Assessment of Easily Accessible Spectroscopic Techniques Coupled with Multivariate Analysis for the Qualitative Characterization and Differentiation of Earth Pigments of Various Provenance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pigments Investigated in This Study
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. X-ray Fluorescence (XRF)
2.4. Raman Spectroscopy
2.5. Multivariate Data Analysis
3. Results
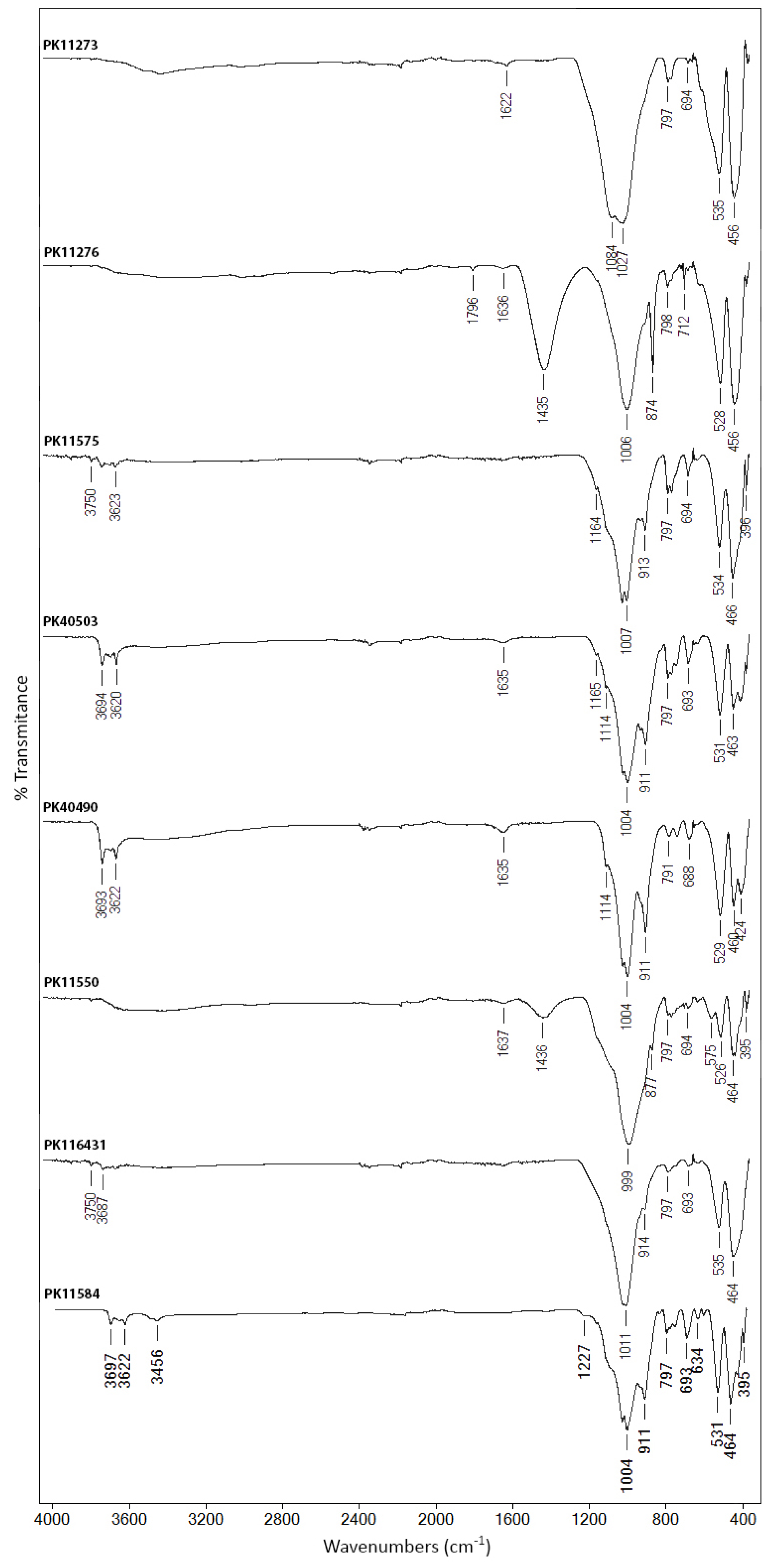
3.1. Molecular Analysis
3.1.1. Red Ochers
3.1.2. Yellow Ochers
3.1.3. Green Earths
3.1.4. Brown and Black Earths
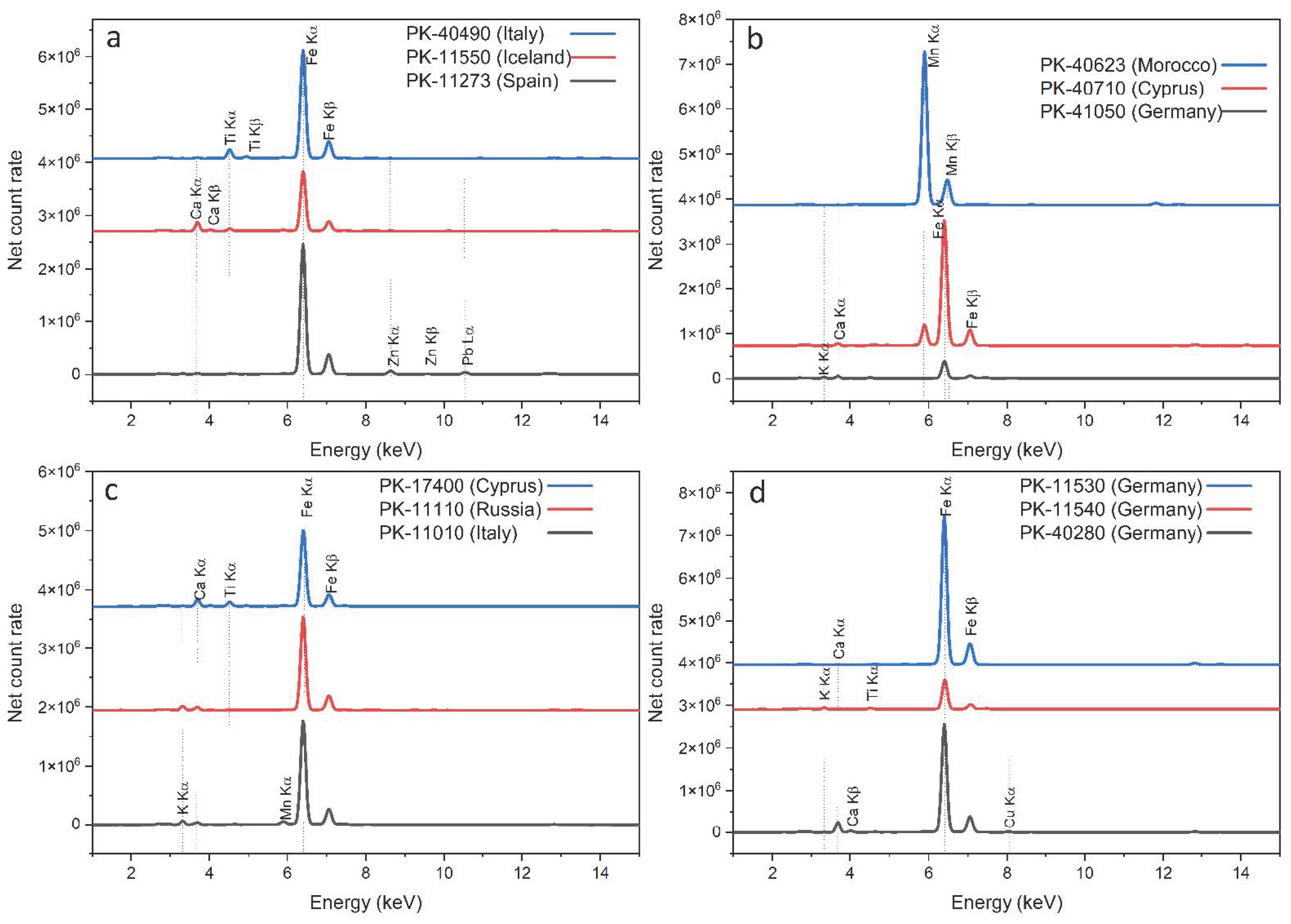
3.2. Elemental Analysis
3.2.1. Red Ochers
3.2.2. Yellow Ochers
3.2.3. Green Earths
3.2.4. Brown and Black Earths
4. Discussion
4.1. Spectral and Chemical Fingerprints
4.1.1. Red Ochers
4.1.2. Yellow Ochers
4.1.3. Green Earths
4.1.4. Brown and Black Earths
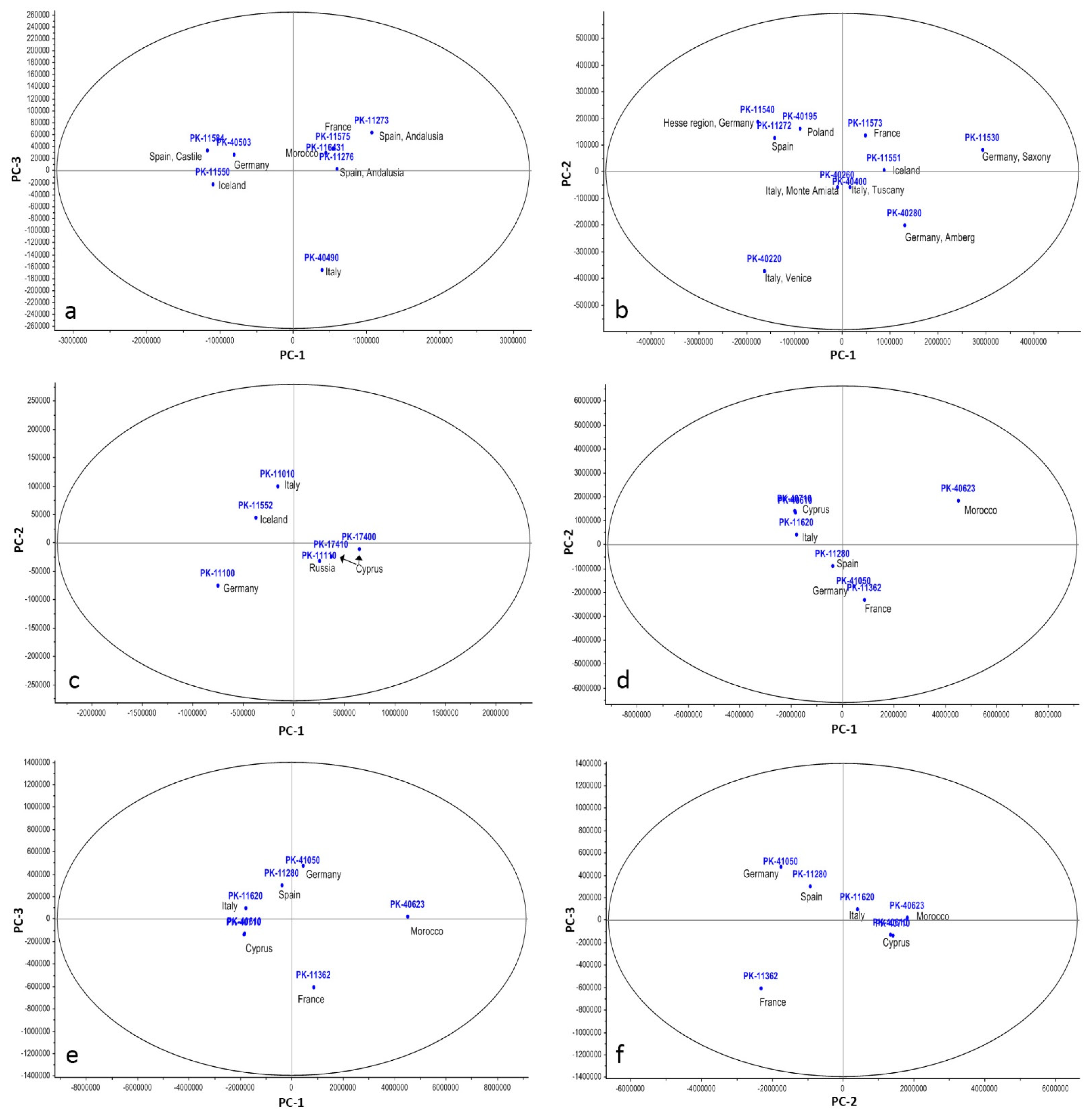
4.2. Discrimination of Earth Pigments of Similar Hue via PCA Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Domingo, I.; Chieli, A. Characterizing the pigments and paints of prehistoric artists. Archaeol. Anthropol. Sci. 2021, 13, 166. [Google Scholar] [CrossRef]
- Berrie, B.H. Artists’ Pigments: A Handbook of Their History and Characteristics; Archetype Publications: London, UK, 2007; Volume 4. [Google Scholar]
- Henshilwood, C.S.; d’Errico, F.; Watts, I. Engraved ochres from the Middle Stone Age levels at Blombos Cave, South Africa. J. Hum. Evol. 2009, 57, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Siddall, R. Mineral pigments in archaeology: Their analysis and the range of available materials. Minerals 2018, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Barham, L.S. Systematic pigment use in the middle pleistocene of south-central Africa. Curr. Anthropol. 2002, 43, 181–190. [Google Scholar] [CrossRef]
- d’Errico, F.; Dayet Bouillot, L.; García-Diez, M.; Pitarch Martí, A.; Garrido Pimentel, D.; Zilhão, J. The technology of the earliest European cave paintings: El Castillo Cave, Spain. J. Archaeol. Sci. 2016, 70, 48–65. [Google Scholar] [CrossRef]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddall, R. Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments, 1st ed.; Routledge: London, UK, 2008. [Google Scholar] [CrossRef]
- Bikiaris, D.; Daniilia, S.; Sotiropoulou, S.; Katsimbiri, O.; Pavlidou, E.; Moutsatsou, A.P.; Chryssoulakis, Y. Ochre-differentiation through micro-Raman and micro-FTIR spectroscopies: Application on wall paintings at Meteora and Mount Athos, Greece. Spectrochim. Acta A 2000, 56, 3–18. [Google Scholar] [CrossRef]
- Popelka-Filcoff, R.S.; Robertson, J.D.; Glascock, M.D.; Descantes, C. Trace element characterization of ochre from geological sources. J. Radioanal. Nucl. Chem. 2007, 272, 17–27. [Google Scholar] [CrossRef]
- Helwig, K. The characterisation of iron earth pigments using infrared spectroscopy. In Proceedings of the Second Infrared and Raman User’s Group (IRUG 2) Conference, Victoria and Albert Museum, London, UK, 12–13 September 1995; pp. 83–92. [Google Scholar]
