Characterisation of the Hydration Products of a Chemically and Mechanically Activated High Coal Fly Ash Hybrid Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Hybrid Coal Fly Ash Cement Paste
2.2.2. Characterisation Techniques
2.2.3. Hybrid Coal Fly Ash Cement Mortar
3. Results and Discussion
3.1. Characterization of the Starting Materials
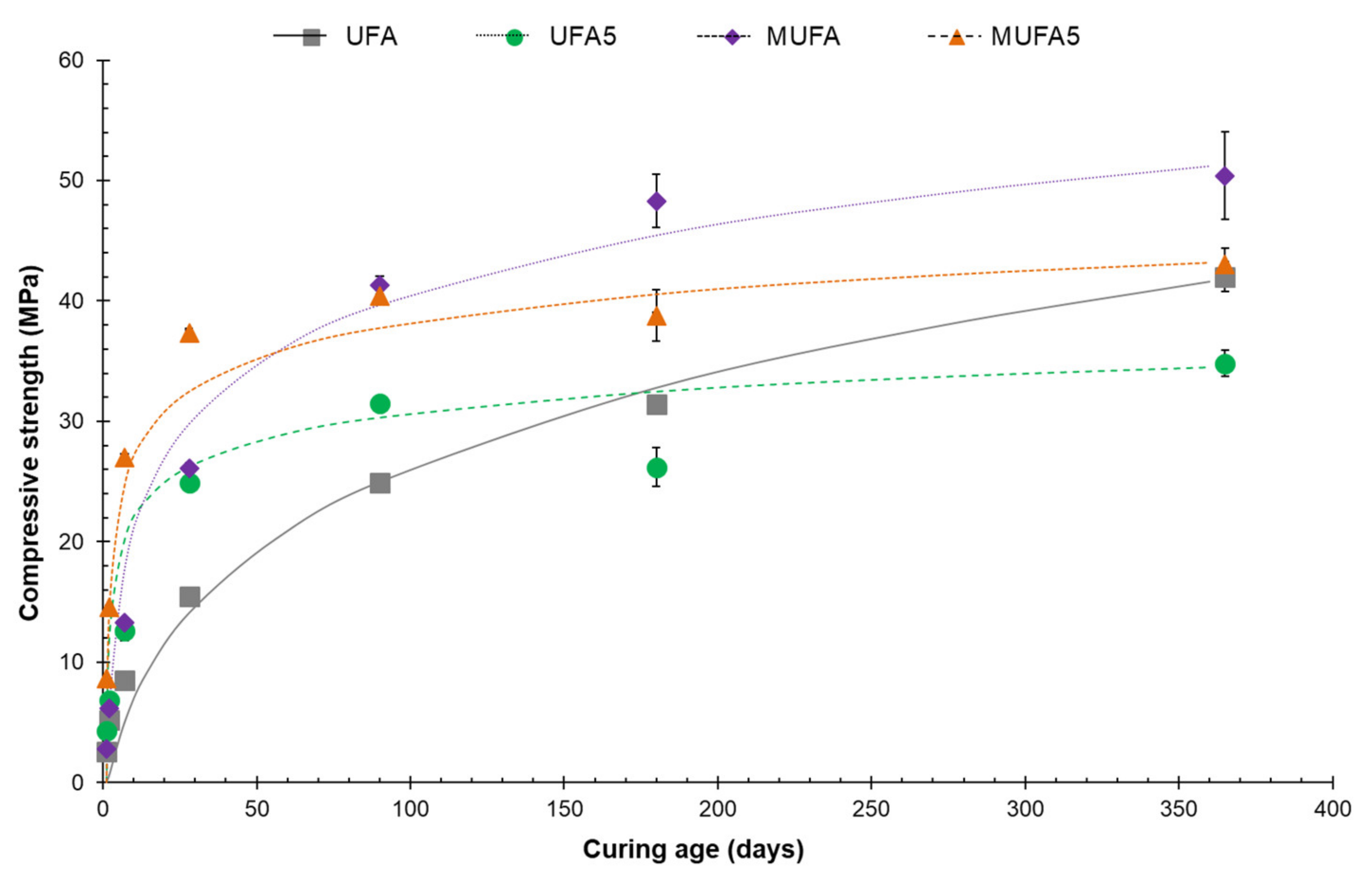
3.2. Mortar Compressive Strength of Hybrid Coal Fly Ash Cement
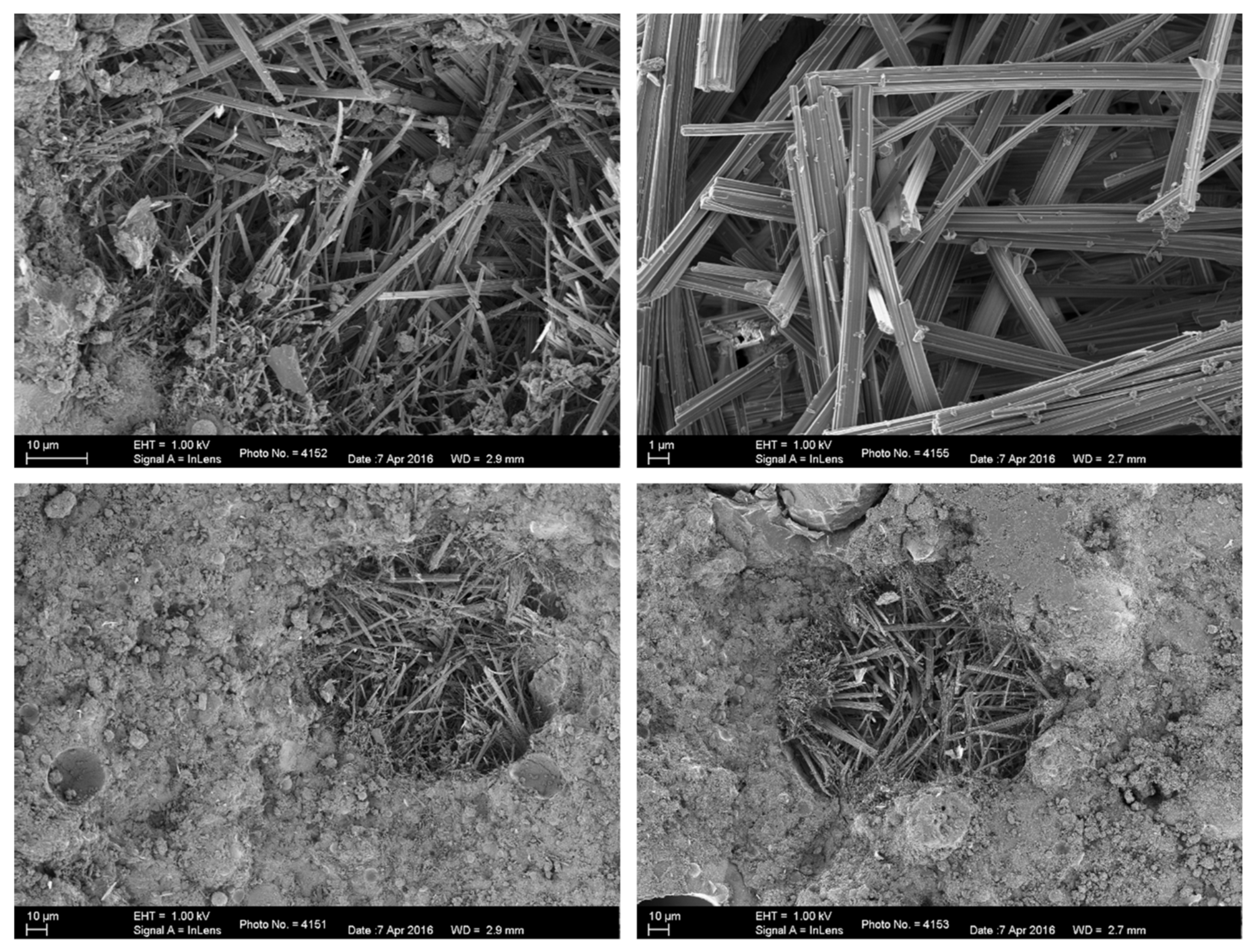
3.3. Characterisation of Hydrating Hybrid Coal Fly Ash Cement Paste
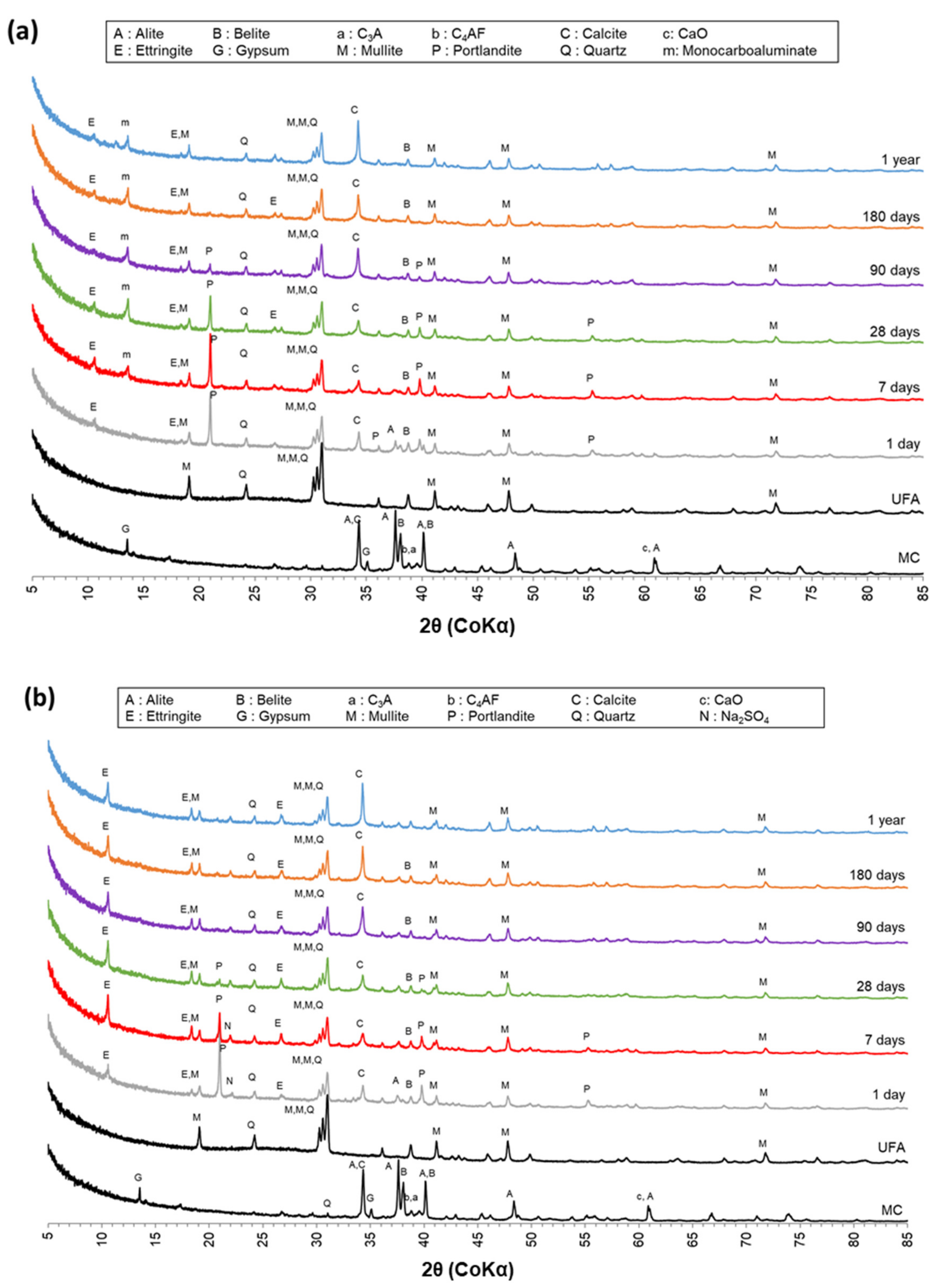
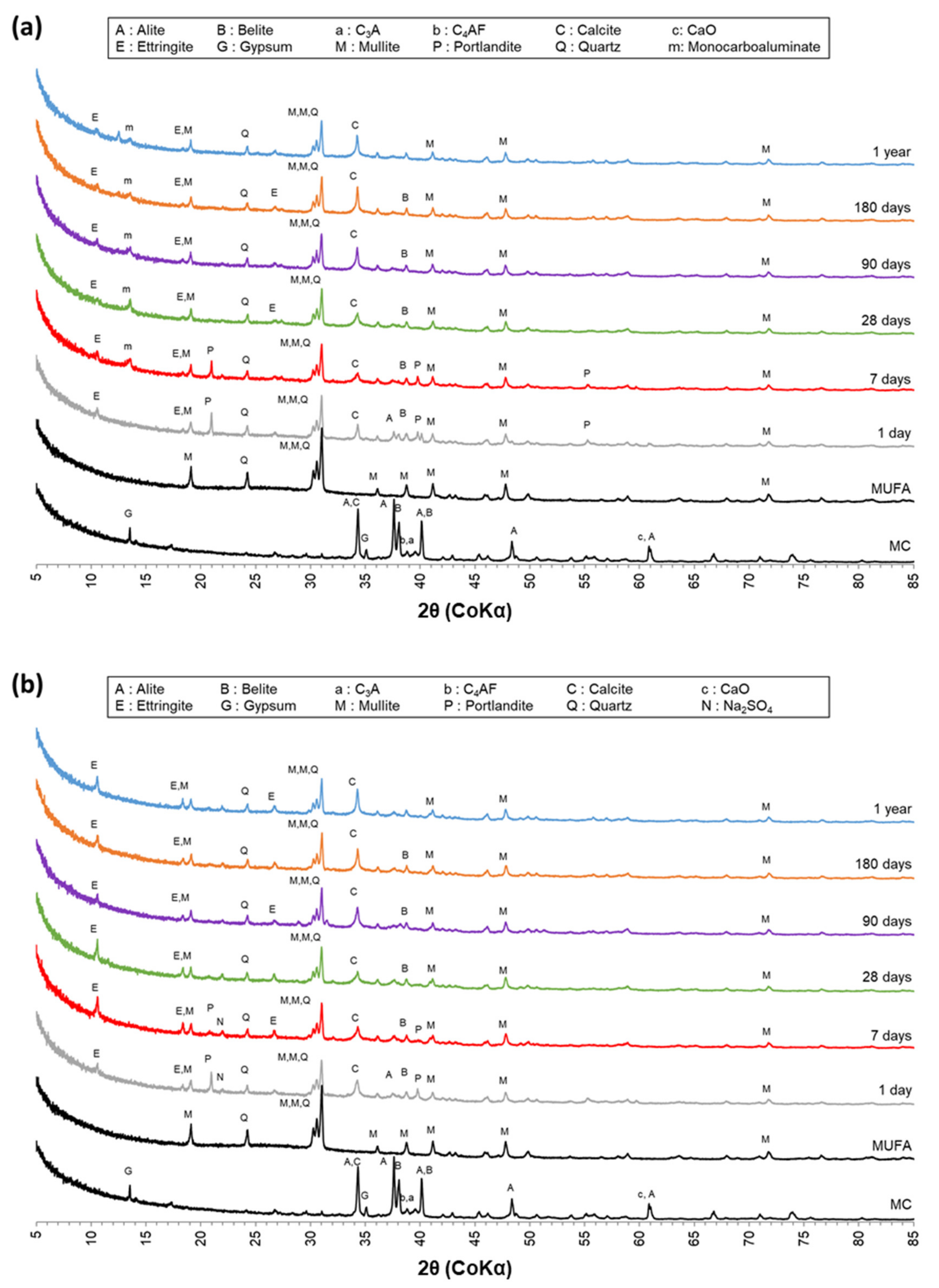
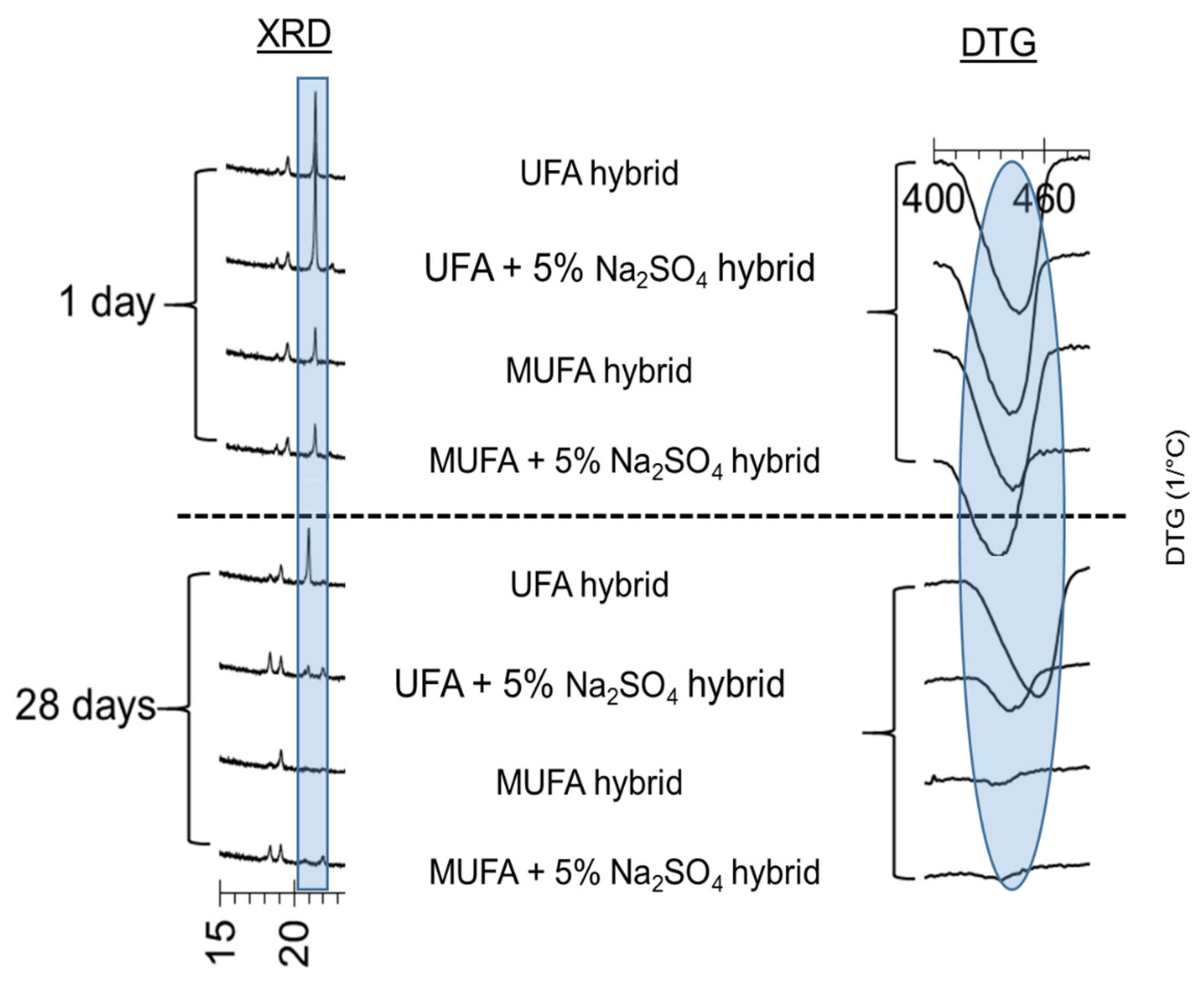
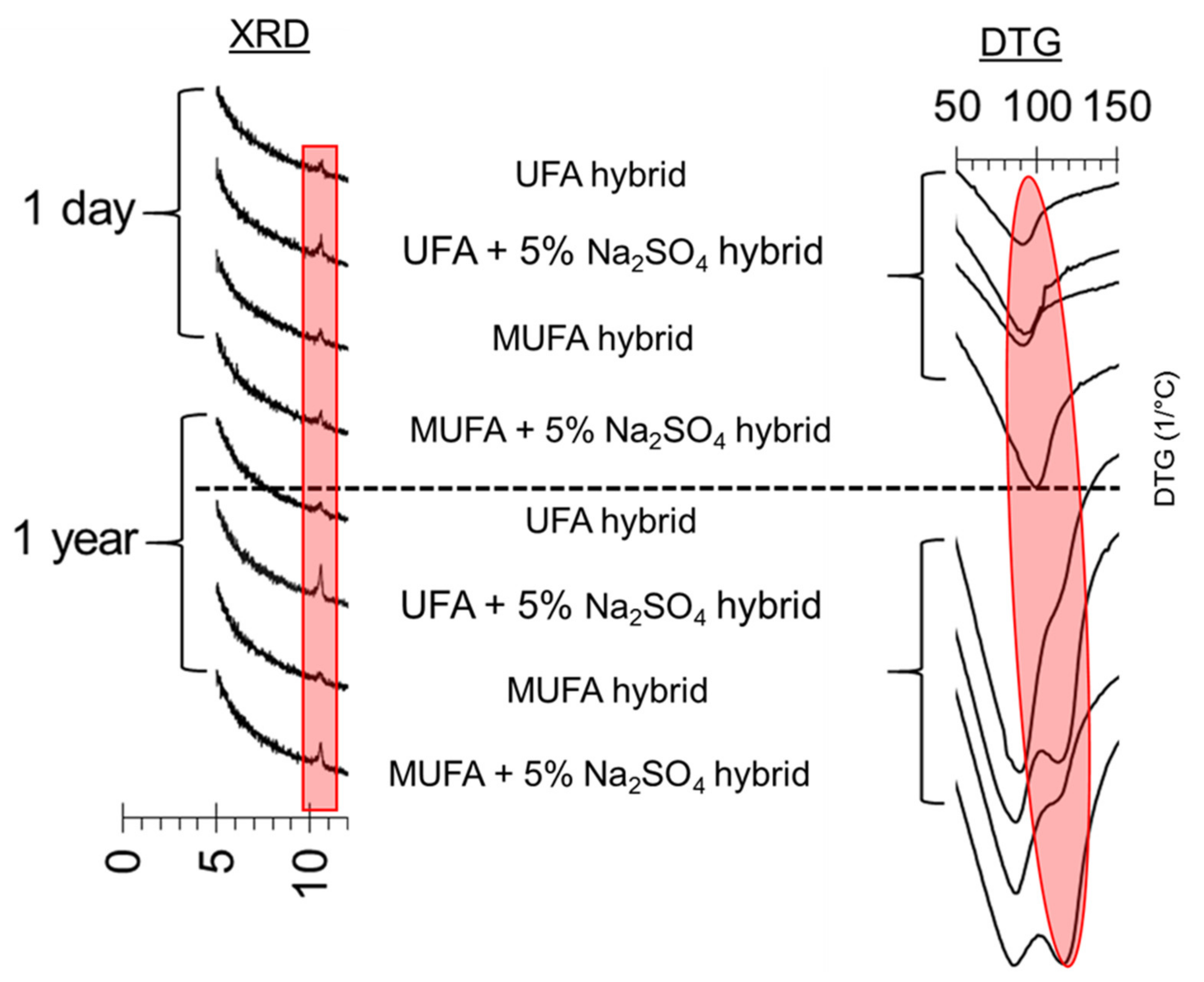
3.3.1. X-ray Powder Diffraction (XRD) Analysis
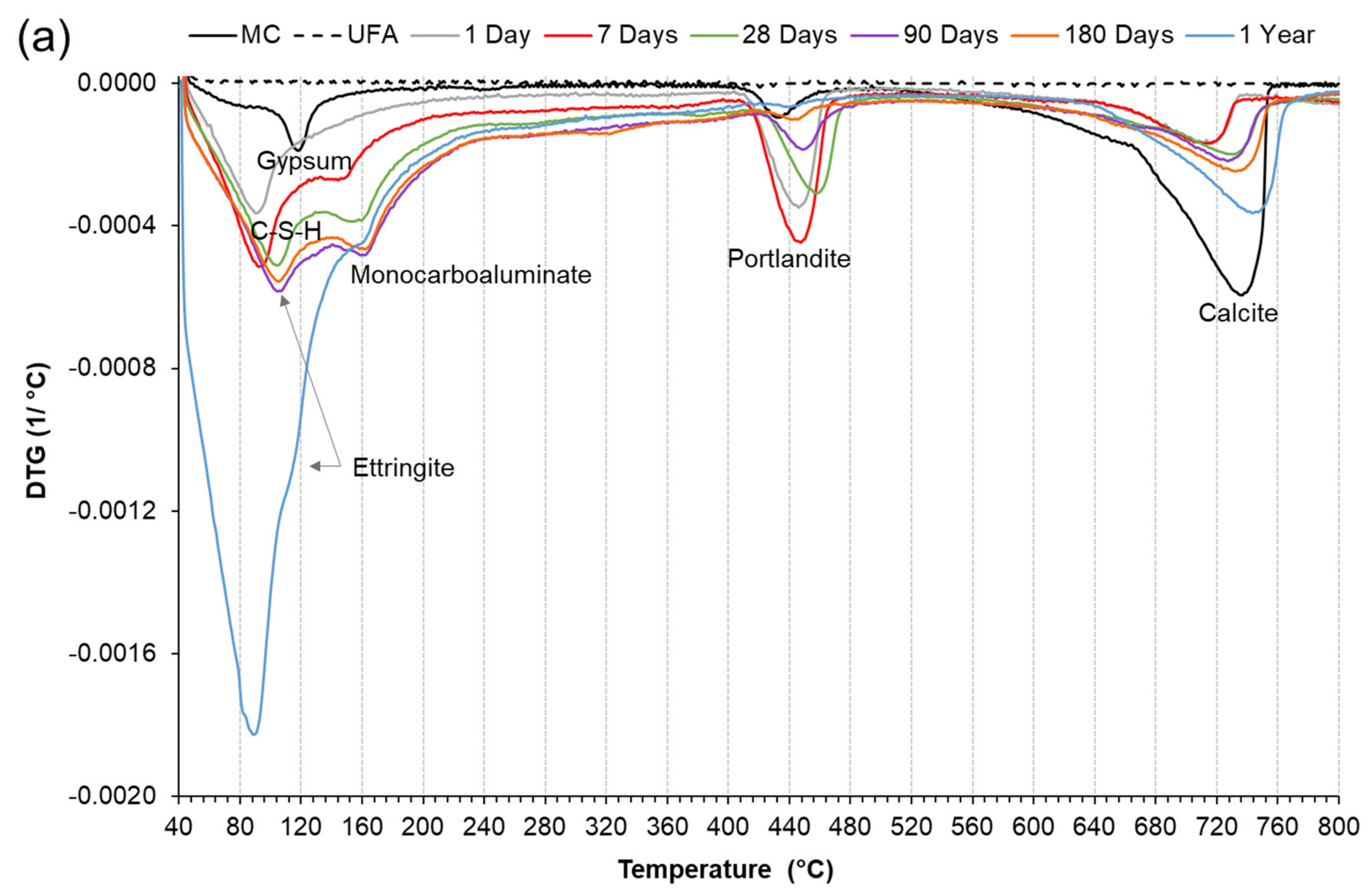
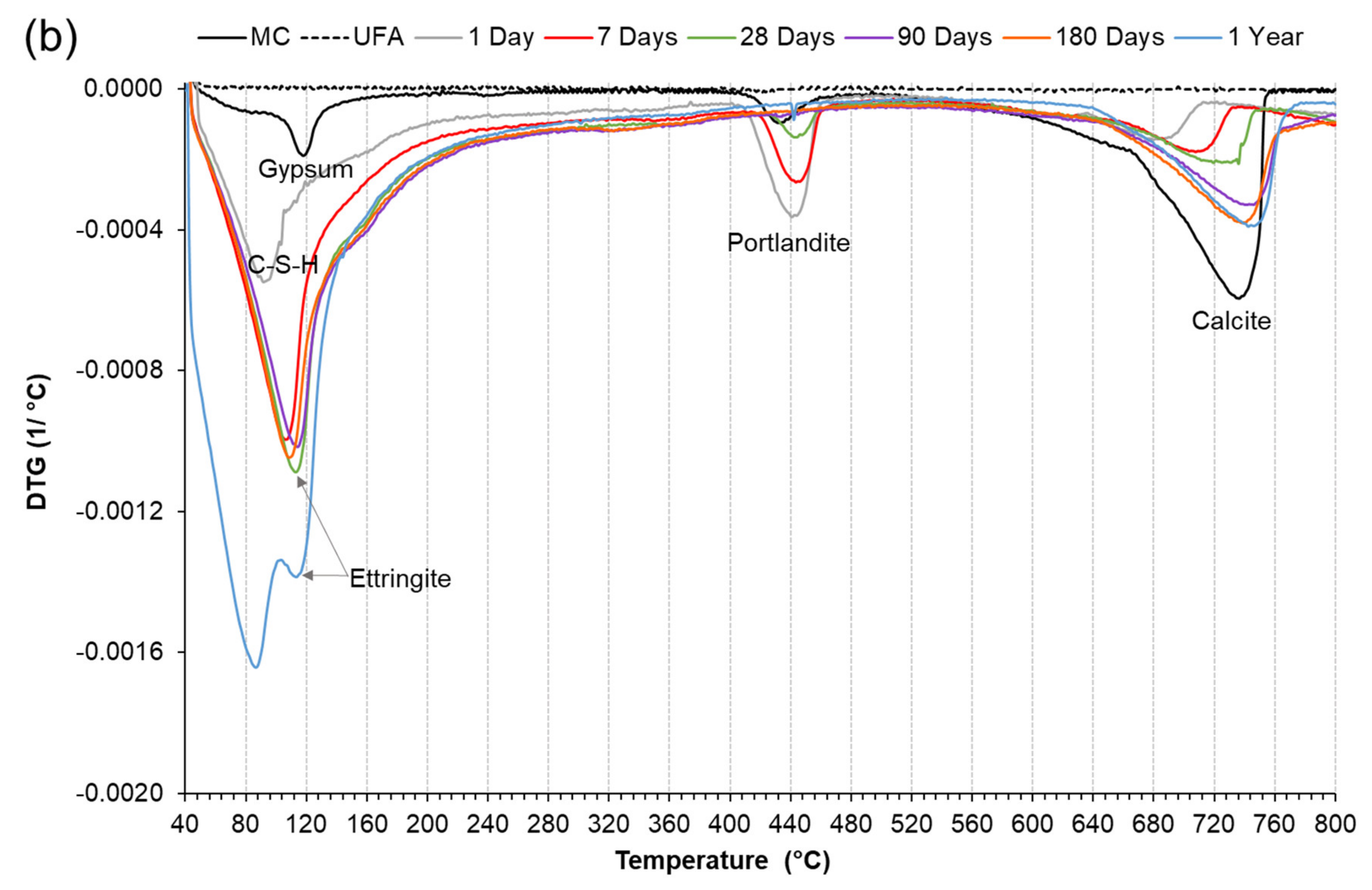
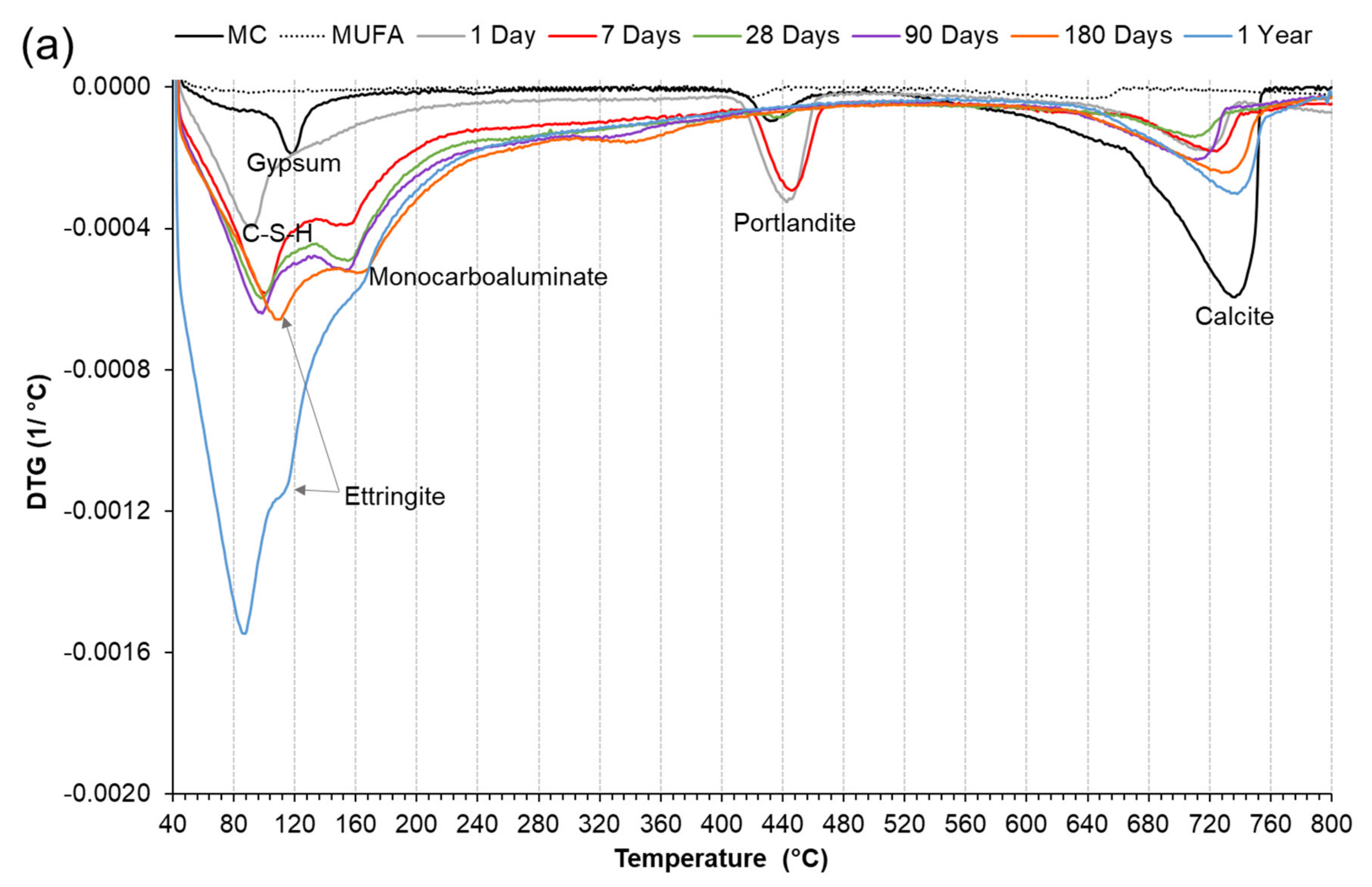
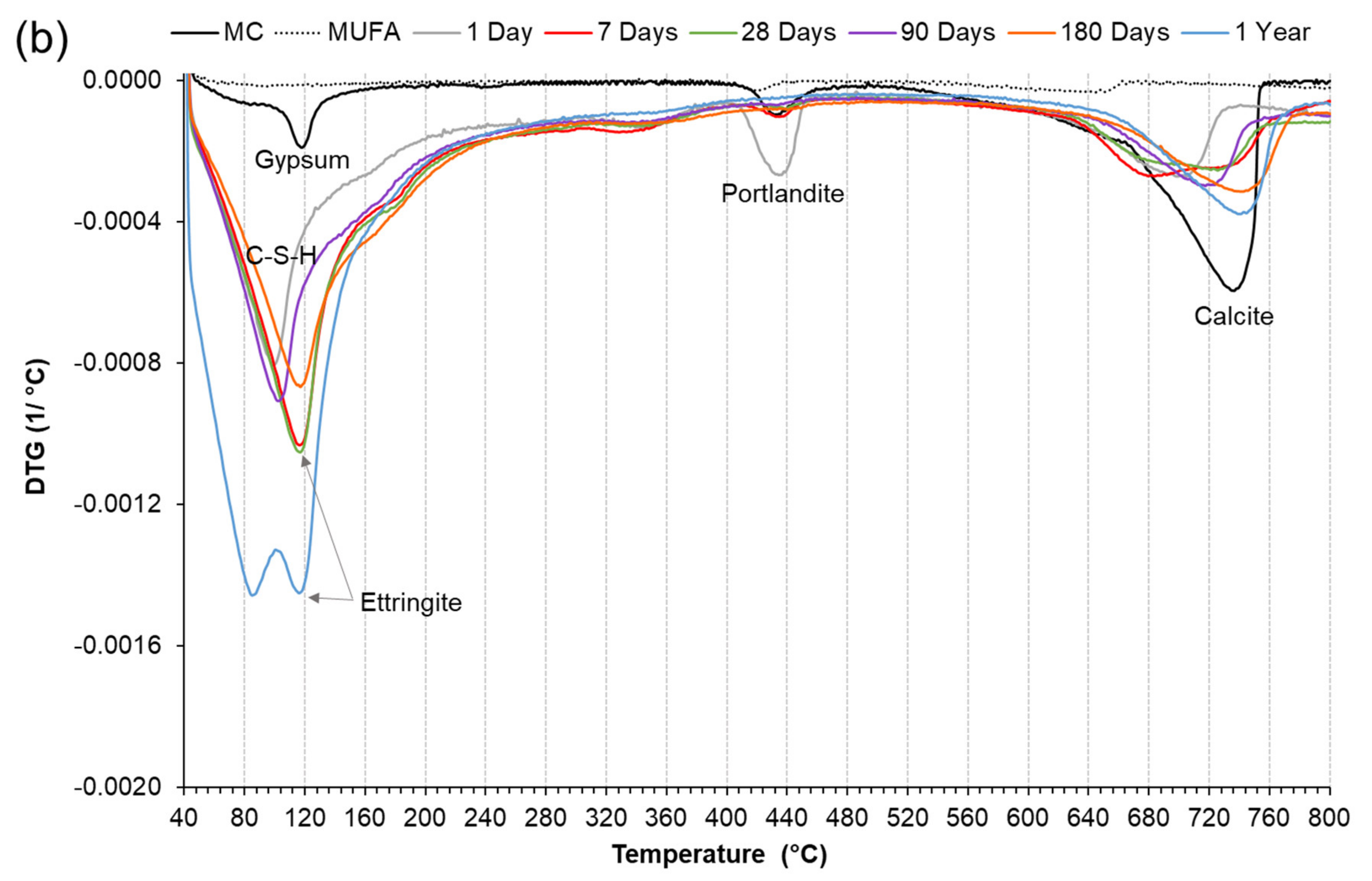
3.3.2. Derivative Thermogravimetric Analysis (DTG)
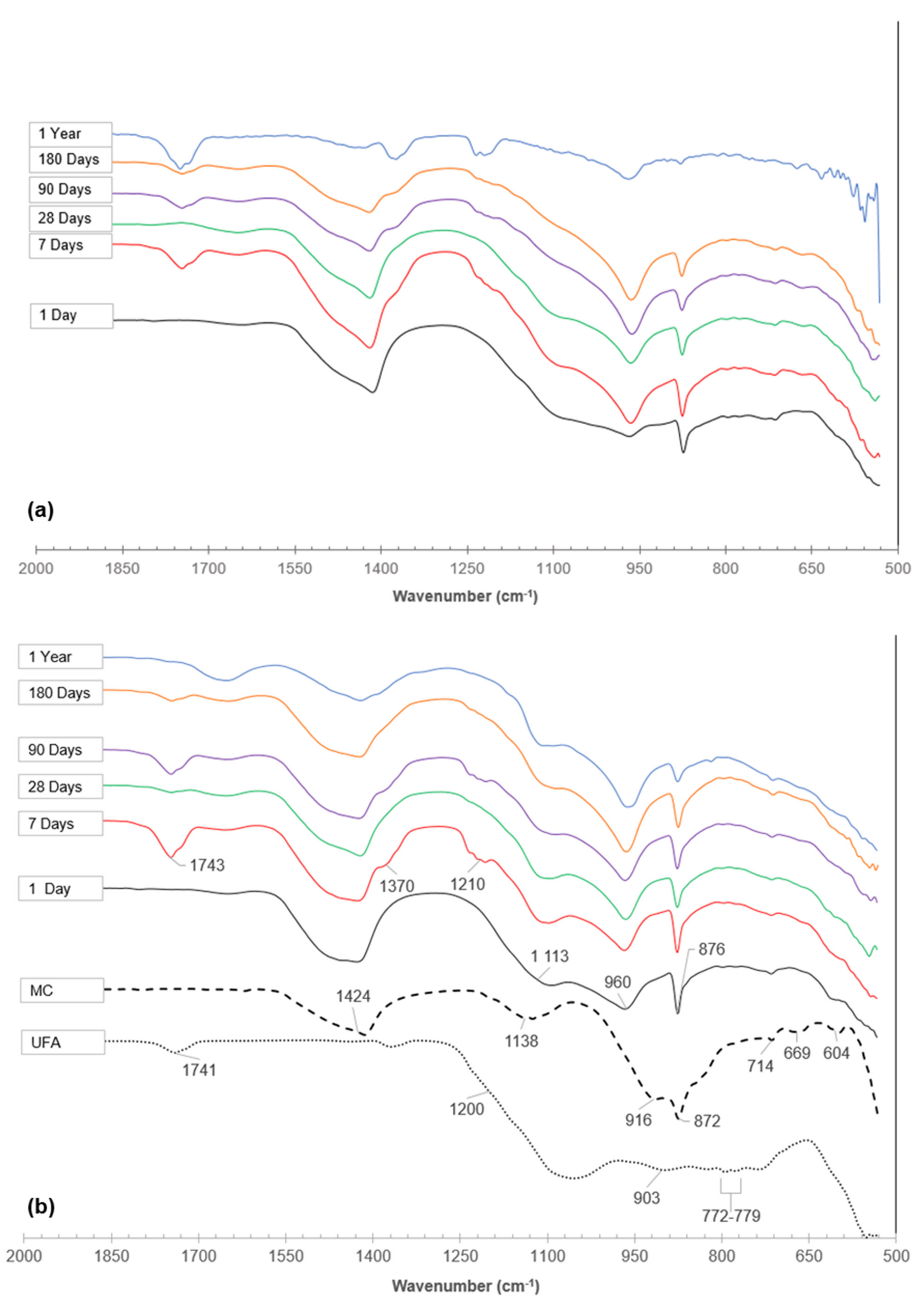
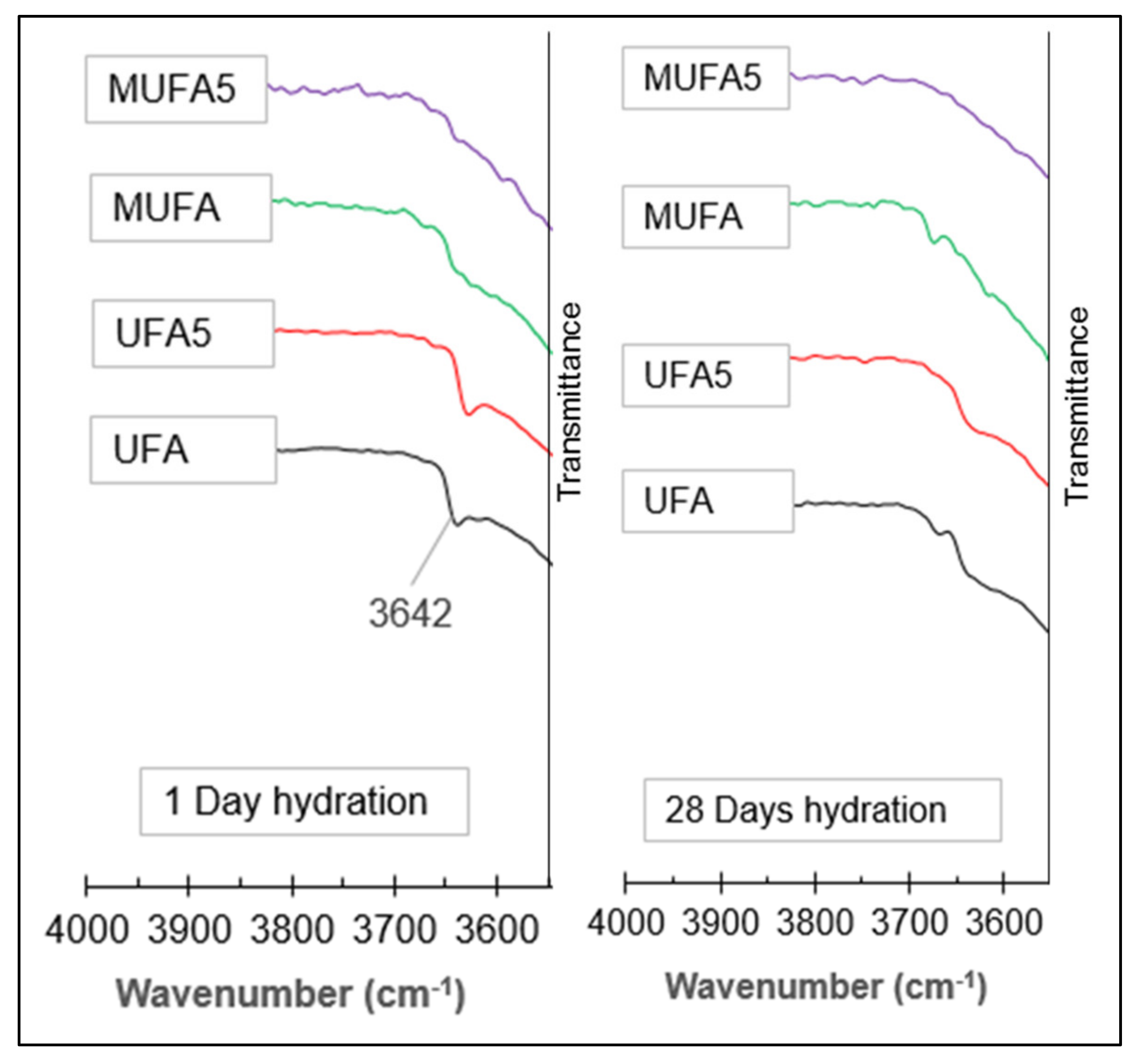
