Abstract
High-chromium vanadium slag (HCVS) is an important by-product generated during the smelting process of high-chromium-vanadium-titanium-magnetite. Direct acid leaching and calcium-roasting acid leaching technology were applied to recover vanadium and chromium from HCVS. The effects of experimental parameters on the leaching process, including concentration of H2SO4, reaction temperature, reaction time, and liquid-to-solid ratio, were investigated. The XRD and UV-Vis DRS results showed that vanadium and chromium existed in low valence with a spinel structure in the HCVS. The Cr-spinel was too stable to leach out; no more than 8% of the chromium could be leached out both in the direct acid leaching process and calcium-roasting acid-leaching process. Most low valence vanadium could be oxidized to high valence with calcium-roasting technology, and the leaching efficiency could be increased from 33.89% to 89.12% at the selected reaction conditions: concentration of H2SO4 at 40 vt.%, reaction temperature of 90 °C, reaction time of 3 h, liquid-to-solid ratio of 4:1 mL/g, and stirring rate of 500 rpm. The kinetics analysis indicated that the leaching behavior of vanadium followed the shrinking core model well, and the leaching process was controlled by the surface chemical reaction, with an Ea of 58.95 kJ/mol and 62.98 kJ/mol for direct acid leaching and roasting acid leaching, respectively.
1. Introduction
Vanadium and chromium are strategic transition elements that have been widely used in some fields such as steel-making, energy-storage, catalysts, the petrochemical industry, and green chemistry owing to their excellence hardness, high corrosion resistance, and other excellent physicochemical properties [1,2,3,4,5]. High-chromium vanadium slag (HCVS) is a by-product generated during the smelting process of high-chromium-vanadium-titanium-magnetite, and it is an important vanadium source in China [6,7,8,9,10]. During the smelting process, the vanadium and chromium are reduced into the molten enriched in the spinel, which is hard to destroy directly and restricted the large-scale utilization of HCVS [11]. Thus, some enhancement technologies were needed to recover vanadium and chromium efficiently.
To date, the basic recovery technology for vanadium has been sodium-roasting leaching technology, which was first proposed by Birck in 1912 and is widely used in the Chinese industries since the 1980s [12,13,14]. The vanadium-containing ores are mixed with the sodium salts (sodium carbonate (800–1000 °C), sodium sulfate (1200–1250 °C), sodium chloride (750–850 °C), and sodium hydroxide (400–800 °C)) at determined mole ratios and then roasted in a vertical kiln under a high temperature atmosphere with O2 [13,15,16]. The structure of vanadium-containing ores is destroyed in the high temperature and low-valence vanadium is exposed and oxidized to a high valence. The high-valence vanadium is formed as sodium vanadate and can be easily leached out with acid, alkaline, or water-leaching [17,18,19,20]. However, some environmental hazards (sulfur dioxide, chlorine, and hydrogen chloride) and large amount of wastewater have limited continuous large-scale industrial application with the high environmental standards of today. To overcome the above problems, a roasting technology called calcium-roasting technology was developed, in which the sodium salts are replaced by calcium salts [21,22,23,24,25]. The roasting process is similar to the sodium-roasting process; the vanadium-containing ores are mixed with lime, limestone, and calcium salts at fixed mole ratios and then roasted at high temperatures, which are higher than with sodium roasting. During the roasting process, the vanadium spinel is decomposed and reacted with calcium salts to form different kinds of calcium-vanadate, which are determined by the mole ratio of the vanadium to calcium salts [26,27,28,29,30]. Usually, some leaching enhancing processes or multiple roasting processes accompany this process to achieve high recovery [16,31,32,33,34].
In this paper, direct acid leaching and calcium-roasting acid leaching technology were applied to leach out chromium and vanadium. The effects of experimental parameters including reaction time, liquid-to-solid ratio, reaction temperature, and concentration of H2SO4 on the leaching process were investigated. The leaching kinetics were also investigated.
2. Materials and Methods
2.1. Materials
The HCVS was collected from Pangang Group Co. Ltd., Panzhihua City, Sichuan Province, China. It was dried and ground to below 75 μm for further experiments. The elemental composition of HCVS was measured by XRF. The results displayed in Table 1 indicate that the vanadium and chromium were about 5.43 wt.% and 6.84 wt.%, respectively.

Table 1.
Elemental accounts of the HCVS (wt.%).
2.2. Experimental Procedure
The batch experiments were conducted in a 300 mL glass beaker placed in a thermostatic mixing water bath. Firstly, the water bath was heated to a determined temperature. Then, a predetermined concentration of H2SO4 solution and a predetermined amount of HCVS or roasting HCVS were added to the beaker. Then, the beaker was placed in the water bath. Finally, the filtrate was collected by vacuum filtration after the required reaction time.
2.3. Analytical Methods
The concentrations of chromium and vanadium in the filtrate were measured by inductively coupled plasma-optical mission spectrometry (ICP-OES, PerkinElmer Optima 6300DV, Kyoto, Japan.) and the leaching efficiency was calculated following Equations (1) and (2):
where CV and CCr, are the concentration of chromium and vanadium in the filtrate in g/L; V, is the volume of the filtrate in liters; ωV, and ωCr, are the percentages of chromium and vanadium in the HCVS; and m, is the mass of the HCVS used in the batch experiments in grams.
2.4. Characterization
The element percentages of HCVS were measured by XRF (Shimadzu Lab Center XRF-1800, Kyoto, Japan) and the main phases were measured by XRD (Shimadzu Lab Center XRD-6000, Kyoto, Japan). The valences of the vanadium and chromium in the HCVS were detected by UV-Vis DRS (Shimadzu Lab Center, Kyoto, Japan) and XPS (ESCALAB-250Xi, Thermo Fisher Scientific, New York, NY, USA). The thermo-gravimetric analysis was conducted by TG-DSC (Shimadzu Lab Center DSC-60H, Kyoto, Japan) with a heating rate of 10 °C/min from 0 °C to 900 °C.