- Huntley, J. Australian Indigenous Ochres: Use, Sourcing, and Exchange. In The Oxford Handbook of the Archaeology of Indigenous Australia and New Guinea; McNiven, I.J., David, B., Eds.; Oxford University Press: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Elias, M.; Chartier, C.; Prévot, G.; Garay, H.; Vignaud, C. The colour of ochres explained by their composition. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2006, 127, 70–80. [Google Scholar] [CrossRef]
- Genestar, C.; Pons, C. Earth pigments in painting: Characterisation and differentiation by means of FTIR spectroscopy and SEM-EDS microanalysis. Anal. Bioanal. Chem. 2005, 382, 269–274. [Google Scholar] [CrossRef]
- Kostomitsopoulou Marketou, A.; Kouzeli, K.; Facorellis, Y. Colourful earth: Iron-containing pigments from the Hellenistic pigment production site of the ancient agora of Kos (Greece). J. Archaeol. Sci. Rep. 2019, 26, 101843. [Google Scholar] [CrossRef]
- Marshall, L.J.R.; Williams, J.R.; Almond, M.J.; Atkinson, S.D.M.; Cook, S.R.; Matthews, W.; Mortimore, J.L. Analysis of ochres from Clearwell Caves: The role of particle size in determining colour. Spectrochim. Acta A 2005, 61, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Rousaki, A.; Vargas, E.; Vázquez, C.; Aldazábal, V.; Bellelli, C.; Carballido Calatayud, M.; Hajduk, A.; Palacios, O.; Moens, L.; Vandenabeele, P. On-field Raman spectroscopy of Patagonian prehistoric rock art: Pigments, alteration products and substrata. Trends Analyt Chem. 2018, 105, 338–351. [Google Scholar] [CrossRef] [Green Version]
- Boucherie, N.; Nowik, W.; Pingaud, N. Characterisation of materials and techniques in first archaeological findings of Nasca wall paintings. Herit. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Cortea, I.M.; Ratoiu, L.; Ghervase, L.; Țentea, O.; Dinu, M. Investigation of ancient wall painting fragments discovered in the roman baths from alburnus maior by complementary non-destructive techniques. Appl. Sci. 2021, 11, 10049. [Google Scholar] [CrossRef]
- Bianchin, S.; Casellato, U.; Favaro, M.; Vigato, P.A. Painting technique and state of conservation of wall paintings at Qusayr Amra, Amman–Jordan. J. Cult. Herit. 2007, 8, 289–293. [Google Scholar] [CrossRef]
- Edreira, M.C.; Feliu, M.J.; Fernández-Lorenzo, C.; Martín, J. Spectroscopic analysis of Roman wall paintings from Casa del Mitreo in Emerita Augusta, Mérida, Spain. Talanta 2003, 59, 1117–1139. [Google Scholar] [CrossRef]
- Mastrotheodoros, G.P.; Beltsios, K.G.; Bassiakos, Y. On the Red and Yellow Pigments of Post-Byzantine Greek Icons. Archaeometry 2020, 63, 753–778. [Google Scholar] [CrossRef]
- Ghervase, L.; Cortea, I.M.; Rădvan, R.; Ratoiu, L.; Chelmuș, A. Complementary investigations of two Lipovan-style icons. Microchem. J. 2018, 138, 509–518. [Google Scholar] [CrossRef]
- Antunes, V.; Candeias, A.; Mirão, J.; Carvalho, M.L.; Dias, C.B.; Manhita, A.; Cardoso, A.; Francisco, M.J.; Lauw, A.; Manso, M. Analytical characterization of the palette and painting techniques of Jorge Afonso, the great 16th century Master of Lisbon painting workshop. Spectrochim. Acta A 2018, 193, 264–275. [Google Scholar] [CrossRef]
