3.1. The Heavy Metals Concentration in Soils and Plants
Initial analyses showed that in the case of Us, the concentrations of Cu and Zn were below the alert threshold, against the background of a higher Zn concentration (
Table 1). In Ps samples, taken from the Sasca Montana mining area, the concentrations of Cu, Zn and Cr are above the alert threshold, while the Pb concentration even exceeds the intervention threshold. According to the values of the Cf
i, much higher contamination with Cr, Cu, Zn and Pb was observed in Ps, and considerable contamination with Cu was observed in Us.
The analysis of the variance components (
Table 2) shows that the three main sources of variation showed a significant influence on the variability of the Cu and Zn concentrations in the
S. perfoliatum plants. The highest contribution to the variability of the Cu concentration was presented by the type of soil, followed by the organ and plant development stage. Significant differences were also observed for the different interactions between the three factors, with a higher contribution in the case of the interaction between the soil type and the plant organs. Regarding the Zn concentration, it was determined that the biggest differences were recorded between analyzed organs, compared to the soil type and the development stage. Additionally, the interactions between the factors had significant contributions to the total variability, with a higher value for the interactions between the soil type and the plant organs.
Analyzing the concentration of Cu in different stages of plant development, it increased significantly by 16.52% from three to five leaves (13 to 15BBCH) and by 49.59% from five to eight leaves (15 to BBCH), respectively (
Table 2). Plants grown in Ps accumulated a significantly higher amount of Cu, by 189%, compared with plants in the Us, which showed small and insignificant variations of 3.16–4.28 mg/kg in the Cu concentration from one development stage to another. As such, only in the eight leaf stage was the amount of Cu in the plant significantly higher, by 7.44 mg kg
−1, compared to the three leaf stage. In the case of plants grown in Ps, a small and insignificant variation in the concentration of Cu was observed between the first two stages, but at the last stage, the concentration of Cu was significantly higher by 12.06–15.51 mg kg
−1. Regardless of the development stages, plants grown in Ps accumulated significantly higher Cu amounts, with differences from 20.88 mg kg
−1 in the three leaf stage to 37.02 mg kg
−1 in the eight leaf stage.
Generally, the Zn amount accumulated by plants grown in Ps was significantly higher by 27.95%. The plants’ Zn concentration increased significantly by 26.75% from three to five leaves, and then, until eight leaves, the variation was reduced and insignificant. In all three development stages, plants grown in Us accumulated a significantly higher Zn amount than those grown in Ps, with differences ranging from 17.29% for the five leaf stage to 41.76% for the three leaf stage. In the conditions of the Us, there was increase in the plants’ Zn concentration increase one stage to another, meaning that only in the last stage was the amount of Zn significantly higher, by 31.67%, compared to that from the first determination. The plants grown in Ps recorded a significantly higher accumulation of Zn by 41.02%–44.92% in the 15th and 18th BBCH stages compared to 13 BBCH.
At the whole experience level, the
S. perfoliatum plants accumulated various Cu amounts in different organs, so that in the lamina, the concentration was significantly higher than in the roots and petioles (
Table 3). Plants grown in Us were recorded as having a higher concentration by 9.3–12.73 mg kg
−1 in the lamina compared to the roots and petioles, which were statistically undifferentiated. The same trend was observed in Ps conditions where the Cu concentration varied from 26.12 mg kg
−1 in the roots to 65.29 mg kg
−1 in the lamina. Cu accumulation in various plant organs was significantly higher when grown in Ps, ranging from 14.1 mg kg
−1 in roots to 43.97 mg kg
−1 for leaf lamina.
The plants showed a significantly higher concentration of Zn in the lamina by 90%-109% compared to the petioles and roots. In the Us, there was a significant variation in the Zn amount determined in different analyzed plant parts, with values between 37.91 mg kg−1 in the roots and 119.25 mg kg−1 in the lamina. In pollution conditions, it was observed that the Zn amount accumulated in the roots was significantly higher by 27.06 mg kg−1 compared to the petioles, against the background of a significantly higher concentration of 86.76 mg kg−1 in the lamina. Plants grown in Ps accumulated in the roots a significantly higher amount of Zn by 60.24% compared to plants grown in Us, while the concentration in petioles and lamina was significantly lower by 27%–55%.
