Abstract
In order to improve the recovery of rare earth elements, finding a collector with a strong selectivity ability had become the focus of research. In this paper, phenylpropyl hydroxamic acid (PHA) was used as a new hydrophobic surfactant collector for the separation of bastnaesite from calcite, and salicylic hydroxamic acid (SHA) was used as a reference collector. The results of a single mineral flotation test with SHA show that the reagent has good collection performance and selectivity. In addition, Zeta potential measurements and FTIR analysis show that PHA is adsorbed on the surface of bastnaesite by chemical adsorption, and the surface state of bastnaesite changes after PHA treatment. By XPS analysis, PHA interacts with Ce, and forms a Ce–O bond with Ce. It is speculated that the hydroxamic acid forms a five-element-chelated hydroxamic group with Ce on bastnaesite surface, so as to improve the hydrophobicity of bastnaesite, and make bastnaesite float more easily out of the pulp. According to DFT calculation, PHA has better adsorption capacity and stronger hydrophobicity than SHA, and shows superior electronic group capacity and chemical reactions that promote its flotation performance.
1. Introduction
Rare earth elements (REE) are widely used in advanced technology fields, such as communication, magnetic materials, biomedical materials, and other fields [1,2,3,4,5]. The unique physical and chemical properties of REE can significantly improve the component parameters when REE are used in the production of components. For example, adding a proper amount of REE can improve the alloys properties during a metallurgy process [6,7,8]. When REE are mixed with nanomaterials that are used to manufacture solar cells, the photoelectric conversion efficiency of solar cells can improve greatly [9,10,11]. REE can also be applied in the synthesis of biodegradable implants, which are degradable within the body [12]. Their wide use in different fields means there is a great demand for rare earth elements. Therefore, how to increase REE production has become the research focus.
Due to the different metallogenic regularity, more than 250 kinds of rare earth ore have been found, which are widely distributed in minerals such as carbonates, phosphates, silicates, and other types of minerals. The variety of minerals and their complex composition result in the exploitation and utilization of REE that has become a very difficult problem. Bastnaesite is one of the few rare earth ores with mining value. The main composition of bastnaesite is CeFCO3, and it often combines calcite and quartz.
Previous studies show that magnetic separation [13,14,15], sulfate roasting [16,17], and oxidized roasting–hydrochloric acid leaching [18,19] are the main methods to obtain bastnaesite concentrate. However, these methods have different disadvantages in industrial production. Magnetic minerals might be selected by a magnetic separator, and this reduces the purity of bastnaesite concentrate. Sulfate roasting generates exhaust gases, such as SO2, which are very harmful to the environment and human beings. The process of oxidized roasting–hydrochloric acid leaching is discontinuous, and leads to radioactive elements being distributed in waste water and tailings, which means long-term ecological pollution. Compared with these methods, flotation is more environmentally friendly in dealing with rare earth ores.
A flotation collector changes the minerals surface state, which means it can realize the selective separation of valuable minerals and gangue minerals; therefore, it is widely used in industry production. The proper kind of collector can improve the production efficiency and greatly reduce the cost of production, hence, a collector with an efficient enrichment capacity has become the research focus. Xanthate is one of the commonly used collectors in the flotation of bastnaesite, but it is easy to decompose into toxic compounds, such as carbon disulfide (CS2), which is harmful to the environment. Studies show that hydroxamic acid has strong stability, and is not easy to hydrolyze, oxidize, or decompose. Not only is it environmentally friendly, but it is also a highly efficient collector [20,21]. Metal ions can chelate with the hydroxamic acid collector C(=O) NHOH to form five-member hydroxamic acid groups, in order to improve selectivity [22,23], but the collection ability is not ideal [18,24]. George Blankson Abaka-Wood et al. study the flotation reaction of rare earth oxide (REO) with major gangue phases, using sodium oleate and hydroxamic acid as collectors. Lanqing Deng et al. investigate the flotation separation of scheine and calcite using alkyl alkylamide surfactants. Mingxia Liu et al. prove the effect of chemical adsorption strength on the fine flotation recovery of pyrolites, by studying the flotation of coarse and fine pyrolites by octyl hydroxamic acid and sodium oleate. Based on the previous application of a hydroxamate collector, it can be inferred that the molecular structure of hydroxamic acid plays an important role in the flotation process of bastnaesite [25,26,27,28].
Therefore, a new type of hydrophobic surfactant phenylpropyl hydroxamic acid (PHA) was used as collector to study the flotation separation performance of bastnaesite and calcite. The flotation separation performance of SHA was compared using a single mineral flotation test and Zeta potential measurements. The adsorption mechanism of PHA on the bastnaesite surface was studied by FTIR and XPS, and DFT calculation explained the strong hydrophobicity of PHA.
2. Materials and Methods
2.1. Materials
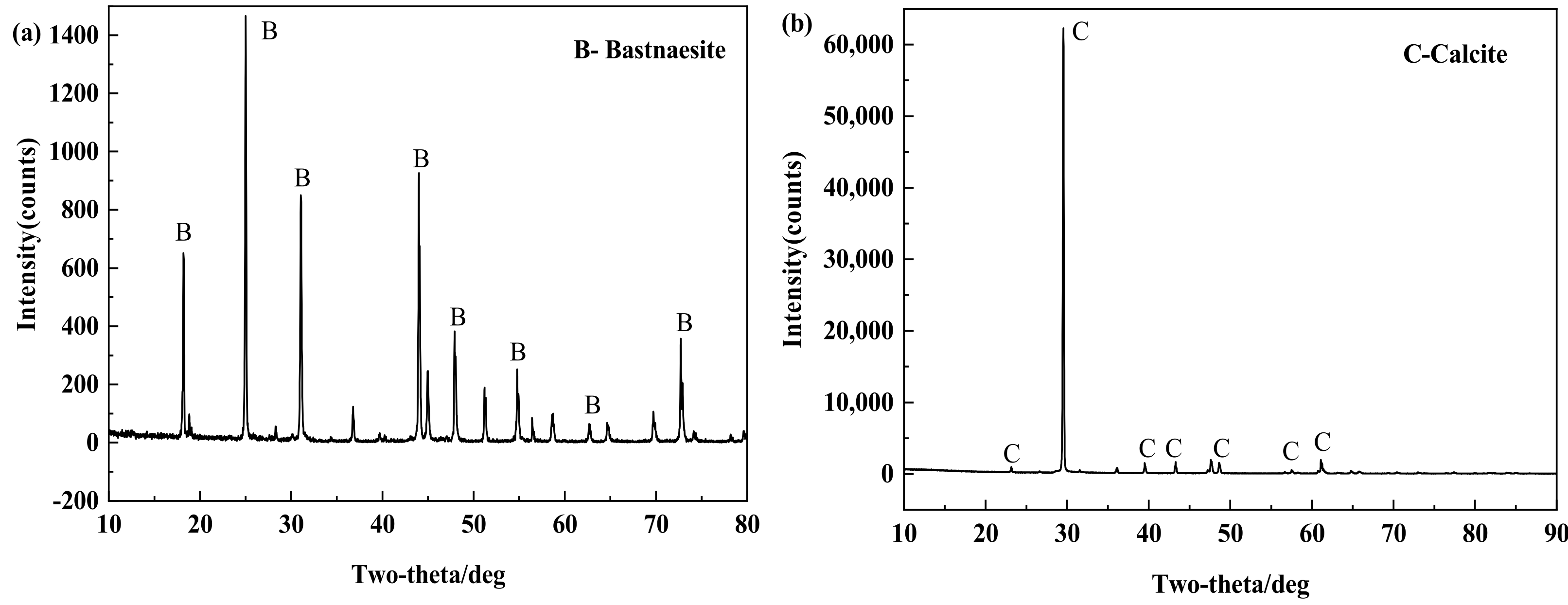
Pure samples of bastnaesite and calcite were purchased online. As shown in Figure 1 and Table 1, the X-ray diffraction (XRD) test patterns and elemental analysis results of the two minerals show that the purity of the samples is sufficient for the flotation test and mechanism test. The two minerals were manually broken, selected, and pulverized by a ceramic ball mill. Flotation experiments were carried out for those with particle size between 35~73 μm, and mechanism tests were carried out for those with particle size less than 5 μm.