- Cortea, I.M.; Ghervase, L.; Ratoiu, L.; Dinu, M.; Rădvan, R. Uncovering hidden jewels: An investigation of the pictorial layers of an 18th-century Taskin harpsichord. Herit. Sci. 2020, 8, 55. [Google Scholar] [CrossRef]
- van Loon, A.; Vandivere, A.; Delaney, J.K.; Dooley, K.A.; De Meyer, S.; Vanmeert, F.; Gonzalez, V.; Janssens, K.; Leonhardt, E.; Haswell, R.; et al. Beauty is skin deep: The skin tones of Vermeer’s Girl with a Pearl Earring. Herit. Sci. 2019, 7, 102. [Google Scholar] [CrossRef]
- Grygar, T.; Hradilová, J.; Hradil, D.; Bezdička, P.; Bakardjieva, S. Analysis of earthy pigments in grounds of Baroque paintings. Anal. Bioanal. Chem. 2003, 375, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Popelka-Filcoff, R.S.; Miksa, E.J.; Robertson, J.D.; Glascock, M.D.; Wallace, H. Elemental analysis and characterization of ochre sources from Southern Arizona. J. Archaeol. Sci. 2008, 35, 752–762. [Google Scholar] [CrossRef]
- MacDonald, B.L.; Fox, W.; Dubreuil, L.; Beddard, J.; Pidruczny, A. Iron oxide geochemistry in the Great Lakes Region (North America): Implications for ochre provenance studies. J. Archaeol. Sci. Rep. 2018, 19, 476–490. [Google Scholar] [CrossRef]
- MacDonald, B.L.; Hancock, R.G.V.; Cannon, A.; Pidruczny, A. Geochemical characterization of ochre from central coastal British Columbia, Canada. J. Archaeol. Sci. 2011, 38, 3620–3630. [Google Scholar] [CrossRef]
- Gil, M.; Carvalho, M.L.; Seruya, A.; Candeias, A.E.; Mirão, J.; Queralt, I. Yellow and red ochre pigments from southern Portugal: Elemental composition and characterization by WDXRF and XRD. Nucl. Instrum. Methods Phys. Res. A Accel. Spectrom. Detect. Assoc. Equip. 2007, 580, 728–731. [Google Scholar] [CrossRef]
- Hradil, D.; Píšková, A.; Hradilová, J.; Bezdička, P.; Lehrberger, G.; Gerzer, S. Mineralogy of bohemian green earth pigment and its microanalytical evidence in historical paintings. Archaeometry 2011, 53, 563–586. [Google Scholar] [CrossRef]
- Weinstein-Evron, M.; Ilani, S. Provenance of ochre in the natufian layers of el-Wad Cave, Mount Carmel, Israel. J. Archaeol. Sci. 1994, 21, 461–467. [Google Scholar] [CrossRef]
- Erlandson, J.M.; Robertson, J.D.; Descantes, C. Geochemical Analysis of Eight Red Ochres from Western North America. Am. Antiq. 1999, 64, 517–526. [Google Scholar] [CrossRef]
- Dayet, L.; Le Bourdonnec, F.X.; Daniel, F.; Porraz, G.; Texier, P.J. Ochre Provenance and Procurement Strategies During The Middle Stone Age at Diepkloof Rock Shelter, South Africa. Archaeometry 2016, 58, 807–829. [Google Scholar] [CrossRef]
- Popelka-Filcoff, R.S.; Zipkin, A.M. The archaeometry of ochre sensu lato: A review. J. Archaeol. Sci. 2022, 137, 105530. [Google Scholar] [CrossRef]
- Dayet, L. Invasive and non-invasive analyses of ochre and iron-based pigment raw materials: A methodological perspective. Minerals 2021, 11, 210. [Google Scholar] [CrossRef]
- Genestar, C.J.; Pons Bonafé, C. The use of natural earths in picture: Study and differentiation by thermal analysis. Thermochim. Acta 2004, 413, 185–192. [Google Scholar] [CrossRef]
