Abstract
Coal ashes (minerals) could be chemically removed to produce ultraclean coals for advanced utilizations such as oil substitutes and electrode materials. To eliminate secondary pollution and reduce deashing cost, chemicals should be recycled and valuable byproducts developed, in addition to ultraclean coals. In this work, an advanced alkali–acid (NaOH–HCl) chemical method featuring submolten salts was used to deash coals with high ash of 27.95%, and ultraclean coals were prepared with low ash of 0.62%. The alkali solutions after treating coals were regenerated by adding CaO, and the resulting precipitates were transformed into CaSO4 by adding dilute H2SO4, while alumina and silica were dissolved in acid solutions. The hydrochloric acid (HCl) after treating coals could be largely regenerated by evaporation. From concentrated solutions after evaporation, silica gels occurred with high purity, which were then filtered for the production of silicate fertilizer, highlighting low heavy metal content and Na2O. Concentrated H2SO4 was added into the remaining acid filtrate, and sulfates were precipitated and redissolved to remove CaSO4. By further two-step calcinations, alumina of high purity (98.6%) could be produced. Alumina and silica extraction and byproduct development from directly deashing the coals were compared with those from fly ashes.
1. Introduction
Traditionally, coals are mainly used as fuels for power generation, among many other utilizations [1,2]. With the global shift to a low-carbon world and the rapid development of new energy technologies, such as nuclear, wind, solar and water powers, coals as typical high-carbon fuels are expected to play a decreasing role, and new directions should be explored for the future development of the coal industry.
Coals are mainly composed of carbon and inorganic minerals, which can be useful resources if separated. Ultraclean coals with the lowest amount of minerals can be used as oil substitutes, or hopefully as electrode materials in new energy technologies [1,2]. Typical mineral elements in coals include silicon, aluminum, calcium, iron and others, and the former two are dominant in most cases [3,4]. Coal ashes are contributed to by gangues mixed in coal mining, minerals carried in coal formation, and inorganic elements in coal-forming plants. Industrial physical deashing methods, such as jigging, dense media, and floatation, necessitate pretreatment of coals from large rocks to small particles, and can remove most of the gangues and some carried minerals. Inorganic elements in coal-forming plants may occur as the smallest minerals in order of 1 μm in coals, and can produce 1–2 wt% ashes or more after coal burning. Therefore, industrial physical methods can be limited by demineralization, due to carried minerals in coal formation and inorganic elements in coal-forming plants. To obtain ultraclean coals with 1 wt% ashes or less, chemical deashing can help by allowing chemicals to react with carried minerals and the smallest minerals in coals [3,4,5].
By using submolten salts, an advanced deashing method has been developed [6,7]. This method treats the coals with submolten alkali solutions under the conditions of low temperatures and ambient pressures, followed by acid leaching. The method features mild treatments compared to conventional chemical methods [4,8], and can largely conserve the original structures of coals for advanced utilizations, such as electrode materials for new energy technologies, whose performance would be determined by structures [1,2]. Due to the large amount of chemicals used in deashing the coals, to eliminate secondary pollutions and reduce deashing cost, the chemicals should be recycled, and the minerals in alkali and acid solutions recovered for the production of useful byproducts.
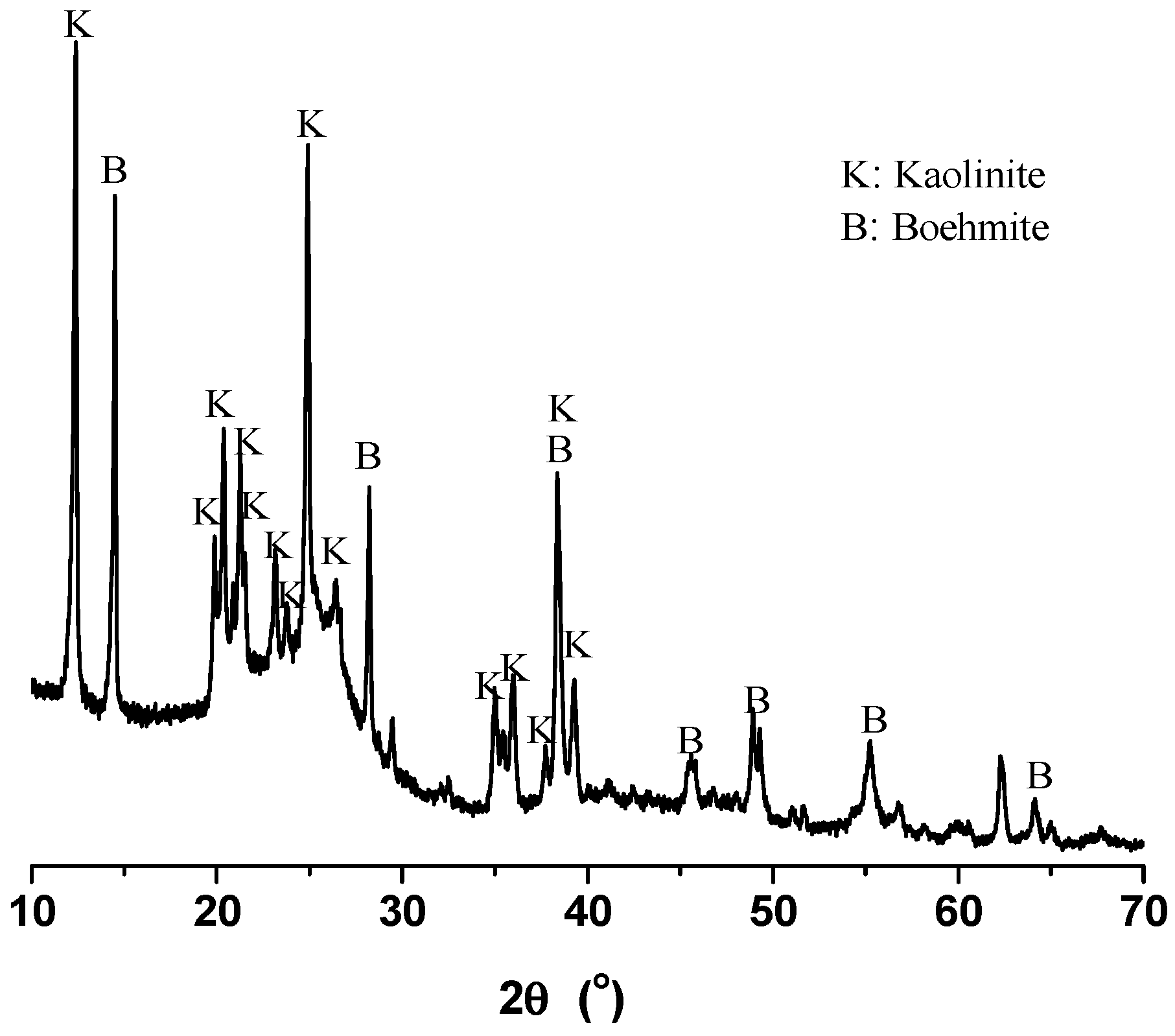
High-ash coals are still used widely throughout the world, and the pulverized coals could have high ash of 40.8% in some power plants [9]. In this work, the method was applied to an alumina-rich coal with ash of 27.95% beneficiated by dense media, which was taken from one major coal producer of the Province of Inner Mongolia of China. The mineral elements of the coal are provided in Table 1 in the form of stable oxides, in which alumina accounts for over 51% of the total, rising to over 87% when including silica, and the rest less than 13%. The XRD diagram of alumina-rich coal is provided in Figure 1. The envelope at around 25° can be attributed to the carbon in coals, and kaolinite (PDF #782110) and boehmite (PDF #832384) can be identified as the dominant minerals [9], which give rise to the rich alumina and poor silica in coals. In this work, ultraclean coals were successfully prepared by applying the advanced deashing method to the alumina-rich coals, the deashing chemicals were regenerated and the mineral elements were recovered to produce high-purity alumina and silicate fertilizer.