Concerning the Cu concentration in various plant organs during the three developmental stages, it was found that the plants accumulated in the lamina a significantly higher amount, with variations from 19.56 mg kg
−1 for 15 BBCH to 33.31 mg kg
−1 for 18 BBCH (
Table 4). Additionally, the concentrations in the roots and petioles were similar and undifferentiated statistically. The degree of plant development had a similar effect on the Cu accumulation in different organs, observing a significant variation only between the values related to the 15 and 18 BBCH stages.
Analyzing the Zn amount in the plant organs, it was found that in all stages, lamina manifested a significantly superior accumulation capacity compared to the roots and petioles, which recorded similar concentrations of this metal. The plant’s stage of development manifested a small and insignificant influence on the Zn accumulation in the roots and petioles. The lamina showed a significant increase in Zn concentration from one stage to another, with variations from 76.64 mg kg−1 in 13 BBCH to 125.95 mg kg−1 in 18 BBCH.
By analyzing the cumulative effects of the three sources of variation (
Table 5), it was observed that the development stage of the plants grown in Us did not significantly influence the Cu concentration in the roots and petioles, while in the lamina, the Cu accumulation in the last analyzed stage was significantly higher than in the previous stages. In the first phase, a reduced and insignificant variation of the Cu concentration of plant organs was observed, with values between 9.52 mg kg
−1 for roots and 14.55 mg kg
−1 for lamina. In the final phases (15 and 18 BBCH), a significantly higher amount of Cu was determined in the lamina.
For plants grown in Ps, it was found that their development was associated with a significant increase in Cu amount, so that in 18BBCH, there were significantly higher values in all plant organs. Regardless of the phenophase, the concentration of this element in the lamina was significantly higher than in the roots and petioles, which showed similar values.
The roots and petioles’ Zn concentration dynamics in plants grown in Us show an insignificant variation during the three phenophases, associated with amplitudes of 7.82–11.39 mg kg−1. The lamina Zn accumulation was influenced by the plant’s development stage, registering a progressive increase from one phase to another. In the first phase, the roots showed a significantly lower Zn content than the other organs, while in the 15 and 18 BBCH phenophases, the lamina accumulated a significantly higher amount than the other organs. Additionally, the petioles had a significantly higher concentration than the roots.
The Zn accumulation in the roots and petioles of plants grown in Ps was not influenced by the development stage but had amplitudes from 9.23 mg kg−1 for the roots to 18.63 mg kg−1 for the petioles. In the lamina, during plant development from 13 to 15 BBCH, a significant increase in Zn concentration to 33.68 mg kg−1 was observed, associated with a low variation between the last two phenophases. In the 13 BBCH stage, the petioles had a significantly higher Zn content than the other two organs. In the 15 and 18 BBCH stages was found a more obvious differentiation between plant organs, manifested by higher Zn concentrations (96.94–100.08 mg kg−1) in the lamina and significantly lower values (38.96–40.37 mg kg−1) in the petioles.
