Geological, Mineralogical and Geochemical Study of the Aquamarine-Bearing Yamrang Pegmatite, Eastern Nepal with Implications for Exploration Targeting
Abstract
:1. Introduction
2. Regional Geological Setting
2.1. Geology
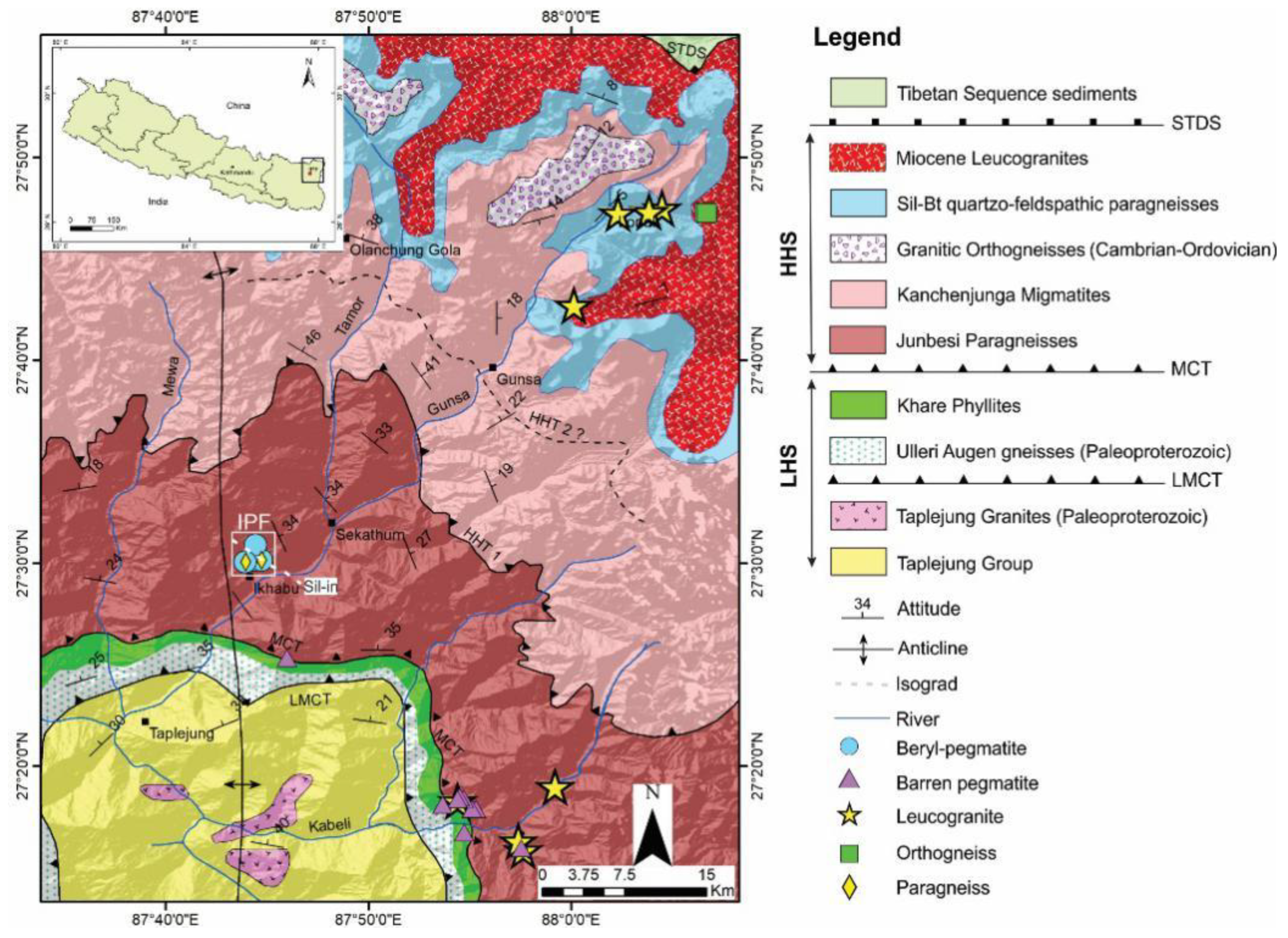
2.2. Granitic Plutons
3. Geology of Yamrang Pegmatite
4. Analytical Methods
4.1. Whole-Rock Major and Trace Element Analysis
4.2. Electron Microprobe Analysis
4.3. Laser Ablation Inductively Coupled Plasma Mass Spectrometry
5. Results
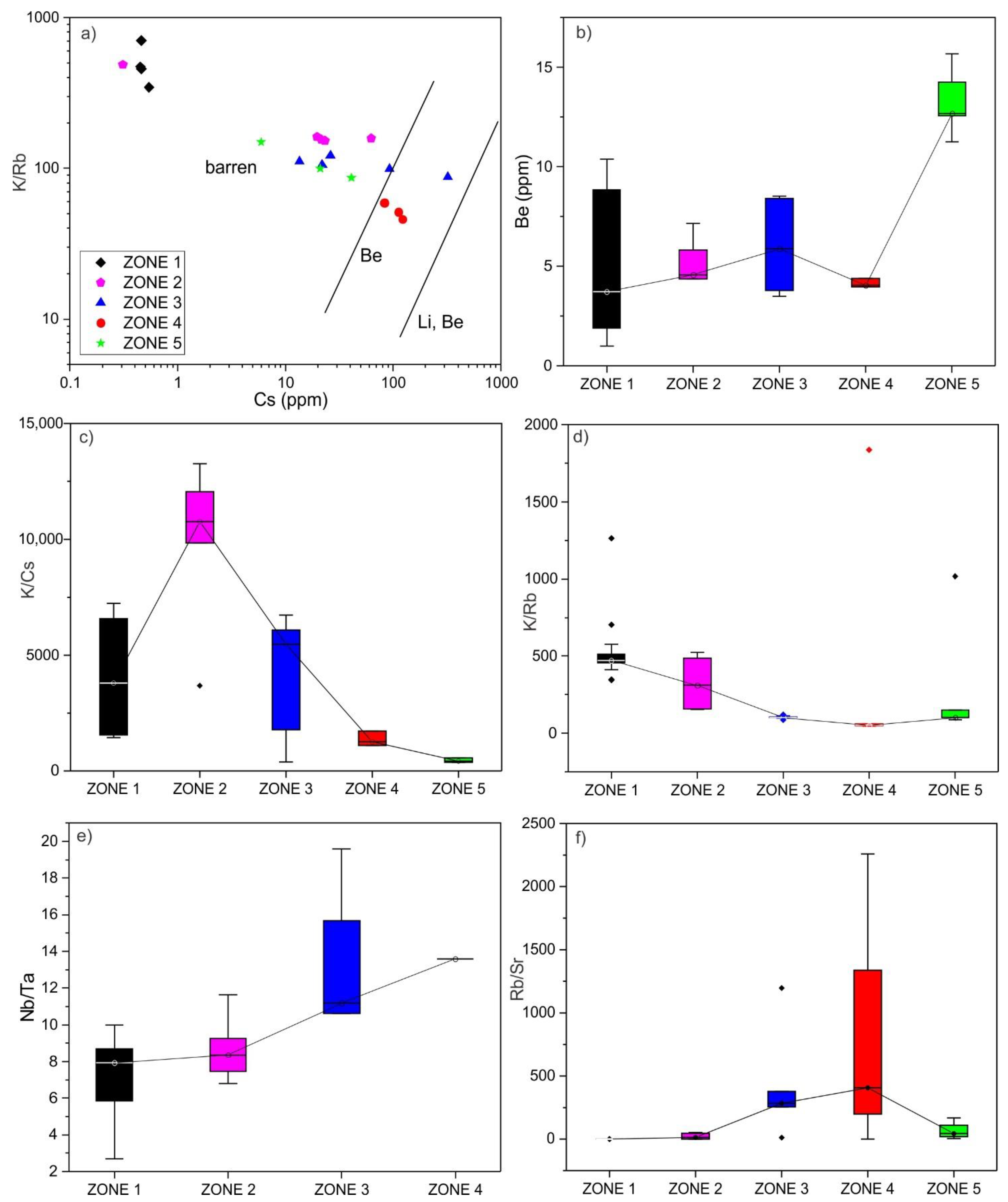
5.1. Whole-Rock Chemistry
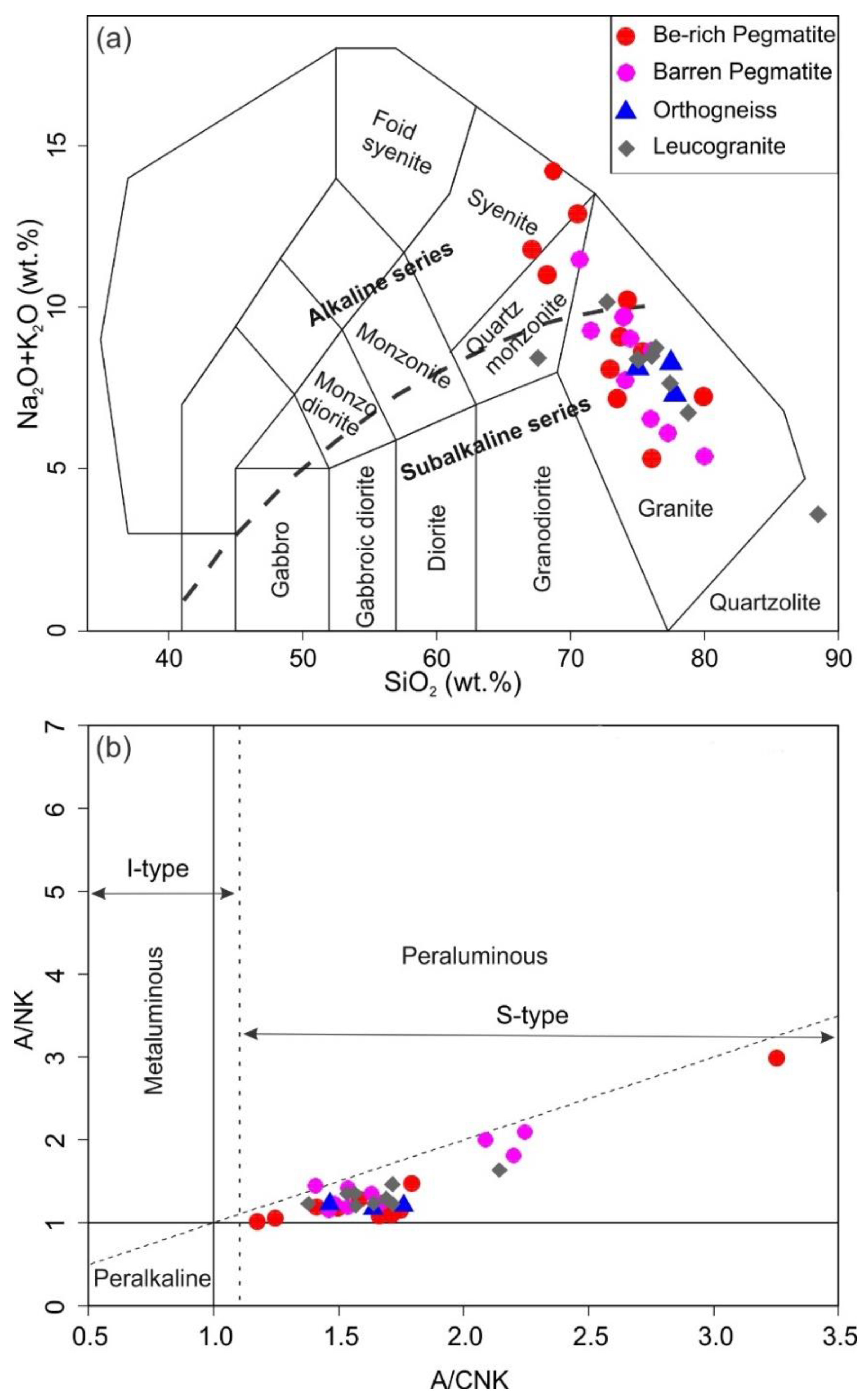
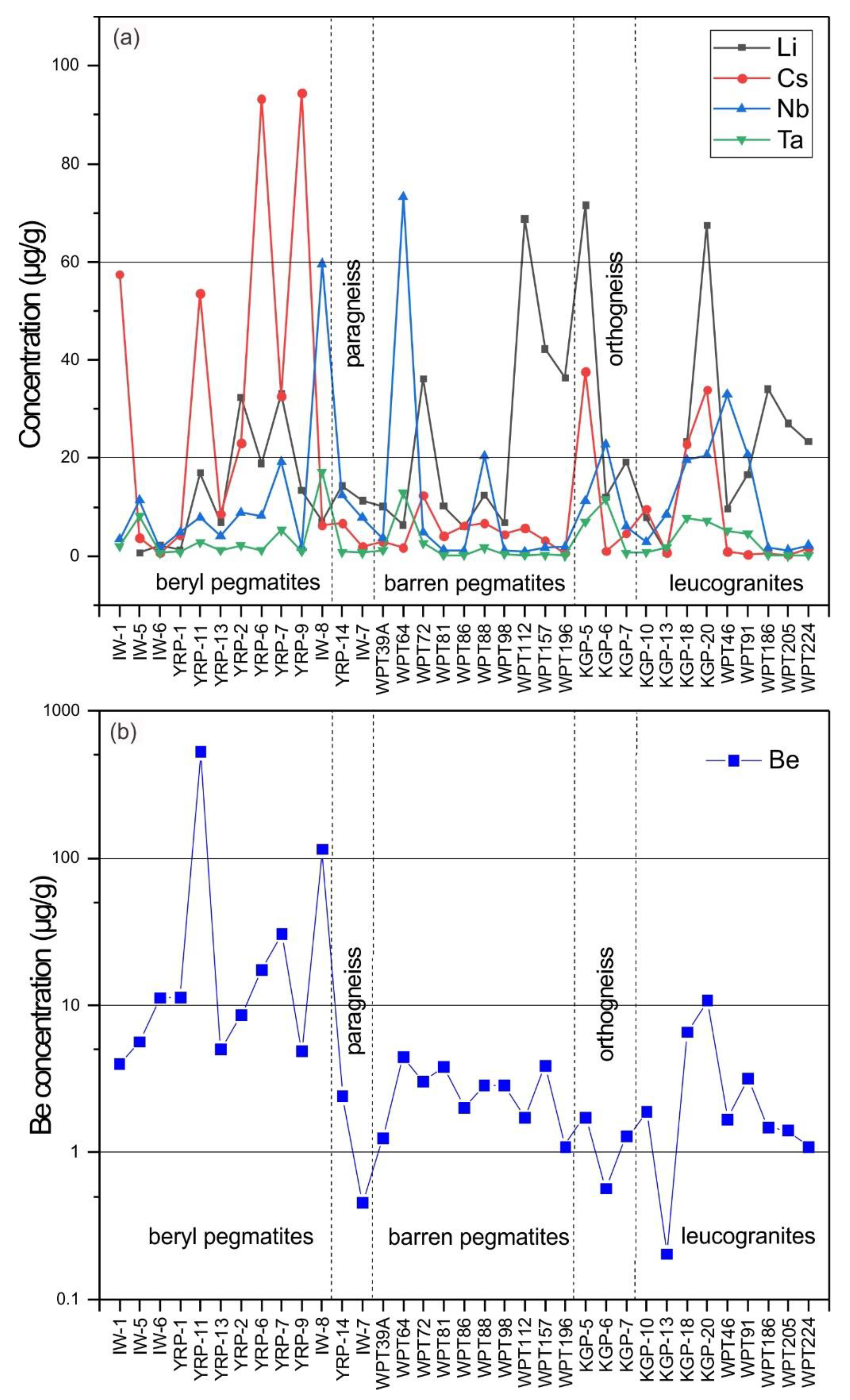
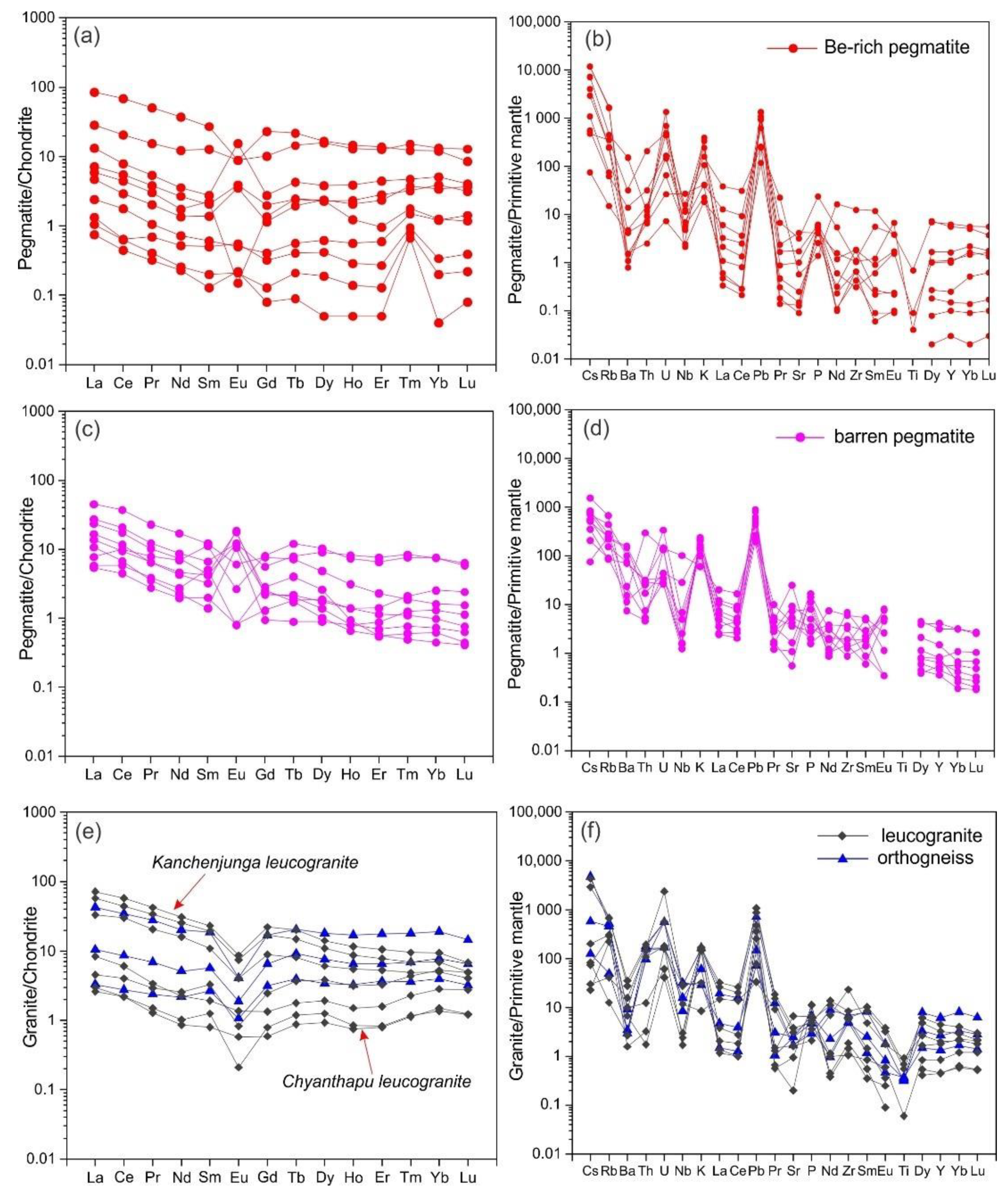
5.2. Feldspar Chemistry
5.3. Muscovite Chemistry
6. Discussion
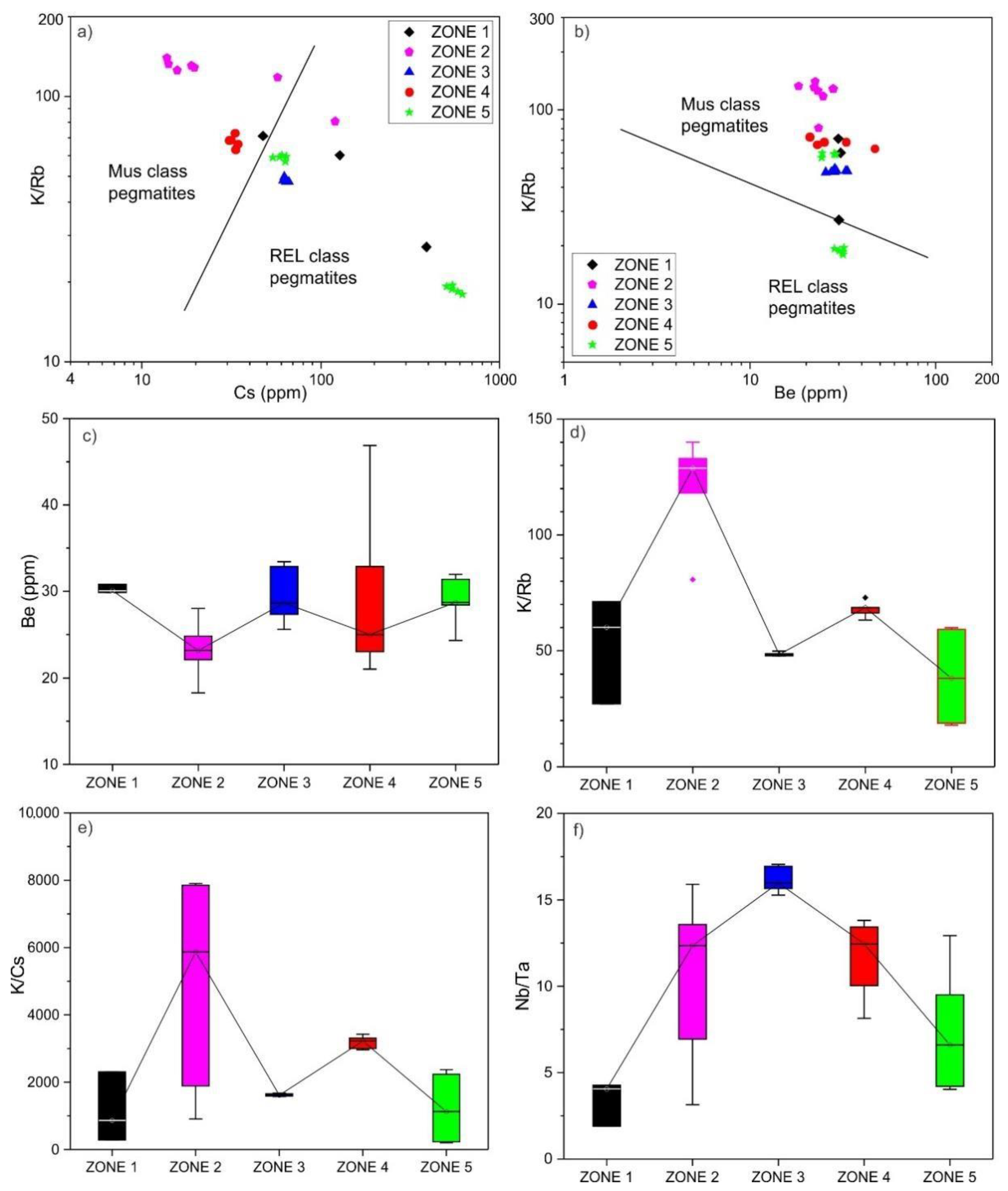
6.1. Classification of the Yamrang Pegmatite
6.2. Beryl Saturation Process in Yamrang Pegmatite
6.2.1. Magmatic Stage
6.2.2. Hydrothermal Stage
6.3. Exploration Implications
7. Conclusions
- The Yamrang Pegmatite can be classified as an intermediate-fractionated REL class, beryl type, beryl-columbite subtype pegmatite of the LCT family.
- High temperature, low fractionation, dominance of Be-compatible mineral phases such as muscovite, calcium-rich alkali feldspar, and tourmaline resulted in beryl undersaturated marginal zones. In contrast, low temperature, high fractional crystallization, and low abundance of Be-compatible mineral phases resulted in beryl saturated inner zones.
- The Be partition sequence among coexisting minerals at the magmatic stage is beryl > muscovite > tourmaline > alkali feldspar > quartz, whereas at the hydrothermal stage, the sequence is beryl > muscovite > albite > tourmaline > quartz.