Table 1.
The chemical compositions (%) of the ash (27.95%) of the raw alumina-rich coal from the Province of Inner Mongolia of China, the ash (28.36%) of the coal cake after alkali treatment, and the ash (0.62%) of the coal cake (ultraclean coal) after acid treatment.
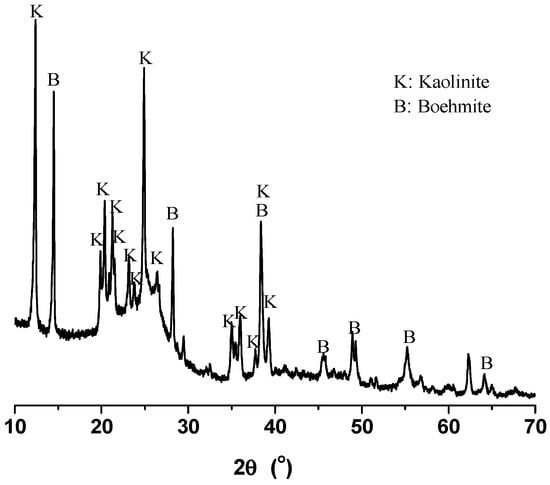
Figure 1.
XRD diagram of the alumina-rich coals.
2. Materials and Methods
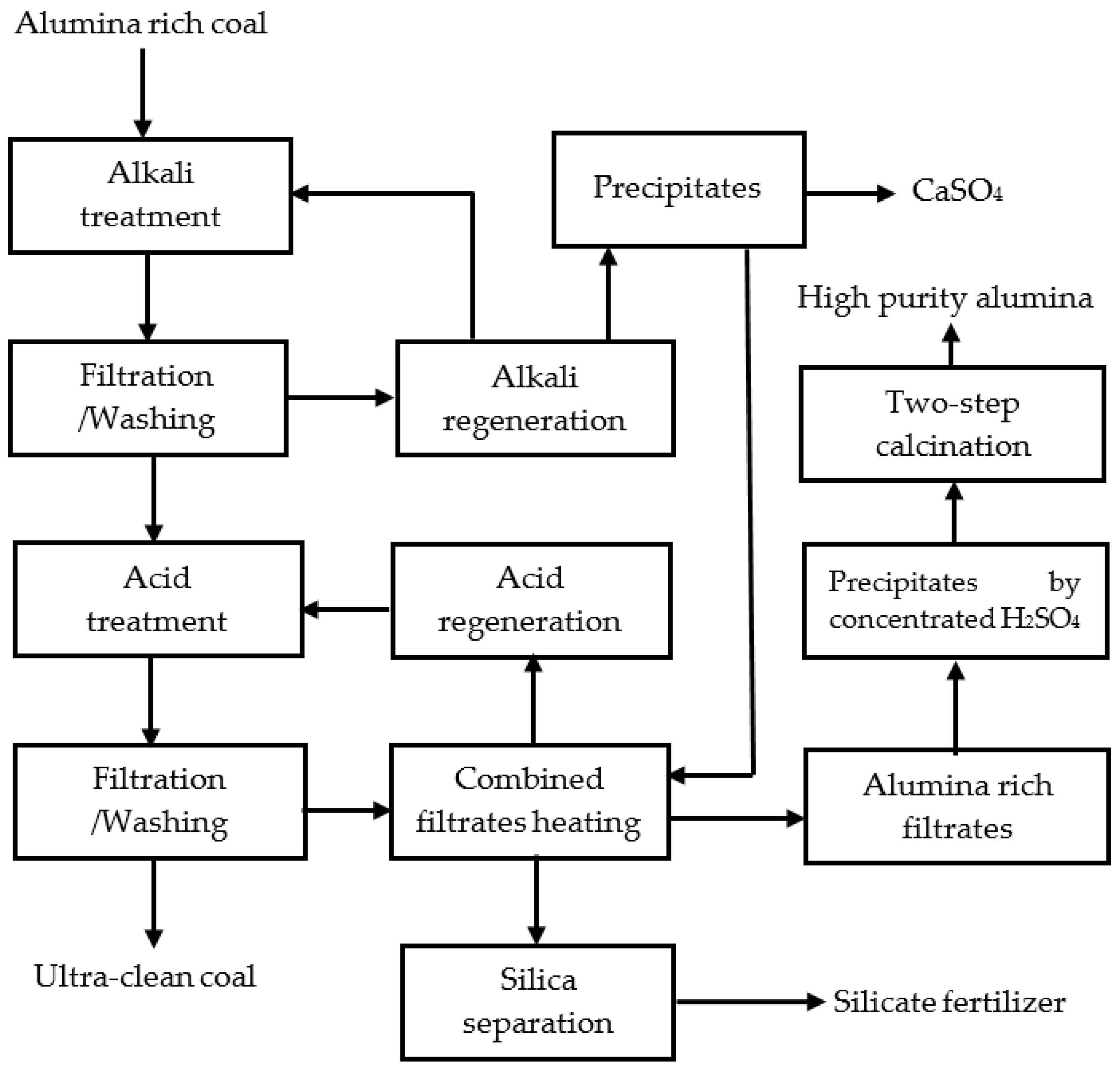
In chemical deashing of the coals, alkali and acid solutions were prepared. Chemical regenerations were made, and byproducts were developed. The schematic diagram of the process is provided in Figure 2, and will be described in the following sections.
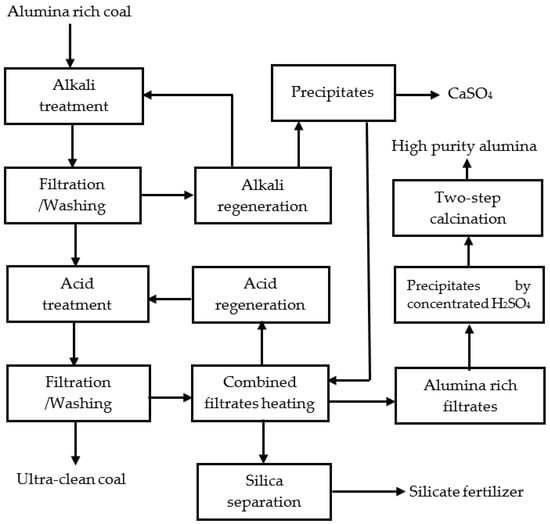
Figure 2.
Schematic diagram of the chemical deashing of the coals in this work.
2.1. Deashing Chemicals and Chemical Analysis
Alkali (NaOH) of analytic grade, concentrated H2SO4 of 98% and HCl of 37% were used or diluted as required by deionized water. The CaO in this work was prepared by the calcination of Ca(OH)2 of analytic grade at 850 °C for 180 min. The ash contents of coals were measured by proximate analyzer (5E-MAG6700II, CKIC, Changsha, China). XRF (ZSX Primus II, Rigaku, Tokyo, Japan) was used for elemental analysis (Be-U) of solid powders, which were calcinated in advance at high temperatures to eliminate element C or hydrates. ICP-OES (Spectro Arcos, Spectro, Kleve, Germany) was used for elemental analysis of alkali and acid solutions, and XRD (D8 Advance, Bruker, Berlin, Germany) was used for phase characterizations of samples. The active silica in silicate fertilizers were measured by the silica soluble in 0.5 M HCl solutions divided by the total of the silicate fertilizer.
