Effects of Al(III) Ions at Magnetite Flotation from Quartz by Dodecylamine Al(III)
Abstract
:1. Introduction
2. Materials and Methods
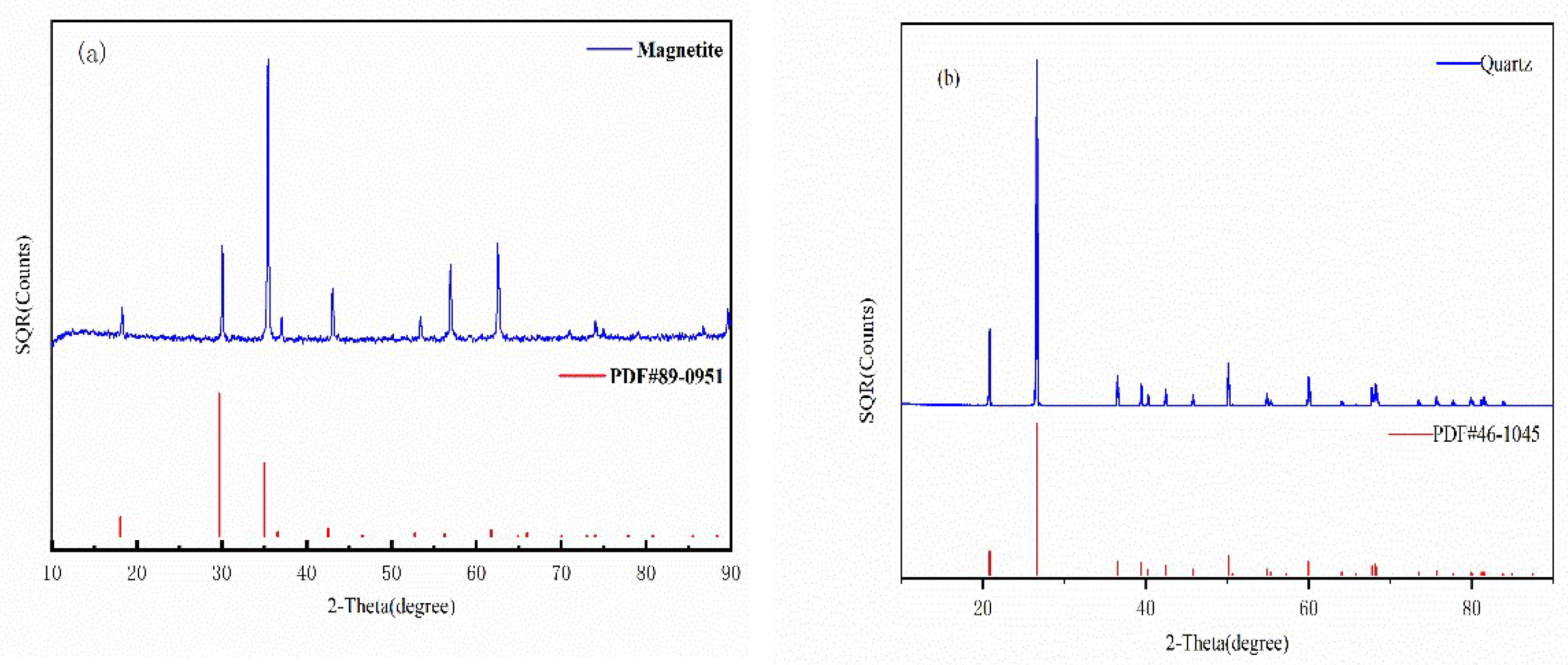
2.1. Materials and Reagents
2.2. Micro-Flotation Experiments
2.3. Solution Chemistry Calculations
2.4. Zeta Potential Analysis
2.5. Contact Angle Measurements
2.6. FT-IR Analysis
3. Results and Discussions
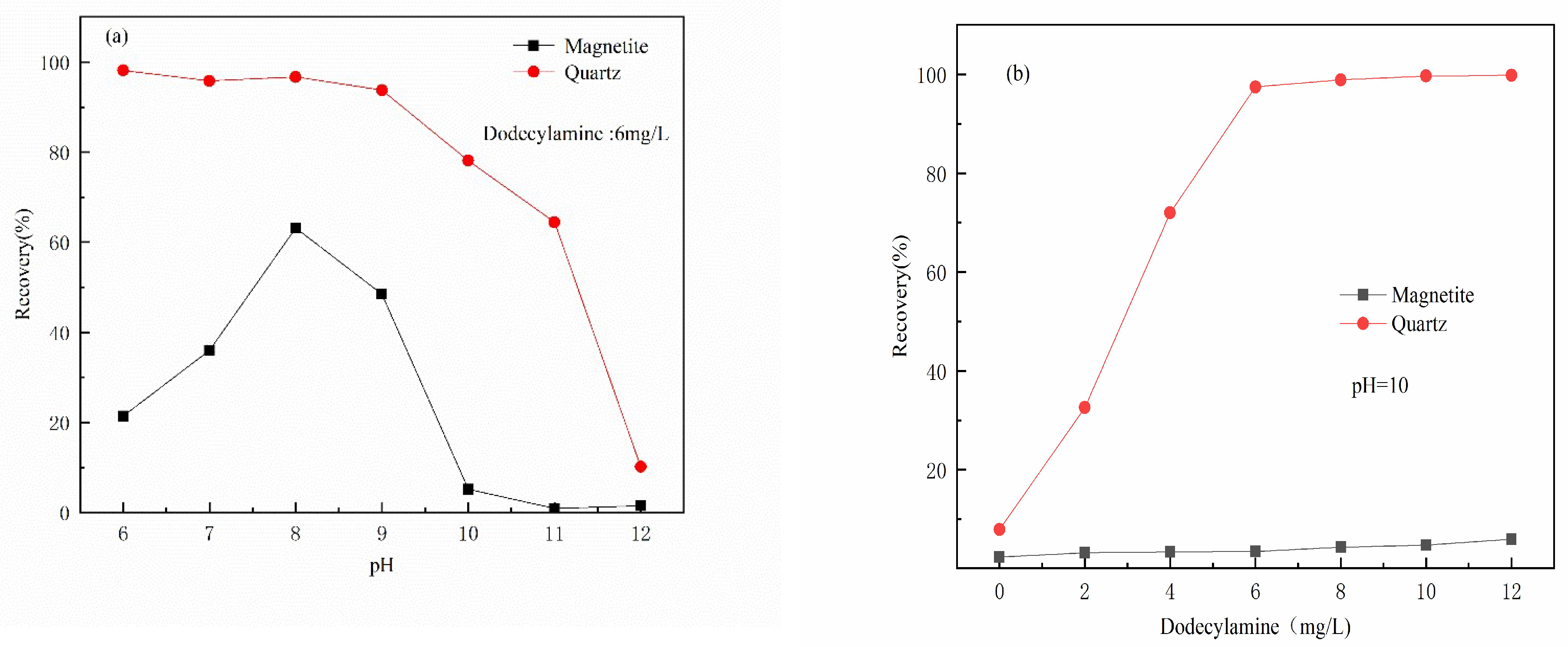
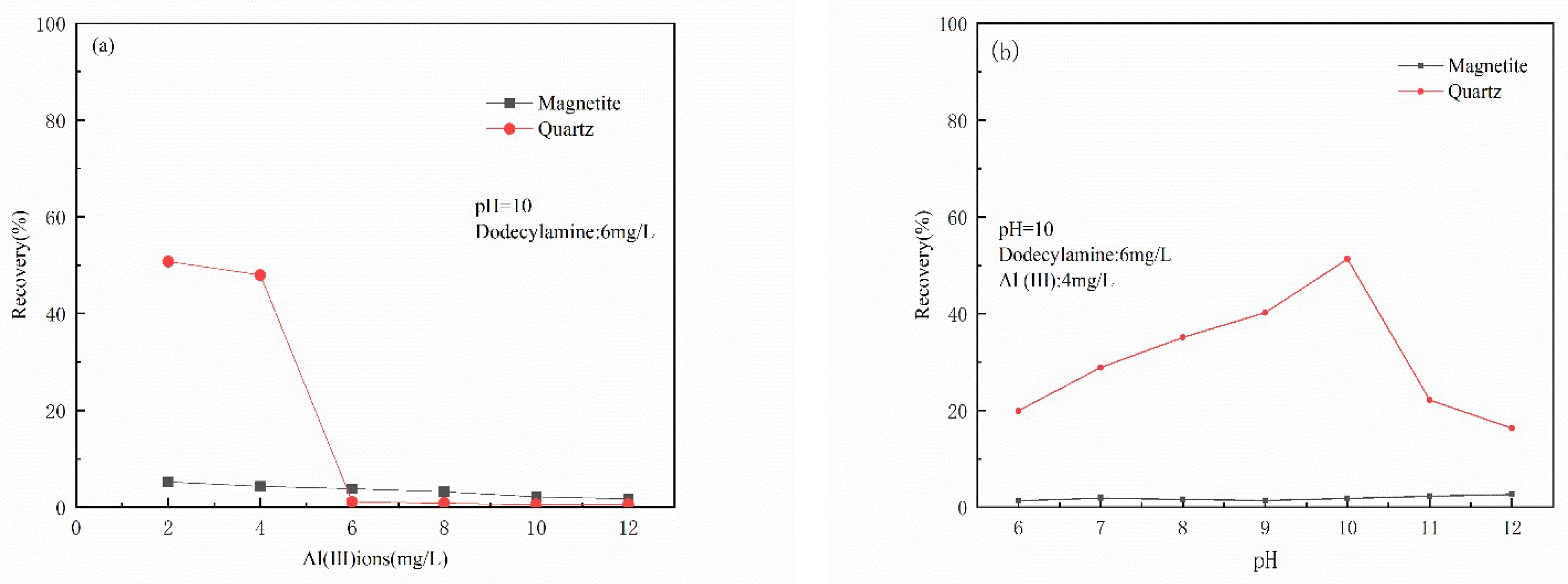
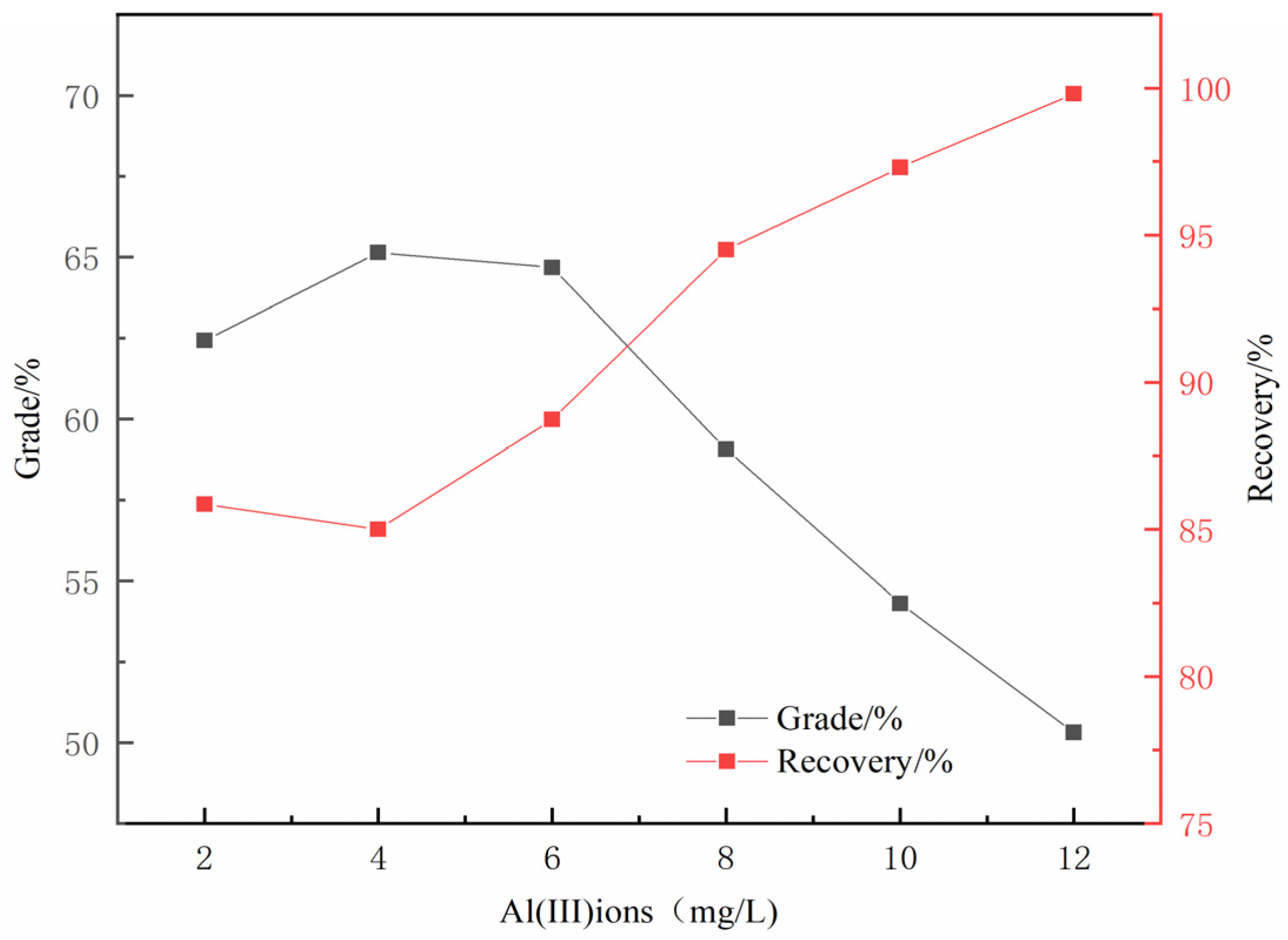
3.1. Micro-Flotation Experiments
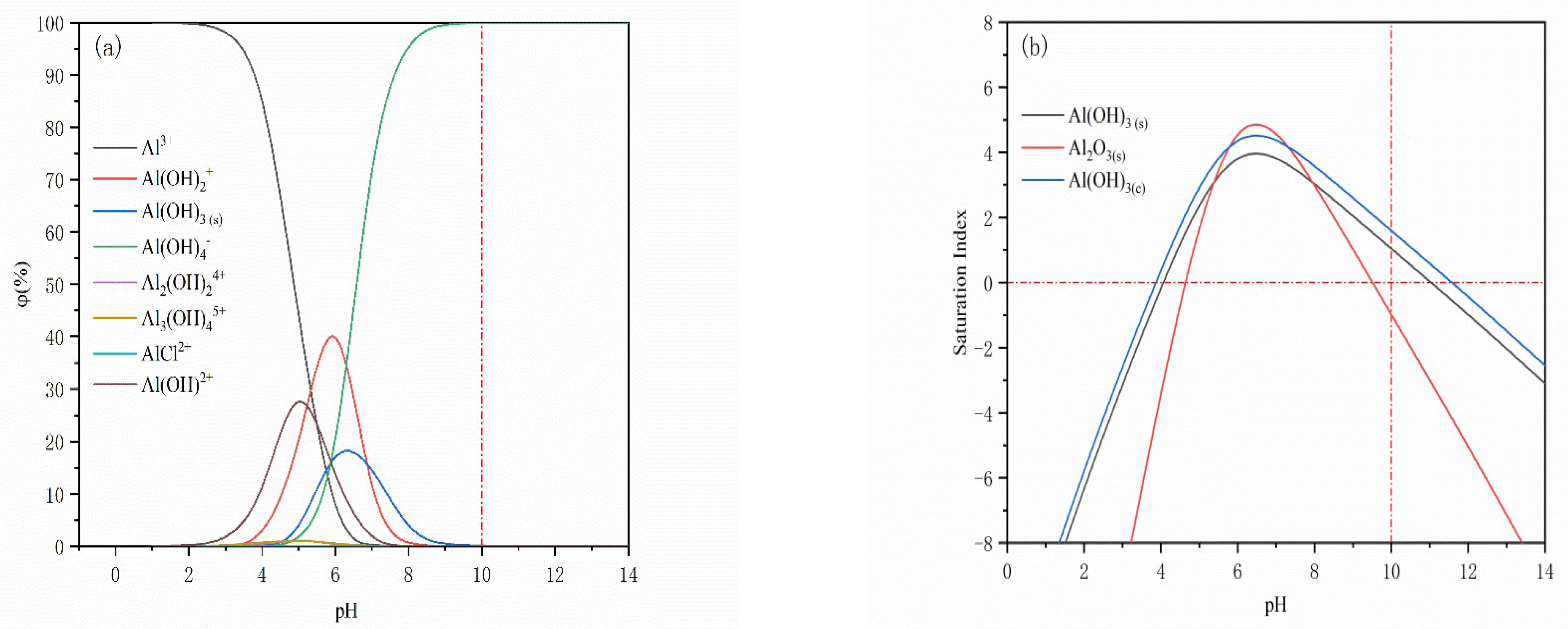
3.2. Solution Chemical Calculations and Analysis
3.3. Zeta Potential Analysis
3.4. Contact Angle Measurements
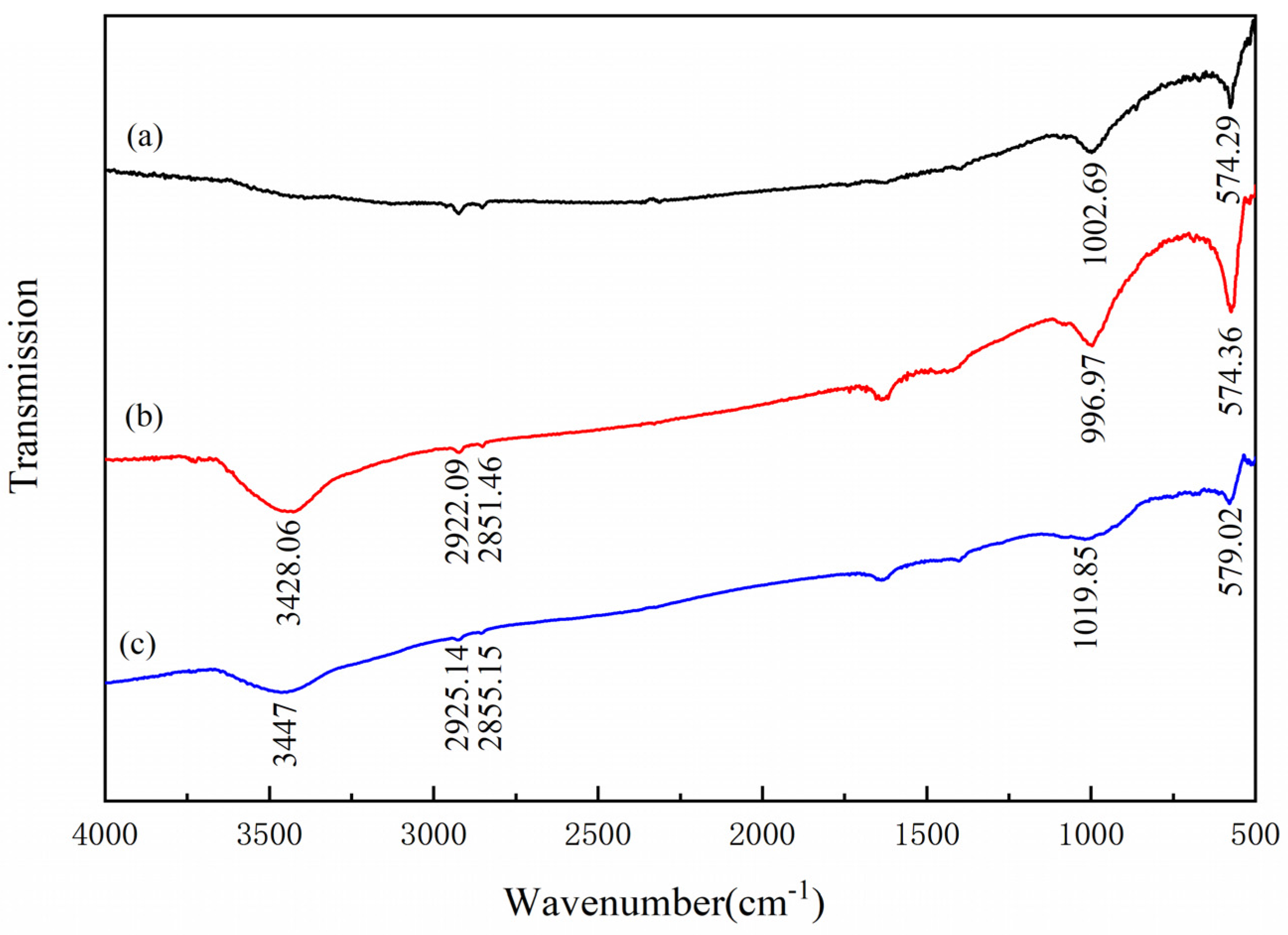
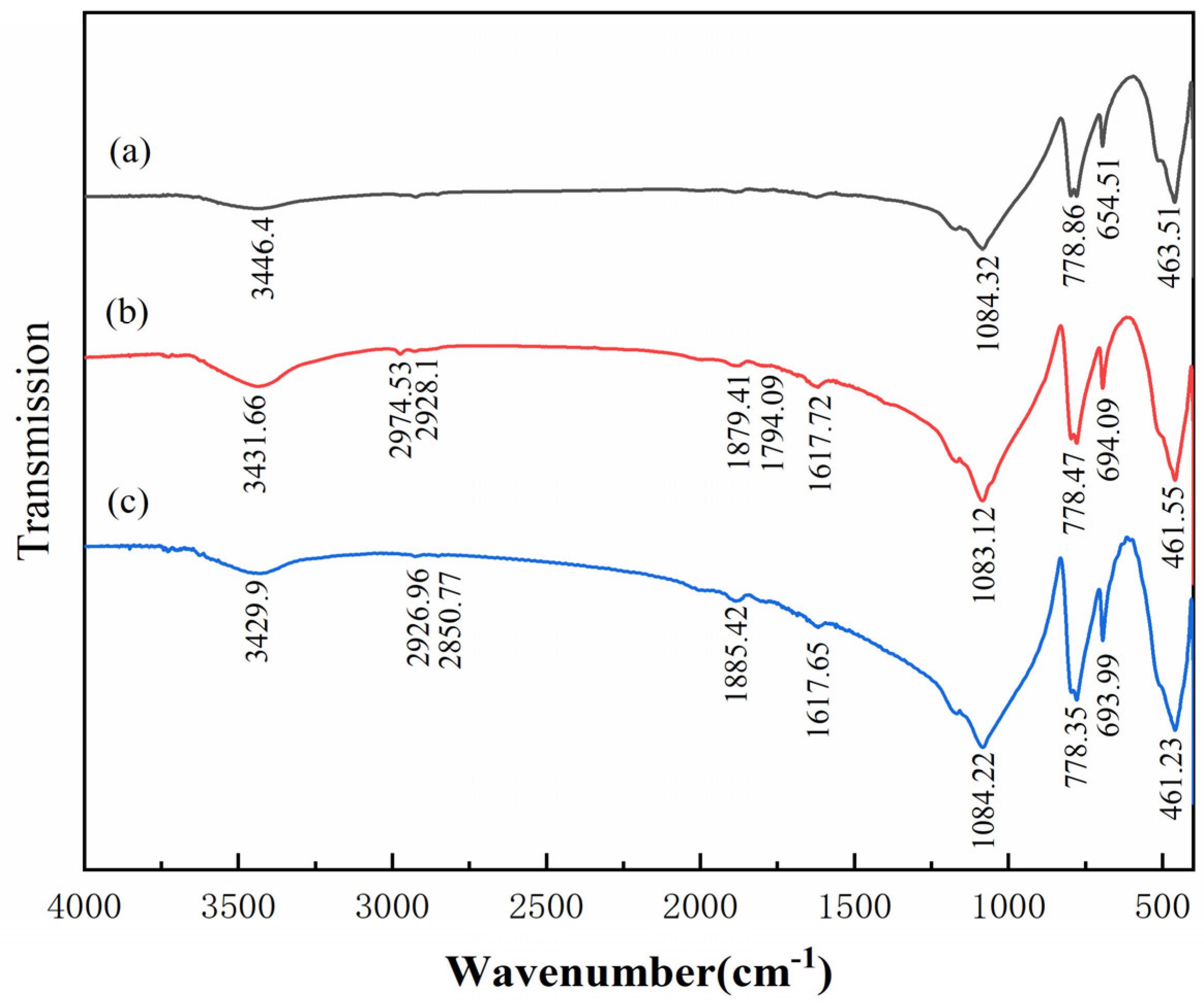
3.5. FTIR Analysis
3.6. Discussion
4. Conclusions
- (1)
- The results of micro-flotation experiments show that Al(III) ions in water have a strong inhibitory effect on magnetite, and the inhibitory effect on quartz is not very obvious.
- (2)
- The solution stoichiometry results show that the phases formed by Al(III) ions on the surface of magnetite and quartz are mainly Al(OH)3(s) and Al(OH)4−.
- (3)
- The results of zeta calculation and analysis show that the precipitation of Al(OH)3 produced by Al(III) ions will cover the surface of magnetite and hinder the direct interaction between minerals and collectors, resulting in a decrease in the floatability of magnetite. However, the subsequent adsorption of dodecylamine was hindered due to the precipitation of Al(III) ions and covering the active sites on the mineral surface.
