α-Fe2O3 Nanoparticles/Iron-Containing Vermiculite Composites: Structural, Textural, Optical and Photocatalytic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Samples Preparation
2.2. Methods
3. Results
3.1. XRF and SEM Analysis
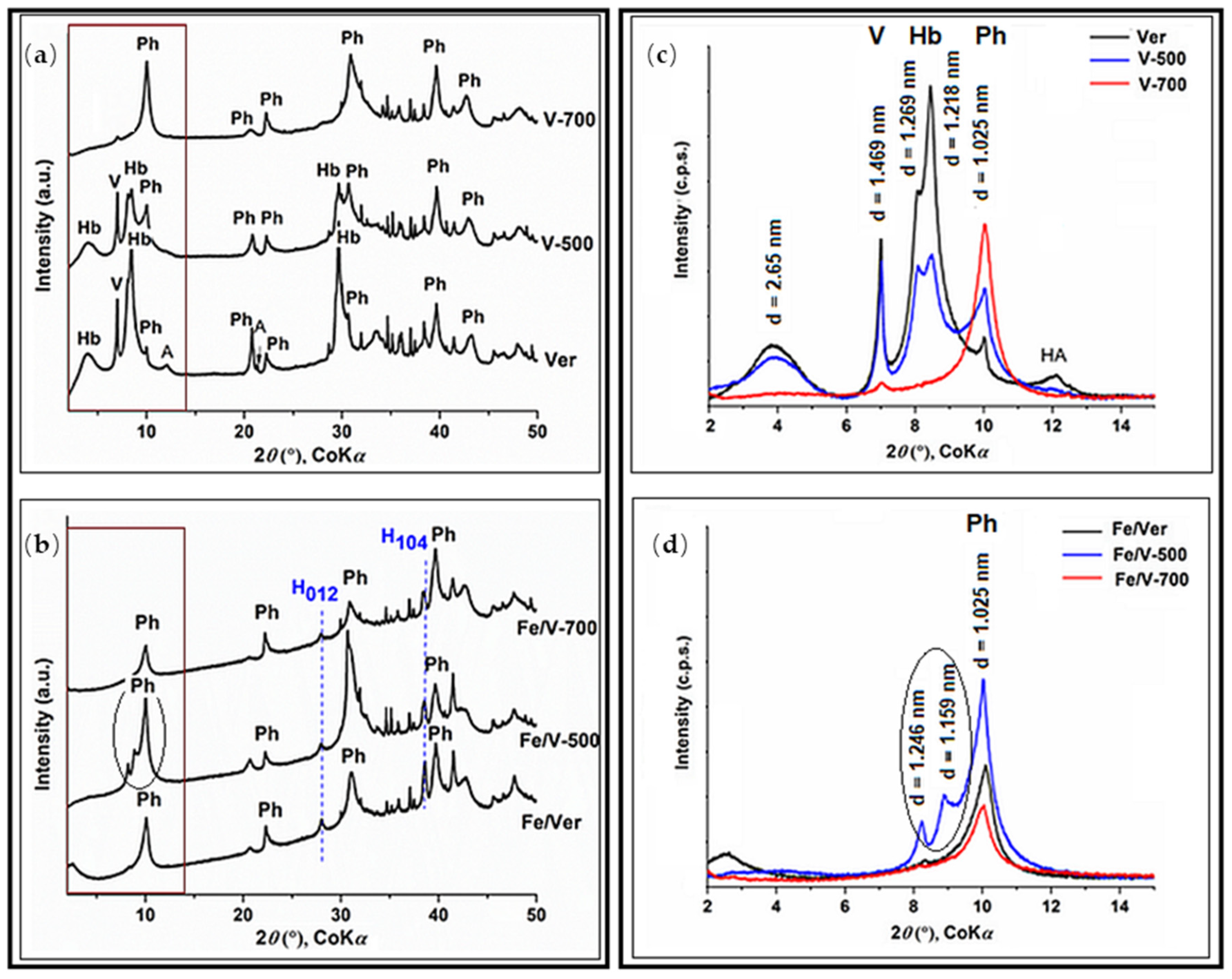
3.2. XRD Phase Analysis
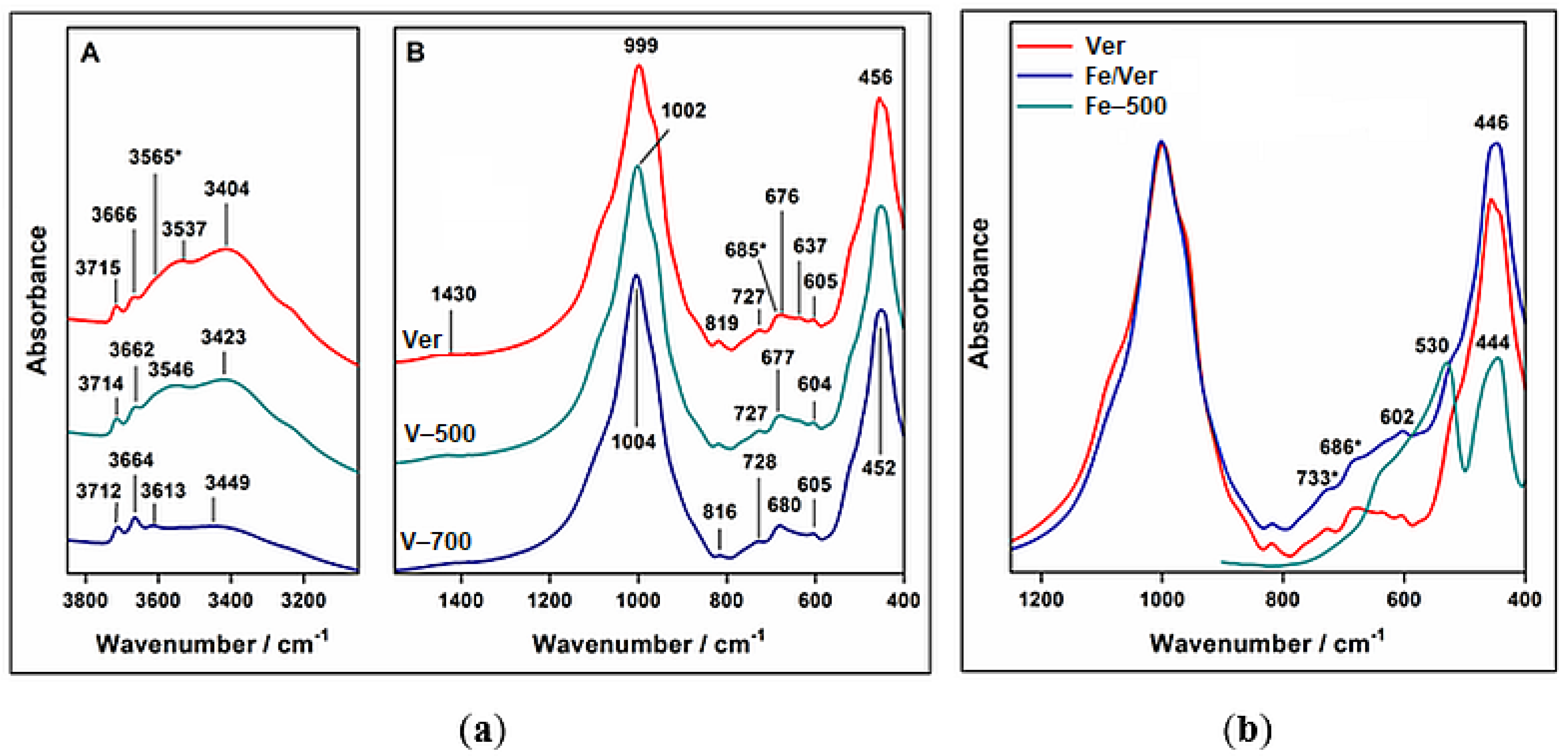