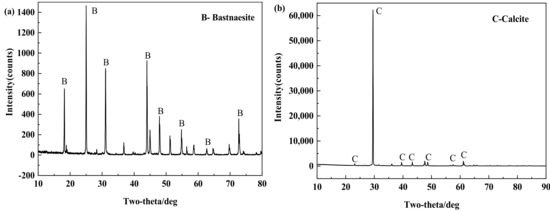
Figure 1.
XRD patterns of bastnaesite (a) and calcite (b).

Table 1.
Elemental analysis results (%) of bastnaesite and calcite sample.
2.2. Synthesis and Characterization of PHA
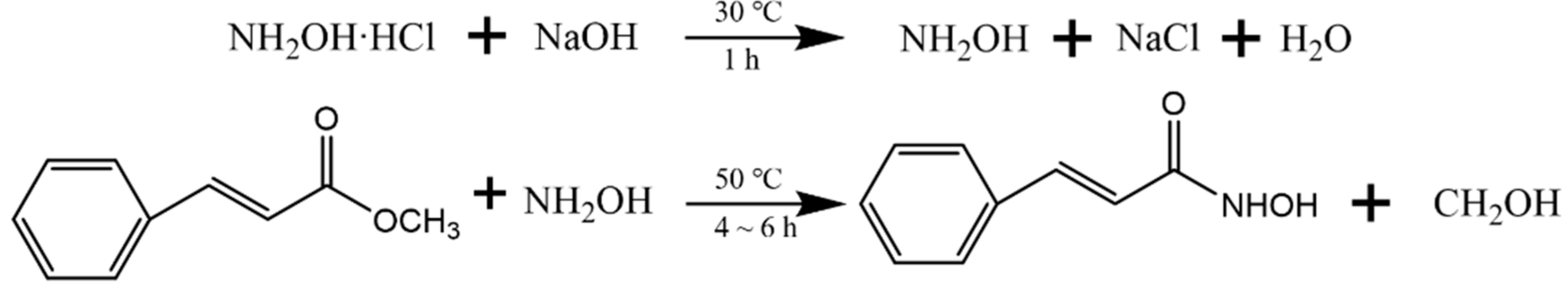
PHA was synthesized from high purity methyl phenylacrylate and hydroxylamine hydrochloride by the hydroxylamine method. The synthesis path is shown in Figure 2. A total of 90 mL DI was added to a 500 mL three-mouth flask, heated by a 30 °C oil bath at constant temperature. First, 35 g hydroxylamine hydrochloride was added, followed by 8.8 g sodium hydroxide in three batches, and the reaction time was 30 min. When hydroxylamine was fully free, methyl phenylacrylate was added. Then the reaction system was heated in a 50 ℃ constant temperature oil bath for 6 h. After the reaction, the reaction solution was acidified with hydrochloric acid to obtain PHA crude product. The crude product was dissolved in as little hot water as possible, cooled, separated out, filtered, and purified repeatedly for 2–3 times, to obtain high purity PHA.
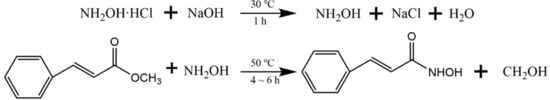
Figure 2.
The synthetic route of PHA.
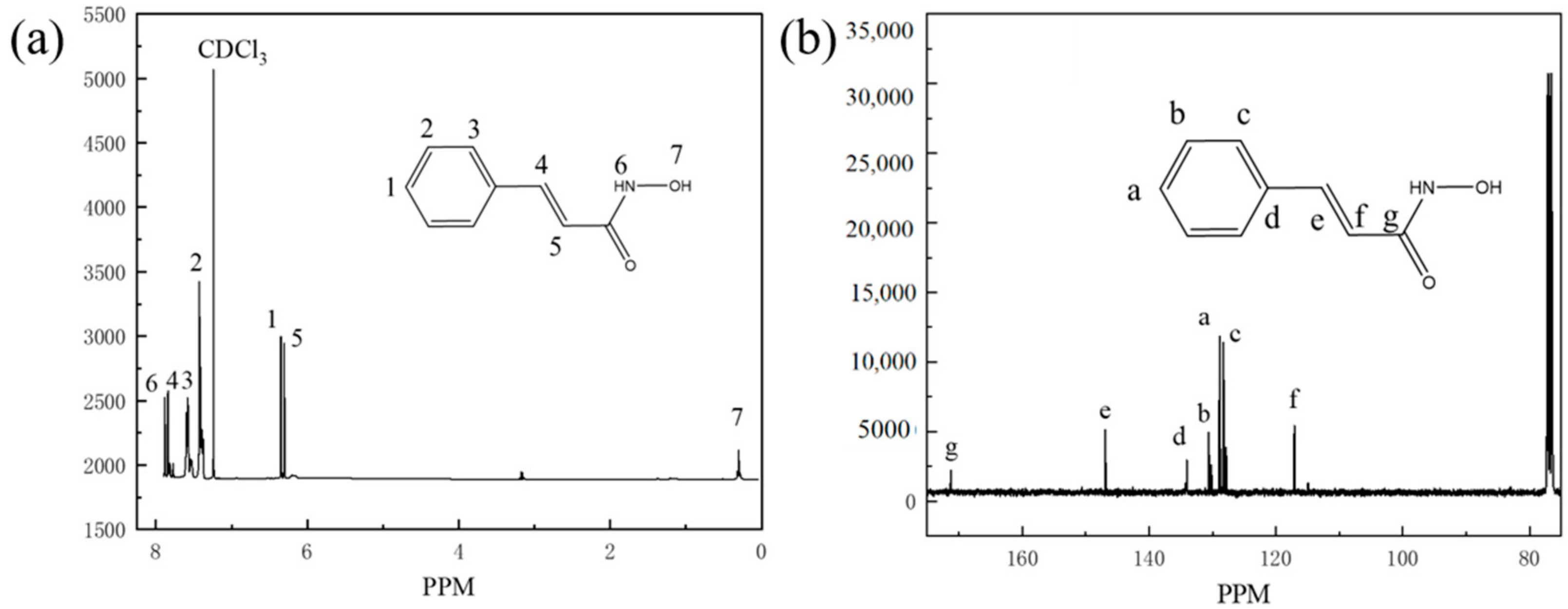
The nuclear magnetic resonance spectroscopy (NMR) is shown in Figure 3. Tetramethylsilane (TMS) was used as internal standard, and deuterium chloroform (CDCl3) was used as solvent. The chemical shifts of hydrogen and carbon atoms in the synthesized product were measured and analyzed using Avance 400 nuclear magnetic resonance instrument, and the structure of the synthesized product was characterized based on the analysis results. The NMR patterns of PHA can be interpreted that 400 MHz 1H NMR (CDCl3, TMS, ppm): δ 1.26 (s, 1H), 6.46 (s, 1H), 6.50 (s, 1H), 7.43 (m, 2H), 7.57 (m, 2H), 7.79 (s, 1H), 7.83 (s, 1H), 100 MHz 13C NMR (CDCl3, TMS, ppm): δ 117.22, 128.33, 128.95, 130.69, 134.1, 146.93, 171.28.
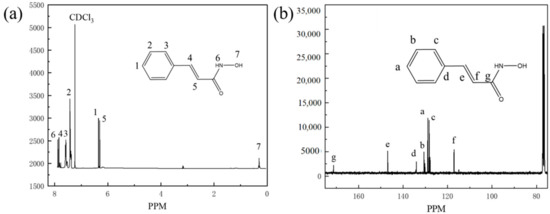
Figure 3.
1H-NMR (a) and 13C-NMR (b) pattern of PHA.
