Na-Alternative to Tinsleyite Obtained under Hydrothermal Conditions: Crystal Structure and Comparative Crystal Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrothermal Synthesis and Crystallization
2.2. X-ray Diffraction and Crystal Structure Determination
3. Results
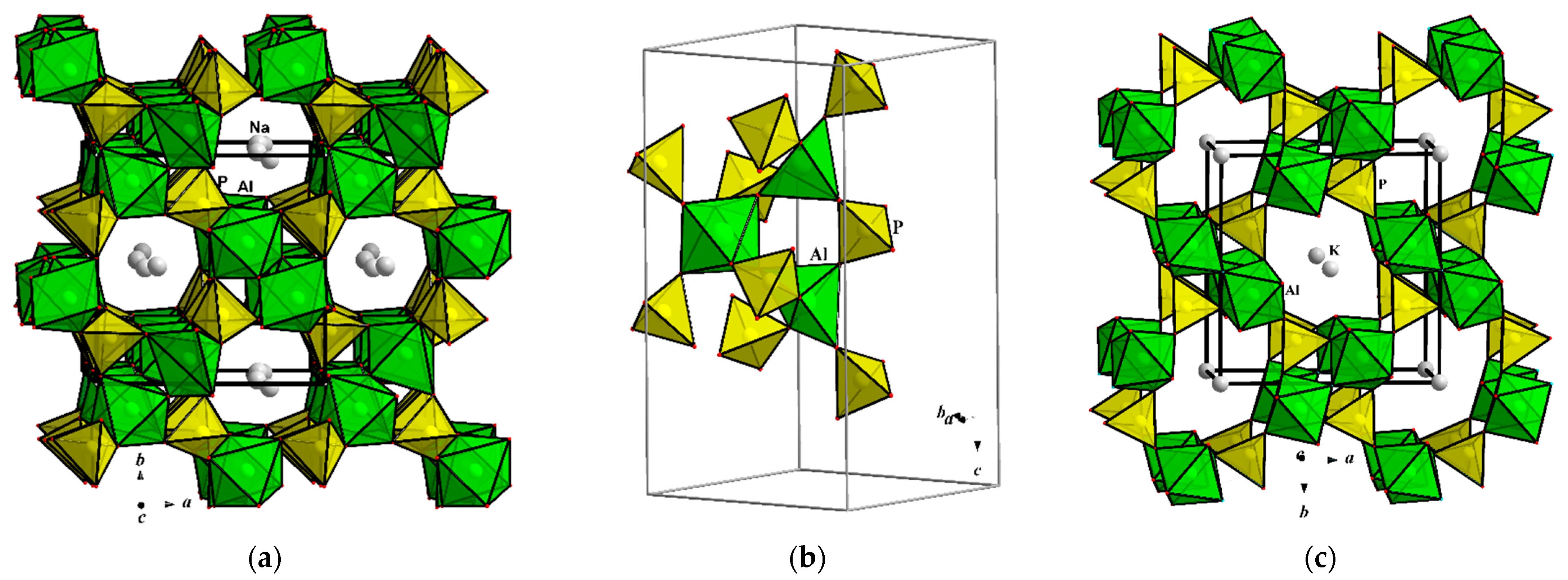
3.1. Interatomic Distances and Crystal Structure Description
3.2. Crystal Chemical Regularities in the Family of Aluminum Phosphates/Aluminophosphates
| Compound/Mineral | Unit Cell Parameters, Å, β, o, V, Å3 | Space Group, Z, ρ, g/cm3 | Clusters of Al-Centered Polyhedra | Anionic Structural Fragment | Reference |
|---|---|---|---|---|---|
| Tinsleyite, synthetic KAl2(OH)(H2O)(PO4)2·H2O | a = 9.499(2) | P21/n | Al4(OH)2(H2O)2O16 | Framework | [5] |
| b = 9.503(2) | 4 | ||||
| c = 9.535(2) | 2.66 | ||||
| β = 103.26(3) | |||||
| V = 837.8(2) | |||||
| Na2Al2O(PO4)2·0.12H2O * | a = 9.9927(3) | P21/c | Al4O18 | Layers | This work |
| b = 8.8811(2) | 4 | ||||
| c = 9.7005(3) | 2.63 | ||||
| β = 116.155(4) | |||||
| V = 772.73(4) | |||||
| Minyulite, KAl2F(H2O)4(PO4)2 | a = 9.337(5) | Pba2 | Al2F(H2O)4O6 | Layers | [35] |
| b = 9.740(5) | 2 | ||||
| c = 5.522(3) | 2.47 | ||||
| V = 502.2(5) | |||||
| Na2Al3(OH)2(PO4)3 | a = 8.475(2) | P212121 | Al3(OH)2O12 | Framework | [32] |
| b = 8.471(2) | 4 | ||||
| c = 14.319(3) | 2.88 | ||||
| V = 1028.0(4) |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, P.B. Octahedral tetramer in the crystal structure of leucophosphite, K2[Fe3+4(OH)2(H2O)2(PO4)4]·2H2O. Am. Miner. 1972, 57, 397–410. [Google Scholar]
- Wilson, M.J.; Bain, D.C. Sphenscidite, a new phosphate mineral from Elephant Island, British Antarctic Territory. Miner. Mag. 1986, 50, 291–293. [Google Scholar] [CrossRef] [Green Version]
- Chukanov, N.V.; Möhn, G.; Pekov, I.V.; Zubkova, N.V.; Ksenofontov, D.A.; Belakovskiy, D.I.; Vozchikova, S.A.; Britvin, S.N.; Desor, J. Ammoniotinsleyite, (NH4)Al2(PO4)2(OH)·2H2O, a new mineral species from guano deposit at Pabellón de Pica, Iquique Province, Chile. Miner. Mag. 2020, 84, 705–711. [Google Scholar] [CrossRef]
- Dunn, P.J.; Rouse, R.C.; Campbell, T.J.; Roberts, W.L. Tinsleyite, the aluminum analogue of leucophosphite, from the Tip Top pegmatite in South Dakota. Am. Miner. 1984, 69, 374–376. [Google Scholar]
- Dick, S. Ueber die Struktur von synthetischem Tinsleyit K(Al2(PO4)2(OH)(H2O))·(H2O). Z. Nat. Teil B. Anorg. Chem. Organ. Chem. 1999, 54, 1385–1390. [Google Scholar]
- Yakubovich, O.V.; Massa, W.; Dimitrova, O.V. A novel potassium-rich variant of tinsleyite, |K1.5(H2O)0.5 |[Al2(OH){(OH)0.5(H2O)0.5}(PO4)2]. Can. Miner. 2012, 50, 559–569. [Google Scholar] [CrossRef]
- Parise, J.B. Preparation and structure of the aluminium ammonium phosphate dihydrate Al2(NH4)(OH)(PO4)2·2H2O: A tunnel structure with ammonium ions in the channels. Acta Crystallogr. 1984, C40, 1641–1643. [Google Scholar]
