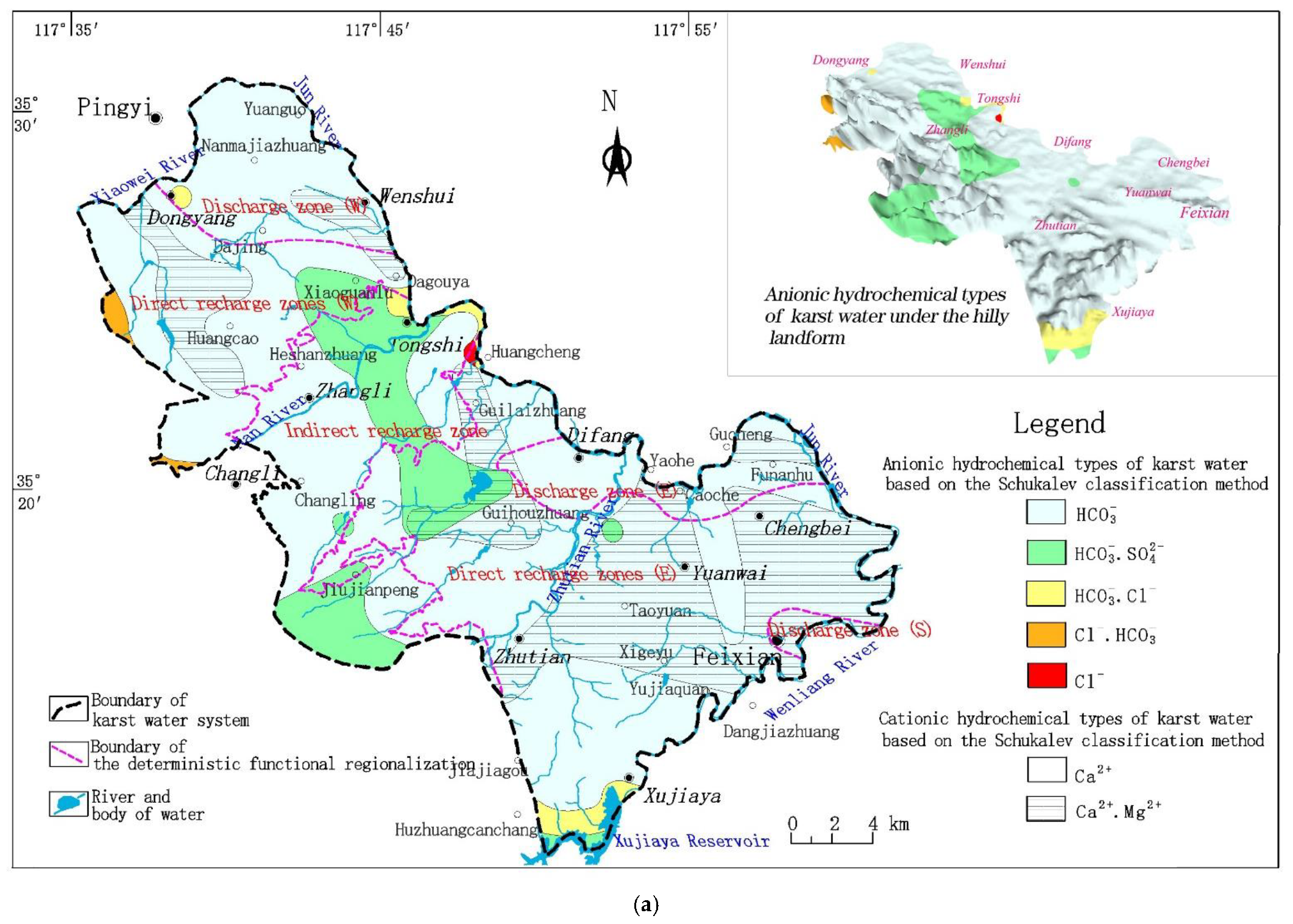
2. Hydrogeological Setting of Karst Water in the Study Region
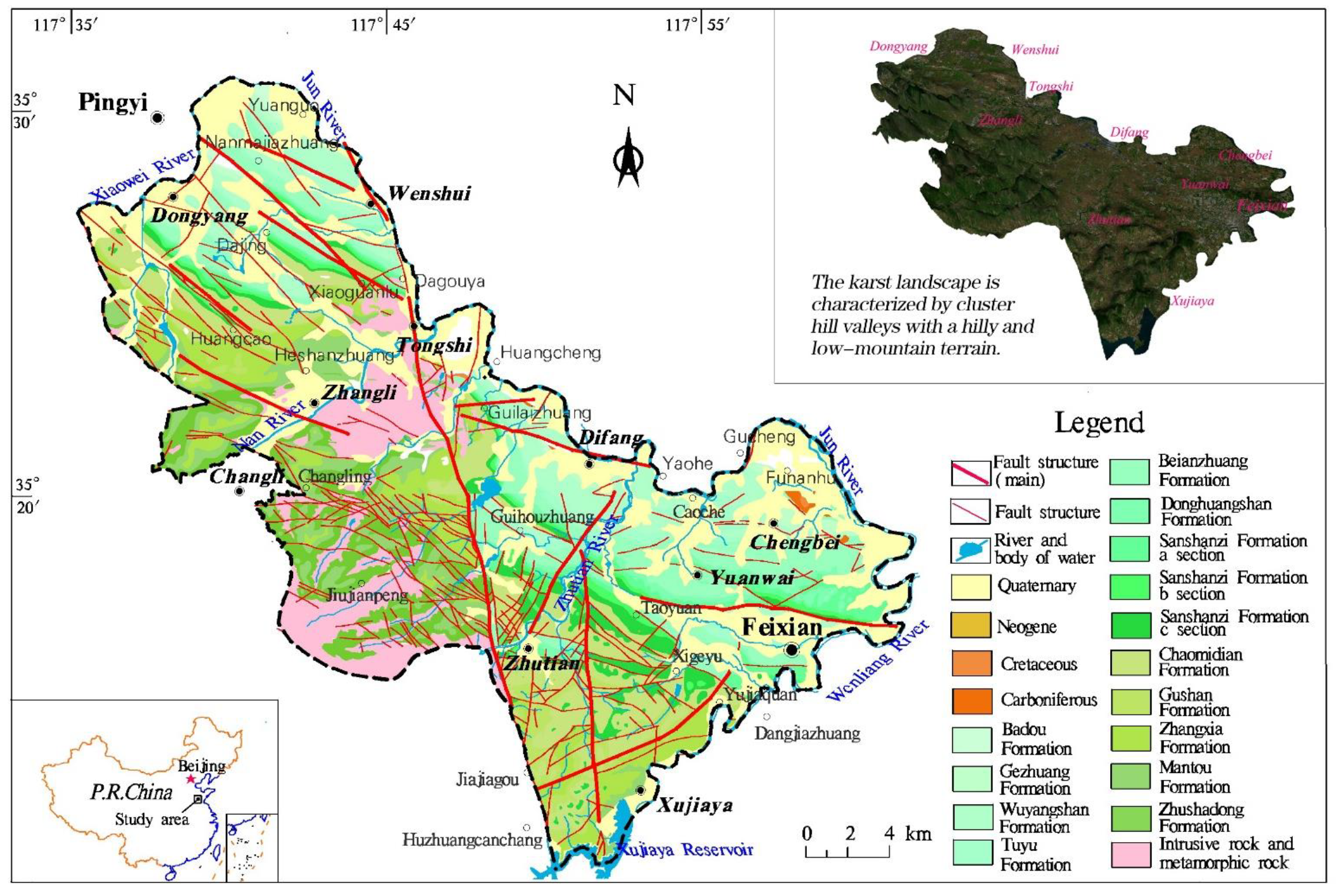
2.1. Geological Setting
2.2. Hydrogeological Characteristics of Karst Aquifers
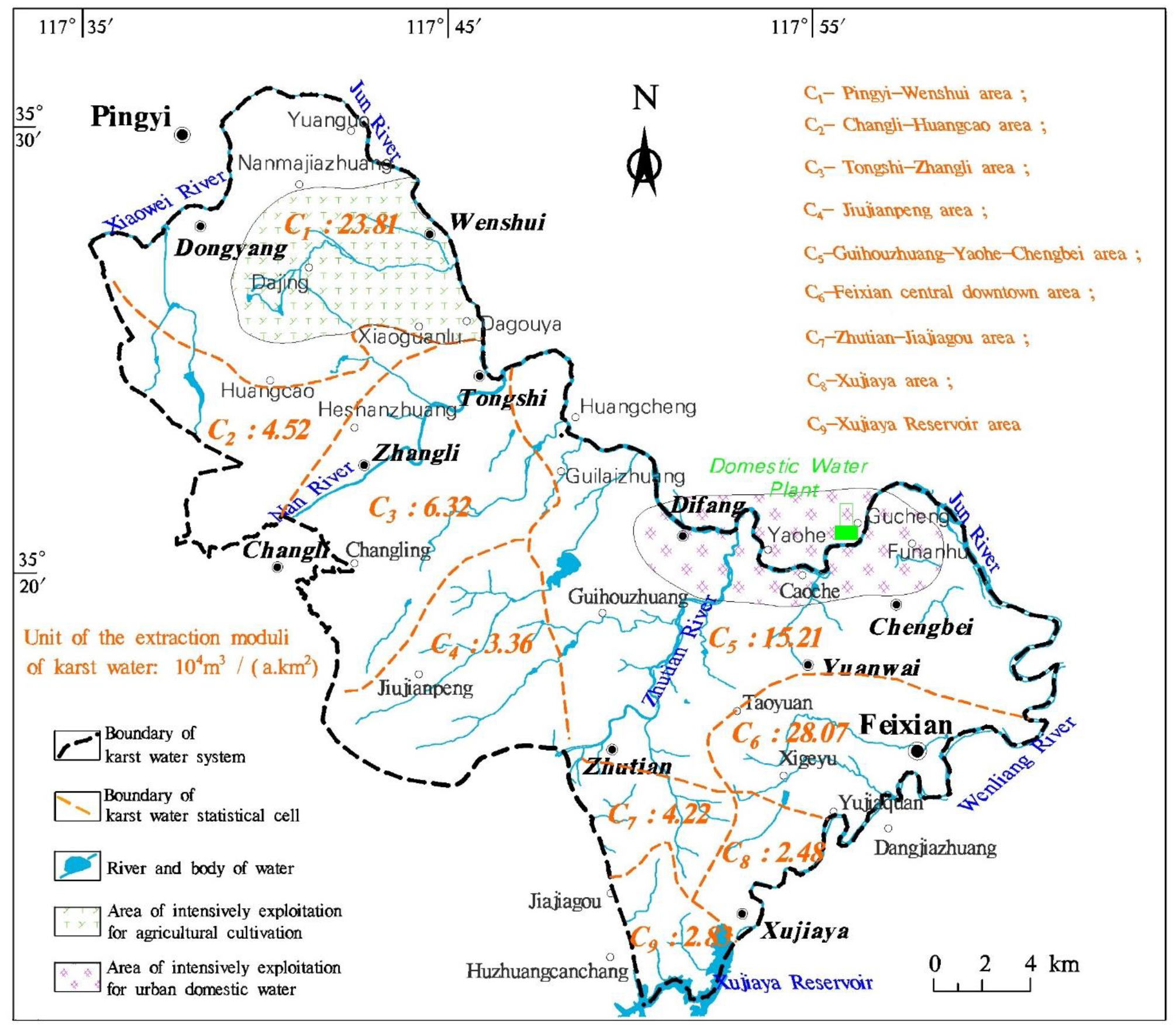
2.3. Exploitation of Karst Water
3. Materials and Methods
4. Results and Discussion
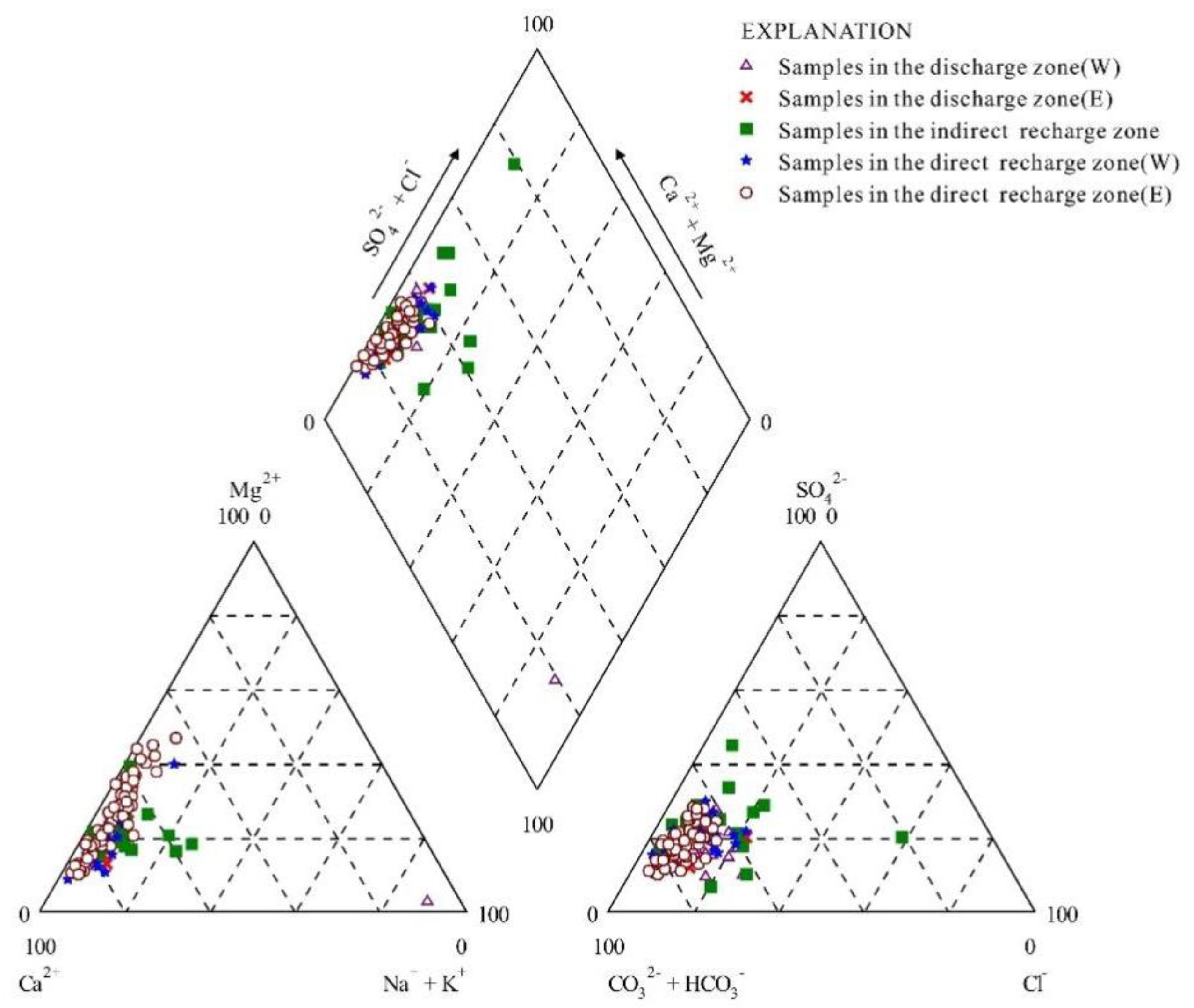
4.1. Hydrochemical Characteristics of Karst Water
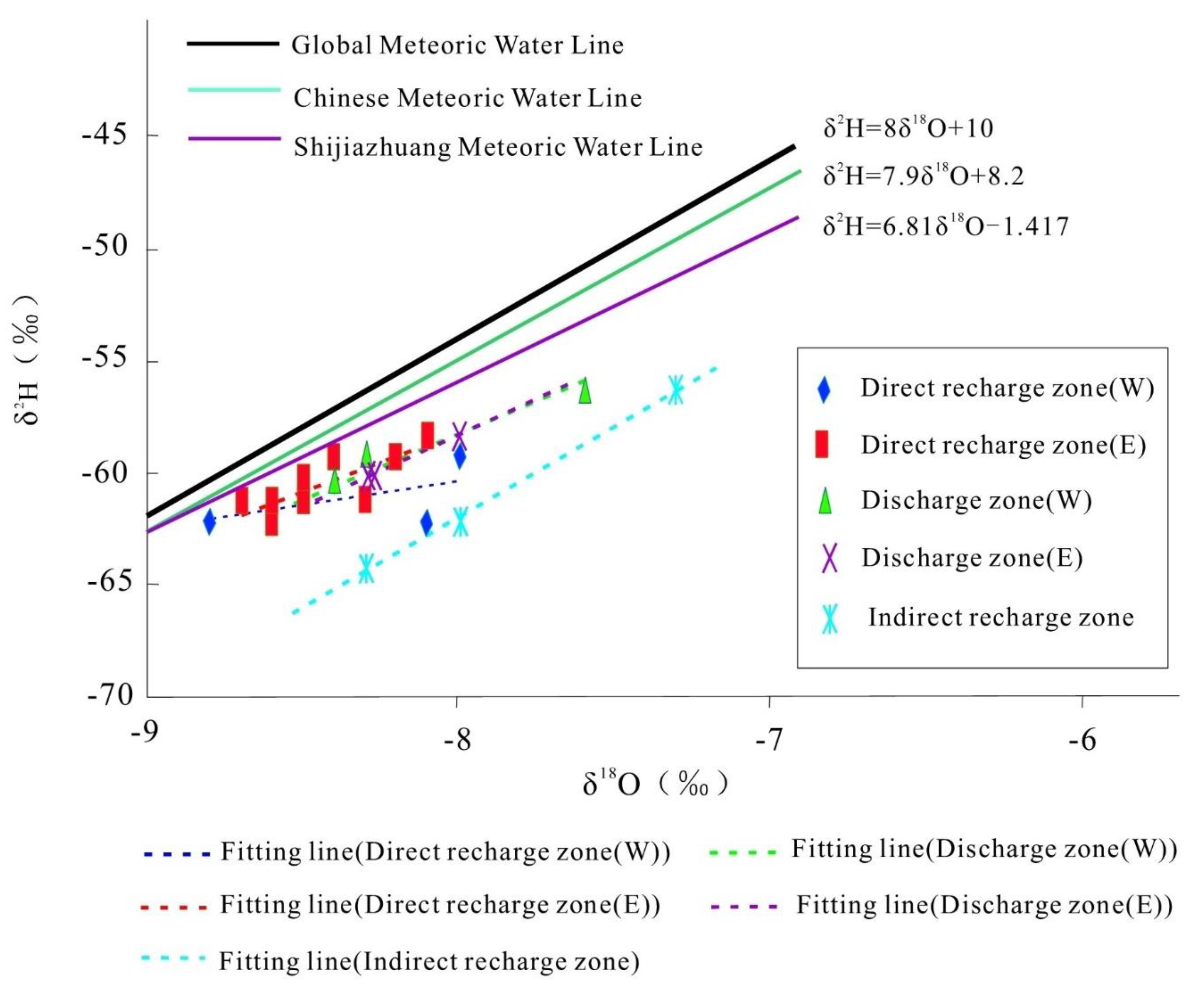