3. Results
3.1. Characterization of HCVS
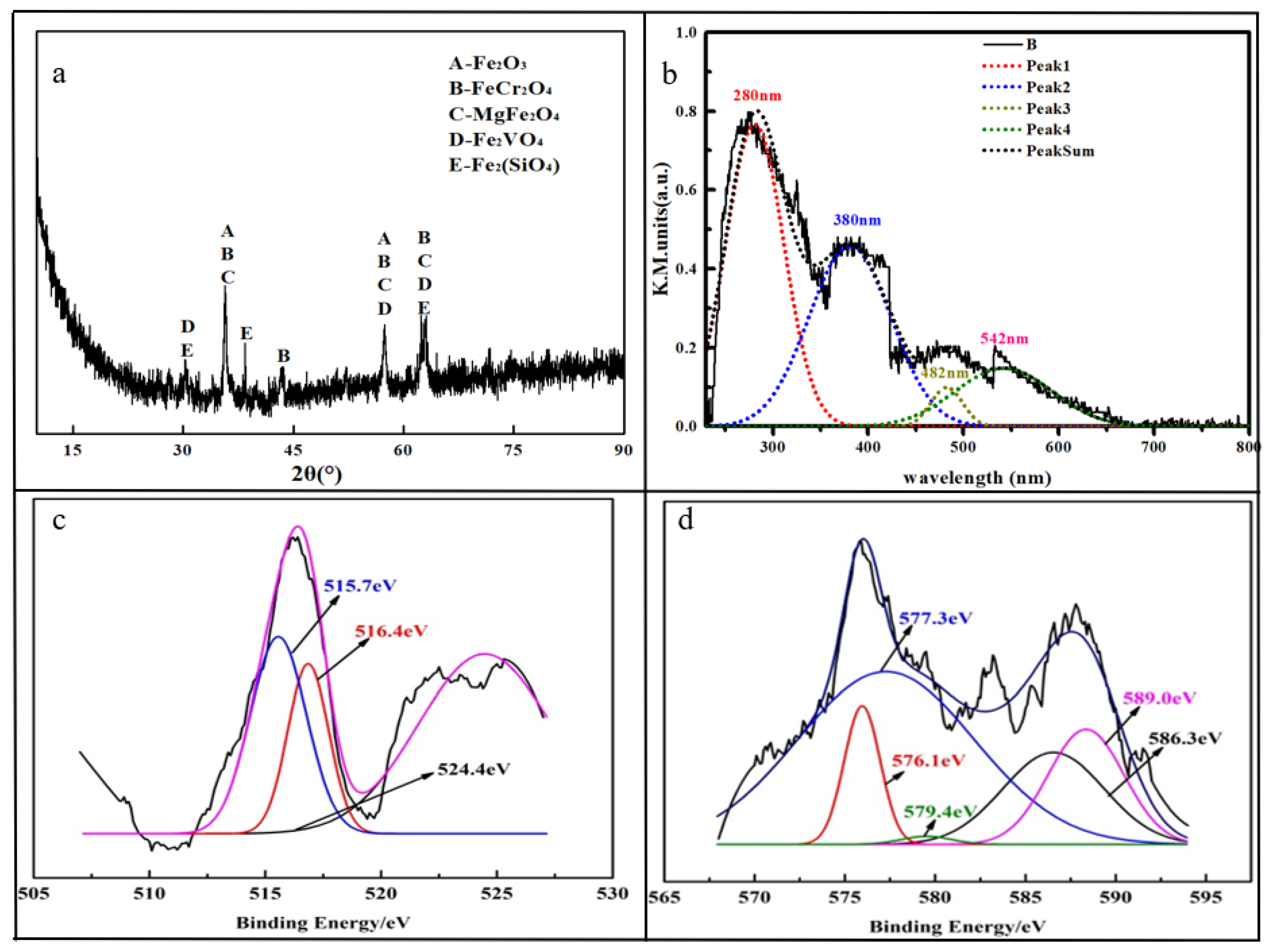
The XRD pattern showed in Figure 1a shows that the main crystal structures in the HCVS were Fe2O3, FeCr2O4, MgFe2O4, Fe2VO4 and Fe2SiO4 [27,28,30]. The vanadium and chromium mainly existed as spinel structures (FeCr2O4 and Fe2VO4), which are hard to destroy. Therefore, the leaching efficiency of vanadium and chromium may not be high and some enhancing technologies are needed in the further experiments.
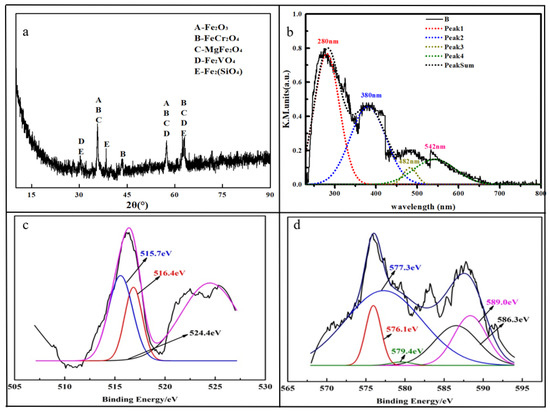
Figure 1.
Characterization of HCVS. (a) XRD pattern of HCVS; (b) UV-Vis DRS of HCVS; (c) XPS of vanadium; (d) XPS of chromium.
The UV-Vis DRS of HCVS was conducted to help understanding the composition of HCVS; the result is displayed in Figure 1b. The original spectrum signal was analyzed and the peaks were fitted to four main peaks: 280 nm, 380 nm, 482 nm, and 542 nm. The peak at 280 nm was assigned to Fe (III) and confirmed the existence of Fe2O3 and MgFe2O4 [35,36]. The peak at 380 nm was assigned to Cr (III) [37], which corresponds to the FeCr2O4 phase. The peak at 482 nm was assigned to V=O stretching, which confirmed the existence of V (IV) and V (V) [38,39].
The XPS results showed that most vanadium existed as V(III) and V(IV) (515.7 eV and 516.4 eV), and the Cr (III) accounted for about 82.84% of the HCVS (576.1 eV, 577.3 eV and 586.3 eV), while there were no V (III) and V (V) phases in the XRD pattern. According to our previous study, V(III), V(IV), and V(V) co-exist in HCVS, which means that some V(III) and V(V) compounds in the HCVS exist amorphously and could not detected by XRD [28].