- Pluth, J.J.; Smith, J.V.; Bennett, J.M.; Cohen, J.P. Structure of NH4Al2(OH)(H2O)(PO4)2·H2O, the ammonium aluminum analog of GaPO4·2H2O and leucophosphite. Acta Crystallogr. 1984, C40, 2008–2011. [Google Scholar]
- Aubert, E.; Porcher, F.; Souhassou, M.; Lecomte, C. Characterization of intra-framework and guest/host interactions in the AlPO4–15 molecular sieve by charge-density analysis. Acta Crystallogr. 2003, B59, 687–700. [Google Scholar] [CrossRef] [Green Version]
- Byrne, P.J.; Warren, J.E.; Morris, R.E.; Ashbrook, S.E. Structure and NMR assignment in AlPO4–15: A combined study by diffraction, MAS NMR and first-principles calculations. Solid State Sci. 2009, 11, 1001–1006. [Google Scholar] [CrossRef]
- Vaughan, D.E.W.; Yennawar, H.; Perrotta, A. Synthesis and structure of a 3D aluminophosphate (PSU-3). Micropor. Mesopor. Mater. 2012, 153, 18–23. [Google Scholar] [CrossRef]
- Dick, S.; Zeiske, T. Leucophosphite K[Fe2(PO4)2(OH)(H2O)]·H2O: Hydrogen bonding and structural relationships. J. Solid State Chem. 1997, 133, 508–515. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Dadashov, M.S. Synthesis and crystal structure of a ammonium analogue of the leucophosphite, NH4{Fe2[PO4]2(OH)(H2O)}·H2O. Sov. Phys. Crystallogr. 1992, 37, 757–760. [Google Scholar]
- Yakubovich, O.V.; Khasanova, N.R.; Antipov, E.V. Mineral-inspired materials: Synthetic phosphate analogues for battery applications. Minerals 2020, 10, 524. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, 41, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Strunz, H.; Nickel, E.H. Strunz Mineralogical Tables; E. Schweizerbart’sche: Stuttgart, Germany, 2001. [Google Scholar]
- Onac, P.B.; Effenberger, H.S. Re-examination of berlinite (AlPO4) from the Cioclovina Cave, Romania. Amer. Miner. 2007, 92, 1998–2005. [Google Scholar] [CrossRef]
- Gatehouse, B.M.; Miskin, B.K. The crystal structure of brazilianite, NaAI3(PO4)2(OH)4. Acta Cryst. 1974, B30, 1311–1317. [Google Scholar] [CrossRef]
- Gatta, G.D.; Vignola, P.; Meven, M.; Rinaldi, R. Neutron diffraction in gemology: Single-crystal diffraction study of brazilianite, NaAl3(PO4)2(OH)4. Amer. Miner. 2013, 98, 1624–1630. [Google Scholar] [CrossRef] [Green Version]
- Fanfani, L.; Nunzi, A.; Zanazzi, P.P. The crystal structure of wardite. Miner. Mag. 1970, 37, 598–605. [Google Scholar] [CrossRef]
- Gatta, G.D.; Guastoni, A.; Fabelo, O.; Fernandez-Diaz, M.T. A single-crystal neutron diffraction study of wardite, NaAl3(PO4)2(OH)4·2H2O. Phys. Chem. Miner. 2019, 46, 427–435. [Google Scholar] [CrossRef]
- Lindsay, W.; Vlek, P.P.; Chien, S.; Dixon, J.B. Minerals in Soil Environments; Dixon, J.B., Weed, S.B., Eds.; SSSA Book Series No. I: Madison, WI, USA, 1989; p. 1089. [Google Scholar]