- Nel, P.; Lynch, P.A.; Laird, J.S.; Casey, H.M.; Goodall, L.J.; Ryan, C.G.; Sloggett, R.J. Elemental and mineralogical study of earth-based pigments using particle induced X-ray emission and X-ray diffraction. Nucl. Instrum. Methods Phys. Res. A Accel. Spectrom. Detect. Assoc. Equip. 2010, 619, 306–310. [Google Scholar] [CrossRef]
- Fanost, A.; Gimat, A.; de Viguerie, L.; Martinetto, P.; Giot, A.C.; Clémancey, M.; Blondin, G.; Gaslain, F.; Glanville, H.; Walter, P.; et al. Revisiting the identification of commercial and historical green earth pigments. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124035. [Google Scholar] [CrossRef]
- Doménech, A.; Doménech-Carbó, M.T.; Edwards, H.G.M. Identification of earth pigments by applying hierarchical cluster analysis to solid state voltammetry. Application to severely damaged frescoes. Electroanalysis 2007, 19, 1890–1900. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Lopes, F.N. Heated goethite and natural hematite: Can Raman spectroscopy be used to differentiate them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Froment, F.; Tournié, A.; Colomban, P. Raman identification of natural red to yellow pigments: Ochre and iron-containing ores. J. Raman Spectrosc. 2008, 39, 560–568. [Google Scholar] [CrossRef]
- Ospitali, F.; Bersani, D.; Di Lonardo, G.; Lottici, P.P. ‘Green earths’: Vibrational and elemental characterization of glauconites, celadonites and historical pigments. J. Raman Spectrosc. 2008, 39, 1066–1073. [Google Scholar] [CrossRef]
- David, B.; Clayton, E.; Watchman, A. Initial Results Of Pixe Analysis On Northern Australian Ochres. Aust. Archaeol. 1993, 36, 50–57. [Google Scholar] [CrossRef]
- Makreski, P.; Jovanovski, G.; Kaitner, B. Minerals from Macedonia. XXIV. Spectra-structure characterization of tectosilicates. J. Mol. Struct. 2009, 924, 413–419. [Google Scholar] [CrossRef]
- Béarat, H. Les Pigments Verts En Peinture Murale Romaine: Bilan Analytique. in Roman Wall Painting: Materials, Techniques, Analysis and Conservation. In Proceedings of the International Workshop on Roman Wall Painintg, Fribourg, Switzerland, 7–9 March 1996; pp. 269–286. [Google Scholar]
- Cavallo, G.; Riccardi, M.P.; Zorzin, R. Powder diffraction of yellow and red natural earths from Lessini Mountains in NE Italy. Powder Diffr. 2015, 30, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Farmer, V.C. The Infrared Spectra of Minerals; Mineralogical Society: London, UK, 1974. [Google Scholar] [CrossRef]
- Mcmillan, P.F.; Hofmeister, A.M. Infrared and Raman spectroscopy. In Spectroscopic Methods in Mineralogy and Geology; Hawthorne, F.C., Ed.; Mineralogical Society of America: Washington, DC, USA, 1988; Volume 18, pp. 99–160. [Google Scholar] [CrossRef]
- Shugar, A.N.; Mass, J.L. Handheld XRF for Art and Archaeology; Leuven University Press: Leuven, Belgium, 2013. [Google Scholar]
- Pozzi, F.; Rizzo, A.; Basso, E.; Angelin, E.M.; Sá, S.F.; Cucci, C.; Picollo, M. Portable Spectroscopy for Cultural Heritage. In Portable Spectroscopy and Spectrometry II—Applications, 1st ed.; Crocombe, R.A., Leary, P.E., Kammrath, B.W., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 499–522. [Google Scholar] [CrossRef]