3.3.3. Fourier Transform Infrared Spectroscopy
3.4. The Effect of Chemical and Mechanical Activation on the Pozzolanic Reactivity of Coal Fly Ash in a High Coal Fly Ash Hybrid Cement
3.5. The effect of Chemical and Mechanical Activation on Stable Ettringite Formation in a High Coal Fly Ash Hybrid Cement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations and Notations
| FA | coal fly ash |
| UFA | unclassified coal fly ash (d50: approximately 60 µm) |
| UFA5 | chemically activated unclassified coal fly ash |
| MUFA | mechanically activated unclassified coal fly ash (d50: approximately 7 µm) |
| MUFA5 | chemically and mechanically activated unclassified coal fly ash |
| MC | anhydrous Portland cement |
| CH | calcium hydroxide (Ca(OH)2) |
| CS | calcium sulphate (CaSO4) |
| (N,C)–A–S–H gel | sodium–calcium–aluminate–silicate hydrate |
| C3A | calcium aluminate phase |
| AFm | monosulfoaluminate |
References
- Miller, S.A.; John, V.M.; Pacca, S.A.; Horvath, A. Carbon dioxide reduction potential in the global cement industry by 2050. Cem. Concr. Res. 2018, 114, 115–124. [Google Scholar] [CrossRef]