3.3. Infrared Spectroscopy Analysis
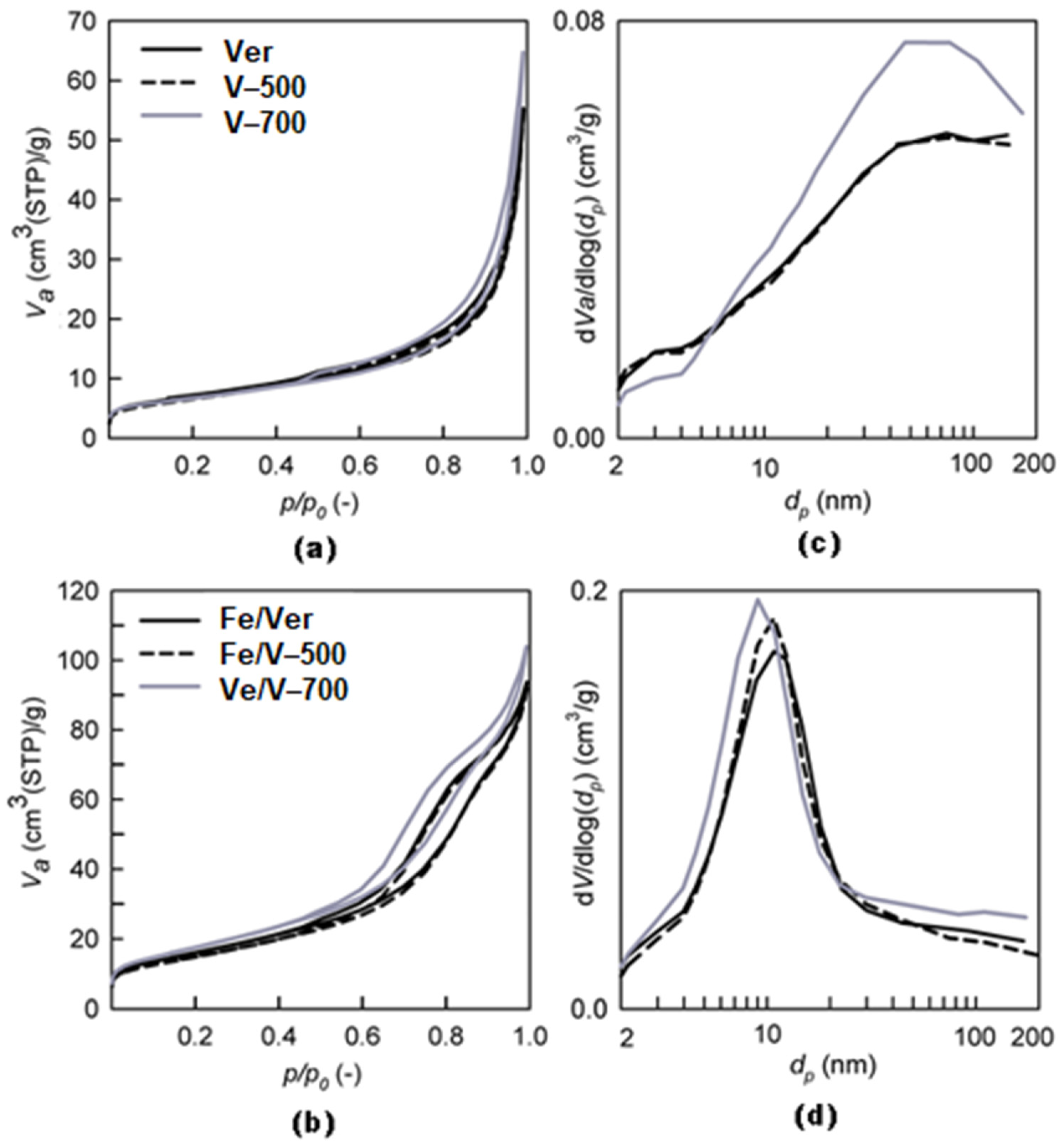
3.4. Textural Properties SBET and Vtot
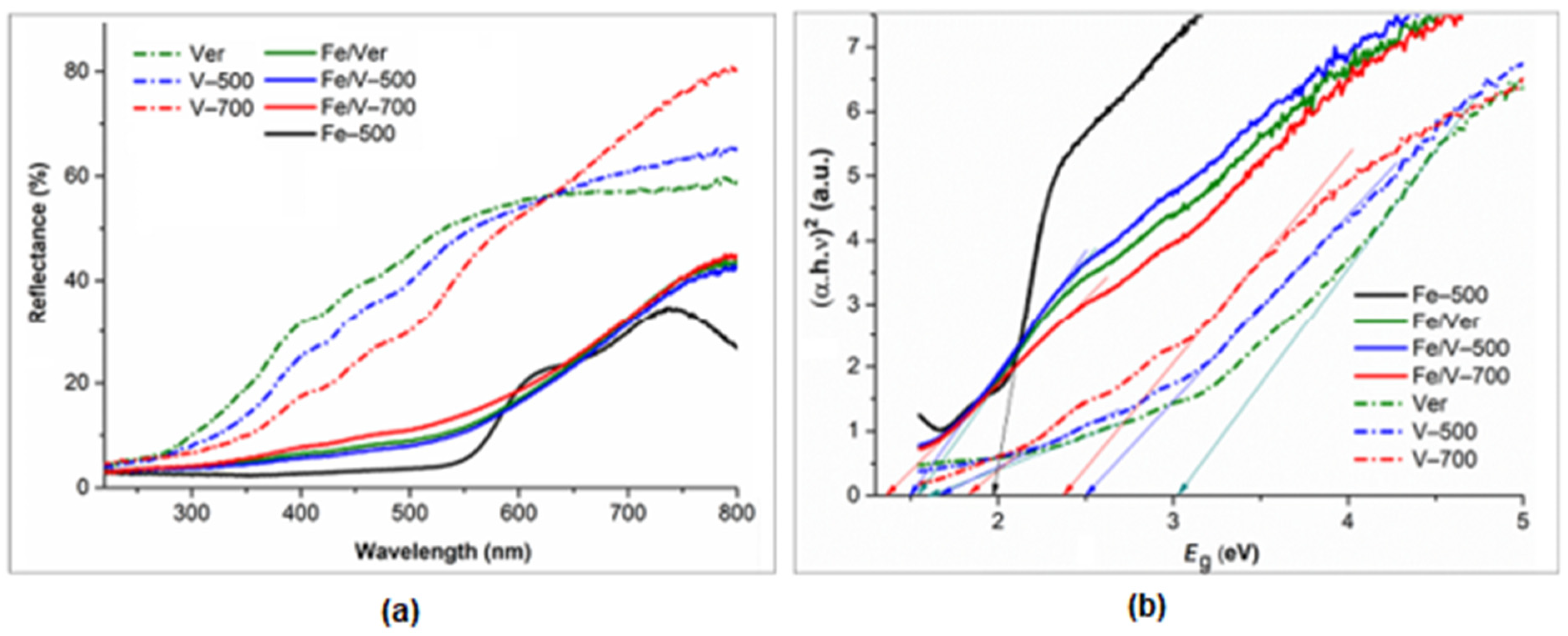
3.5. UV-Vis Diffuse Reflectance (DR) Spectroscopy
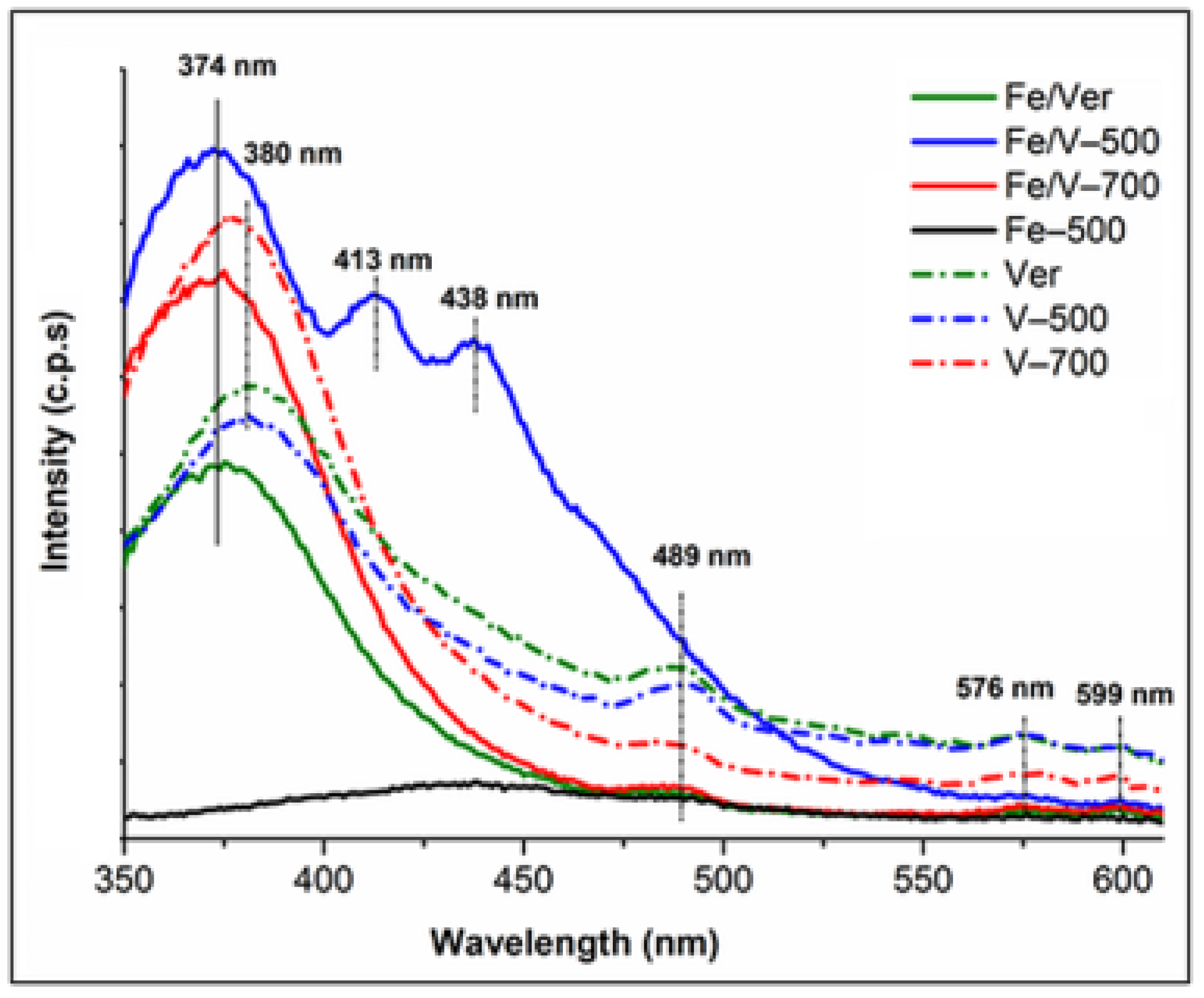
3.6. Photoluminescence (PL) Study
3.7. Photocatalytic Hydrogen Generation from Methanol–Water Mixture
4. Discussion
5. Conclusions
- Calcination decreased LOI = 12.3 mass% in Ver to 5.5 and 5.3 mass% in V–500 and V–700, respectively.
- Change in the hydration state of vermiculite was observed in the interlayer space of the V-Hb-Ph phases. The intensity of the basal peaks allows the representation of V-Hb-Ph (%) phases in Ver: V(30)-Hb(60)-Ph(10), in V–500: V(35)-Hb(37)-Ph(28) and in V–700: V(6)-Hb(0)-Ph(94) to be estimated.
- Infrared spectroscopy, confirmed the calcination effect on the presence of Al or Fe in addition to Si in the tetrahedral sheet.
- Calcination at 700 °C increased the pores volume with the formation of slit-like pores between the particles.