3.2. Direct-Acid-Leaching Process
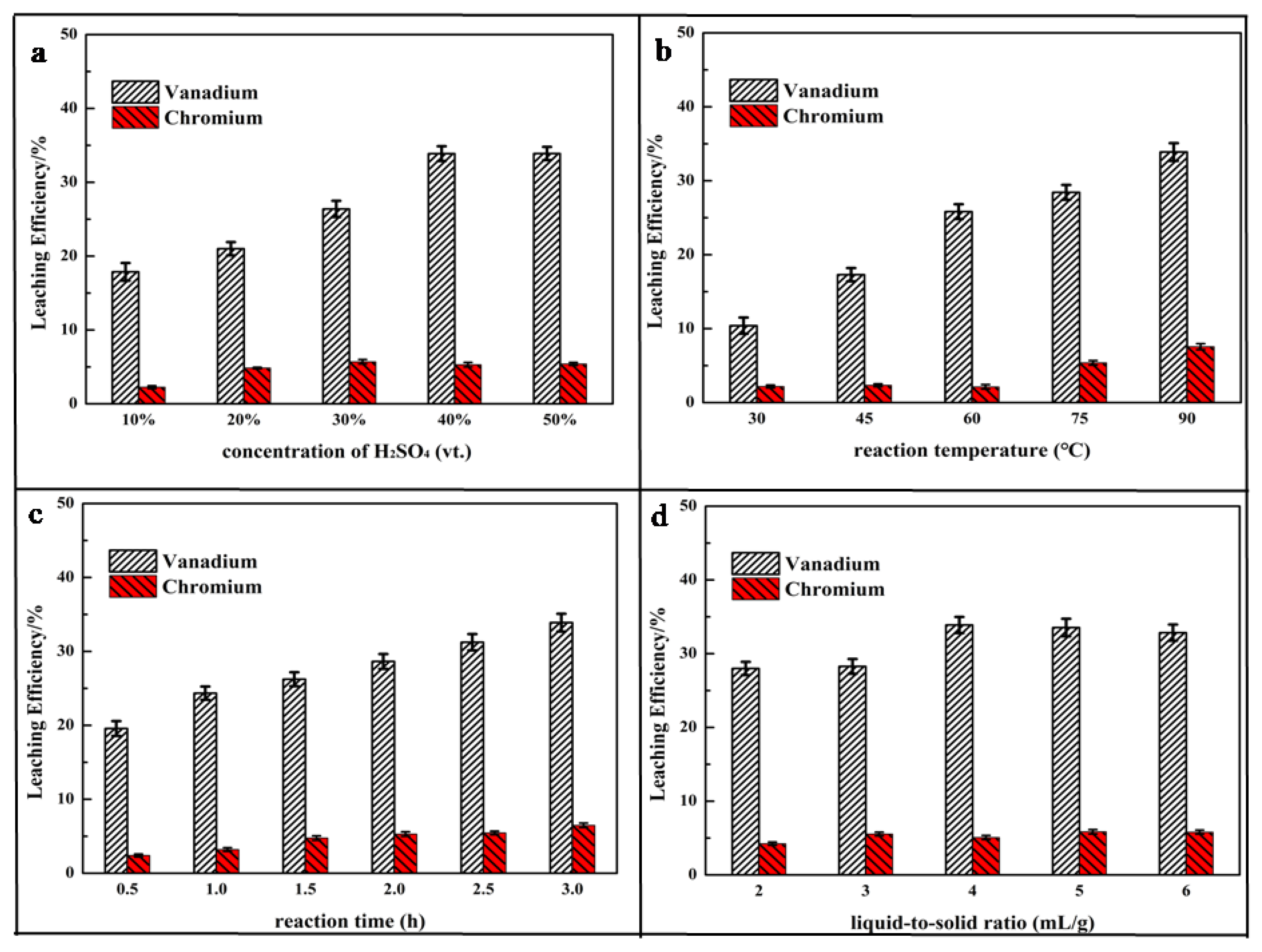
The direct acid leaching process was conducted to leach out vanadium and chromium from the HCVS. Figure 2 summarizes the effects of the liquid-to-solid ratio, reaction time, concentration of H2SO4, and reaction temperature on the leaching process. The leaching efficiencies of vanadium and chromium were relatively low (<35% for vanadium and 8% for chromium), which is consistent with the results of XRD.
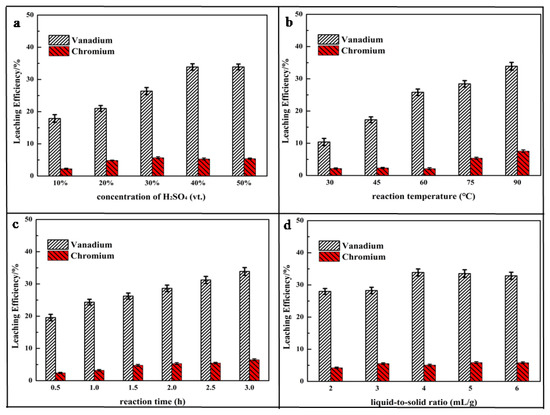
Figure 2.
Effect of parameters on leaching efficiency of vanadium and chromium: (a) concentration of H2SO4; (b) reaction temperature; (c) reaction time; (d) liquid-to-solid ratio.
Figure 2a shows that the leaching efficiency of vanadium increased with the increase of the concentration of H2SO4. During the leaching process, the H+ attacks the spinel and destroys the spinel structure to release vanadium and chromium. With an increasing of concentration of H2SO4, the corrosion process of the spinel by the highly concentrated H+ was intensified and the leaching efficiency of vanadium was increased from 17.87% to 33.90%, as the concentration of H2SO4 increased from 10 vt.% to 50 vt.%. As the chromium spinel was more stable than the vanadium spinel, the chromium was harder to leach out, with a leaching efficiency below 8%. Otherwise, the leaching efficiency showed no obvious increase when the concentration of H2SO4 increased from 40 vt.% to 50 vt.%, and high concentrations bring high impurities [40]; thus, the concentration of 40 vt.% was selected as optimal for further experiments.
The results showed in Figure 2b indicate that only 10.40% vanadium and 2.18% chromium can be leached out at 30 °C. Higher temperatures enhances the activity of vanadium and chromium ions and further favors the reaction intensity [41]. The leaching efficiency increased to 33.89% for vanadium and 7.56% for chromium at 90 °C. In other words, high reaction temperature was beneficial to the leaching process.
Usually, in order to produce more products, long reaction times are utilized. Figure 2c shows that the leaching efficiency of vanadium and chromium increased with the reaction time, but the increase amplitude was slow. Longer reaction times may not make any contribution to the leaching process; thus, the reaction time of 3 h was selected in the following experiments.
During the leaching process, the HCVS particles was ground fine enough for good contact with the concentrated H2SO4 solution. The leaching process was most controlled by parameters such as the reaction temperature and concentration of H2SO4, but less by the liquid-to-solid ratio, as the liquid-to-solid ratio had no obvious effect on the leaching efficiency (seen in Figure 2d).
As the spinel structure was hard to destroy directly, the leaching efficiencies of vanadium and chromium were 33.89% and 7.56%, respectively, at the selected optimum conditions: reaction time of 3 h, liquid-to-solid ratio of 4: 1 mL/g, concentration of H2SO4 of 40 vt.%, reaction temperature of 90 °C, and stirring rate of 500 rpm.
