Thermal Transformation of Natural Schwertmannite in the Presence of Chromium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site and Sampling
2.2. Preparation of Cr(VI)-AMD Samples
2.3. Thermal Transformation Products
2.4. Solid Characterization
2.5. Selective Extractions
2.6. Arsenic Adsorption Experiments
3. Results and Discussion
3.1. Characterization of AMD
3.1.1. AMD Aqueous Chemistry
3.1.2. Chemical and Mineralogical Composition of AMD Precipitate
3.2. Characterization of Cr(VI)-AMD Samples
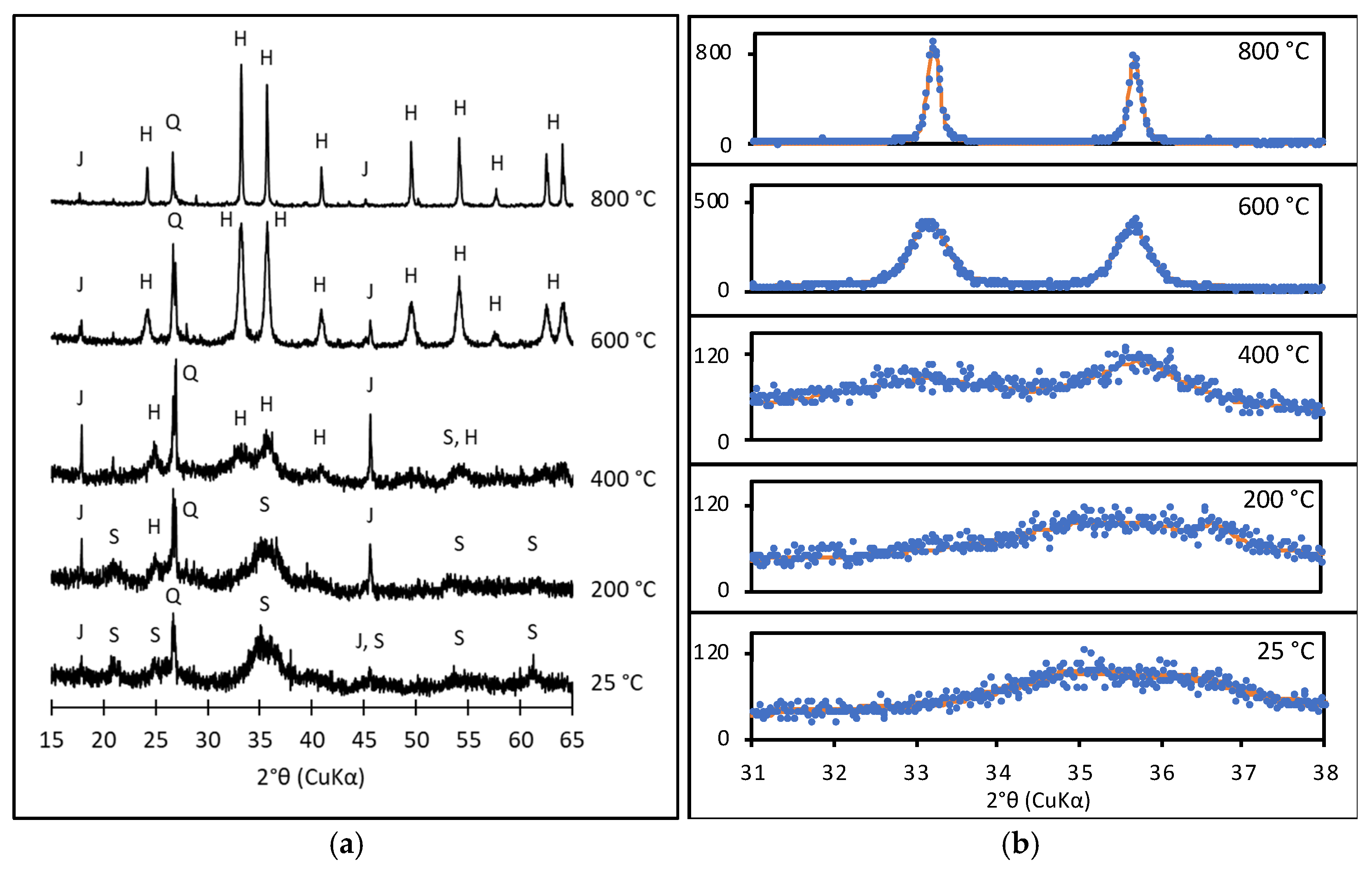
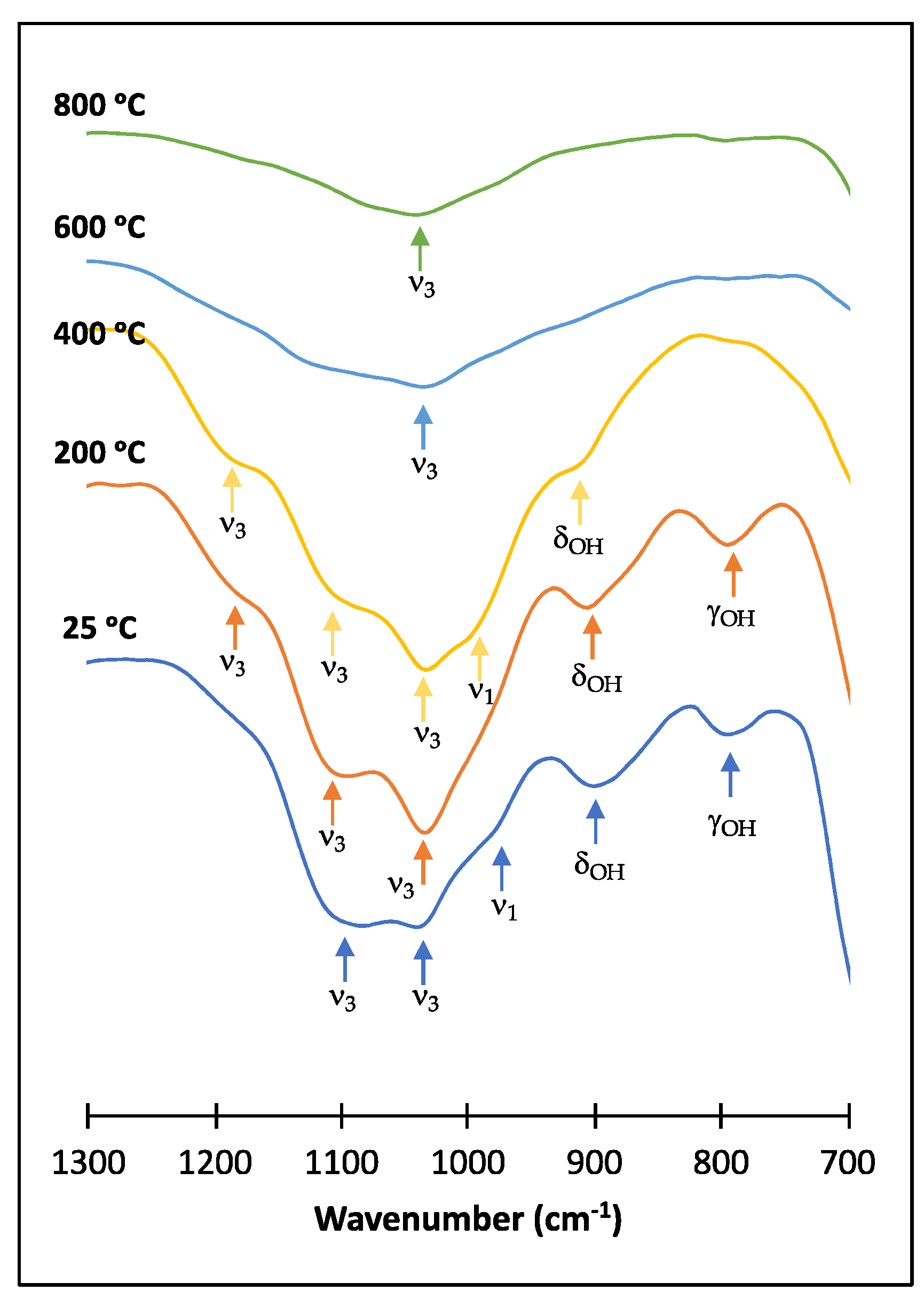
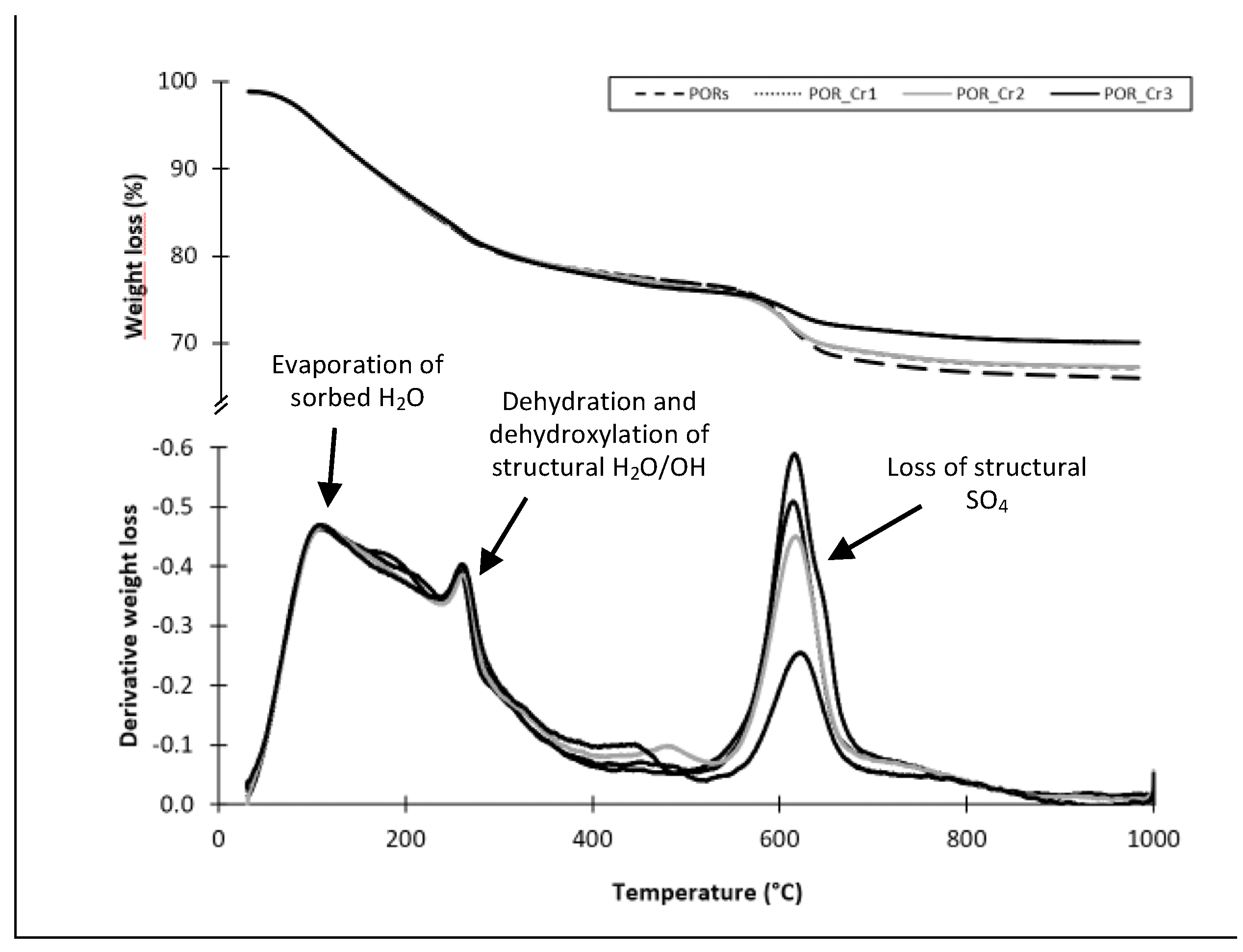
3.3. Thermal Transformation Products
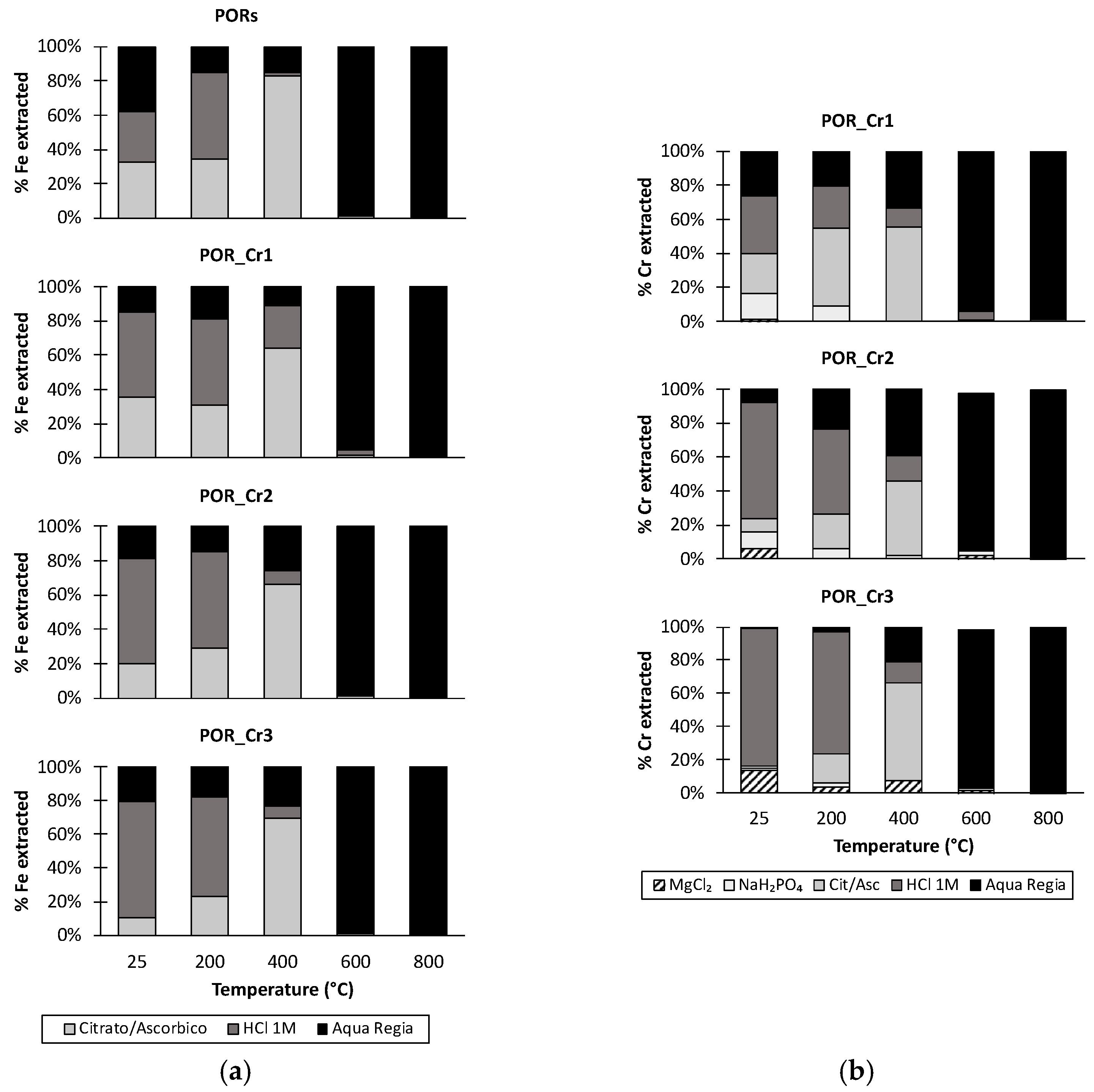
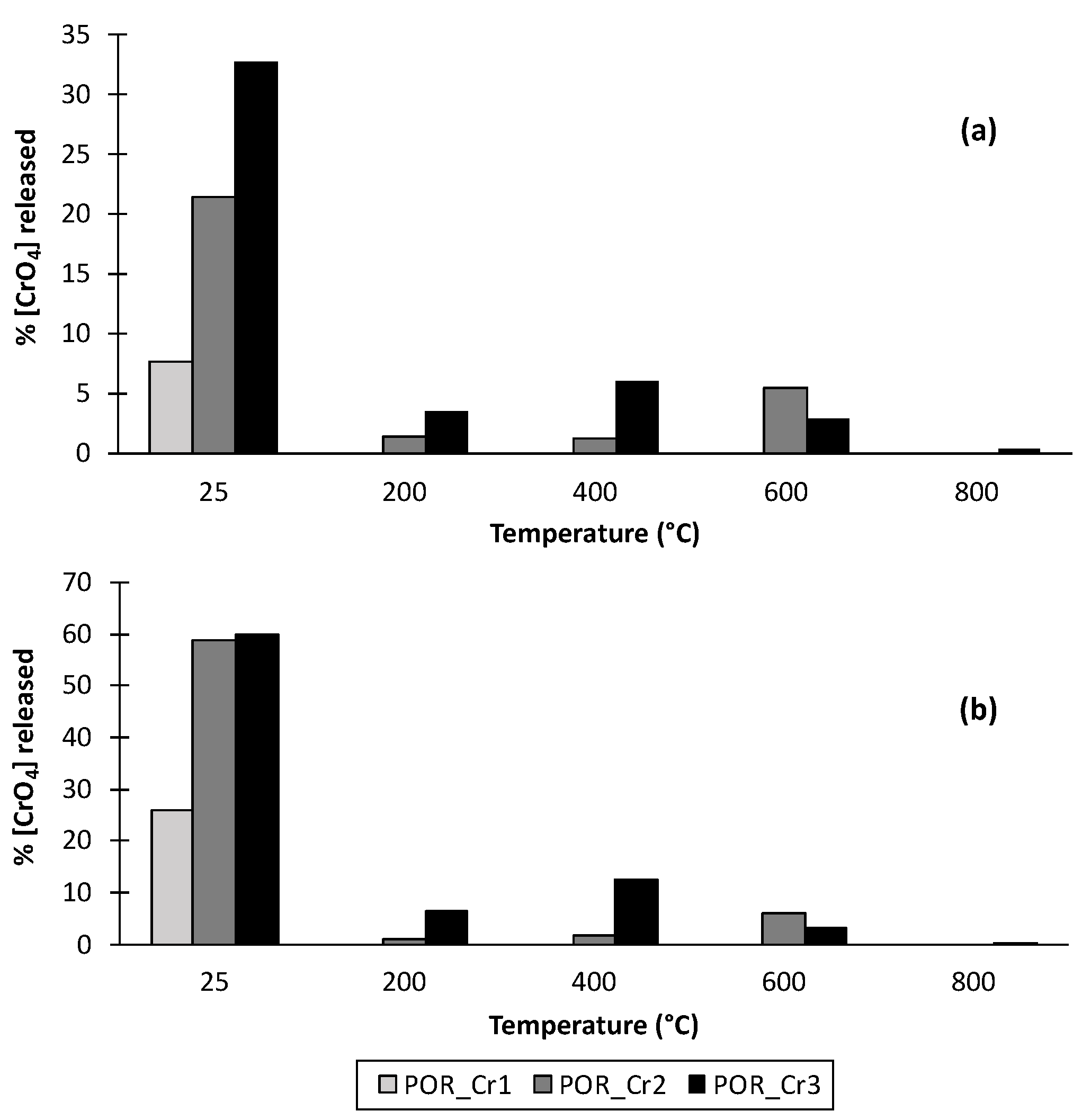
3.4. Partitioning of Iron and Chromium in Thermal Transformation Products
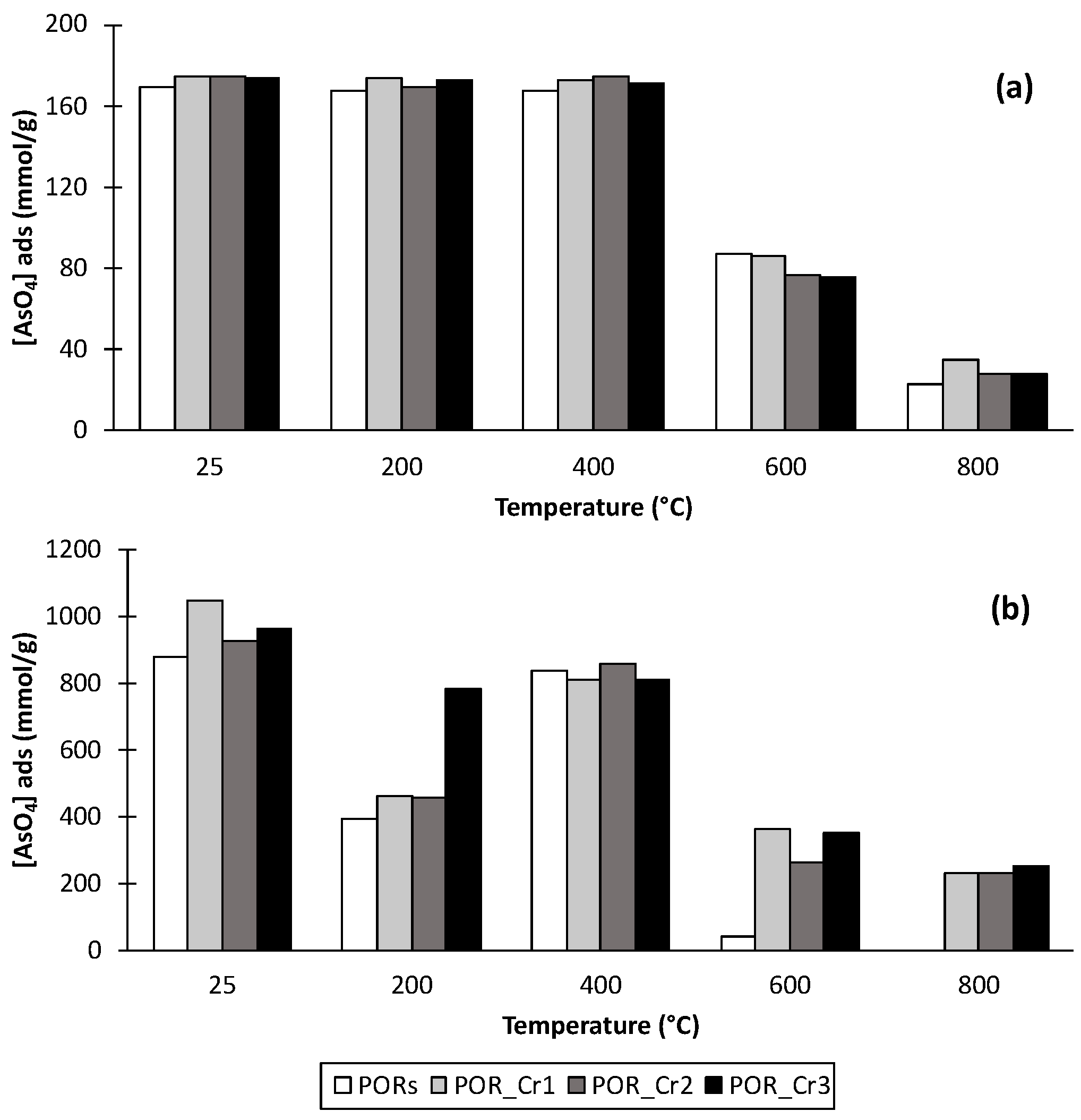
3.5. Arsenate Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoepfer, V.A.; Burton, E.D. Schwertmannite: A review of its occurrence, formation, structure, stability and interactions with oxyanions. Earth-Sci. Rev. 2021, 221, 103811. [Google Scholar] [CrossRef]
- Bigham, J.M.; Schwertmann, U.; Carlson, L.; Murad, E. A poorly crystallized oxyhydroxysulfate of iron formed by bacterial oxidation of Fe(II) in acid mine waters. Geochim. Cosmochim. Acta 1990, 54, 2743–2758. [Google Scholar] [CrossRef]
- Bigham, J.M.; Nordstrom, D.K. Iron and aluminium hydroxysulfates from acid sulphate waters. In Reviews in Mineralogy and Geochemistry—Sulfate Minerals: Crystallography, Geochemistry, and Environmental Significance; Alpers, C.N., Jambor, J.L., Nordstrom, D.K., Eds.; The Mineralogical Society of America: Washington, DC, USA, 2000; pp. 351–403. [Google Scholar]