- Calcination supported increasing the amount of Fe(III) bound in the structure, which increases the direct band energy gap (Eg), while reducing the Fe2+/Fe3+ ratio in octahedra and PL intensity.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matějka, V.; Tokarský, J. Photocatalytical nanocomposites: A Review. J. Nanosci. Nanotechnol. 2014, 14, 1597–1616. [Google Scholar] [CrossRef] [PubMed]
- Holešová, S.; Štembírek, J.; Bartošová, L.; Pražanová, G.; Valášková, M.; Samlíková, M.; Pazdziora, E. Antibacterial efficiency of vermiculite/chlorhexidine nanocomposites and results of the in vivo test of harmlessness of vermiculite. Mater. Sci. Eng. C 2014, 42, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.D. Interpretation of the composition of vermiculites and hydrobiotites. Clays Clay Miner. 1963, 10, 70–89. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galan, E.; Theng, B.K.G. Structures and mineralogy of clay minerals. In Handbook of Clay Science. Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 19–86. [Google Scholar]
- Stucki, J. Properties and behaviour of iron in clay minerals. In Handbook of Clay Science. Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 423–475. [Google Scholar]
- Neumann, A.; Olson, T.L.; Scherer, M.M. Spectroscopic evidence for Fe(II)–Fe(III) electron transfer at clay mineral edge and basal sites. Environ. Sci. Technol. 2013, 47, 6969–6977. [Google Scholar] [CrossRef] [PubMed]
- Banfield, J.F.; Eggleton, R.A. Transmission electron microscope study of biotite weathering. Clays Clay Miner. 1988, 36, 47–60. [Google Scholar] [CrossRef]
- Suzuki, M.; Wada, N.; Hines, D.R.; Whittingham, M.S. Hydration states and phase transitions in vermiculite intercalation compounds. Phys. Rev. B 1987, 36, 2844–2851. [Google Scholar] [CrossRef]
- Ruiz-Conde, A.; Ruiz-Amil, A.; Pérez-Rodríguez, J.L.; Sánchez-Soto, P.J. Dehydration-rehydration in magnesium vermiculite: Conversion from two-one and one-two water hydration states through the formation of interstratified phases. J. Mater. Chem. 1996, 6, 1557–1566. [Google Scholar] [CrossRef]
- Marcos, C.; Argüelles, A.; Ruíz-Conde, A.; Sánchez-Soto, P.J.; Blanco, J.A. Study of the dehydration process of vermiculites by applying a vacuum pressure: Formation of interstratified phases. Miner. Mag. 2003, 67, 1253–1268. [Google Scholar] [CrossRef]
- Kikuchi, R.; Kogue, T. Structural and compositional variances in “hydrobiotite” sample from Palabora, South Africa. Clay Sci. J.-Stage 2018, 22, 39–52. [Google Scholar]
- Badreddine, R.; Grandjean, F.; Vandormael, D.; Fransolet, A.M.; Long, G.J. An Fe-57 Mossbauer spectral study of vermiculitization in the Palabora Complex, Republic of South Africa. Clay Miner. 2000, 35, 653–663. [Google Scholar] [CrossRef]
- Grandjean, J. Water sites at a clay interface. J. Colloid Interface Sci. 1997, 185, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Theng, B.K.G. Surface acidity and catalytic activity. In Clay Mineral Catalysis of Organic Reactions, 1st ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 85–130. [Google Scholar]
- Ying, S. Preparation and properties of vermiculite supported TiO2 photocatalyst. Chin. J. Inorg. Chem. 2011, 27, 40–46. [Google Scholar]
- Li, P.; Wen, B.; Yu, F.; Zhu, M.; Guo, X.; Han, Y.; Kang, L.; Huang, X.; Dan, J.; Ouyang, F.; et al. High efficient nickel/vermiculite catalyst prepared via microwave irradiation-assisted synthesis for carbon monoxide methanation. Fuel 2016, 171, 263–269. [Google Scholar] [CrossRef]
- Valášková, M.; Kupková, J.; Kočí, K.; Ambrožová, N.; Klemm, V.; Rafaja, D. Structural properties and photocatalytic activity of ceria nanoparticles on vermiculite matrix. J. Nanosci. Nanotechnol. 2016, 16, 7844–7848. [Google Scholar] [CrossRef]
- Valášková, M.; Kupková, J.; Simha Martynková, G.; Seidlerová, J.; Tomášek, V.; Ritz, M.; Kočí, K.; Klemm, V.; Rafaja, D. Comparable study of vermiculites from four commercial deposits prepared with fixed ceria nanoparticles. Appl. Clay Sci. 2018, 151, 164–174. [Google Scholar] [CrossRef]