2.3. Experiments Methods
2.3.1. Flotation Experiments
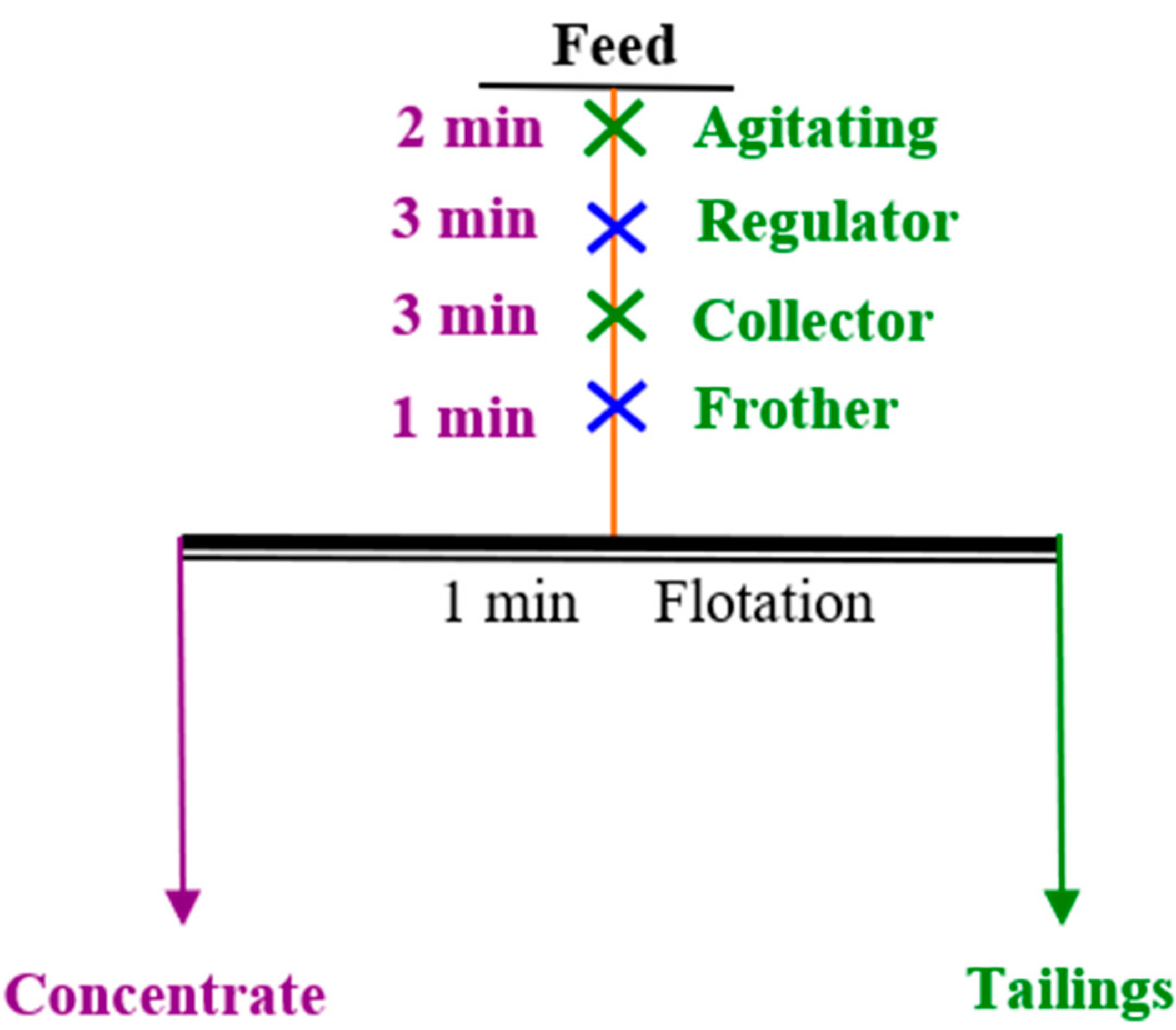
The micro-flotation experiment was carried out using a XFG-II flotation machine with rotation speed of 1980 r/min. The flotation process is shown in Figure 4. A total of 2 g of pure mineral was mixed with 35 mL of DI in a flotation cell. After being stirred for 2 min, pH regulator was added, stirred for 3 min, then the corresponding collector was added and stirred for 3 min. After foaming agent for 1 min, the concentrate and tailings were collected, and recovery calculated. The average of three independent tests were taken as the final result.
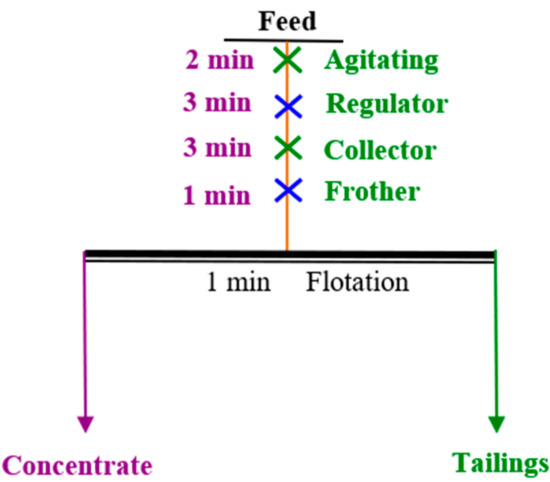
Figure 4.
Flotation experiments process.
2.3.2. Zeta Potential Measurements
Zeta potential measurements were carried out by a JS94H potential tester. The sample was made into slurry with a concentration of 0.01%. After standing for 48 h, the supernatant was taken to test the Zeta potential. The supernatant pH was adjusted by adding diluted HCl or NaOH solution. The average of three independent tests was taken as the final result.
2.3.3. FTIR Measurements
FTIR of bastnaesite and calcite, treated with and without PHA, in the range of 400–4000 cm−1 was scanned by UV-2350 infrared spectrometer. Then, 2 g pure mineral samples were mixed with 40 mL DI in a beaker. The slurry was stirred for 1.5 h, filtered, then the mineral particles were washed with DI 3 times, and dried in a vacuum drying oven at 35 °C for FTIR measurement.
2.3.4. XPS Measurements
Escalab 250Xi was used to obtain XPS spectra of bastnaesite before and after PHA treatment. The adsorption mechanism of PHA on bastnaesite surface was elucidated. The sample preparation method is the same as FTIR. The obtained test results were corrected, by referring to the charge effect of C 1s at 284.8 eV, and the spectral results were processed by Thermo Advantage software.
2.3.5. DFT Calculation
The hydrophobic collector was optimized using DFT calculation. The Gauss 09 software package, with function B3LYP in the basis set of 6-311+G(d) was used. The polarized continuum model (IEF-PCM) was used for calculation, with water as reference solvent.
3. Results
3.1. Micro-Flotation Experiment Results
3.1.1. Single Mineral Flotation Test
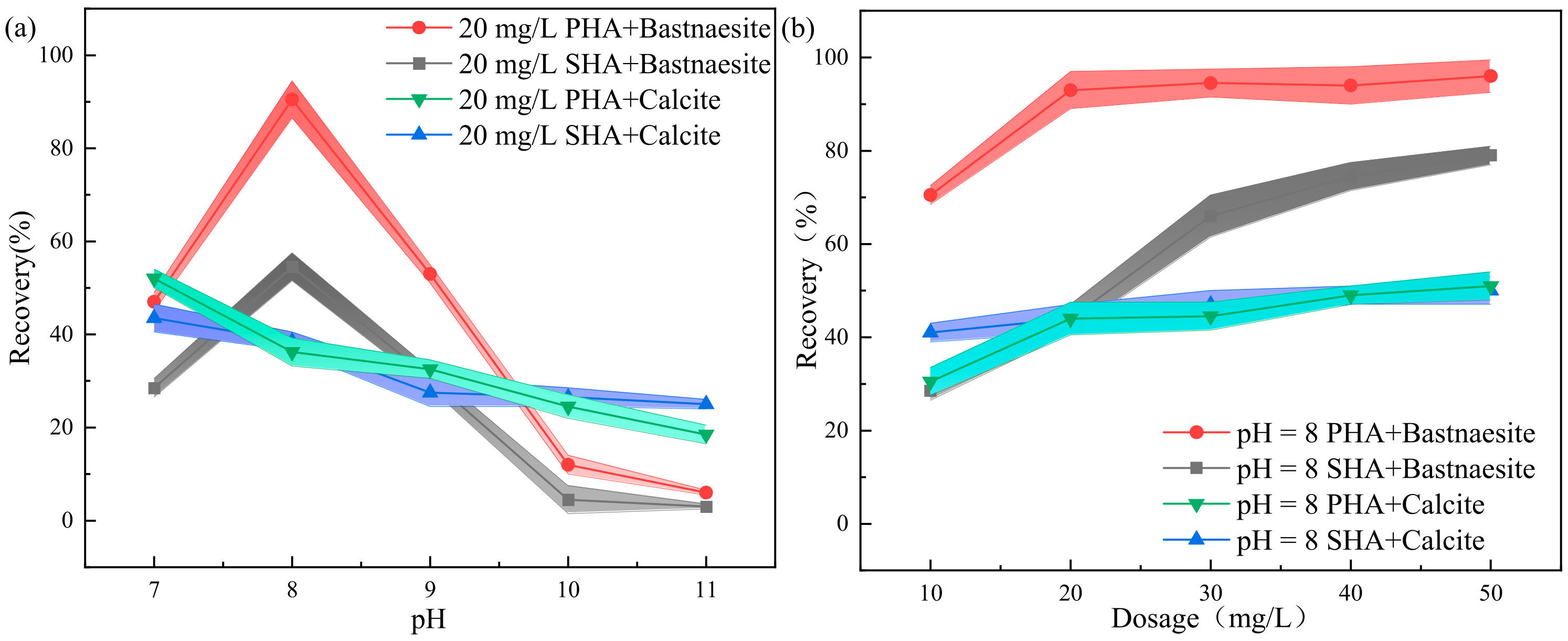
As shown in Figure 5a,b, the dosages of the foaming agent MIBC remain unchanged in the flotation experiment, which is 20 mg/L. When the amounts of PHA and SHA are 20 mg/L, the collector affects the pulp pH of bastnaesite and calcite (Figure 5a). When the pH is 8, the collector affects the dosages of PHA and SHA on pure bastnaesite and pure calcite (Figure 5b).