- Wei, C.; Wang, J.; Ji, J. Forensic Classification of Pigments by Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy and Chemometrics. Anal. Lett. 2021, 54, 1309–1328. [Google Scholar] [CrossRef]
- Festa, G.; Scatigno, C.; Armetta, F.; Saladino, M.L.; Ciaramitaro, V.; Nardo, V.M.; Ponterio, R.C. Chemometric Tools to Point Out Benchmarks and Chromophores in Pigments through Spectroscopic Data Analyses. Molecules 2022, 27, 163. [Google Scholar] [CrossRef] [PubMed]
- Mauran, G.; Caron, B.; Détroit, F.; Nankela, A.; Bahain, J.J.; Pleurdeau, D.; Lebon, M. Data pretreatment and multivariate analyses for ochre sourcing: Application to Leopard Cave (Erongo, Namibia). J. Archaeol. Sci. Rep. 2021, 35, 102757. [Google Scholar] [CrossRef]
- Esbensen, K.H.; Geladi, P. Principal Component Analysis: Concept, Geometrical Interpretation, Mathematical Background, Algorithms, History, Practice. In Comprehensive Chemometrics, 1st ed.; Brown, S.D., Tauler, R., Walczak, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 1, pp. 211–226. [Google Scholar] [CrossRef]
- Peris-Díaz, M.D.; Krężel, A. A guide to good practice in chemometric methods for vibrational spectroscopy, electrochemistry, and hyphenated mass spectrometry. Trends Analyt Chem. 2021, 135, 116157. [Google Scholar] [CrossRef]
- Aida, S.; Matsuno, T.; Hasegawa, T.; Tsuji, K. Application of principal component analysis for improvement of X-ray fluorescence images obtained by polycapillary-based micro-XRF technique. Nucl. Instrum. Meth. B 2017, 402, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Invernizzi, C.; Fiocco, G.; Iwanica, M.; Targowski, P.; Piccirillo, A.; Vagnini, M.; Licchelli, M.; Malagodi, M.; Bersani, D. Surface and interface treatments on wooden artefacts: Potentialities and limits of a non-invasive multi-technique study. Coatings 2021, 11, 29. [Google Scholar] [CrossRef]
- Andrić, V.; Gajić-Kvaščev, M.; Korolija Crkvenjakov, D.; Marić-Stojanović, M.; Gadžurić, S. Evaluation of pattern recognition techniques for the attribution of cultural heritage objects based on the qualitative XRF data. Microchem. J. 2021, 167, 106267. [Google Scholar] [CrossRef]
- Salama, W.; El Aref, M.; Gaupp, R. Spectroscopic characterization of iron ores formed in different geological environments using FTIR, XPS, Mössbauer spectroscopy and thermoanalyses. Spectrochim. Acta A 2015, 136, 1816–1826. [Google Scholar] [CrossRef]
- Saikia, B.J.; Parthasarathy, G.; Sarmah, N.C. Fourier transform infrared spectroscopic estimation of crystallinity in SiO2 based rocks. Bull. Mater. Sci. 2008, 31, 775–779. [Google Scholar] [CrossRef] [Green Version]
- Miliani, C.; Rosi, F.; Daveri, A.; Brunetti, B.G. Reflection infrared spectroscopy for the non-invasive in situ study of artists’ pigments. Appl. Phys. A Mater. Sci. Process. 2012, 106, 295–307. [Google Scholar] [CrossRef]
- Roy, A. Artists’ Pigments. A Handbook of Their History and Characteristics; National Gallery of Art: Washington, DC, USA, 1993; Volume 2.