- Valášková, M.; Tokarský, J.; Pavlovský, J.; Prostějovský, T.; Kočí, K. α–Fe2O3 nanoparticles/vermiculite clay material: Structural, optical and photocatalytic properties. Materials 2019, 12, 1880. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Yu, F.; Zhu, M.; Dan, J.; Wang, X.; Zhang, J.; Dai, B. Enhanced low temperature NO reduction performance via MnOx-Fe2O3/vermiculite monolithic honeycomb catalysts. Catalysts 2018, 88, 100. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Tian, J.; Qian, G.; Liu, Z.; Li, W.; Dan, J.; Dai, B.; Yu, F. Ultralow specific surface area vermiculite supporting Mn-Ce-Fe mixed oxides as “curling catalysts” for selective catalytic reduction of NO with NH3. Green Chem. Eng. 2021, 2, 284–293. [Google Scholar] [CrossRef]
- Reli, M.; Ambrožová, N.; Valášková, M.; Edelmannová, M.; Čapek, L.; Schimpf, C.; Motylenko, M.; Rafaja, K.; Kočí, K. Photocatalytic water splitting over CeO2/Fe2O3/Ver photocatalysts. Energy Convers. Manag. 2021, 238, 114156. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Chen, R.; Liu, X. Vermiculite as a natural silicate crystal for hydrogen generation from photocatalytic splitting of water under visible light. RSC Adv. 2014, 4, 406–408. [Google Scholar] [CrossRef]
- Valášková, M.; Madejová, J.; Inayat, A.; Matějová, L.; Ritz, M.; Martaus, A.; Leštinský, P. Vermiculites from Brazil and Palabora: Structural changes upon heat treatment and influence on the depolymerization of polystyrene. Appl. Clay Sci. 2020, 192, 105639. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, A.; Wang, L.; Li, X.; Huang, W. Striving toward visible light photocatalytic water splitting based on natural silicate clay mineral: The interface modification of attapulgite at the atomic-molecular level. ACS Sustain. Chem. Eng. 2016, 4, 4601–4607. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Ma, H.; Cheng, L.; Ar, S.; Yang, J.; Zhang, Q. A new natural layered clay mineral applicable to photocatalytic hydrogen production and/or degradation of dye pollutant. Environ. Prog. Sustain. Energy 2018, 37, 1003–1010. [Google Scholar] [CrossRef]
- Boumaza, S.; Boudjemaa, A.; Omeiri, S.; Bouarab, R.; Bouguelia, A.; Trari, M. Physical and photoelectrochemical characterizations of hematite α-Fe2O3: Application to photocatalytic oxygen evolution. Sol. Energy 2010, 84, 715–721. [Google Scholar] [CrossRef]
- Mekatel, E.; Trari, M.; Nibou, D.; Sebai, I.; Amorkrane, S. Preparation and characterization of a-Fe 2O 3 supported clay as a novel photocatalyst for hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 10309–10315. [Google Scholar] [CrossRef]
- Ibrahim, N.S.; Leaw, W.L.; Mohamad, D.; Alias, S.H.; Nur, H. A critical review of metal-doped TiO2 and its structure–physical properties–photocatalytic activity relationship in hydrogen production. Int. J. Hydrogen Energy 2020, 45, 28553–28565. [Google Scholar] [CrossRef]
- Kazi, M.-K.; Eljack, F.; El-Halwagi, M.M.; Haouari, M. Green hydrogen for industrial sector decarbonization: Costs and impacts on hydrogen economy in Qatar. Comput. Chem. Eng. 2021, 145, 107144. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Otha, T.; Masegi, H.; Noda, K. Photocatalytic decomposition of gaseous methanol over anodized iron oxide nanotube arrays in high vacuum. Mater. Res. Bull. 2018, 99, 367–376. [Google Scholar]
- Lassoued, A.; Dkhil, B.; Gardi, A.; Ammar, S. Control of the shape and size of iron oxide (α–Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 2017, 7, 3007–3015. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Broekhoff, J.C.P.; de Boer, J.H. Studies on pore systems in catalysts: XI. Pore distribution calculations from the adsorption branch of a nitrogen adsorption isotherm of open cylindrical pores A. Fundamental equations. J. Catal. 1967, 9, 8–14. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Horvath, G.; Kawazoe, K. Method for the calculation of effective pore-size distribution in molecular-sieve carbon. J. Chem. Eng. J. 1983, 16, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Kubelka, P.; Munk, F. Ein Beitrag zur Optik der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Brindley, G.W.; Zalba, P.E.; Bethke, C.M. Hydrobiotite, a regular 1:1 interstratification of biotite and vermiculite layers. Am. Mineral. 1983, 68, 420–425. [Google Scholar]
