Efficient Separation of Ultrafine Coal Assisted by Selective Adsorption of Polyvinylpyrrolidone
Abstract
:1. Introduction
2. Materials and Methods
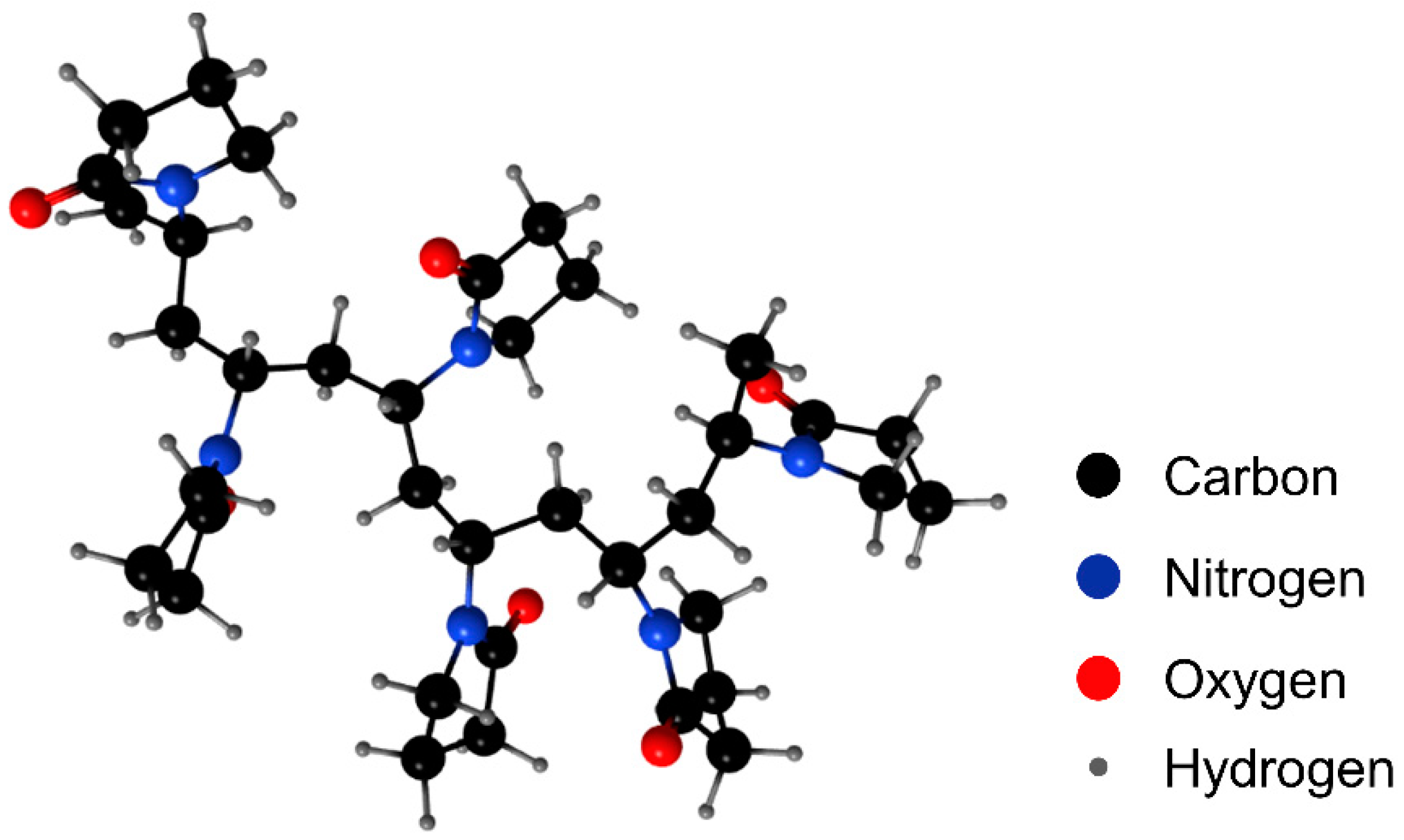
2.1. Materials
2.2. Laboratory Flotation Test
2.3. Adsorption Amount and Equilibrium Test
2.4. Zeta Potential Test
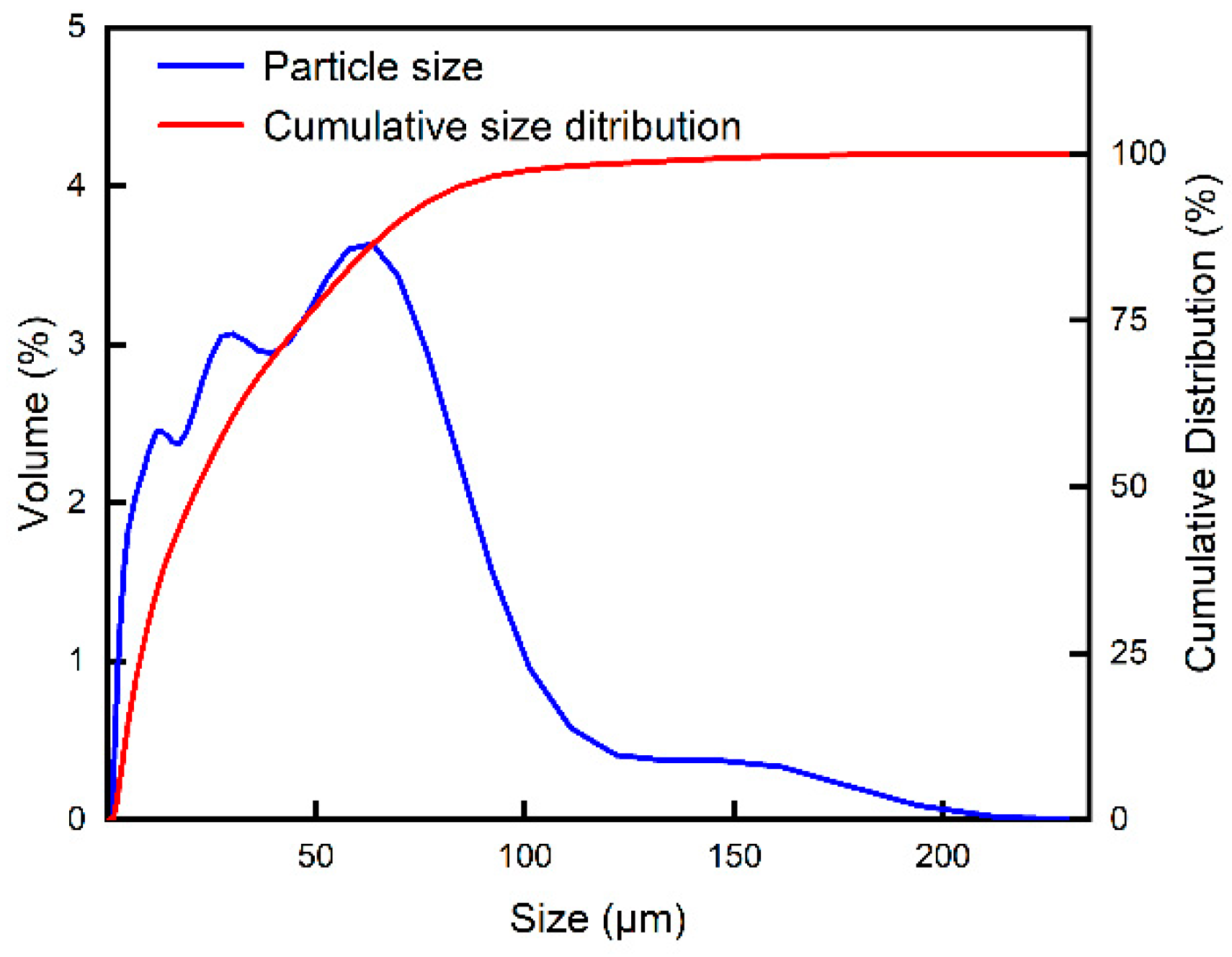
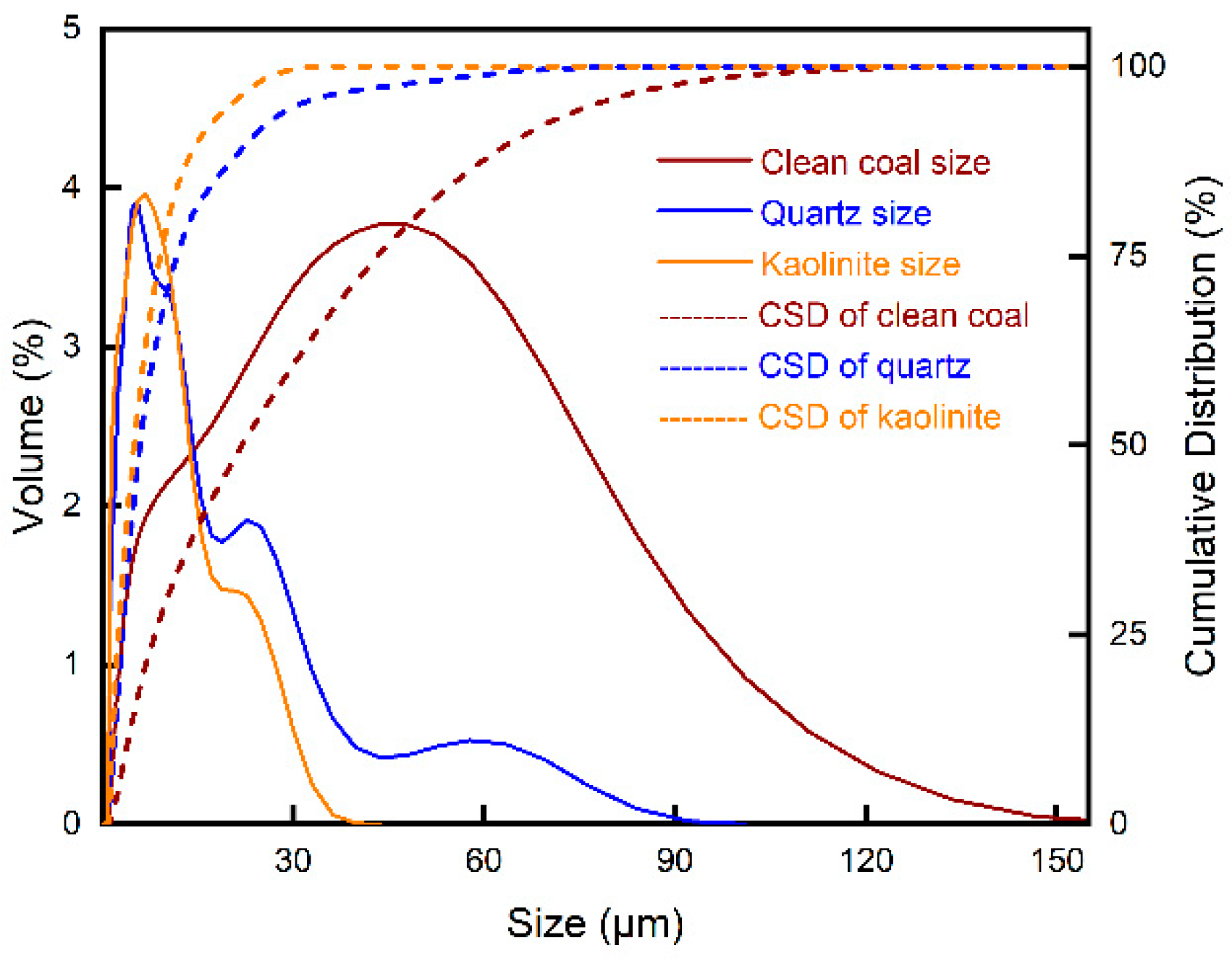
2.5. Particle Size Distribution Test
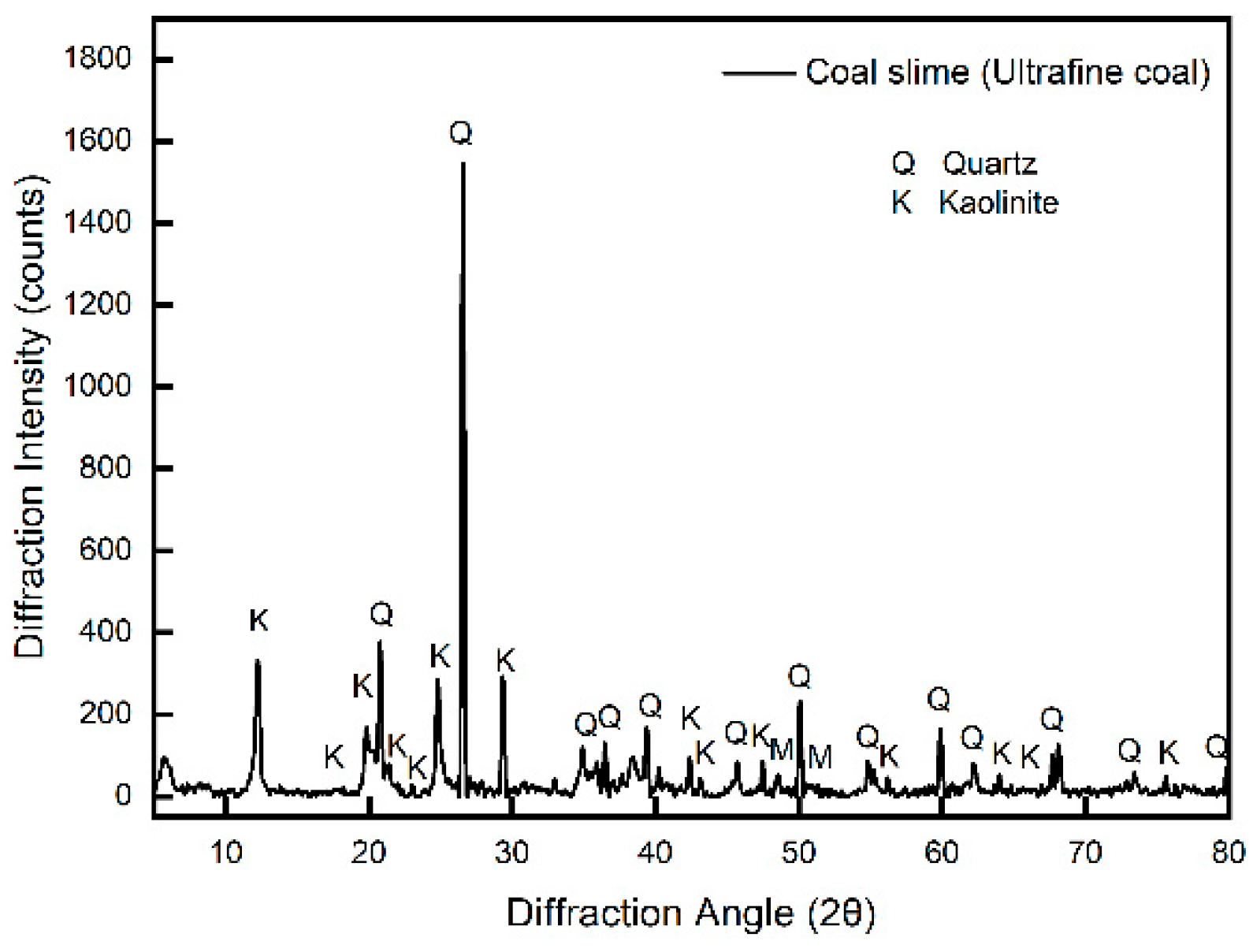
2.6. XPS Test
3. Results and Discussion
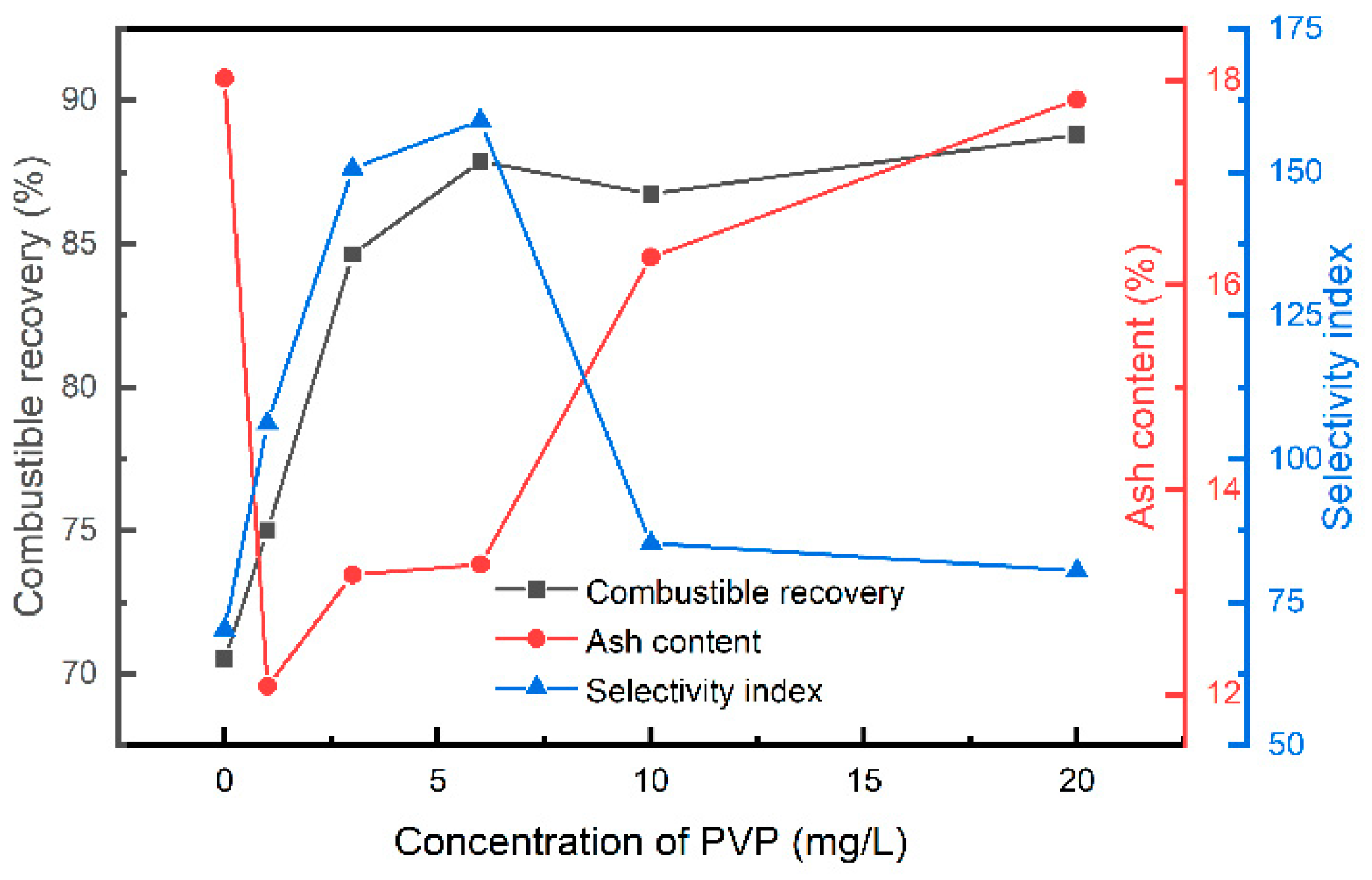
3.1. Laboratory Flotation
3.2. Adsorption Amount and Equilibrium
3.3. Zeta Potential
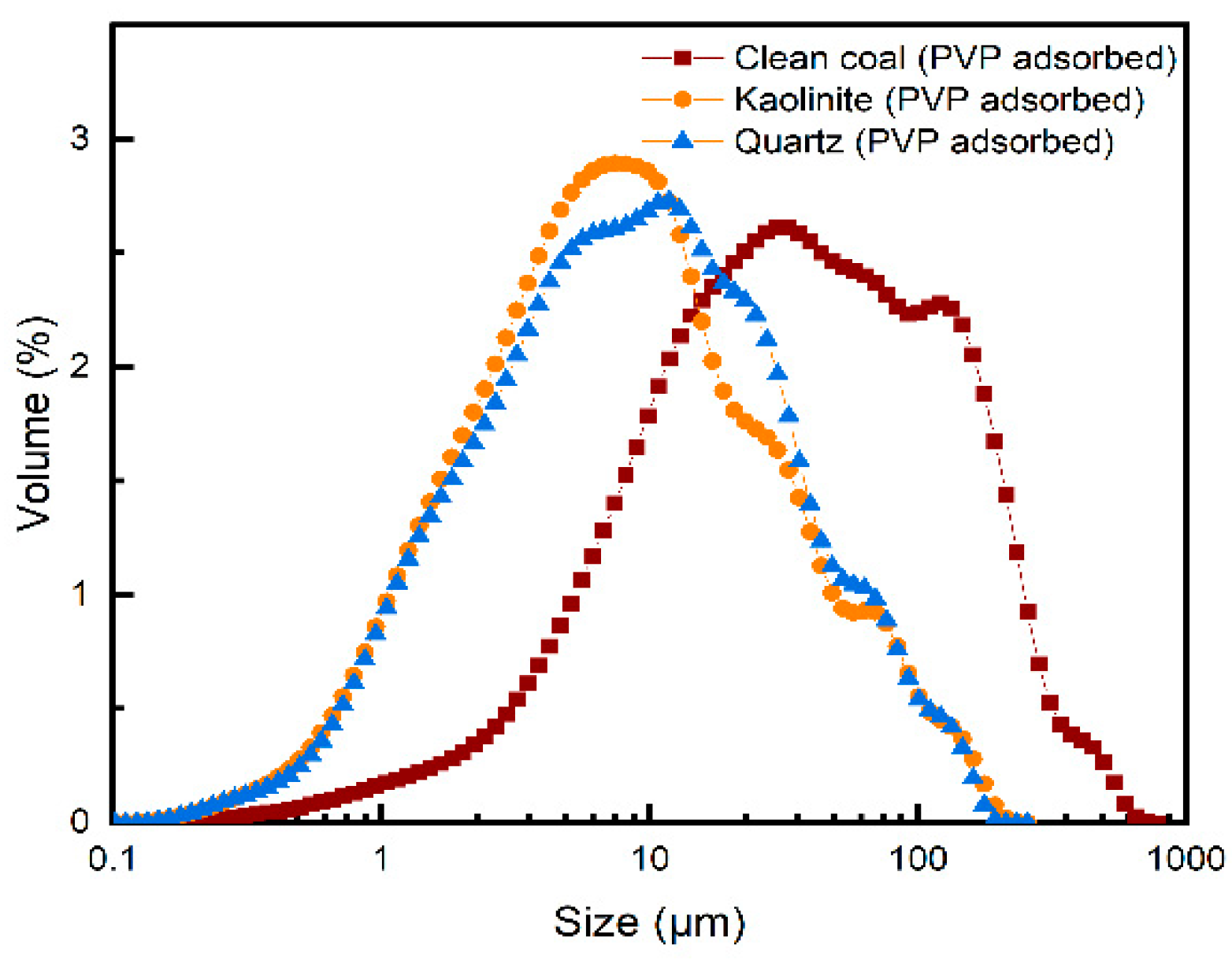
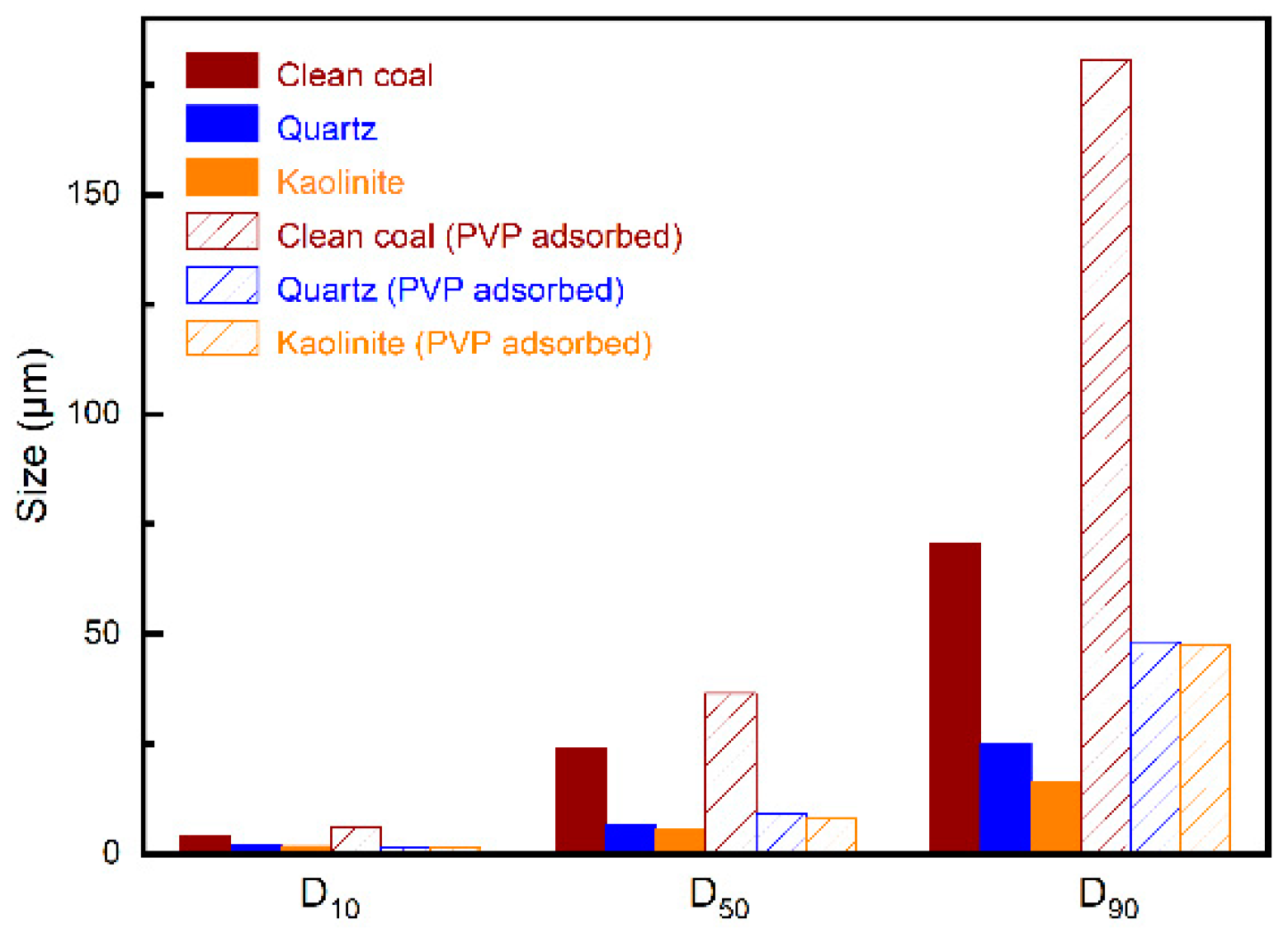
3.4. Particle Size Distribution
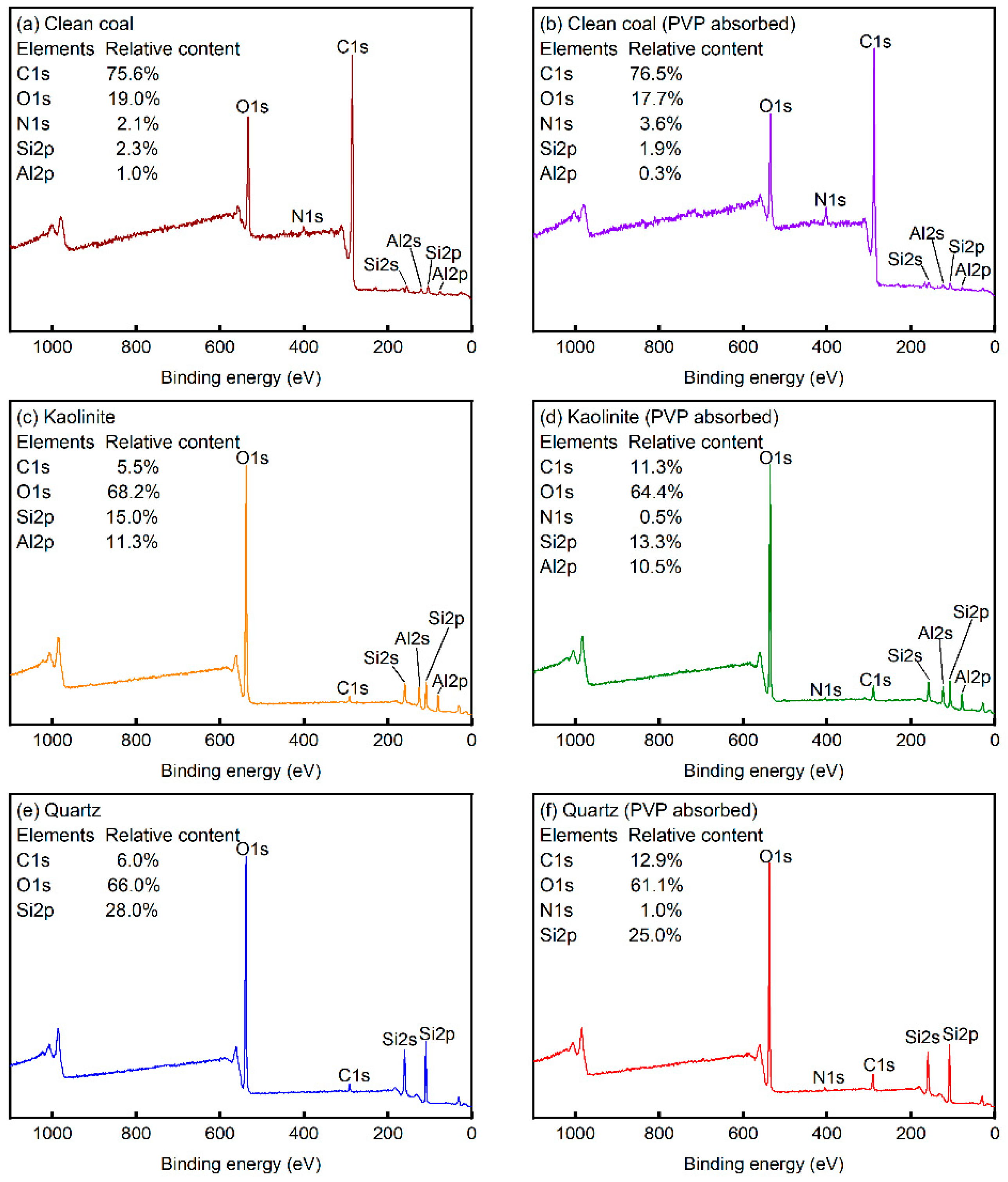
3.5. XPS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cai, Y.; Du, M.; Wang, S.; Liu, L. Determination of oxidation properties and flotation parameters of low-rank coal slimes. Powder Technol. 2019, 353, 20–26. [Google Scholar] [CrossRef]
- Wang, G.; Bai, X.; Wu, C.; Li, W.; Liu, K.; Kiani, A. Recent advances in the beneficiation of ultrafine coal particles. Fuel Process. Technol. 2018, 178, 104–125. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, Y.; Zhao, B.; Wu, C.; Li, J.; Chu, C. Collecting behaviors of high internal phase (HIP) emulsion in flotation of ultrafine high-ash content coal slime. Int. J. Coal Prep. Util. 2021, 41. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L. Improving column flotation of oxidized or ultrafine coal particles by changing the flow pattern of air supply. Miner. Eng. 2018, 124, 98–102. [Google Scholar] [CrossRef]
- Norori, M.A.; Brito, P.P.R.; Hadler, K.; Cole, K.; Cilliers, J.J. The effect of particle size distribution on froth stability in flotation. Sep. Purif. Technol. 2017, 184, 240–247. [Google Scholar] [CrossRef]
- Tao, X.; Cao, Y.; Liu, J.; Shi, K.; Liu, J.; Fan, M. Studies on characteristics and flotation of a hard-to-float high-ash fine coal. In Proceedings of the 6th International Conference on Mining Science & Technology, Xuzhou, China, 18 October 2009. [Google Scholar]
- Akdemir, Ü.; Sönmez, I. Investigation of coal and ash recovery and entrainment in flotation. Fuel Process. Technol. 2003, 82, 1–9. [Google Scholar] [CrossRef]
- Li, C.; Xing, Y.; Gui, X.; Cao, Y. Enhancement of oxidized coal flotation by preconditioning with positive-charged microbubbles. Int. J. Coal Prep. Util. 2020, 40, 553–563. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, L.; Zhang, Y. Role of nanobubbles in the flotation of fine rutile particles. Miner. Eng. 2021, 172, 107140. [Google Scholar] [CrossRef]