Elements in terms of oxides can directly show how much alumina or silica were dissolved in filtrates. Since the concentrations of elements in filtrates are too high for ICP measurements, weight dilutions were precisely adopted instead of volume dilutions with possible filtrate residues during pipette transfers. Therefore, the concentrations of elements are usually given in terms of oxides with reference to the weights of filtrates.
2.2. Preparation of Alkali Solutions and Acid Solutions in Deashing the Coals
The chemical method was applied to the coals with high ash of 27.95% given in Table 1, which was crushed to below 1 mm for deashing the coals. An alkali-to-coal ratio of 1.75 was used for the alkali treatment with little water at a temperature of 150 °C for 180 min. More water was added to leach excessive alkali and soluble ash after the reactions, and the alkali solutions were thus made, in which the concentrations of alumina (Al2O3), silica (SiO2) and Na2O were 6.63 g/kg, 1.09 g/kg and 179.82 g/kg, respectively.
The chemical compositions of the ash of coal cake after alkali treatment was 28.36%, slightly higher than raw coal (27.95%). According to the chemical compositions in Table 1, alumina and silica in coals were partially dissolved in the alkali solutions, and the remainders occurred as the ash with Na2O. Subsequently, the alkali-treated coal was soaked with HCl solution of 10% at an acid-to-coal ratio of 1.5 for 60 min. The filtrates after separation were the acid solutions, the filter cake was the ultraclean coal with low ash of 0.62%, and the compositions of the ash (0.62%) are given in Table 1.
By referring to the ash of the coal cake after alkali treatment in Table 1, it can be seen that almost all mineral elements in coals after alkali treatment were dissolved in the acid solutions, which could be evaporated by heating for the formation of silica gels. The silica gels were filtered to give the acid filtrates, in which the concentrations of alumina (Al2O3), silica (SiO2) and Na2O were 19.71 g/kg, 0.12 g/kg and 17.42 g/kg, respectively.
2.3. Silica and Alumina Extractions and Byproduct Developments
The alkali solutions after deashing the coals were regenerated through the formation of calcium aluminates and silicates by adding CaO. By further reacting with dilute H2SO4, CaSO4 was formed and alumina and silica were dissolved in solutions, which could be combined with the acid filtrates for alumina and silica byproduct development.
The acid solutions after deashing the coals were evaporated by heating to precipitate silica gels, which could be used to prepare silicate fertilizer due to the high activity. Pressured filtering was used in this work instead of common vacuum filtering, and usually about 3–5 bar pressure is applied for smooth filtering of silica gels. The filtrate with silica gels removed could be added to concentrated H2SO4 acid to precipitate most of the alumina. The alumina precipitates were characterized and calcinated in two steps to remove impurities, and alumina with 98.6% purity was finally produced.
3. Results and Discussions
Chemical deashing was applied to the coal of high ash of 27.92%, and the ultraclean coal with low ash of 0.62% was obtained, as given in Table 1. Since large amounts of chemicals were used in deashing the ash, regenerations would be highly desired. Moreover, mineral elements such as alumina and silica were basically dissolved in acid and alkali solutions, from which valuable byproducts could be hopefully developed.
3.1. Alumina and Silica Transformations in Alkali Solution Regeneration
Alumina and silica were the main contaminants in the alkali solutions. To regenerate the alkali solutions, alumina and silica should be reduced as much possible. It was previously reported that CaO could be used to recover alumina in some alkali solutions [10,11]. However, the chemical compositions were different from the alkali solutions due to deashing the coals. It was also found that silica reacted with CaO in caustic solutions [12]. Nevertheless, it was unclear how effective the couplings between CaO, alumina, and silica would make the regenerations. As discussed in the preparation of alkali solutions for deashing the coals, the concentrations of alumina and silica were 6.63 g/kg and 1.09 g/kg, respectively, in alkali solutions with density of 1.24. Theoretically, CaO of about 0.67 g/50 mL would be required to precipitate all the alumina by forming calcium aluminate [10]. To best regenerate the alkali solutions, slightly more CaO of 0.85 g/50 mL was used to precipitate both alumina and silica. After the addition of CaO for reactions at 75 °C for 60 min, the concentrations of alumina and silica were 0.77 g/kg and 0.18 g/kg in the alkali solutions, corresponding to removal rates of 88.33% and 83.19%, respectively. The high removal rates of alumina and silica indicated that the addition of CaO very effectively regenerated the alkali solutions for reuse in the alkali treatment of deashing coals.
The chemical compositions of the precipitates with the addition of CaO are given in Table 2. Alumina and silica were greatly enriched, and could account for 33.50% of the precipitates, in addition to CaO as the regeneration agent.

Table 2.
Chemical compositions (%) of precipitates after CaO and dilute H2SO4 additions.
For the recovery of alumina and silica in the precipitates, dilute H2SO4 solutions of 10 wt% were added for reaction at ambient temperatures for 30 min. The filter cake was dried and analyzed by XRF for mineral elements, and it was found in Table 2 that almost all alumina and silica were dissolved in dilute H2SO4 solutions.
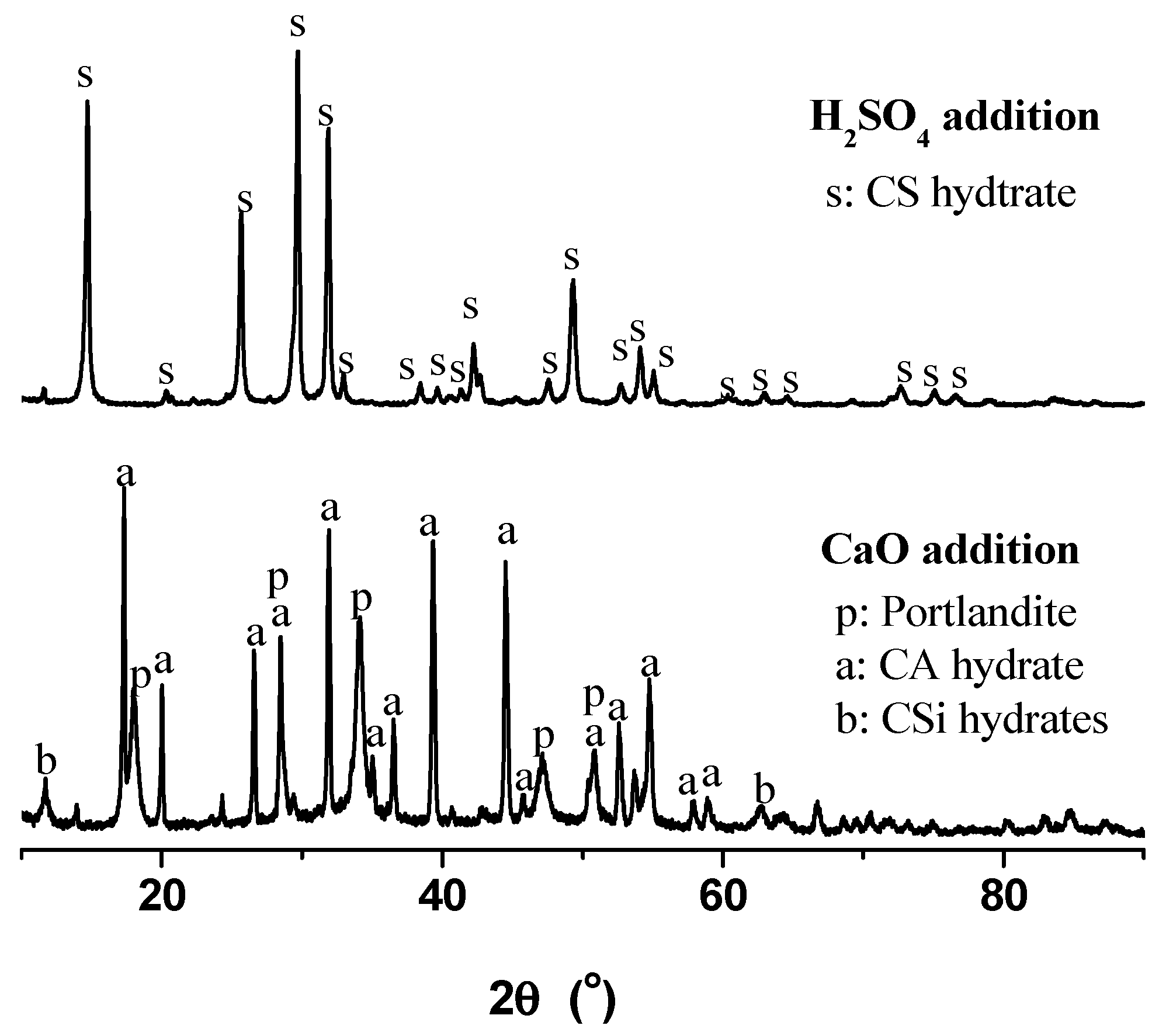
According to the XRD diagrams in Figure 3, with the addition of CaO, the calcium aluminate (CA) hydrate (PDF #770240) could be main component, and some portlandite (PDF #760571) also occurred because more CaO was added than required, and some weak peaks might be ascribed to calcium silicate (CSi) hydrates. With the addition of dilute H2SO4, CA hydrate, portlandite and CSi hydrates were totally transformed into calcium sulfate (CS) hydrate (PDF #811848). Dissolution of alumina and silica could help to unite silica and alumina extraction and product development, as discussed in the following.