3.3. Characterization of Roasting HCVS
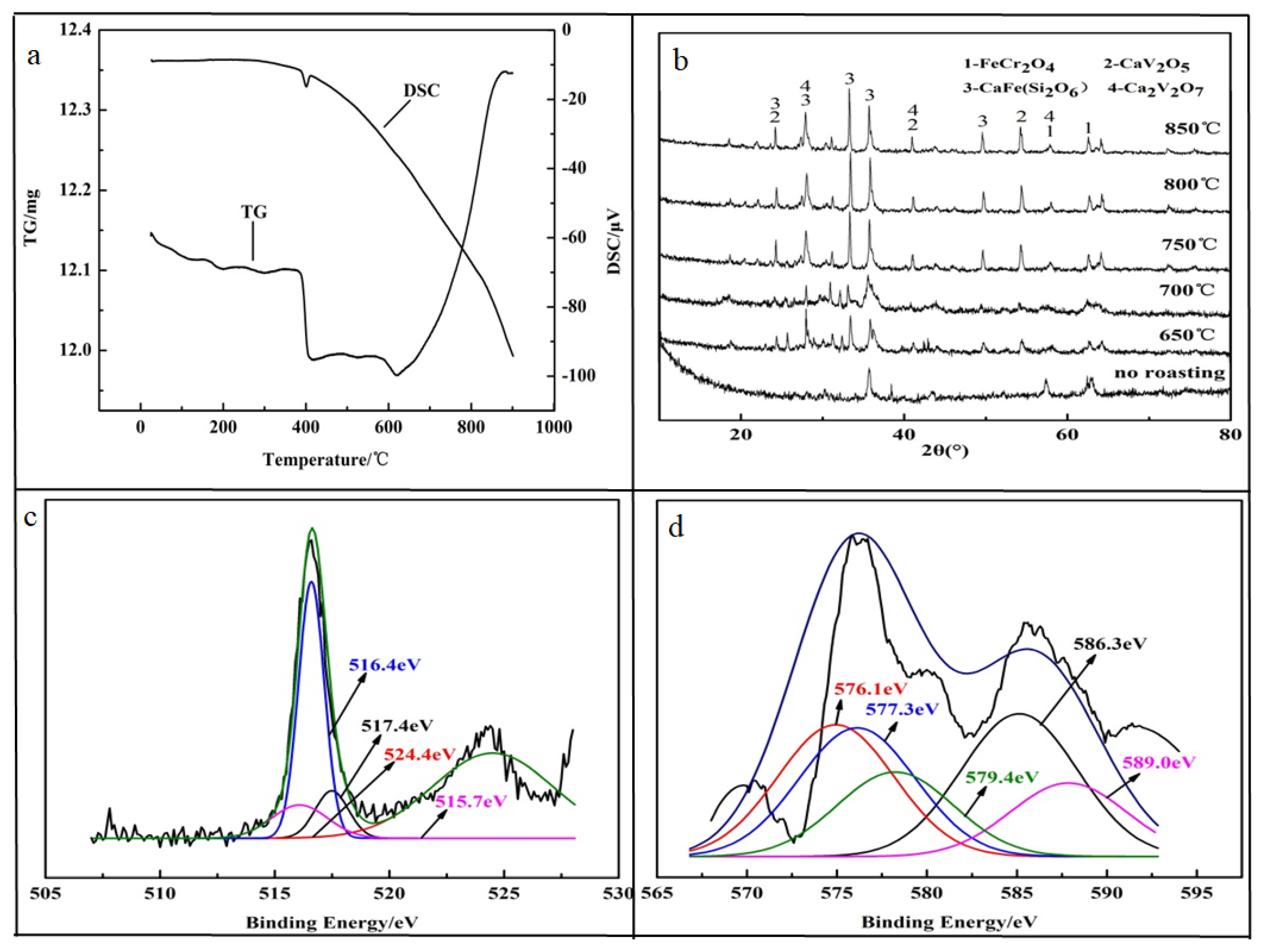
In order to achieve efficient leaching performance of HCVS, calcium-roasting technology was applied to oxidize the low valence compounds. The obtained TG-DSC curves shown in Figure 3a indicate that there was a dehydration step, with weight loss of 1.23% from 0 °C to 400 °C, and an obvious exothermic peak of the DSC curve at 400 °C was observed, which corresponds to the decomposition of the spinel structure. After the temperature increased to 620 °C, a dramatic mass gain of 6.46% was obtained due to the oxidative decomposition of the vanadium spinel phase. This means that the oxidative roasting of vanadium spinel should be conducted above 620 °C. Thus, the calcium-roasting process was conducted at 650–850 °C and the HCVS was mixed with CaO at a mole ratio of n(CaO)/n(V2O5) = 1.1
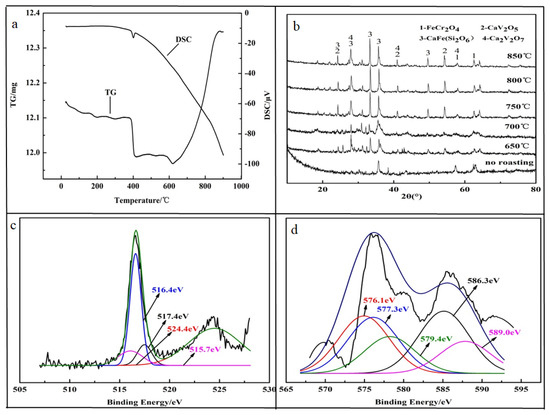
Figure 3.
Characterization of roasting HCVS. (a) TG and DSC; (b) XRD pattern of roasted HCVS; (c) XPS of vanadium; (d) XPS of chromium.
The XRD pattern was used to analyze the phase changes during the calcium-roasting process. The results showed in Figure 3b indicate that some new peaks appeared, corresponding to the new phases of Ca2V2O5, CaFe (Si2O6), and Ca2V2O7. These three new phases appeared at 650 °C, and the crystal structures became more stable as the roasting temperature increased from 650 °C to 850 °C. During the calcium-roasting process, the Fe2VO4 decomposed (seen in Equation (3)) to form V2O4 at nearly 400 °C according to DG-TSC results, and then reacted with CaO to form Ca2V2O5. With the increasing roasting temperature, partial Ca2V2O5 was further oxidized to Ca2V2O7, which means that in the calcium roasting of HCVS, the V(IV) and V(V) co-existed.
After roasting, some V(III) and V(IV) were oxidized to V(V). The XPS results indicate that only 9.55% V(III) was retained in the roasted HCVS, while Cr(III) still accounted for about 80.32%. As the Cr spinel was more stable than the V spinel [13], the Cr was not oxidized and still existed in FeCr2O4, according to the XRD results. It was concluded that the chromium was still hard to leach out.
3.4. Acid Leaching for Roasting HCVS
The acid-leaching experiments with calcium roasting of HCVS were conducted to investigate the effect of calcium roasting on the leaching process under the same reaction conditions as the direct acid leaching process described above. As the Cr spinel was still stable under the roasting temperature, the leaching efficiency of chromium showed no obvious increase compared with the direct acid leaching process; therefore, the leaching behavior of chromium is not discussed in this part.