- A whole rock Be content of >10 ppm may indicate beryl mineralization in the region.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- London, D. A Petrologic Assessment of Internal Zonation in Granitic Pegmatites. Lithos 2014, 184–187, 74–104. [Google Scholar] [CrossRef]
- Černý, P. Rare-Element Granitic Pegmatites. Part I: Anatomy and Internal Evolution of Pegmatite Deposits. Geosci. Can. 1991, 18, 49–67. [Google Scholar]
- Černý, P.; Ercit, T.S. The Classification of Granitic Pegmatites Revisited. Can. Mineral. 2005, 43, 2005–2026. [Google Scholar] [CrossRef] [Green Version]
- Goscombe, B.; Gray, D.; Hand, M. Crustal Architecture of the Himalayan Metamorphic Front in Eastern Nepal. Gondwana Res. 2006, 10, 232–255. [Google Scholar] [CrossRef]
- Imayama, T.; Takeshita, T.; Arita, K. Metamorphic P-T Profile and P-T Path Discontinuity across the Far-Eastern Nepal Himalaya: Investigation of Channel Flow Models. J. Metamorph. Geol. 2010, 28, 527–549. [Google Scholar] [CrossRef]
- Schelling, D. The Tectonostratigraphy and Structure of the Eastern Nepal Himalaya. Tectonics 1992, 11, 925–943. [Google Scholar] [CrossRef]
- Le Fort, P. Himalayas: The Collided Range. Present Knowledge of the Continental Arc. Am. J. Sci. 1975, 275-A, 1–44. [Google Scholar]
- Larson, K.P.; Camacho, A.; Cottle, J.M.; Coutand, I.; Buckingham, H.M.; Ambrose, T.K.; Rai, S.M. Cooling, Exhumation, and Kinematics of the Kanchenjunga Himal, Far East Nepal. Tectonics 2017, 36, 1037–1052. [Google Scholar] [CrossRef]
- Imayama, T.; Takeshita, T.; Yi, K.; Cho, D.-L.; Kitajima, K.; Tsutsumi, Y.; Kayama, M.; Nishido, H.; Okumura, T.; Yagi, K.; et al. Two-Stage Partial Melting and Contrasting Cooling History within the Higher Himalayan Crystalline Sequence in the Far-Eastern Nepal Himalaya. Lithos 2012, 134–135, 1–22. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for Names of Rock-Forming Minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- DeCelles, P.G.; Gehrels, G.E.; Quade, J.; LaReau, B.; Spurlin, M. Tectonic Implications of U-Pb Zircon Ages of the Himalayan Orogenic Belt in Nepal. Science 2000, 288, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Hodges, K.V. Tectonics of the Himalaya and Southern Tibet from Two Perspectives. GSA Bull. 2000, 112, 324–350. [Google Scholar] [CrossRef]
- Parrish, R.R.; Hodges, V. Isotopic Constraints on the Age and Provenance of the Lesser and Greater Himalayan Sequences, Nepalese Himalaya. Geol. Soc. Am. Bull. 1996, 108, 904–911. [Google Scholar] [CrossRef]
- Harrison, M.T.; Grove, M.; Mckeegan, K.D.; Coath, C.D.; Lovera, O.M.; Fort, P.L. Origin and Episodic Emplacement of the Manaslu Intrusive Complex, Central Himalaya. J. Petrol. 1999, 40, 3–19. [Google Scholar] [CrossRef]
- Searle, M.P.; Simpson, R.L.; Law, R.D.; Parrish, R.R.; Waters, D.J. The Structural Geometry, Metamorphic and Magmatic Evolution of the Everest Massif, High Himalaya of Nepal-South Tibet. J. Geol. Soc. London. 2003, 160, 345–366. [Google Scholar] [CrossRef] [Green Version]
- Simpson, R.L.; Parrish, R.R.; Searle, M.P.; Waters, D.J. Two Episodes of Monazite Crystallization during Metamorphism and Crustal Melting in the Everest Region of the Nepalese Himalaya. Geology 2000, 28, 403. [Google Scholar] [CrossRef]
- Searle, M.P.; Szulc, A.G. Channel Flow and Ductile Extrusion of the High Himalayan Slab-the Kangchenjunga-Darjeeling Profile, Sikkim Himalaya. J. Asian Earth Sci. 2005, 25, 173–185. [Google Scholar] [CrossRef]
- Liu, X.C.; Wu, F.Y.; Yu, L.J.; Liu, Z.C.; Ji, W.Q.; Wang, J.G. Emplacement Age of Leucogranite in the Kampa Dome, Southern Tibet. Tectonophysics 2016, 667, 163–175. [Google Scholar] [CrossRef]
- Liu, Z.C.; Wu, F.Y.; Ji, W.Q.; Wang, J.G.; Liu, C.Z. Petrogenesis of the Ramba Leucogranite in the Tethyan Himalaya and Constraints on the Channel Flow Model. Lithos 2014, 208, 118–136. [Google Scholar] [CrossRef]
- Wu, F.; Liu, X.-C.; Liu, Z.-C.; Wang, R.-C.; Xie, L.; Wang, J.; Ji, W.; Yang, L.; Liu, C.; Khanal, G.P.; et al. Highly Fractionated Himalayan Leucogranites and Associated Rare-Metal Mineralization. Lithos 2020, 352–353, 105319. [Google Scholar] [CrossRef]
- Visona, D.; Carosi, R.; Montomoli, C.; Tiepolo, M.; Peruzzo, L. Lithos Miocene Andalusite Leucogranite in Central-East Himalaya (Everest–Masang Kang Area): Low-Pressure Melting during Heating. Lithos 2012, 144–145, 194–208. [Google Scholar] [CrossRef]
- Le Fort, P. Metamorphism and Magmatism during the Himalayan Collision. Geol. Soc. Lond. Spec. Publ. 1986, 19, 159–172. [Google Scholar] [CrossRef]
- Smith, C.P.; Gübelin, E.J.; Bassett, A.M.; Manandhar, M.N. Rubies and Fancy-Color Sapphires from Nepal. Gems Gemol. 1997, 33, 24–41. [Google Scholar] [CrossRef]
- Dill, H.G. Pegmatites and Aplites: Their Genetic and Applied Ore Geology. Ore Geol. Rev. 2015, 69, 417–561. [Google Scholar] [CrossRef]
- ESCAP. Atlas of Mineral Resources of the ESCAP Region. Geology and Mineral Resources of Nepal. Explanatory Brochure. V. 9; United Nations: New York, NY, USA, 1993; Volume 9.
- DMG. Mineral Resources of Nepal, 2nd ed.; Department of Mines and Geology: Kathmandu, Nepal, 2017.
- Li, X.; Liu, Y.; Tu, X.; Hu, G.; Zeng, W. Precise Determination of Chemical Compositions in Silicate Rocks Using ICP-AES and ICP-MS: A Comparative Study of Sample Digestion Techniques of Alkali Fusion and Acid Dissolution. Geochimica 2002, 31, 289–294. [Google Scholar] [CrossRef]
- Monier, G.; Robert, J.L. Muscovite Solid Solutions in the System K2O-MgO-FeO-Al2O3-SiO2-H2O: An Experimental Study at 2 Kbar PH2O and Comparison with Natural Li-Free White Micas. Mineral. Mag. 1986, 50, 257–266. [Google Scholar] [CrossRef]
- Tindle, A.G.; Webb, P.C. Estimation of Lithium Contents in Trioctahedral Micas Using Microprobe Data: Application to Micas from Granitic Rocks. Eur. J. Mineral. 1990, 2, 595–610. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, Y.; Zhang, H.; Sun, J.; Wu, F. In Situ Simultaneous Determination of Trace Elements, U-Pb and Lu-Hf Isotopes in Zircon and Baddeleyite. Chin. Sci. Bull. 2008, 53, 1565–1573. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Karius, V.; Schmidt, B.C.; Simon, K.; Wörner, G. Comparison of Ultrafine Powder Pellet and Flux-Free Fusion Glass for Bulk Analysis of Granitoids by Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry. Geostand. Geoanalytical Res. 2018, 42, 575–591. [Google Scholar] [CrossRef]