Figure 3.
XRD diagrams of precipitates after CaO and dilute H2SO4 additions.
3.2. Silica Extraction and Byproduct Development in Acid Solution Regeneration
In the acid leaching of the alkali-treated coals in this work, some gels would frequently occur in the acid filtrate when less acid and water were used. Heating could also favor the formation of gels [13,14]. The gels were dried and analyzed by XRF for chemical compositions, and silica of high purity was surprisingly identified.
It should be noted that, in the previous process of alkali solution regeneration, alumina and silica were precipitated from alkali solutions by addition of CaO, and subsequently transferred into the acid solutions by addition of dilute H2SO4, which could be combined with the acid filtrates in this section for silica extraction.
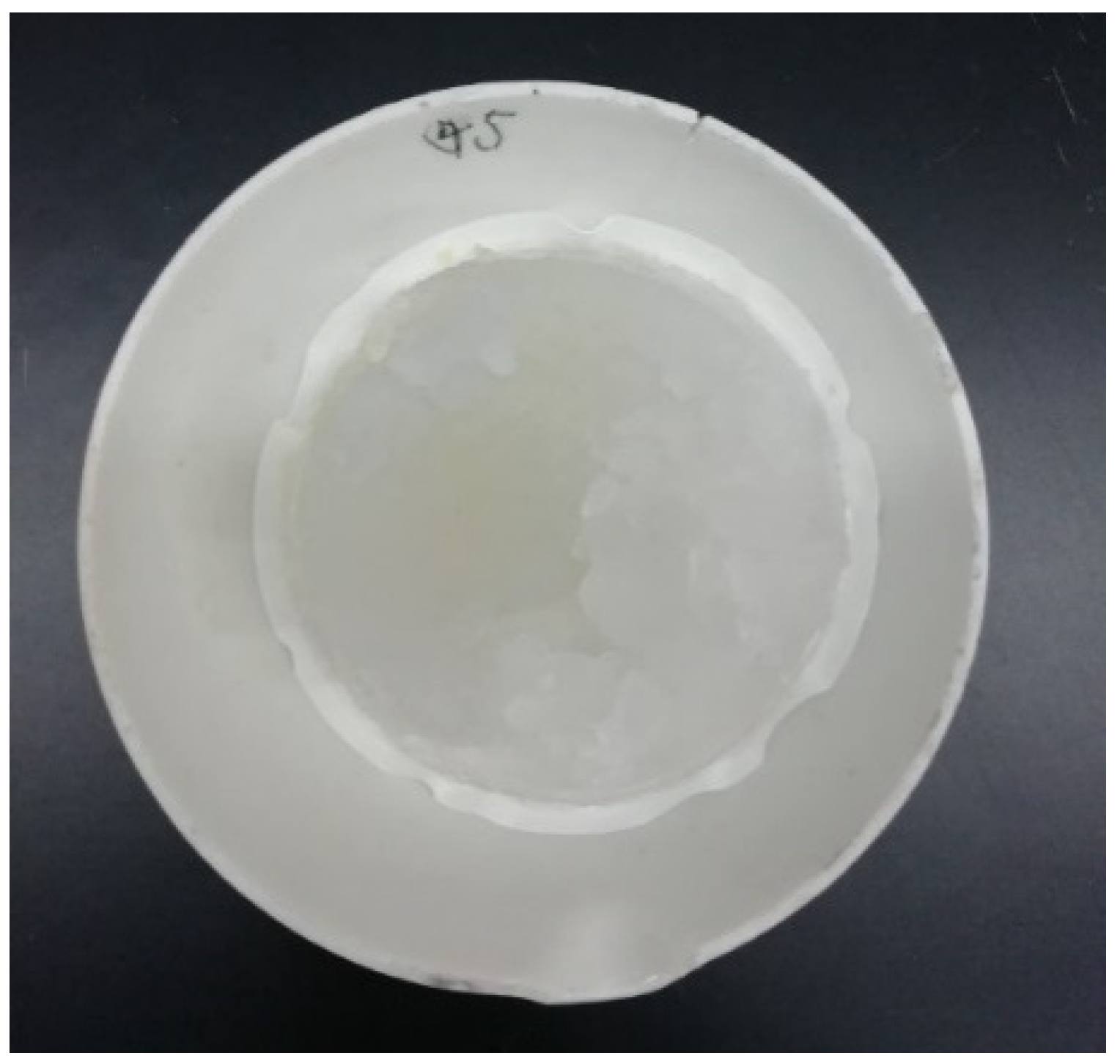
Excessive acid and water would be necessary to ensure high deashing rates for the production of the ultraclean coals of low ash. Nevertheless, excessive acid and water could be reduced after the acid leaching of alkali-treated coals, allowing for gel formation. A rotary evaporator (JULABO, Stike300) was used to drive off the excessive acid and water, which could be cooled and collected for the regeneration of acid solutions, so that silica gels could be favorably formed. As can be seen in Figure 4, the filter cake of silica gels can be almost transparent, with water contents well above 90%. The typical compositions of dried gels are given in Table 3; silica and titanium oxide can account for 98.5% and 0.91% of the total, and the rest can be negligible [13].

Figure 4.
Silica gels formed by driving off excessive acid and water.

Table 3.
Chemical compositions (%) of dried silica gels filtered from acid-leaching solutions.