- Vahur, S.; Teearu, A.; Leito, I. ATR-FT-IR spectroscopy in the region of 550–230 cm−1 for identification of inorganic pigments. Spectrochim. Acta A 2010, 75, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Cambier, P. Infrared study of goethites of varying crystallinity and particle size: I. Interpretation of OH and lattice vibration frequencies. Clay Miner. 1986, 21, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Schwertmann, U.; Cambier, P.; Murad, E. Properties of goethites of varying crystallinity. Clays Clay Miner. 1985, 33, 369–378. [Google Scholar] [CrossRef]
- Jovanovski, G.; Makreski, P.; Kaitner, B.; Šoptrajanov, B. Minerals from Macedonia. X-ray powder diffraction vs. vibrational spectroscopy in mineral identification. Contrib. Sect. Nat. Math. Biotech. Sci. 2009, 30, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Equeenuddin, S.M.; Tripathy, S.; Sahoo, P.K.; Panigrahi, M.K. Geochemistry of ochreous precipitates from coal mine drainage in India. Environ. Earth Sci. 2010, 61, 723–731. [Google Scholar] [CrossRef]
- Bigham, J.M.; Nordstrom, D.K. Iron and aluminum hydroxysulfates from acid sulfate waters. Rev. Mineral. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Madejová, J.; Gates, W.P.; Petit, S. IR Spectra of Clay Minerals. In Developments in Clay Science; Gates, W.P., Kloprogge, J.T., Madejová, J., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, pp. 107–149. [Google Scholar] [CrossRef]
- Buckley, H.A.; Bevan, J.C.; Brown, K.M.; Johnson, L.R.; Farmer, V.C. Glauconite and celadonite: Two separate mineral species. Mineral. Mag. 1978, 42, 373–382. [Google Scholar] [CrossRef]
- Feller, R.L. Artists’ Pigments: A Handbook of Their History and Characteristics; National Gallery of Art: Washington, DC, USA, 1986; Volume 1.
- Hradil, D.; Grygar, T.; Hrušková, M.; Bezdička, P.; Lang, K.; Schneeweiss, O.; Chvátal, M. Green earth pigment from the Kadaň region, Czech Republic: Use of rare Fe-rich smectite. Clays Clay Miner. 2004, 52, 767–778. [Google Scholar] [CrossRef]
- Frost, R.L.; López, A.; Xi, Y.; Scholz, R.; Souza, L.; Lana, C. The molecular structure of the borate mineral rhodizite (K, Cs)Al 4Be4(B, Be)12O28—A vibrational spectroscopic study. Spectrochim. Acta A 2014, 128, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hradil, D.; Grygar, T.; Hradilová, J.; Bezdička, P. Clay and iron oxide pigments in the history of painting. Appl. Clay Sci. 2003, 22, 223–236. [Google Scholar] [CrossRef]
- McKeown, D.A.; Post, J.E.; Etz, E.S. Vibrational analysis of palygorskite and sepiolite. Clays Clay Miner. 2002, 50, 667–680. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhang, Y.; Sun, J.; Wang, Z. The thermal transmission behavior analysis of two coal gangues selected from Inner Mongolia In China. Therm. Sci. 2018, 22, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Tomasini, E.; Siracusano, G.; Maier, M.S. Spectroscopic, morphological and chemical characterization of historic pigments based on carbon. Paths for the identification of an artistic pigment. Microchem. J. 2012, 102, 28–37. [Google Scholar] [CrossRef]
- Fitz Hugh, E.W. Artists’ Pigments: A Handbook of Their History and Characteristics; National Gallery of Art: Washington, DC, USA, 1997; Volume 3.
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Bernardini, S.; Bellatreccia, F.; Della Ventura, G.; Ballirano, P.; Sodo, A. Raman spectroscopy and laser-induced degradation of groutellite and ramsdellite, two cathode materials of technological interest. RSC Adv. 2020, 10, 923–929. [Google Scholar] [CrossRef] [Green Version]
- Rogerio-Candelera, M.Á.; Herrera, L.K.; Miller, A.Z.; García Sanjuán, L.; Mora Molina, C.; Wheatley, D.W.; Justo, Á.; Saiz-Jimenez, C. Allochthonous red pigments used in burial practices at the Copper Age site of Valencina de la Concepción (Sevilla, Spain): Characterisation and social dimension. J. Archaeol. Sci. 2013, 40, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Bibi, I.; Icenhower, J.; Niazi, N.K.; Naz, T.; Shahid, M.; Bashir, S. Clay Minerals: Structure, Chemistry, and Significance in Contaminated Environments and Geological CO2 Sequestration. In Environmental Materials and Waste: Resource Recovery and Pollution Prevention, 1st ed.; Prasad, M.N.V., Shih, K., Eds.; Academic Press: London, UK, 2016; pp. 543–567. [Google Scholar] [CrossRef]