- Wu, S.; Wörner, G.; Jochum, K.P.; Stoll, B.; Simon, K.; Kronz, A. The Preparation and Preliminary Characterisation of Three Synthetic Andesite Reference Glass Materials (ARM-1, ARM-2, ARM-3) for In Situ Microanalysis. Geostand. Geoanalytical Res. 2019, 43, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Griffin, W.L. GLITTER: Data Reduction Software for Laser Ablation ICP-MS. Laser Ablation ICP-MS Earth Sci. Curr. Pract. Outst. Issues 2008, 308–311. [Google Scholar]
- Rushmer, T.; Knesel, K. Defining Geochemical Signatures and Timescales of Melting Processes in the Crust: An Experimental Tale of Melt Segregation, Migration and Emplacement. In Timescales of Magmatic Processes; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 181–211. [Google Scholar] [CrossRef]
- Middlemost, E.A.K. Naming Materials in the Magma/Igneous Rock System. Earth-Sci. Rev. 1994, 37, 215–224. [Google Scholar] [CrossRef]
- Maniar, P.D.; Piccoli, P.M. Tectonic Discrimination of Granitoids. GSA Bull. 1989, 101, 635–643. [Google Scholar] [CrossRef]
- Boynton, W.V. Cosmochemistry of the Rare Earth Elements: Meteorite Studies. Dev. Geochem. 1984, 2, 63–114. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.F. Chemical and Isotopic Systematics of Oceanic Basalts: Implications for Mantle Composition and Processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Trueman, D.L.; Černý, P. Exploration for Rare-Element Granitic Pegmatites. In Proceedings of the Short Course in Granitic Pegmatites in Science and Industry; Mineralogical Association of Canada: Quebec, QC, Canada, 1982; pp. 463–493. [Google Scholar]
- Morteani, G.; Preinfalk, C.; Horn, A.H. Classification and Mineralization Potential of the Pegmatites of the Eastern Brazilian Pegmatite Province. Miner. Depos. 2000, 35, 638–655. [Google Scholar] [CrossRef]
- Tischendorf, G.; Gottesmann, B.; Förster, H.-J.; Trumbull, R.B. On Li-Bearing Micas: Estimating Li from Electron Microprobe Analyses and an Improved Diagram for Graphical Representation. Mineral. Mag. 1997, 61, 809–834. [Google Scholar] [CrossRef]
- Miller, C.F.; Stoddard, E.F.; Bradfish, L.J.; Dollase, W.A. Composition of Plutonic Muscovite: Genetic Implications. Can. Mineral. 1981, 19, 25–34. [Google Scholar]
- Cerny, P.; Burt, D.M. Paragenesis, Crystallochemical Characteristics, and Geochemical Evolution of the Micas in Granite Pegmatites. Rev. Mineral. Geochem. 1984, 13, 257–297. [Google Scholar]
- Cameron, E.N.; Jahns, R.H.; Mcnair, A.H.; Page, L.R. Internal Structure of Granitic Pegmatites, No. 2. Econ. Geol. Urbana. Ill. 1949, 2, 115. [Google Scholar]
- Ginsburg, A.I.; Timofeyev, I.N.; Feldman, L.G. Principles of Geology of the Granitic Pegmatites. Nedra. Moscow 1979, 296. [Google Scholar]
- Zagorsky, V.Y.; Makagon, V.M.; Shmakin, B.M. The Systematics of Granitic Pegmatites. Can. Mineral. 1999, 37, 800–802. [Google Scholar]
- Černý, P. Anatomy and Classification of Granitic Pegmatites. In Granitic Pegmatites in Science and Industry; Černý, P., Ed.; Mineralogical Association of Canada: Québec, QC, Canada, 1982; Volume 8, pp. 1–39. [Google Scholar]
- Selway, J.B.; Breaks, F.W.; Tindle, A.G. A Review of Rare-Element (Li-Cs-Ta) Pegmatite Exploration Techniques for the Superior Province, Canada, and Large Worldwide Tantalum Deposits. Explor. Min. Geol. 2005, 14, 1–30. [Google Scholar] [CrossRef]
- Barton, M.D.; Young, S. Non-Pegmatitic Deposits of Beryllium: Mineralogy, Geology, Phase Equilibria and Origin. Rev. Mineral. Geochem. 2002, 50, 591–691. [Google Scholar] [CrossRef]
- London, D. Pegmatites; Martin, R.F., Ed.; Mineralogical Association of Canada: Quebec, QC, Canada, 2008; Volume 10. [Google Scholar]
- London, D. Ore-Forming Processes within Granitic Pegmatites. Ore Geol. Rev. 2018, 101, 349–383. [Google Scholar] [CrossRef]
- Černý, P.; London, D.; Novák, M. Granitic Pegmatites as Reflections of Their Sources. Elements 2012, 8, 289–294. [Google Scholar] [CrossRef]
- Duc-Tin, Q.; Keppler, H. Monazite and Xenotime Solubility in Granitic Melts and the Origin of the Lanthanide Tetrad Effect. Contrib. Mineral. Petrol. 2015, 169, 1–26. [Google Scholar] [CrossRef]
- Gehrels, G.E.; DeCelles, P.G.; Martin, A.; Ojha, T.P.; Pinhassi, G.; Upreti, B.N. Initiation of the Himalayan Orogen as an Early Paleozoic Thin-Skinned Thrust Belt. GSA Today 2003, 13, 4. [Google Scholar] [CrossRef]
- Cawood, P.A.; Johnson, M.R.W.; Nemchin, A.A. Early Palaeozoic Orogenesis along the Indian Margin of Gondwana: Tectonic Response to Gondwana Assembly. Earth Planet. Sci. Lett. 2007, 255, 70–84. [Google Scholar] [CrossRef]
- Hopkinson, T.N.; Harris, N.B.W.; Warren, C.J.; Spencer, C.J.; Roberts, N.M.W.; Horstwood, M.S.A.; Parrish, R.R.; EIMF. The Identification and Significance of Pure Sediment-Derived Granites. Earth Planet. Sci. Lett. 2017, 467, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-G.; Liu, X.-C.; Liu, Z.-C.; Ji, W.-Q.; Wu, F.-Y.; Ding, L. Highly Fractionated Late Eocene (~35 Ma) Leucogranite in the Xiaru Dome, Tethyan Himalaya, South Tibet. Lithos 2015, 240–243, 337–354. [Google Scholar] [CrossRef]
- Liu, C.; Wang, R.-C.; Wu, F.-Y.; Xie, L.; Liu, X.-C.; Li, X.-K.; Yang, L.; Li, X.-J. Spodumene Pegmatites from the Pusila Pluton in the Higher Himalaya, South Tibet: Lithium Mineralization in a Highly Fractionated Leucogranite Batholith. Lithos 2020, 358–359, 105421. [Google Scholar] [CrossRef]
- Qin, K.; Zhao, J.; He, C.; Shi, R. Discovery of the Qongjiagang Giant Lithium Pegmatite Deposit in Himalaya, Tibet, China. Acta Petrol. Sin. 2021, 37, 3277–3286. [Google Scholar] [CrossRef]
- Zhou, Q.; Qin, K.; He, C.; Wu, H.; Liu, Y.; Niu, X.; Mo, L.; Liu, X.; Zhao, J. Li-Be-Nb-Ta Mineralogy of the Kuqu Leucogranite and Pegmatite in the Eastern Himalaya, Tibet, and Its Implication. Acta Petrol. Sin. 2021, 37, 3305–3324. [Google Scholar] [CrossRef]
- London, D.; Evensen, J.M. Beryllium in Silicic Magmas and the Origin of Beryl-Bearing Pegmatites. Rev. Mineral. Geochem. 2002, 50, 445–486. [Google Scholar] [CrossRef]
- Zhao, J.; He, C.; Qin, K.; Shi, R.; Liu, X.; Hu, F.; Yu, K.; Sun, Z. Geochronology, Source Features and the Characteristics of Fractional Crystallization in Pegmatite at the Qongjiagang Giant Pegmatite-Type Lithium Deposit, Himalaya, Tibet. Acta Petrol. Sin. 2021, 37, 3325–3347. [Google Scholar] [CrossRef]
- Müller, A.; Keyser, W.; Simmons, W.B.; Webber, K.; Wise, M.; Beurlen, H.; Garate-Olave, I.; Roda-Robles, E.; Galliski, M.Á. Quartz Chemistry of Granitic Pegmatites: Implications for Classification, Genesis and Exploration. Chem. Geol. 2021, 584, 120507. [Google Scholar] [CrossRef]