With regard to the whole experiment, the dynamics of Zn accumulation in the S. perfoliatum organs were on average 153% higher than the concentration of Cu.
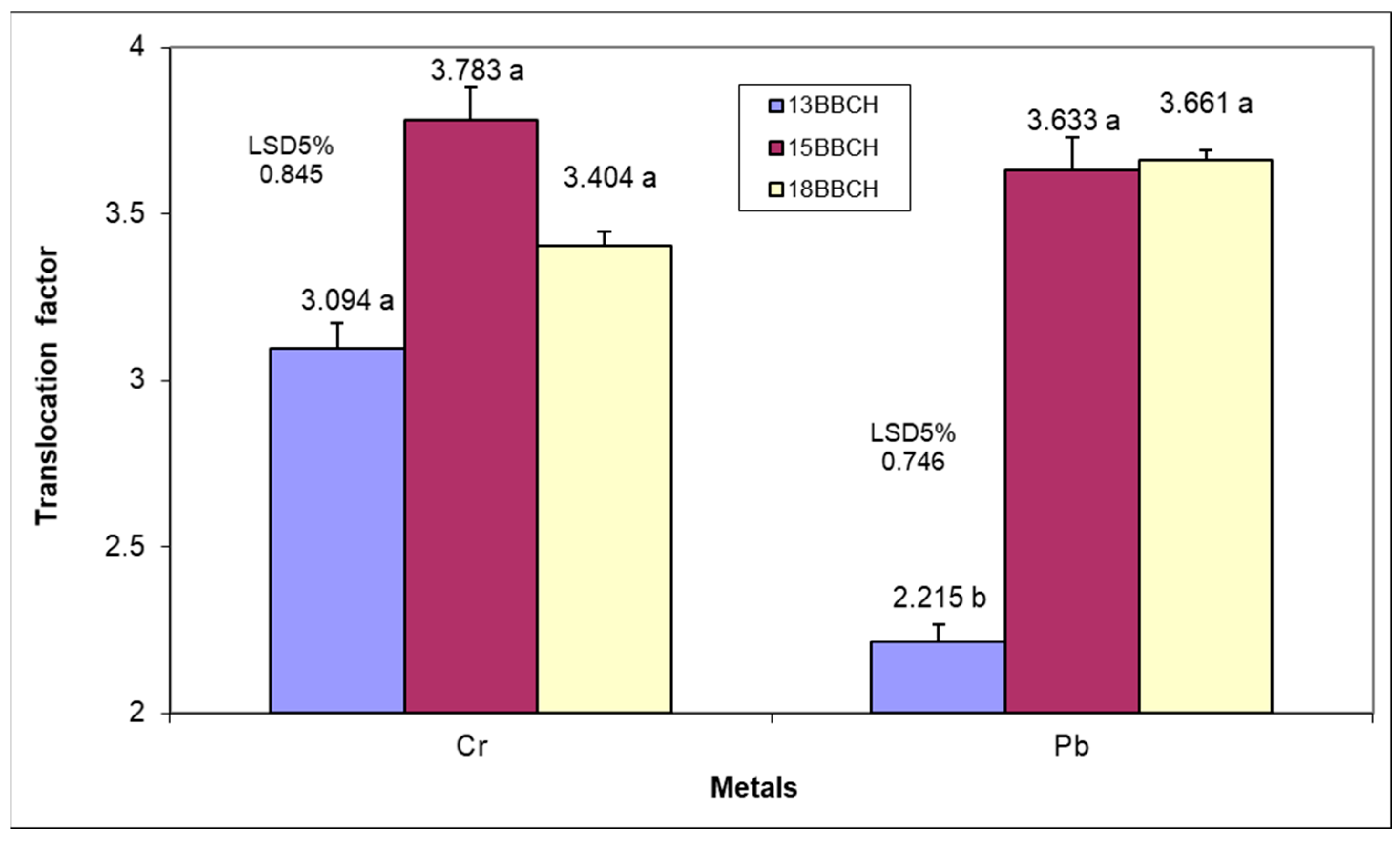
Analysis of the data in
Table S2 (Supplementary Material) indicates that there are significant variations in the Pb and Cr content in plants both between stages and between organs. Phenophase showed a lower contribution to the variability of Pb and Cr content compared to plant organs. A significant contribution was also for the interaction between phenophases and analyzed organs with regard to the quantity of these elements.
The Cr content of plant roots grown in Ps demonstrated positive but insignificant variations, between 18.03 and 24.19 mg kg
−1 for the first two phenophases (
Table 6). Thus, only in the last stage was the Cr concentration significantly higher, by 13.08 mg kg
−1, than in the first one. The petioles’ Cr content demonstrated an insignificant variation during the plant development, associated with an amplitude between 21.55 and 30.21 mg kg
−1. The developmental stage of the plants showed a significant influence on the Cr accumulation in the lamina, based on the background of significant differences from one stage to another, between 13.31 mg kg
−1 in the last two and 28.45 mg kg
−1 in the first two, respectively. Throughout the whole experiment, the lamina’s Cr accumulation was significantly higher than that of the roots or petioles, respectively.
Therefore, the plant development stage determined a gradual increase in the accumulated Cr amount in different organs, associated with a variation of 57.93% between the first two phenophases and 18.39% between the last two. Analyzing all stages, it was found that the Cr content of the lamina was significantly higher than that of the other two organs.
The Pb content analysis shows that the roots’ accumulation of this element was not significantly influenced by the developmental stage, based on the background of small variations of 4.16–4.98 mg kg−1 between phenophases. In the first stage, the amount of Pb in petioles was significantly lower by 12.7–21.72 mg kg−1 than in the other two stages, which were statistically undifferentiated. In the leaf lamina, there is a higher Pb accumulation in the 15 and 18BBCH stages, associated with deviations of 38.53–46.25 mg kg−1 compared to 13 BBCH. Lead analyses in the last two stages show that there is a significantly higher concentration at the level of the lamina, associated with similar values in other plant organs. In the first stage, based on the background of smaller variations between organs, it was observed that the lamina showed a significantly higher Pb concentration by 17.77 mg kg−1 compared to the petioles.