- (4)
- The contact angle measurement results showed that Al(III) ions weakened the hydrophobicity of magnetite and quartz by reducing the contact angle of magnetite and quartz, and the floatability of magnetite and quartz after treatment decreased. Therefore, with the addition of Al(III) ions, the floatability of magnetite is lower than that of quartz.
- (5)
- The results of FTIR analysis showed that the addition of Al(III) ions could hinder the adsorption of dodecylamine on the magnetite surface. Meanwhile, the addition of Al(III) ions has no obvious effect on the adsorption of dodecylamine on the quartz surface.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, S.; Wang, L. Measurement of froth zone and collection zone recoveries with various starch depressants in anionic flotation of hematite and quartz. Miner. Eng. 2019, 138, 31–42. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L. Structural and functional insights into starches as depressant for hematite flotation. Miner. Eng. 2018, 124, 149–157. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Xie, Y.; Shang, Y.; Zheng, G. A novel macromolecular depressant for reverse flotation: Synthesis and depressing mechanism in the separation of hematite and quartz. Sep. Purif. Technol. 2017, 186, 175–181. [Google Scholar] [CrossRef]
- Luo, L.; Wu, H.; Xu, L.; Meng, J.; Huang, L.; Lu, J.; Zhou, H.; Huo, X. An in situ ATR-FTIR study of mixed collectors BHA/DDA adsorption in ilmenite-titanaugite flotation system. International J. Min. Sci. Technol. 2021, 10, 689–697. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Wen, S.; Ma, M.; Sun, C.; Yin, W.; Ma, Y. Effect of carbonate minerals on quartz flotation behavior under conditions of reverse anionic flotation of iron ores. Int. J. Miner. Process. 2016, 152, 1–6. [Google Scholar] [CrossRef]
- Lima, N.P.; Valadao, G.; Peres, A.M.E. Effect of amine and starch dosages on the reverse cationic flotation of an iron ore. Miner. Eng. 2013, 45, 180–184. [Google Scholar] [CrossRef]
- Mowla, D.; Karimi, G.; Ostadnezhad, K.J.S. Removal of hematite from silica sand ore by reverse flotation technique. Sep. Purif. Technol. 2008, 58, 419–423. [Google Scholar] [CrossRef]
- Scott, J.L.; Smith, R.W. Calcium ion effects in amine flotation of quartz and magnetite. Miner. Eng. 1993, 6, 1245–1255. [Google Scholar] [CrossRef]
- Vieira, A.M.; Peres, A.E.C. The effect of amine type, pH, and size range in the flotation of quartz. Miner. Eng. 2007, 20, 1008–1013. [Google Scholar] [CrossRef]
- Ma, X.; Marques, M.; Gontijo, C. Comparative studies of reverse cationic/anionic flotation of Vale iron ore. Int. J. Miner. Process. 2011, 100, 179–183. [Google Scholar] [CrossRef]