- Shi, C.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Donatello, S.; Garcia-Lodeiro, I.; Fernandez-Jimenez, A.; Palomo, A. Some durability aspects of hybrid alkaline cements. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2014; Volume 11, p. 01008. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Fernández-Jimenez, A.; Palomo, A. Cements with a low clinker content: Versatile use of raw materials. J. Sustain. Cem.-Based Mater. 2015, 4, 140–151. [Google Scholar] [CrossRef]
- Donatello, S.; Fernández-Jimenez, A.; Palomo, A.; Jantzen, C. Very High Volume Fly Ash Cements. Early Age Hydration Study Using Na2SO4 as an Activator. J. Am. Ceram. Soc. 2013, 96, 900–906. [Google Scholar] [CrossRef]
- Donatello, S.; Maltseva, O.; Fernandez-Jimenez, A.; Palomo, A.; Klein, L. The Early Age Hydration Reactions of a Hybrid Cement Containing a Very High Content of Coal Bottom Ash. J. Am. Ceram. Soc. 2014, 97, 929–937. [Google Scholar] [CrossRef] [Green Version]
- Kovtun, M.; Kearsley, E.P.; Shekhovtsova, J. Dry powder alkali-activated slag cements. Adv. Cem. Res. 2015, 27, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Shekhovtsova, J.; Kovtun, M.; Kearsley, E.P. Temperature rise and initial shrinkage of alkali-activated fly ash cement pastes. Adv. Cem. Res. 2016, 28, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Blanco, F.; Garcia, M.P.; Ayala, J.; Mayoral, G.; Garcia, M.A. The effect of mechanically and chemically activated fly ashes on mortar properties. Fuel 2006, 85, 2018–2026. [Google Scholar] [CrossRef]
- Heinz, D.; Göbel, M.; Hilbig, H.; Urbonas, L.; Bujauskaite, G. Effect of TEA on fly ash solubility and early age strength of mortar. Cem. Concr. Res. 2010, 40, 392–397. [Google Scholar] [CrossRef]
- Wilińska, I.; Pacewska, B.; Ostrowski, A. Investigation of different ways of activation of fly ash–cement mixtures. J. Therm. Anal. Calorim. 2019, 138, 4203–4213. [Google Scholar] [CrossRef] [Green Version]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC 3). Cem. Concr. Res. 2017, 114, 49–56. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A review on alkaline activation: New analytical perspectives. Mater. Constr. 2014, 64, e022. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Lodeiro, I.; Donatello, S.; Fernández-Jiménez, A.; Palomo, Á. Hydration of Hybrid Alkaline Cement Containing a Very Large Proportion of Fly Ash: A Descriptive Model. Materials 2016, 9, 605. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.C.; Poon, C.S.; Cheung, E. Comparative studies of three methods for activating rejected fly ash. Adv. Cem. Res. 2006, 18, 165–170. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Taboada, V.C.; Fernández-Jiménez, A.; Palomo, Á. Recycling Industrial By-Products in Hybrid Cements: Mechanical and Microstructure Characterization. Waste Biomass Valorization 2016, 8, 1433–1440. [Google Scholar] [CrossRef]
- Qian, J.; Shi, C.; Wang, Z. Activation of blended cements containing fly ash. Cem. Concr. Res. 2001, 31, 1121–1127. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Sobrados, I.; Sanz, J.; Palomo, A. Hybrid cements with very low OPC content. In Proceedings of the International Congress on the Chemistry of Cement ICCC XIII, Madrid, Spain, 3–8 July 2011. [Google Scholar]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.; Mehrotra, S.P. Towards sustainable solutions for fly ash through mechanical activation. Resour. Conserv. Recycl. 2007, 52, 157–179. [Google Scholar] [CrossRef]
- Pacewska, B.; Wilińska, I. Comparative investigations of influence of chemical admixtures on pozzolanic and hydraulic activities of fly ash with the use of thermal analysis and infrared spectroscopy. J. Therm. Anal. Calorim. 2014, 120, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Velandia, D.F.; Lynsdale, C.J.; Provis, J.L.; Ramirez, F.; Gomez, A.C. Evaluation of activated high volume fly ash systems using Na2SO4, lime and quicklime in mortars with high loss on ignition fly ashes. Constr. Build. Mater. 2016, 128, 248–255. [Google Scholar] [CrossRef]
- Joseph, S.; Snellings, R.; Cizer, Ö. Activation of Portland cement blended with high volume of fly ash using Na2SO4. Cem. Concr. Compos. 2019, 104, 103417. [Google Scholar] [CrossRef]
- Du Toit, G.; Kearsley, E.P.; Mc Donald, J.M.; Kruger, R.A.; van der Merwe, E.M. Chemical and mechanical activation of hybrid fly ash cement. Adv. Cem. Res. 2018, 30, 399–412. [Google Scholar] [CrossRef]
- Du Toit, G. Chemical and Mechanical Activation of Hybrid Fly Ash Cement. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2018. [Google Scholar]
- Deschner, F.; Winnefeld, F.; Lothenbach, B.; Seufert, S.; Schwesig, P.; Dittrich, S.; Goetz-Neunhoeffer, F.; Neubauer, J. Hydration of Portland cement with high replacement by siliceous fly ash. Cem. Concr. Res. 2012, 42, 1389–1400. [Google Scholar] [CrossRef]
- Scholer, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydrate formation in quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 2015, 55, 374–382. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; Scrivener, K., Snellings, R., Lothenbach, B., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- EN 196-1:2016; Methods of Testing Cemen—Part 1: Determination of Strength. CEN: Brussels, Belgium, 2016.