The Pb content showed a progressive and significant increase from one stage to another, being more intense (70%) between the first two, compared to 18.64% in the last two. The roots and the petioles showed similar accumulations of Pb, while in the lamina, the capacity for storing this element was considerably higher.
Against the background of significant effects of different sources of variation, the results (
Table S3) indicate that heavy metal soil pollution had a greater influence on the accumulation of Zn in
S. perfoliatum, while the concentration of Cu showed higher variations between the organs.
3.5. Heavy Metals Content in Different Plant Organs and Developmental Stages
In the case of plants grown in Us, in the first stage, the lamina showed a significantly higher Cu content compared to the other two organs (
Figure 4). Additionally, the roots had a significantly higher concentration than the petioles. In the 15 BBCH stage, a significantly higher content of Cu in the lamina was observed, associated with a significantly lower value in the petioles. In the last stage, against the background of a high content at the level of the lamina, a more intense Cu accumulation occurs in the petioles compared to the roots. Regarding the Cu content of the lamina, a significant increase in this element from 4.28 to 7.58 mg kg
−1 was observed from 15 to 18 BBCH. For the other organs, the variations between different stages were smaller.
In plants grown in the soil with heavy metals, at the first determination, a significantly higher accumulation of Cu was found in the lamina, and a lower accumulation was observed in the roots (
Figure 4). In the 15 BBCH stage, the accumulation of Cu at the root level was intensified, so that it reached values similar to those of the petioles, against the background of a significantly higher amount in the lamina. The intensification of copper accumulation in the root continued in the last stage, reaching a higher value than in the petioles, but both are significantly lower than the amount in the lamina. Plants grown in Ps accumulated significantly higher Cu amounts compared to those in Us.
In the plants that were grown in Us, at the first determination, the lamina Zn amount was significantly higher by over 7.03 mg kg
−1 compared to the values of the root and petioles (
Figure 5). In the 15 BBCH stage, significant variations, between 7.45 mg kg
−1 in the roots and 27.24 mg kg
−1 in the lamina, were observed. In the last stage, it was determined that the lamina Zn content was significantly higher by over 21.14 mg kg
−1 than in the petioles and by 27.24 mg kg
−1 than in the roots.