4.2. 2H and 18O Isotope Characteristics of Karst Water
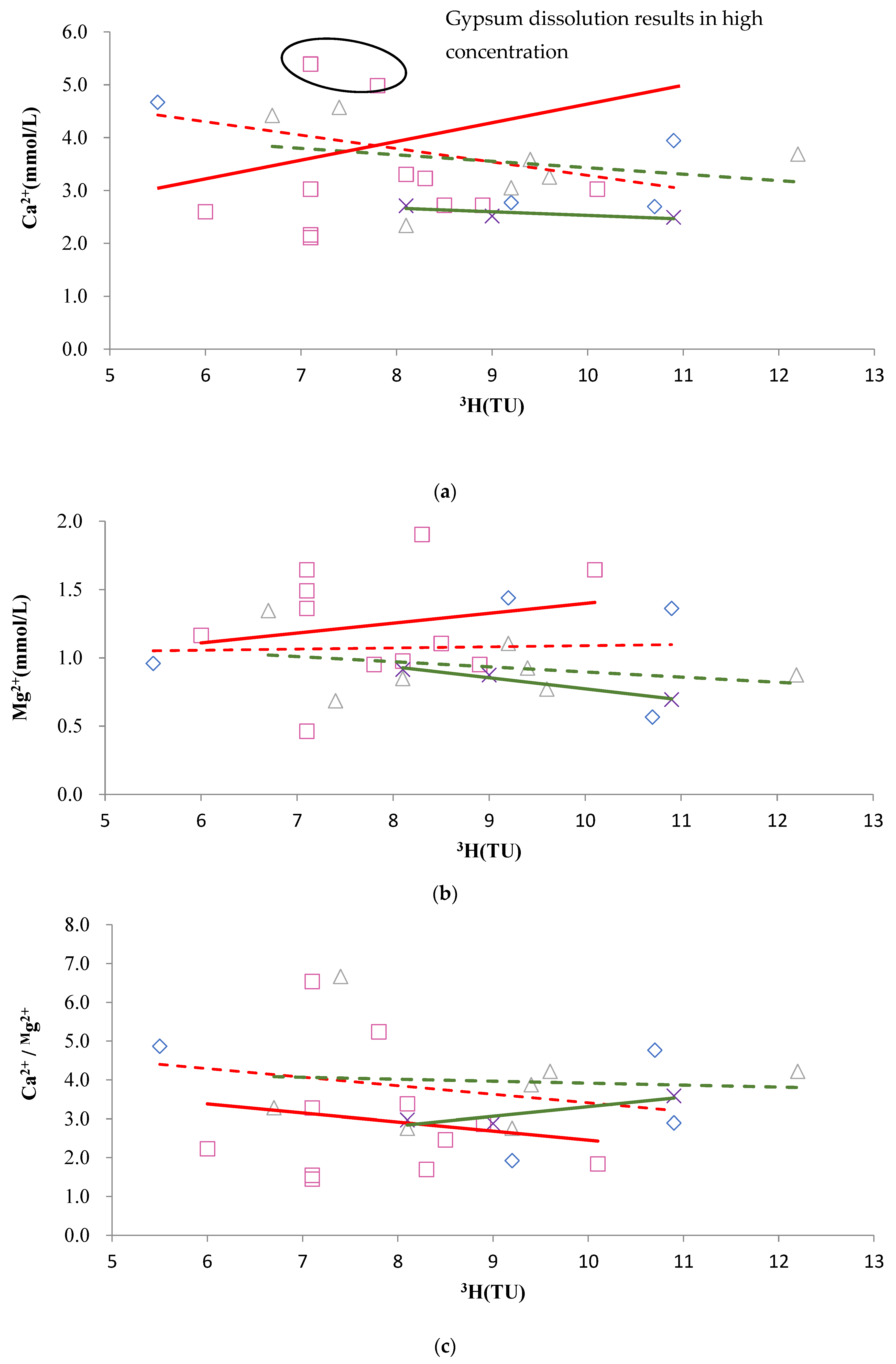
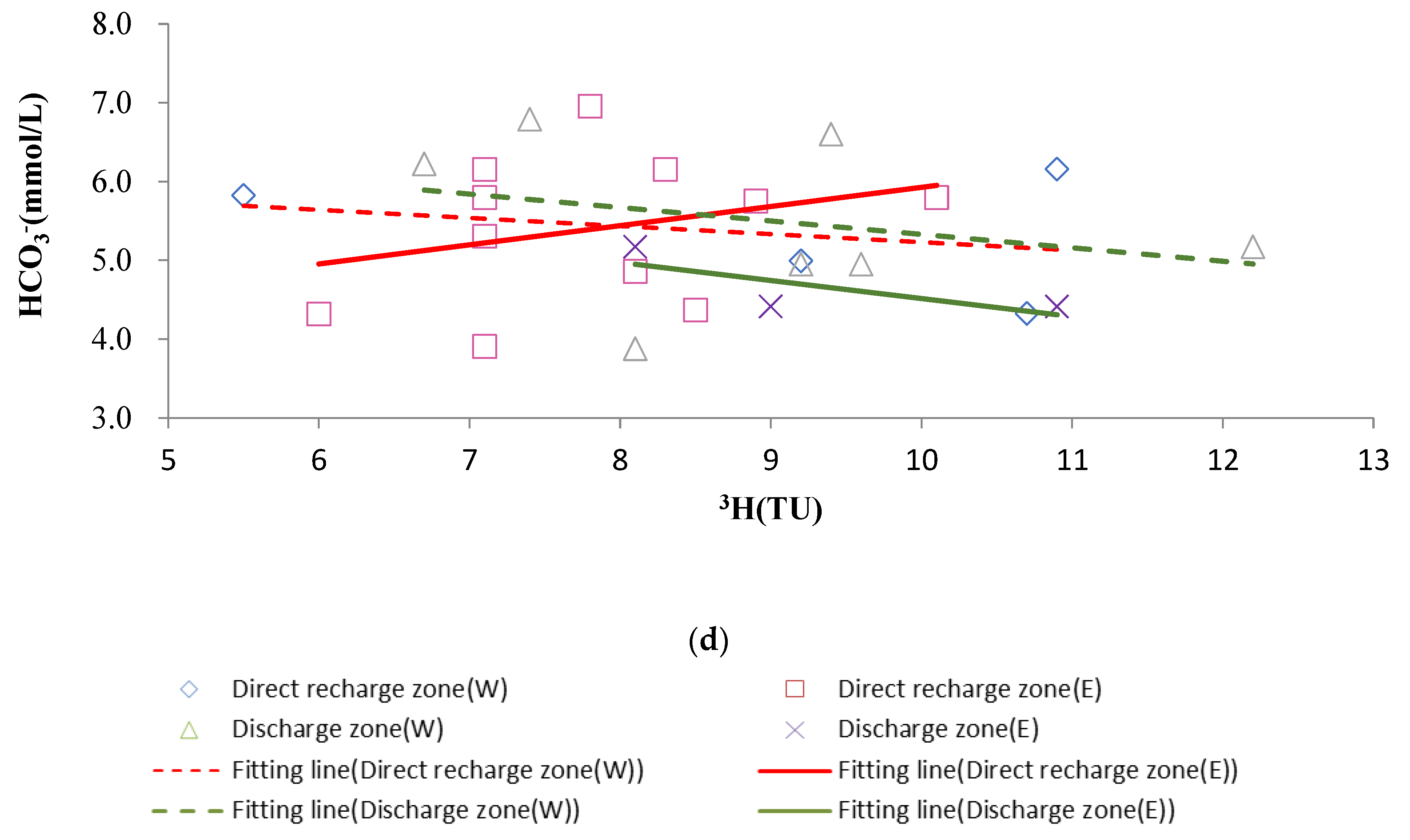
4.3. 3H Isotope Characteristics of Karst Water
- (1)
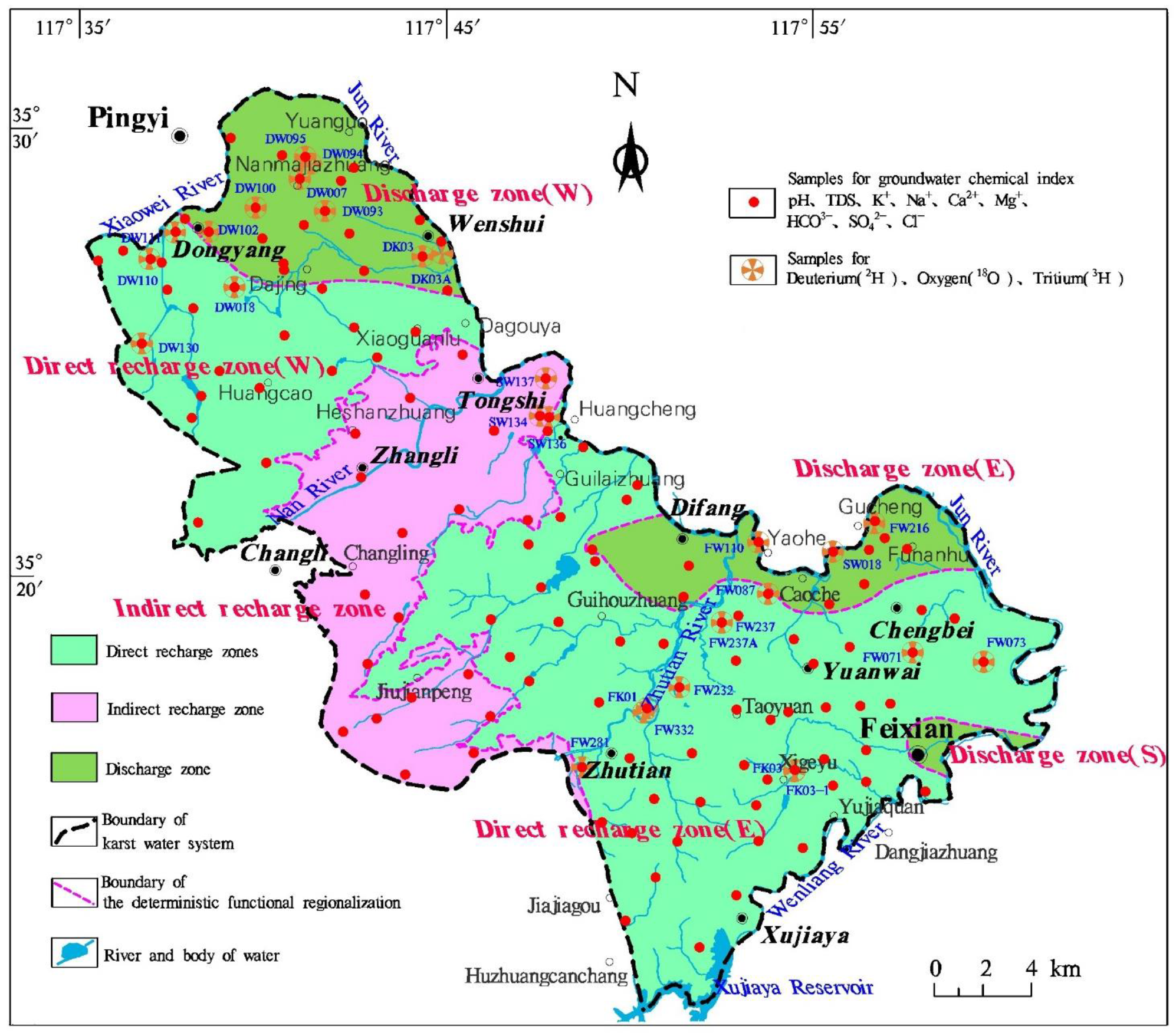
- The 3H concentration generally increased with an increase in runoff distance. The lowest concentration was observed at the boundaries of karst watersheds (i.e., sampling sites DW130 and FW071). Sampling sites with 3H concentrations <7.5 TU were found to be mainly located at the front edge of the direct recharge zones (i.e., sampling sites FW281, FW232, FW332, and FK01). Sampling sites with 3H concentrations of 7.5–8.5 TU were located primarily inside the direct recharge zones (i.e., sampling sites FW237A, FW237, FK03-1, and FW087), while sampling sites with 3H concentrations of 9.0–12.5 TU were principally inside the discharge zones and at some near-boundary areas of the direct recharge zones relative to the discharge zones.
- (2)
- Discharge zones W and E both had significantly lower 3H concentrations distributed in a belt-like pattern. These low-value areas coincided with areas featuring the large-scale artificial extraction of karst water, indicating that karst water extraction induced old karst water that flowed from the aquifer to the extraction areas.
- (3)
- Changes in 3H concentrations were larger closer to large-scale extraction areas.