- Breiter, K.; Ďurišová, J.; Dosbaba, M. Chemical Signature of Quartz from S- and A-Type Rare-Metal Granites – A Summary. Ore Geol. Rev. 2020, 125, 103674. [Google Scholar] [CrossRef]
- Evensen, J.M.; London, D. Experimental Silicate Mineral/Melt Partition Coefficients for Beryllium and the Crustal Be Cycle from Migmatite to Pegmatite. Geochim. Cosmochim. Acta 2002, 66, 2239–2265. [Google Scholar] [CrossRef]
- Evensen, J.M.; London, D.; Wendlandt, R.F. Solubility and Stability of Beryl in Granitic Melts. Am. Mineral. 1999, 84, 733–745. [Google Scholar] [CrossRef]
- Hezel, D.C.; Angelika, K.; Marschall, H.R.; Ludwig, T.; Meyer, H.P. Major-Element and Li, Be Compositional Evolution of Tourmaline in an S-Type Granite-Pegmatite System and Its Country Rocks: An Example from Ikaria, Aegean Sea, Greece. Can. Mineral. 2011, 49, 321–340. [Google Scholar] [CrossRef]
- Simmons, W.B.; Pezzotta, F.; Shigley, J.E.; Beurlen, H. Granitic Pegmatites as Sources of Colored Gemstones. Elements 2012, 8, 281–287. [Google Scholar] [CrossRef]
- Jahns, R.H.; Burnham, C.W. Experimental Studies of Pegmatite Genesis; l, A Model for the Derivation and Crystallization of Granitic Pegmatites. Econ. Geol. 1969, 64, 843–864. [Google Scholar] [CrossRef]
- London, D.; Hervig, R.L.; Morgan, G.B. Melt-Vapor Solubilities and Elemental Partitioning in Peraluminous Granite-Pegmatite Systems: Experimental Results with Macusani Glass at 200 MPa. Contrib. Mineral. Petrol. 1988, 99, 360–373. [Google Scholar] [CrossRef]
- Cerny, P.; Meintzer, R.E.; Anderson, A.J. Extreme Fractionation in Rare-Element Granitic Pegmatites: Selected Examples of Data and Mechanisms. Can. Mineralogist. 1985, 23, 381–421. [Google Scholar]
- Zhou, Q.; Qin, K.; Tang, D.; Ding, J.; Guo, Z. Mineralogy and Significance of Micas and Feldspars from the Koktokay No. 3 Pegmatitic Rare-Element Deposit, Altai. Acta Petrol. Sin. 2013, 29, 3004–3022. [Google Scholar]
- Michallik, R.M.; Wagner, T.; Fusswinkel, T.; Heinonen, J.S.; Heikkilä, P. Chemical Evolution and Origin of the Luumäki Gem Beryl Pegmatite: Constraints from Mineral Trace Element Chemistry and Fractionation Modeling. Lithos 2017, 274–275, 147–168. [Google Scholar] [CrossRef]
- Küster, D.; Romer, R.L.; Tolessa, D.; Zerihun, D.; Bheemalingeswara, K.; Melcher, F.; Oberthür, T. The Kenticha Rare-Element Pegmatite, Ethiopia: Internal Differentiation, U–Pb Age and Ta Mineralization. Miner. Depos. 2009, 44, 723–750. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Chen, Z.; Liu, X.; Huang, Z.; Zhou, F. Compositional Evolution of the Muscovite of Renli Pegmatite-Type Rare-Metal Deposit, Northeast Hunan, China: Implications for Its Petrogenesis and Mineralization Potential. Ore Geol. Rev. 2021, 138, 104380. [Google Scholar] [CrossRef]

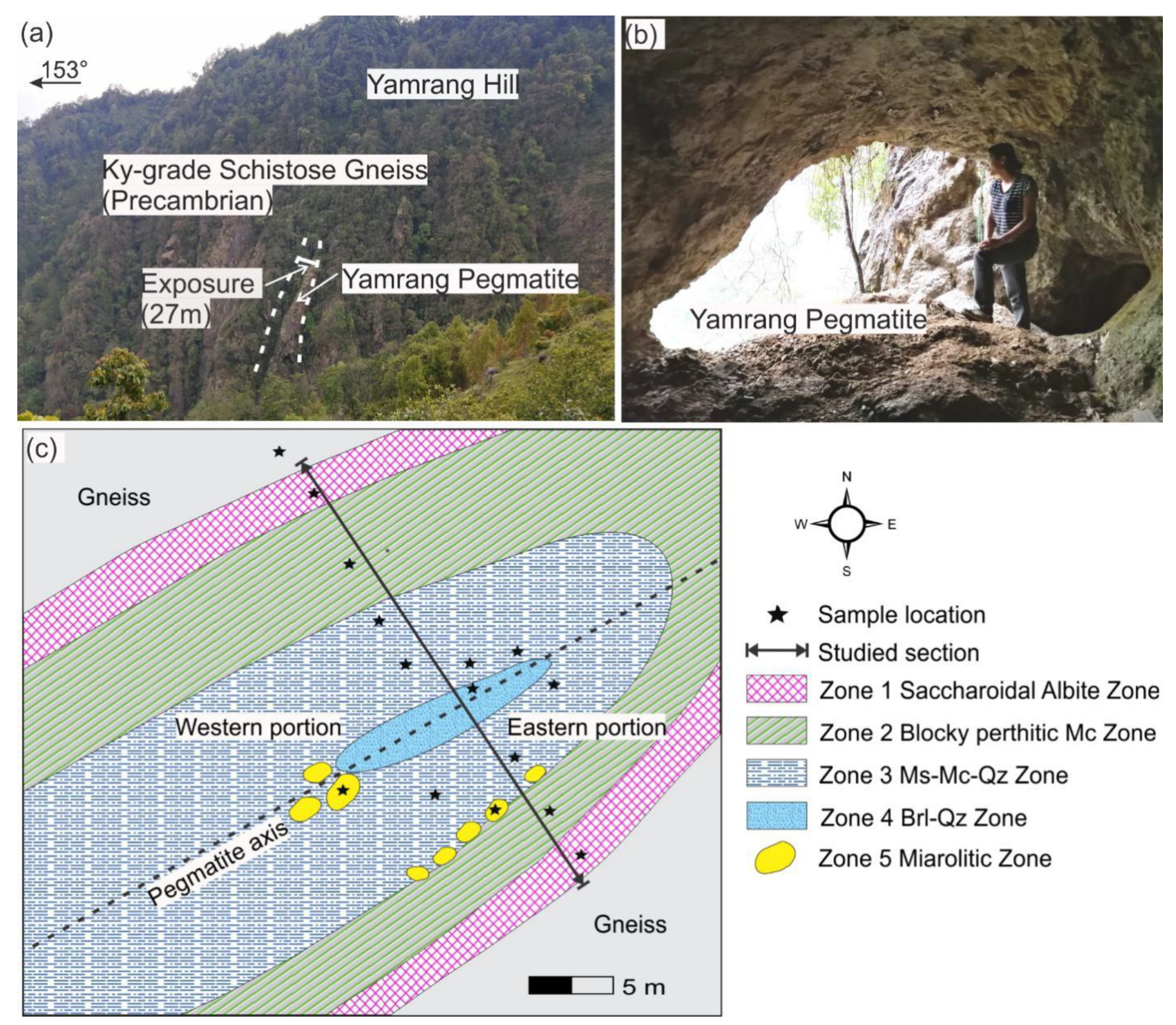

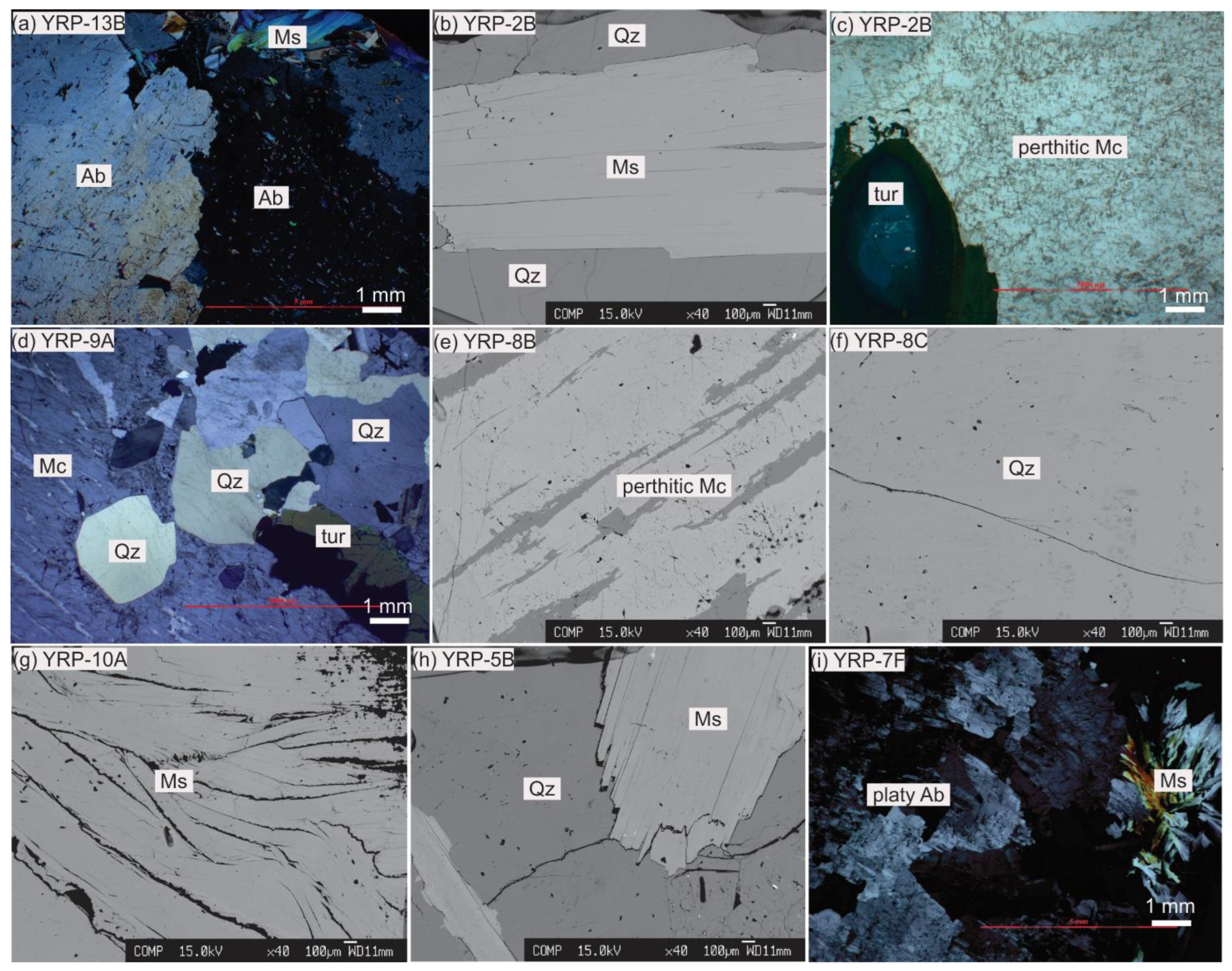


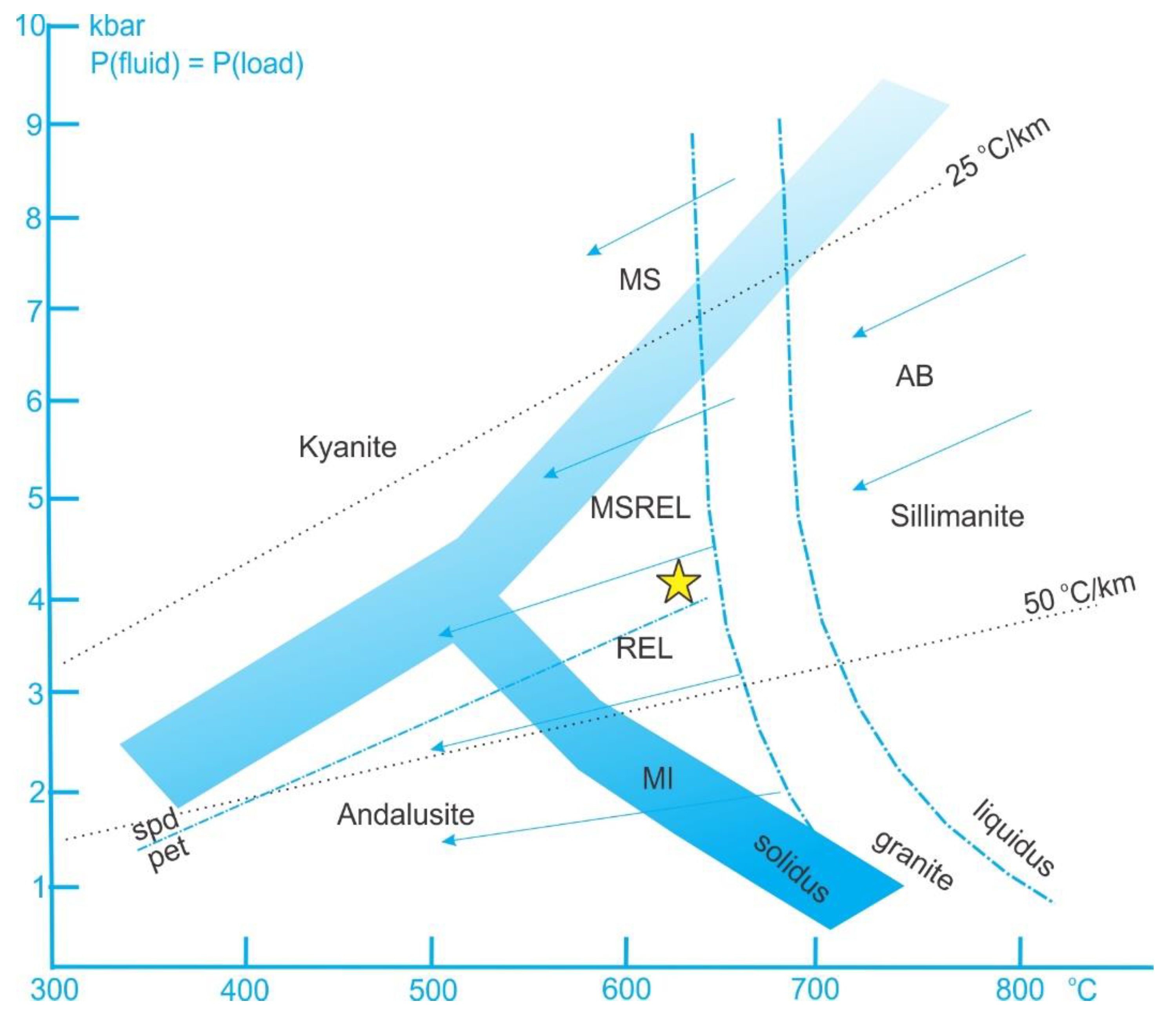
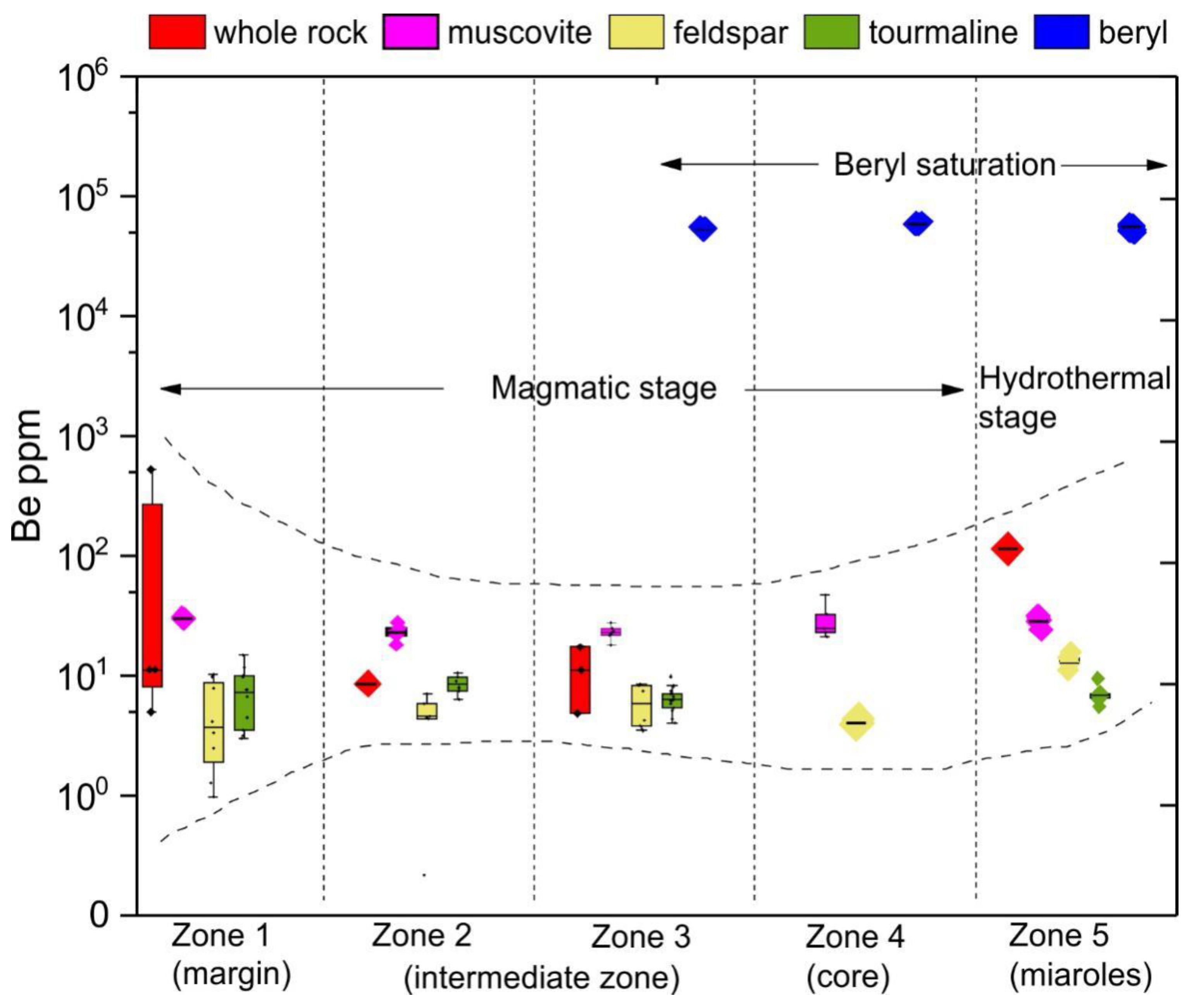
| Zone Number | Zone Name (Sequential) | Zone Name (Mineralogical) | Thickness | Main Mineral Assemblage (Relative Abundances wt.%) | Accessory and (Rare) Minerals | Descriptive Features | Texture |
|---|---|---|---|---|---|---|---|
| Zone 1 | Marginal Zone | Saccharoidal albite | 2 m | Ab (56), Mc (8), Qz (30), Ms (4) | Drv-Srl, (Fl, Zrn, Mnz) | saccharoidal albite, inwardly oriented tourmaline layers | Fine to medium-grained |
| Zone 2 | Outer Intermediate Zone | Blocky perthitic microcline | 4–7 m | Ab (10), Mc (70), Qz (15), Ms (3) | Sps, Srl-Drv, (Zrn, Mnz) | Massive microcline, graphic pegmatite, platy albite, quartz lenses, muscovite clusters | monomineralic, massive |
| Zone 3 | Inner Intermediate Zone | Muscovite-microcline-quartz | 5–6 m | Ab (15), Mc (40), Qz (25), Ms (15) | Brl, Sps, Rt, Srl, (Col-Tan, Zrn, Mnz, Xtm) | coarse graphic pegmatite, platy albite, book muscovite veins, quartz-beryl pods, quartz-muscovite nests | coarse-grained to massive |
| Zone 4 | Core Zone | Beryl-quartz | 1–1.5 m | Mc (5), Qz (60), Ms (1), Brl (33) | Srl, (Zrn, Mnz) | quartz-beryl 90%, microcline patches at core margin | medium to coarse-grained |
| Zone 5 | Miarolitic Zone | Albite-quartz | few to tens of cm | Ab (40), Mc (15), Qz (25), Ms (15) | Brl, Aqm, Col-Tan, Rt, Srl, Hem, (Zrn, Mnz, Xtm) | clay filled pockets, platy albite, dipyramidal smoky quartz, aquamarine, muscovite books, overprinted on zone 2 and 3, | fine to coarse-grained |
| Lithology | Beryl Pegmatite | Paragneiss | Barren Pegmatite | Leucogranite | Orthogneiss | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Zone 1 | Zone 2 | Zone 3 | Zone 5 | WP | SP | YRP-14 | IW-7 | WPT-88 | WPT-112 | KGP-20 | WPT-205 | KGP-6 |
| wt.% | |||||||||||||
| SiO2 | 73.15 | 79.16 | 70.22 | 75.10 | 73.24 | 72.36 | 71.83 | 81.73 | 78.28 | 73.39 | 73.11 | 75.76 | 76.49 |
| Al2O3 | 16.28 | 11.76 | 16.14 | 15.06 | 14.75 | 16.63 | 12.11 | 7.92 | 13.05 | 15.15 | 14.52 | 14.10 | 13.62 |
| TiO2 | 0.02 | 0.05 | 0.01 | 0.01 | 0.03 | 0.05 | 0.50 | 0.22 | 0.06 | 0.02 | 0.14 | 0.01 | 0.11 |
| TFe2O3 | 0.39 | 0.58 | 0.19 | 0.27 | 0.15 | 0.16 | 2.21 | 2.32 | 0.67 | 0.47 | 1.13 | 0.45 | 0.47 |
| MgO | 0.07 | 0.05 | 0.00 | 0.01 | 0.06 | 0.10 | 2.74 | 3.15 | 0.22 | 0.13 | 0.23 | 0.11 | 0.22 |
| MnO | 0.03 | 0.02 | 0.09 | 0.26 | 0.00 | 0.00 | 0.03 | 0.00 | 0.02 | 0.05 | 0.03 | 0.03 | 0.01 |
| CaO | 0.51 | 0.70 | 0.13 | 0.47 | 0.36 | 2.21 | 1.58 | 0.46 | 0.55 | 0.57 | 1.10 | 0.30 | 0.56 |
| Na2O | 7.43 | 3.98 | 2.73 | 8.31 | 2.63 | 6.52 | 1.71 | 2.46 | 0.92 | 3.39 | 3.42 | 4.22 | 6.32 |
| K2O | 1.59 | 3.18 | 10.11 | 0.28 | 7.46 | 0.54 | 2.69 | 1.12 | 4.35 | 6.24 | 4.74 | 4.47 | 0.86 |
| P2O5 | 0.16 | 0.12 | 0.12 | 0.13 | 0.14 | 0.09 | 0.05 | 0.06 | 0.37 | 0.17 | 0.12 | 0.24 | 0.15 |
| LOI | 0.36 | 0.39 | 0.25 | 0.08 | 0.51 | 0.47 | 4.42 | 0.51 | 1.47 | 0.29 | 0.84 | 0.27 | 0.27 |
| TOTAL | 100.0 | 100.0 | 100.0 | 100.0 | 99.3 | 99.1 | 99.9 | 100.0 | 100.0 | 99.9 | 99.4 | 100.0 | 99.1 |
| A/CNK | 1.71 | 1.50 | 1.25 | 1.66 | 1.41 | 1.79 | 2.03 | 1.96 | 2.24 | 1.5 | 1.57 | 1.57 | 1.76 |
| μg/g | |||||||||||||
| Li | 16.96 | 32.39 | 18.86 | 7.06 | bdl | 2.20 | 14.29 | 11.33 | 12.43 | 68.76 | 67.51 | 27.05 | 12.07 |
| Be | 531 | 8.56 | 17.37 | 115 | 4.01 | 11.26 | 2.42 | 0.45 | 2.85 | 1.71 | 10.82 | 1.41 | 0.57 |
| B | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 20.46 | 76.07 | 0.00 | 187 | 0.00 |