- Ren, L.; Qiu, H.; Zhang, Y.; Nguyen, A.V.; Ming, Z.; Wei, P.; Long, Q. Effects alkyl ether amine and calcium ions on fine quartz flotation and its guidance for upgrading vanadium from stone coal. Powder Technol. 2018, 338, 180–189. [Google Scholar] [CrossRef]
- Flood, C.; Cosgrove, T.; Howell, I.; Revell, P. Effects of Electrolytes on Adsorbed Polymer Layers: Poly(ethylene oxide)Silica System. Langmuir 2006, 22, 6923–6930. [Google Scholar] [CrossRef] [PubMed]
- Nevskaia, D.M.; Guerrero-Ruiz, A.; López-González, J.d.D. Adsorption of Polyoxyethylenic Nonionic and Anionic Surfactants from Aqueous Solution: Effects Induced by the Addition of NaCl and CaCl2. Interface 1998, 205, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Choi, S.Q.; Park, K.; Han, Y.; Kim, H. Flotation behaviour of malachite in mono- and di-valent salt solutions using sodium oleate as a collector. Int. J. Miner. Process. 2016, 146, 38–45. [Google Scholar] [CrossRef]
- Johnston, D.L. The Flotation of Apatite and Dolomite in Orthophosphate Solution. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1969. [Google Scholar]
- Palmer, B.R.; Gutierrez, B.G.; Fuerstenau, M.C. Mechanisms involved in the flotation of oxides and silicates with anionic collectors-1, 2. Trans. AIME 1975, 258, 257–263. [Google Scholar]
