Extraction of Aluminum and Iron Ions from Coal Gangue by Acid Leaching and Kinetic Analyses
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
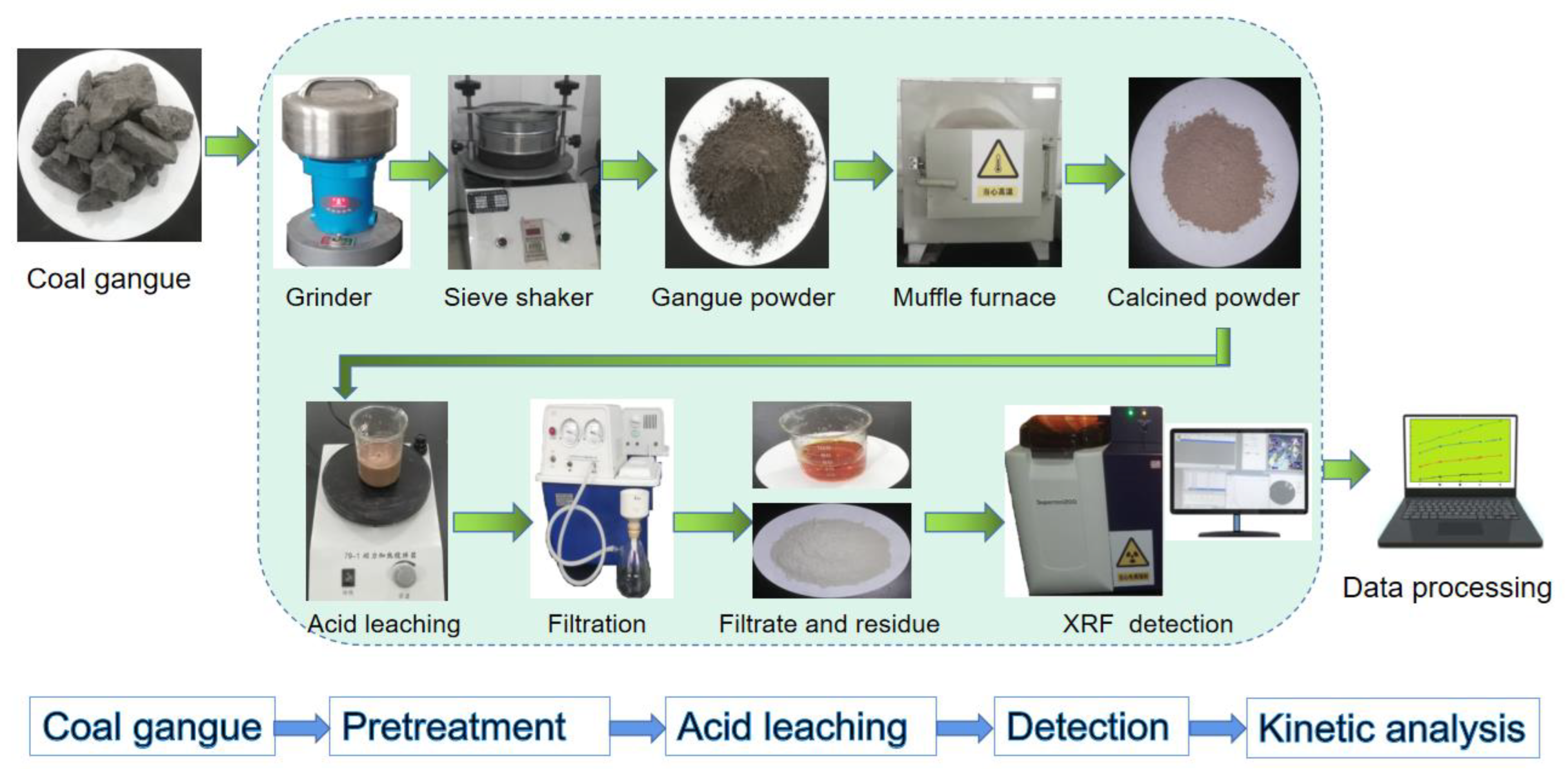
2.2. Procedure
2.3. Instrumentation and Characterization
3. Results and Discussion
3.1. Chemical Components of the Coal Gangue
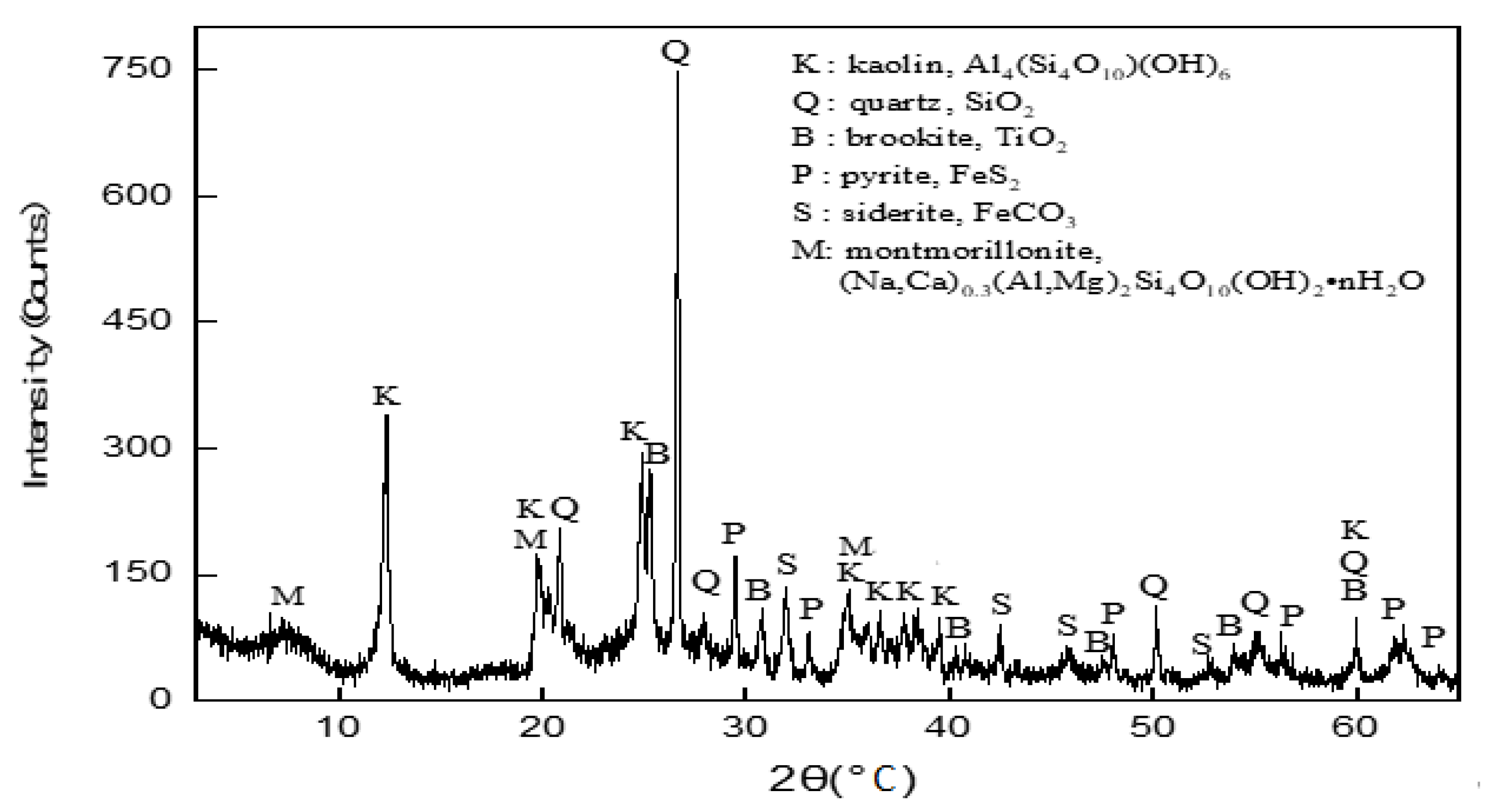
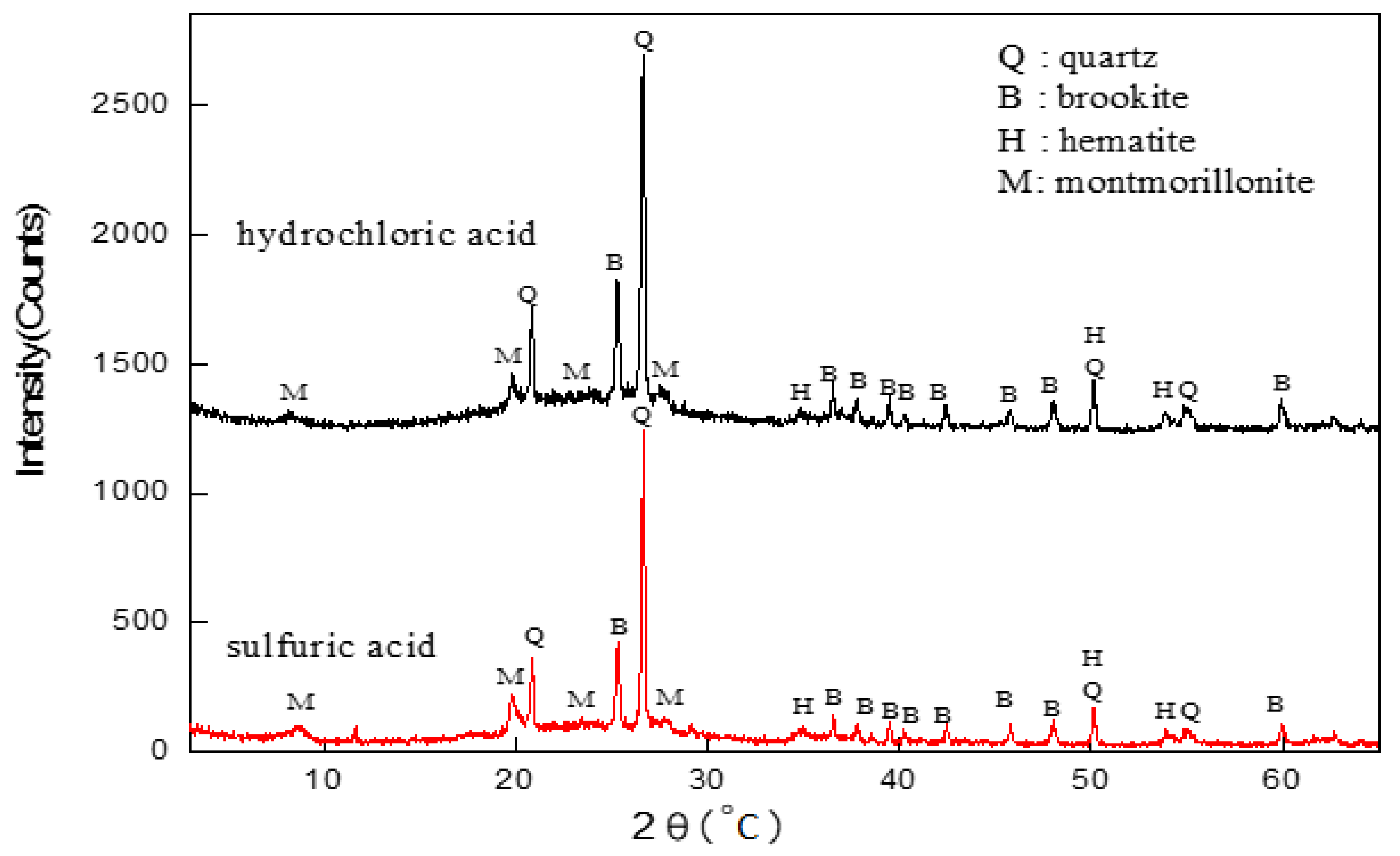
3.2. XRD Analysis of the Coal Gangue
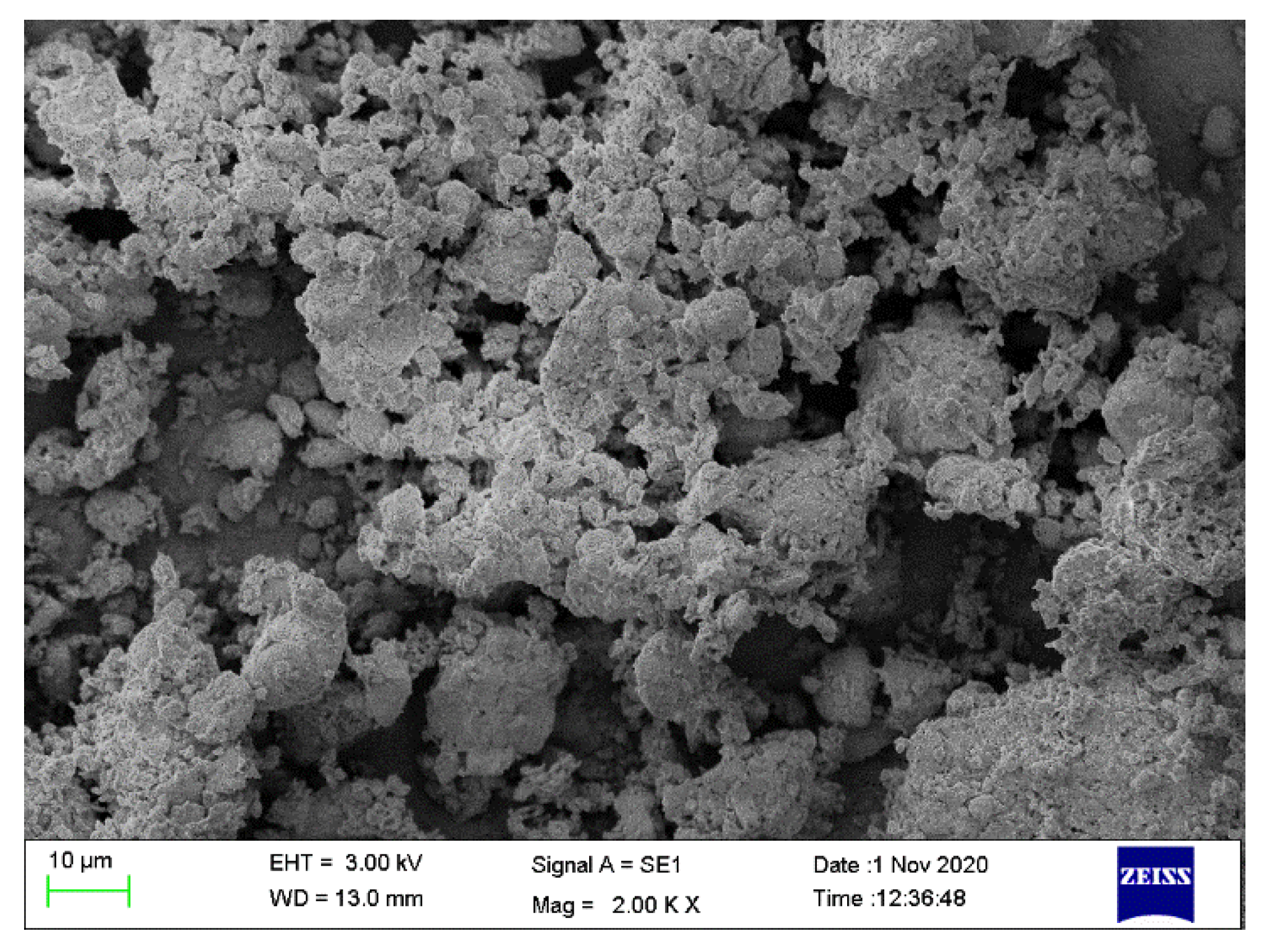
3.3. Morphology Analysis of the Coal Gangue
3.4. TG-DSC Analyses of the Coal Gangue
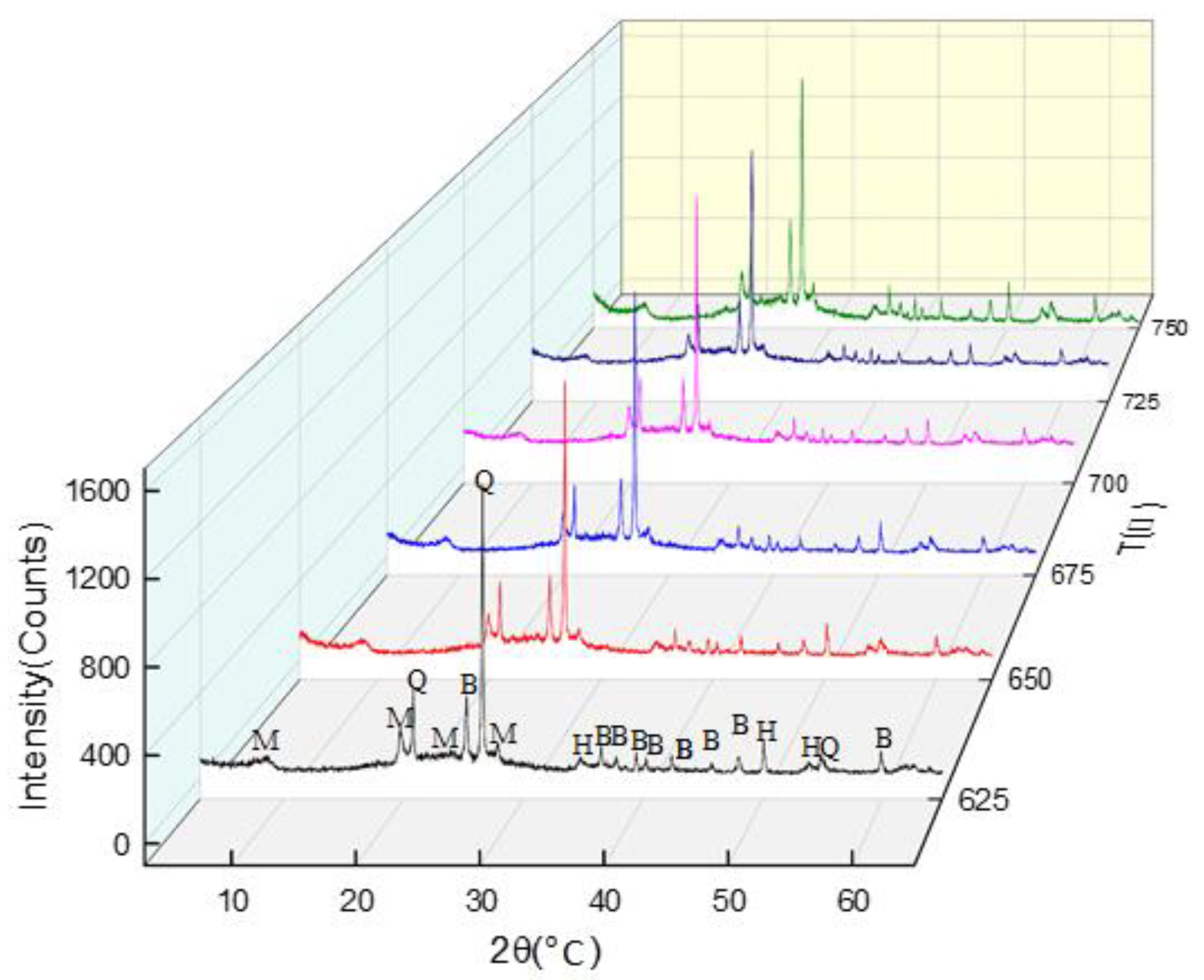
3.5. Phase Analyses of the Calcined Powders at Different Temperatures
3.6. Analyses of the Leaching Effects with Different Acids
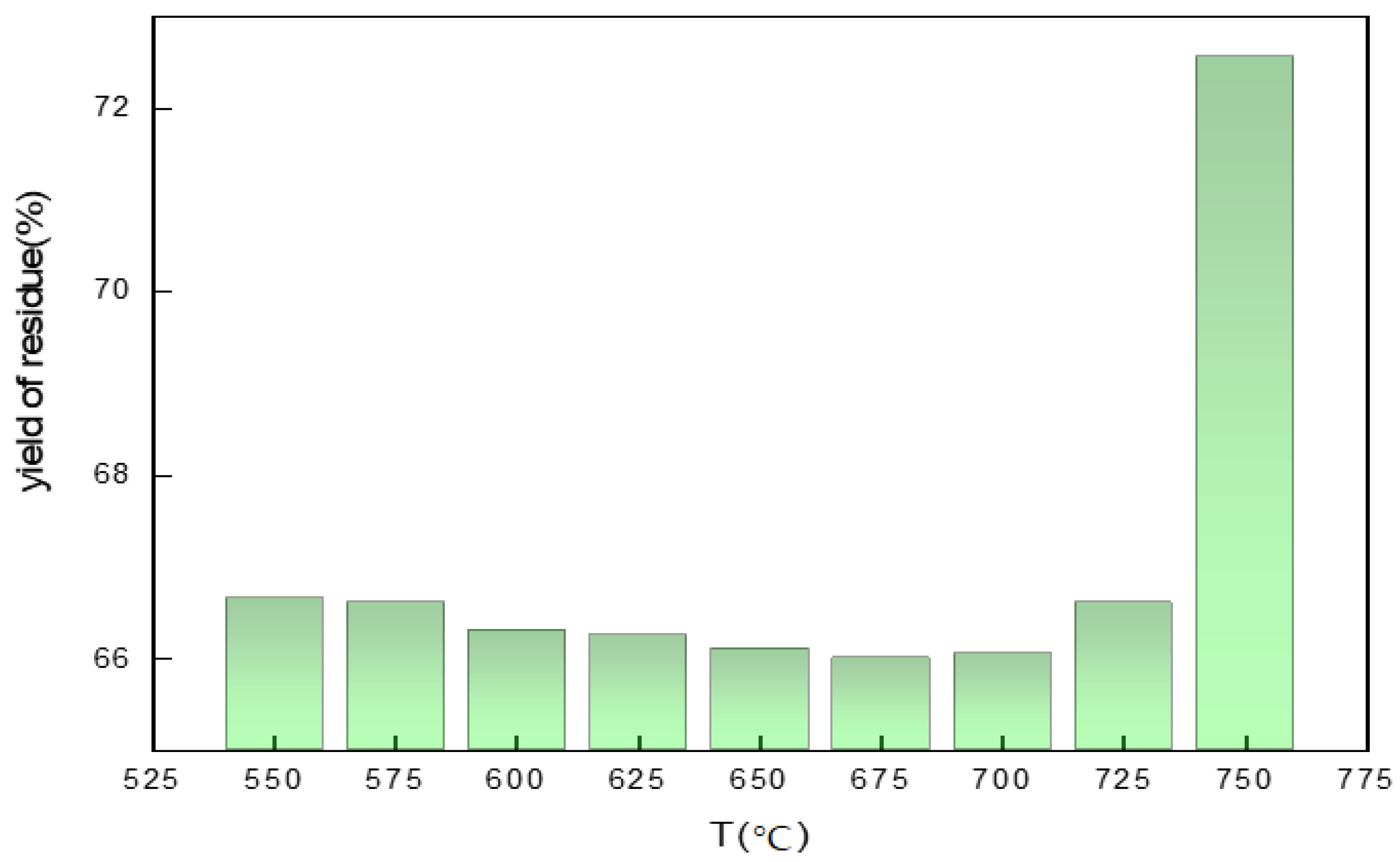
3.7. Effects of Calcination Temperature on the Yield of Residue
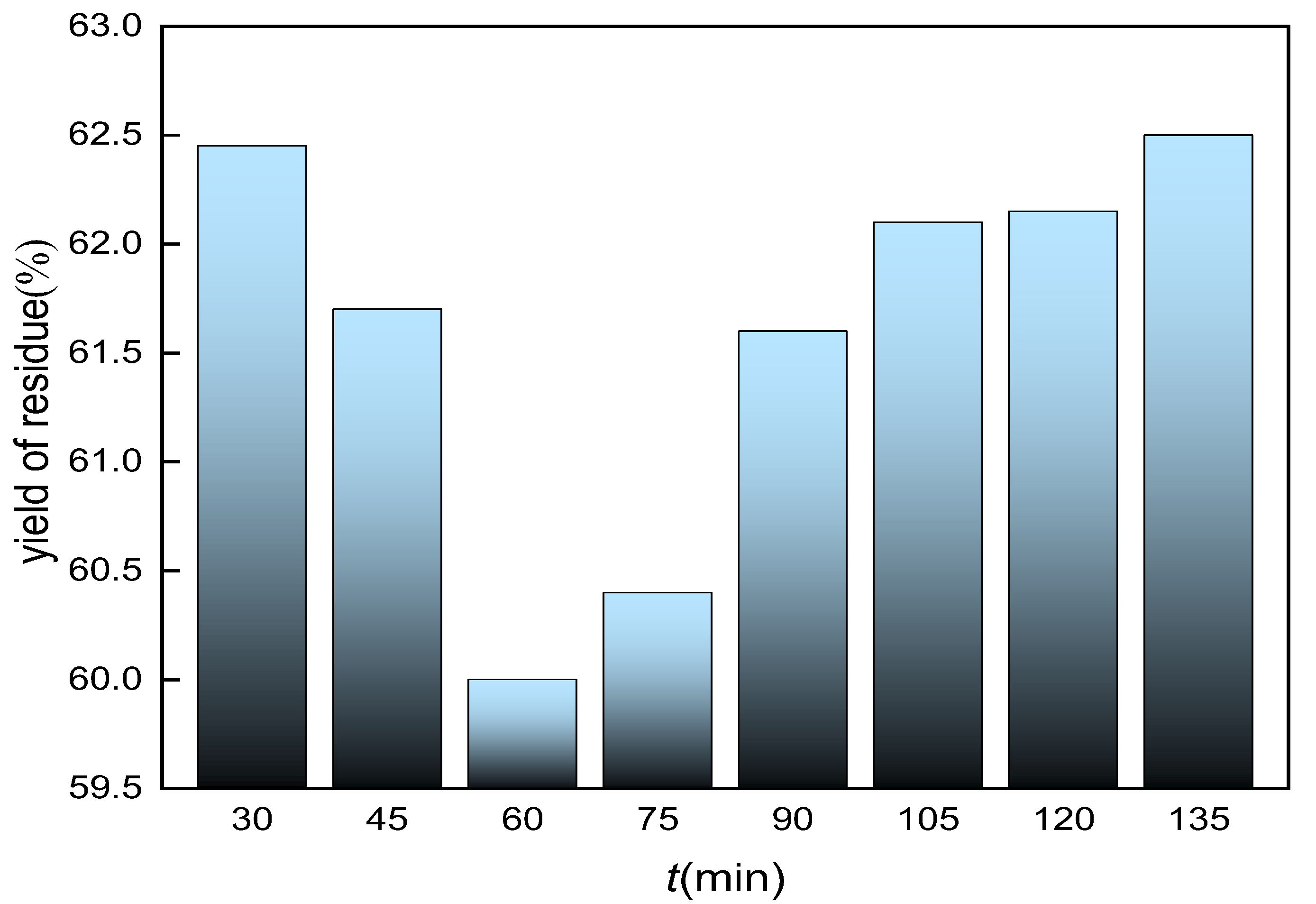
3.8. Effect of Calcination Time on the Yield of Residue
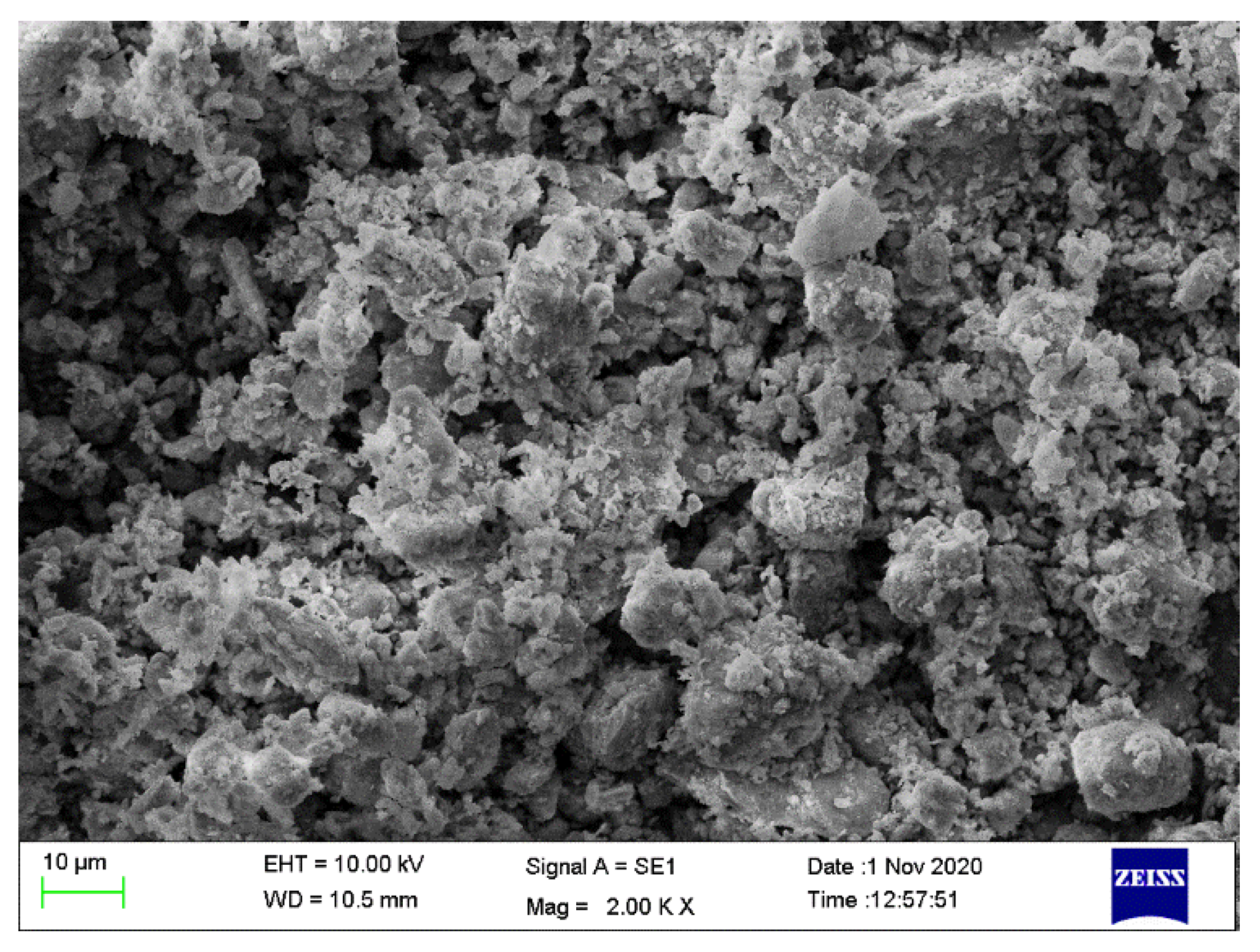
3.9. Morphology Analysis of Filter Residue
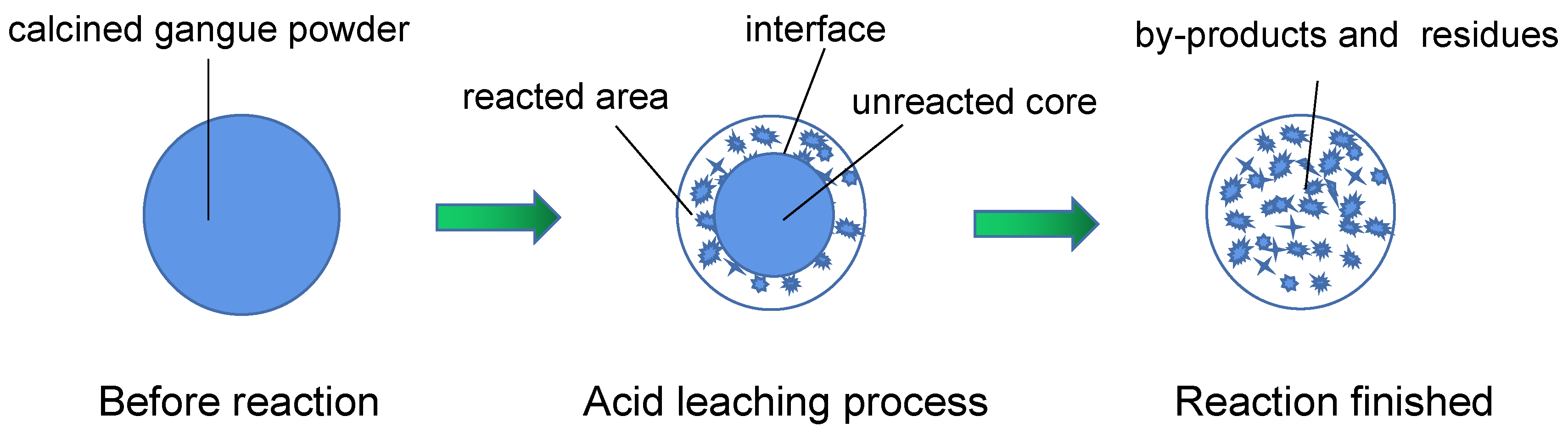
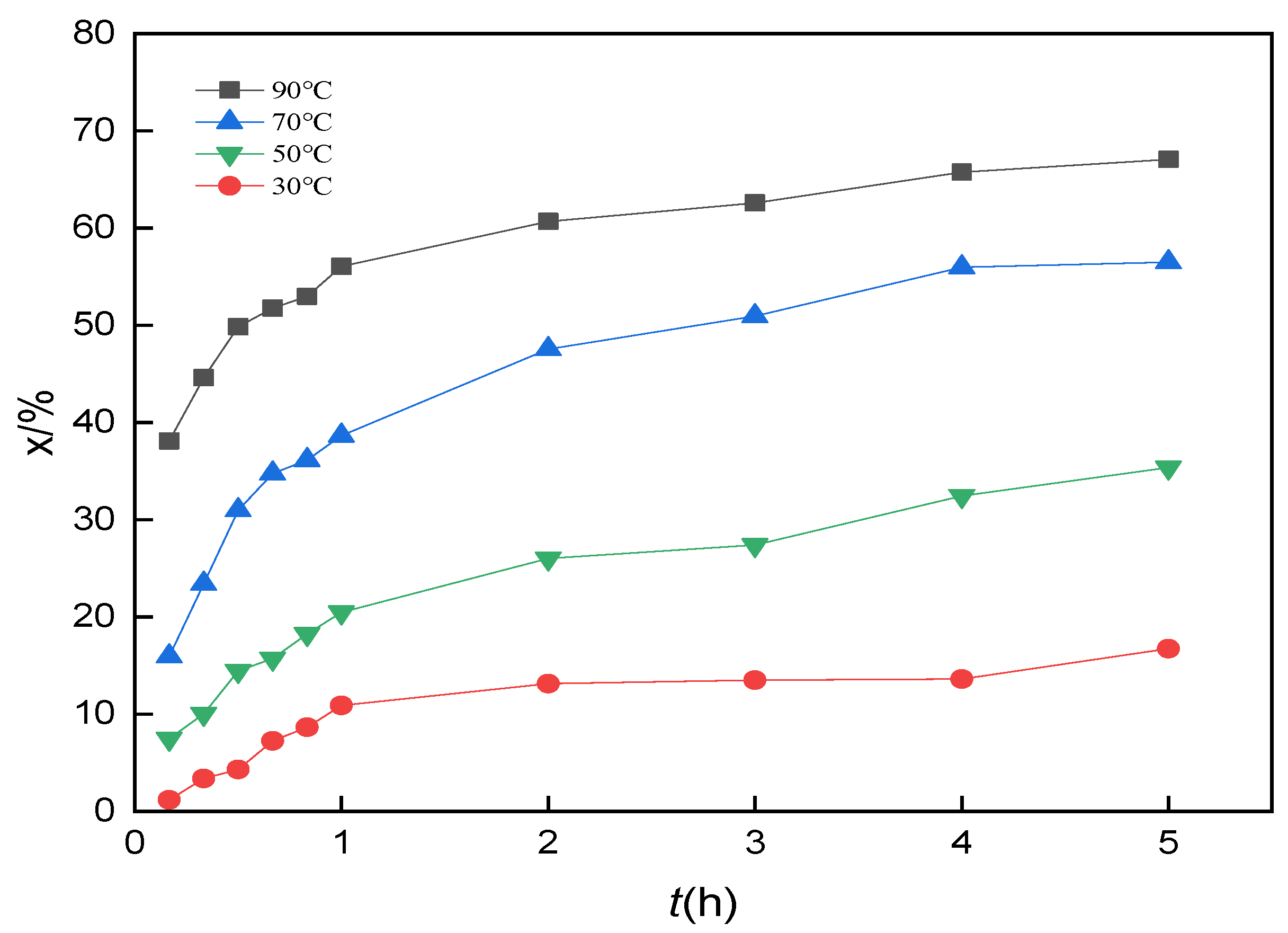
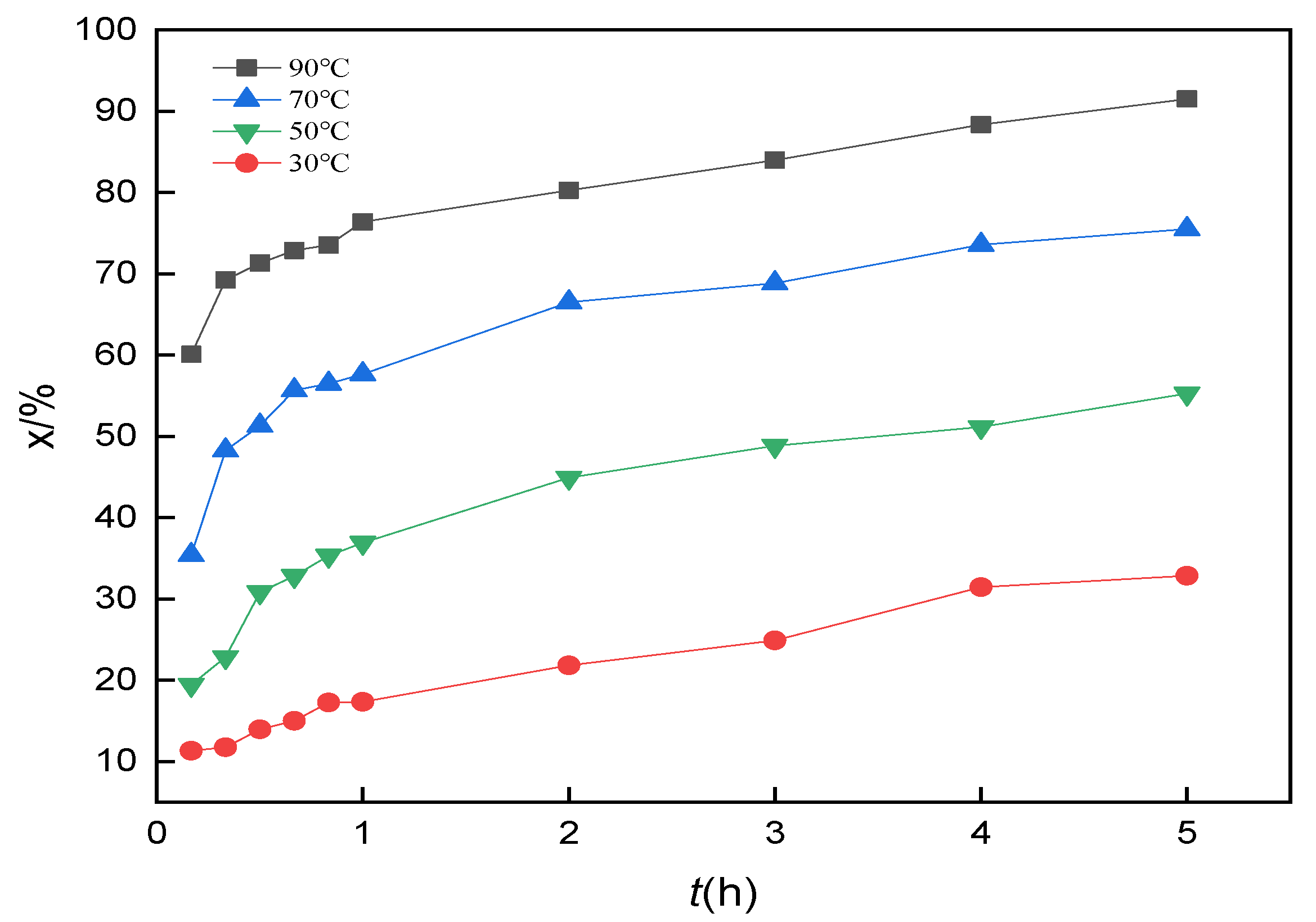
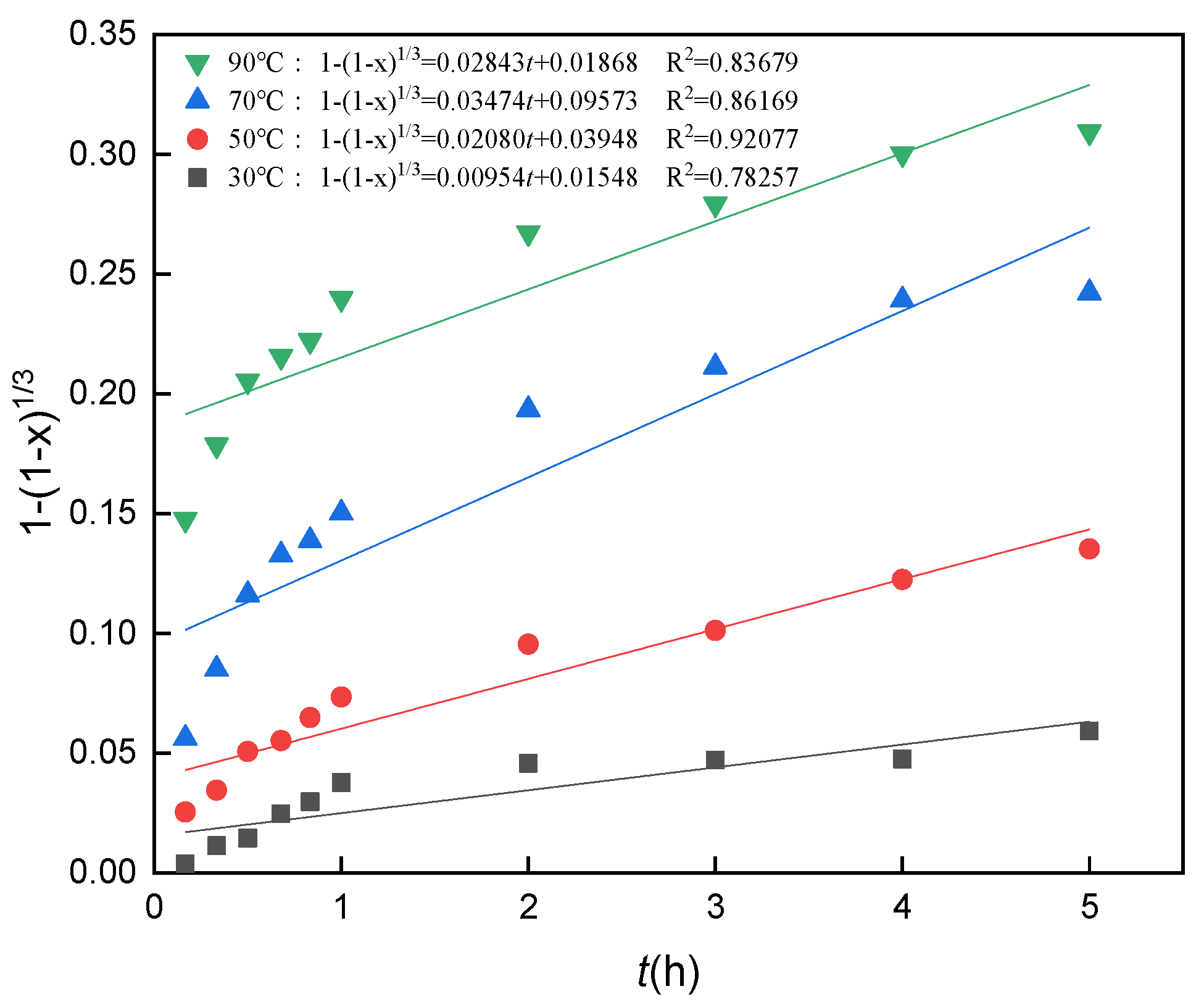
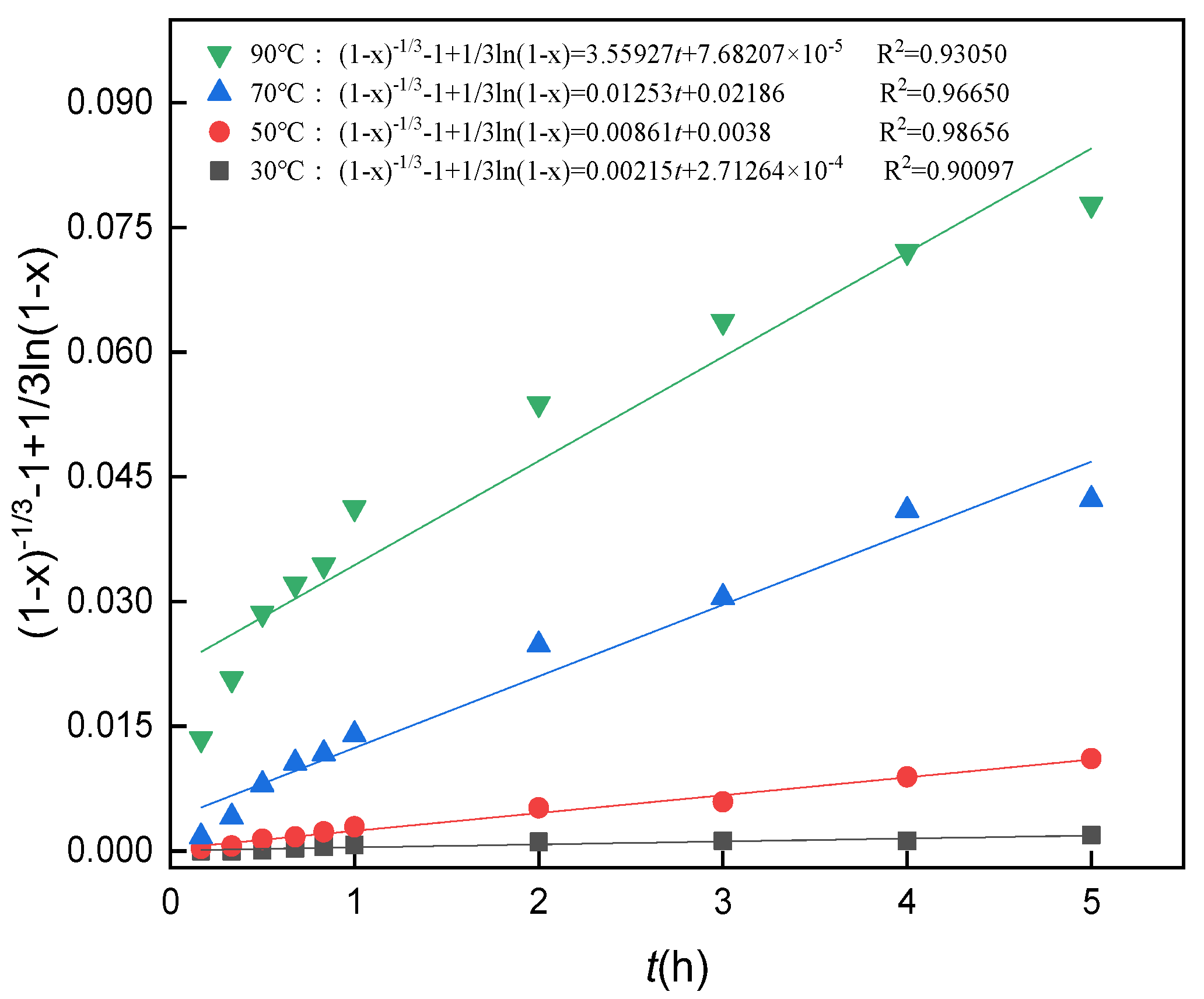
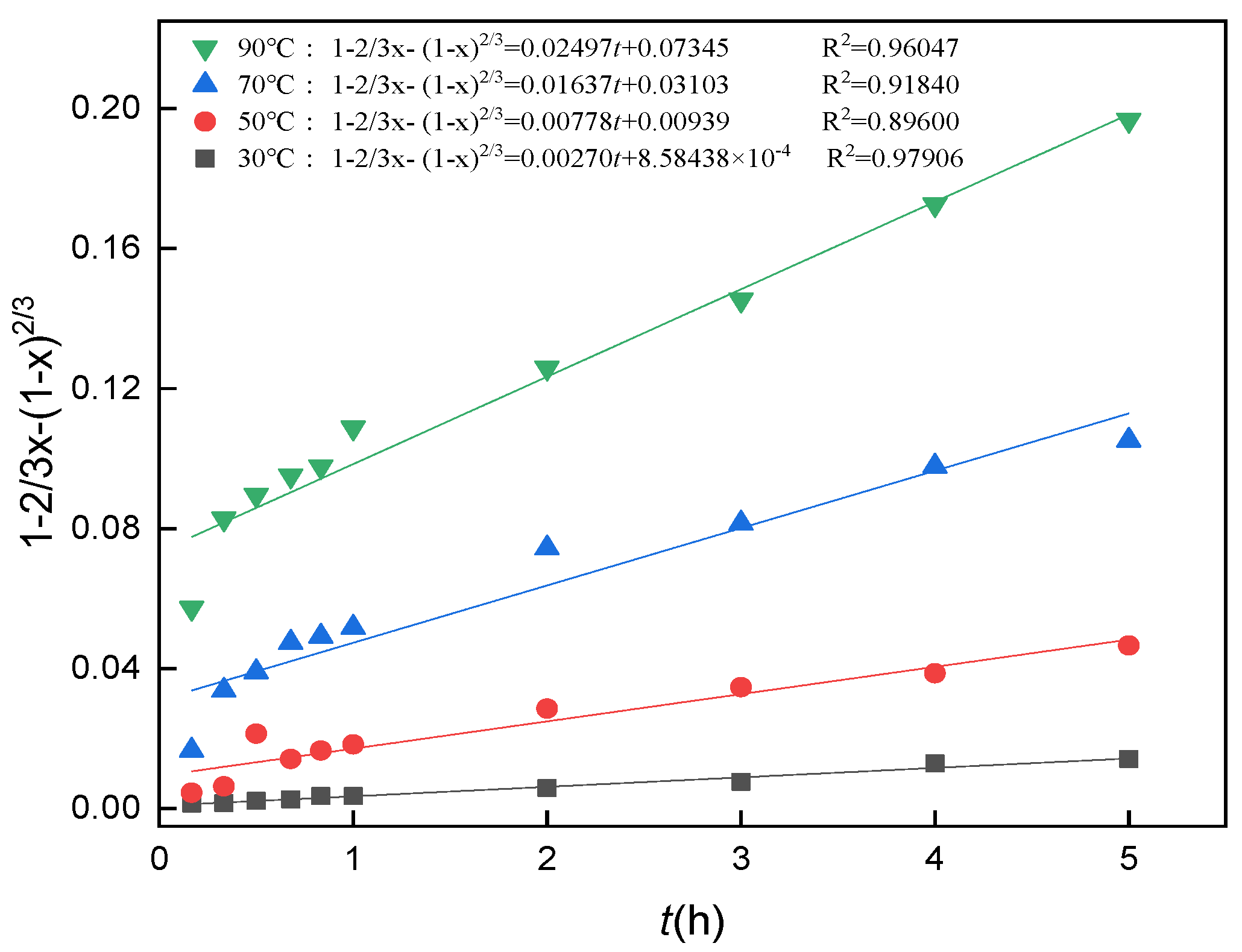
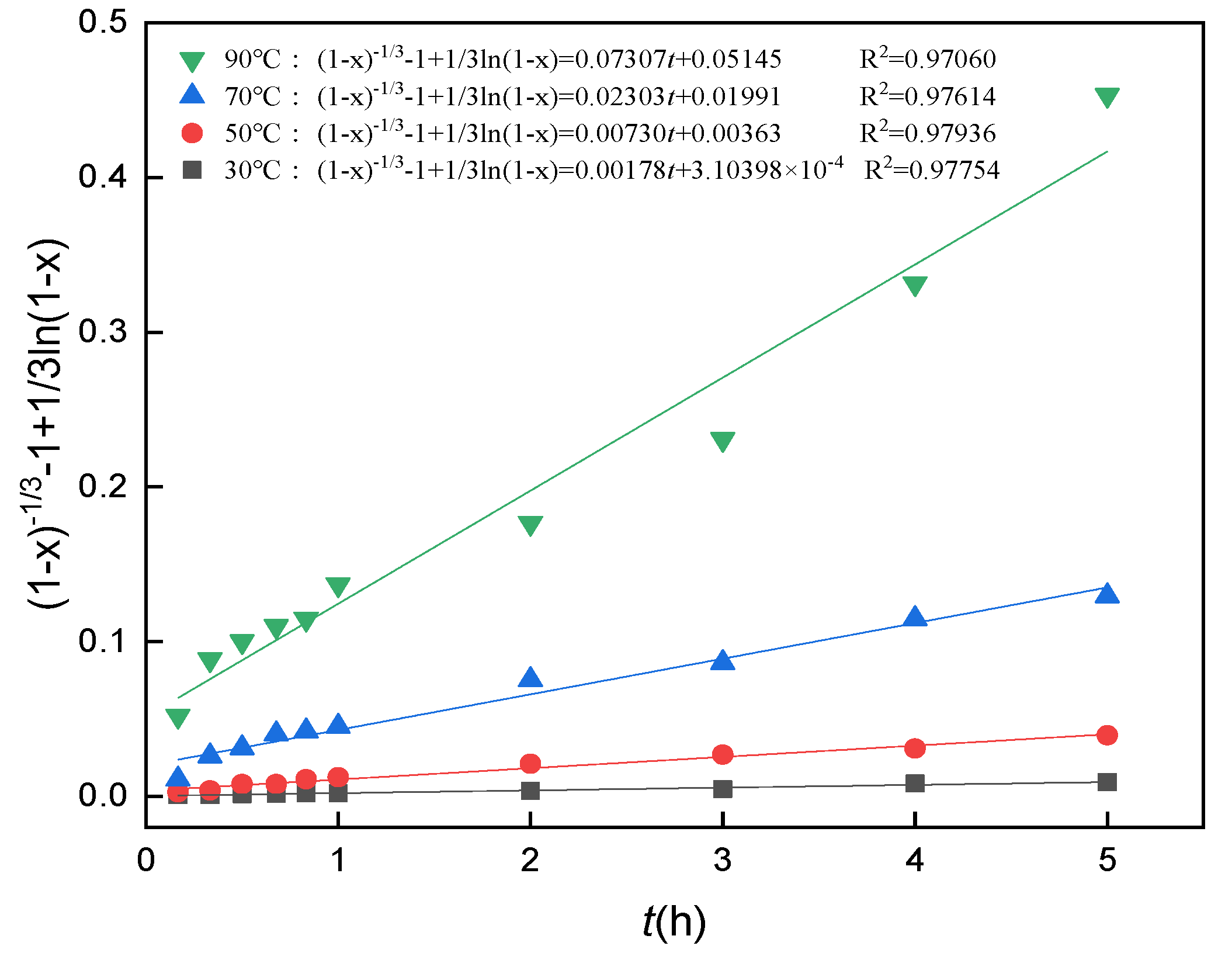
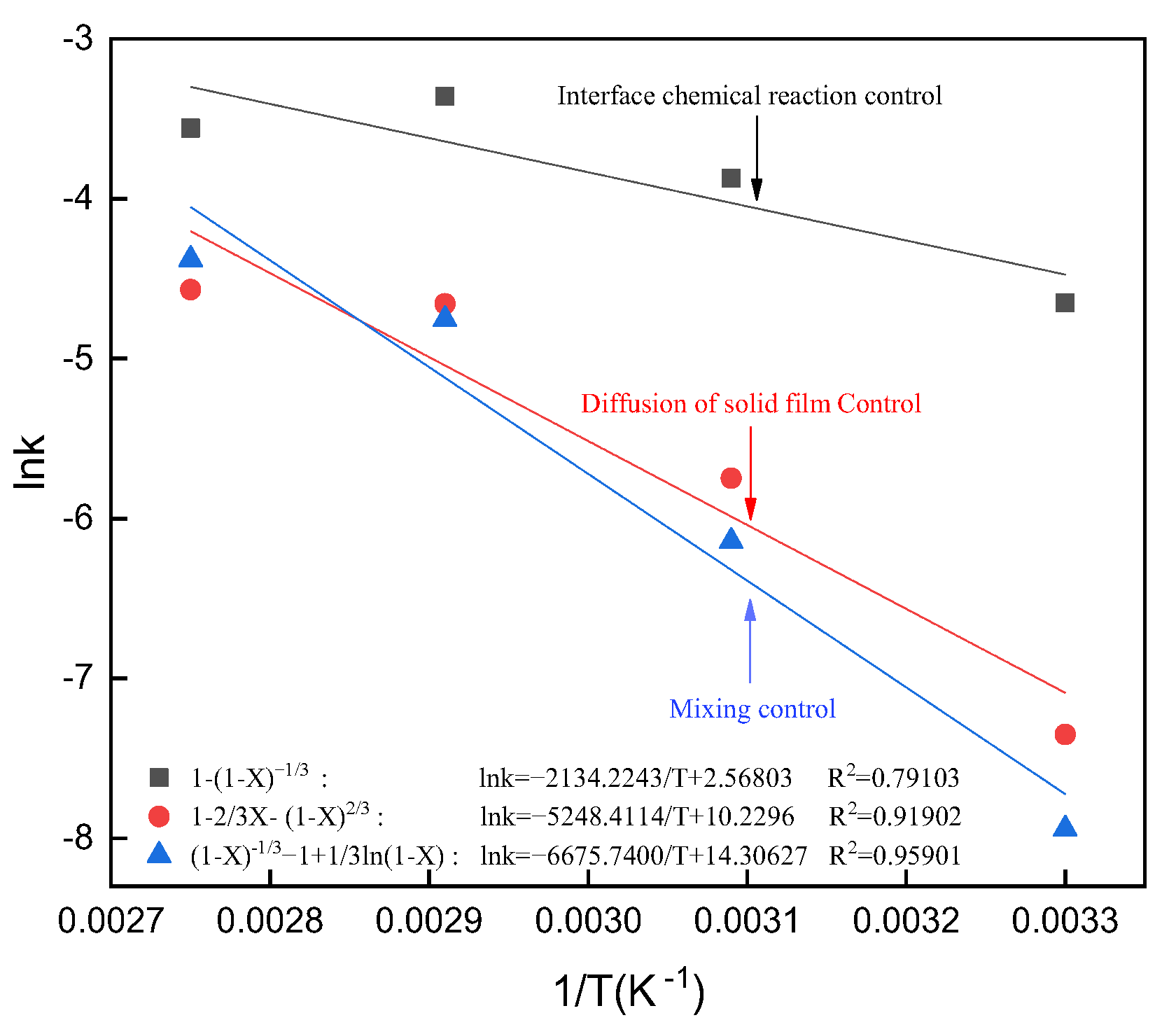
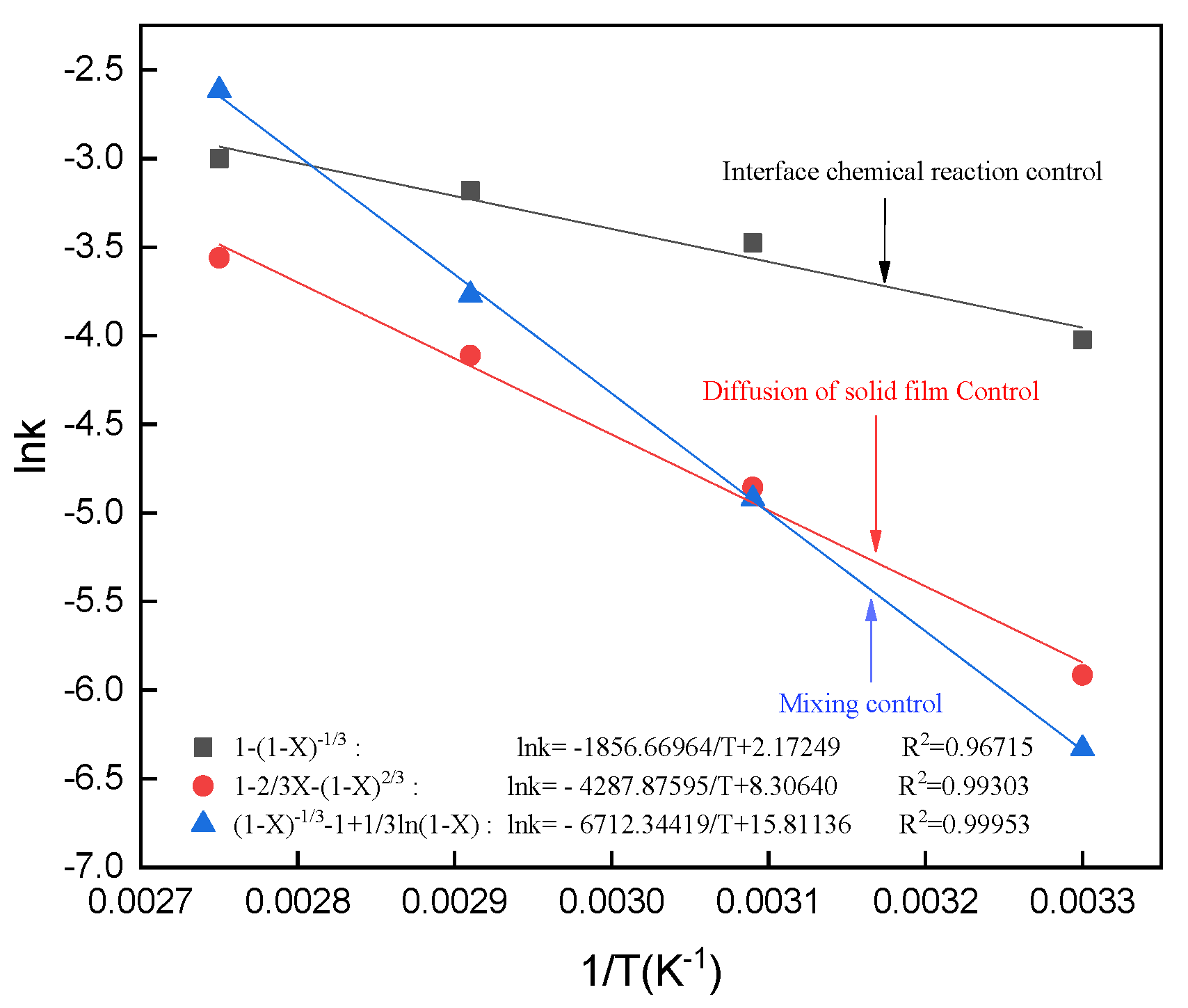
3.10. Analysis of Leaching Kinetics
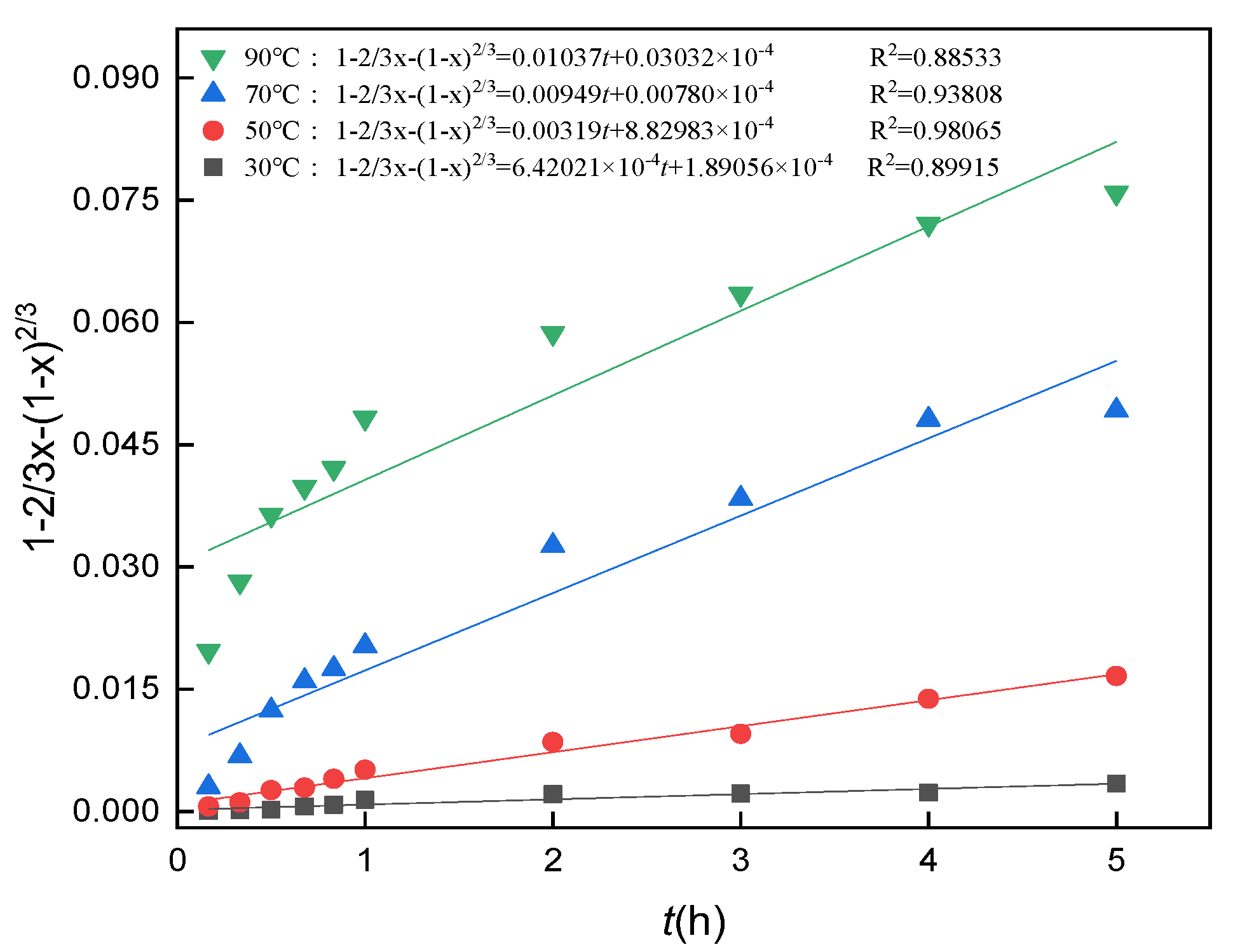
3.11. Calculation of the Apparent Activation Energy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, H.Q.; Zhu, H.G.; Wu, C.; Chen, H.Y.; Sun, J.W.; Liu, J.Y. Study on compressive strength and durability of alkali-activated coal gangue-slag concrete and its mechanism. Powder Technol. 2020, 368, 112–124. [Google Scholar] [CrossRef]