- Regenspurg, S.; Brand, A.; Peiffer, S. Formation and stability of schwertmannite in acidic mining lakes. Geochim. Cosmochim. Acta 2004, 68, 1185–1197. [Google Scholar] [CrossRef]
- Antelo, J.; Fiol, S.; Gondar, D.; López, R.; Arce, F. Comparison of arsenate, chromate and molybdate binding on schwertmannite: Surface adsorption vs anion-exchange. J. Colloid Interface Sci. 2012, 386, 338–343. [Google Scholar] [CrossRef]
- Marouane, B.; Klug, M.; As, K.S.; Enge, J.; Reichel, S.; Janneck, E.; Peiffer, S. The potential of granulated schwertmannite adsorbents to remove oxyanions (SeO23−, SeO24−, MoO24−, PO34−, Sb(OH)−6) from contaminated water. J. Geochem. Explor. 2021, 223, 107708. [Google Scholar] [CrossRef]
- Burton, E.D.; Karimian, N.; Johnston, S.G.; Schoepfer, V.A.; Choppala, G.; Lamb, D. Arsenic-imposed effects on schwertmannite and jarosite formation in acid mine drainage and coupled impacts on arsenic mobility. ACS Earth Space Chem. 2021, 5, 1418–1435. [Google Scholar] [CrossRef]
- Paikaray, S.; Peiffer, S. Lepidocrocite formation kinetics from schwertmannite in Fe(II)-rich anoxic alkaline medium. Mine Water Environ. 2014, 34, 213–222. [Google Scholar] [CrossRef]
- Antelo, J.; Fiol, S.; Carabante, I.; Arroyo, A.; Lezama-Pacheco, J.S.; Josevska, N.; Protopapa, C.; Kumpiene, J. Stability of naturally occurring AMD–schwertmannite in the presence of arsenic and reducing agents. J. Geochem. Explor. 2021, 220, 106677. [Google Scholar] [CrossRef]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250, 272–291. [Google Scholar] [CrossRef]
- Xie, J.; Gu, X.; Tong, F.; Zhao, Y.; Tan, Y. Surface complexation modeling of Cr(VI) adsorption at the goethite–water interface. J. Colloid Interf. Sci. 2015, 455, 55–62. [Google Scholar] [CrossRef]