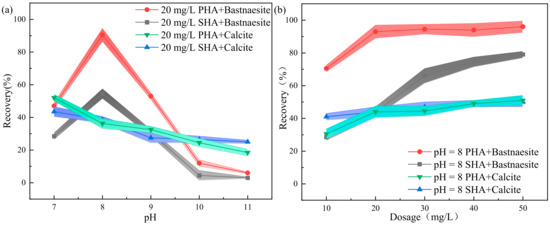
Figure 5.
Effects of the (a) pH and (b) collector dosage on the flotation performance.
The collection effect of PHA and SHA on pure bastnaesite is greatly affected by pH. When the pH is 7~8, the recovery of bastnaesite increases. The maximum recovery of bastnaesite is achieved at pH 8. When PHA is used as the collector, the maximum recovery is 93%, but when SHA is used as the collector, the maximum recovery is only 54.5%. The recovery rate of calcite decreases with the increase in alkalinity, which may be caused by reagent inactivation. When PHA and SHA are used as collectors, the calcite recovery at pH 8 are 36.2% and 38.5%, respectively (Figure 5a).
When pH is 8 and PHA is the collector, the recovery of bastnaesite increases from 70.5% to 93%, with the collector dosage increasing from 10 mg/L to 50 mg/L, and then stabilizing. The maximum recovery is achieved when the dosage of the collector is 20 mg/L. However, when SHA is used as the collector, the recovery of bastnaesite increases from 28.5% to 79%, and the trend still increases. Even when the dosage of SHA is 2.5 times that of PHA, the recovery is still below 90%. Under the same conditions, the recovery of calcite increases slightly with the increase of the collector. When the collector dosage is 20 mg/L, the recovery of the two kinds of collectors are about 45% (Figure 5b).
It is worth noting that PHA collector is a high-molecular-weight compound, and when the concentration is too high, it forms micelles. It is, therefore, advisable not to exceed the CMC point during flotation, as micelle formation means the useless consumption of collector [20].
In conclusion, when pH is 8 and collector dosage is 20 mg/L, PHA efficiently collects bastnaesite. It is proven that PHA is a more efficient collector than SHA.
3.1.2. Artificially Mixed Minerals Flotation Test
In order to further evaluate the flotation performance of PHA, artificial mineral mixing (bastnaesite: calcite = 1:1) was carried out. The recovery and grade of the obtained concentrate product of the minerals are listed in Table 2. Under the optimum condition of single mineral flotation, sodium humate (2 mg/L) was added as an inhibitor. When PHA is used as the collector, the bastnaesite recovery rate is 82.73%, and the grade of bastnaesite is 75.12%. When SHA is used as the collector, the bastnaesite recovery rate is 29.14%, but the bastnaesite grade is 74.48%. Compared with SHA, PHA not only has good selection performance, but also has good collection performance.

Table 2.
Flotation separation of artificial mixed minerals results.
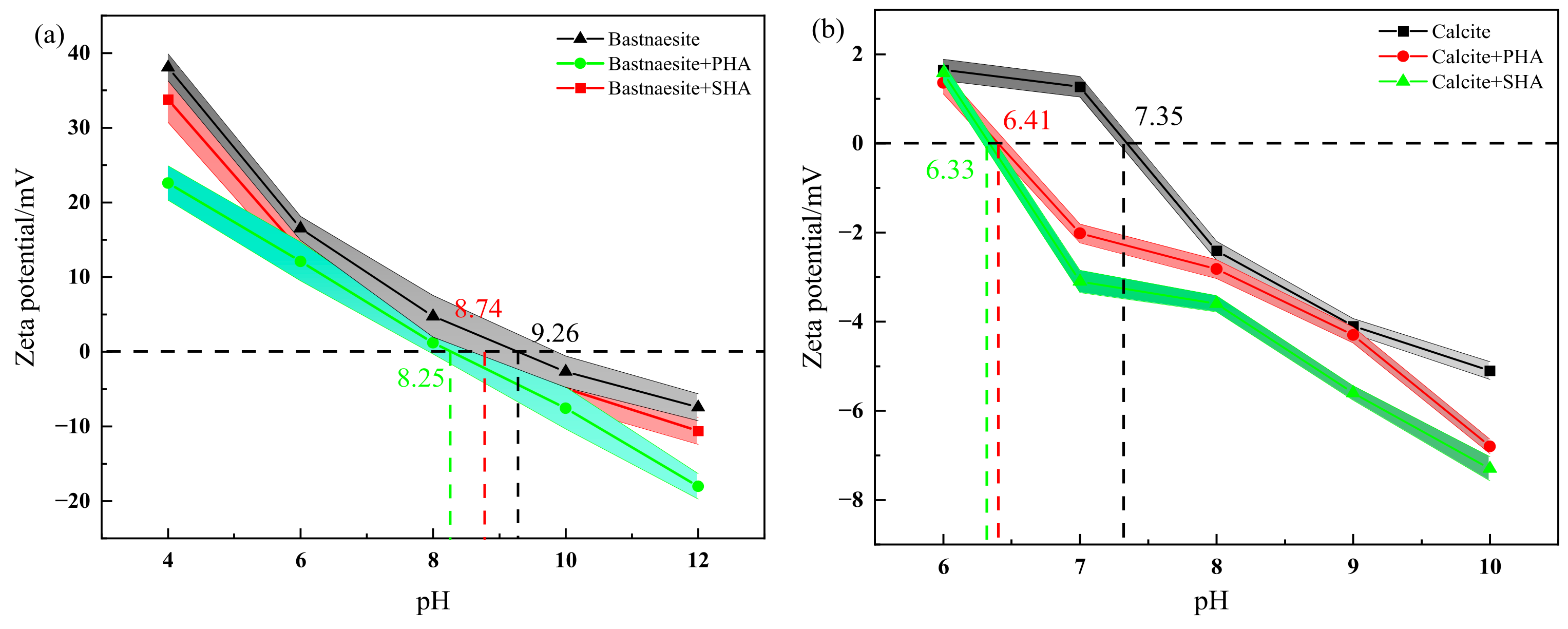
3.2. Zeta Potential Measurements Result
The results of Zeta potential measurements are shown in Figure 6. The PZC (zero charge point) of bastnaesite and calcite are 9.26 and 7.35, respectively, which is close to the previous research [29]. The PZC of bastnaesite treated with collector (20 mg/L) increases to 8.74 (SHA) and 8.25 (PHA). The PZC of calcite treated with collector (20 mg/L) is 6.41 (SHA) and 6.33 (PHA), respectively. The results show that both PHA and SHA are adsorbed on the surfaces of bastnaesite and calcite. However, under the same conditions, the Zeta potential of bastnaesite treated with PHA is lower than that of bastnaesite treated with SHA, indicating that PHA is more likely to adsorb on the surface of bastnaesite than SHA. The addition of the collector has little effect on the Zeta potential of calcite. In a word, these results demonstrate that PHA is more readily adsorbed on the surface of bastnaesite than SHA.