| Sc | 1.64 | 0.66 | 0.31 | 0.31 | 0.09 | 0.69 | 11.85 | 4.57 | 18.31 | 1.85 | 1.47 | 2.95 | 4.05 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 113 | 56.32 | 0.00 | 0.00 | 0.00 | 0.00 | 721 | 0.00 | 437 |
| V | 0.25 | 0.27 | 0.11 | 0.26 | 4.25 | 7.09 | 295 | 24.53 | 0.80 | 1.67 | 9.83 | 0.44 | 5.39 |
| Cr | 3.31 | 1.69 | 0.93 | 2.19 | 0.00 | 0.00 | 58.43 | 25.38 | 2.83 | 4.23 | 0.00 | 5.28 | 0.00 |
| Mn | 0.00 | 0.00 | 0.00 | 0.00 | 21.19 | 29.87 | 0.00 | 0.00 | 0.00 | 0.00 | 200 | 0.00 | 56.14 |
| Co | 0.33 | 0.70 | 0.08 | 0.09 | 0.44 | 0.18 | 2.19 | 3.71 | 0.50 | 0.48 | 0.92 | 0.18 | 0.30 |
| Ni | 1.56 | 0.75 | 0.63 | 0.67 | 0.75 | 0.82 | 7.72 | 9.85 | 0.47 | 0.73 | 0.78 | 0.47 | 0.70 |
| Cu | 2.32 | 7.78 | 1.13 | 2.16 | 1.12 | 1.08 | 4.77 | 1.36 | 2.27 | 2.37 | 0.43 | 2.80 | 0.15 |
| Zn | 12.25 | 21.78 | 2.75 | 3.54 | 0.07 | 5.37 | 123 | 14.23 | 10.40 | 4.63 | 44.00 | 7.86 | 7.65 |
| Ga | 12.46 | 7.52 | 9.32 | 14.15 | 3.98 | 11.95 | 17.26 | 10.10 | 20.40 | 11.38 | 12.90 | 10.51 | 13.16 |
| Rb | 160 | 161 | 1083 | 17.20 | 289.10 | 9.58 | 133 | 41.01 | 181 | 285 | 440 | 141 | 31.46 |
| Sr | 2.02 | 21.14 | 1.95 | 0.61 | 36.49 | 88.43 | 76.74 | 22.58 | 35.16 | 154 | 65.21 | 19.94 | 52.06 |
| Zr | 170 | 3.51 | 4.86 | 10.71 | 20.85 | 11.80 | 56.46 | 57.12 | 16.59 | 16.95 | 69.89 | 16.37 | 54.16 |
| Nb | 7.90 | 8.85 | 8.20 | 59.61 | 3.38 | 1.53 | 12.48 | 7.76 | 20.39 | 0.89 | 20.51 | 1.20 | 22.88 |
| Mo | 0.05 | 0.01 | 0.01 | 0.02 | 0.05 | 0.02 | 11.73 | 0.07 | 0.02 | 0.06 | 0.16 | 0.03 | 0.19 |
| Sn | 0.00 | 0.00 | 0.00 | 0.00 | 6.06 | 1.64 | 0.00 | 0.00 | 0.00 | 0.00 | 21.23 | 0.00 | 7.28 |
| Cd | 0.24 | 0.12 | 0.09 | 0.34 | 0.00 | 0.00 | 0.10 | 0.01 | 0.02 | 0.09 | 0.00 | 0.09 | 0.00 |
| In | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.07 | 0.02 | 0.15 | 0.04 | 0.00 | 0.03 | 0.00 |
| Cs | 53.63 | 23.10 | 93.32 | 6.22 | 57.45 | 0.60 | 6.72 | 1.84 | 6.73 | 5.66 | 33.95 | 0.18 | 1.00 |
| Ba | 2.93 | 32.97 | 5.51 | 3.13 | 1069 | 28.96 | 438 | 93.56 | 165.47 | 711.31 | 193 | 47.06 | 24.26 |
| Hf | 8.63 | 0.15 | 0.28 | 0.71 | 1.01 | 0.50 | 1.61 | 1.74 | 0.57 | 0.44 | 3.40 | 0.44 | 3.00 |
| Ta | 2.83 | 2.11 | 1.19 | 17.12 | 2.06 | 0.66 | 0.86 | 0.69 | 1.67 | 0.13 | 7.18 | 0.10 | 11.52 |
| W | 0.86 | 1.33 | 0.69 | 1.34 | 8.74 | 3.38 | 1.46 | 0.37 | 22.66 | 0.23 | 6.17 | 0.11 | 3.26 |
| Tl | 1.10 | 0.83 | 5.25 | 0.20 | 1.52 | 0.06 | 1.40 | 0.15 | 0.96 | 1.74 | 2.63 | 0.87 | 0.19 |
| Pb | 29.00 | 66.32 | 77.25 | 23.05 | 68.49 | 93.78 | 27.65 | 3.07 | 17.64 | 39.65 | 76.52 | 25.87 | 5.18 |
| Bi | 2.52 | 11.99 | 4.18 | 0.76 | 0.00 | 0.00 | 0.44 | 0.01 | 0.10 | 0.06 | 0.00 | 0.06 | 0.00 |
| Th | 3.11 | 1.25 | 0.80 | 2.22 | 1.01 | 0.62 | 12.48 | 15.08 | 0.47 | 0.64 | 14.88 | 0.15 | 8.20 |
| U | 25.39 | 3.09 | 14.76 | 5.87 | 9.39 | 28.66 | 7.05 | 3.45 | 0.93 | 0.58 | 50.10 | 0.86 | 11.85 |
| μg/g | |||||||||||||
| Y | 37.27 | 5.06 | 0.45 | 4.14 | 4.59 | 7.49 | 17.28 | 10.71 | 14.38 | 2.41 | 14.88 | 1.98 | 12.03 |
| La | 6.70 | 1.46 | 0.33 | 1.85 | 2.24 | 4.13 | 38.23 | 25.62 | 2.42 | 3.34 | 17.83 | 0.93 | 3.26 |
| Ce | 12.25 | 2.38 | 0.51 | 3.60 | 4.47 | 6.44 | 65.32 | 46.62 | 8.00 | 5.50 | 35.60 | 1.76 | 6.94 |
| Pr | 1.40 | 0.25 | 0.05 | 0.37 | 0.47 | 0.65 | 8.95 | 6.22 | 0.96 | 0.43 | 4.18 | 0.16 | 0.85 |
| Nd | 4.50 | 0.81 | 0.16 | 1.04 | 1.61 | 2.15 | 29.84 | 20.04 | 4.19 | 1.37 | 15.22 | 0.52 | 3.09 |
| Sm | 1.61 | 0.27 | 0.04 | 0.44 | 0.40 | 0.54 | 4.94 | 3.60 | 2.19 | 0.27 | 3.79 | 0.16 | 1.11 |
| Eu | 0.04 | 0.26 | 0.02 | 0.01 | 0.29 | 1.13 | 0.89 | 0.68 | 0.19 | 0.78 | 0.55 | 0.04 | 0.14 |
| Gd | 1.79 | 0.30 | 0.03 | 0.36 | 0.51 | 0.71 | 4.19 | 2.97 | 2.08 | 0.24 | 4.37 | 0.15 | 1.70 |
| Tb | 0.63 | 0.09 | 0.01 | 0.12 | 0.11 | 0.20 | 0.61 | 0.41 | 0.57 | 0.04 | 0.71 | 0.04 | 0.43 |
| Dy | 5.70 | 0.77 | 0.06 | 0.75 | 0.74 | 1.23 | 3.05 | 1.98 | 3.34 | 0.29 | 3.53 | 0.30 | 2.44 |
| Ho | 1.19 | 0.15 | 0.01 | 0.09 | 0.17 | 0.28 | 0.60 | 0.35 | 0.53 | 0.06 | 0.63 | 0.05 | 0.47 |
| Er | 4.24 | 0.49 | 0.03 | 0.20 | 0.59 | 0.94 | 1.64 | 0.96 | 1.37 | 0.18 | 1.64 | 0.17 | 1.36 |
| Tm | 1.11 | 0.12 | 0.03 | 0.06 | 0.10 | 0.15 | 0.25 | 0.12 | 0.25 | 0.04 | 0.22 | 0.04 | 0.22 |
| Yb | 9.18 | 0.72 | 0.04 | 0.26 | 0.81 | 1.06 | 1.55 | 0.63 | 1.57 | 0.27 | 1.44 | 0.31 | 1.63 |
| Lu | 1.61 | 0.12 | 0.01 | 0.04 | 0.10 | 0.13 | 0.22 | 0.09 | 0.20 | 0.04 | 0.16 | 0.04 | 0.21 |
| ∑REE | 89.2 | 13.2 | 1.76 | 13.3 | 17.2 | 27.2 | 178 | 121 | 42.3 | 15.3 | 105 | 6.65 | 35.9 |
| Zone 1 (Marginal Zone) | Zone 2 (Outer IZ) | Zone 3 (Inner IZ) | Zone 4 (Core) | Zone 5 (Miarolitic Zone) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Albite | Microcline | Microcline | Microcline | Albite | ||||||||
| Sample | YRP-1A-F1 | YRP-1A-F2 | YRP-1A-F3 | YRP-12A-F1 | YRP-12A-F5 | YRP-6A-F1 | YRP-4-F2 | YRP-9A-F1 | YRP-8B-F2 | YRP-8B-F5 | YRP-7B-F2 | YRP-7B-F3 |
| SiO2 (wt%) | 65.16 | 65.04 | 64.86 | 63.82 | 64.44 | 64.60 | 65.36 | 64.47 | 64.30 | 64.32 | 67.86 | 68.22 |
| TiO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al2O3 | 20.93 | 21.02 | 20.91 | 17.72 | 18.23 | 17.99 | 18.19 | 17.71 | 18.01 | 17.91 | 19.56 | 18.86 |
| FeO | 0.01 | 0.00 | 0.06 | 0.00 | 0.00 | 0.02 | 0.01 | 0.00 | 0.01 | 0.02 | 0.01 | 0.00 |
| MnO | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.03 | 0.00 | 0.00 | 0.02 | 0.03 | 0.00 | 0.00 |
| MgO | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.02 |
| CaO | 2.56 | 2.50 | 2.49 | 0.01 | 0.00 | 0.04 | 0.04 | 0.00 | 0.05 | 0.01 | 0.78 | 0.36 |
| Na2O | 10.65 | 10.59 | 10.66 | 1.47 | 1.00 | 1.06 | 1.16 | 1.32 | 1.58 | 1.30 | 11.67 | 12.03 |
| K2O | 0.20 | 0.13 | 0.30 | 13.60 | 14.57 | 14.54 | 14.12 | 14.04 | 13.87 | 13.94 | 0.17 | 0.09 |
| Total | 99.52 | 99.28 | 99.28 | 96.61 | 98.26 | 98.30 | 98.89 | 97.58 | 97.84 | 97.54 | 100.07 | 99.59 |
| Oxygen atoms = 8 | ||||||||||||
| Si (apfu) | 2.870 | 2.873 | 2.863 | 3.042 | 3.027 | 3.033 | 3.051 | 3.045 | 3.023 | 3.040 | 2.960 | 2.984 |
| Ti | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 |
| Al | 1.087 | 1.094 | 1.088 | 0.995 | 1.009 | 0.995 | 1.001 | 0.986 | 0.998 | 0.998 | 1.005 | 0.972 |
| Fe | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 |
| Mn | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 |
| Mg | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.001 |
| Ca | 0.121 | 0.118 | 0.118 | 0.001 | 0.000 | 0.002 | 0.002 | 0.000 | 0.002 | 0.001 | 0.036 | 0.017 |
| Na | 0.910 | 0.907 | 0.912 | 0.136 | 0.091 | 0.097 | 0.105 | 0.121 | 0.144 | 0.119 | 0.987 | 1.020 |
| K | 0.011 | 0.007 | 0.017 | 0.827 | 0.872 | 0.870 | 0.841 | 0.846 | 0.831 | 0.840 | 0.009 | 0.005 |