- Huang, G.D.; Ji, Y.S.; Li, J.; Hou, Z.H.; Dong, Z.C. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Construct. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Yang, F.L.; Yang, Y.; Li, G.K.; Sang, N. Heavy metals in soil from gangue stacking areas increases children health risk and causes developmental neurotoxicity in zebrafish larvae. Sci. Total Environ. 2021, 794, 148629. [Google Scholar] [CrossRef] [PubMed]
- Gomo, M.; Vermeulen, D. Hydrogeochemical characteristics of a flooded underground coal mine groundwater system. J. Afr. Earth. Sci. 2014, 92, 68–75. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.X.; Li, A.L.; Zhou, N. Reutilisation of coal gangue and fly ash as underground back fill materials for surface subsidence control. J. Clean. Prod. 2020, 254, 120113. [Google Scholar] [CrossRef]
- Xu, H.L.; Song, W.J.; Cao, W.B.; Shao, G.; Lu, H.X.; Yang, D.Y.; Chen, D.; Zhang, R. Utilization of coal gangue for the production of brick. J. Mater. Cycles Waste Manag. 2017, 19, 1270–1278. [Google Scholar] [CrossRef]
- Xu, B.H.; Liu, Q.F.; Ai, B.; Ding, S.L.; Frost, R.L. Thermal decomposition of selected coal gangue. J. Therm. Anal. Calorim. 2018, 131, 1–10. [Google Scholar] [CrossRef]
- Guan, J.; Lu, M.; Yao, X.H.; Wang, Q.; Wang, D.C.; Yang, B.; Liu, H. An experimental study of the road performance of cement stabilized coal gangue. Crystals 2021, 11, 993. [Google Scholar] [CrossRef]
- Zhou, M.; Dou, Y.W.; Zhang, Y.Z.; Zhang, Y.Q.; Zhang, B.Q. Effects of the variety and content of coal gangue coarse aggregate on the mechanical properties of concrete. Construct. Build. Mater. 2019, 220, 386–395. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Liu, Y.Q.; Ma, H.W.; Feng, W.W.; Zeng, C. Synthesis of ultrafine silica powder using the silica source alkali-leached from coal gangue. Integr. Ferroelectr. 2015, 161, 10–17. [Google Scholar] [CrossRef]