- Kupka, D.; Pállová, Z.; Horňáková, A.; Achimovičová, M.; Kavečanský, V. Effluent water quality and the ochre deposit characteristics of the abandoned Smolník mine, East Slovakia. Acta Montan. Slovaca 2012, 17, 56–64. [Google Scholar]
- Odin, G.S.; Desprairies, A.; Fullagar, P.D.; Bellon, H.; Decarreau, A.; Frohlich, F.; Zelvelder, M. Nature and Geological Significance of Celadonite. In Developments in Sedimentology; Odin, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 45, pp. 337–398. [Google Scholar] [CrossRef]






| Group | Alternative Names | Color | Main Coloring Components |
|---|---|---|---|
| Ochers | ocher, flesh ochers, variations given by the geographical location of the source (e.g., Bristol ocher) | varying from browns and reds though yellows | iron oxide- and hydroxide-rich earths |
| Siennas | terre de Sienne, terra di Siena, Siena-erde | yellowinvestigated in this study. Pig-brown | iron hydroxide-rich earths + minor amounts of manganese oxides (<5%) |
| Umbers | ombra, terre d’ombre, terra d’ombra, tierra de sombra | warm brown to greenish brown | iron oxides + manganese oxides (between 5 and 20%) |
| Wads | bog manganese, black wad, black earth, manganese ochers | dark brown to black | iron oxides + manganese oxides (c. 50%) |
| Green earths | terra verde, terre vert, green stone | green to bluish green | clay minerals, celadonite or glauconite |
| Humic earths | Cassel earth, Cologne earth, Vandyke brown | rich brown | low-grade coal deposits or lignites |
| Sample ID | Pigment (Commercial Name) | Hue | Color Index | Provenance (Origin) |
|---|---|---|---|---|
| PK11273 | Red ocher from Andalusia | light-red | PR 102 | Andalusia, Spain |
| PK11272 | Yellow ocher from Andalusia | deep yellow | PY 43 | Andalusia, Spain |
| PK11276 | Brown ocher from Andalusia | reddish brown | PR 102 | Andalusia, Spain |
| PK11280 | Black earth from Andalusia | warm black | — | Andalusia, Spain |
| PK11584 | Spanish red ocher | orange-red | PR 102 | Castile, Spain |
| PK11575 | Burgundy red ocher | orange-red | PR 102 | Burgundy, France |
| PK11573 | Burgundy yellow ocher | warm yellow | PY 43 | Burgundy, France |
| PK11362 | Gray from Burgundy | warm gray | — | Burgundy, France |
| PK11100 | Bavarian green earth | light green | PG 23 | Bavaria, Germany |
| PK40280 | Amberg yellow | deep yellow | PY 43 | Bavaria, Germany |
| PK40503 | Red bole | orange-red | PR 102 | Germany |
| PK11530 | Lusatian ocher | brown-gold | PY 43 | Saxony, Germany |
| PK11540 | Taunus ocher | light warm yellow | PY 43 | Hesse region, Germany |
| PK41050 | Cassel brown | deep brown | NB 8 | Cologne, Germany |
| PK40490 | Rosso Sartorius | deep red | PR 102 | Sardinia, Italy |
| PK40220 | Italian gold ocher light | light gold | PY 43 | Venice, Italy |
| PK40400 | Raw Sienna Italian | deep yellow | PY 43 | Tuscany, Italy |
| PK40260 | Satin ocher | gold-orang | PY 43 | Monte Amiata, Italy |
| PK11620 | Brown earth from Otranto | sanguine-rust brown | PBr 7 | Otranto, Italy |
| PK11010 | Verona green earth | warm green | PG 23 | Monte Baldo, Italy |
| PK11550 | Snaefellsjoekull Red | reddish brown-violet | — | Snaefellsjoekull, Iceland |
| PK11551 | Heydalsvegur Yellow | brown-gold | — | Snaefellsjoekull, Iceland |
| PK11552 | Brimisvellir Green | moss green | — | Snaefellsjoekull, Iceland |
| PK17400 | Green earth from Cyprus | brilliant green | PG 23 | Cyprus |
| PK17410 | Bluish green earth from Cyprus | brilliant bluish green | PG 23 | Cyprus |
| PK40610 | Raw umber from Cyprus | light warm brown | PBr 8 | Cyprus |
| PK40710 | Burnt umber from Cyprus | reddish deep brown | PBr 8 | Cyprus |
| PK116431 | Red Moroccan ocher | warm orange-red | PR 102 | Midelt, Morocco |
| PK40623 | Caledonian brown | intense brown | PBr 8 | Morocco |
| PK11110 | Russian green earth | light green | PG 23 | Russia |
| PK40195 | Gold ocher from Poland | light gold | PY 43 | Carpathian Mts., Poland |
| Sample ID | Detected Elements |
|---|---|
| PK11273 | Fe (ma), Zn (mi), Pb, Ca, Mn, K, Ti, As, Cu, Sr, Cr, S, Zr, Si, Ga (tr) |
| PK11272 | Fe (ma), Ca, Pb, As, Cu, Ti, Cr, K, Zn, Si, S, Sr, Zr, Rb, Se, Ga (tr) |
| PK11276 | Fe (ma), Ca (mi), Mn, K, As, Ti, Zn, Sr, Cu, Zr, Si, S, Ga (tr) |
| PK11280 | Fe (ma), Ca, Mn, K, Ti (mi), Rb, Zr, Zn, Cr, Sr, Cu, Pb, Si, Ga, S, As (tr) |