| Total | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 |
| End members | ||||||||||||
| Ab | 87.29 | 87.85 | 87.12 | 14.08 | 9.48 | 9.97 | 11.06 | 12.51 | 14.75 | 12.38 | 95.59 | 97.91 |
| An | 11.61 | 11.44 | 11.26 | 0.06 | 0.01 | 0.19 | 0.20 | 0.00 | 0.25 | 0.05 | 3.51 | 1.63 |
| Or | 1.10 | 0.70 | 1.62 | 85.85 | 90.51 | 89.84 | 88.74 | 87.49 | 85.00 | 87.57 | 0.90 | 0.46 |
| Zone 1 (Marginal Zone) | Zone 2 (Outer IZ) | Zone 3 (Inner IZ) | Zone 4 (Core) | Zone 5 (Miarolitic Zone) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase | Albite | Microcline | Microcline | Microcline | Albite | |||||||||||||||
| n = 16 | n = 5 | n = 5 | n = 3 | n = 5 | ||||||||||||||||
| Element | Avg | Min | Max | σ | Avg | Min | Max | σ | Avg | Min | Max | σ | Avg | Min | Max | σ | Avg | Min | Max | σ |
| Li (ppm) | 2.31 | 1.27 | 4.27 | 0.81 | 6.30 | 3.66 | 11.1 | 2.88 | 24.2 | 3.47 | 48.7 | 19.8 | 48.6 | 19.9 | 69.0 | 25.6 | - | bdl | bdl | - |
| Be | 5.61 | 1.27 | 10.4 | 3.42 | 5.29 | 4.36 | 7.15 | 1.19 | 5.51 | 3.48 | 8.52 | 2.32 | 4.12 | 3.94 | 4.40 | 2.28 | 13.3 | 11.3 | 15.7 | 1.71 |
| B | 22.5 | 11.4 | 43.8 | 8.7 | 26.7 | 21.1 | 37.5 | 7.5 | 38.1 | 27.3 | 49.0 | 15.4 | - | bdl | bdl | - | - | bdl | bdl | - |
| P | 84.5 | 41.5 | 215 | 52.3 | 376 | 307 | 405 | 40.2 | 479 | 308 | 599 | 108 | 695 | 671 | 709 | 263 | 841 | 794 | 906 | 40.5 |
| K/103 | 2.33 | 0.65 | 3.91 | 1.18 | 148 | 143 | 154 | 4.19 | 132 | 90.8 | 165 | 30.7 | 139 | 134 | 143 | 65.5 | 6.19 | 1.19 | 15.4 | 5.94 |
| Ti | 98.2 | 5.5 | 156 | 40.9 | 6.8 | 5.10 | 9.7 | 1.99 | 20.2 | 3.6 | 47.7 | 24.0 | 5.91 | 5.91 | 5.91 | 49.8 | 4.46 | 3.81 | 5.10 | 0.91 |
| Ga | 8.24 | 1.21 | 18.7 | 5.81 | 12.6 | 11.6 | 14.0 | 0.94 | 13.0 | 10.3 | 14.9 | 1.7 | 18.6 | 14.0 | 21.1 | 6.93 | 28.8 | 27.6 | 30.2 | 1.13 |
| Rb | 5.87 | 0.94 | 11.3 | 3.11 | 937 | 884 | 977 | 33.8 | 1272 | 816 | 1669 | 320 | 2697 | 2417 | 2925 | 980 | 72.8 | 2.08 | 177 | 78.9 |
| Sr | 119 | 6.33 | 237 | 83.7 | 22.7 | 17.0 | 34.3 | 6.7 | 17.0 | 1.0 | 69.2 | 29.2 | 5.01 | 1.07 | 7.35 | 64.6 | 0.94 | 0.52 | 1.74 | 0.49 |
| Nb | 0.55 | 0.40 | 0.79 | 0.14 | 0.25 | 0.22 | 0.28 | 0.04 | - | bdl | bdl | - | - | bdl | bdl | - | - | bdl | bdl | - |
| Sn | 2.95 | 0.76 | 6.92 | 1.79 | 4.30 | 3.46 | 4.89 | 0.75 | 4.56 | 4.14 | 4.99 | 0.37 | 7.76 | 5.57 | 9.06 | 2.18 | 2.16 | 2.16 | 2.16 | - |
| Sb | 1.06 | 0.35 | 2.70 | 0.73 | - | bdl | bdl | - | - | bdl | bdl | - | 0.92 | bdl | bdl | - | 1.63 | 1.47 | 1.78 | 0.22 |
| Cs | 0.48 | 0.45 | 0.54 | 0.04 | 19.1 | 12.7 | 40.6 | 12.1 | 95.4 | 13.5 | 323 | 131 | 106 | 82.9 | 122 | 104 | 22.7 | 5.95 | 41.0 | 17.6 |
| Ba | 217 | 0.92 | 462 | 151 | 71 | 43.4 | 95 | 19.0 | 41.1 | 1.72 | 145 | 59.6 | 29.9 | 29.8 | 30.0 | 112 | 21.1 | bdl | bdl | - |
| La | 5.04 | 2.80 | 8.00 | 1.90 | 0.14 | 0.12 | 0.17 | 0.02 | 0.68 | 0.11 | 2.11 | 0.96 | - | bdl | bdl | - | 0.71 | 0.32 | 1.66 | 0.55 |
| Ce | 7.93 | 3.76 | 13.0 | 3.24 | 0.13 | 0.11 | 0.15 | 0.03 | 0.88 | 0.06 | 3.81 | 1.64 | 0.07 | 0.07 | 0.07 | 3.23 | 0.85 | 0.25 | 2.21 | 0.78 |
| Ta | 0.08 | 0.05 | 0.19 | 0.05 | - | bdl | bdl | - | - | bdl | bdl | - | - | bdl | bdl | - | - | bdl | bdl | - |
| Pb | 23.5 | 1.02 | 153 | 44.7 | 186 | 167 | 236 | 28.4 | 158 | 118 | 194 | 35.8 | 133 | 113 | 167 | 64.1 | 58.7 | 38.9 | 98.3 | 24.1 |
| K/Rb | 540 | 345 | 1265 | 227 | 158 | 153 | 162 | 3.98 | 105 | 87.7 | 121 | 12.6 | 52.1 | 45.9 | 59.0 | 42.6 | 338 | 86.7 | 1017 | 454 |
| K/Cs | 4067 | 1439 | 7238 | 2554 | 9426 | 3683 | 12,052 | 3315 | 4091 | 387 | 6731 | 2824 | 1361 | 1101 | 1721 | 2309 | 455 | 375 | 566 | 99.5 |
| Nb/Ta | 7.16 | 2.70 | 10.0 | 2.23 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Rb/Sr | 0.06 | 0.01 | 0.23 | 0.05 | 43.5 | 27.6 | 52.0 | 9.39 | 425 | 11.8 | 1196 | 452 | 1024 | 398 | 2259 | 627 | 65.0 | 4.00 | 169 | 71.9 |
| Sample | Zone 1 (Marginal Zone) | Zone 2 (Outer IZ) | Zone 3 (Inner IZ) | Zone 4 (Core) | Zone 5 (Miarolitic Zone) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| YRP-13B-M1 | YRP-13B-M2 | YRP-2B-M1 | YRP-2B-M2 | YRP-6A-M1 | YRP-6A-M2 | YRP-10A-M1 | YRP-10A-M2 | YRP-5B-M1 | YRP-7F-M2 | |
| SiO2 | 47.08 | 47.95 | 46.98 | 47.15 | 48.25 | 47.87 | 46.19 | 47.00 | 47.29 | 47.77 |
| TiO2 | 0.13 | 0.22 | 0.20 | 0.24 | 0.11 | 0.12 | 0.16 | 0.13 | 0.13 | 0.51 |
| Al2O3 | 32.19 | 32.10 | 33.12 | 32.88 | 34.88 | 34.90 | 32.94 | 33.35 | 33.55 | 32.69 |
| FeO | 2.41 | 2.29 | 2.36 | 2.30 | 2.01 | 1.94 | 1.91 | 1.93 | 1.90 | 2.15 |
| MnO | 0.12 | 0.07 | 0.05 | 0.09 | 0.05 | 0.09 | 0.05 | 0.05 | 0.04 | 0.04 |
| MgO | 0.87 | 1.17 | 1.12 | 1.10 | 0.47 | 0.42 | 0.94 | 0.83 | 0.85 | 0.94 |
| CaO | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.04 | 0.00 | 0.04 | 0.04 |
| Na2O | 0.33 | 0.42 | 0.74 | 0.64 | 0.87 | 0.82 | 0.84 | 0.71 | 0.57 | 0.56 |
| K2O | 9.53 | 9.39 | 9.08 | 9.13 | 9.09 | 9.13 | 9.26 | 9.40 | 9.30 | 9.34 |
| F | 0.25 | 0.51 | 0.18 | 0.21 | 0.23 | 0.30 | 0.41 | 0.23 | 0.30 | 0.19 |
| Cl | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cr2O3 | 0.02 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.02 |
| NiO | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 | 0.05 | 0.00 | 0.00 | 0.01 | 0.03 |
| Li2O* | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 0.02 | 0.05 | 0.00 | 0.02 | 0.00 |
| H2O* | 4.27 | 4.21 | 4.36 | 4.34 | 4.45 | 4.40 | 4.19 | 4.33 | 4.31 | 4.38 |
| Subtotal | 97.24 | 98.43 | 98.22 | 98.10 | 100.45 | 100.08 | 96.98 | 97.96 | 98.31 | 98.66 |
| O=F,Cl | 0.11 | 0.22 | 0.08 | 0.09 | 0.10 | 0.12 | 0.17 | 0.10 | 0.13 | 0.08 |
| Total | 97.13 | 98.21 | 98.15 | 98.02 | 100.35 | 99.95 | 96.81 | 97.86 | 98.18 | 98.58 |
| Structural formulae based on O, OH, F = 24 | ||||||||||
| Si | 6.43 | 6.46 | 6.34 | 6.37 | 6.34 | 6.32 | 6.32 | 6.35 | 6.36 | 6.41 |
| Al iv | 1.57 | 1.54 | 1.66 | 1.63 | 1.66 | 1.68 | 1.68 | 1.65 | 1.64 | 1.59 |
| Y | ||||||||||
| Al vi | 3.61 | 3.56 | 3.61 | 3.60 | 3.74 | 3.75 | 3.63 | 3.67 | 3.68 | 3.58 |
| Ti | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.05 |
| Cr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fe | 0.28 | 0.26 | 0.27 | 0.26 | 0.22 | 0.21 | 0.22 | 0.22 | 0.21 | 0.24 |
| Mn | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 |
| Mg | 0.18 | 0.23 | 0.23 | 0.22 | 0.09 | 0.08 | 0.19 | 0.17 | 0.17 | 0.19 |
| Ni | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Li* | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.01 | 0.03 | 0.00 | 0.01 | 0.00 |
| X | ||||||||||
| Ca | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 |