- Komárek, M.; Koretsky, C.M.; Stephen, K.J.; Alessi, D.S.; Chrastný, V. Competitive adsorption of Cd(II), Cr(VI), and Pb(II) onto nanomaghemite: A spectroscopic and modeling approach. Environ. Sci. Technol. 2015, 49, 12851–12859. [Google Scholar] [CrossRef] [PubMed]
- Bompoti, N.M.; Chrysochoou, M.; Machesky, M. A unified surface complexation modeling approach for chromate adsorption on iron oxides. Environ. Sci. Technol. 2019, 53, 6352–6361. [Google Scholar] [CrossRef] [PubMed]
- Dabizha, A.; Kersten, M. Exothermic adsorption of chromate by goethite. Appl. Geochem. 2020, 121, 104785. [Google Scholar] [CrossRef]
- Regenspurg, S.; Peiffer, S. Arsenate and chromate incorporation in schwertmannite. Appl. Geochem. 2005, 20, 1226–1239. [Google Scholar] [CrossRef]
- Li, X.; Guo, C.; Jin, X.; He, C.; Yao, Q.; Lu, G.; Dang, Z. Mechanisms of Cr(VI) adsorption on schwertmannite under environmental disturbance: Changes in surface complex structures. J. Haz. Mat. 2021, 416, 125781. [Google Scholar] [CrossRef]
- Fan, C.; Guo, C.; Zeng, Y.; Tu, Z.; Ji, Y.; Reinfelder, J.R.; Chen, M.; Huang, W.; Lu, G.; Yi, X.; et al. The behavior of chromium and arsenic associated with redox transformation of schwertmannite in AMD environment. Chemosphere 2019, 222, 945–953. [Google Scholar] [CrossRef]
- Choppala, G.; Karimian, N.; Burton, E.D. An X-ray absorption spectroscopic study of the Fe(II)-induced transformation of Cr(VI)-substituted schwertmannite. J. Haz. Mat. 2022, 431, 128580. [Google Scholar] [CrossRef]
- Johnston, S.G.; Burton, E.D.; Moon, E.M. Arsenic mobilization is enhanced by thermal transformation of schwertmannite. Environ. Sci. Technol. 2016, 50, 8010–8019. [Google Scholar] [CrossRef]
