Arsenopyrite Dissolution and Bioscorodite Precipitation by Acidithiobacillus ferrivorans ACH under Mesophilic Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. Bio-Oxidation Experiments
2.3. Mineralogical Characterization
2.4. Search for Arsenic Resistance Genetic Determinants in the ACH Strain Genome
3. Results
3.1. Characterization of Arsenopyrite Concentrate
3.2. Growth of A. ferrivorans ACH Cells Using Arsenopyrite as Energy Source
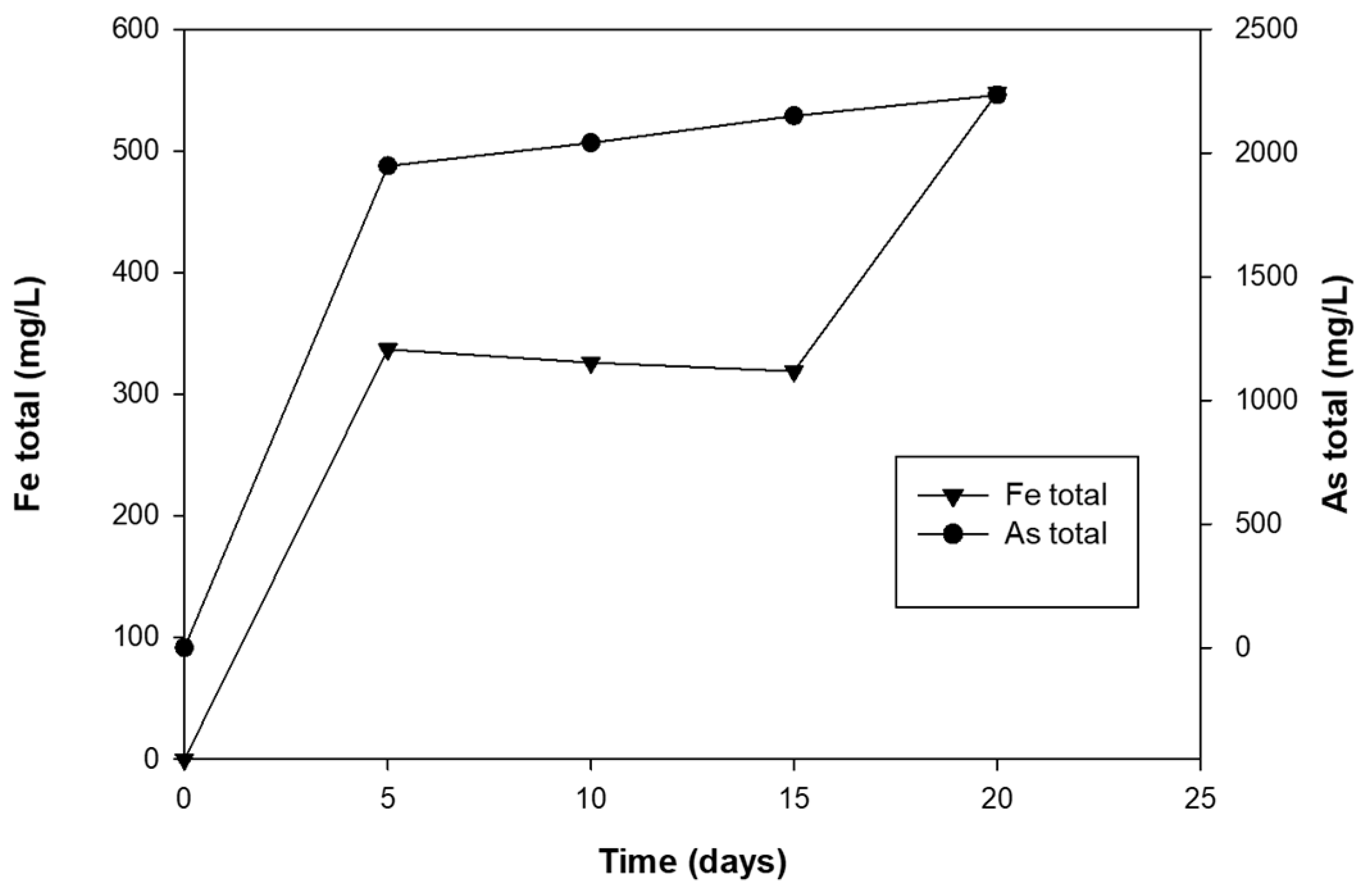
3.3. Arsenopyrite Dissolution and Bioscorodite Precipitation by A. ferrivorans ACH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brierley, C.L. Biohydrometallurgical prospects. Hydrometallurgy 2010, 104, 324–328. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A.; Pradhan, N.; Deveci, H. Current scenario of chalcopyrite bioleaching: A review on the recent advances to its heap-leach technology. Bioresour. Technol. 2015, 196, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Cassity, W.; Pesic, B. Interactions of Thiobacillus ferrooxidans with arsenite, arsenate and arsenopyrite. In Proceedings of Biohydrometallurgy and the Environment toward the Mining of the 21st Century, Part I; Amils, R., Ballester, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 521–532. [Google Scholar]
- Ndlovu, S. Biohydrometallurgy for sustainable development in the African minerals industry. Hydrometallurgy 2008, 91, 20–27. [Google Scholar] [CrossRef]
- Fashola, M.; Ngole-Jeme, V.; Babalola, O. Review Heavy metal pollution from gold mines: Environmental effects and bacterial strategies for resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef] [Green Version]
- Tuffin, I.; de Groot, P.; Deane, S.; Rawlings, D. An unusual Tn21-like transposon containing an ars operon is present in highly arsenic resistant strains of the biomining bacterium Acidithiobacillus caldus. Microbiology 2005, 151, 3027–3039. [Google Scholar] [CrossRef] [Green Version]