| Na | 0.09 | 0.11 | 0.19 | 0.17 | 0.22 | 0.21 | 0.22 | 0.19 | 0.15 | 0.15 |
| K | 1.66 | 1.61 | 1.56 | 1.57 | 1.52 | 1.54 | 1.62 | 1.62 | 1.60 | 1.60 |
| OH* | 3.89 | 3.78 | 3.92 | 3.91 | 3.90 | 3.88 | 3.82 | 3.90 | 3.87 | 3.92 |
| F | 0.11 | 0.22 | 0.08 | 0.09 | 0.10 | 0.12 | 0.18 | 0.10 | 0.13 | 0.08 |
| TOTAL | 17.84 | 17.85 | 17.88 | 17.86 | 17.82 | 17.83 | 17.94 | 17.88 | 17.84 | 17.82 |
| Y total | 4.09 | 4.13 | 4.13 | 4.12 | 4.07 | 4.08 | 4.09 | 4.07 | 4.09 | 4.07 |
| X total | 1.75 | 1.73 | 1.76 | 1.74 | 1.75 | 1.75 | 1.84 | 1.81 | 1.75 | 1.75 |
| Al total | 5.18 | 5.10 | 5.27 | 5.23 | 5.40 | 5.43 | 5.31 | 5.31 | 5.32 | 5.17 |
| Fe/Fe+Mg | 0.61 | 0.52 | 0.54 | 0.54 | 0.70 | 0.72 | 0.53 | 0.57 | 0.56 | 0.56 |
| Fe+Mg+Aliv+Ti | 4.07 | 4.07 | 4.12 | 4.11 | 4.07 | 4.06 | 4.06 | 4.07 | 4.08 | 4.06 |
| Mg-Li | 0.18 | 0.19 | 0.23 | 0.22 | 0.09 | 0.07 | 0.16 | 0.17 | 0.16 | 0.19 |
| Fe+Mg+Ti-Alvi | −3.14 | −3.04 | −3.09 | −3.10 | −3.42 | −3.44 | −3.21 | −3.27 | −3.28 | −3.10 |
| Element | Zone 1 (Marginal Zone) | Zone 2 (Outer IZ) | Zone 3 (Inner IZ) | Zone 4 (Core) | Zone 5 (Miarolitic Zone) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 16 | n = 5 | n = 5 | n = 3 | n = 5 | ||||||||||||||||
| Avg | Min | Max | σ | Avg | Min | Max | σ | Avg | Min | Max | σ | Avg | Min | Max | σ | Avg | Min | Max | σ | |
| Li (ppm) | 526 | 432 | 593 | 84.0 | 360 | 329 | 392 | 20.1 | 282 | 256 | 327 | 24.3 | 535 | 519 | 558 | 16.5 | 567 | 480 | 696 | 62.9 |
| Be | 30.3 | 29.9 | 30.8 | 0.49 | 23.2 | 18.3 | 28.0 | 2.94 | 29.4 | 25.6 | 33.4 | 2.84 | 29.8 | 21.0 | 46.9 | 10.6 | 28.8 | 24.3 | 32.0 | 2.71 |
| B | 154 | 109 | 199 | 44.7 | 167 | 114 | 195 | 29.1 | 200.5 | 193.5 | 205.4 | 4.57 | 162 | 131 | 200 | 28.5 | 140 | 119 | 167 | 14.5 |
| Na | 4237 | 3562 | 4928 | 683 | 4145 | 3473 | 4488 | 325 | 5459 | 5367 | 5546 | 63.6 | 5436 | 4114 | 6869 | 981 | 4259 | 3238 | 5081 | 599 |
| Mg | 4521 | 3939 | 4847 | 505 | 4883 | 4553 | 5219 | 248 | 2233 | 2159 | 2393 | 75.7 | 5079 | 4543 | 5738 | 486 | 5184 | 4512 | 5851 | 498 |
| P | 77.5 | 67.3 | 88.5 | 10.6 | 65.2 | 55.1 | 72.8 | 9.12 | 63.2 | 52.6 | 81.8 | 11.0 | 82.6 | 57.5 | 101 | 22.4 | 87.9 | 71.6 | 113 | 14.6 |
| K/103 | 110 | 110 | 110 | 0.29 | 109 | 108 | 111 | 1.22 | 103 | 100 | 105 | 2.04 | 104 | 99 | 113 | 5.51 | 127 | 122 | 136 | 5.02 |
| Sc | 14.2 | 13.9 | 14.9 | 0.55 | 18.4 | 13.5 | 20.3 | 2.3 | 15.6 | 15.0 | 16.2 | 0.48 | 15.0 | 11.3 | 18.0 | 2.88 | 10.5 | 2.64 | 20.8 | 7.95 |
| Ti | 1009 | 749 | 1248 | 250 | 1197 | 1128 | 1286 | 57.0 | 706 | 678 | 732 | 16.1 | 1016 | 902 | 1240 | 146 | 2574 | 1176 | 4491 | 1485 |
| Mn | 460 | 410 | 489 | 44.0 | 275 | 248 | 297 | 22.9 | 421 | 406 | 439 | 10.5 | 428 | 360 | 524 | 70.5 | 674 | 515 | 986 | 171 |
| Fe | 15,337 | 14,085 | 15,997 | 1086 | 15,394 | 14,500 | 16,072 | 544 | 15,229 | 14,521 | 15,752 | 402 | 14,695 | 12,833 | 16,168 | 1575 | 21,384 | 16,703 | 29,575 | 4730 |
| Zn | 73.1 | 54.4 | 89.3 | 17.5 | 55.6 | 48.4 | 65.4 | 6.3 | 104 | 95.0 | 120 | 7.8 | 62.6 | 18.9 | 75.3 | 24.4 | 98.8 | 59.7 | 203 | 41.3 |
| Ga | 128 | 112 | 159 | 26.5 | 90.5 | 87.4 | 92.5 | 1.8 | 146 | 141 | 151 | 3.1 | 117 | 106 | 125 | 8.29 | 155 | 134 | 181 | 18.2 |
| Rb | 2475 | 1540 | 4057 | 1377 | 920 | 774 | 1354 | 196 | 2125 | 2056 | 2202 | 49 | 1525 | 1480 | 1561 | 40.8 | 4398 | 2172 | 6881 | 2288 |
| Sr | 0.73 | 0.42 | 0.97 | 0.28 | 1.5 | 0.9 | 2.0 | 0.4 | 0.47 | 0.18 | 0.77 | 0.30 | - | bdl | bdl | - | 0.44 | 0.35 | 0.56 | 0.07 |
| Zr | 1.60 | 0.54 | 3.28 | 1.47 | 2.5 | 1.4 | 3.1 | 0.6 | 1.29 | 1.00 | 1.85 | 0.29 | 2.75 | 1.84 | 4.20 | 0.93 | 2.22 | 1.74 | 2.55 | 0.25 |
| Nb | 296 | 186 | 367 | 96.5 | 87.6 | 68.7 | 142 | 24.4 | 245 | 236 | 252 | 6.64 | 158 | 136 | 182 | 18.0 | 450 | 213 | 701 | 234 |
| In | 1.15 | 0.45 | 1.95 | 0.75 | 0.5 | 0.3 | 0.8 | 0.2 | 0.73 | 0.50 | 0.96 | 0.15 | - | bdl | bdl | - | 2.05 | 0.61 | 3.58 | 1.41 |
| Sn | 246 | 73.5 | 473 | 205 | 87.4 | 63.3 | 154 | 31.4 | 140 | 133 | 144 | 4.48 | 68.4 | 58.7 | 84.5 | 12.8 | 491 | 113 | 880 | 385 |
| Cs | 188 | 47.6 | 390 | 179 | 37.1 | 13.8 | 121 | 39.9 | 63.6 | 61.9 | 66.2 | 1.48 | 32.6 | 30.7 | 34.4 | 1.46 | 310 | 54.0 | 621 | 264.8 |
| Ba | 29.3 | 21.1 | 34.1 | 7.16 | 26.3 | 18.8 | 49.6 | 10.5 | - | bdl | bdl | - | 10.6 | 2.68 | 17.7 | 7.85 | 3.32 | 1.14 | 6.77 | 2.14 |
| Ta | 103 | 43.6 | 176 | 66.9 | 12.4 | 4.9 | 45.0 | 14.6 | 15.1 | 13.9 | 15.9 | 0.80 | 14.5 | 9.8 | 22.4 | 5.13 | 90.8 | 17.9 | 174 | 72.1 |
| W | 38.3 | 28.4 | 44.4 | 8.68 | 46.6 | 42.6 | 49.8 | 2.7 | 42.1 | 38.1 | 44.6 | 2.26 | 41.5 | 32.2 | 61.8 | 11.6 | 49.0 | 45.4 | 53.4 | 2.7 |
| Pb | 9.7 | 6.9 | 11.9 | 2.6 | 13.7 | 12.2 | 15.2 | 0.9 | 7.52 | 6.97 | 7.95 | 0.36 | 12.1 | 9.7 | 15.5 | 2.34 | 16.3 | 12.8 | 20.5 | 2.4 |
| K/Rb | 52.8 | 27.2 | 71.2 | 22.9 | 122 | 80.7 | 140 | 19.6 | 48.4 | 47.8 | 49.9 | 0.71 | 68.0 | 63.2 | 72.9 | 3.55 | 38.9 | 18.0 | 59.9 | 21.2 |
| K/Cs | 1150 | 282 | 2305 | 1042 | 5270 | 906 | 7900 | 2801 | 1618 | 1573 | 1675 | 36.5 | 3184 | 2961 | 3423 | 199 | 1207 | 199 | 2375 | 1044 |
| Nb/Ta | 3.4 | 1.9 | 4.3 | 1.3 | 11.0 | 3.1 | 15.9 | 4.4 | 16.2 | 15.3 | 17.0 | 0.69 | 11.6 | 8.15 | 13.8 | 2.42 | 7.3 | 4.0 | 12.9 | 3.4 |
| Rb/Sr | 3369 | 2258 | 4183 | 997 | 624 | 389 | 937 | 182 | 6434 | 2751 | 11,837 | 4781 | - | - | - | - | 10,070 | 4309 | 19,660 | 5700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandari, S.; Qin, K.; Zhou, Q.; Evans, N.J. Geological, Mineralogical and Geochemical Study of the Aquamarine-Bearing Yamrang Pegmatite, Eastern Nepal with Implications for Exploration Targeting. Minerals 2022, 12, 564. https://doi.org/10.3390/min12050564
Bhandari S, Qin K, Zhou Q, Evans NJ. Geological, Mineralogical and Geochemical Study of the Aquamarine-Bearing Yamrang Pegmatite, Eastern Nepal with Implications for Exploration Targeting. Minerals. 2022; 12(5):564. https://doi.org/10.3390/min12050564
Chicago/Turabian StyleBhandari, Sushmita, Kezhang Qin, Qifeng Zhou, and Noreen J. Evans. 2022. "Geological, Mineralogical and Geochemical Study of the Aquamarine-Bearing Yamrang Pegmatite, Eastern Nepal with Implications for Exploration Targeting" Minerals 12, no. 5: 564. https://doi.org/10.3390/min12050564
APA StyleBhandari, S., Qin, K., Zhou, Q., & Evans, N. J. (2022). Geological, Mineralogical and Geochemical Study of the Aquamarine-Bearing Yamrang Pegmatite, Eastern Nepal with Implications for Exploration Targeting. Minerals, 12(5), 564. https://doi.org/10.3390/min12050564






