Effect of Freeze–Thaw Cycles on Mechanical and Microstructural Properties of Tailings Reinforced with Cement-Based Material
Abstract
:1. Introduction
2. Design of Experiment
2.1. Geology of the Mine and Test Materials
2.2. Test Specimens
2.3. Tests on Specimens
3. Results and Analysis
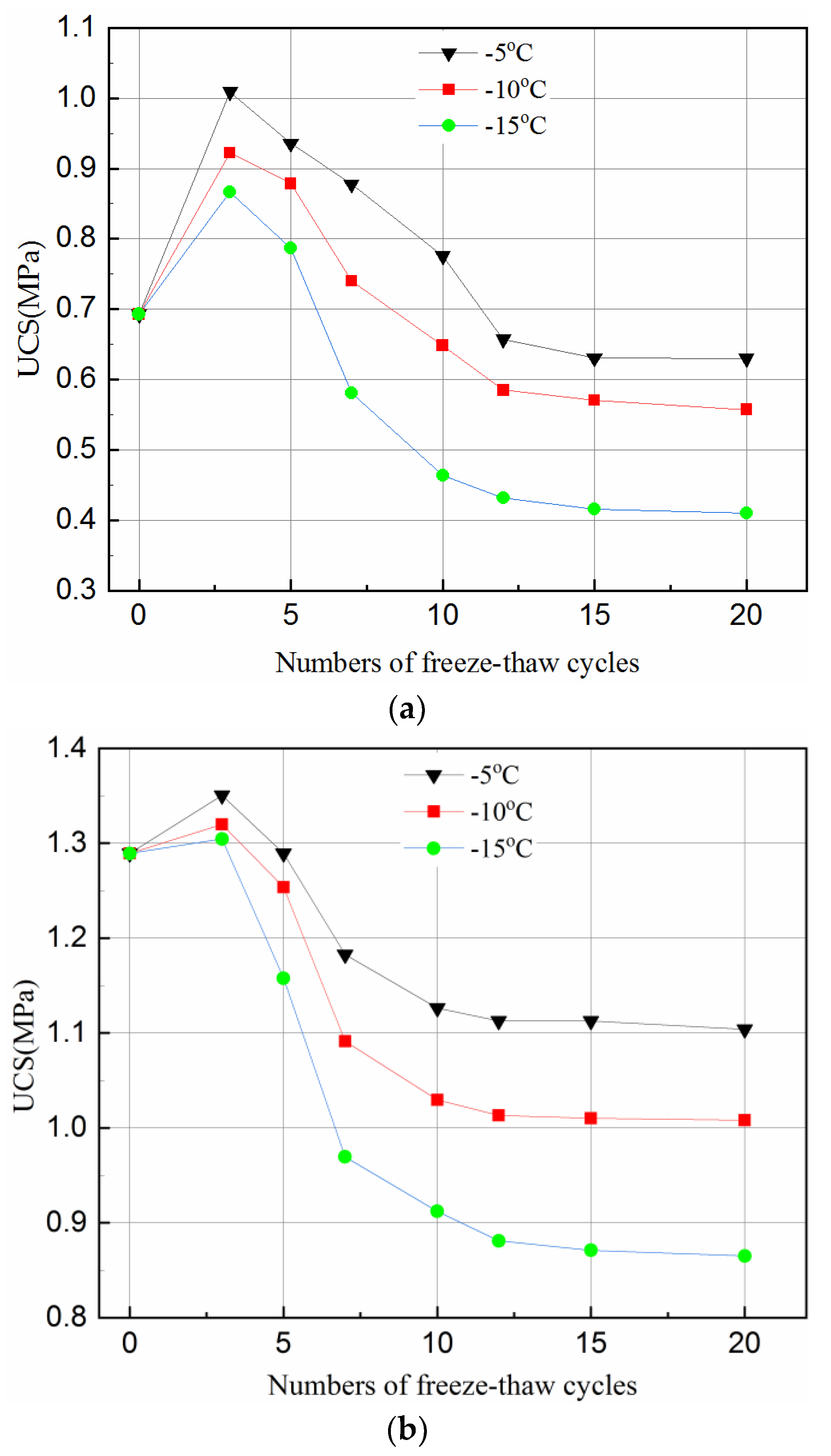
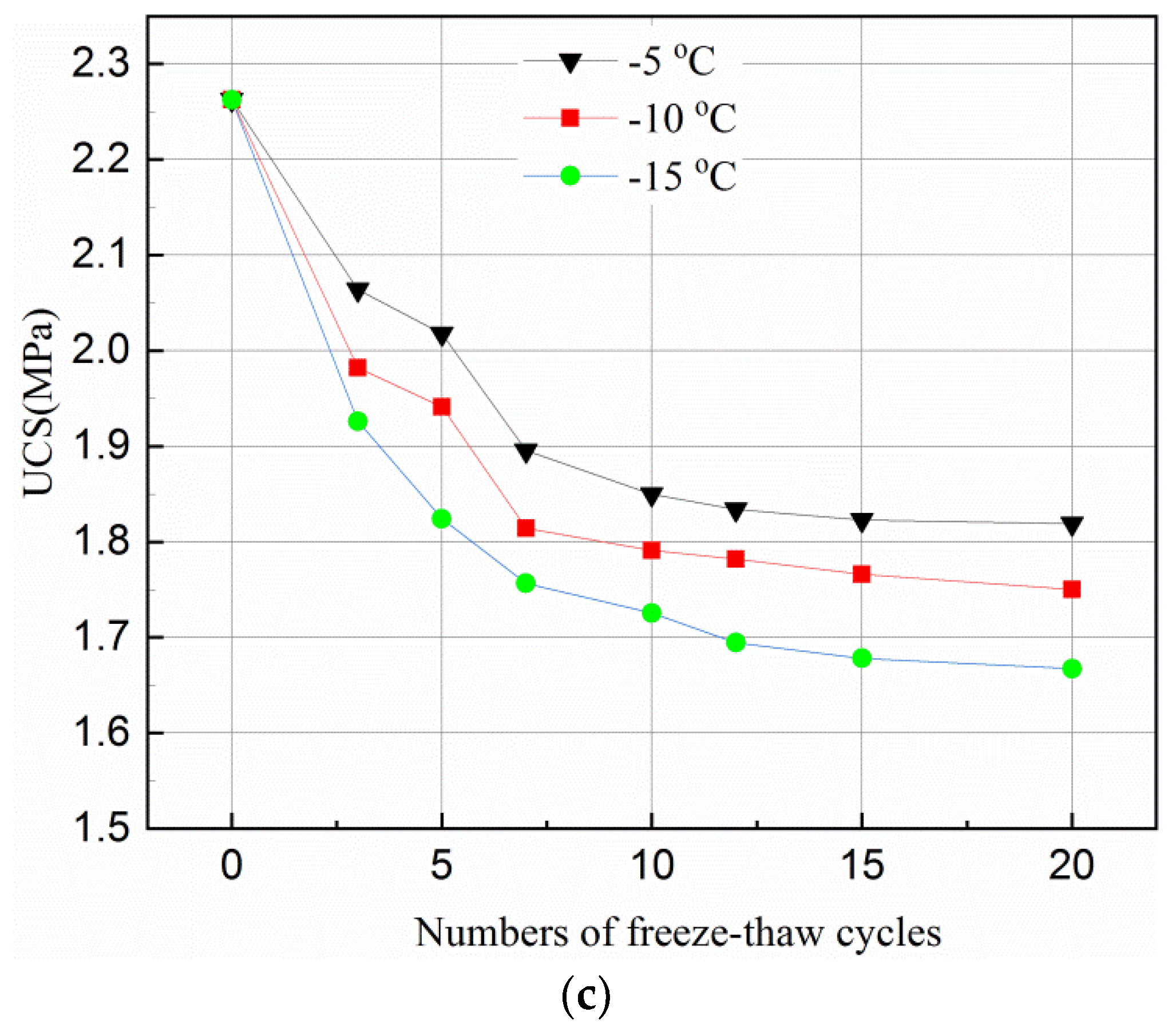
3.1. Effects of Freeze–Thaw Cycles on Strength of Tailings Samples
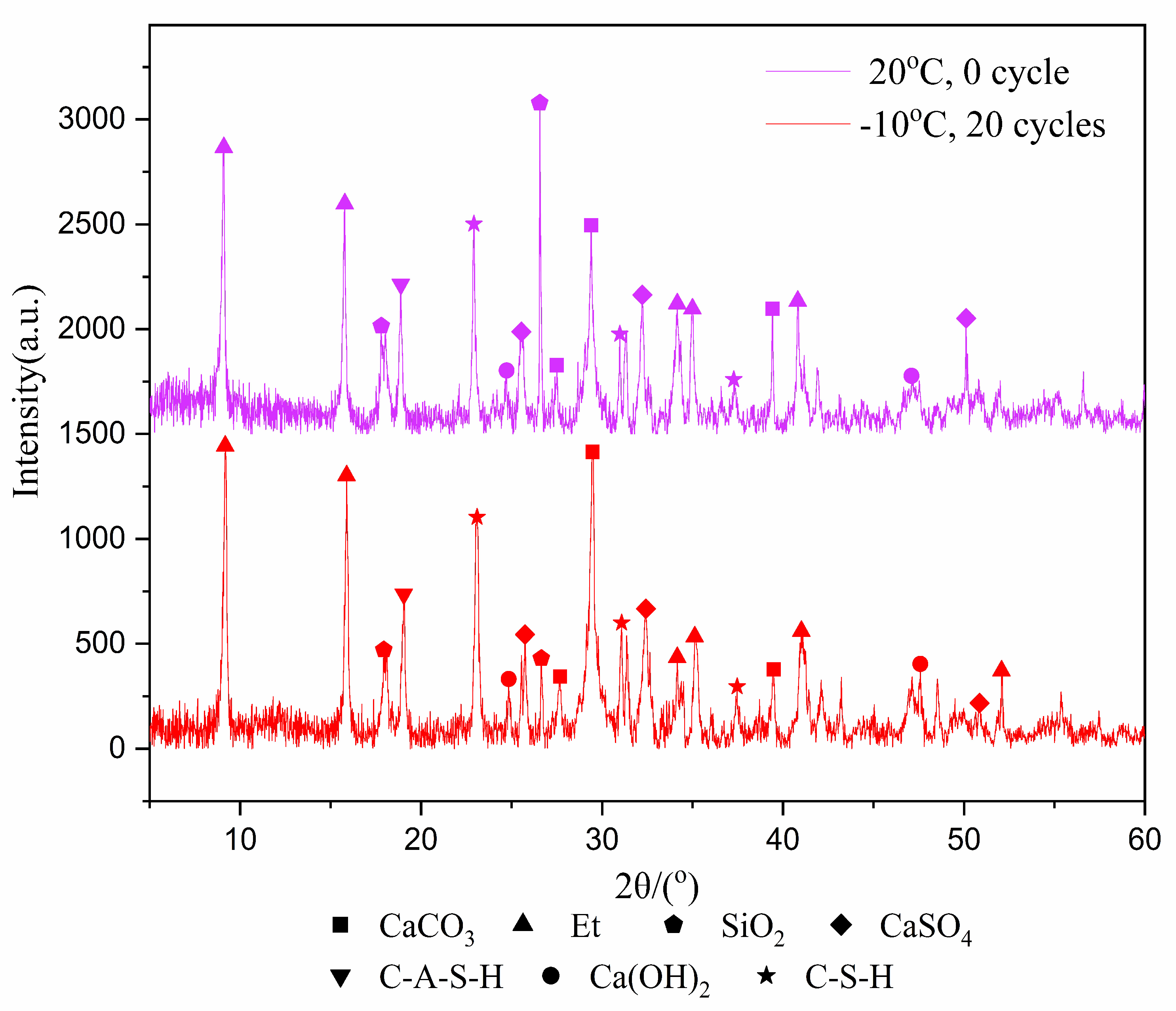
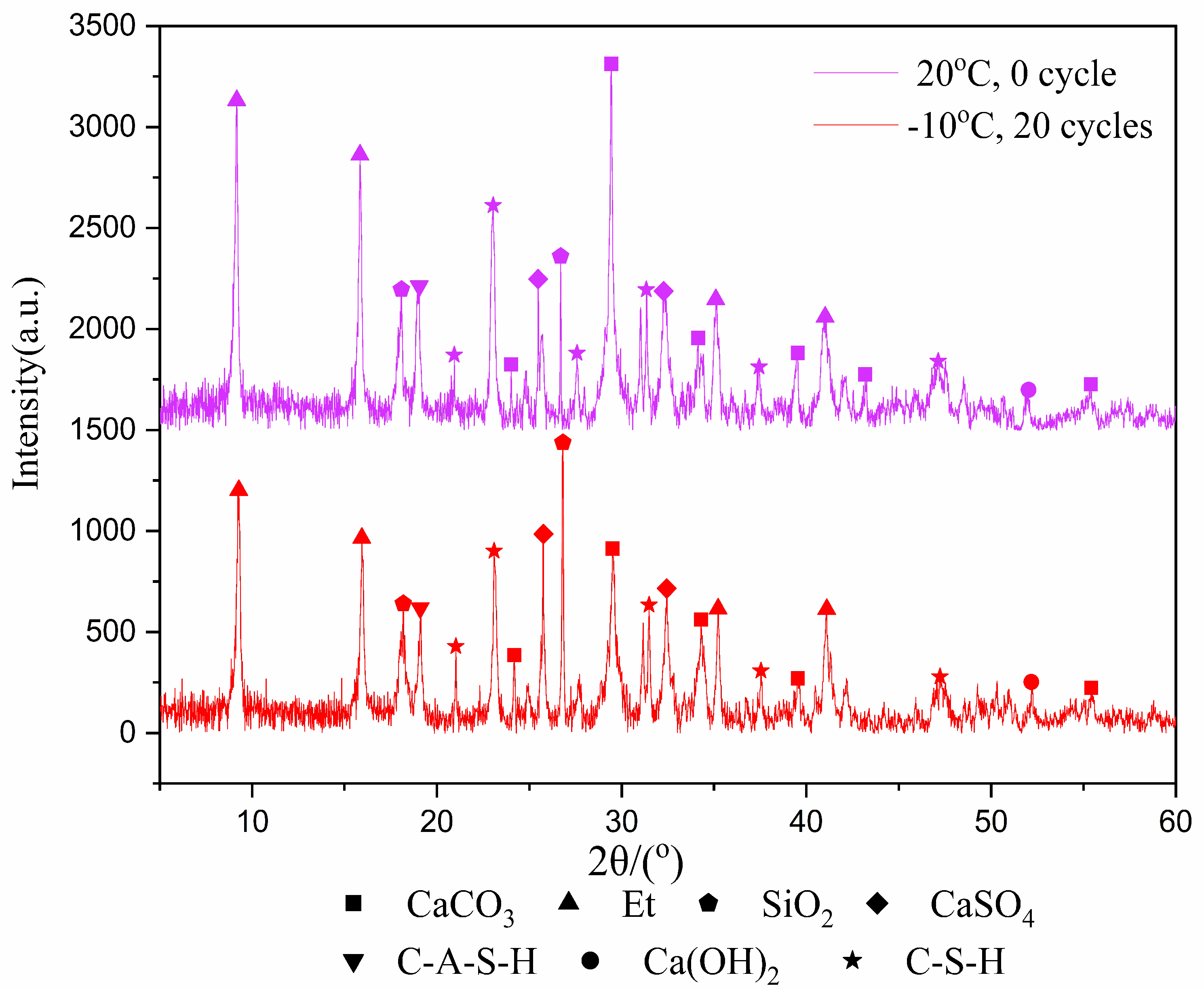
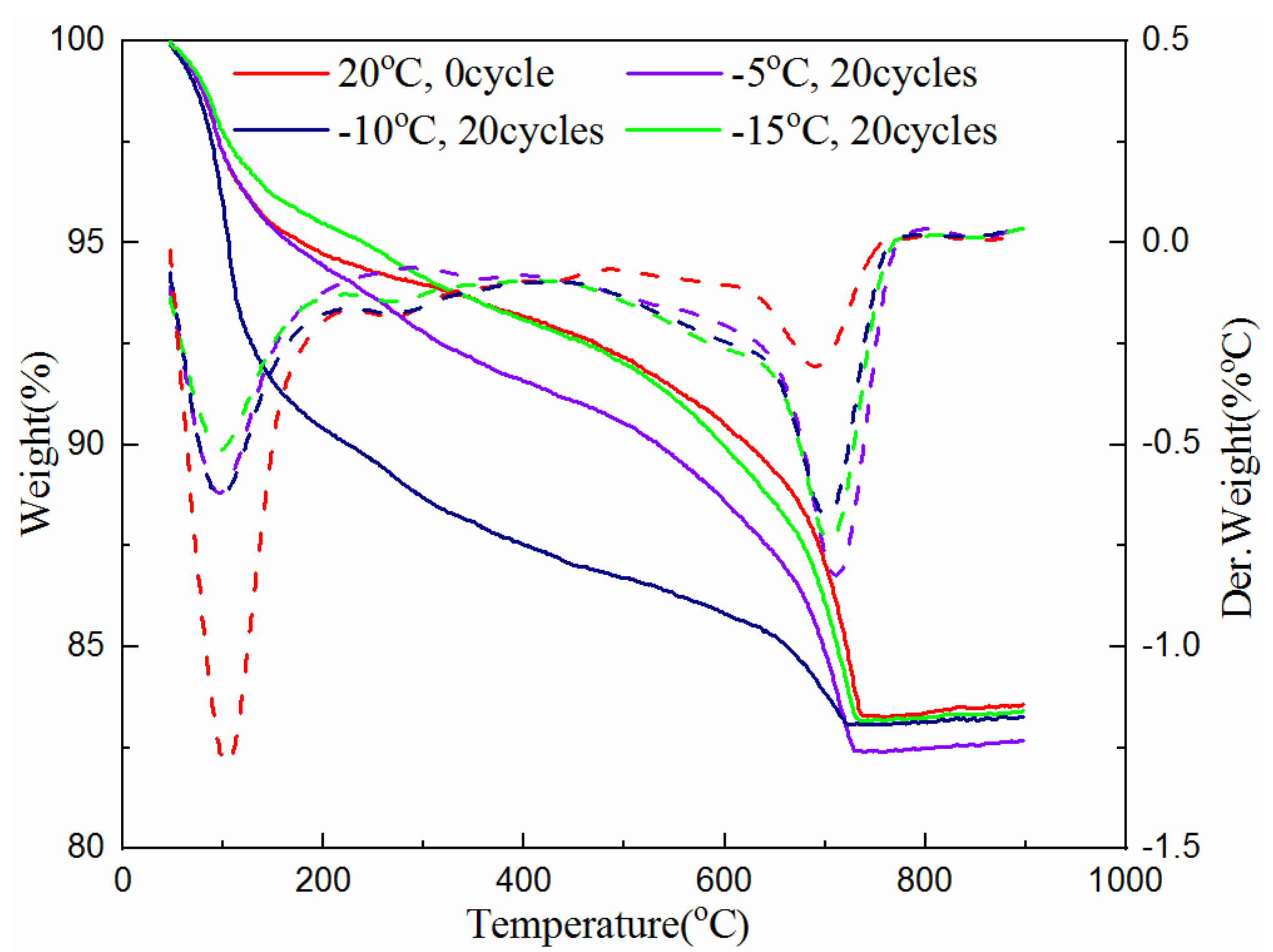
3.2. Crystalline Phases and Amount of Hydration Products of NCM