- Shibata, E.; Onodera, N.; Fujita, T.; Nakamura, T. Elusion tests of scorodite synthesized by oxidation of ferrous ions. Resour. Process. 2012, 59, 42–48. [Google Scholar] [CrossRef]
- Caetano, M.L.; Ciminelli, V.S.T.; Rocha, S.D.F.; Spitale, M.C.; Caldeira, C.L. Batch and continuous precipitation of scorodite from dilute industrial solutions. Hydrometallurgy 2009, 95, 44–52. [Google Scholar] [CrossRef]
- Paktunc, D.; Dutrizac, J.; Gertsman, V. Synthesis and phase transformations involving scorodite, ferric arsenate and arsenical ferrihydrite: Implications for arsenic mobility. Geochim. Cosmochim. Acta 2008, 72, 2649–2672. [Google Scholar] [CrossRef]
- Dutrizac, J.; Jambor, J. The synthesis of crystalline scorodite, FeAsO2x 2H2O. Hydrometallurgy 1988, 19, 377–384. [Google Scholar] [CrossRef]
- Monhemius, A.; Swash, P. Removing and stabilizing as from copper refining circuits by hydrothermal processing. JOM 1999, 51, 30–33. [Google Scholar] [CrossRef]
- Tanaka, M.; Okibe, N. Factors to Enable Crystallization of Environmentally Stable Bioscorodite from Dilute As(III) Contaminated Waters. Minerals 2018, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Contreras, P.; Weijma, J.; van der Weijden, R.; Buisman, C.J.N. Biogenic scorodite crystallization by Acidianus sulfidivorans for arsenic removal. Environ. Sci. Technol. 2010, 44, 675–680. [Google Scholar] [CrossRef]
- Gonzalez-Contreras, P.; Weijma, J.; Buisman, C.J.N. Bioscorodite crystallization in an airlift reactor for arsenic removal. Cryst. Growth Des. 2012, 12, 2699–2706. [Google Scholar] [CrossRef]
- Okibe, N.; Koga, M.; Morishita, S.; Tanaka, M.; Heguri, S.; Asano, S.; Sasaki, K.; Hirajima, T. Microbial formation of crystalline scorodite for treatment of As(III)-bearing copper refinery process solution using Acidianus brierleyi. Hydrometallurgy 2014, 143, 34–41. [Google Scholar] [CrossRef]