4.4. Hydrochemical Evolution of Karst Water
4.4.1. Dissolution and Precipitation of Carbonate Rocks
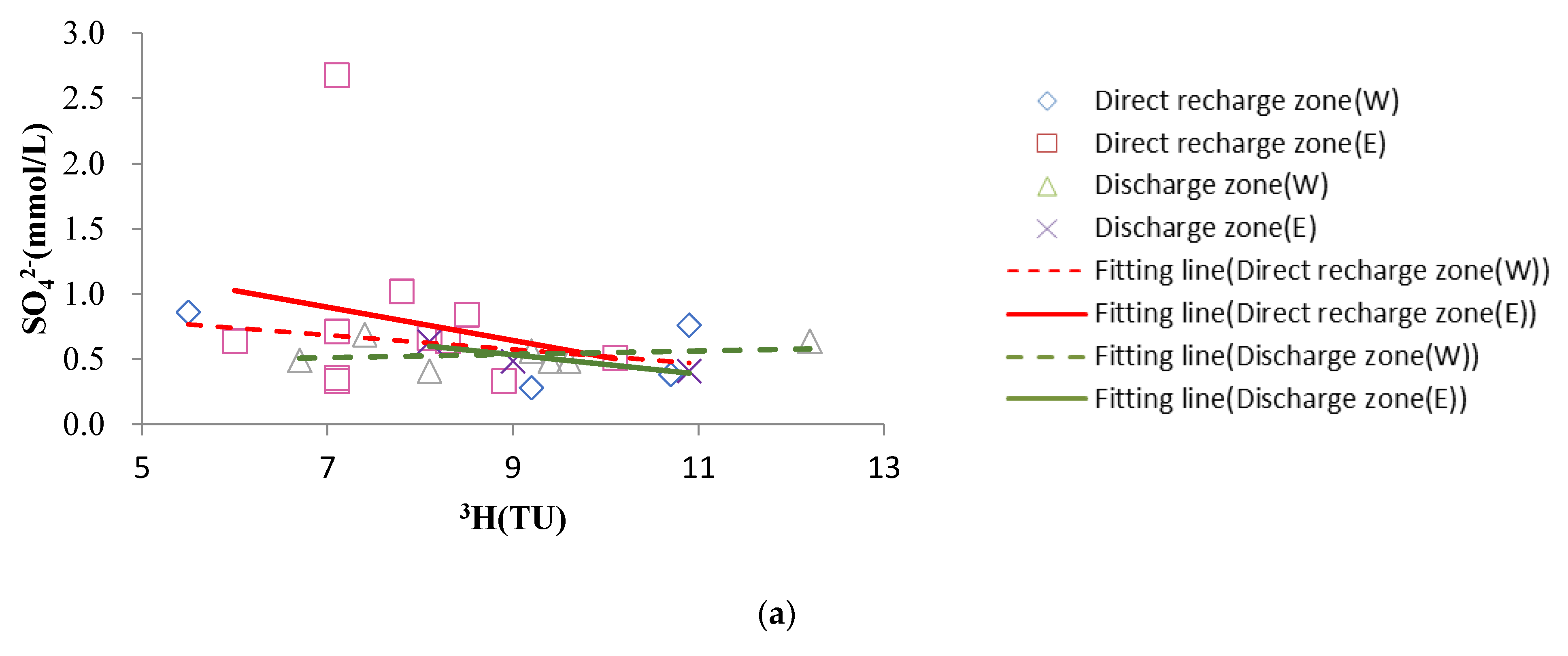
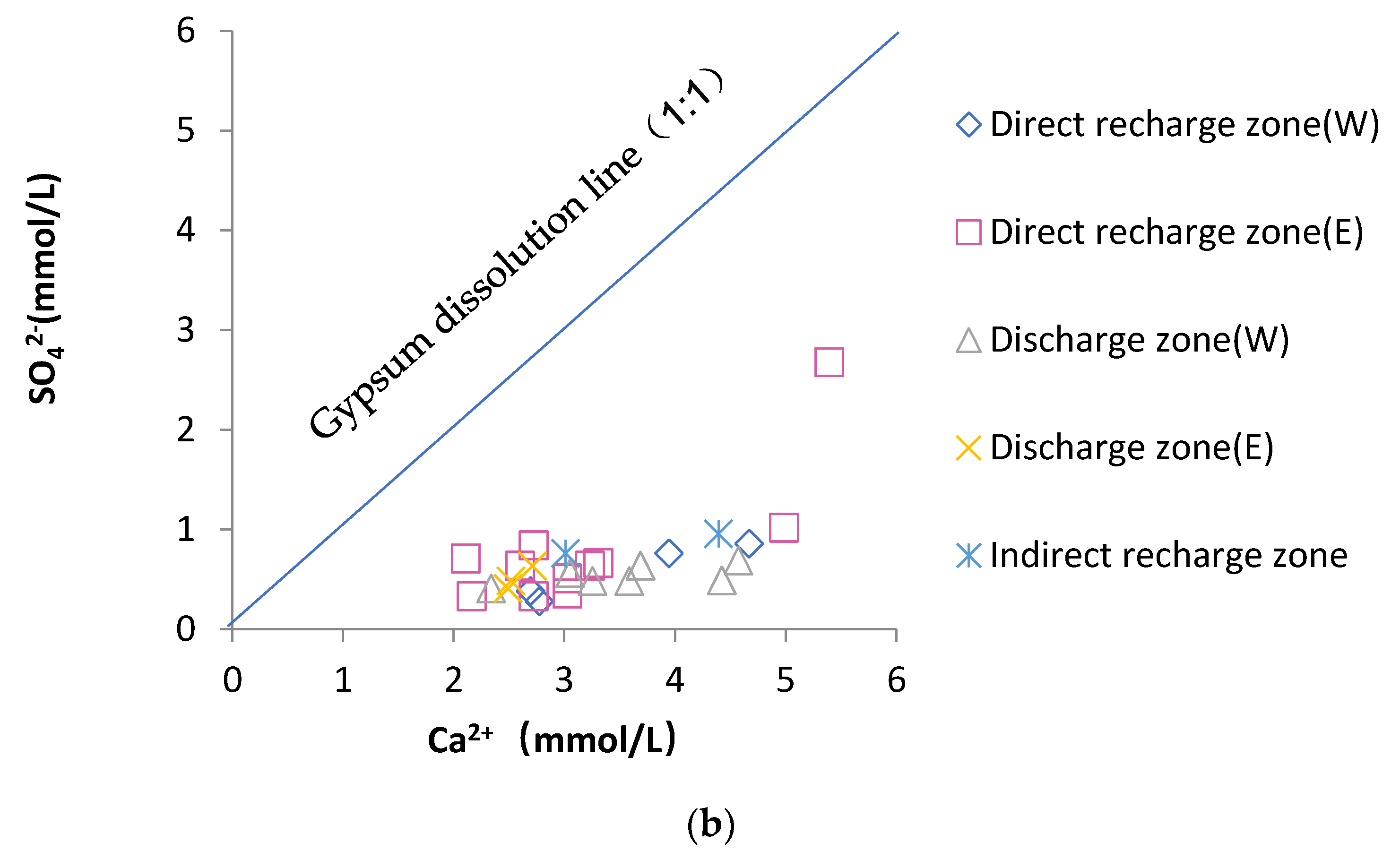
4.4.2. Dissolution of Sulfate Rocks
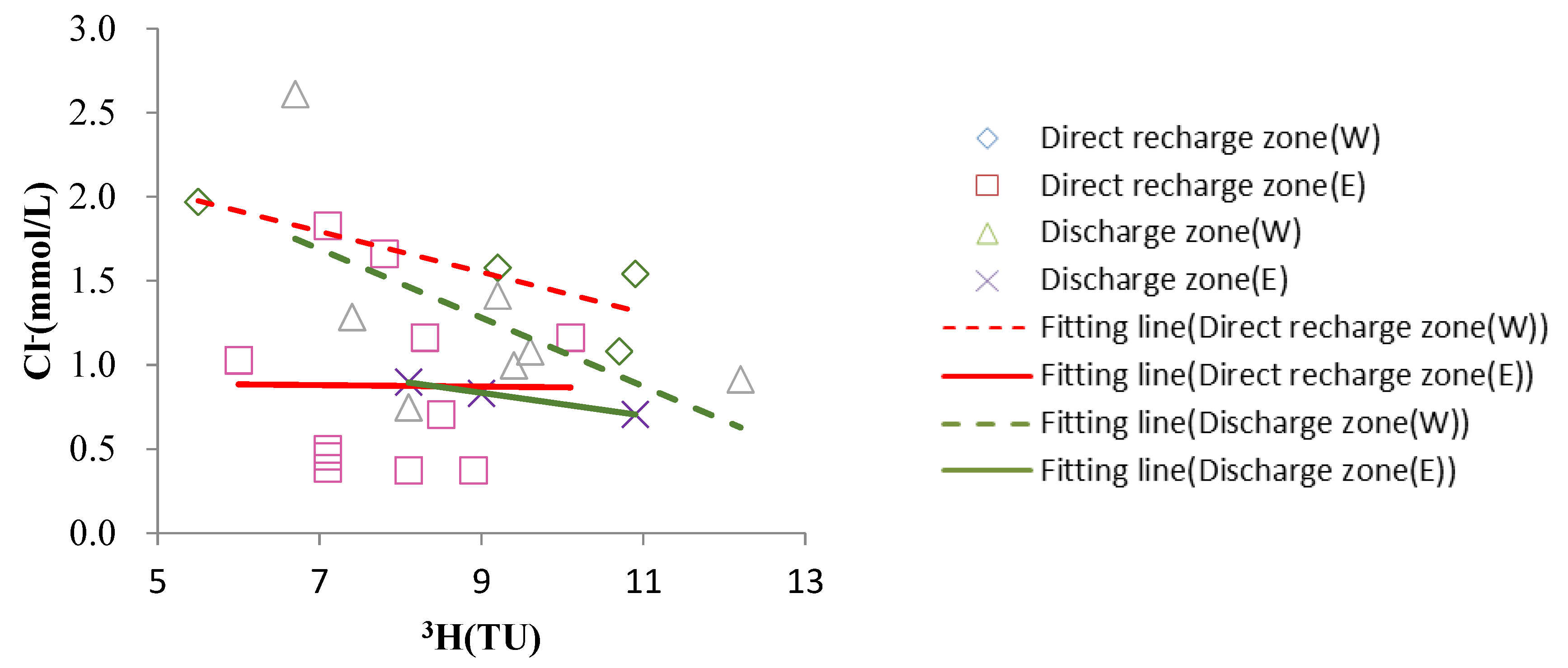
4.4.3. Dissolution of Chlorides
5. Conclusions
- (1)
- Shallow karst water of the HCO3-Ca type with a short flow path is ubiquitous in the study region, while karst water of the HCO3-Ca·Mg type with a long flow path is present in both the direct recharge and discharge zones. The interactions of karst water with carbonate, sulfate, and halite result in a variety of hydrochemical processes such as dissolution, precipitation, mixing, and dilution. Overall, karst water has a slightly lower degree of mineralization in the direct recharge zones than in the discharge zones. In contrast, the indirect recharge zone suffers from a relatively closed condition with TDS up to 1949 mg/L, indicative of long-term water–rock interactions.