- Huo, X.; Zuo, W.; Shi, F.; Huang, W. Coal middling retreatment using high voltage pulse technique. Part 1: Experimental findings. Fuel 2022, 314, 123066. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Bu, X.; Wang, M.; An, B.; Shao, H. A novel flotation technique combining carrier flotation and cavitation bubbles to enhance separation efficiency of ultra-fine particles. Ultrason. Sonochem. 2020, 64, 105005. [Google Scholar] [CrossRef]
- Capes, C.E.; Darcovich, K. A survey of oil agglomeration in wet fine coal processing. Powder Technol. 1984, 40, 43–52. [Google Scholar] [CrossRef]
- Kadagala, M.R.; Nikkam, S.; Tripathy, S.K. A review on flotation of coal using mixed reagent systems. Miner. Eng. 2021, 173, 107217. [Google Scholar] [CrossRef]
- Dey, S. Enhancement in hydrophobicity of low rank coal by surfactants: A critical overview. Fuel Process. Technol. 2012, 94, 151–158. [Google Scholar] [CrossRef]
- Vamvuka, D.; Agridiotis, V. The effect of chemical reagents on lignite flotation. Int. J. Miner. Process. 2001, 61, 209–224. [Google Scholar] [CrossRef]
- Ozer, M.; Basha, O.M.; Morsi, B. Coal-Agglomeration Processes: A Review. Int. J. Coal Prep. Util. 2016, 37, 131–167. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, B.; Zhang, X.; Yang, S.; Huang, W. Understanding the role of kerosene on the coal particle and bubble attachment process. Fuel 2022, 307, 121915. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, X.; Wang, J.; Song, G.; Wang, K. Influence of ultra-fine grinding mode on ultra-clean coal separation effect. J. China Coal Soc. 2016, 41, 3108–3114. [Google Scholar]
- Xia, Y.; Zhang, R.; Xing, Y.; Gui, X. Improving the adsorption of oily collector on the surface of low-rank coal during flotation using a cationic surfactant: An experimental and molecular dynamics simulation study. Fuel 2019, 235, 687–695. [Google Scholar] [CrossRef]
- Marsalek, R.; Pospisil, J.; Taraba, B. The influence of temperature on the adsorption of CTAB on coals. Colloids Surf. A Physicochem. Eng. Asp. 2011, 383, 80–85. [Google Scholar] [CrossRef]
- Wen, B.; Xia, W.; Sokolovic, J.M. Recent advances in effective collectors for enhancing the flotation of low rank/oxidized coals. Powder Technol. 2017, 319, 1–11. [Google Scholar] [CrossRef]
- Zou, W.; Gong, L.; Huang, J.; Zhang, Z.; Sun, C.; Zeng, H. Adsorption of hydrophobically modified polyacrylamide P(AM-NaAA-C(16)DMAAC) on model coal and clay surfaces and the effect on selective flocculation of fine coal. Miner. Eng. 2019, 142, 105887. [Google Scholar] [CrossRef]