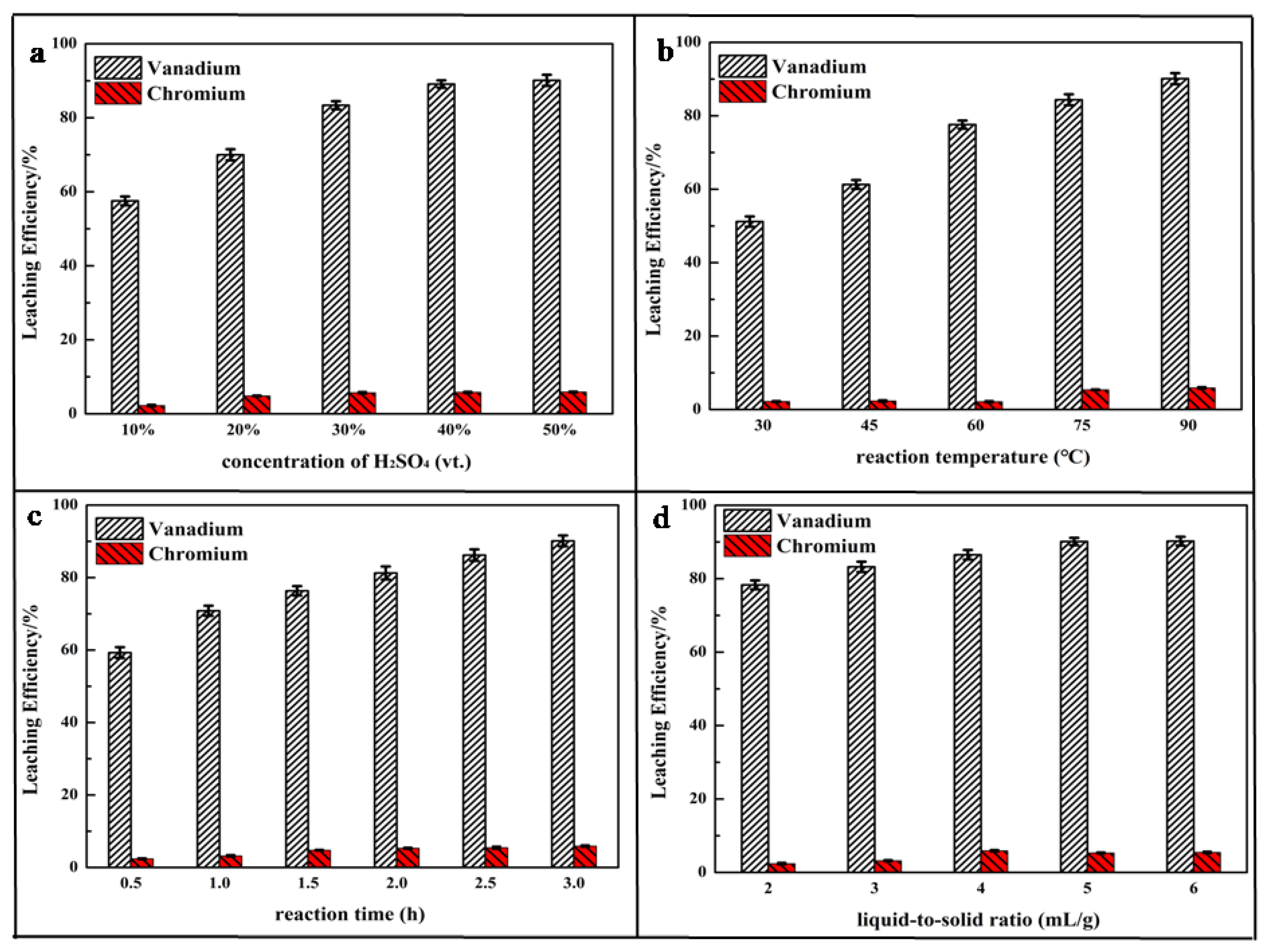
Figure 4a shows that the calcium roasting made a great contribution to the leaching process. The leaching efficiency of vanadium was increased by nearly 40 percentage (up to 57.54%) after roasting, compared with the direct acidic leaching process (at the concentration of 10 vt.% H2SO4). During the roasting process, most V(III) and V(IV) were oxidized to V(V), making a contribution to the great leaching performance of roasting HCVS. The leaching efficiency increased quickly at the beginning and then smoothly with the increase of H2SO4 concentration. The highest leaching efficiency was up to 90.12% at a concentration of 50 vt.%, which showed nearly a 58% improvement compared to the direct acid leaching process. Compared to our previous study, the leaching efficiency might be increased more with some enhancing technologies, such as oxidative leaching and electro-oxidative leaching [28]. Otherwise, the formation of the by-product CaSO4, which is a villous particle, might adsorb on the surface of leaching residue and have negative effects on the leaching process [40]; thus, a too high concentration of H2SO4 is not suitable for leaching while calcium roasting HCVS. Meanwhile, the leaching efficiency showed little increase as the concentration increased from 40 vt.% to 50 vt.%; thus, a concentration of H2SO4 of 40 vt.% was selected for further experiments.
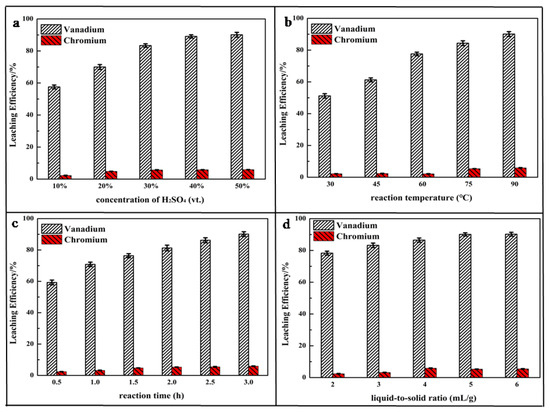
Figure 4.
Effect of parameters on leaching efficiency of vanadium and chromium: (a) concentration of H2SO4; (b) reaction temperature; (c) reaction time; (d) liquid-to-solid ratio.
The same phenomenon can also be observed in Figure 4b. The leaching process was greatly enhanced by calcium roasting; the leaching efficiency was increased from 10.4% to 89.12% as the reaction temperature increased from 30 °C to 90 °C. Usually, metal ions have high solubility at high temperatures, accompanied by high activity; thus, 90 °C was chose in further experiments.
The results displayed in Figure 4c,d indicate that the liquid-to-solid ratio and reaction time showed similar effects on the leaching process, and a suitable liquid-to-solid ratio and a long reaction time could achieve high leaching efficiency. As can be seen, the calcium-roasting process can oxidize low valence vanadium to high valence vanadium and enhance the leaching process to achieve high leaching efficiency of vanadium, but has no influence on the change trend of leaching efficiency affected by the experimental parameters.
To summarize, low valence vanadium in V spinel was decomposed and oxidized to V(V) during the calcium-roasting process, but Cr spinel was too stable to decompose. For vanadium, 89.12% was leached out under the optimal reaction conditions: reaction time of 3 h, reaction temperature of 90 °C, liquid-to-solid ratio at 4:1 mL/g, concentration of H2SO4 at 40 vt.%, and stirring rate at 500 rpm. Most chromium existing as FeCr2O4 was hard to leach out and was retained in the leaching residue.