- Vithana, C.L.; Johnston, S.G.; Dawson, N. Divergent repartitioning of copper, antimony and phosphorus following thermal transformation of schwertmannite and ferrihydrite. Chem. Geol. 2018, 483, 530–543. [Google Scholar] [CrossRef]
- Das, S.; Hendry, M.J.; Essilfie-Dughan, J. Transformation of two-line ferrihydrite to goethite and hematite as a function of pH and temperature. Environ. Sci. Technol. 2012, 45, 268–275. [Google Scholar] [CrossRef]
- Grogan, K.L.; Gilkes, R.J.; Lottermoser, B.G. Maghemite formation in burnt plant litter at East Trinity, north Queensland, Australia. Clays Clay Miner. 2003, 51, 390–396. [Google Scholar] [CrossRef]
- Otero-Fariña, A.; Gago, R.; Antelo, J.; Fiol, S.; Arce, F. Surface complexation modelling of arsenic and copper immobilization by iron oxide precipitates derived from acid mine drainage. Bol. Soc. Geol. Mex. 2015, 67, 493–508. [Google Scholar] [CrossRef]
- Baleeiro, A.; Fiol, S.; Otero-Fariña, A.; Antelo, J. Surface chemistry of iron oxides formed by neutralization of acidic mine waters: Removal of trace metals. App. Geochem. 2018, 89, 129–137. [Google Scholar] [CrossRef]
- Álvarez, E.; Fernández-Sanjurjo, M.; Otero, X.L.; Macías, F. Aluminum speciation in the bulk and rhizospheric soil solution of the species colonizing an abandoned copper mine in Galicia (NW Spain). J. Soil Sediment. 2011, 11, 221–230. [Google Scholar] [CrossRef]
- Asensio, V.; Vega, F.A.; Andrade, M.L.; Covelo, E.F. Tree vegetation and waste amendments to improve the physical condition of copper mine soils. Chemosphere 2013, 90, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Burgos, W.D.; Borch, T.; Troyer, L.D.; Luan, F.; Larson, L.N.; Brown, J.F.; Lambson, J.; Shimizu, M. Schwertmannite and Fe oxides formed by biological low-pH Fe(II) oxidation versus abiotic neutralization: Impact on trace metal sequestration. Geochim. Cosmochim. Acta 2012, 76, 29–44. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Bartlett, R.J.; James, B.R. Chromium. In Methods of Soils Analysis, Part 3: Chemical Methods, 1st ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Summer, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 683–701. [Google Scholar]
- Certini, G. Effects of fire on properties of forest soils: A review. Oceologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Blake, D.; Lu, K.; Horwitz, P.; Boyce, M.C. Fire suppression and burnt sediments: Effects on the water chemistry of fire-affected wetlands. Int. J. Wildland Fire 2012, 21, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Claff, S.R.; Sullivan, L.A.; Burton, E.D.; Bush, R.T. A sequential extraction procedure for acid sulfate soils: Partitioning of iron. Geoderma 2010, 2010 155, 224–230. [Google Scholar] [CrossRef]
- Keon, N.E.; Swartz, C.H.; Brabander, D.J.; Harvey, C.; Hemond, H.F. Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environ. Sci. Technol. 2001, 35, 2778–2784. [Google Scholar] [CrossRef]
- Paul, C.J.; Ford, R.G.; Wilkin, R.T. Assessing the selectivity of extractant solutions for recovering labile arsenic associated with iron (hydr)oxides and sulfides in sediments. Geoderma 2009, 152, 137–144. [Google Scholar] [CrossRef]
- Lenoble, V.; Deluchat, V.; Serpaud, B.; Bollinger, J.C. Arsenite oxidation and arsenate determination by the molybdene blue method. Talanta 2003, 61, 267–276. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Mine waters: Acid to circumneutral. Elements 2011, 7, 393–398. [Google Scholar] [CrossRef]