- Wu, R.D.; Dai, S.B.; Jian, S.W.; Huang, J.; Tan, H.B.; Li, B.D. Utilization of solid waste high-volume calcium coal gangue in autoclaved aerated concrete: Physico-mechanical properties, hydration products and economic costs. J. Clean. Prod. 2021, 278, 123416. [Google Scholar] [CrossRef]
- Bu, N.J.; Liu, X.M.; Song, S.L.; Liu, J.H.; Yang, Q.; Li, R.; Zheng, F.; Yan, L.; Zhen, Q.; Zhang, J. Synthesis of NaY zeolite from coal gangue and its characterization for lead removal from aqueous solution. Adv. Powder Technol. 2020, 31, 2699–2710. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, J.D.; Li, M.; Dai, Z.R.; Jiang, R.L.; Zhang, J.X. Chemical modification of combusted coal gangue for U(VI) adsorption: Towards a waste control by waste strategy. Sustainability 2021, 13, 8421. [Google Scholar] [CrossRef]
- Han, L.; Ren, W.; Wang, B.; He, X.; Ma, L.; Huo, Q.; Wang, J.; Bao, W.; Chang, L. Extraction of SiO2 and Al2O3 from coal gangue activated by supercritical water. Fuel 2019, 253, 1184–1192. [Google Scholar] [CrossRef]
- Tang, Z.H. Preparation of nanometer Al(OH)3 powder using coal gangue by polyacrylamide dispersant-carbonization method. Adv. Mater. Res. 2011, 160–162, 307–313. [Google Scholar] [CrossRef]
- Dong, L.; Liang, X.; Song, Q.; Gao, G.; Song, L.; Shu, Y.; Shu, X. Study on Al2O3 extraction from activated coal gangue under different calcination atmospheres. J. Therm. Sci. 2017, 26, 570–576. [Google Scholar] [CrossRef]
- Guo, Y.X.; Zhao, Q.; Yan, K.Z.; Cheng, F.Q.; Lou, H.H. Novel process for alumina extraction via the coupling treatment of coal gangue and bauxite red mud. Ind. Eng. Chem. Res. 2014, 53, 4518–4521. [Google Scholar] [CrossRef]