- Li, C.; Xu, M.; Zhang, H. Efficient separation of high-ash fine coal by the collaboration of nanobubbles and polyaluminum chloride. Fuel 2020, 260, 116325. [Google Scholar] [CrossRef]
- Lv, S.; Peng, W.; Cao, Y.; Liu, S.; Wang, W.; Fan, G. Synthesis and characterisation of a novel pH-sensitive flocculant and its flocculation performance. J. Mol. Liq. 2022, 348, 118480. [Google Scholar] [CrossRef]
- Palchoudhury, S.; Lead, J.R. A Facile and Cost-Effective Method for Separation of Oil-Water Mixtures Using Polymer-Coated Iron Oxide Nanoparticles. Environ. Sci. Technol. 2014, 48, 14558–14563. [Google Scholar] [CrossRef]
- Alabresm, A.; Chen, Y.P.; Decho, A.W.; Lead, J. A novel method for the synergistic remediation of oil-water mixtures using nanoparticles and oil-degrading bacteria. Sci. Total Environ. 2018, 630, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Mei, Y.; Huang, W.; Zhang, B.; Liu, K. Understanding the Role of Polyvinylpyrrolidone on Ultrafine Low-Rank Coal Flotation. ACS Omega 2022, 7, 10196–10204. [Google Scholar] [CrossRef] [PubMed]
- Barbian, N.; Ventura, M.E.; Cilliers, J.J. Dynamic froth stability in froth flotation. Miner. Eng. 2003, 16, 1111–1116. [Google Scholar] [CrossRef]