- Boily, J.F.; Gassman, P.L.; Peretyazhko, T.; Szanyi, J.; Zachara, J.M. FTIR spectral components of schwertmannite. Environ. Sci. Technol. 2010, 44, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Tresintsi, S.; Simeonidis, K.; Pliatsikas, N.; Vourlias, G.; Patsalas, P.; Mitrakas, M. The role of SO2− surface distribution in arsenic removal by iron oxy-hydroxides. J. Solid State Chem. 2014, 213, 145–151. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; Diez-Ercilla, M. Schwermannite and hydrobasaluminite: A re-evaluation of their solubility and control on the iron and aluminium concentration in acidic pit lakes. Appl. Geochem 2011, 26, 1752–1774. [Google Scholar] [CrossRef]
- Caraballo, M.A.; Rimstidt, J.D.; Macías, F.; Nieto, J.M.; Hochella, M.F., Jr. Metastability, nanocrystallinity and pseudo-solid solution effects on the understanding of schwertmannite solubility. Chem. Geol. 2013, 360, 22–31. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Pamo, E.L.; Santofimia, E.; Aduvire, O.; Reyes, J.; Barettino, D. Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershec, Huelva, SW Spain): Geochemistry, mineralogy and environmental implications. Appl. Geochem 2005, 20, 1320–1356. [Google Scholar] [CrossRef]
- Antelo, J.; Fiol, S.; Gondar, D.; Pérez, C.; López, R.; Arce, F. Cu(II) incorporation to schwertmannite: Effect on stability and reactivity under AMD conditions. Geochim. Cosmochim. Acta 2013, 119, 149–163. [Google Scholar] [CrossRef]
- Majzlan, J.; Alpers, C.N.; Kock, C.B.; McCleskey, R.B.; Mynenie, S.C.B.; Neil, J.M. Vibrational, X-ray absorption, and Mössbauer spectra of sulfate minerals from the weathered massive sulfide deposit at Iron Mountain, California. Chem. Geol. 2011, 284, 296–305. [Google Scholar] [CrossRef]