- Guo, Y.X.; Lv, H.B.; Yang, X.; Cheng, F.Q. AlCl3·6H2O recovery from the acid leaching liquor of coal gangue by using concentrated hydrochloric inpouring. Sep. Purif. Technol. 2015, 151, 177–183. [Google Scholar] [CrossRef]
- Cheng, Y.; Hongqiang, M.; Hongyu, C.; Jiaxin, W.; Jing, S.; Zonghui, L.; Mingkai, Y. Preparation and characterization of coal gangue geopolymers. Construct. Build. Mater. 2018, 187, 318–326. [Google Scholar] [CrossRef]
- Wang, R.G.; Yun, L.X. Preparation and wastewater treatment of polymeric aluminum chloride from coal gangue. Adv. Mater. Res. 2012, 518–523, 780–783. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, N.; Zhao, W.; Lan, D.; Hao, G.; Yi, X.; Yang, Z.; Hu, J.; He, W.; Liu, Y.; et al. Green preparation of hierarchical porous C/SiOx composites from coal gangue as anodes for Li-ion batteries. Solid State Ionics 2021, 371, 115772. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.J.; Hu, Y.X.; Yan, S.; Yang, J.L. Adsorption removal of cationic dyes from aqueous solutions using ceramic adsorbents prepared from industrial waste coal gangue. J. Environ. Manag. 2019, 234, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Li, J.G.; Liu, X.Y.; Du, Z.P.; Cheng, F.Q. Effect of silicon content on preparation and coagulation performance of poly-silicic-metal coagulants derived from coal gangue for coking wastewater treatment. Sep. Purif. Technol. 2018, 202, 149–156. [Google Scholar] [CrossRef]
- Luo, K.J.; Ren, G.M. Coagulant prepared by gangue and its application in treatment of coal washing wastewater. Adv. Mater. Res. 2010, 142, 270–273. [Google Scholar] [CrossRef]