- Chen, Y.; Tong, X.; Feng, D.; Xie, X. Minerals effect of al (iii) ions on the separation of cassiterite and clinochlore through reverse flotation. Minerals 2018, 8, 347. [Google Scholar] [CrossRef] [Green Version]
- Ofor, O. Effect of Inorganic Ions on Oleate Adsorption at a Nigerian Hematite–Water Interface. Science 1996, 180, 323–328. [Google Scholar] [CrossRef]
- Tang, M.; Tong, X. The relationship between anion distribution in process water and flotation properties of iron oxides. Miner. Eng. 2020, 154, 106378. [Google Scholar] [CrossRef]
- Min, T.; Wen, S.J.M. Effects of Cations/Anions in Recycled Tailing Water on Cationic Reverse Flotation of Iron Oxides. Minerals 2019, 9, 161. [Google Scholar]
- Chen, Q.; Tian, M.; Kasomo, R.M.; Li, H.; Zheng, H.; Song, S.; Luo, H.; He, D. Depression effect of Al(III) and Fe(III) on rutile flotation using dodecylamine polyxyethylene ether as collector. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125269. [Google Scholar] [CrossRef]
- Zyga, B.; Zyja, B.; Wei, S.; Ysg, C. Typical roles of metal ions in mineral flotation: A review. Trans. Nonferrous Met. Soc. China 2021, 31, 2081–2101. [Google Scholar]
- Qiang, L.; Li, Y.; Zhang, J.; Ying, C.; Ruan, X.; Liu, J.; Qian, G. Effective removal of zinc from aqueous solution by hydrocalumite. Chem. Eng. J. 2011, 175, 33–38. [Google Scholar]