Plants grown in Ps accumulated, in the first stage, a significantly higher amount of Zn in the lamina compared to the roots and petioles. In the 15 BBCH stage, the same trend and variation level between the analyzed organs was maintained, but in the last stage, the Zn accumulation in the roots and lamina was intensified, with a variation between 8.84 mg kg−1 in the petioles and 26.32 mg kg−1 in the lamina. Thus, there was an increase in the Zn accumulation in the roots in parallel with its decrease in the petioles, compared to the plants grown in Us.
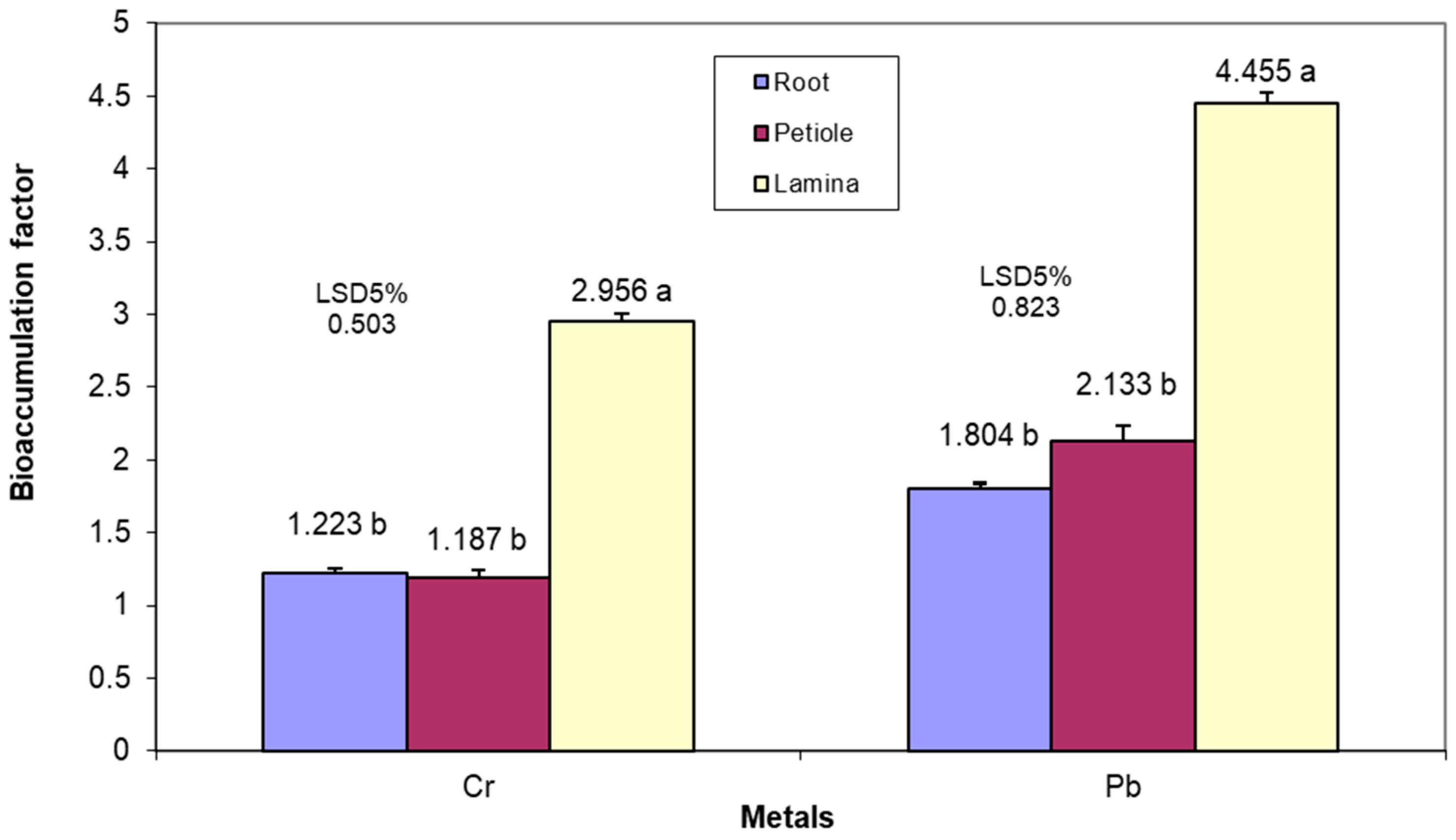
The analysis of Cr concentration (
Figure 6) of the three organs shows a significant variation from one stage to another, with an amplitude between 10.32 mg kg
−1 for the petioles and 13.78 mg kg
−1 for the lamina. In the first and second stage, the lamina had a significantly higher Cr concentration compared to the other two organs, but for the last phenophase, the high Cr content of the lamina was also associated with a high concentration in the roots, significantly higher than in the petioles.
The Pb concentration in the lamina was significantly higher in the first phenophase, by 3.22–5.13 mg kg
−1, than in the other two organs, which showed significantly different values and higher concentration in the roots, respectively. In the second stage, there was an obvious differentiation of the Pb concentration in the lamina compared to the roots and petioles, as in the last stage (
Figure 7).
3.6. The Effects of Heavy Metals on Free Proline, Total Chlorophyll and Plant Biomass
Thus, it was noted that the three main sources of variation demonstrated a very significant influence on the variability of the free proline amount in the
S. perfoliatum plants (
Table S6). The highest contribution to this trait’s variability was manifested by the plant organs, followed by the type of soil and phenophase. Significant differences were also observed for different interactions between the three factors, with a higher contribution in the case of the interaction between soil type and phenophases.
The variation in the free proline amount in different stages resulted in a significant increase of 0.2 mg g
−1 from 13 to 15 BBCH, set against the background of an insignificant difference from 15 to 18 BBCH (
Table 10). Plants grown in Ps conditions synthesized a significantly higher amount of free proline, by 30.6%, compared to those grown in Us. The plant development stage showed an insignificant influence on the free proline content of plants grown in Us, but in the case of plants grown in Ps, there were significant variations in the proline content from one phenophase to another, with values from 1.41 mg g
−1 in 13 BBCH to 1.98 mg g
−1 for 18 BBCH. In the last two phenophases, plants grown in Ps synthesized significantly higher amounts of free proline, by 38.80%–51.14%.