- Marcos, C.; Arango, Y.C.; Rodriguez, I. X-ray diffraction studies of the thermal behaviour of commercial vermiculites. Appl. Clay Sci. 2009, 42, 368–378. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Gött. Nachr. 1918, 2, 98–100. [Google Scholar]
- Farmer, V.C. The layer silicates. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society: London, UK, 1974; pp. 331–363. [Google Scholar]
- Madejova, J.; Gates, W.P.; Petit, S. IR spectra of clay minerals. In Infrared and Raman Spectroscopies of Clay Minerals. Developments in Clay Science; Gates, W.P., Klopproge, J.T., Madejova, J., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, pp. 107–149. [Google Scholar]
- Swayze, G.A.; Lowers, H.A.; Benzel, W.M.; Clark, R.N.; Driscoll, R.L.; Perlman, Z.S.; Hoefen, T.M.; Dyar, M.D. Characterizing the source of potentially asbestosbearing commercial vermiculite insulation using in situ IR spectroscopy. Am. Mineral. 2018, 103, 517–549. [Google Scholar] [CrossRef]
- Heller-Kallai, L. Chapter 10.2—Thermally modified clay minerals. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands; The Hebrew University of Jerusalem: Jerusalem, Israel, 2013; Volume 5411–5433. [Google Scholar]
- Huang, C.; Fan, E.; Xu, H.; Li, M.; Shao, G.; Wang, H.; Lu, H.; Zhang, R. Effect of particle size of vermiculite on the microstructure and photocatalytic performance of g-C3N4/vermiculite composite. Solid State Sci. 2021, 113, 106533. [Google Scholar] [CrossRef]
- Martínez-Costa, J.I.; Rivera-Utrilla, J.; Leyva-Ramos, R.; Sánchez-Polo, M.; Velo-Gala, I. Individual and simultaneous degradation of antibiotics sulfamethoxazole and trimethoprim by UV and solar radiation in aqueous solution using bentonite and vermiculite as photocatalysts. Appl. Clay Sci. 2018, 160, 217–225. [Google Scholar] [CrossRef]
- Yamanoi, Y.; Nakashima, S.; Katsura, M. Temperature dependence of reflectance spectra and color values of hematite by in situ, high-temperature visible micro-spectroscopy. Am. Mineral. 2009, 94, 90–97. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α–Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 49, 126–141. [Google Scholar] [CrossRef]
- Vayssieres, L.; Sathe, C.; Butorin, S.M.; Shuh, D.K.; Nordgren, J.; Guo, J. One-dimensional quantum-confinement effect in α–Fe2O3 ultrafine nanorod arrays. Adv. Mater. 2005, 17, 2320–2323. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Wang, G.; Ling, Y.; Li, Y.; Zhang, J.Z. Nanostructured hematite: Synthesis, characterization, charge carrier dynamics, and photoelectrochemical properties. Energy Environ. Sci. 2012, 5, 6682–6702. [Google Scholar] [CrossRef]
- Mahadik, M.A.; Shinde, S.S.; Mohite, V.S.; Kumbhar, S.S.; Rajpure, K.Y.; Moholkar, A.V.; Bhosale, C.H. Photoelectrocatalytic activity of ferric oxide nanocatalyst: A synergestic effect of thickness. Ceram. Int. 2014, 40, 9463–9471. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Liston, D.B.; Lovejoy, J.A.; Deng, H.; Zhang, J.Z. Ultrafast studies of photoexcited electron dynamics in γ– and α–Fe2O3 semiconductor nanoparticles. J. Phys. Chem. B 1998, 102, 770–776. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, W.; He, B.; Zhang, J.; Anpo, M. Characterization of Fe–TiO2 photocatalysts synthesized by hydrothermal method and their photocatalytic reactivity for photodegradation of XRG dye diluted in water. J. Mol. Catal. A Chem. 2004, 216, 35–43. [Google Scholar] [CrossRef]
- Mathevula, L.E.; Noto, L.L.; Mothudi, B.M.; Chithambo, M.; Dhlamini, M.S. Structural and optical properties of sol-gel derived α–Fe2O3 nanoparticles. J. Lumin. 2017, 192, 879–887. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Oxidation of hydrogen and reduction of methanol to methane is the sole energy source for a methanogen isolated from human feces. J. Bacteriol. 1983, 153, 1051–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, A.B.; Jadhav, B.D.; Bhoir, P. Optical band gap modification of Ce/ZnO for visible light photocatalytic H2 production from aqueous methanol solution. Opt. Mater. 2021, 121, 111503. [Google Scholar] [CrossRef]
- Sadale, S.B.; Noda, K.; Kobayashi, K.; Yamada, H. Real-time investigation on photocatalytic oxidation of gaseous methanol with nanocrystalline WO3–TiO2 composite films. Thin Solid Films 2012, 520, 3847–3851. [Google Scholar] [CrossRef]