- (2)
- The δ18O-δ2H fitting line of karst water in the indirect recharge zone has the closest slope to the meteoric water lines, while the slopes of the fitting lines in the direct recharge zones are significantly lower than those of the meteoric water lines. The δ18O-δ2H fitting line of recharge zone E coincides with that of discharge zone E, indicating rapid karst water recharge. Moreover, δ18O of karst water is relatively high in discharge zone W, which may be attributed to the dissolution and leaching of 18O from carbonate rocks.
- (3)
- The 3H value of karst water increases along the flow path and it is generally higher than 9.0 TU in the discharge zones, where intensive exploitation of karst water has produced increased karst water flow in the surrounding areas, led to significant mixing between karst water receiving surface infiltration recharge and karst water receiving lateral recharge, and yielded larger changes in 3H concentrations.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klimchouk, A.B.; Aksem, S.D. Gypsum karst in the western Ukraine: Hydrochemistry and solution rates. Carbonates Evaporites 2002, 17, 142–153. [Google Scholar] [CrossRef]
- White, W.B. Karst hydrology: Recent developments and open questions. Eng. Geol. 2002, 65, 85–105. [Google Scholar] [CrossRef]
- Escolero, O.A.; Marin, L.E.; Steinich, B.; Pacheco, A.J.; Cabrera, S.A.; Alcocer, J. Development of a Protection Strategy of Karst Limestone Aquifers: The Merida Yucatan, Mexico Case Study. Water Resour. Manag. 2002, 16, 351–367. [Google Scholar] [CrossRef]
- Valerie, P.; Michel, B. The protection of a karst resource from example of the Larzac karst plateau (south of France): A matter of regulations or a matter of process knowledge. Eng. Geol. 2002, 65, 107–116. [Google Scholar]
- Hess, J.W. Methods in Karst Hydrogeology. Ground Water 2008, 46, 172. [Google Scholar] [CrossRef]
- Turpaud, P.; Zini, L.; Ravbar, N.; Cucchi, F.; Petrič, M.; Urbanc, J. Development of a Protocol for the Karst Water Source Protection Zoning: Application to the Classical Karst Region (NE Italy and SW Slovenia). Water Resour. Manag. 2018, 32, 1953–1968. [Google Scholar] [CrossRef]
- Brkić, Ž.; Kuhta, M.; Hunjak, T. Groundwater flow mechanism in the well-developed karst aquifer system in the western Croatia: Insights from spring discharge and water isotopes. Catena 2018, 161, 14–26. [Google Scholar] [CrossRef]
- He, X.; Wu, J.; Guo, W. Karst Spring Protection for the Sustainable and Healthy Living: The Examples of Niangziguan Spring and Shuishentang Spring in Shanxi, China. Expo. Health 2019, 11, 153–165. [Google Scholar] [CrossRef]
- Chang, Y.; Wu, J.; Jiang, G.; Zhao, X.; Zhang, Q. Investigating the appropriate model structure for simulation of a karst catchment from the aspect of spatial complexity. Environ. Earth Sci. 2019, 78, 13. [Google Scholar] [CrossRef]
- Zaree, M.; Javadi, S.; Neshat, A. Potential detection of water resources in karst formations using APLIS model and modification with AHP and TOPSIS. J. Earth Syst. Sci. 2019, 128, 76. [Google Scholar] [CrossRef] [Green Version]
- Narany, T.S.; Bittner, D.; Disse, M.; Chiogna, G. Spatial and temporal variability in hydrochemistry of a small-scale dolomite karst environment. Environ. Earth Sci. 2019, 78, 273. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, X.; Hou, B.; Zhang, S.; Zhang, J.; Li, C.; Wang, W. Occurrence and environmental impact of coal mine goaf water in karst areas in China. J. Clean. Prod. 2020, 275, 123813. [Google Scholar] [CrossRef]
- Gao, X.; Wang, W.; Hou, B.; Gao, L.; Zhang, J.; Zhang, S.; Li, C.; Jiang, C. Analysis of karst groundwater pollution in Northern China. Carpol. Sin. 2020, 39, 287–298. [Google Scholar]
- Eftimi, R. Karst and karst water recourses of Albania and their management. Carbonates Evaporites 2020, 35, 69. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Wu, P.; Guo, Q. Trace elements and environmental isotopes as tracers of surface water–groundwater interaction: A case study at Xin’an karst water system, Shanxi Province, Northern China. Environ. Earth Sci. 2010, 59, 1223–1234. [Google Scholar] [CrossRef]
- Rao, P.N.; Prasad, K.M.; Madhusudhan, B.J.; Krishna, V.S.R.; Anand, A.V.S.S.; Madhnure, P. Impact of urbanization on groundwater quality in Vijayawada urban agglomeration, the new capital region of Andhra Pradesh, India—A baseline study. J. Geol. Soc. India 2016, 87, 539–552. [Google Scholar] [CrossRef]
- Keshavarzi, M.; Baker, A.; Kelly, B.F.J.; Andersen, M.S. River–groundwater connectivity in a karst system, Wellington, New South Wales, Australia. Hydrogeol. J. 2017, 25, 557–574. [Google Scholar] [CrossRef]
- Lesser, L.E.; Mora, A.; Moreau, C.; Mahlknecht, J.; Hernández-Antonio, A.; Ramírez, A.I.; Barrios-Piña, H. Survey of 218 organic contaminants in groundwater derived from the world’s largest untreated wastewater irrigation system: Mezquital Valley, Mexico. Chemosphere 2018, 198, 510–521. [Google Scholar] [CrossRef]
- Jampani, M.; Hülsmann, S.; Liedl, R.; Sonkamble, S.; Ahmed, S.; Amerasinghe, P. Spatio-temporal distribution and chemical characterization of groundwater quality of a wastewater irrigated system: A case study. Sci. Total Environ. 2018, 636, 1089–1098. [Google Scholar] [CrossRef]
- Hamad, A.; Baali, F.; Hadji, R.; Zerrouki, H.; Besser, H.; Mokadem, N.; Legrioui, R.; Hamed, Y. Hydrogeochemical characterization of water mineralization in Tebessa-Kasserine karst system (Tuniso-Algerian Transboundry basin). Euro-Mediterr. J. Environ. Integr. 2017, 3, 7. [Google Scholar] [CrossRef]
- Shamsi, A.; Karami, G.H.; Hunkeler, D.; Taheri, A. Isotopic and hydrogeochemical evaluation of springs discharging from high-elevation karst aquifers in Lar National Park, northern Iran. Hydrogeol. J. 2019, 27, 655–667. [Google Scholar] [CrossRef]