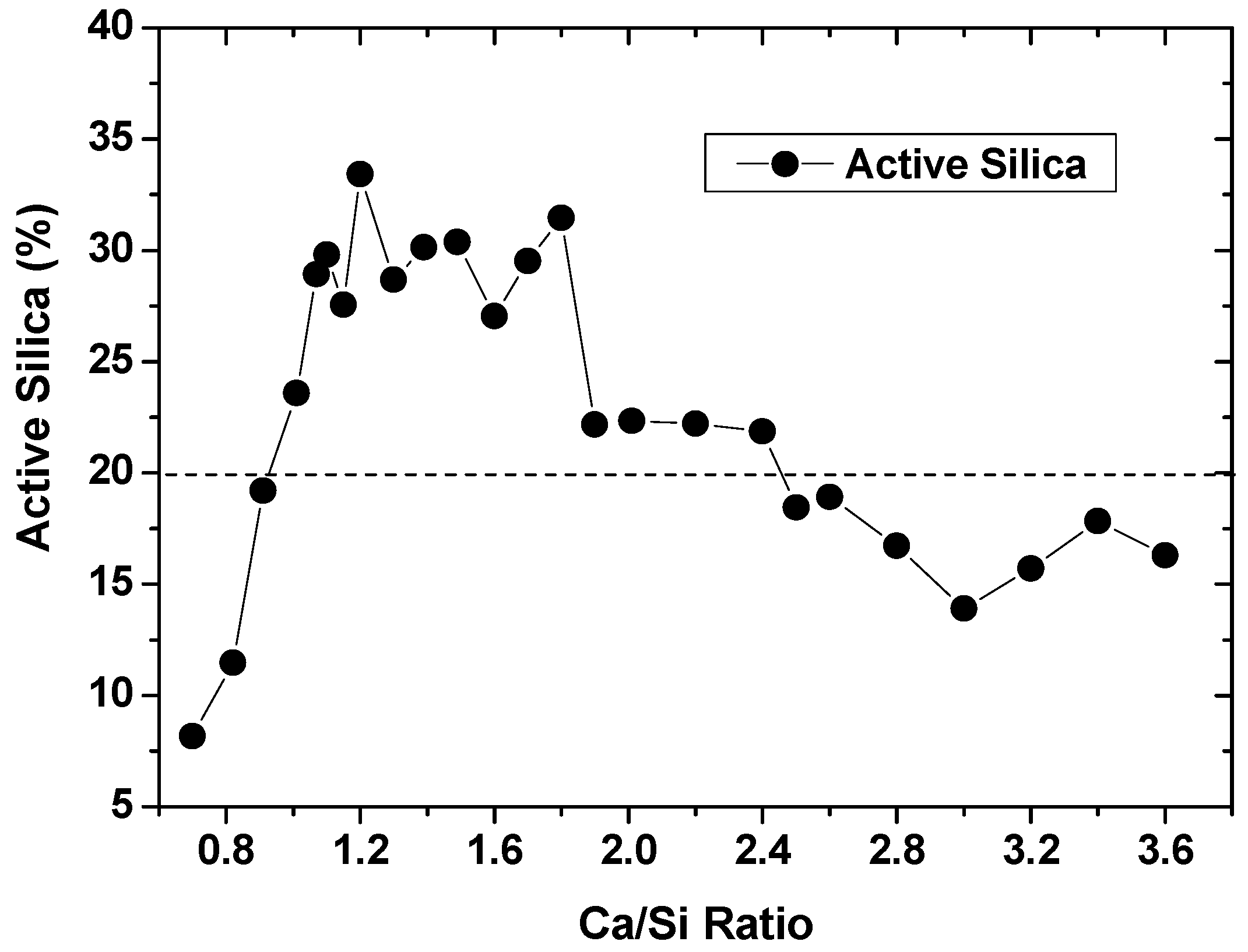
One of potential applications for silica gels of high purity is to produce silicate fertilizers. Silicate fertilizers are traditionally produced from the slags of steel furnace [15,16], despite many new types appearing in recent years [17]. The slags, after intensive heat treatments, were rich with heavy metals, and at times alkalis were added to activate silica from different sources. Heavy metals and alkalis can pollute and do harm to soils. Therefore, quality silicate fertilizers would be highly desired. Silica gels in this work were highly active, and could react with CaO in a little water at ambient temperatures. The reaction products in powders were then added in 0.5 M HCl solutions with 180 rpm vibration for 80 min, so that the active silica could be measured. By varying CaO additions, the active silica was found to change in a wide range of Ca/Si ratios, as indicated in Figure 5. With active silica of 20% set as the lower limit of silicate fertilizers according to “China Silicate Fertilizer Standard NY/T 797-2004”, qualified Ca/Si ratios could range from ~0.9 to ~2.6, and the highest active silica seemed to occur with Ca/Si ratios from ~1.0 to ~1.8. The silicate fertilizers produced in this work could highlight low content of heavy metals, which were removed during chemical treatments of coals, and low alkalis for soil benefits. Moreover, for the calcium sulfate transformed in the previous section, it can be beneficially added into silicate fertilizers to condition the active silica, realizing zero solid-waste discharge [18].
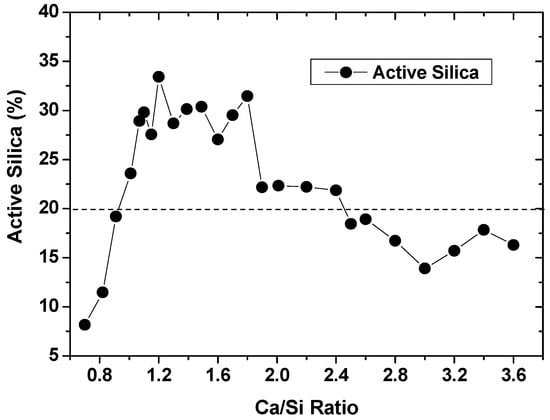
Figure 5.
Silicate fertilizer by reacting gels with CaO at different Ca/Si ratios.