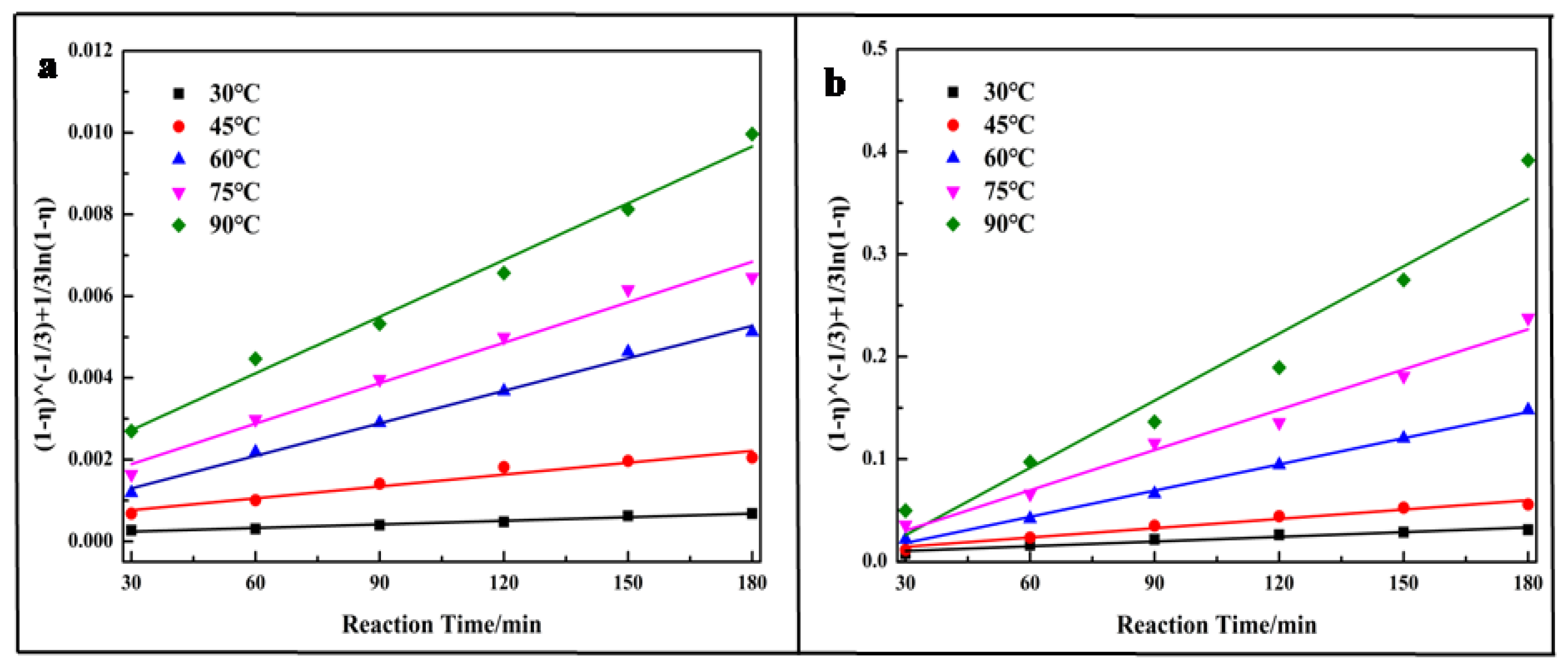
3.5. Leaching Kinetics
In order to understand the reaction mechanism, the leaching kinetics of vanadium were analyzed (leaching out chromium was very difficult; thus, it is not analyzed here). Usually, the leaching kinetics followed the shrinking core model described in Equation (6), which was used to describe the liquid-solid reaction [40,42,43,44,45]:
where η is the leaching efficiency of vanadium, in percentage.
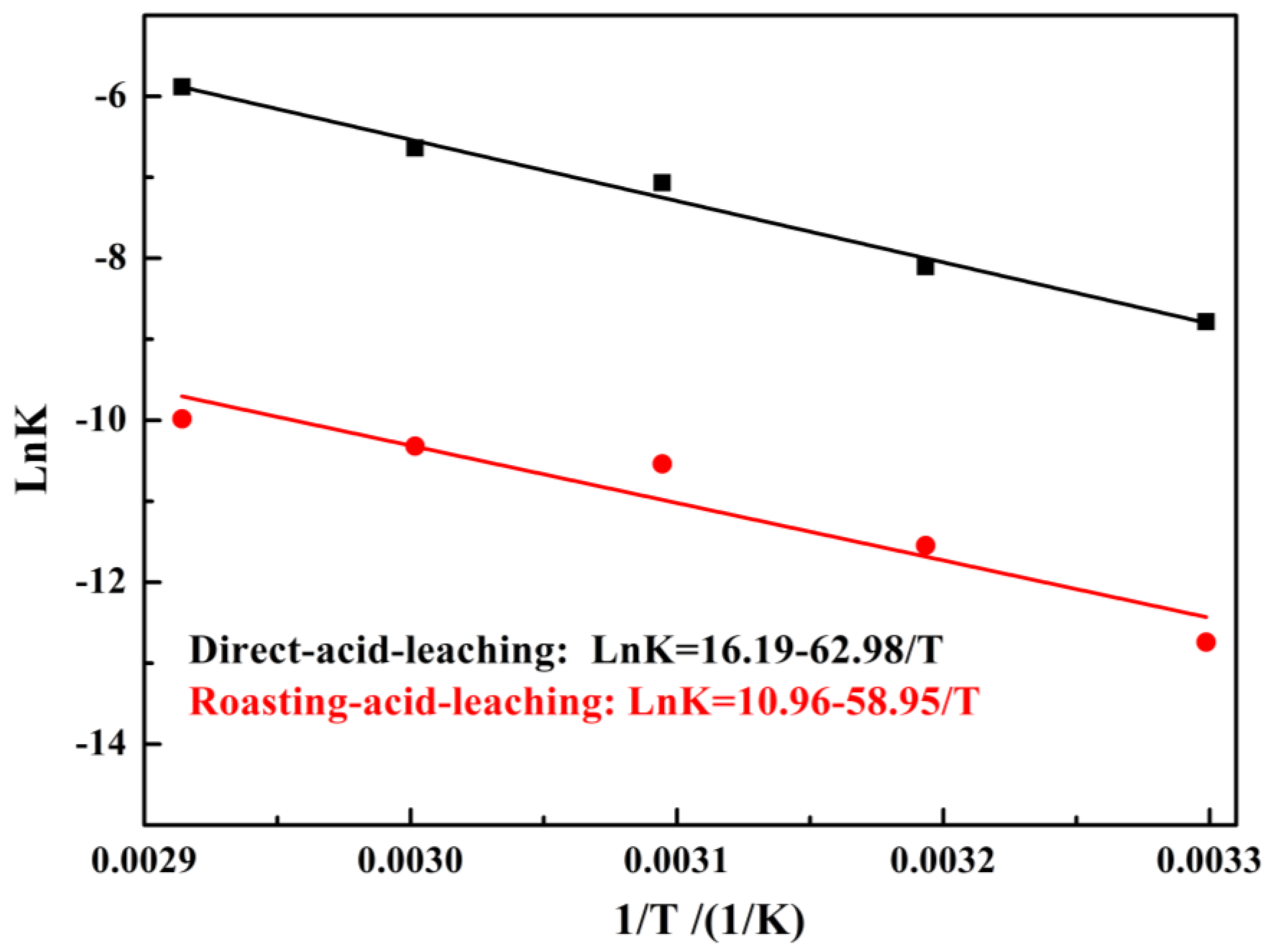
The experimental data was fitted to Equation (6) and the results are shown in Figure 5. Based on the fitting results, the Ea for vanadium leached out was calculated following the Arrhenius equations (Equation (7)). Figure 6 shows that the Ea for vanadium leached out was 58.95 kJ/mol for the direct acid leaching process and 62.98 kJ/mol for the calcium-roasting acid leaching process, which indicates that the controlling step for vanadium leaching is the surface chemical reaction [40,43,44,45]. Compared with the references [40,43,45,46], the Ea was much larger, indicating that the vanadium in the HCVS was hard to leach out by both direct acid leaching technology and calcium-roasting acid leaching technology. In order to improve the leaching efficiency and enhance the leaching process, some more efficient pre-treatment technologies are needed.
where Ea is the apparent activation energy, A is the pre-exponential factor, and R is the mole gas constant.
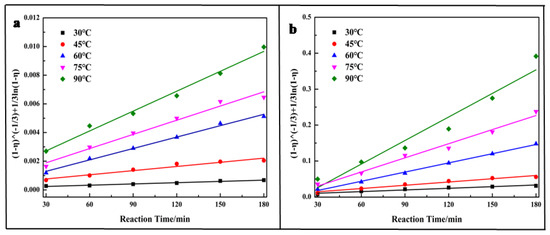
Figure 5.
Plot of leaching kinetics of vanadium at various reaction temperatures. (a) direct acid leaching process; (b) calcium-roasting acid leaching process.