- Xiao, J.; Li, F.; Zhong, Q.; Bao, H.; Wang, B.; Huang, J.; Zhang, Y. Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC. Hydrometallurgy 2015, 155, 118–124. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, F.; Deng, X.; Guo, H.; Zhang, C.; Shi, C.; Zeng, M. Extraction of alumina from alumina rich coal gangue by a hydro-chemical process. Roy. Soc. Open Sci. 2020, 7, 192132. [Google Scholar] [CrossRef]
- Johnston, C.J.; Pepper, R.A.; Martens, W.N.; Couperthwaite, S. Improvement of aluminium extraction from low-grade kaolinite by iron oxide impurities: Role of clay chemistry and morphology. Miner. Eng. 2022, 176, 107346. [Google Scholar] [CrossRef]
- Remya, P.N.; Kazantzis, N.K.; Emmert, M.H. Selective process steps for the recovery of scandium from jamaican bauxite residue (red mud). ACS Sustain. Chem. Eng. 2018, 6, 1478–1488. [Google Scholar]
- Ma, H.Q.; Yi, C.; Zhu, H.G.; Dong, Z.C.; Chen, H.Y.; Wang, J.X.; Li, D.Y. Compressive strength and durability of coal gangue aggregate concrete. Mater. Rep. 2018, 32, 2390–2395. [Google Scholar]
- Zhang, M.S.; Xiao, L.W. Preparation of a gangue-based X-type zeolite molecular sieve as a multiphase fenton catalyst and its catalytic performance. ACS Omega 2021, 6, 18414–18425. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, Z.Z.; Zhu, M.M.; Cheng, F.Q.; Zhang, D.K. Decomposition of key minerals in coal gangues during combustion in O2/N2 and O2/CO2 atmospheres. Appl. Therm. Eng. 2019, 148, 977–983. [Google Scholar] [CrossRef]
- Gasparini, E.; Tarantino, S.; Ghigna, P.; Riccardi, M.P.; Cedillo-González, E.I.; Siligardi, C.; Zema, M. Thermal dehydroxylation of kaolinite under isothermal conditions. Appl. Clay Sci. 2013, 80–81, 417–425. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ge, X.L.; Nakano, J.; Liu, L.L.; Wang, X.D.; Zhang, Z.T. Pyrite transformation and sulfur dioxide release during calcination of coal gangue. RSC Adv. 2014, 4, 42506–42513. [Google Scholar] [CrossRef]