- Higashidani, N.; Kaneta, T.; Takeyasu, N.; Shoji Motomizu, S.; Okibe, N.; Sasaki, K. Speciation of arsenic in a thermos acidophilic iron-oxidizing archaeon, Acidianus brierleyi, and its culture medium by inductively coupled plasma–optical emission spectroscopy combined with flow injection pretreatment using an anion-exchange mini-column. Talanta 2014, 122, 240–245. [Google Scholar] [CrossRef]
- Johnson, D.B.; Dybowska, A.; Schofield, P.F.; Herrington, R.J.; Smith, S.L.; Santos, A.L. Bioleaching of arsenic-rich cobalt mineral resources, and evidence for concurrent biomineralisation of scorodite during oxidative bio-processing of skutterudite. Hydrometallurgy 2020, 195, 105395. [Google Scholar] [CrossRef]
- Hallberg, K.; González-Toril, E.; Johnson, D. Acidithiobacillus ferrivorans, sp. nov.; facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles 2010, 14, 9–19. [Google Scholar] [CrossRef]
- Kimura, S.; Bryan, C.; Hallberg, K.; Johnson, D. Biodiversity and geochemistry of an extremely acidic, low-temperature subterranean environment sustained by chemolithotrophy. Environ. Microbiol. 2011, 13, 2092–2104. [Google Scholar] [CrossRef]
- Barahona, S.; Dorador, C.; Remonsellez, F. Identification and characterization of a psychrotolerant Acidithiobacillus strain from Chilean Altiplano. Adv. Mater. Res. 2013, 825, 74–78. [Google Scholar] [CrossRef]
- Talla, E.; Hedrich, S.; Mangenot, S.; Ji, B.; Johnson, D.; Barbe, V.; Bonnefoy, V. Insights into the pathways of iron- and sulfur-oxidation, and biofilm formation from the chemolithotrophic acidophile Acidithiobacillus ferrivorans CF27. Res. Microbiol. 2014, 165, 753–760. [Google Scholar] [CrossRef]
- Ccorahua-Santos, R.; Eca, A.; Abanto, M.; Guerra, G.; Ramírez, P. Physiological and comparative genomic analysis of Acidithiobacillus ferrivorans PQ33 provides psychrotolerant fitness evidence for oxidation at low temperature. Res. Microbiol. 2017, 168, 482–492. [Google Scholar] [CrossRef]