- Pulišová, P.; Máša, B.; Michalková, E.; Večerníková, E.; Maříková, M.; Bezdička, P.; Murafa, N.; Šubrt, J. Thermal behaviour of natural and synthetic iron precipitates from mine drainage. J. Therm. Anal. Calorim. 2014, 116, 625–632. [Google Scholar] [CrossRef]
- Burton, E.D.; Bush, R.T.; Johnston, S.G.; Watling, K.M.; Hocking, R.K.; Sullivan, L.A.; Parker, G.K. Sorption of arsenic(V) and arsenic(III) to schwertmannite. Environ. Sci. Technol. 2009, 43, 9202–9207. [Google Scholar] [CrossRef] [PubMed]
- Cismasu, A.C.; Michel, F.M.; Tcaciuc, A.P.; Tyliszczak, T.; Brown, G.E., Jr. Composition and structural aspects of naturally occurring ferrihydrite. Compt. Rendus Geosci. 2011, 343, 210–218. [Google Scholar] [CrossRef]
- Yue, P.; Chen, N.; Peak, D.; Bompoti, N.M.; Chrysochoou, M.; Onnis-Hayden, A.; Larese-Casanova, P. Oxygen atom release during selenium oxyanion adsorption on goethite and hematite. Appl. Geochem. 2020, 117, 104605. [Google Scholar] [CrossRef]
- Waiman, C.V.; Arroyave, J.M.; Chen, H.; Tan, W.; Avena, M.J.; Zanini, G.P. The simultaneous presence of glyphosate and phosphate at the goethite surface as seen by XPS, ATR-FTIR and competitive adsorption isotherms. Colloid. Surf. A 2016, 498, 121–127. [Google Scholar] [CrossRef]
- Burton, E.D.; Choppala, G.; Vithana, C.L.; Karimina, N.; Hockman, K.; Johnston, S.G. Chromium(VI) formation via heating of Cr(III)-Fe(III)-(oxy)hydroxides: A pathway for fire-induced soil pollution. Chemosphere 2019, 222, 440–444. [Google Scholar] [CrossRef]
| Extractant | Reaction Time | Phase Justification | Reference |
|---|---|---|---|
| 1 M MgCl2 | 1 h | Exchangeable or ionically bound to the mineral surface (Crexc) | [32] |
| 1 M NaH2PO4 | 16 h | Strongly adsorbed on the mineral surface (Crads) | [33] |
| 0.2 M citrate/0.4 M ascorbate | 24 h | Associated with poorly crystalline mineral phases (Crcit and Fecit) | [34] |