| PK11584 | Fe (ma), Ti, K, Mn, Sr, Ca, Zr, Rb, Cr, Cu, Zn, Si, Pb, S, Ga, Nb (tr) |
| PK11575 | Fe (ma), Ti, K, Zr, Zn, Cr, Ca, Si, Cu, As, Y, Ga, Nb, Ge, S (tr) |
| PK11573 | Fe (ma), Ti, K, Zr, Zn, Cr, Sr, Cl, Cu, Ca, As, Si, Ga, Y, S (tr) |
| PK11362 | Ca (ma), Fe (mi), K, Sr, Ti, Mn, Rb, Cu, Si, S, Cr, S, Ga, Zn (tr) |
| PK11100 | Fe (ma), Ca, K, Mn, Ti, Rb, Sr, Zr, Cr, Si, Cu, Pb, Zn, Ga (tr) |
| PK40280 | Fe (ma), Ca (mi), Cu, Mn, Zn, S, Sr, Ti, V, As, K, Se, Ga, Si, Al (tr) |
| PK40503 | Fe (ma), Ti, K, Zr, Rb, Zn, Ca, Y, Cr, Sr, Cu, Si, As, Ga, S (tr) |
| PK11530 | Fe (ma), Ca, S, Ti, Cu, Cr, Ga, Y (tr) |
| PK11540 | Fe (ma), K, Ti, Zr, Rb, Mn, Cr, Si, Cu, Sr, Zn, As, Ca, Ga, Nb, S (tr) |
| PK41050 | Fe (ma), Ca, K, Ti (mi), Cu, Sr, As, Cr, Zr, Zn, S, Ga, Rb, Si, Nb, Al (tr) |
| PK40490 | Fe (ma), Ti (mi), Mn, Ca, Rb, Sr, Zr, Cr, Cu, Nb, K, Si, Zn, Ga, S, Y (tr) |
| PK40220 | Fe (ma), Ca (mi), Sr, S, Ti, Mn, K, Cu, Cr, Si, Zn, Ga (tr) |
| PK40400 | Fe (ma), Ca, Ti (mi), Mn, Sr, Zr, Cr, Cu, As, Zn, Nb, K, Si, Ga, Y, S (tr) |
| PK40260 | Fe (ma), Ca, Ti (mi), Mn, Sr, S, Zr, Cr, Cu, As, Zn, K, Nb, Si, Al (tr) |
| PK11620 | Fe (ma), Ti (mi), Mn, Cr, Ca, Zr, Zn, Pb, Cu, Ga, Nb, Y, Al, Si (tr) |
| PK11010 | Fe (ma), Ca, Ti (mi), Mn, Sr, K, Zr, Cr, Cu, Zn, Si, Nb, Y, Ga, S (tr) |
| PK11550 | Fe (ma), Ca (mi), Ti, Mn, Sr, K, Cu, Si, Zr, Cr, Zn, Ga, Y, Nb (tr) |
| PK11551 | Fe (ma), Ca (mi), Ti, Mn, Sr, Cr, K, Cu, Zr, Si, Zn, Nb (tr) |
| PK11552 | Fe (ma), Ca, Ti, Mn (mi), Cr, Sr, K, Cu, Si, Zr, Zn, Ga, Y, Nb, S, Al (tr) |
| PK17400 | Fe (ma), Mn, K, Ca, Ti, Cu, Cr, Si, Au, Zn, Y, Pb, Zr, Al, S (tr) |
| PK17410 | Fe (ma), K, Mn, Ca, Ti, Rb, Zn, Cr, Cu, Sr, Si, Ga, Zr, S (tr) |
| PK40610 | Fe (ma), Mn (mi), Ca, Sr, Ti, V, As, Cu, K, Zn, Si, Al (tr) |
| PK40710 | Fe (ma), Mn (mi), Ca, Sr, V, Ti, Cu, Pb, K, Zn, Y, Mo, Si, As (tr) |
| PK116431 | Fe (ma), Ti, Mn, K, Ca, Ba, Sr, Zn, Cu, Zr, Cr, Y, As, Si, Ga, S (tr) |
| PK40623 | Mn (ma), Fe (mi), Ti, Pb, As, Cu, Ca, Al, Zn (tr) |
| PK11110 | Fe (ma), K, Ca, Mn, Ti, Rb, Cr, Si, Sr, Cu, Zr, Au, Ga, S, Al, Zn, As (tr) |
| PK40195 | Fe (ma), Ti, K, Mn, Rb, Sr, Zr, Ca, Cr, Cu, Si, Zn, As, Ga, Al (tr) |
| Group of Pigments | PC | Variance Account (%) | Variance Accumulated (%) | ||
|---|---|---|---|---|---|
| PCA FTIR | PCA XRF | PCA FTIR | PCA XRF | ||
| Red ochers | PC1 | 96.2 | 97.17 | 96.2 | 97.17 |
| PC2 | 3.4 | 2.04 | 99.6 | 99.21 | |
| PC3 | 0.3 | 0.65 | 99.9 | 99.86 | |
| Yellow ochers | PC1 | 78.9 | 98.45 | 78.9 | 98.45 |
| PC2 | 15.4 | 1.42 | 94.3 | 99.87 | |
| PC3 | 3.8 | 0.11 | 98.1 | 99.98 | |
| PC4 | 0.8 | — | 98.9 | — | |
| PC5 | 0.3 | — | 99.2 | — | |
| Green earths | PC1 | 91.4 | 98.45 | 91.4 | 98.45 |
| PC2 | 7.2 | 1.39 | 98.6 | 99.84 | |
| PC3 | 0.8 | 0.12 | 99.4 | 99.96 | |
| PC4 | 0.4 | — | 99.8 | — | |
| Brown and black earths | PC1 | 90.1 | 64.51 | 90.1 | 64.51 |
| PC2 | 9.2 | 33.93 | 99.3 | 98.46 | |
| PC3 | 0.4 | 1.52 | 99.7 | 99.98 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortea, I.M.; Ghervase, L.; Rădvan, R.; Serițan, G. Assessment of Easily Accessible Spectroscopic Techniques Coupled with Multivariate Analysis for the Qualitative Characterization and Differentiation of Earth Pigments of Various Provenance. Minerals 2022, 12, 755. https://doi.org/10.3390/min12060755
Cortea IM, Ghervase L, Rădvan R, Serițan G. Assessment of Easily Accessible Spectroscopic Techniques Coupled with Multivariate Analysis for the Qualitative Characterization and Differentiation of Earth Pigments of Various Provenance. Minerals. 2022; 12(6):755. https://doi.org/10.3390/min12060755
Chicago/Turabian StyleCortea, Ioana Maria, Luminița Ghervase, Roxana Rădvan, and George Serițan. 2022. "Assessment of Easily Accessible Spectroscopic Techniques Coupled with Multivariate Analysis for the Qualitative Characterization and Differentiation of Earth Pigments of Various Provenance" Minerals 12, no. 6: 755. https://doi.org/10.3390/min12060755
APA StyleCortea, I. M., Ghervase, L., Rădvan, R., & Serițan, G. (2022). Assessment of Easily Accessible Spectroscopic Techniques Coupled with Multivariate Analysis for the Qualitative Characterization and Differentiation of Earth Pigments of Various Provenance. Minerals, 12(6), 755. https://doi.org/10.3390/min12060755