- Paul, K.T.; Satpathy, S.K.; Manna, I.; Chakraborty, K.K.; Nando, G.B. Preparation and Characterization of nano structured materials from fly ash: A waste from thermal power stations, by high energy ball milling. Nanoscale Res. Lett. 2007, 2, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Patil, A.G.; Anandhan, S. Ball milling of class-f indian fly ash obtained from a thermal power station. Int. J. Energy Eng. 2012, 2, 57–62. [Google Scholar]
- Kearsley, E.P.; Wainwright, P.J. Effect of fly ash properties on concrete strength. J. South Afr. Inst. Civ. Eng. 2003, 45, 19–24. [Google Scholar]
- Hewlett, P. Lea’s Chemistry of Cement and Concrete, 4th ed.; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2004. [Google Scholar]
- EN 197-1; CEN; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. CEN: Brussels, Belgium, 2011.
- Fernández-Jiménez, A.; Palomo, A. Characterisation of fly ashes. Potential reactivity as alkaline cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Criado, M.; Jiménez, A.F.; Palomo, A. Effect of sodium sulfate on the alkali activation of fly ash. Cem. Concr. Compos. 2010, 32, 589–594. [Google Scholar] [CrossRef]
- Palomo, A.; Fernández-Jiménez, A.; Kovalchuk, G.; Ordoñez, L.M.; Naranjo, M.C. Opc-fly ash cementitious systems: Study of gel binders produced during alkaline hydration. J. Mater. Sci. 2007, 42, 2958–2966. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Hydration kinetics in hybrid binders: Early reaction stages. Cem. Concr. Compos. 2013, 39, 82–92. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Variation in hybrid cements over time. Alkaline activation of fly ash–portland cement blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lee, H.K.; Leeb, K.M. Strength and microstructural characteristics of chemically activated fly ash–cement systems. Cem. Concr. Res. 2003, 33, 425–431. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Acceleration of the reactivity of fly ash by chemical activation. Cem. Concr. Res. 1995, 25, 15–21. [Google Scholar] [CrossRef]
- Pacewska, B.; Wilińska, I. Usage of supplementary cementitious materials: Advantages and limitations. J. Therm. Anal. Calorim. 2020, 142, 371–393. [Google Scholar] [CrossRef]
- Wilińska, I.; Pacewska, B. Influence of selected activating methods on hydration processes of mixtures containing high and very high amount of fly ash. J. Therm. Anal. Calorim. 2018, 133, 823–843. [Google Scholar] [CrossRef] [Green Version]
- Ubbrìaco, P.; Bruno, P.; Traini, A.; Calabrese, D. Fly ash reactivity. Formation of hydrate phases. J. Therm. Anal. Calorim. 2001, 66, 293–305. [Google Scholar] [CrossRef]
- Alahrache, S.; Winnefeld, F.; Champenois, J.-B.; Hesselbarth, F.; Lothenbach, B. Chemical activation of hybrid binders based on siliceous fly ash and Portland cement. Cem. Concr. Compos. 2016, 66, 10–23. [Google Scholar] [CrossRef]
- Herath, C.; Gunasekara, C.; Law, D.W.; Setunge, S. Long term mechanical performance of nano-engineered high volume fly ash concrete. J. Build. Eng. 2021, 43, 103168. [Google Scholar] [CrossRef]
- Duvallet, T.; Rathbone, R.F.; Henke, K.R.; Jewell, R.B. Low-Energy, Low CO2-Emitting Cements Produced from Coal Combustion By-Products and Red Mud. In Proceedings of the 2009 World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 22–25 April 2009. [Google Scholar]
- Kontoleontos, F.; Katsiotisa, N.; Tsakiridisb, P.; Kaloidasc, V.; Marinosa, A.; Katsiotia, M. Dry-grinded Ultrafine Cements Hydration. Physicochemical and Microstructural Characterization. Mater. Res. 2013, 16, 404–416. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Yu, W.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Dermatas, D. Evaluation of ettringite and hydrocalumite formation for heavy metal immobilization: Literature review and experimental study. J. Hazard Mater. 2006, 136, 20–33. [Google Scholar] [CrossRef] [PubMed]



| Diameter | |||
|---|---|---|---|
| d10 | d50 | d90 | |
| UFA | 4.4 | 52.5 | 224.2 |
| MUFA | 0.5 | 4.8 | 19.0 |
| MC | 1.3 | 12.7 | 31.6 |
| UFA | MUFA | Cement | UFA | MUFA | Cement | ||
|---|---|---|---|---|---|---|---|
| SiO2 | 54.83 | 54.87 | 20.36 | Anhydrite | - | - | 1.3 |
| Al2O3 | 30.86 | 31.11 | 4.73 | Belite | - | - | 7.7 |
| CaO | 4.84 | 5.06 | 65.08 | Alite | - | - | 43.2 |
| Fe2O3 | 3.62 | 3.83 | 2.80 | Brownmillerite | - | - | 12.9 |
| MgO | 1.17 | 1.19 | 1.78 | Tricalcium aluminate | - | - | 3.4 |
| K2O | 0.63 | 0.63 | 0.46 | Calcite | - | - | 9.9 |
| Na2O | 0.16 | 0.17 | 0.07 | Gypsum | - | - | 1.3 |
| TiO | 1.57 | 1.55 | 0.45 | Hematite | 1.0 | 0.8 | - |
| Mn2O3 | 0.03 | 0.03 | 0.10 | Mullite | 31.5 | 27.5 | - |
| P2O5 | 0.63 | 0.63 | 0.07 | Quartz | 12.2 | 10.3 | 0.8 |
| SO3 | 0.40 | 0.31 | 2.73 | Amorphous | 55.3 | 61.5 | 19.3 |
| LOI | 1.19 | 1.64 | 5.67 |
| Cement Phase | Chemical Formula | Temperature Range (°C) | |
|---|---|---|---|
| Dehydration <300 °C | C-S-H (Calsium silicate hydrates) | - | 50–600 |
| Gypsum | CaSO4·2H2O | 100–120 | |
| Ettringite | 3CaO·Al2O3·3CaSO4·32H2O | 90–140 | |
| Hemihydrate | CaSO4·0.5H2O | 120–130 | |
| Monocarboaluminate | 4CaO·Al2O3·CO3·11H2O | 125–175 | |
| Dehydroxylation 400–500 °C | Portlandite | Ca(OH)2 | 400–500 |
| Decarbonation >600 °C | Calcium Carbonate | CaCO3 | 600–800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
du Toit, G.; van der Merwe, E.M.; Kruger, R.A.; McDonald, J.M.; Kearsley, E.P. Characterisation of the Hydration Products of a Chemically and Mechanically Activated High Coal Fly Ash Hybrid Cement. Minerals 2022, 12, 157. https://doi.org/10.3390/min12020157
du Toit G, van der Merwe EM, Kruger RA, McDonald JM, Kearsley EP. Characterisation of the Hydration Products of a Chemically and Mechanically Activated High Coal Fly Ash Hybrid Cement. Minerals. 2022; 12(2):157. https://doi.org/10.3390/min12020157
Chicago/Turabian Styledu Toit, Grizelda, Elizabet M. van der Merwe, Richard A. Kruger, James M. McDonald, and Elsabé P. Kearsley. 2022. "Characterisation of the Hydration Products of a Chemically and Mechanically Activated High Coal Fly Ash Hybrid Cement" Minerals 12, no. 2: 157. https://doi.org/10.3390/min12020157