- Dick, S.; Goẞner, U.; Weiẞ, A.; Robl, C.; Groẞmann, G.; Ohms, G.; Zeiske, T. Taranakite—The mineral with the longest crystallographic axis. Inorg. Chim. Acta. 1998, 269, 47–57. [Google Scholar] [CrossRef]
- Dick, S.; Zeiske, T. Francoanellit K3A15HPO4)6(PO4)2·12H2O: Struktur und Synthese durch topochemische Entwässerung von Taranakit. Z. Nat. 1998, 53, 711–719. [Google Scholar]
- Yakubovich, O.V.; Kiriukhina, G.V.; Volkov, A.S.; Dimitrova, O.V.; Borovikova, E.Y. Novel aluminophosphate Na6[Al3P5O20] with the original microporous crystal structure established in the study of a pseudomerohedric microtwin. Acta Cryst. 2021, 77, 232–240. [Google Scholar] [CrossRef]
- Duan, M.; Kong, B.; Li, R. Molten salt synthesis of an open-frame aluminum phosphate Ba3Al2P4O16 with a rare-pyramidal AlO5 group. Inorg. Chem. 2020, 59, 12978–12982. [Google Scholar] [CrossRef] [PubMed]
- Bouchevreau, B.; Charlotte Martineau, C.; Mellot-Draznieks, C.; Dutour, J.; Tuel, A.; Suchomel, M.R.; Trébosc, J.; Lafon, O.; Amoureux, J.-P.; Taulelle, F. High-resolution structural characterization of two layered aluminophosphates by synchrotron powder diffraction and NMR crystallographies. Chem. Mater. 2013, 25, 2227–2242. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Dimitrova, O.V.; Urusuv, V.S. Crystal structure of a new microporous Na2{Al3(OH)2[PO4]3} aluminum phosphate. Dokl. Phys. 2003, 48, 209–215. [Google Scholar] [CrossRef]
- Haseman, J.F.; Lehr, J.R.; Smith, J.P. Mineralogical character of some iron and aluminum phosphates containing potassium and ammonium. Soil Sci. Soc. Am. Proc. 1950, 15, 76–84. [Google Scholar]
- Dick, S.; Großmann, G.; Ohms, G.; Zeiske, T. Aluminiumphosphate mit nichtzentrosymmetrischen Schicht- und Raumnetzstrukturen aus topologisch verwandten Motiven: 1. KA12(PO4)2(OH)·4H2O. Z. Nat. 1997, 52, 1439–1446. [Google Scholar]
- Kampf, A.R. Minyulite: Its atomic arrangement. Amer. Miner. 1977, 62, 256–262. [Google Scholar]





| Crystal Data | |
|---|---|
| Absorption µ (mm−1) | 0.933 |
| Space group, Z | P21/c, 4 |
| a, b, c (Å) | 9.9927(3), 8.8811(2), 9.7005(3) |
| β (o) | 116.155(4) |
| V (Å3) | 772.73(4) |
| Dcalc (g/cm3) | 2.629 |
| Crystal size (mm) | 0.046 × 0.147 × 0.262 |
| Data Collection | |
| Diffractometer | Oxford Diffraction Gemini, CCD detector |
| Radiation | Mo Kα (λ = 0.71073 Å) |
| Temperature (K) | 150(2) |
| Scanning mode | Omega scans |
| Measuring range, Θ (o) | 2.271–29.996 |
| Reflections (total) | 24749 |
| Rint | 0.0365 |
| Rσ | 0.0162 |
| Refinement | |
| Reflections unique | 2254 |
| Reflections observed [I > 2σ(I)] | 2063 |
| Parameters | 147 |
| Absorption correction | Numerical |
| Tmax, Tmin | 0.958,0.792 |
| Residuals | |
| R (observed reflections) | 0.0221 |
| R, wR (all reflections) | 0.0253, 0.0630 |
| S | 1.177 |