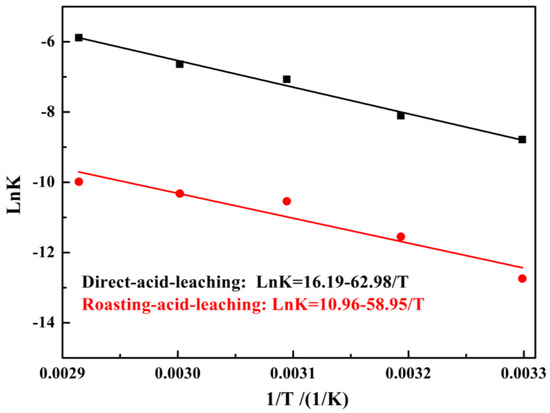
Figure 6.
Natural logarithm of reaction rate constant versus reciprocal temperature of vanadium.
4. Conclusions
A direct acid leaching process and a calcium-roasting acid leaching process on HCVS were conducted. The following conclusions were obtained:
- (1)
- The chromium and vanadium existed as spinel structure in the HCVS, which are too stable to destroy directly; only 33.89% of vanadium and 7.56% of chromium could be leached out at the selected conditions during the direct acid leaching process: reaction time of 3 h, liquid-to-solid ratio at 4:1 mL/g, concentration of H2SO4 at 40 vt.%, reaction temperature of 90 °C, and stirring rate at 500 rpm. The Ea of the vanadium leached out was 62.98 kJ/mol, which indicates that the vanadium was hard to leach out directly;
- (2)
- Most low valence vanadium could be oxidized to high valence during the calcium-roasting process, and the leaching efficiency could achieve 89.12% under the optimal conditions: reaction time of 3 h, liquid-to-solid ratio at 4:1 mL/g, reaction temperature of 90 °C, concentration of H2SO4 at 40 vt.%, and stirring rate at 500 rpm. The leaching behavior followed the shrinking core model well, and the controlling step was the surface chemical reaction, with an Ea of 58.95 kJ/mol for the calcium-roasting acid leaching process.
- (3)
- Chromium was hard to leach out both in the direct acid leaching process and the calcium-roasting acid leaching process; the leaching efficiency was below 8%. Higher roasting temperatures and new additive agents will be needed for efficient chromium recovery in our future works.
Author Contributions
Methodology, B.L.; supervision, W.S. and Z.L.; other contributions were made by H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Chongqing Science and Technology Commission (No. cstc2021jcyj-msxmX0129) and the Science and Technology Research Program of Chongqing Municipal Education Commission (No. CXQT20026).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, H.; Xiao, X.; Guo, Z.; Han, X.; Liang, Y.; Zhang, Y.; Zhou, C. Adsorption of vanadium (V) on natural kaolinite and montmorillonite: Characteristics and mechanism. Appl. Clay Sci. 2018, 161, 310–316. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, D.; Hsu, C.; Liu, F. All-vanadium redox photoelectrochemical cell: An approach to store solar energy. Electrochem. Commun. 2014, 45, 79–82. [Google Scholar] [CrossRef]
- Nicholas, N.J.; da Silva, G.; Kentish, S.; Stevens, G.W. Use of Vanadium(V) Oxide as a Catalyst for CO2 Hydration in Potassium Carbonate Systems. Ind. Eng. Chem. Res. 2014, 53, 3029–3039. [Google Scholar] [CrossRef]
- Wen, J.; Ning, P.; Cao, H.; Zhao, H.; Sun, Z.; Zhang, Y. Novel method for characterization of aqueous vanadium species: A perspective for the transition metal chemical speciation studies. J. Hazard. Mater. 2018, 364, 91–99. [Google Scholar] [CrossRef]
- Peng, H. A literature review on leaching and recovery of vanadium. J. Environ. Chem. Eng. 2019, 7, 103313. [Google Scholar] [CrossRef]
- Yan, B.; Wang, D.; Wu, L.; Dong, Y. A novel approach for pre-concentrating vanadium from stone coal ore. Miner. Eng. 2018, 125, 231–238. [Google Scholar] [CrossRef]
- Xue, N.; Zhang, Y.; Huang, J.; Liu, T.; Wang, L. Separation of impurities aluminum and iron during pressure acid leaching of va-nadium from stone coal. J. Clean. Prod. 2017, 166, 1265–1273. [Google Scholar] [CrossRef]
- Hu, X.; Yue, Y.; Peng, X. Release kinetics of vanadium from vanadium titano-magnetite: The effects of pH, dissolved oxygen, temperature and foreign ions. J. Environ. Sci. 2018, 64, 298–305. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Zhou, W.; Gao, H.; Xue, X. A cleaner and efficient process for extraction of vanadium from high chromium vanadium slag: Leaching in (NH4)2SO4-H2SO4 synergistic system and NH4+ recycle. Sep. Purif. Technol. 2019, 216, 126–135. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Xu, Y.; Liu, J.; Xue, X. Efficient Separation and Extraction of Vanadium and Chromium in High Chromium Vanadium Slag by Selective Two-Stage Roasting–Leaching. Metall. Mater. Trans. A 2018, 49, 1471–1481. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, T.; Xu, Y.; Wen, J.; Xue, X. Change in phase, microstructure, and physical-chemistry properties of high chromium vanadium slag during microwave calcification-roasting process. Powder Technol. 2018, 340, 520–527. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Bao, S. The Effects of Sodium Ions, Phosphorus, and Silicon on the Eco-Friendly Process of Vanadium Precipitation by Hydrothermal Hydrogen Reduction. Minerals 2018, 8, 294. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-Y.; Fang, H.-X.; Wang, K.; Zhou, W.; Yang, Z.; Yan, X.-M.; Ge, W.-S.; Li, Q.-W.; Xie, B. Asynchronous extraction of vanadium and chromium from vanadium slag by stepwise sodium roasting–water leaching. Hydrometallurgy 2015, 156, 124–135. [Google Scholar] [CrossRef]
- Qiu, S.; Wei, C.; Li, M.; Zhou, X.; Li, C.; Deng, Z. Dissolution kinetics of vanadium trioxide at high pressure in sodium hydroxide–oxygen systems. Hydrometallurgy 2011, 105, 350–354. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y. Phase transformations of vanadium recovery from refractory stone coal by novel NaOH molten roasting and water leaching technology. RSC Adv. 2017, 7, 36917–36922. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Shen, S.; Liu, J.; Xue, Y. Cleaner and effective process for extracting vanadium from vanadium slag by using an innovative three-phase roasting reaction. J. Clean. Prod. 2017, 149, 1068–1078. [Google Scholar] [CrossRef]
- Crundwell, F. The mechanism of dissolution of the feldspars: Part II dissolution at conditions close to equilibrium. Hydrometallurgy 2015, 151, 163–171. [Google Scholar] [CrossRef]
- Crundwell, F. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part IV equilibrium and near-equilibrium behaviour. Hydrometallurgy 2015, 153, 46–57. [Google Scholar] [CrossRef]
- Crundwell, F. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part III. Application to oxide, hydroxide and sulfide minerals. Hydrometallurgy 2014, 149, 71–81. [Google Scholar] [CrossRef]