- Xie, M.Z.; Liu, F.Q.; Zhao, H.L.; Ke, C.Y.; Xu, Z.Q. Mineral phase transformation in coal gangue by high temperature calcination and high-efficiency separation of alumina and silica minerals. J. Mater. Res. Technol. 2021, 14, 2281–2288. [Google Scholar] [CrossRef]
- Ptáček, P.; Šoukal, F.; Opravil, T.; Nosková, M.; Havlica, J.; Brandštetr, J. The kinetics of Al-Si spinel phase crystallization from calcined kaolin. J. Solid State Chem. 2010, 183, 2565–2569. [Google Scholar] [CrossRef]
- Kenne Diffo, B.B.; Elimbi, A.; Cyr, M.; Dika Manga, J.; Tchakoute Kouamo, H. Effect of the rate of calcination of kaolin on the properties of metakaolin-based geopolymers. J. Asian Ceram. Soc. 2015, 42, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Cao, Y.D.; Dong, H.J.; Zhang, J.S.; Sun, C.B. Effect of calcination condition on the microstructure and pozzolanic activity of calcined coal gangue. Int. J. Miner. Process. 2016, 146, 23–28. [Google Scholar] [CrossRef]
- Valeev, D.; Kunilova, I.; Shoppert, A.; Salazar-Concha, C.; Kondratiev, A. High-pressure HCl.leaching of coal ash to extract Al into a chloride solution with further use as a coagulant for water treatment. J. Clean. Prod. 2020, 276, 123206. [Google Scholar] [CrossRef]
- Valeev, D.; Mikhailova, A.; Atmadzhidi, A. Kinetics of iron extraction from coal fly ash by hydrochloric acid leaching. Metals 2018, 8, 533. [Google Scholar] [CrossRef] [Green Version]
- Valeev, D.V.; Lainer, Y.A.; Mikhailova, A.B.; Dorofievich, I.V.; Zheleznyi, M.V.; Gol’dberg, M.A. Reaction of bauxite with hydrochloric acid under autoclave conditions. Metallurgist 2016, 60, 204–211. [Google Scholar] [CrossRef]