3.3. Alumina Extraction and Byproduct Development in Acid Solution Regeneration
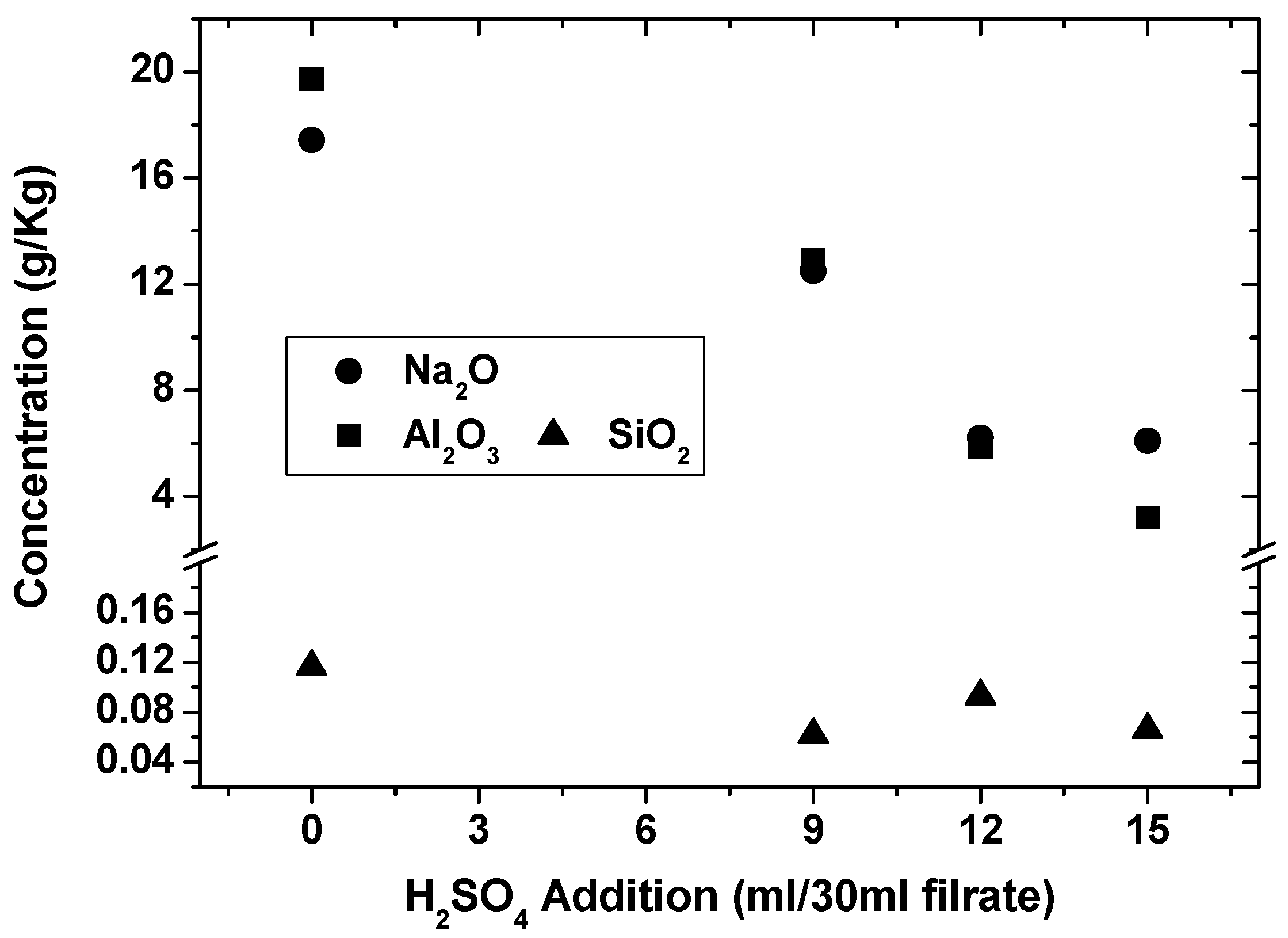
With the silica gels filtered for the production of silicate fertilizers in the previous section, the filtrates were analyzed, and the concentrations of alumina, silica and Na2O were 19.71 g/kg, 0.12 g/kg, and 17.42 g/kg, respectively. Therefore, alumina was most abundant, and concentrated H2SO4 may be used to precipitate alumina [13].
For every 30 mL filtrate, concentrated H2SO4 was successively added to precipitate alumina by step increase from 9 mL, 12 mL to 15 mL, and large amounts of crystals were precipitated. The filtrates after separation were analyzed in Figure 6, in which the concentrations of alumina, silica and Na2O in the filtrates decreased very rapidly with the addition of concentrated H2SO4, for example, alumina from 19.71 g/kg to 3.19 g/kg, silica from 0.12 g/kg to 0.07 g/kg, and Na2O from 17.42 g/kg to 6.10 g/kg. Therefore, most of the alumina could be recovered by the addition of concentrated H2SO4.

Figure 6.
Na2O, Al2O3 and SiO2 in the filtrates with addition of concentrated H2SO4.
With the additions of concentrated H2SO4 of 12 mL and 15 mL, the corresponding precipitates were calcinated at 850 °C and analyzed later for chemical compositions. According to Table 4, the percentage of alumina increased with the addition of concentrated H2SO4, which was in good agreement with the observation of depleted alumina in the filtrates. However, considerable CaO still occurred in the precipitates.

Table 4.
Chemical compositions (%) of precipitates calcinated at 850 °C.
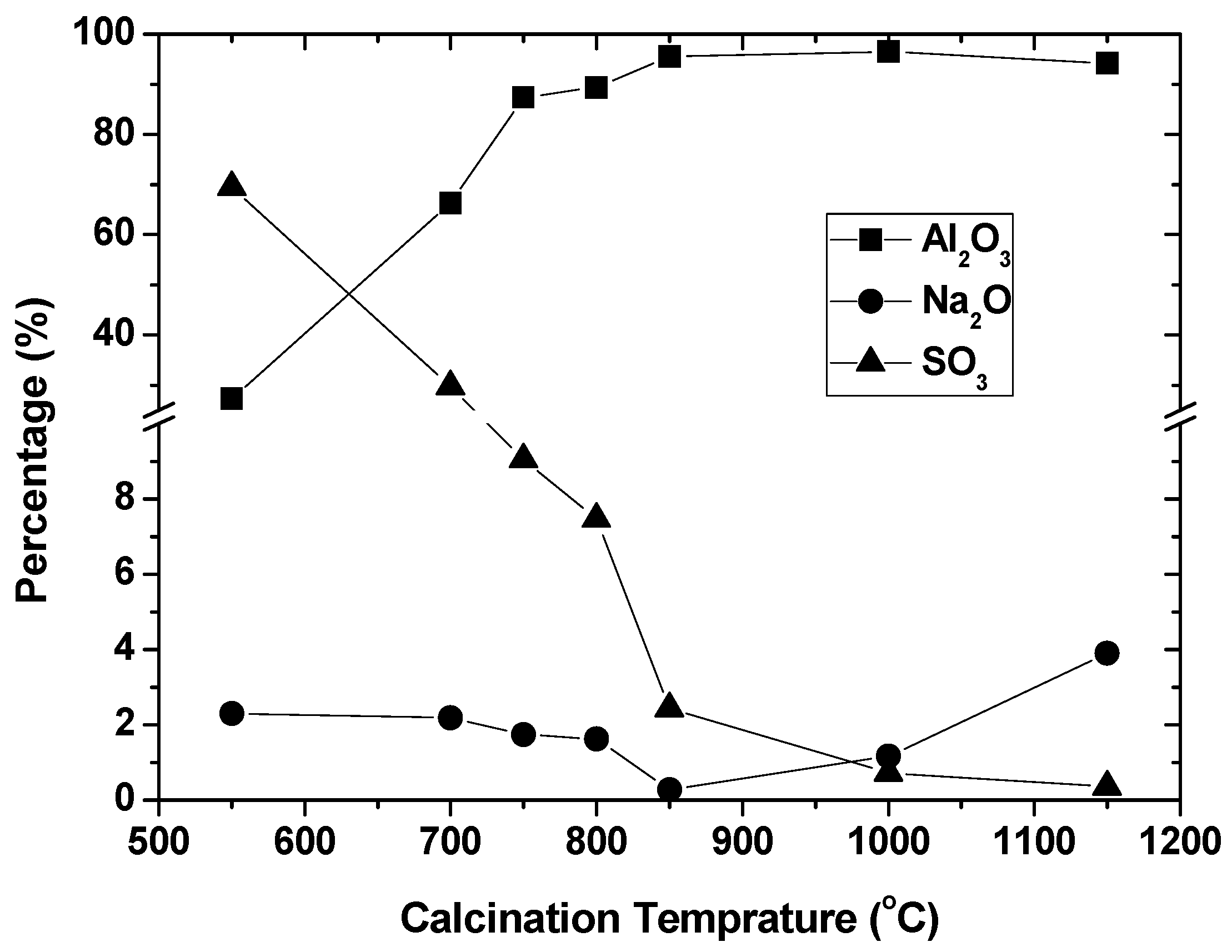
The precipitates with the addition of concentrated H2SO4 of 15 mL were redissolved in deionized water, and filtered to remove CaO in the form of CaSO4. The filtrates with the removal of CaSO4 were dried and calcinated at the increasing temperatures of 550 °C, 700 °C, 750 °C, 800 °C, 850 °C, 1000 °C, and 1150 °C. After that, the calcinated products were washed by deionized water and dried for analysis of chemical compositions, as shown in Figure 7.