- Peng, T.; Ma, L.; Feng, X.; Tao, J.; Nan, M.; Liu, Y.; Li, J.; Shen, L.; Wu, X.; Yu, R.; et al. Genomic and transcriptomic analyses reveal adaptation mechanisms of an Acidithiobacillus ferrivorans strain YL15 to alpine acid mine drainage. PLoS ONE 2017, 12, e0178008. [Google Scholar] [CrossRef]
- Barahona, S.; Dorador, C.; Riuyong, Z.; Aguilar, P.; Sand, W.; Vera, M.; Remonsellez, F. Isolation and characterization of a novel Acidithiobacillus ferrivorans strain from the Chilean Altiplano: Attachment and biofilm formation on pyrite at low temperature. Res. Microbiol. 2014, 165, 782–793. [Google Scholar] [CrossRef]
- Barahona, S.; Castro-Severyn, J.; Dorador, C.; Saavedra, C.; Remonsellez, F. Determinants of copper resistance in Acidithiobacillus ferrivorans ACH isolated from the Chilean Altiplano. Genes 2020, 11, 844. [Google Scholar] [CrossRef]
- Mackintosh, M. Nitrogen fixation by Thiobacillus ferrooxidans. J. Gen. Microbiol. 1978, 105, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.; Mustin, C.; de Donato, P.; Barres, O.; Marion, P.; Berthelin, J. Occurrences at mineral-bacterial interface during oxidation of arsenopyrite by Thiobacillus ferrooxidans. Biotechnol. Bioeng. 1995, 46, 13–21. [Google Scholar] [CrossRef]
- Corkhill, C.; Vaughan, D. Arsenopyrite oxidation—A review. Appl. Geochem. 2009, 24, 2342–2361. [Google Scholar] [CrossRef]
- Kaksonen, A.; Mudunuru, B.; Hackl, R. The role of microorganisms in gold processing and recovery—A review. Hydrometallurgy 2014, 142, 70–83. [Google Scholar] [CrossRef]
- Dave, S.; Gupta, K.; Tipre, D. Characterization of arsenic resistant and arsenopyrite oxidizing Acidithiobacillus ferrooxidans from Hutti gold leachate and effluents. Bioresour. Technol. 2008, 99, 7514–7520. [Google Scholar] [CrossRef]