During the whole experience, the
S. perfoliatum plants synthesized different free proline amounts in different organs, so that in the lamina, the concentration was significantly higher than in the roots and petioles (
Table 11). The plants grown in Us recorded displayed the lamina a higher concentration by 34.81%–111.62% compared to the roots and petioles, statistically differentiated in favor of the petioles. The same trend was observed in Ps, where the free proline content ranged from 1.39 mg g
−1 in the roots to 2.14 mg g
−1 in the lamina. The biosynthesis and accumulation of proline in various plant organs were significantly higher under pollution conditions, with variations ranging from 0.37 mg g
−1 in the petioles to 0.53 mg g
−1 in the roots.
The analysis of the cumulative effect of the three sources of variation (
Table 12) showed that the developmental stage of the plants grown in the Us did not significantly influence the amount of free proline in the plant organs.
In the case of plants grown in Ps, it was found that the development from 13 BBCH to 15 BBCH was associated with an increase in the free proline amount, from 0.20 mg g−1 in the roots to 0.66 mg g−1 in the petioles. Regardless of phenophase, the proline amount in the lamina was significantly higher than in the roots and petioles, statistically differentiated for petioles.
The root biomass analysis in plants grown in Us shows a significant increase of 54.04% in the first two phenophases, while from 15 to 18 BBCH the increase is only 14.68% (
Figure 8). The biomass of the petioles showed a variation from 9.02 g in 13 BBCH to 20.43 g in 18 BBCH, which means increases of 47.08%–53.99% from one phenophase to another. The lamina also demonstrated a significant increase of 43.08% between 13 and 15 BBCH and 25.90% from 15 to 18 BBCH, respectively.
The biomass dynamics for roots were significantly higher than in the other two organs, but the lamina had a significantly superior value to the petioles. In the last stage, the roots’ biomass achieved increases of 31.66% compared to the lamina and 106.41% compared to the petioles.
In the case of plants grown in Ps, the roots’ biomass recorded positive variation between 23.06 and 32.67 g from one stage to another (
Figure 8). Thus, in the last phenophase, the roots’ biomass was significantly higher by 41.67% than in the first phenophase. Petiole biomass showed a gradual increase of 32.86%–54.15% from one stage to another, associated with a difference of 8.71 g between 13 and 15 BBCH. The biomass of the lamina recorded values between 17.23 and 22.14 g, against the background of a higher variation between the last phenophases. In 13 BBCH, the root biomass was significantly higher by 5.83–14.75 g compared to the other two organs. For 15BBCH, high root values of 51.31%–12.56% were determined, while in the last phenophase, compared to the biomass of the lamina and petioles, increases of 47.56%–91.95% were recorded.