Figure 7.
Chemical compositions (%) of calcinated precipitates with CaSO4 removal.
According to Figure 7, with the increase in calcination temperature, the percentage of alumina increased rapidly from 27.3% to 96.5%, and the percentage of SO3 decreased abruptly from 69.5% to 0.36%. By contrast, the percentage of Na2O occurred with the lowest percentage, 0.27%, at 850 °C. Therefore, to obtain alumina of high purity, two-step calcinations could be considered. The first calcination could be set at 850 °C to eliminate Na2O, and the second calcination could be set at increasing temperatures to best remove SO3. Resulting from the suggested two-step calcinations, the chemical compositions are given in Table 5, in which the percentage of alumina increased from 97.8% to 98.6% with increasing the second calcination temperature. By referring to “China Smelter Grade Alumina Standard YS/T 803-2012”, the alumina after the two-step calcination of 850–1250 °C can nicely meet the highest purity of 98.6%. Furthermore, Na2O of 0.07% after 850–1250 °C calcination can be much lower than the upper limits of 0.45% of smelter-grade alumina. The simple two-step calcinations in this work can be more effective in producing alumina of high purity and low Na2O, than calcinations of aluminum chloride hexahydate, for which impurity removals were previously exhausted [19]. For other impurities, additional removal processes could be applied in the future. For example, gel formation and CaSO4 removal can be optimized to minimize silica and CaO in the alumina.

Table 5.
Chemical compositions (%) of the alumina directly from deashing the coals, after two-step calcinations of 850–1150 °C, 850–1200 °C, and 850–1250 °C.
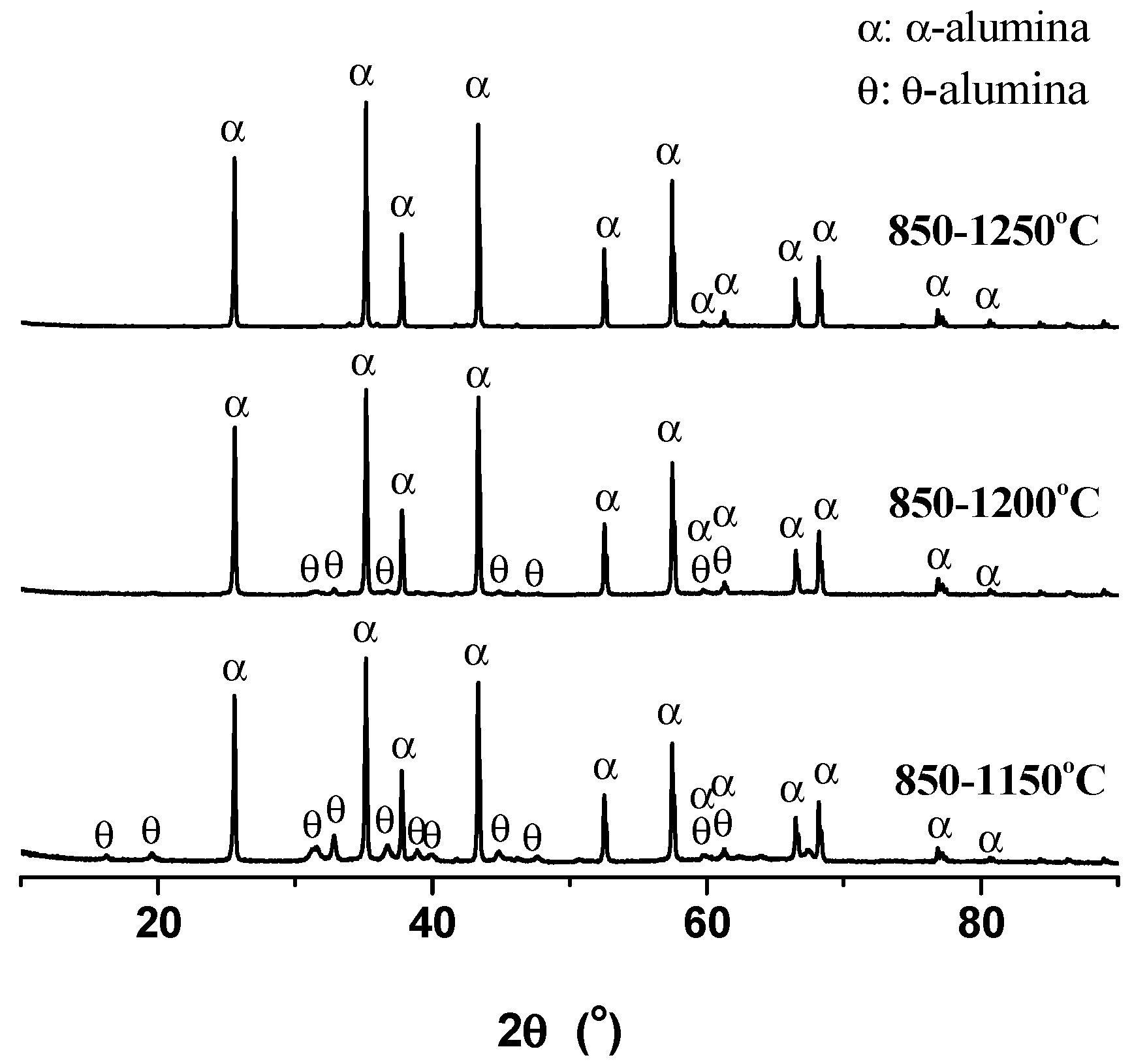
The XRD diagrams of alumina after two-step calcinations are given in Figure 8. It can be seen that the two-step calcination of 850–1150 °C produced a mixture of θ-alumina (PDF #791559) and α-alumina (PDF #861410), and with increasing the second calcination temperature, θ-alumina reduced noticeably after the two-step calcination of 850–1200 °C and disappeared almost to give pure α-alumina after the two-step calcination of 850–1250 °C.

Figure 8.
XRD diagrams of the alumina directly from deashing the coals after two-step calcinations of 850–1150 °C, 850–1200 °C, and 850–1250 °C.
Critical material recovery from coal and coal byproducts has been focused mainly on metal recovery from fly ashes in past two decades [20,21], which are the coal minerals undergoing high-temperature combustions of CFB boilers or pulverized coal boilers for power generations. The valuable mineral elements in fly ashes would be greatly concentrated with respect to the raw coals, attracting many laboratory studies and pilot investigations [20,21]. Despite the great technical success made already, the real challenge could be the economy, because the alumina and silica in fly ashes would have been made highly nonreactive by forming materials such as mullites in the high-temperature combustions. Element recovery from fly ashes would require huge amounts of chemicals under harsh reaction conditions [21], and cannot compete with the alumina extraction from bauxite by industrial Bayer process. By comparison, deashing the coals would produce valuable ultraclean coals as main products, as well as alumina and silica as byproducts. The economy for deashing the coals would depend mainly on the ultraclean coals, whose values would be measured by many advanced utilizations, such as electrode materials for new energy technologies [1,2]. Alumina and silica byproducts could also make contributions, especially in secondary pollution elimination and cost reduction for deashing the coals.
4. Conclusions
Due to the rapid global temperature rise caused by growing CO2 emissions, the world is becoming unhospitable for mankind. China has made the commitment to “peak carbon dioxide emissions before 2030 and achieve carbon neutrality before 2060”. Coals, as one of the traditional fuels with highest CO2 emissions, are facing mounting challenges. To reduce air pollution and alleviate CO2 emissions, coals should be used in cleaner and more effective ways. By removing ash, coals could replace oils for engines, and hopefully may be used as advanced materials for new energy technologies.