- Deng, S.; Gu, G.; Wu, Z.; Xu, X. Bioleaching of arsenopyrite by mixed cultures of iron-oxidizing and sulfur-oxidizing microorganisms. Chemosphere 2017, 185, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Butcher, B.; Deane, S.; Rawling, D. The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl. Environ. Microbiol. 2000, 66, 1826–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duqesne, K.; Lebrun, C.; Casiot, O.; Bruneel, J.; Personne, C.; Leblanc, M.; Elbaz-Poulichet, F.; Morin, G.; Bonnefoy, V. Immobilization of arsenite and ferric iron by Acidithiobacillus ferrooxidans and its relevance to acid mine drainage. Appl. Environ. Microbiol. 2003, 69, 6165–6173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngoma, E.; Borja, D.; Smart, M.; Shaik, K.; Kim, H.; Petersen, J.; Harrison, S.T.L. Bioleaching of arsenopyrite from Janggun mine tailings (South Korea) using an adapted mixed mesophilic culture. Hydrometallurgy 2018, 181, 21–28. [Google Scholar] [CrossRef]
- Borja, D.; Nguyen, K.M.; Silva, R.A.; Ngoma, E.; Petersen, J.; Harrison, S.T.L.; Park, J.H.; Kim, H. Continuous bioleaching of arsenopyrite from mine tailings using an adapted mesophilic microbial culture. Hydrometallurgy 2019, 187, 187–194. [Google Scholar] [CrossRef]
- Rohwerder, T.; Sand, W. Oxidation of inorganic sulfur compounds in acidophilic prokaryotes. Eng. Life. Sci. 2007, 7, 301–309. [Google Scholar] [CrossRef]
- Fomchenko, N.V.; Muravyov, M.I. Thermodynamic and XRD analysis of arsenopyrite biooxidation and enhancement of oxidation efficiency of gold-bearing concentrates. Int. J. Miner. Process. 2014, 133, 112–118. [Google Scholar] [CrossRef]
- Tuovinen, O.; Bhatti, T.; Bigham, J.; Garcia, O.; Lindstrom, E. Oxidative dissolution of arsenopyrite by mesophilic and moderately thermophilic acidophiles. Appl. Environ. Microbiol. 1994, 60, 3268–3274. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Rene ASilva, R.; Kim, S.; Kim, H. Bioleaching of arsenic from highly contaminated mine tailings using Acidithiobacillus thiooxidans. J. Environ. Manag. 2015, 147, 124–131. [Google Scholar] [CrossRef]