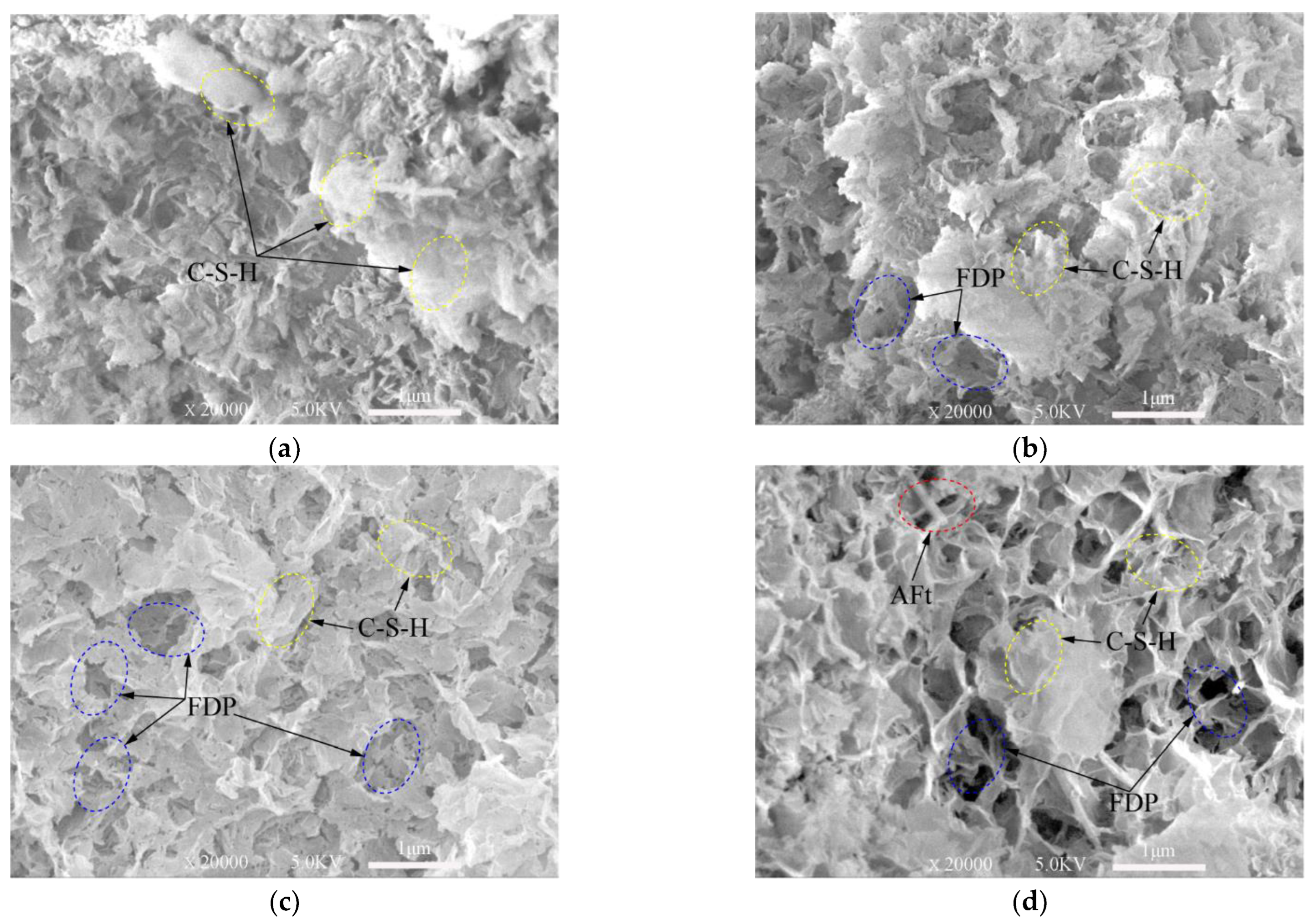
3.3. Surface Morphology Destroyed by Freeze–Thaw Cycles of the Samples
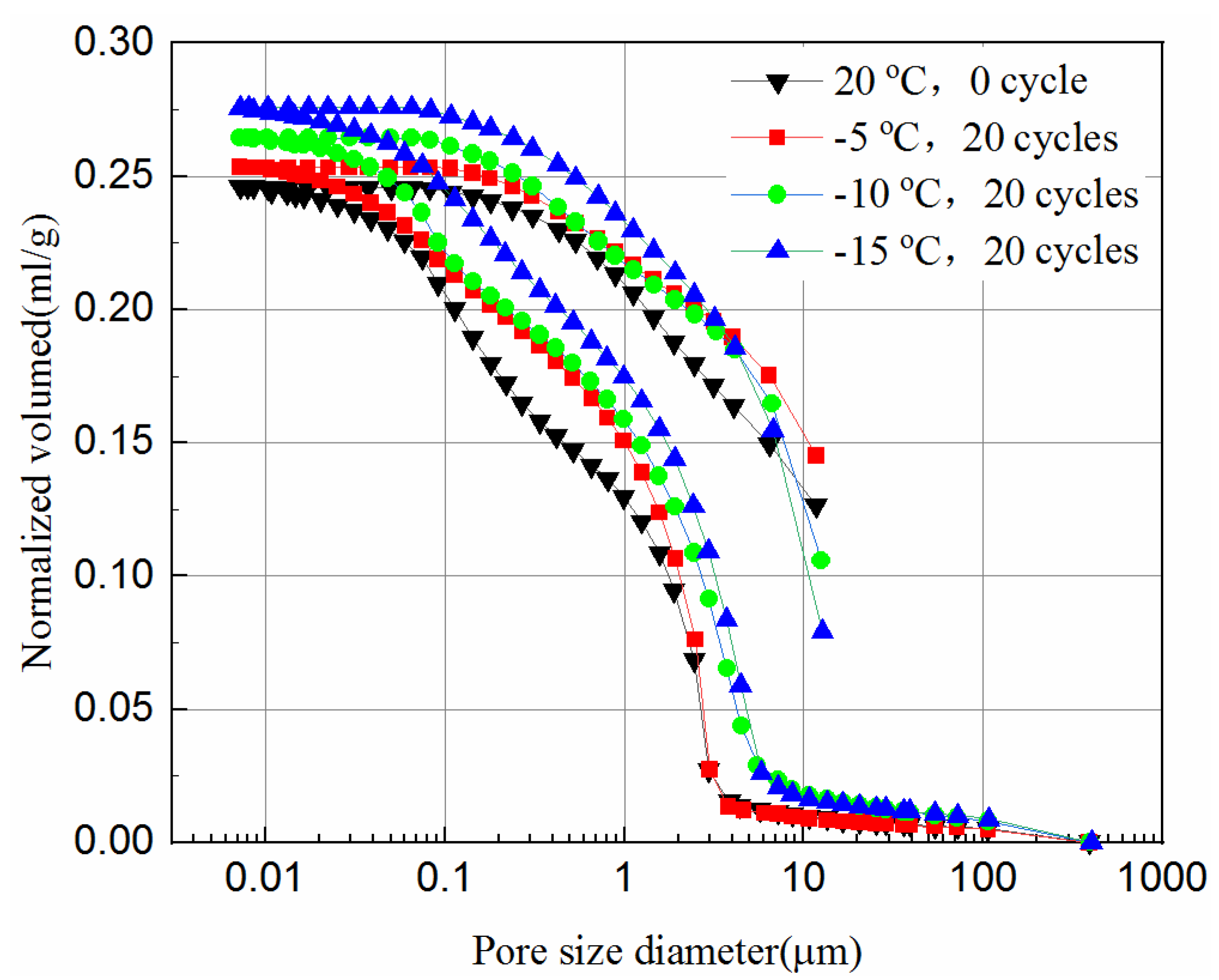
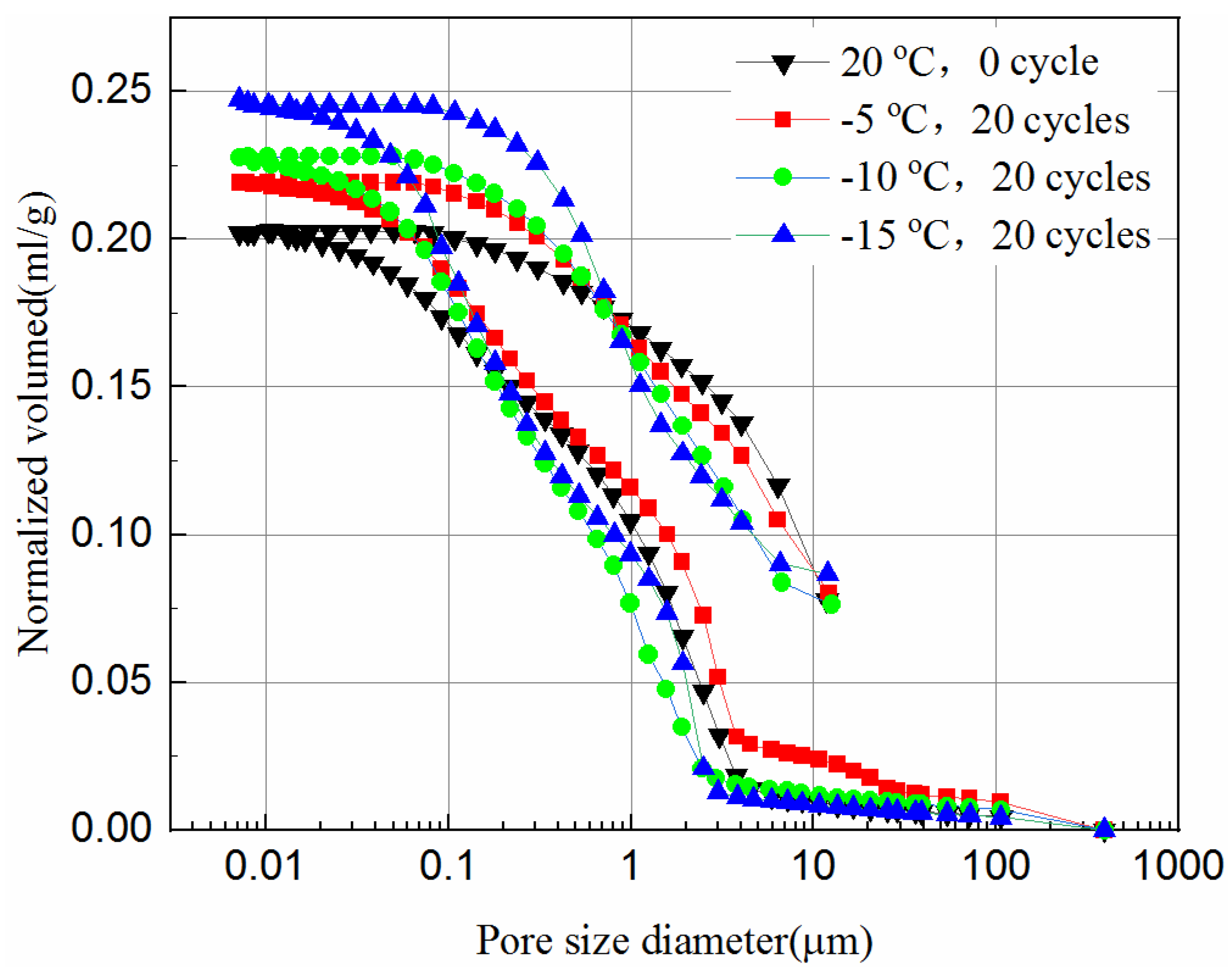
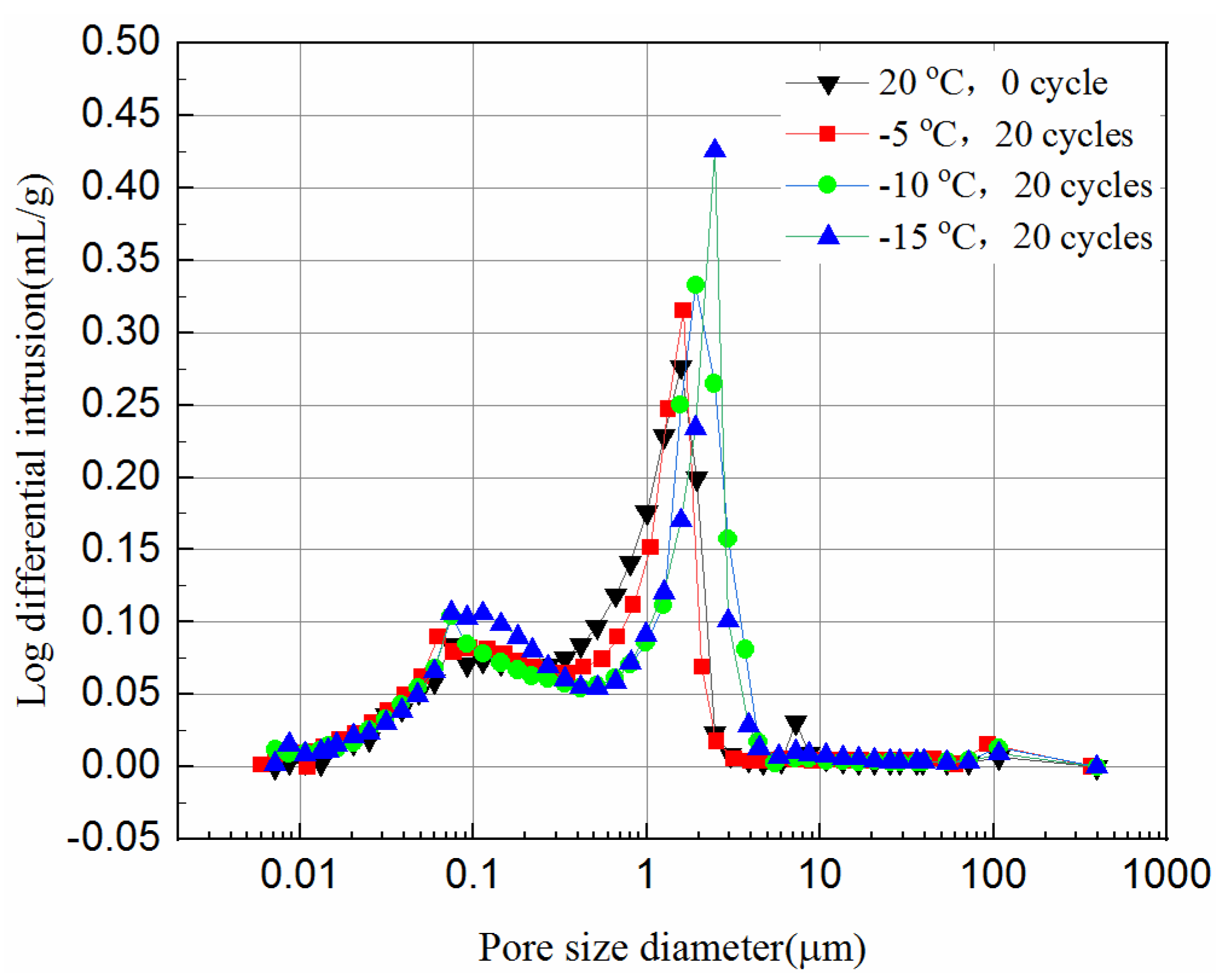
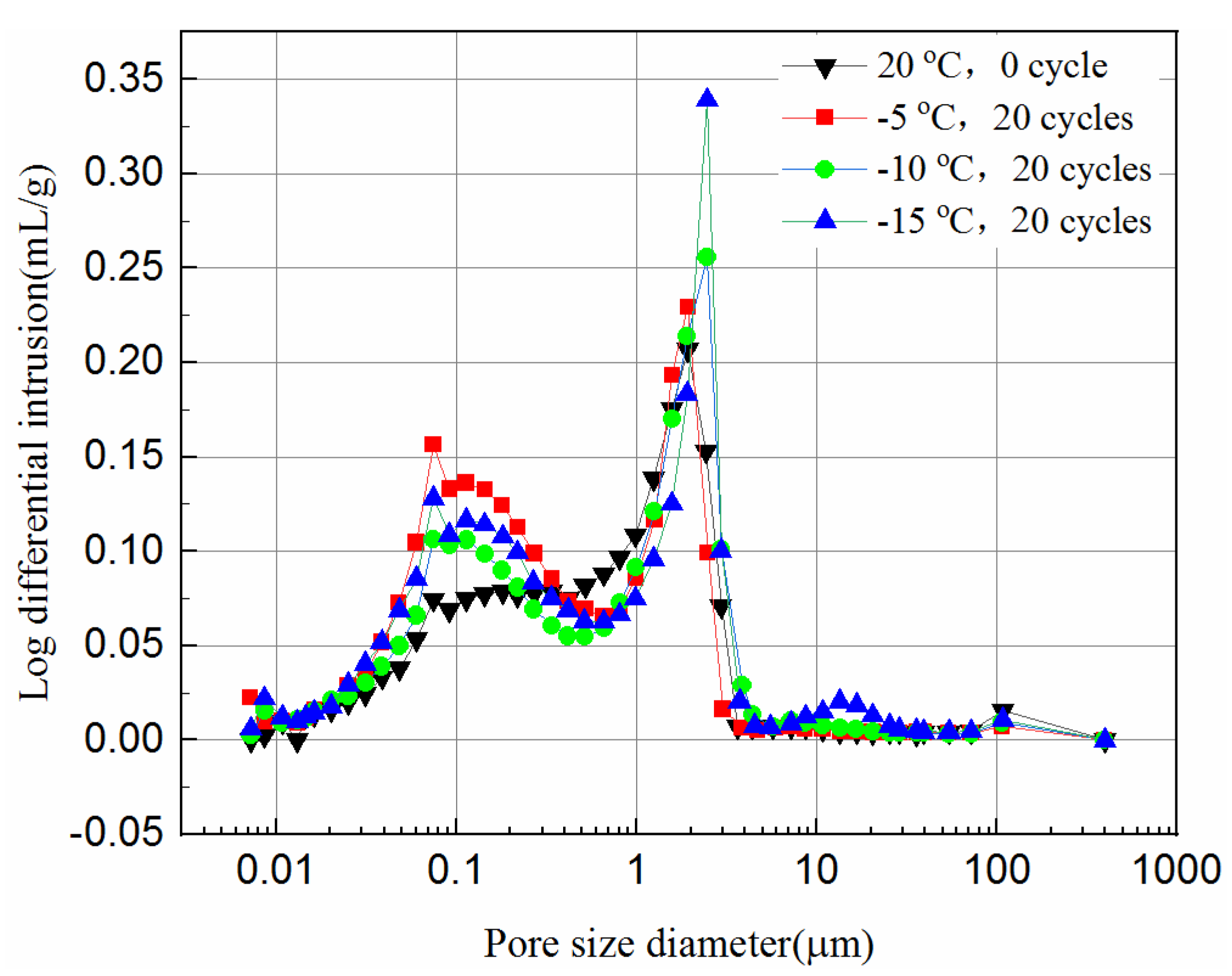
3.4. Pore Size Distribution of the Samples
4. Conclusions
- (1)
- Freeze–thaw has a positive effect on UCS of tailings samples in the first three cycles for short curing times of 3- and 7-days but has a negative effect on the UCS for a normal curing time of 28-days under all freeze–thaw cycles. The frozen temperature has slight effect on UCS reduction for short curing time but has little effect for normal curing time.
- (2)
- The larger the number of freeze–thaw cycles are, the more damage there is to the surface morphology and the matrix of the tailings, and the more severe the surface morphology damage is, the lower the UCS of the samples is.
- (3)
- The freeze–thaw cycles have no effect on phases of the hydration products. The higher the freezing temperature is, the greater the amount of hydration products. Fuller hydration would result in a higher UCS of the samples.