- Markovski, J.S.; Marković, D.; Đokić, V.; Mitrić, M.; Marinković, A. Arsenate adsorption on waste eggshell modified by goethite, α-MnO2 and goethite/α-MnO2. Chem. Eng. J. 2014, 237, 430–442. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liu, T.; Cai, Z. Influence of metal ions on muscovite and calcite flotation: With respect to the pre-treatment of vanadium bearing stone coal. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 564, 89–94. [Google Scholar] [CrossRef]
- Li, C.; Bai, S.; Ding, Z.; Yu, P.; Wen, S. Visual MINTEQ model, ToF–SIMS, and XPS study of smithsonite surface sulfidation behavior: Zinc sulfide precipitation adsorption. J. Taiwan Inst. Chem. Eng. 2018, 96, 53–62. [Google Scholar] [CrossRef]
- Liu, A.; Fan, M.; Li, Z.; Fan, J. Non-polar oil assisted DDA flotation of quartz I: Interfacial interaction between dodecane oil drop and mineral particle. Int. J. Miner. Process. 2017, 168, 1–8. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Q.; Liu, X.; Li, W.; Chi, R.A. Surface Electrical Behaviors of Apatite, Dolomite, Quartz, and Phosphate Ore. Front. Mater. 2020, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, G.; Ge, P.; Sun, W.; Tang, H.; Hu, W. Activation mechanisms of quartz flotation with calcium ions and cationic/anionic mixed collectors under alkalescent conditions. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 632, 127771. [Google Scholar] [CrossRef]