- Jiang, Y.; Cao, M.; Yuan, D.; Zhang, Y.; He, Q. Hydrogeological characterization and environmental effects of the deteriorating urban karst groundwater in a karst trough valley: Nanshan, SW China. Appl. Hydrogeol. 2018, 26, 1487–1497. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, D.; Chen, Q.; Tang, Y.; Chen, F. Large heterogeneity of water and nutrient supply derived from runoff of nearby rock outcrops in karst ecosystems in SW China. Catena 2018, 172, 125–131. [Google Scholar] [CrossRef]
- Doney, S.C.; Glover, D.M.; Jenkins, W.J. A model function of the global bomb tritium distribution in precipitation, 1960–1986. J. Geophys. Res. 1992, 97, 5481–5492. [Google Scholar] [CrossRef]
- Glynn, P.D.; Plummer, L.N. Geochemistry and the understanding of ground-water systems. Appl. Hydrogeol. 2005, 13, 263–287. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Xu, G.; Shu, L. Use of hydrologic time-series data for identification of hydrodynamic function and behavior in a karstic water system in China. Appl. Hydrogeol. 2011, 19, 1577–1585. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, Y.; Zeng, C.; Liu, Z.; Yang, R.; Chen, B. Effects of land cover on variations in stable hydrogen and oxygen isotopes in karst groundwater: A comparative study of three karst catchments in Guizhou Province, Southwest China. J. Hydrol. 2018, 565, 374–385. [Google Scholar] [CrossRef]
- Cardona, A.; Carlos, G.O.; Manuel, M.M.; Gerardo, O.F.; Luis, G.H. Hydrogeochemical characterization and evolution of a regional karst aquifer in the Cuatrociénegas area, Mexico. Environ. Earth Sci. 2018, 77, 785. [Google Scholar] [CrossRef]
- Eltarabily, M.G.; Negm, A.; Yoshimura, C.; Takemura, J. Groundwater Modeling in Agricultural Watershed under Different Recharge and Discharge Scenarios for Quaternary Aquifer Eastern Nile Delta, Egypt. Environ. Model. Assess. 2017, 23, 289–308. [Google Scholar] [CrossRef]
- Luiz, T.B.P.; da Silva, J.L.S.; Filho, L.L.V.D. Hydrogeochemical modeling of fluoride contents in groundwater in outcrop area of Guarani Aquifer System, southern Brazil. Geol. USP-Ser. Cient. 2019, 19, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Viaroli, S.; Di Curzio, D.; Lepore, D.; Mazza, R. Multiparameter daily time-series analysis to groundwater recharge assessment in a caldera aquifer: Roccamonfina Volcano, Italy. Sci. Total Environ. 2019, 676, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, Z.H.; Wang, T. Groundwater source identification incarbonate-hosted deposit using hydrogeochemistry, hydrogen and oxygen isotope method. Hydrogeol. Eng. Geol. 2019, 46, 19–26. [Google Scholar]
- Guo, Y.; Qin, D.; Sun, J.; Li, L.; Li, F.; Huang, J. Recharge of River Water to Karst Aquifer Determined by Hydrogeochemistry and Stable Isotopes. Water 2019, 11, 479. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Luo, D.; Hong, A.; Ham, B.; Xie, S.; Ming, X.; Wang, Z.; Pang, Z. Hydrogeochemistry and geothermometry of the carbonate-evaporite aquifers controlled by deep-seated faults using major ions and environmental isotopes. J. Hydrol. 2019, 579, 124116. [Google Scholar] [CrossRef]
- Li, C.; Gao, X. Assessment of Groundwater Quality at Yuncheng Basin: Denotation for the Water Management in China. Ground Water 2018, 57, 492–503. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Wang, W.; Zhang, X.; Zhang, X.; Jiang, C.; Wang, Y. Hydro-biogeochemical processes of surface water leakage into groundwater in large scale karst water system: A case study at Jinci, northern China. J. Hydrol. 2021, 596, 125691. [Google Scholar] [CrossRef]
- Iacurto, S.; Grelle, G.; De Filippi, F.M.; Sappa, G. Karst Spring Recharge Areas and Discharge Relationship by Oxygen-18 and Deuterium Isotopes Analyses: A Case Study in Southern Latium Region, Italy. Appl. Sci. 2020, 10, 1882. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.G.; Veláusqez, L.; Fleming, P. Origin of spring waters employing a multiparametric approach with special focus on stable isotopes 2H and 18O in the Lagoasanta Karst region, Southern Brazil. Isot. Environ. Health Stud. 2020, 56, 158–169. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Lian, J.; Fu, Z.; Nie, Y. Seasonal recharge of spring and stream waters in a karst catchment revealed by isotopic and hydrochemical analyses. J. Hydrol. 2020, 591, 125595. [Google Scholar] [CrossRef]
- Wang, W. Functional Regionalization and Renewable Capacity of Karst Water System in the Semi-humid Area, Central Southern Shandong Province. Ph.D. Thesis, China University of Geosciences (Beijing), Beijing, China, 2019. [Google Scholar]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, M.J.; Wang, S.J.; Li, Z.Q.; Li, X.F. Spatial distribution of δ18O in China’s precipitation based on a secondary variable of temperature. Prog. Geogr. 2011, 30, 1387–1394. [Google Scholar]



| Sample No. | Sampling Date | Sampling Location | Sampling Depth (m) | Notes |
|---|---|---|---|---|
| Fxj | November 2013 | Feixian County | Precipitation | |
| SW134 | November 2013 | Pingyi County | 85–200 | Indirect recharge zone |
| SW136 | November 2013 | Pingyi County | 9–88 | |
| SW137 | November 2013 | Pingyi County | 8–27 | |
| DW018 | November 2013 | Pingyi County | 60–187 | Direct recharge zone(W) |
| DW110 | November 2013 | Pingyi County | 18–26 | |
| DW111 | November 2013 | Pingyi County | 0–141 | |
| DW130 | November 2013 | Pingyi County | 14–170 | |
| FK03-1 | October 2013 | Feixian County | 4–32 | Direct recharge zone(E) |
| FW281 | November 2013 | Feixian County | 30–73 | |
| FW237A | October 2013 | Pingyi County | 80–100 | |
| FW332 | October 2013 | Feixian County | 18–80 | |
| FW071 | October 2013 | Feixian County | 50–120 | |
| FW073 | October 2013 | Feixian County | 0–88 | |
| FW237 | October 2013 | Pingyi County | 80–100 | |
| FW087 | November 2013 | Feixian County | 40–170 | |
| FK03 | October 2013 | Feixian County | 29–202 | |
| FK01 | October 2013 | Feixian County | 80–325 | |
| FW232 | November 2013 | Feixian County | 200–370 | |
| DW093 | November 2013 | Pingyi County | 28–40 | Discharge zone(W) |
| DW094 | October 2013 | Pingyi County | 16–20 | |
| DK03A | October 2013 | Pingyi County | 0–301 | |
| DW007 | October 2013 | Pingyi County | 40–150 | |
| DW100 | November 2013 | Pingyi County | 180 | |