- (4)
- The mercury intrusion pressure is inversely related to the pore diameter of the samples. The lower the freezing temperature is the more mercury ingresses, and the most probable pore sizes increase after the freeze–thaw cycles, which in turn reduces the UCS of the samples.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- David, J.W. Lessons from Tailings Dam Failures—Where to Go from Here? Minerals 2021, 11, 853. [Google Scholar] [CrossRef]
- Liu, R.; Huang, F.; Du, R.; Zhao, C.; Li, Y.; Yu, H. Recycling and utilisation of industrial solid waste: An explorative study on gold deposit tailings of ductile shear zone type in China. Waste Manag. Res. 2015, 33, 570–577. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J. The Statistics and Causes of Dam Break and Leakage in Chinese Tailings Pond. Chin. Molybdenum Ind. 2019, 4, 10–14. [Google Scholar] [CrossRef]
- Yu, M.; Kong, X.; Huang, J.; Liu, J.; Li, J. Status of disposal of tailings as a solid waste and suggestions in China. Ind. Miner. Processing 2022, 1, 34–38, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Bolaños-Benítez, V.; Van Hullebusch, E.; Lens, P.L.; Quantin, C.; Van De Vossenberg, J.; Subramanian, S.; Sivry, Y. (Bio)leaching Behavior of Chromite Tailings. Minerals 2018, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Anning, C.; Wang, J.; Chen, P.; Batmunkh, I.; Lyu, X. Determination and detoxification of cyanide in gold mine tailings: A review. Waste Manag. Res. 2019, 37, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 5, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wei, X.; Li, T.; Zhang, L. Strengthening Behavior of Cemented Paste Backfill Using Alkali-Activated Slag Binders and Bottom Ash Based on the Response Surface Method. Materials 2020, 13, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, X.B.; Liu, L.; Wang, P.; Wang, M.; Xin, J. Microscopic Parameter Extraction and Corresponding Strength Prediction of Cemented Paste Backfill at Different Curing Times. Adv. Civ. Eng. 2018, 4, 2837571. [Google Scholar] [CrossRef]
- Nasir, O.; Fall, M. Shear behavior of cemented pastefill–rock interfaces. Eng. Geol. 2008, 101, 146–153. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Chen, Q.S.; Wang, X.M. Cemented Backfilling Technology of Paste-Like Based on Aeolian Sand and Tailings. Minerals 2016, 6, 132. [Google Scholar] [CrossRef] [Green Version]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Park, I.; Villacorte-Tabelin, M. Synthesis and characterization of coal fly ash and palm oil fuel ash modified artisanal and small-scale gold mine (ASGM) tailings based geopolymer using sugar mill lime sludge as Ca-based activator. Heliyon 2021, 7, e06654. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.B.; Tang, J.; Wei, S.X. Study on Tailings Consolidation Emissions Technology. Chin. J. Met. Mine 2011, 40, 59–62, (In Chinese with English Abstract). [Google Scholar]
- Michael, P.; Arnel, B.; Aileen, O.; Bernardo-Arugay, I.; Resabal, V.J.; Villacorte-Tabelin, M.; Dalona, I.M.; Opiso, E.; Alloro, R.; Alonzo, D.; et al. Systems Approach toward a Greener Eco-efficient Mineral Extraction and Sustainable Land Use Management in the Philippines. Chem. Eng. Trans. 2021, 88, 1171–1176. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; Nyambe, I.; et al. Detoxification of lead-bearing zinc plant leach residues from Kabwe, Zambia by coupled extraction-cementation method. J. Environ. Chem. Eng. 2020, 8, 104197. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, W.; Zhang, S.; Mu, Y.; Li, G. Effect of freeze-thaw cycles in mechanical behaviors of frozen loess. Cold Reg. Sci. Technol. 2018, 146, 9–18. [Google Scholar] [CrossRef]
- Qiao, Y.F.; Sun, W.; Jiang, J. Damage process of concrete subjected to coupling fatigue load and freeze/thaw cycles. Constr. Build. Mater. 2015, 93, 806–811. [Google Scholar] [CrossRef]
- Polat, R. The effect of antifreeze additives on fresh concrete subjected to freezing and thawing cycles. Cold Reg. Sci. Technol. 2016, 127, 10–17. [Google Scholar] [CrossRef]
- Polat, R.; Demirboga, R.; Karakoc, M.B.; Türkmen, I. The influence of light weight aggregate on the physical-mechanical properties of concrete exposed to freeze–thaw cycles. Cold Reg. Sci. Technol. 2010, 60, 51–56. [Google Scholar] [CrossRef]
- Cao, D.F.; Fu, L.Z.; Yang, Z.W. Experimental study on tensile properties of concrete after freeze-thaw cycles. J. Build. Mater. 2012, 15, 42–52. [Google Scholar] [CrossRef]
- Qureshi, A.; Bussière, B.; Benzaazoua, M.; Lessard, F.; Boulanger-Martel, V. Geochemical Assessment of Desulphurized Tailings as Cover Material in Cold Climates. Minerals 2021, 11, 280. [Google Scholar] [CrossRef]
- Hanjari, K.Z.; Utgenannt, P.; Lundgren, K. Experimental study of the material and bond properties of frost-damaged concrete. Cem. Concr. Res. 2011, 41, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Hanjari, K.; PerKettil, K.L. Modelling the structural behaviour of frost-damaged reinforced concrete structures. Struct. Infrastruct. Eng. 2013, 9, 416–431. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.Q.; Li, J.; Wan, P.; Yang, P. Resilient and plastic strain behavior of freezing-thawing mucky clay under subway loading in Shanghai. Nat. Hazards 2014, 72, 771–787. [Google Scholar] [CrossRef]
- Xie, S.B.; Qu, J.J.; Xu, X.T.; Pang, Y. Interactions between freeze–thaw actions, wind erosion desertification, and permafrost in the Qinghai–Tibet Plateau. Nat. Hazards 2017, 85, 829–850. [Google Scholar] [CrossRef]
- Liu, L.; Ye, G.; Schlangen, E.; Chen, H.; Qian, Z.; Sun, W.; van Breugel, K. Modeling of the internal damage of saturated cement paste due to ice crystallization pressure during freezing. Cem. Concr. Comp. 2011, 33, 562–571. [Google Scholar] [CrossRef]
- Liu, L.; Shen, D.; Chen, H.; Sun, W.; Qian, Z.; Zhao, H.; Jiang, J. Analysis of damage development in cement paste due to ice nucleation at different temperatures. Cem. Concr. Comp. 2014, 53, 1–9. [Google Scholar] [CrossRef]
- Liu, L.; Wu, S.; Chen, H.; Haitao, Z. Numerical investigation of the effects of freezing on micro-internal damage and macro-mechanical properties of cement pastes. Cold Reg. Sci. Technol. 2014, 106, 141–152. [Google Scholar] [CrossRef]