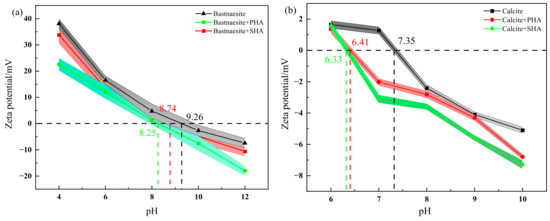
Figure 6.
Zeta-potential results of bastnaesite (a) and calcite (b).
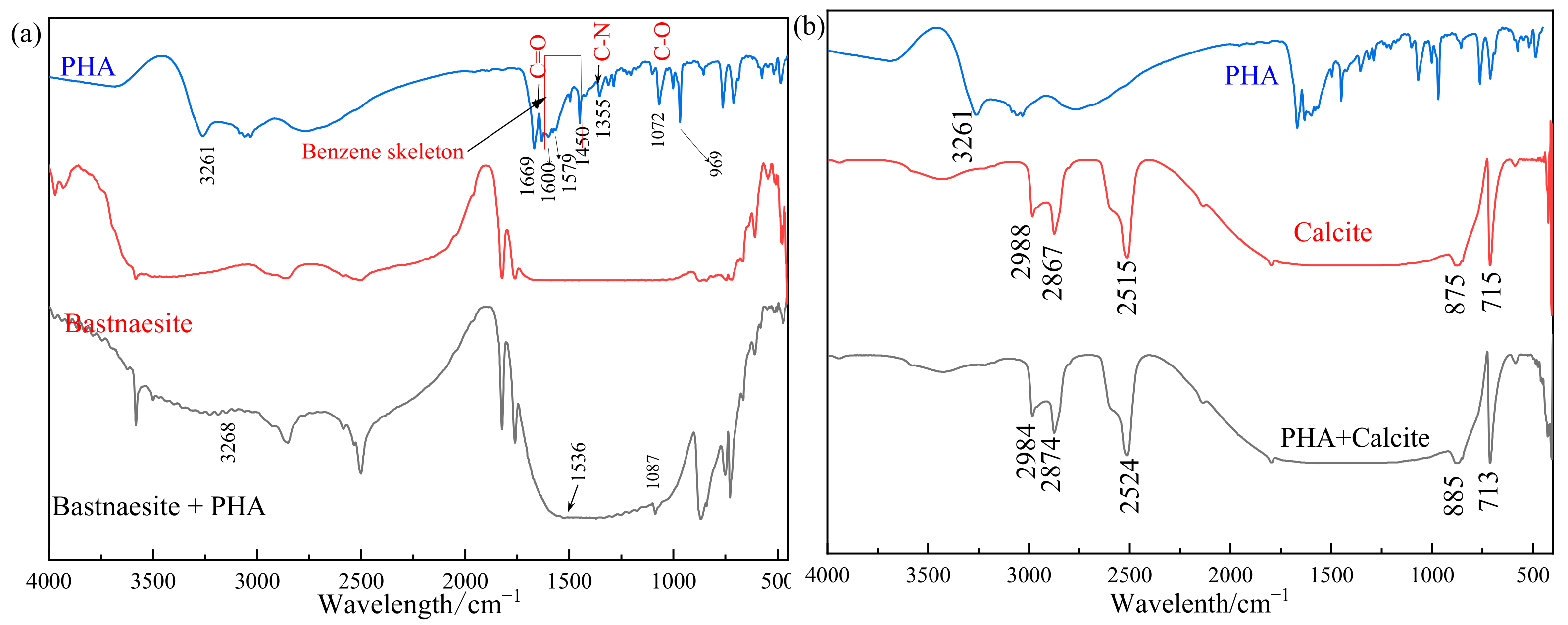
3.3. FTIR Measurements Result
The results of the FTIR measurements are shown in Figure 7. The possible attribution of peaks in the FTIR spectra of CIHA is as follows: the strong peak around 3261 cm−1 may be ascribed to the superimposed stretching vibration peak of O–H and N–H; 1669 cm−1 to the stretching vibration peak of carbonyl C=O; 1600 cm−1, 1579 cm−1, 1497 cm−1, and 1450 cm−1 to the benzene ring skeleton structure; 1355 cm−1 is the tensile vibration peak of C–N; and 1072 cm−1 and 969 cm−1 are the stretching vibration peak of C–O and N–O, respectively. The peak at 1632 cm−1 is part of the C=C stretching vibration peak of carbon–carbon double bond. The characteristic peaks in the spectrogram are distinct, which suggests that the synthesized product contains PHA [30].
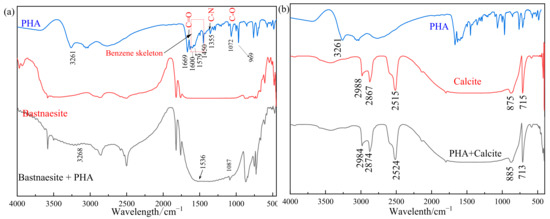
Figure 7.
Infrared spectrum results of bastnaesite (a) and calcite (b).
The FTIR analysis of bastnaesite before and after 20 mg/L PHA treatment shows that the range of 500–1000 cm−1 has obvious changes, and the corresponding C–O fluctuation peak appears at 1087 cm−1. The corresponding fluctuation peak of the benzene ring fluctuates near 1536 cm−1. There are also significant fluctuations near 3268 cm−1. It is proven that PHA is adsorbed on the surface of bastnaesite, by chemical adsorption (Figure 7a).
However, the FTIR spectra of calcite treated with 20 mg/L PHA shows no characteristic peak of PHA, and their spectra are almost the same as those of untreated calcite. Therefore, it is considered that the collector is not adsorbed on the calcite surface (Figure 7b).
Combined with the results of flotation and Zeta potential measurements, it is found that PHA could change the surface properties of bastnaesite by chemical adsorption on its surface.