- Deng, S.; Gu, G.; Xu, B.; Li, L.; Wu, B. Surface characterization of arsenopyrite during chemical and biological oxidation. Sci. Total Environ. 2018, 626, 349–356. [Google Scholar] [CrossRef]
- Meruane, G.; Vargas, T. Bacterial oxidation of ferrous iron by Acidithiobacillus ferrooxidans in the pH range 2.5–7.0. Hydrometallurgy 2003, 71, 149–158. [Google Scholar] [CrossRef]
- Wang, X.; Qingzhu, L.; Qi, L.; Yuchen, Y.; Xia, J.; Qiuhong, L.; Qingwei, W.; Yanjie, L. Arsenic(III) biotransformation to tooeleite associated with the oxidation of Fe(II) via Acidithiobacillus ferrooxidans. Chemosphere 2020, 248, 126080. [Google Scholar] [CrossRef]
- Egal, M.; Casiot, C.; Morin, G.; Parmentier, M.; Bruneel, O.; Lebrun, S.; Elbaz-Poulichet, F. Kinetic control on the formation of tooeleite, schwertmannite and jarosite by Acidithiobacillus ferrooxidans strains in an As(III)-rich acid mine water. Chem. Geol. 2009, 265, 432–441. [Google Scholar] [CrossRef]
- Slyemi, D.; Bonnefoy, V. How prokaryotes deal with arsenic. Env. Microbiol. Rep. 2012, 4, 571–586. [Google Scholar] [CrossRef]
- Wiertz, J.; Magda, M.; Escobar, B. Mechanism of pyrite catalysis of As(III) oxidation in bioleaching solutions at 30 °C and 70 °C. Hydrometallurgy 2006, 83, 35–39. [Google Scholar] [CrossRef]
- Tao, J.; Qian, L.; Yong-bin, Y. Bio-oxidation of arsenopyrite. Trans. Nonferrous Met. Soc. China 2008, 18, 1433–1438. [Google Scholar]
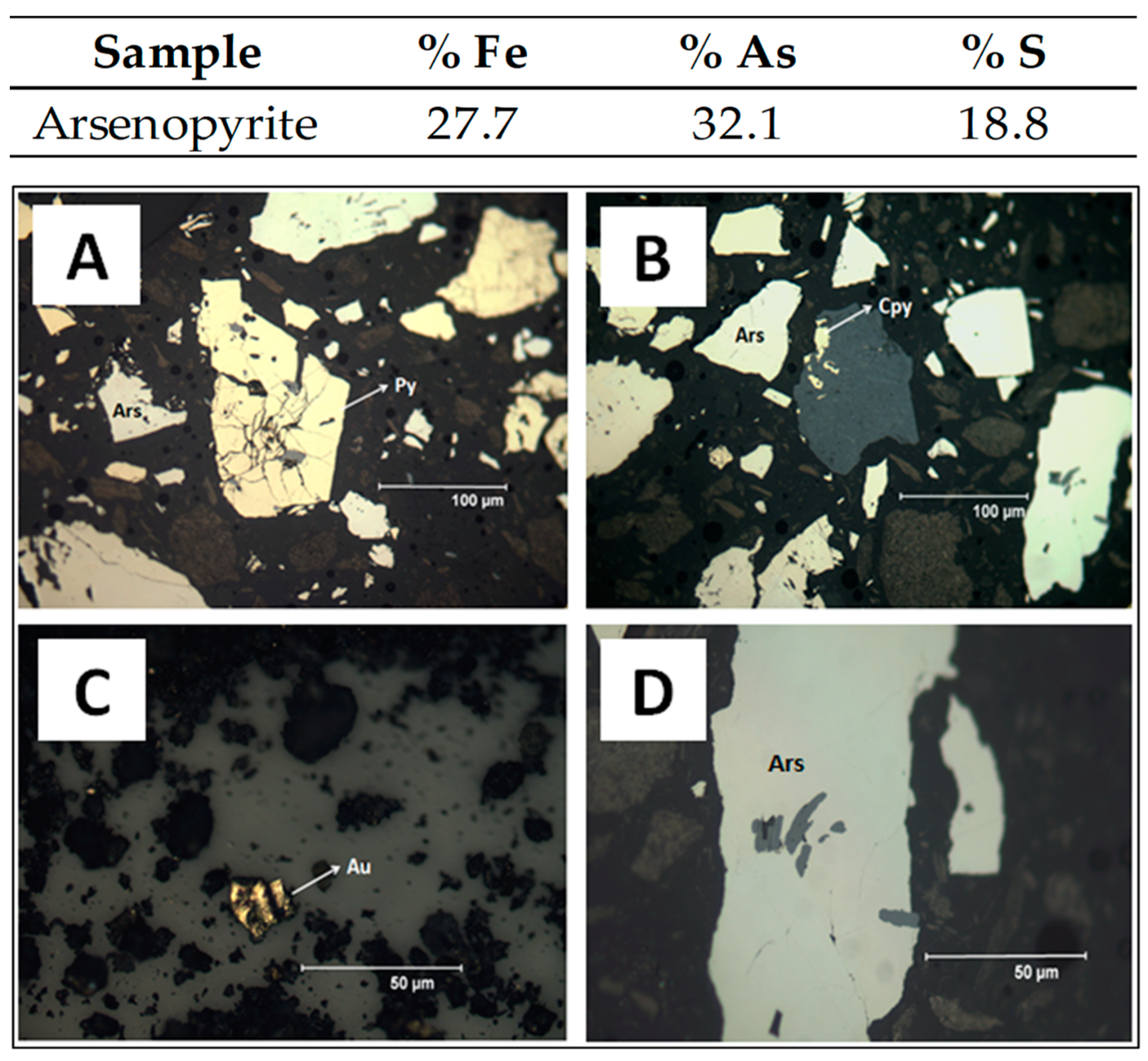




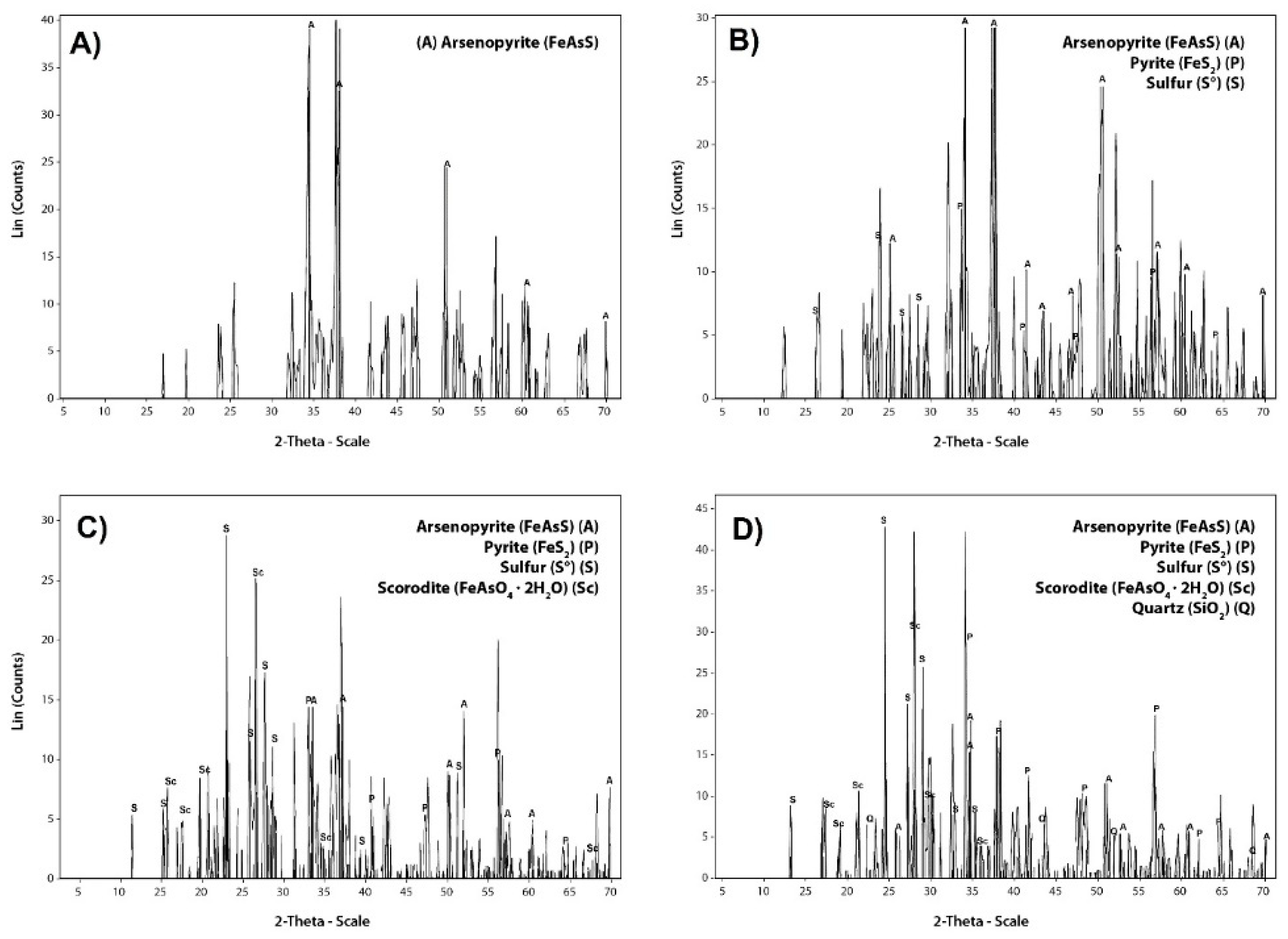
| Trace Elements | Ni | Co | Sb | Cu | Zn | Pb | Bi |
|---|---|---|---|---|---|---|---|
| Amounts (ppm) | 16.71 | 7.08 | 671.00 | 165.00 | 605.00 | 138.00 | 582.00 |
| Mineral Mass (%) | Arsenopyrite Pulp without Bacteria | Bio-Oxidation Process | |||
|---|---|---|---|---|---|
| 5 Days | 10 Days | 15 Days | 20 Days | ||
| Alloys As-Fe | 2.2 | 0.7 | 0.5 | 0.9 | 0.8 |