- Yukselen-Aksoy, Y.; Kaya, A. A study of factors affecting on the zeta potential of kaolinite and quartz powder. Environ. Earth Sci. 2011, 62, 697–705. [Google Scholar] [CrossRef]
- Wang, D.; Liu, D.; Mao, Y.; Sun, R.; Wen, S. Effect of Fluoride Ion on the Separation of Fluorite from Calcite Using Flotation with Acidified Water Glass. Minerals 2021, 11, 1203. [Google Scholar] [CrossRef]
- Liu, C.; Mei, G.; Yu, M.; Cheng, Q.; Yang, S. New applications of deep eutectic solvents for separation of quartz and magnetite. Chem. Phys. Lett. 2021, 762, 138152. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Jurga-Stopa, J.; Coy, E.; Peplinska, B.; Pietralik, Z.; Jurga, S. Fourier transform infrared and Raman spectroscopy studies on magnetite/Ag/antibiotic nanocomposites. Appl. Surface Sci. 2016, 364, 400–409. [Google Scholar] [CrossRef]
- El-Mahdy, G.; Atta, A.; Al-Lohedan, H. Synthesis and Evaluation of Poly(Sodium 2-Acrylamido-2-Methylpropane Sulfonate-co-Styrene)/Magnetite Nanoparticle Composites as Corrosion Inhibitors for Steel. Molecules 2014, 19, 1713–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuel, J.; Kim, J.K.; Ahn, J.H.; Cheruvally, G.; Chauhan, G.S.; Choi, J.W.; Kim, K.W. Surface-modified maghemite as the cathode material for lithium batteries. J. Power Sources 2008, 184, 527–531. [Google Scholar] [CrossRef]
- Drzymala, J.; Fuerstenau, D.W. Selective flocculation of hematite in the hematite-quartz-ferric ion-polyacrylic acid system. Part 1, activation and deactivation of quartz. Int. J. Miner. Process. 1981, 8, 265–277. [Google Scholar]