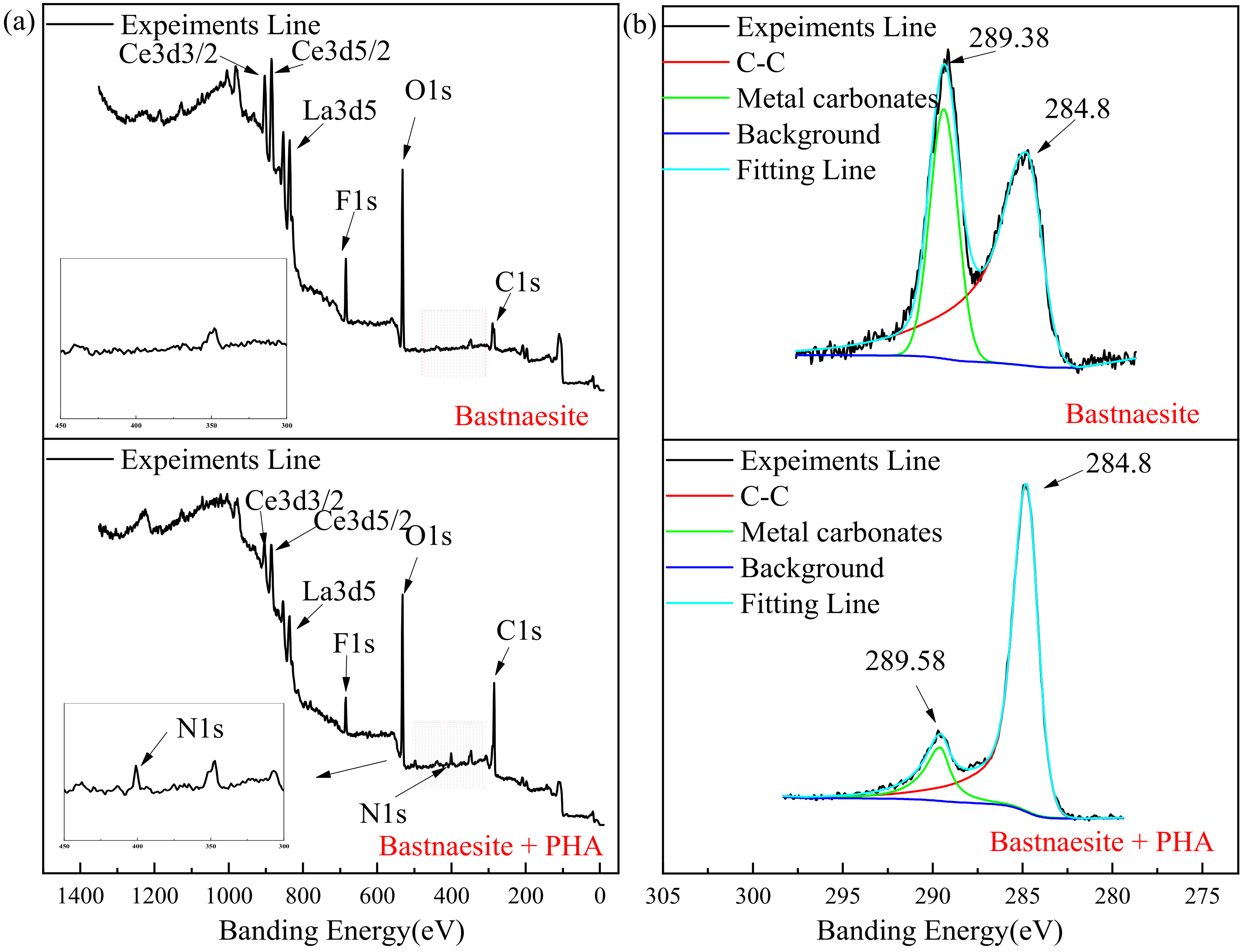
3.4. XPS Measurement Results
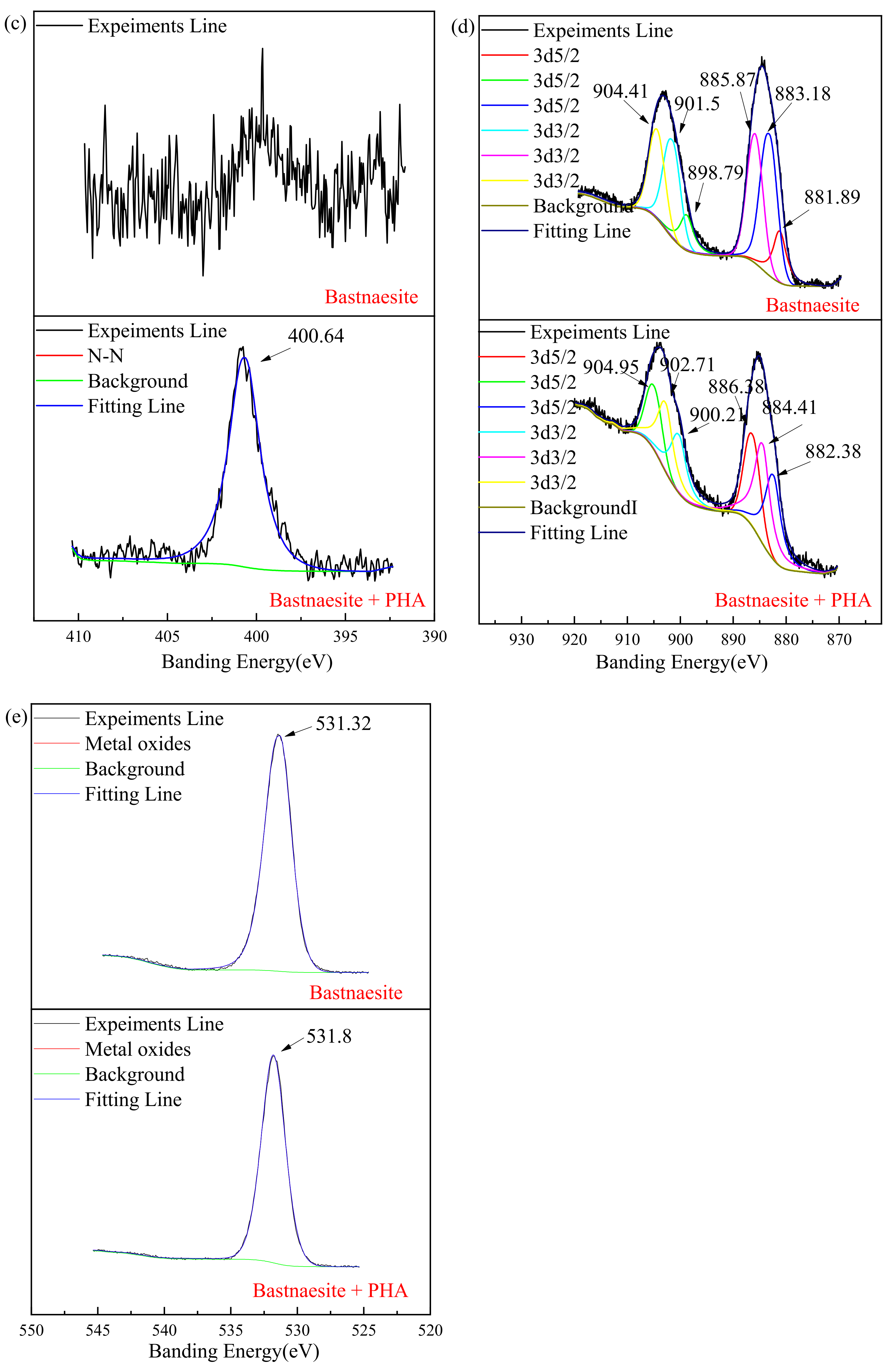
The full scan spectra result of the XPS measurement are shown in Figure 8a, and the element concentration that exists on the surface of bastnaesite are shown in Table 3. A new characteristic peak N 1s (400.64 eV) is evident on the surface of the bastnaesite. The concentration of Ce and O decreases significantly, while the concentration of C increases significantly.
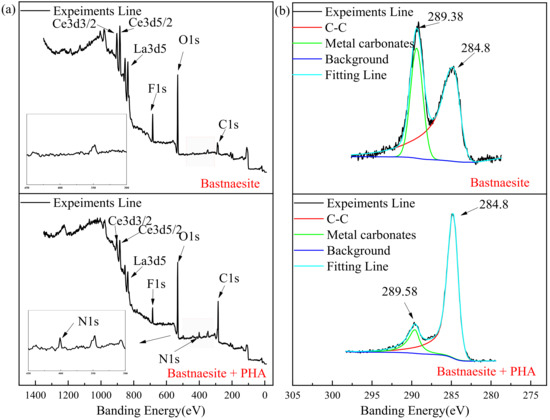
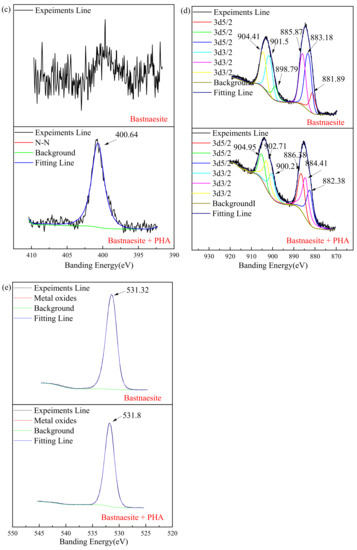
Figure 8.
XPS analysis spectrum of wolframite before and after treating with PHA. ((a) survey, (b) C 1s, (c) N 1s, (d) Ce, (e) O).

Table 3.
Element concentration exist on bastnaesite surface.
As shown in Figure 8b, due to the unavoidable external carbon pollution of the environment or equipment, correction can be made by referring to the C 1s at 284.8 eV, while the existing 289.38 eV and 289.58 eV before and after the reaction, respectively, are due to the presence of metal carbonate. Figure 8c shows the spectrum of N on the bastnaesite surface. The characteristic peak of N 1s on the bastnaesite surface before the reaction is disordered, mainly due to the nitrogen in the air. However, a new characteristic peak appears at 400.64 eV (N 1s), after the reaction. This is mainly due to the chemisorption on the surface of bastnaesite by PHA. As shown in Figure 8d, the binding energies of Ce are 881.89, 883.18, and 885.87 eV (3d5/2), and 898.79, 901.5, and 904.41 eV (3d3/2).After 20 mg/L PHA treatment, the above binding energies are 883.23, 884.41, and 886.38 eV(3d5/2), and 900.21, 902.71, and 904.95 eV (3d3/2), respectively. Compared with pure bastnaesite samples, all Ce characteristic peaks of bastnaesite samples in XPS after PHA treatment shift by >0.2 eV. It indicates that bastnaesite and PHA have chemisorption, and the binding site is Ce. [31] The XPS spectrum of O is shown in Figure 8e, and the results show that the banding energy shifts from 531.32 eV to 531.80 eV. This proves, once again, that there is chemisorption between PHA and bastnaesite.