- Tang, S.; Yao, Y.; Andrade, C.; Li, Z. Recent durability studies on concrete structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, Q.; Wang, L.; Li, K.; Xu, S.; Yao, Y. Characterizing frost damages of concrete with flatbed scanner. Constr. Build. Mater. 2016, 102, 872–883. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, D.; Yao, Z. Influence of freeze-thaw cycles on dynamic compressive strength and energy distribution of soft rock specimen. Cold Reg. Sci. Technol. 2018, 153, 10–17. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, J.; Shen, S. Effects of curing methods of concrete after steam curing on mechanical strength and permeability. Constr. Build. Mater. 2020, 256, 119441. [Google Scholar] [CrossRef]
- Shin, M.; Park, D.; Seo, Y. Response of subsea pipelines to anchor impacts considering pipe–soil–rock interactions. Int. J. Impact Eng. 2020, 143, 103590. [Google Scholar] [CrossRef]
- Kalonji-Kabambi, A.; Bussière, B.; Demers, I. Hydrogeochemical Behavior of Reclaimed Highly Reactive Tailings, Part 2: Laboratory and Field Results of Covers Made with Mine Waste Materials. Minerals 2020, 10, 589. [Google Scholar] [CrossRef]
- Pokharel, M.; Fall, M. Combined influence of sulphate and temperature on the saturated hydraulic conductivity of hardened cemented paste backfill. Cem. Concr. Comp. 2013, 38, 21–28. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M. Modeling the effect of sulphate on strength development of paste backfill and binder mixture optimization. Cem. Concr. Res. 2005, 35, 301–314. [Google Scholar] [CrossRef]
- Hou, Y.; Ding, P.; Han, D.; Zhang, X.; Cao, S. Study on the Preparation and Hydration Properties of a New Cementitious Material for Tailings Discharge. Processes 2019, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Fall, M. Sulphate effect on the early age strength and self-desiccation of cemented paste backfill. Constr. Build. Mater. 2016, 106, 296–304. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, C.; Sun, C.; Zheng, X.; Ji, Y.; Yuan, X. Experimental Investigation on the Freeze–Thaw Resistance of Steel Fibers Reinforced Rubber Concrete. Materials 2020, 13, 1260. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.P.; Yang, L.; Sun, X.G.; Xing, J.; Li, S. Strength Characteristics and Failure Mechanism of Cemented Super-Fine Unclassified Tailings Backfill. Minerals 2017, 7, 58. [Google Scholar] [CrossRef] [Green Version]
- You, Z.M.; Lai, Y.M.; Zhang, M.Y.; Liu, E. Quantitative analysis for the effect of microstructure on the mechanical strength of frozen silty clay with different contents of sodium sulfate. Environ. Earth Sci. 2017, 4, 143. [Google Scholar] [CrossRef]
- Wu, S.Y.; Yang, J.; Yang, R.C.; Zhu, J.; Liu, S.; Wang, C. Investigation of microscopic air void structure of anti-freezing asphalt pavement with X-ray CT and MIP. Constr. Build. Mater. 2018, 178, 473–483. [Google Scholar] [CrossRef]
- Lee, J.K.; Shang, J.Q. Micropore Structure of Cement-Stabilized Gold Mine Tailings. Minerals 2018, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.l; Cui, Z.D. Effects of freezing-thawing and cyclic loading on pore size distribution of silty clay by mercury intrusion porosimetry. Cold Reg. Sci. Technol. 2018, 145, 185–196. [Google Scholar] [CrossRef]
- Cui, Z.D.; Tang, Y.Q. Microstructures of different soil layers caused by the high-rise building group in Shanghai. Environ. Earth Sci. 2011, 63, 109–119. [Google Scholar] [CrossRef]
- Guo, B.; Zhou, Y.; Zhu, J.F.; Liu, W.; Wang, F.; Wang, L.; Jiang, L. An estimation method of soil freeze-thaw erosion in the Qinghai-Tibet Plateau. Nat. Hazards. 2015, 78, 1843–1857. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Ni, W.; Yan, Q.; Gao, W.; Li, Y. Immobilisation of high-arsenic-containing tailings by using metallurgical slag-cementing materials. Chemosphere 2019, 223, 117–123. [Google Scholar] [CrossRef] [PubMed]




| Element Unit | d10/μm | d30/μm | d50/μm | d60/μm | d90/μm | Cu | Cc |
|---|---|---|---|---|---|---|---|
| slag | 9.32 | 18.07 | 31.23 | 50.68 | 226.28 | 2.80 | 5.44 |
| clinker | 7.48 | 12.26 | 20.39 | 25.31 | 29.62 | 2.06 | 4.59 |
| gypsum | 2.47 | 9.19 | 18.73 | 24.86 | 74.81 | 2.71 | 3.09 |
| lime | 3.13 | 5.91 | 10.82 | 21.74 | 33.32 | 6.95 | 1.42 |
| Tailings | 14.55 | 26.61 | 38.32 | 54.27 | 82.33 | 3.73 | 0.89 |
| Element Unit | MgO (wt.%) | Al2O3 (wt.%) | SiO2 (wt.%) | CaO (wt.%) | SO3 (wt.%) | Fe2O3 (wt.%) | Total |
|---|---|---|---|---|---|---|---|
| slag | 8.38 | 14.79 | 33.81 | 36.95 | 0.28 | 0.89 | 95.09 |
| clinker | 2.45 | 4.47 | 22.01 | 64.31 | 2.45 | 3.45 | 99.14 |
| gypsum | 2.14 | 0.12 | 0.98 | 45.85 | 42.45 | 0.11 | 91.66 |
| lime | 0.56 | 0.23 | 0.38 | 72.29 | 0.13 | 0.26 | 73.84 |
| Tailings | 2.41 | 3.85 | 82.05 | 2.46 | 0.18 | 8.01 | 98.96 |
| Tests | Cured Time/Days | Cured Temperature/°C | Freeze–Thaw Times | Number of Samples |
|---|---|---|---|---|
| UCS | 3, 7, 28 | −5, −10, −15 | 0, 3, 5, 7, 10, 12, 15, 20 | 198 |
| XRD | 7, 28 | −10 | 0, 20 | 4 |
| TG | 7, 28 | −5, −10, −15 | 0, 20 | 8 |
| SEM | 3, 7, 28 | −10 | 0, 5, 10, 20 | 12 |
| MIP | 7, 28 | −5, −10, −15 | 0, 20 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, P.; Hou, Y.; Han, D.; Zhang, X.; Cao, S.; Li, C. Effect of Freeze–Thaw Cycles on Mechanical and Microstructural Properties of Tailings Reinforced with Cement-Based Material. Minerals 2022, 12, 413. https://doi.org/10.3390/min12040413
Ding P, Hou Y, Han D, Zhang X, Cao S, Li C. Effect of Freeze–Thaw Cycles on Mechanical and Microstructural Properties of Tailings Reinforced with Cement-Based Material. Minerals. 2022; 12(4):413. https://doi.org/10.3390/min12040413
Chicago/Turabian StyleDing, Pengchu, Yunbing Hou, Dong Han, Xing Zhang, Shuxiong Cao, and Chunqing Li. 2022. "Effect of Freeze–Thaw Cycles on Mechanical and Microstructural Properties of Tailings Reinforced with Cement-Based Material" Minerals 12, no. 4: 413. https://doi.org/10.3390/min12040413
APA StyleDing, P., Hou, Y., Han, D., Zhang, X., Cao, S., & Li, C. (2022). Effect of Freeze–Thaw Cycles on Mechanical and Microstructural Properties of Tailings Reinforced with Cement-Based Material. Minerals, 12(4), 413. https://doi.org/10.3390/min12040413