Plants grown in Ps do not express, at the first determination, major differences between the biomass of different organs compared to those in Us. In the second determination, however, the biomass of plants grown in the Us was higher by 35.52% compared to those grown in Ps, especially due to higher values of the roots and lamina. In the last determination, the plants grown in Us accumulated a superior fresh biomass, with an increase of 31.76%, associated with increases in the roots and the lamina (29.07%–44.67%).
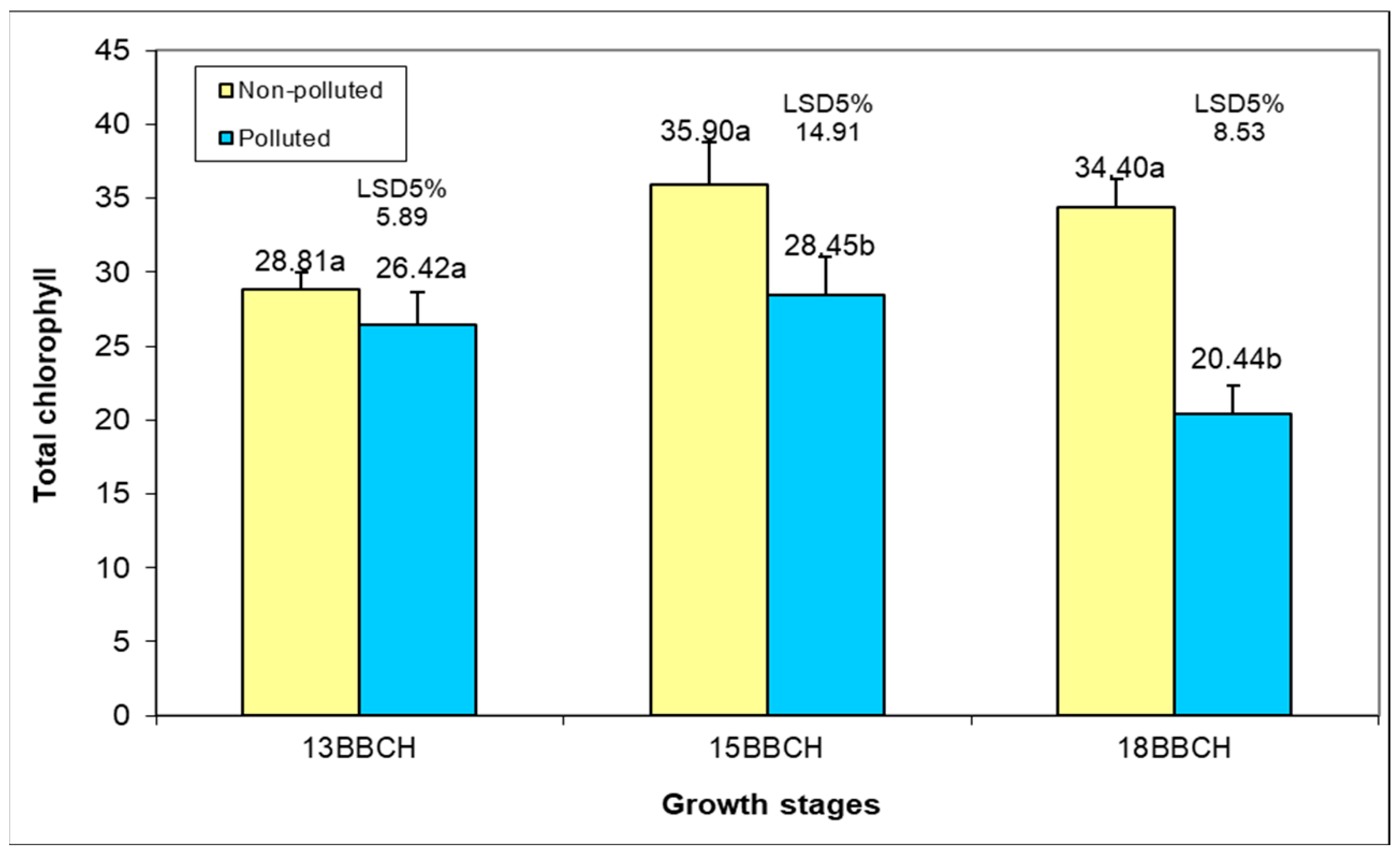
Soil pollution had a small and insignificant influence on the biosynthesis of total chlorophyll in the foliar apparatus in the 13 BBCH stage, with variation from 26.42 to 28.81 μg cm
−2 (
Figure 9). At the five leaf stage, the pollution effect was significant, with a reduction in the total chlorophyll by 20.75% in plants grown in Ps. The negative influence of Ps on the total chlorophyll was intensified in plants at the eight leaf stage, when there was a decrease in the chlorophyll content by 60.59%. In both soil conditions, an increase in the chlorophyll content was observed from 13 to 15 BBCH, which was more intense (24.61%) in the case of plants grown in Us compared to those grown in Ps (7.68%). Plant development from five to eight leaves has been associated with a reduction in chlorophyll content, ranging from 4.18% in Us to 28.16% in Ps conditions. These results are according to other studies that demonstrated that elevated levels of pollution with various heavy metals caused a reduction in the amount of chlorophyll pigments [
84,
85] associated with the inhibition of photosynthesis [
86,
87].
The existence of significant correlations between the plant biomass and the heavy metal content of the soil is evident (
Table 13). The increase in the Cu and Zn concentration in Ps led to an increase in the free proline content of plant organs, while in Us, a negative correlation was observed between the free proline and these two elements. Additionally, the content of Cr and Pb showed a strong correlation with the free proline, indicating that under the Ps conditions, the plants synthesized a superior amount of free proline. The chlorophyll content showed low and insignificant correlations with all metals, against the background of positive relations with the Cu and Zn concentration in Us.
At the end of the research, there was a major significant reduction in the soil concentration of different heavy metals compared to the initial values, so that in all cases the soil metal concentration was within the normal allowable limits. According to the Cf
i, a low contamination level of Cu and Pb was observed, while a moderate level of contamination was observed for the rest of the elements (
Table 14).