| Arsenopyrite | 35.5 | 37.7 | 31.0 | 23.0 | 16.8 * |
| Arsenopyrite (Ni+Co) | 13.5 | 12.8 | 9.2 | 4.2 | 1.1 * |
| As-Ni-Fe Sulfides | 3.6 | 2.4 | 2.8 | 0.8 | 1.0 * |
| As-Fe-Ni Sulfides | 15.1 | 12.3 | 8.0 | 2.2 | 0.3 * |
| As-bearing Pyrite | 1.0 | 1.5 | 1.8 | 2.3 | 2.9 |
| As-bearing sulfates | 7.4 | 11.4 | 17.4 | 32.0 | 32.1 + |
| As-bearing silicates | 4.1 | 3.9 | 5.8 | 7.0 | 9.8 + |
| Scorodite | 1.9 | 2.5 | 3.3 | 4.7 | 5.4 + |
| Others As minerals | 0.1 | 0.2 | 0.5 | 0.9 | 2.1 + |
| Pyrite | 10.6 | 9.4 | 12.1 | 13.8 | 15.8 |
| Others | 5.0 | 5.0 | 8.0 | 8.0 | 12.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barahona, S.; Herrera, E.; Jara, A.; Castro-Severyn, J.; Gallardo, K.; Fuentes, G.; Dorador, C.; Saavedra, C.; Remonsellez, F. Arsenopyrite Dissolution and Bioscorodite Precipitation by Acidithiobacillus ferrivorans ACH under Mesophilic Condition. Minerals 2022, 12, 520. https://doi.org/10.3390/min12050520
Barahona S, Herrera E, Jara A, Castro-Severyn J, Gallardo K, Fuentes G, Dorador C, Saavedra C, Remonsellez F. Arsenopyrite Dissolution and Bioscorodite Precipitation by Acidithiobacillus ferrivorans ACH under Mesophilic Condition. Minerals. 2022; 12(5):520. https://doi.org/10.3390/min12050520
Chicago/Turabian StyleBarahona, Sergio, Erick Herrera, Andrea Jara, Juan Castro-Severyn, Karem Gallardo, Gerardo Fuentes, Cristina Dorador, Claudia Saavedra, and Francisco Remonsellez. 2022. "Arsenopyrite Dissolution and Bioscorodite Precipitation by Acidithiobacillus ferrivorans ACH under Mesophilic Condition" Minerals 12, no. 5: 520. https://doi.org/10.3390/min12050520
APA StyleBarahona, S., Herrera, E., Jara, A., Castro-Severyn, J., Gallardo, K., Fuentes, G., Dorador, C., Saavedra, C., & Remonsellez, F. (2022). Arsenopyrite Dissolution and Bioscorodite Precipitation by Acidithiobacillus ferrivorans ACH under Mesophilic Condition. Minerals, 12(5), 520. https://doi.org/10.3390/min12050520