- Masegi, H.; Goto, H.; Sadale, S.B.; Noda, K. Real-time monitoring of photocatalytic methanol decomposition over Cu2O-loaded TiO2 nanotube arrays in high vacuum. J. Vac. Sci. Technol. B 2020, 38, 052401. [Google Scholar] [CrossRef]
- Jung, K.Y.; Park, S.B.; Ihm, S.-K. Linear relationship between the crystallite size and the photoactivity of non-porous titania ranging from nanometer to micrometer size. Appl. Catal. A 2002, 224, 229–237. [Google Scholar] [CrossRef]
- Jung, K.Y.; Park, S.B. Photoactivity of SiO2/TiO2 and ZrO2/TiO2 mixed oxides prepared by sol–gel method. Mater. Lett. 2004, 58, 2897–2900. [Google Scholar] [CrossRef]
- Tanwar, K.S.; Lo, C.S.; Eng, P.J.; Catalano, J.G.; Walko, D.A.; Brown, G.E., Jr.; Waychunas, G.A.; Chaka, A.M.; Trainor, T.P. Surface diffraction study of the hydrated hematite (1ī02) surface. Surf. Sci. 2007, 601, 460–474. [Google Scholar] [CrossRef]



| Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 FeO | P2O5 | CaO | MgO | K2O | Na2O | L.O.I. 1 | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ver | 35.53 ± 0.02 | 1.26 ± 0.01 | 7.59 ± 0.01 | 9.17 ± 0.01 | 1.16 ± 0.01 | 3.67 ± 0.01 | 23.02 ± 0.05 | 5.60 ± 0.01 | 0.08 ± 0.01 | 12.30 | 99.91 |
| 0.53 | |||||||||||
| V–500 | 38.27 ± 0.03 | 1.34 ± 0.01 | 8.11 ± 0.02 | 10.20 ± 0.01 | 1.22 ± 0.01 | 3.74 ± 0.01 | 24.46 ± 0.05 | 5.92 ± 0.01 | 0.09 ± 0.01 | 5.53 | 99.50 |
| 0.62 | |||||||||||
| V–700 | 37.39 ± 0.03 | 1.35 ± 0.01 | 7.73 ± 0.02 | 11.12 ± 0.01 | 1.61 ± 0.01 | 4.53 ± 0.01 | 23.36 ± 0.05 | 5.85 ± 0.01 | 0.07 ± 0.01 | 5.27 | 98.41 |
| 0.13 | |||||||||||
| Fe/Ver | 25.94 ± 0.01 | 0.97 ± 0.01 | 5.40 ± 0.01 | 37.38 ± 0.02 | 1.15 ± 0.01 | 3.33 ± 0.01 | 16.55 ± 0.05 | 3.86 ± 0.01 | 1.40 ± 0.01 | 3.87 | 99.85 |
| Fe/V–500 | 27.26 ± 0.02 | 0.96 ± 0.01 | 5.82 ± 0.01 | 35.16 ± 0.02 | 1.28 ± 0.01 | 3.19 ± 0.01 | 17.12 ± 0.05 | 3.83 ± 0.01 | 1.41 ± 0.01 | 3.86 | 99.89 |
| Fe/V–700 | 24.97 ± 0.02 | 0.92 ± 0.01 | 5.23 ± 0.01 | 39.15 ± 0.02 | 1.21 ± 0.01 | 3.26 ± 0.01 | 16.34 ± 0.05 | 3.60 ± 0.01 | 1.23 ± 0.01 | 3.87 | 99.78 |
| Atoms | Si | Al | Fe3+ | Tet.ch. | Al | Fe3+ | Fe2+ | Mg | Ti | Oct.ch. | Tot.ch. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ver | 2.887 | 0.726 | 0.387 | −1.113 | 0.00 | 0.173 | 0.063 | 2.687 | 0.077 | +0.327 | −0.786 |
| V–500 | 2.900 | 0.723 | 0.377 | −1.100 | 0.00 | 0.204 | 0.039 | 2.681 | 0.076 | +0.366 | −0.734 |
| V–700 | 2.902 | 0.707 | 0.391 | −1.098 | 0.00 | 0.258 | 0.010 | 2.653 | 0.079 | +0.416 | −0.682 |
| Samples | Fe–500 | Fe/Ver | Fe/V–500 | Fe/V–700 |
|---|---|---|---|---|
| D(012) (nm) | 29 | 31 | 27 | 34 |
| Ver | V–500 | V–700 | Fe–500 | Fe/Ver | Fe/V–500 | Fe/V–700 | |
|---|---|---|---|---|---|---|---|
| SBET (m2/g) | 25 | 23 | 24 | 36 | 58 | 53 | 63 |
| Vtot (mm3liq/g) | 86 | 85 | 100 | 197 | 145 | 143 | 161 |
| Ver | V–500 | V–700 | Fe–500 | Fe/Ver | Fe/V–500 | Fe/V–700 | |
|---|---|---|---|---|---|---|---|
| Eg (eV) | 1.64 | 1.70 | 1.86 | 1.99 | 1.55 | 1.50 | 1.35 |
| 3.03 | 2.50 | 2.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valášková, M.; Kočí, K.; Madejová, J.; Matějová, L.; Pavlovský, J.; Barrocas, B.T.; Klemencová, K. α-Fe2O3 Nanoparticles/Iron-Containing Vermiculite Composites: Structural, Textural, Optical and Photocatalytic Properties. Minerals 2022, 12, 607. https://doi.org/10.3390/min12050607
Valášková M, Kočí K, Madejová J, Matějová L, Pavlovský J, Barrocas BT, Klemencová K. α-Fe2O3 Nanoparticles/Iron-Containing Vermiculite Composites: Structural, Textural, Optical and Photocatalytic Properties. Minerals. 2022; 12(5):607. https://doi.org/10.3390/min12050607
Chicago/Turabian StyleValášková, Marta, Kamila Kočí, Jana Madejová, Lenka Matějová, Jiří Pavlovský, Beatriz Trindade Barrocas, and Kateřina Klemencová. 2022. "α-Fe2O3 Nanoparticles/Iron-Containing Vermiculite Composites: Structural, Textural, Optical and Photocatalytic Properties" Minerals 12, no. 5: 607. https://doi.org/10.3390/min12050607
APA StyleValášková, M., Kočí, K., Madejová, J., Matějová, L., Pavlovský, J., Barrocas, B. T., & Klemencová, K. (2022). α-Fe2O3 Nanoparticles/Iron-Containing Vermiculite Composites: Structural, Textural, Optical and Photocatalytic Properties. Minerals, 12(5), 607. https://doi.org/10.3390/min12050607