| Δρ (max)/(min) (e/Å3) | 0.495/−0.373 |
| P1—Tetrahedron | Al1—Bipyramid | Na1—Octahedron | |||||
|---|---|---|---|---|---|---|---|
| P1—O2 | 1.5289(11) | Al1—O1 | 1.7744(11) | Na1—O8 | 2.190(2) | Na1—O5 | 2.448(3) |
| O4 | 1.5291(11) | O9 | 1.7985(11) | O3 | 2.316(1) | O9 | 2.996(3) |
| O3 | 1.5402(11) | O5 | 1.8313(11) | O4 | 2.366(1) | O2 | 3.033(3) |
| O5 | 1.5466(11) | O1’ | 1.8798(11) | <Na1—O> 2.556 | |||
| O4 | 1.8980(11) | ||||||
| <P1—O> | 1.536 | <Al1—O> | 1.836 | Na1’—octahedron | |||
| Na1′—O8 | 2.18(2) | Na1′—O2 | 2.69(3) | ||||
| P2—tetrahedron | Al2—bipyramid | O3 | 2.30(1) | O5 | 2.85(3) | ||
| P2—O8 | 1.5050(11) | Al2—O7 | 1.8158(11) | O4 | 2.36(2) | O7 | 3.09(3) |
| O6 | 1.5206(11) | O3 | 1.8248(11) | <Na1′—O> 2.58 | |||
| O7 | 1.5465(11) | O1 | 1.8277(11) | ||||
| O9 | 1.5543(11) | O2 | 1.8426(11) | Na2—eight-vertex polyhedron | |||
| O6 | 1.8438(11) | Na2—O9 | 2.309(1) | Na2—O10 * | 2.689(1) | ||
| <P2—O> | 1.532 | <Al2—O> | 1.831 | O7 | 2.337(10 | O4 | 2.693(1) |
| O6 | 2.470(1) | O8′ | 2.935(1) | ||||
| O8 | 2.500(1) | O8″ | 3.086(1) | ||||
| <Na2—O> 2.627 | |||||||
| Atom | P1 | P2 | Al1 | Al2 | Na1 | Na2 | Ʃ |
|---|---|---|---|---|---|---|---|
| O1 | 0.716; 0.539 | 0.620 | 1.88 | ||||
| O2 | 1.269 | 0.596 | 0.036 | 1.90 | |||
| O3 | 1.231 | 0.625 | 0.250 | 2.11 | |||
| O4 | 1.268 | 0.513 | 0.218 | 0.090 | 2.09 | ||
| O5 | 1.210 | 0.614 | 0.175 | 2.00 | |||
| O6 | 1.298 | 0.594 | 0.165 | 2.06 | |||
| O7 | 1.210 | 0.640 | 0.236 | 2.09 | |||
| O8 | 1.354 | 0.352 | 0.152; 0.047; 0.036 | 1.94 | |||
| O9 | 1.185 | 0.671 | 0.040 | 0.255 | 2.15 | ||
| Ʃ | 4.98 | 5.05 | 3.05 | 3.07 | 1.07 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakubovich, O.; Kiriukhina, G.; Verchenko, P.; Simonov, S.; Volkov, A.; Dimitrova, O. Na-Alternative to Tinsleyite Obtained under Hydrothermal Conditions: Crystal Structure and Comparative Crystal Chemistry. Minerals 2022, 12, 542. https://doi.org/10.3390/min12050542
Yakubovich O, Kiriukhina G, Verchenko P, Simonov S, Volkov A, Dimitrova O. Na-Alternative to Tinsleyite Obtained under Hydrothermal Conditions: Crystal Structure and Comparative Crystal Chemistry. Minerals. 2022; 12(5):542. https://doi.org/10.3390/min12050542
Chicago/Turabian StyleYakubovich, Olga, Galina Kiriukhina, Polina Verchenko, Sergey Simonov, Anatoly Volkov, and Olga Dimitrova. 2022. "Na-Alternative to Tinsleyite Obtained under Hydrothermal Conditions: Crystal Structure and Comparative Crystal Chemistry" Minerals 12, no. 5: 542. https://doi.org/10.3390/min12050542
APA StyleYakubovich, O., Kiriukhina, G., Verchenko, P., Simonov, S., Volkov, A., & Dimitrova, O. (2022). Na-Alternative to Tinsleyite Obtained under Hydrothermal Conditions: Crystal Structure and Comparative Crystal Chemistry. Minerals, 12(5), 542. https://doi.org/10.3390/min12050542