| 1 M HCl | 4 h | Coprecipitated with carbonates, Mn oxides, and poorly crystalline Fe oxyhydroxides (CrHCl and FeHCl) | [32] |
| Aqua regia | 16 h | Total concentration of iron and chromium present in solid phases (Crtot and Fetot) | [28] |
| pH | EC | Eh | T | Fetot | Fe(II) | Al | Cu | Zn | Ni | Mn | SO4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| µS/cm | mV | °C | mg/L | ||||||||
| 3.34 | 1856 | 297 | 16.7 | 365.4 | 309 | 12.9 | 0.09 | 0.16 | 0.20 | 13.5 | 2485 |
| Sample | Fe | SO4 | Cr |
|---|---|---|---|
| mmol/g | mmol/g | μmol/g | |
| PORS | 8.59 | 1.79 | - |
| POR-Cr1 | 6.98 | 1.50 | 5.64 |
| POR-Cr2 | 6.71 | 1.28 | 47.88 |
| POR-Cr3 | 7.27 | 0.71 | 194.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lázaro, C.; Antelo, J.; Carabante, I.; Otero-Fariña, A.; Verdes, P.V.; Dacunha-Marinho, B.; Fiol, S. Thermal Transformation of Natural Schwertmannite in the Presence of Chromium. Minerals 2022, 12, 726. https://doi.org/10.3390/min12060726
Lázaro C, Antelo J, Carabante I, Otero-Fariña A, Verdes PV, Dacunha-Marinho B, Fiol S. Thermal Transformation of Natural Schwertmannite in the Presence of Chromium. Minerals. 2022; 12(6):726. https://doi.org/10.3390/min12060726
Chicago/Turabian StyleLázaro, Carlos, Juan Antelo, Ivan Carabante, Alba Otero-Fariña, Pedro V. Verdes, Bruno Dacunha-Marinho, and Sarah Fiol. 2022. "Thermal Transformation of Natural Schwertmannite in the Presence of Chromium" Minerals 12, no. 6: 726. https://doi.org/10.3390/min12060726
APA StyleLázaro, C., Antelo, J., Carabante, I., Otero-Fariña, A., Verdes, P. V., Dacunha-Marinho, B., & Fiol, S. (2022). Thermal Transformation of Natural Schwertmannite in the Presence of Chromium. Minerals, 12(6), 726. https://doi.org/10.3390/min12060726