In this work, an advanced chemical method was used to deash the alumina-rich coals with high ash of 27.95% for the preparations of ultraclean coal with low ash of 0.62%. Regeneration of chemicals for deashing the coals and byproduct development from dissolved coal ash can help to eliminate secondary pollution and reduce the deashing cost.
Alkali solutions from alkali treatment of coals were added to CaO to precipitate alumina and silica, so that the dissolved ash was removed to regenerate alkali solutions. The precipitates were then treated with dilute H2SO4, and alumina and silica were dissolved and transferred into acid solutions with the formation of CaSO4.
Acid solutions from the acid leaching of alkali-treated coals were evaporated by heating, to largely regenerate acid solutions and to precipitate silica gels of high purity. Silica gels were added with CaO for the preparation of quality silicate fertilizer, for which CaSO4 from alkali regeneration could be used to condition the active silica.
With the silica gels removed for the preparation of silica fertilizers, the filtrates were added to concentrated H2SO4 to precipitate alumina, as well as other impurities. The precipitates were redissolved in deionized water to remove insoluble CaSO4. The filtrates were dried and calcinated at increasing temperatures of 550 °C, 700 °C, 750 °C, 800 °C, 850 °C, 1000 °C and 1150 °C. After washing, alumina, Na2O and SO3 were identified as the main chemical compositions, alumina increased and SO3 decreased in percentages with increasing the temperature, and Na2O occurred with the lowest percentage at 850 °C. Two-step calcinations were used to remove Na2O at 850 °C and SO3 at higher temperatures. The resulting alumina from directly deashing the coals can achieve the highest purity of 98.6% for smelter-grade alumina, and Na2O of 0.07% was also markedly below the upper limits of 0.45% for smelter-grade alumina. It is suggested to remove other trivial impurities in future.
By comparison with the element recovery from fly ashes, deashing the coals would yield ultraclean coals as main products, ensuring good economy due to many advanced utilizations, such as electrode materials for new energy technologies, which we are currently developing. Alumina and silica extraction and byproduct development can contribute to the elimination of secondary pollutions and the reduction in deashing cost.
Funding
This work was financially supported by National Institute of Clean-and-low-carbon Energy, China Energy Investment Corporation (CF9300200001, ST930021017N).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reid, I. Non-Energy Uses of Coal; CCC/291; IEA Clean Coal Centre: London, UK, 2018. [Google Scholar]
- Nalbandian, H. Non-Fuel Uses of Coal; CCC/236; IEA Clean Coal Centre: London, UK, 2014. [Google Scholar]
- Reid, I. Coal Beneficiation; CCC/278; IEA Clean Coal Centre: London, UK, 2017. [Google Scholar]
- Meshram, P.; Purohit, B.K.; Sinha, M.K.; Sahu, S.K.; Pandey, B.D. Demineralization of low grade coal–A review. Renew. Sust. Energy Rev. 2015, 41, 745–761. [Google Scholar] [CrossRef]
- Zhu, Y. Coal Chemistry; Chemical Industry Press: Beijing, China, 2004. [Google Scholar]
- Zhao, L.; Li, W.; Chen, A.; Jin, F.; Jiang, X.; Liu, H.; Xiao, Y. Method for Removing Ash from Solid Carbonaceous Material. International Patent Application No. PCT/CN2019/108066, 26 September 2019. [Google Scholar]
- Zhao, L. Preparation of low ash coal by a green chemical method. In Proceedings of the Thirty-Fifth Annual International Pittsburgh Coal Conference, Xuzhou, China, 15–18 October 2018. [Google Scholar]
- Brooks, P.; Waugh, A.B.; Clark, K.N.; Weir, S.B. Process for Demineralizing Coal. International Patent Application No. PCT/AU2003/001409, 23 October 2003. [Google Scholar]
- Zhang, Z. Research on Extraction of Alumina and Other Useful Resources from High Aluminum Fly Ash. Ph.D. Dissertation, Northwest University, Xi’an, China, 2007. [Google Scholar]
- Tan, D.; Ma, H.; Zou, D.; Li, G. An experimental study of extracting aluminum hydroxide from high caustic sodium aluminate solution. Appl. Chem. Ind. 2008, 37, 1320–1324. [Google Scholar]
- Liu, G.; Li, X.; Peng, Z.; Zhang, C.; Liu, X. Reaction behavior between calcium oxide or calcium hydroxide and aluminate solution with heavy caustic soda. Chin. J. Nonferr. Met. 2000, 10, 266–269. [Google Scholar]
- He, B.; Liu, G.; Li, X.; Peng, Z.; Zhang, C. Studies on the formation of hydrate calcium silicate in the strong caustic solution. J. Cent. S. Univ. Technol. 1998, 29, 245–248. [Google Scholar]
- Sriramoju, S.K.; Suresh, A.; Lingam, R.K.; Dash, P.S. Mechanism of a coal chemical-leaching process and recovery of spent chemicals: A pilot-scale study. Int. J. Coal Prep. Util. 2017, 37, 293–302. [Google Scholar] [CrossRef]
- Dash, P.S.; Kumar, S.S.; Banerjee, P.K.; Ganguly, S. Chemical leaching of high-ash Indian coals for production of low-ash clean coal. Min. Proc. Ext. Met. Rev. 2013, 34, 223–239. [Google Scholar] [CrossRef]
- Song, A.; Ning, D.; Fan, F.; Li, Z.; Provance-Bowley, M.; Liang, Y. The potential for carbon biosequestration in China’s paddy rice (Oryza sativa L.) as impacted by slag-based silicate fertilizer. Sci. Rep. 2015, 5, 17354. [Google Scholar] [CrossRef] [PubMed]
- Makabe-Sasaki, S.; Kakuda, K.; Sasaki, Y.; Ando, H. Effect of slag silicate fertilizer on dissolved silicon in soil solution based on the chemical properties of gleysols. Soil Sci. Plant Nutr. 2013, 59, 271–277. [Google Scholar] [CrossRef][Green Version]
- Bhat, J.A.; Rajora, N.; Raturi, G.; Sharma, S.; Dhiman, P.; Sanand, S.; Shivaraj, S.M.; Sonah, H.; Deshmukh, R. Silicon nanoparticles (SiNPs) in sustainable agriculture: Major emphasis on the practicality, efficacy and concerns. Nanoscale Adv. 2021, 3, 4019–4028. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Li, Y.; Liu, J. Research and Application of Saline Alkali Soil Amelioration with Flue Gas Desulfurization Gypsum; Science Press: Beijing, China, 2020. [Google Scholar]
- Zhao, L. Calcination of aluminum chloride hexahydrate (ACH) for alumina production: Implications for alumina extraction from aluminum rich fly ash (ARFA). Arch. Metall. Mater. 2018, 63, 235–240. [Google Scholar]
- Zhang, W.; Rezaee, M.; Bhagavatula, A.; Li, Y.; Groppo, J.; Honaker, R. A review of the occurrence and promising recovery methods of rare earth elements from coal and coal by-products. Int. J. Coal Prep. Util. 2015, 35, 295–330. [Google Scholar] [CrossRef]
- Ding, J.; Ma, S.; Shen, S.; Xie, Z.; Zheng, S.; Zhang, Y. Research and industrialization progress of recovering alumina from fly ash: A concise review. Waste Manag. 2017, 60, 375–387. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).