- Crundwell, F. The mechanism of dissolution of minerals in acidic and alkaline solutions: Part II Application of a new theory to silicates, aluminosilicates and quartz. Hydrometallurgy 2014, 149, 265–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.-A.; Dreisinger, D.; Lv, C.; Lv, G.; Zhang, W. Recovery of vanadium from calcification roasted-acid leaching tailing by enhanced acid leaching. J. Hazard. Mater. 2019, 369, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Liao, X.; Zhang, X.; Teng, A.; Xue, X. A novel resource utilization of the calcium-based semi-dry flue gas desulfurization ash: As a reductant to remove chromium and vanadium from vanadium industrial wastewater. J. Hazard. Mater. 2018, 342, 436–445. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Xue, Z. Oxidation Kinetics of Vanadium Slag Roasting in the Presence of Calcium Oxide. Miner. Process. Extr. Metall. Rev. 2017, 38, 265–273. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, T.; Zhou, M.; Wen, J.; Li, X.; Wang, Y.; Xue, X. Effect of microwave irradiation and conventional calcification roasting with calcium hydroxide on the extraction of vanadium and chromium from high-chromium vanadium slag. Int. J. Miner. Process. 2017. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Zhou, M.; Gao, H.; Liu, J.; Xue, X. Roasting and leaching behaviors of vanadium and chromium in calcification roasting–acid leaching of high-chromium vanadium slag. Int. J. Miner. Process. 2018, 25, 515–526. [Google Scholar] [CrossRef]
- Xiaoyong, Z.; Qingjing, P.; Yuzhu, O.; Renguo, T. Research on the Roasting Process with Calcium Compounds for Silica Based Vanadium Ore. Chin. J. Process Eng. 2001, 2, 189–192. [Google Scholar]
- Yue, H.-R.; Xue, X.-X.; Zhang, W.-J. Reaction Mechanism of Calcium Vanadate Formation in V-slag/CaO Diffusion System. Metall. Mater. Trans. B 2021, 52, 944–955. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Zhang, X. Leaching Kinetics of Vanadium from Calcium-Roasting High-Chromium Vanadium Slag Enhanced by Electric Field. ACS Omega 2020, 5, 17664–17671. [Google Scholar] [CrossRef]
- Peiyang, S.; Bo, Z.; Maofa, J. Kinetics of the Carbonate Leaching for Calcium Metavanadate. Minerals 2016, 6, 102. [Google Scholar]
- Juhua, Z.; Wei, Z.; Li, Z.; Songqing, G. Mechanism of vanadium slag roasting with calcium oxide. Int. J. Miner. Process. 2015, 138, 20–29. [Google Scholar]
- Liu, B.; Du, H.; Wang, S.-N.; Zhang, Y.; Zheng, S.-L.; Li, L.-J.; Chen, D.-H. A novel method to extract vanadium and chromium from vanadium slag using molten NaOH-NaNO3binary system. AIChE J. 2012, 59, 541–552. [Google Scholar] [CrossRef]
- Liu, H.-B.; DU, H.; Wang, D.-W.; Wang, S.-N.; Zheng, S.-L.; Zhang, Y. Kinetics analysis of decomposition of vanadium slag by KOH sub-molten salt method. Trans. Nonferrous Met. Soc. China 2013, 23, 1489–1500. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, X.; Tian, X.; Yang, Y.; Chen, Y. Leaching of vanadium from chromium residue. Hydrometallurgy 2010, 103, 7–11. [Google Scholar] [CrossRef]
- Peng, H.; Yang, L.; Chen, Y.; Guo, J. A Novel Technology for Recovery and Separation of Vanadium and Chromium from Vana-dium-Chromium Reducing Residue. Appl. Sci. 2019, 10, 198. [Google Scholar] [CrossRef] [Green Version]
- Chai, L.; Yang, J.; Zhang, N.; Wu, P.J.; Li, Q.; Wang, Q.; Liu, H.; Yi, H. Structure and spectroscopic study of aqueous Fe(III)-As(V) complexes using UV-Vis, XAS and DFT-TDDFT. Chemosphere 2017, 182, 595–604. [Google Scholar] [CrossRef]
- Bunnag, N.; Kasri, B.; Setwong, W.; Sirisurawong, E.; Chotsawat, M.; Chirawatkul, P.; Saiyasombat, C. Study of Fe ions in aquamarine and the effect of dichroism as seen using UV–Vis, NIR and X-ray. Radiat. Phys. Chem. 2020, 177, 109107. [Google Scholar] [CrossRef]
- Yu, S.; Wang, B.; Pan, Y.; Chen, Z.; Meng, F.; Duan, S.; Cheng, Z.; Wu, L.; Wang, M.; Ma, W. Cleaner production of spherical nanostructure chromium oxide (Cr2O3) via a facile combination membrane and hydrothermal approach. J. Clean. Prod. 2018, 176, 636–644. [Google Scholar] [CrossRef]
- Nagaraju, G.; Chithaiahb, P.; Ashokac, S.; Mahadevaiah, N. Vanadium pentoxide nanobelts: One pot synthesis and its lithium storage behavior. Cryst. Res. Technol. 2012, 47, 868–875. [Google Scholar] [CrossRef]
- Shin, K.-H.; Jin, C.-S.; So, J.-Y.; Park, S.-K.; Kim, D.-H.; Yeon, S.-H. Real-time monitoring of the state of charge (SOC) in vanadium redox-flow batteries using UV–Vis spectroscopy in operando mode. J. Energy Storage 2019, 27, 101066. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Zheng, X.; Liu, Z.; Tao, C. Leaching kinetics of vanadium from calcification roasting converter vanadium slag in acidic medium. J. Environ. Chem. Eng. 2018, 6, 5119–5124. [Google Scholar] [CrossRef]
- Deng, R.; Xie, Z.; Liu, Z.; Tao, C. Leaching kinetics of vanadium catalyzed by electric field coupling with sodium persulfate. J. Electroanal. Chem. 2019, 854, 113542. [Google Scholar] [CrossRef]
- Peng, H.; Liu, Z.; Tao, C. Leaching Kinetics of Vanadium with Electro-oxidation and H2O2 in Alkaline Medium. Energy Fuels 2016, 30, 7802–7807. [Google Scholar] [CrossRef]
- Peng, H.; Yang, L.; Chen, Y.; Guo, J.; Li, B. Recovery and Separation of Vanadium and Chromium by Two-Step Alkaline Leaching Enhanced with an Electric Field and H2O2. ACS Omega 2020, 5, 5340–5345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Jia, Y.; Ma, S.; Zheng, S.; Sun, Q. Effect of mechanical activation on the leaching kinetics of niobium-bearing minerali-sation in KOH hydrothermal system. Hydrometallurgy 2018, 181, 123–129. [Google Scholar] [CrossRef]
- Luo, M.-J.; Liu, C.-L.; Xue, J.; Li, P.; Yu, J.-G. Leaching kinetics and mechanism of alunite from alunite tailings in highly concentrated KOH solution. Hydrometallurgy 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Huang, H.; Li, B.; Zhang, X. Novel Technology for Vanadium and Chromium Extraction with KMnO4 in an Alkaline Medium. ACS Omega 2021, 6, 27478–27484. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).