- Valeev, D.V.; Lainer, Y.A.; Pak, V.I. Autoclave leaching of boehmite-kaolinite bauxites by hydrochloric acid. Inorg. Mater. Appl. Res. 2016, 7, 272–277. [Google Scholar] [CrossRef]
- Zinoveev, D.; Grudinsky, P.; Zhiltsova, E.; Grigoreva, D.; Volkov, A.; Dyubanov, V.; Petelin, A. Research on high-pressure hydrochloric acid leaching of scandium, aluminum and other valuable components from the non-magnetic tailings obtained from red mud after iron removal. Metals 2021, 11, 469. [Google Scholar] [CrossRef]
- Cheng, F.; Cui, L.; Miller, J.D.; Wang, X. Aluminum leaching from calcined coal waste using hydrochloric acid solution. Miner. Procoss. Extr. Metall. Rev. 2012, 33, 391–403. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Li, Y. Research on the extract Al2O3 from coal gangue. Adv. Mater. Res. 2012, 524–527, 1947–1950. [Google Scholar] [CrossRef]
- Kong, D.S.; Jiang, R.L. Preparation of NaA zeolite from high iron and quartz contents coal gangue by acid leaching-alkali melting activation and hydrothermal synthesis. Crystals 2021, 11, 1198. [Google Scholar] [CrossRef]
- Gui, Q.H.; Khan, M.I.; Wang, S.X.; Zhang, L.B. The ultrasound leaching kinetics of gold in the thiosulfate leaching process catalysed by cobalt ammonia. Hydrometallurgy 2020, 196, 105426. [Google Scholar] [CrossRef]
- Dickinson, C.F.; Heal, G.R. Solid-liquid diffusion controlled rate equations. Thermochim. Acta 1999, 340, 89–103. [Google Scholar] [CrossRef]
- Rao, S.; Yang, T.; Zhang, D.; Liu, W.; Chen, L.; Hao, Z.; Xiao, Q.; Wen, J. Leaching of low grade zinc oxide ores in NH4Cl-NH3 solutions with nitrilotriacetic acid as complexing agents. Hydrometallurgy 2015, 158, 101–106. [Google Scholar] [CrossRef]