These changes indicate that chemisorption can occur between PHA and bastnaesite, and the main binding site is Ce.
3.5. DFT Results
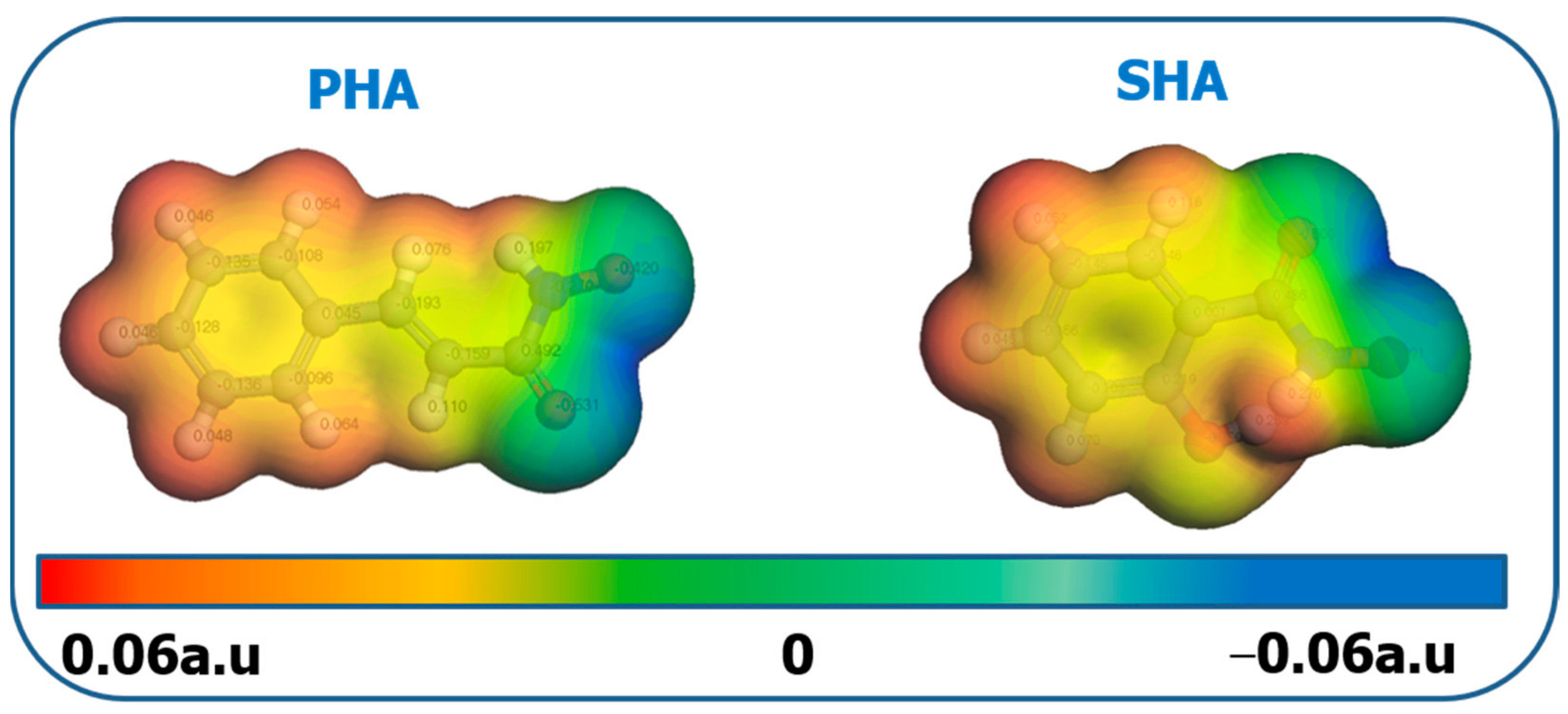
Molecular electrostatic potential (MEP) is a widely used method to describe the electron density in a target molecule, and to predict nucleophilic and electrophilic active sites in various reactions. In the MEP diagram, the red region represents the part with the most negative charge in the molecule, which is also the active site of the electrophilic attack, while the blue region corresponds to the part with the most positive charge in the nucleophilic attack. The MEP diagram of PHA and SHA is shown in Figure 9. The blue region shown by PHA and SHA appears in the C=O and N–OH groups in hydroxamic acid molecules. They form five-element-chelated hydroxamic groups through electron conjugation, and adsorb on bastnaesite surface. The hydrophobicity of the bastnaesite surface can be greatly improved when the hydrophobicity of the benzene ring is outwards. By comparing the hydrophobic constants (CLogP) of PHA and SHA, the CLogP of PHA and SHA are 1.448 and 0.72, respectively. The CLogP of PHA is almost twice that of SHA. Adsorption of PHA on the bastnaesite surface greatly improves the hydrophobicity of the bastnaesite surface. Therefore, in the flotation test, when the dosage of PHA is 20 mg/L, the recovery of bastnaesite reaches 93%. However, when SHA dosage is 50 mg/L, the recovery of bastnaesite is only 79% [32].
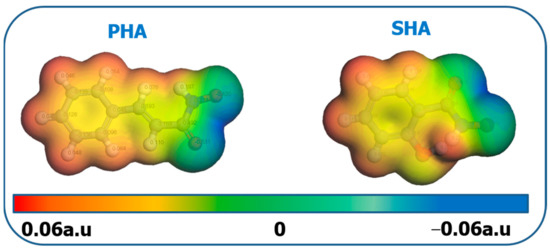
Figure 9.
MEP map of PHA and SHA.
4. Conclusions
A novel surfactant phenylpropyl hydroxamic acid (PHA) was synthesized using the hydroxylamine method, and its molecular structure was characterized by FTIR and NMR. Its application in separation flotation of bastnaesite and calcite was studied. In the single mineral test, the flotation effect can reach the peak value (pH = 8, PHA = 20 mg/ L). In the artificial mixed ore test, bastnaesite can be selectively collected with high efficiency (recovery 82.13%, grade: 75.12%).
Zeta potential measurement results show that PHA can change the potential of bastnaesite by chemical adsorption on the surface of bastnaesite. According to FTIR results, the surface of bastnaesite treated with PHA shows characteristic groups only found in PHA, which is also a phenomenon that can only be produced by chemisorption. XPS results show that PHA molecules combine with bastnaesite through chemical adsorption, and form five-element-chelated hydroxamic groups with PHA, which changes the hydrophobicity of bastnaesite and improves its floatability. The DFT calculation explains that PHA interacts with metal element (Ce) on the bastnaesite surface through C=O and N–O groups to form a five-element-chelated hydroxamic group. At the same time, the non-polar tail chain moves outward, transforming the bastnaesite particles into hydrophobic ones. Combined with the flotation test results, the hydrophobicity of the bastnaesite surface is improved after PHA treatment, that is, bastnaesite particles are more likely to be attached to the pulp surface by bubbles, so as to improve the pulp recovery.
In conclusion, PHA can change the hydrophobicity of bastnaesite, and make it easier to emerge from pulp by combining with Ce to form a five-element-chelated hydroxamic group through chemical adsorption.
Author Contributions
Writing—original draft preparation, X.Y. (Xiang Yao); writing—review and editing, X.Y. (Xinyang Yu) and X.Y. (Xiang Yao); methodology, Y.Z.; software, L.M.; validation, H.X. and S.L.; formal analysis, G.H.; investigation, Z.H.; data curation, H.W.; supervision, Z.L.; funding acquisition, G.H. and Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Nature Science Foundation of China (No. 51774152) and Jiangxi Provincial Education Department (GJJ200816).
Acknowledgments
The author would like to acknowledge the National Nature Science Foundation of China (No. 51774152, funder is Guichun He) and Jiangxi Provincial Education Department (GJJ200816, funder is Zhilin Liu).