- Bu, X.; Wang, X.; Zhou, S.; Li, B.; Zhan, H.; Xie, G. Discrimination of Six Flotation Kinetic Models Used in the Conventional Flotation and Carrier Flotation of −74 μm Coal Fines. ACS Omega 2020, 5, 13813–13821. [Google Scholar] [CrossRef]
- Xu, M. Modified flotation rate constant and selectivity index. Miner. Eng. 1998, 11, 271–278. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y.; Wu, C.; Li, J.; Liu, K. The performance and dispersing mechanism of anionic dispersants in slurries prepared by upgraded coal. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125450. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Yang, X.; Jiang, F.; Wu, C.; Li, J. Performance and mechanism of polyacrylamide stabilizers in coal water slurry. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127544. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Ma, C.; Wang, J.; Cao, X.; Wang, P. Molecular dynamics simulations of nonionic surfactant adsorbed on subbituminous coal model surface based on XPS analysis. Mol. Simul. 2019, 45, 736–742. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, C.; Bu, X.; Bilal, M.; Ni, C.; Peng, Y. Enhancement of Flotation Performance of Oxidized Coal by the Mixture of Laurylamine Dipropylene Diamine and Kerosene. Minerals 2021, 11, 1271. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Karakas, F.; Cao, Y. Role of Collectors and Depressants in Mineral Flotation: A Theoretical Analysis Based on Extended DLVO Theory. Minerals 2017, 7, 223. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.A.; Demirci, S.; Ozbayoglu, G. Zeta potential measurements on three clays from Turkey and effects of clays on coal flotation. J. Colloid Interface Sci. 1996, 184, 535–541. [Google Scholar] [CrossRef]
- Sahoo, H.; Sinha, N.; Rath, S.S.; Das, B. Ionic liquids as novel quartz collectors: Insights from experiments and theory. Chem. Eng. J. 2015, 273, 46–54. [Google Scholar] [CrossRef]
- Tian, M.; Khoso, S.A.; Wang, L.; Sun, W.; Zhang, C.; Hu, Y. Selective separation behavior and its molecular mechanism of cassiterite from quartz using cupferron as a novel flotation collector with a lower dosage of Pb2+ ions. Appl. Surf. Sci. 2019, 486, 228–238. [Google Scholar] [CrossRef]


| Industrial Analysis/% | Elemental Analysis/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| chemical | Mad | Aad | Vdaf | FCdaf | Cdaf | Hdaf | Ndaf | Sdaf | Odaf * |
| results | 2.03 | 55.0 | 23.41 | 64.58 | 65.19 | 5.34 | 1.04 | 1.61 | 26.82 |
| Name | Purity | Molecule Weight | Abbreviation |