- Valeev, D.; Pankratov, D.; Shoppert, A.; Sokolov, A.; Kasikov, A.; Mikhailova, A.; Salazar-Concha, C.; Rodionov, I. Mechanism and kinetics of iron extraction from high silica boehmite–kaolinite bauxite by hydrochloric acid leaching. Trans. Nonferrous Met. Soc. China 2021, 31, 3128–3149. [Google Scholar] [CrossRef]



| Components | wt/% |
|---|---|
| SiO2 | 42.18 |
| Al2O3 | 20.43 |
| Fe2O3 | 11.94 |
| CaO | 2.95 |
| MgO | 1.61 |
| MnO | 0.25 |
| P2O5 | 0.30 |
| TiO2 | 3.77 |
| S | 0.54 |
| K2O | 1.24 |
| Na2O | 0.40 |
| FC and Loss | 14.39 |
| Components | wt/% |
|---|---|
| SiO2 | 76.95 |
| Al2O3 | 10.37 |
| Fe2O3 | 1.00 |
| CaO | 0.05 |
| MgO | 0.22 |
| TiO2 | 6.22 |
| MnO | 0.04 |
| P2O5 | 0.06 |
| S | 0.01 |
| K2O | 1.01 |
| Na2O | 0.38 |
| FC and others | 3.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Zhou, Z.; Jiang, R.; Song, S.; Feng, S.; Lian, M. Extraction of Aluminum and Iron Ions from Coal Gangue by Acid Leaching and Kinetic Analyses. Minerals 2022, 12, 215. https://doi.org/10.3390/min12020215
Kong D, Zhou Z, Jiang R, Song S, Feng S, Lian M. Extraction of Aluminum and Iron Ions from Coal Gangue by Acid Leaching and Kinetic Analyses. Minerals. 2022; 12(2):215. https://doi.org/10.3390/min12020215
Chicago/Turabian StyleKong, Deshun, Zihan Zhou, Rongli Jiang, Shuojiang Song, Shan Feng, and Minglei Lian. 2022. "Extraction of Aluminum and Iron Ions from Coal Gangue by Acid Leaching and Kinetic Analyses" Minerals 12, no. 2: 215. https://doi.org/10.3390/min12020215
APA StyleKong, D., Zhou, Z., Jiang, R., Song, S., Feng, S., & Lian, M. (2022). Extraction of Aluminum and Iron Ions from Coal Gangue by Acid Leaching and Kinetic Analyses. Minerals, 12(2), 215. https://doi.org/10.3390/min12020215