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sudarshan, K.; Kadam, R.M. Optical nanomaterials with focus on rare earth doped oxide: A Review. Mater. Today Commun. 2021, 27, 102277. [Google Scholar] [CrossRef]
- Huang, G.; Lou, L.; Song, W.; Li, M.; Hou, F.; Li, X. Microstructure and magnetic properties of (SmCo+FeCo)/NdFeB multicomponent nanocomposite magnets fabricated by HPTC with change in heating temperature and composition. J. Rare Earths 2020, 38, 742. [Google Scholar] [CrossRef]
- Madavali, B.; Shin, D.W.; Song, S.H.; Kim, D.S.; Lee, J.K.; Hong, S.J. Preparation and thermoelectric performance of nano rare-earth oxides dispersed p-type BiSbTe alloys by mechanical milling and spark plasma sintering. Mater. Chem. Phys. 2020, 253, 123378. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Wei, Y.; Liu, J.; Zhao, Z. Research Advances of Rare Earth Catalysts for the Catalytic Purification of Vehicle Exhausts. J. Rare Earths 2021, 39, 1151–1180. [Google Scholar] [CrossRef]
- Wang, C.; Ma, R.; Zhou, Y.; Liu, Y.; Daniel, E.F.; Li, X.; Wang, P.; Dong, J.; Ke, W. Effects of rare earth modifying inclusions on the pitting corrosion of 13Cr4Ni martensitic stainless steel. J. Mater. Sci. Technol. 2021, 93, 232. [Google Scholar] [CrossRef]
- Sharma, V.K.; Kumar, V. Development of rare-earth oxides based hybrid AMCs reinforced with SiC/Al2O3: Mechanical & metallurgical characterization. J. Mater. Res. Technol. 2019, 8, 1971. [Google Scholar]
- Fan, X.; Ding, G.; Chen, K.; Guo, S.; You, C.; Chen, R.; Lee, D.; Yan, A. Whole process metallurgical behavior of the high-abundance rare-earth elements LRE (La, Ce and Y) and the magnetic performance of Nd0.75LRE0.25-Fe-B sintered magnets. Acta Mater. 2018, 154, 343. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, S.K.; Verma, S.; Sharma, V.; Kumar, V. A Short Review on Rare Earth Doped NaYF4 Upconverted Nanomaterials for Solar Cell Applications. Mater. Today Proc. 2020, 21, 1868. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, J.; Yang, Y.; Liu, X.; Sun, W.; Wei, Y.; Lan, Z.; Lin, J.; Huang, M.; Chen, H.; et al. Low-temperature processed rare-earth doped brookite TiO2 scaffold for UV stable, hysteresis-free and high-performance perovskite solar cells. Nano Energy 2020, 77, 105183. [Google Scholar] [CrossRef]
- Chen, X.; Shi, Z.; Pan, G.; Zhu, J.; Hu, J.; Wu, Y.; Tian, Y.; Li, X.; Xu, W. Boosting interfacial charge transfer by constructing rare earth–doped WOx nanorods/SnO2 hybrid electron transport layer for efficient perovskite solar cells. Mater. Today Energy 2021, 21, 100724. [Google Scholar] [CrossRef]
- Kujur, M.S.; Deshpande, A.; Mallick, A.; Gupta, M. Development of rare-earth oxide reinforced magnesium nanocomposites targeting biomedical applications. Mater. Today Proc. 2020, 33, 5414. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Zanin, M.; Addai-Mensah, J.; Skinner, W. Recovery of rare earth elements minerals from iron oxide–silicate rich tailings—Part 1: Magnetic separation. Miner. Eng. 2019, 136, 50. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Z.; Wang, H.; Liu, R.; Cheng, C.; Guo, Z.; Fu, W. Separation of wolframite ore by froth flotation using a novel “crab” structure sebacoyl hydroxamic acid collector without Pb(NO3)2 activation. Powder Technol. 2021, 389, 96–103. [Google Scholar] [CrossRef]
- Wu, Y.; Song, M.; Zhang, Q.; Wang, W. Review of rare-earths recovery from polishing powder waste. Resour. Conserv. Recycl. 2021, 171, 105660. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; van Gerven, T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151. [Google Scholar] [CrossRef]
- Zou, D.; Li, H.; Deng, Y.; Chen, J.; Bai, Y. Recovery of lanthanum and cerium from rare earth polishing powder wastes utilizing acid baking-water leaching-precipitation process. Sep. Purif. Technol. 2021, 261, 118244. [Google Scholar] [CrossRef]
- Marion, C.; Li, R.; Waters, K.E. A review of reagents applied to rare-earth mineral flotation. Adv. Colloid Interface Sci. 2020, 279, 102142. [Google Scholar] [CrossRef]
- Meng, D.; Wang, M.; Feng, Z.; Xia, C.; Zhao, Y.; Huang, X. Behavior of phase transformation of Baotou mixed rare earth concentrate during oxidation roasting. J. Rare Earths 2021, 40, 981–987. [Google Scholar] [CrossRef]
- Elizondo-Alvarez, M.A.; Uribe-Salas, A.; Bello-Teodoro, S. Chemical stability of xanthates, dithiophosphinates and hydroxamic acids in aqueous solutions and their environmental implications. Ecotoxicol. Environ. Saf. 2021, 207, 111509. [Google Scholar] [CrossRef]
- Shen, Y.; Nagaraj, D.R.; Farinato, R.; Somasundaran, P. Study of xanthate decomposition in aqueous solutions. Miner. Eng. 2016, 93, 10. [Google Scholar] [CrossRef] [Green Version]
- Espiritu ER, L.; da Silva, G.R.; Azizi, D.; Larachi, F.; Waters, K.E. Flotation behavior and electronic simulations of rare earth minerals in the presence of dolomite supernatant using sodium oleate collector. J. Rare Earths 2019, 37, 101. [Google Scholar] [CrossRef]
- Espiritu, E.R.L.; Naseri, S.; Waters, K.E. Surface chemistry and flotation behavior of dolomite, monazite and bastnäsite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 254. [Google Scholar] [CrossRef]
- Duan, H.; Liu, W.; Wang, X.; Gu, X.; Sun, W.; Peng, X.; Yue, H. Investigation on flotation separation of bastnaesite from calcite and barite with a novel surfactant: Octylamino-bis-(butanohydroxamic acid). Sep. Purif. Technol. 2021, 256, 117792. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Zanin, M.; Addai-Mensah, J.; Skinner, W. The upgrading of rare earth oxides from iron-oxide silicate rich tailings: Flotation performance using sodium oleate and hydroxamic acid as collectors. Adv. Powder Technol. 2018, 29, 3163. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G. Investigation on the selectivity of N-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation. J. Ind. Eng. Chem. 2016, 33, 131. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.; Jiang, T.; Liu, Q. Flotation of coarse and fine pyrochlore using octyl hydroxamic acid and sodium oleate. Miner. Eng. 2019, 132, 191. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Li, L.; Lu, J.; Yang, J. Modification mechanism of lead ions and its response to wolframite flotation using salicylhydroxamic acid. Powder Technol. 2020, 366, 477–487. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Luo, H.; Cheng, R.; Liu, F. Selective reverse flotation of apatite from dolomite in collophanite ore using saponified gutter oil fatty acid as a collector. Int. J. Miner. Processing 2017, 165, 20. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, H. Determination of hydroxamic acids by direct spectrophotometry of colored complex in acidic solution. Res. Chem. Intermed. 2010, 36, 495. [Google Scholar] [CrossRef]
- Guo, Z.; Khoso, S.A.; Wang, J.; Zhang, C.; Gao, Z.; Sun, W.; Tian, M.; Liu, Y. Interaction mechanism of 2-hydroxy-3-naphthyl hydroxamic acid and 1-hydroxy-2-naphthyl hydroxamic acid in the flotation separation of bastnaesite/fluorite: Experiments and first-principles calculations. Sep. Purif. Technol. 2022, 285, 120307. [Google Scholar] [CrossRef]
- Duan, H.; Huang, X.; Cao, X.; Cao, Z.; Zhong, H.; Zeng, J.; Zhou, H.; Xue, J.; Liu, Y. Investigating the flotation performance and interfacial adsorption mechanism of N-benzoyl-N’,N’-diethyl thiourea on chalcopyrite and pyrite. Miner. Eng. 2021, 172, 107178. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).