|---|---|---|---|
| dodecane | ≥99% | 170 | DD |
| polyvinylpyrrolidone | 27,000 | PVP | |
| 2-octanol | ≥99% | 130 | 2-octanol |
| PVP (mg/L) | Combustible Matter | Ash | ||||
|---|---|---|---|---|---|---|
| Rmax | kf | Km,com | Rmax | kf | Km,ash | |
| 0 | 41.36 | 3.13 | 1.29 | 28.37 | 0.14 | 0.04 |
| 1 | 43.93 | 3.28 | 1.44 | 23.88 | 0.11 | 0.03 |
| 3 | 59.77 | 4.16 | 2.49 | 21.51 | 0.08 | 0.02 |
| 6 | 84.58 | 3.23 | 2.73 | 22.08 | 0.08 | 0.02 |
| 10 | 78.76 | 3.67 | 2.89 | 28.54 | 0.12 | 0.03 |
| 20 | 80.01 | 3.62 | 2.90 | 35.69 | 0.10 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, Y.; Lin, Q.; Wu, C.; Huang, W.; Cao, D.; Liu, K. Efficient Separation of Ultrafine Coal Assisted by Selective Adsorption of Polyvinylpyrrolidone. Minerals 2022, 12, 725. https://doi.org/10.3390/min12060725
Mei Y, Lin Q, Wu C, Huang W, Cao D, Liu K. Efficient Separation of Ultrafine Coal Assisted by Selective Adsorption of Polyvinylpyrrolidone. Minerals. 2022; 12(6):725. https://doi.org/10.3390/min12060725
Chicago/Turabian StyleMei, Yujie, Qiuyu Lin, Changning Wu, Wei Huang, Daofan Cao, and Ke Liu. 2022. "Efficient Separation of Ultrafine Coal Assisted by Selective Adsorption of Polyvinylpyrrolidone" Minerals 12, no. 6: 725. https://doi.org/10.3390/min12060725