| DW102 | November 2013 | Pingyi County | 150 | |
| DK03 | October 2013 | Pingyi County | 0–301 | |
| SW018 | October 2013 | Feixian County | 15–88 | Discharge zone(E) |
| FW110 | October 2013 | Feixian County | 20–200 | |
| SW216 | October 2013 | Feixian County | 15–200 |
| Sample No. | pH | δ2H (‰) | δ18O (‰) | 3H (TU) | TDS (mg/L) | K+ (mg/L) | K+ (mmol/L) | Na+ (mg/L) | Na+ (mmol/L) | Ca2+ (mg/L) | Ca2+ (mmol/L) | Mg2+ (mg/L) | Mg2+ (mmol/L) | HCO3 (mg/L) | HCO3 (mmol/L) | SO42 (mg/L) | SO42 (mmol/L) | Cl (mg/L) | Cl (mmol/L) | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fxj | / | −60 | −9.2 | 12.6 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | Precipitation |
| SW134 | 7.6 | −64 | −8.3 | 7.2 | 678.2 | 0.89 | 0.023 | 16.7 | 0.72 | 120.4 | 3.01 | 26.5 | 1.10 | 288.7 | 4.73 | 73.1 | 0.76 | 36.5 | 1.03 | Indirect recharge zone |
| SW136 | 7.2 | −62 | −8.0 | 8.9 | 1949.2 | 1.56 | 0.040 | 66.7 | 2.90 | 415.5 | 10.39 | 71.1 | 2.96 | 254.2 | 4.17 | 188.6 | 1.96 | 410.3 | 11.56 | |
| SW137 | 7.4 | −56 | −7.3 | 8.0 | 929.6 | 1.11 | 0.028 | 32.0 | 1.39 | 175.8 | 4.39 | 32.3 | 1.35 | 389.1 | 6.38 | 91.9 | 0.96 | 86.6 | 2.44 | |
| DW018 | 7.4 | −60 | −8.3 | 10.9 | 859.9 | 1.11 | 0.028 | 20.0 | 0.87 | 157.8 | 3.95 | 32.7 | 1.36 | 375.6 | 6.16 | 73.1 | 0.76 | 54.7 | 1.54 | Direct recharge zone(W) |
| DW110 | 7.3 | −62 | −8.1 | 9.2 | 695.4 | 1.11 | 0.028 | 10.0 | 0.43 | 110.9 | 2.77 | 34.6 | 1.44 | 304.7 | 4.99 | 26.9 | 0.28 | 56.0 | 1.58 | |
| DW111 | 7.8 | −59 | −8.0 | 10.7 | 519.7 | 0.44 | 0.011 | 7.5 | 0.33 | 107.9 | 2.70 | 13.6 | 0.57 | 263.9 | 4.33 | 36.7 | 0.38 | 38.3 | 1.08 | |
| DW130 | 7.5 | −62 | −8.8 | 5.5 | 898.7 | 0.67 | 0.017 | 26.2 | 1.14 | 186.8 | 4.67 | 23.0 | 0.96 | 355.2 | 5.82 | 82.5 | 0.86 | 69.9 | 1.97 | |
| FK03–1 | 7.8 | −58 | −8.1 | 8.3 | 793.2 | 0.33 | 0.008 | 14.2 | 0.62 | 129.2 | 3.23 | 45.7 | 1.90 | 375.4 | 6.15 | 61.2 | 0.64 | 41.3 | 1.16 | Direct recharge zone(E) |
| FW281 | 7.4 | −61 | −8.7 | 7.1 | 554.2 | 0.44 | 0.011 | 2.5 | 0.11 | 121.1 | 3.03 | 11.1 | 0.46 | 323.7 | 5.31 | 34.3 | 0.36 | 17.7 | 0.50 | |
| FW237A | 7.6 | −60 | −8.5 | 7.8 | 964.2 | 0.33 | 0.008 | 20.0 | 0.87 | 199.4 | 4.99 | 22.8 | 0.95 | 424.4 | 6.96 | 97.9 | 1.02 | 59.0 | 1.66 | |
| FW332 | 7.9 | −59 | −8.4 | 7.1 | 1167.3 | 1.22 | 0.031 | 36.0 | 1.57 | 215.7 | 5.39 | 39.5 | 1.65 | 375.4 | 6.15 | 257.1 | 2.68 | 64.9 | 1.83 | |
| FW071 | 7.7 | −60 | −8.5 | 6.0 | 596.8 | 0.33 | 0.008 | 13.3 | 0.58 | 104.0 | 2.60 | 28.0 | 1.16 | 263.6 | 4.32 | 61.3 | 0.64 | 36.5 | 1.03 | |
| FW073 | 7.4 | −62 | −8.6 | 8.9 | 579.8 | 0.33 | 0.008 | 3.3 | 0.14 | 108.9 | 2.72 | 22.8 | 0.95 | 350.9 | 5.75 | 31.8 | 0.33 | 13.3 | 0.37 | |
| FW237 | 7.4 | −61 | −8.5 | 8.1 | 662.6 | 0.22 | 0.006 | 4.2 | 0.18 | 132.3 | 3.31 | 23.5 | 0.98 | 296.5 | 4.86 | 63.7 | 0.66 | 13.3 | 0.37 | |
| FW087 | 8.0 | −61 | −8.3 | 8.5 | 586.8 | 0.44 | 0.011 | 7.5 | 0.33 | 108.9 | 2.72 | 26.5 | 1.11 | 266.6 | 4.37 | 80.8 | 0.84 | 25.1 | 0.71 | |
| FK03 | 7.5 | −59 | −8.2 | 10.1 | 718.9 | 0.44 | 0.011 | 11.7 | 0.51 | 121.1 | 3.03 | 39.5 | 1.65 | 353.6 | 5.80 | 49.0 | 0.51 | 41.3 | 1.16 | |
| FK01 | 7.7 | −61 | −8.6 | 7.1 | 579.5 | 1.33 | 0.034 | 8.8 | 0.38 | 86.5 | 2.16 | 35.8 | 1.49 | 353.6 | 5.80 | 31.8 | 0.33 | 16.2 | 0.46 | |
| FW232 | 7.9 | −62 | −8.6 | 7.1 | 533.9 | 0.44 | 0.011 | 5.0 | 0.22 | 84.4 | 2.11 | 32.7 | 1.36 | 238.5 | 3.91 | 68.4 | 0.71 | 13.7 | 0.39 | |
| DW093 | 7.2 | −70 | −8.9 | 7.4 | 868.1 | 0.22 | 0.006 | 13.3 | 0.58 | 183.0 | 4.57 | 16.5 | 0.69 | 414.2 | 6.79 | 66.0 | 0.69 | 45.6 | 1.28 | Discharge zone(W) |
| DW094 | 7.5 | −59 | −8.2 | 9.2 | 681.7 | 1.44 | 0.037 | 15.0 | 0.65 | 122.1 | 3.05 | 26.5 | 1.11 | 302.0 | 4.95 | 53.9 | 0.56 | 50.1 | 1.41 | |
| DK03A | 7.4 | −59 | −8.3 | 8.4 | 725.6 | 0.44 | 0.011 | 10.8 | 0.47 | 143.5 | 3.59 | 22.2 | 0.93 | 402.6 | 6.60 | 46.5 | 0.48 | 35.4 | 1.00 | |
| DW007 | 7.6 | −61 | −8.5 | 12.2 | 714.3 | 0.44 | 0.011 | 7.9 | 0.34 | 147.5 | 3.69 | 21.0 | 0.87 | 315.6 | 5.17 | 61.2 | 0.64 | 32.4 | 0.91 | |
| DW100 | 7.7 | −56 | −7.6 | 9.6 | 656.0 | 0.44 | 0.011 | 11.7 | 0.51 | 130.2 | 3.26 | 18.5 | 0.77 | 302.0 | 4.95 | 46.5 | 0.48 | 38.3 | 1.08 | |
| DW102 | 7.5 | −59 | −8.3 | 6.7 | 880.5 | 0.56 | 0.014 | 15.0 | 0.65 | 176.9 | 4.42 | 32.3 | 1.35 | 379.7 | 6.22 | 47.1 | 0.49 | 92.7 | 2.61 | |
| DK03 | 7.9 | −60 | −8.4 | 8.1 | 500.1 | 0.33 | 0.008 | 5.8 | 0.25 | 93.6 | 2.34 | 20.4 | 0.85 | 236.7 | 3.88 | 39.2 | 0.41 | 26.5 | 0.75 | |
| SW018 | 7.6 | −60 | −8.3 | 10.9 | 530.5 | 0.56 | 0.014 | 9.2 | 0.40 | 99.7 | 2.49 | 16.7 | 0.69 | 269.3 | 4.41 | 39.2 | 0.41 | 25.1 | 0.71 | Discharge zone(E) |
| FW110 | 7.4 | −58 | −8.0 | 8.1 | 606.4 | 1.7 | 0.044 | 15.0 | 0.65 | 108.5 | 2.71 | 21.9 | 0.91 | 315.5 | 5.17 | 60.8 | 0.63 | 31.9 | 0.90 | |
| SW216 | 7.6 | −58 | −8.0 | 9.0 | 535.7 | 1.22 | 0.031 | 9.2 | 0.40 | 100.7 | 2.52 | 21.0 | 0.87 | 269.3 | 4.41 | 46.5 | 0.48 | 29.5 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wang, W.; Zhang, G.; Zhu, H.; Wang, J.; Guo, Y. Hydrochemical and Isotope (18O, 2H and 3H) Characteristics of Karst Water in Central Shandong Province: A Case Study of the Pingyi-Feixian Region. Minerals 2022, 12, 154. https://doi.org/10.3390/min12020154
Liu C, Wang W, Zhang G, Zhu H, Wang J, Guo Y. Hydrochemical and Isotope (18O, 2H and 3H) Characteristics of Karst Water in Central Shandong Province: A Case Study of the Pingyi-Feixian Region. Minerals. 2022; 12(2):154. https://doi.org/10.3390/min12020154
Chicago/Turabian StyleLiu, Chunhua, Wei Wang, Guanghui Zhang, Henghua Zhu, Jingjing Wang, and Yan Guo. 2022. "Hydrochemical and Isotope (18O, 2H and 3H) Characteristics of Karst Water in Central Shandong Province: A Case Study of the Pingyi-Feixian Region" Minerals 12, no. 2: 154. https://doi.org/10.3390/min12020154
APA StyleLiu, C., Wang, W., Zhang, G., Zhu, H., Wang, J., & Guo, Y. (2022). Hydrochemical and Isotope (18O, 2H and 3H) Characteristics of Karst Water in Central Shandong Province: A Case Study of the Pingyi-Feixian Region. Minerals, 12(2), 154. https://doi.org/10.3390/min12020154