- Yang, B.; Yin, W.; Yao, J.; Zhu, Z.; Cao, S. Selective collection and differential adsorption of pentaethoxylated laurylamine for the flotation recovery of magnesite from quartz. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 625, 126991. [Google Scholar] [CrossRef]
- Liu, W.G.; Liu, W.B.; Dai, S.J.; Wang, B.Y. Adsorption of bis(2-hydroxy-3-chloropropyl) dodecylamine on quartz surface and its implication on flotation. Results Phys. 2018, 9, 1096–1101. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Hou, B.; Ye, J.; Mao, S.; Li, X. Flotation separation of quartz from collophane using an amine collector and its adsorption mechanisms. Powder Technol. 2017, 318, 224–229. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Tang, M.; Wu, Y.; Niu, X. Effects of Al(III) Ions at Magnetite Flotation from Quartz by Dodecylamine Al(III). Minerals 2022, 12, 613. https://doi.org/10.3390/min12050613
Wang D, Tang M, Wu Y, Niu X. Effects of Al(III) Ions at Magnetite Flotation from Quartz by Dodecylamine Al(III). Minerals. 2022; 12(5):613. https://doi.org/10.3390/min12050613
Chicago/Turabian StyleWang, Dong, Min Tang, Yan Wu, and Xiaoying Niu. 2022. "Effects of Al(III) Ions at Magnetite Flotation from Quartz by Dodecylamine Al(III)" Minerals 12, no. 5: 613. https://doi.org/10.3390/min12050613




