Genetic Association between Granites and Mineralization at the Gindi Akwati Cassiterite–Sulfide Deposit, North-Central Nigeria: Insights from Mineralogy, Fluid Inclusions, and Sulfur Isotopes
Abstract
1. Introduction
2. Geological Background
2.1. Regional Geology
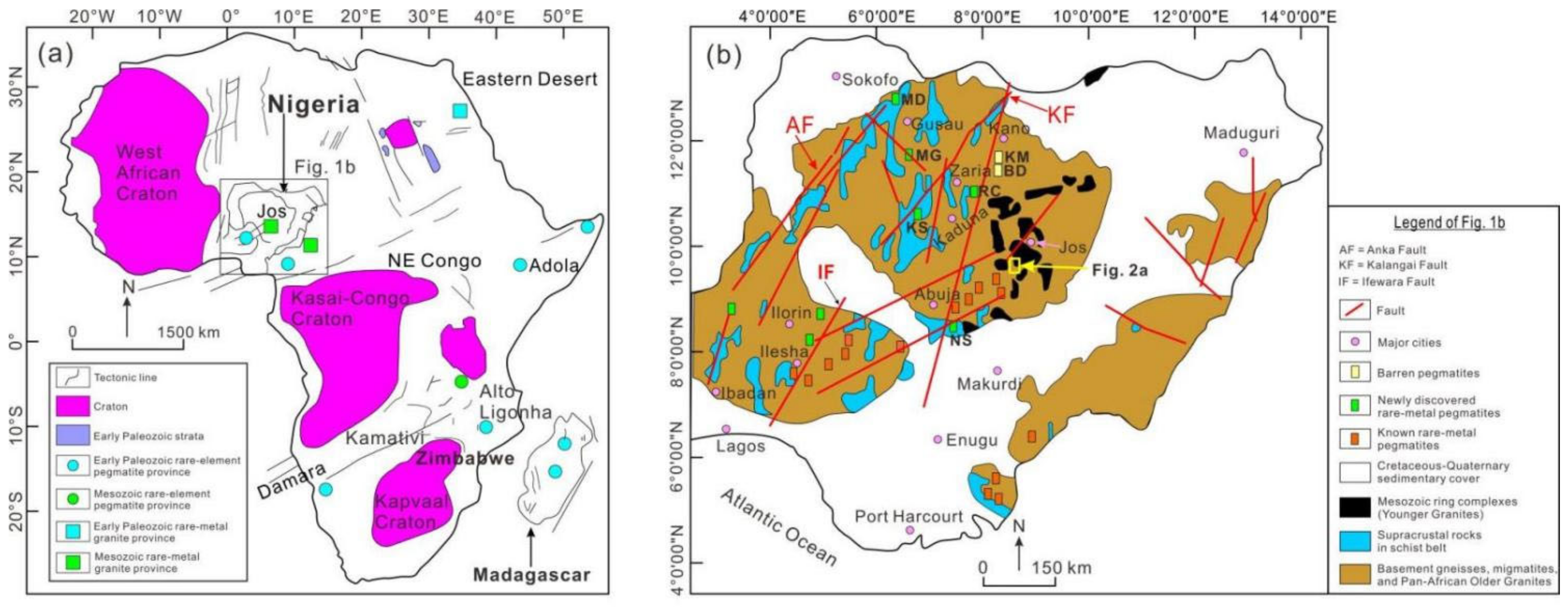
2.2. Local Geology
2.2.1. Occurrence of Plutons and Mineralization
2.2.2. Deposit Morphology, Alteration, and Ore Mineralogy
3. Petrographic Characteristics
3.1. Older Granite, Greisenized Granite, and Quartz Veins
3.2. Granite Porphyry
3.3. Biotite granite
3.4. Ore Microscopy
4. Analytical Methods
4.1. Whole-Rock Major and Trace Element Analysis
4.2. Electron Probe Microanalysis (EPMA)
4.3. In Situ LA-MC-ICP-MS Analysis of Sulfur Isotope
4.4. Fluid Inclusion Analysis
5. Results
5.1. Geochemical Characteristics of Gindi Akwati Granites
5.2. Mass Changes during Greisenization



5.3. Hydrothermal Alteration and Mineral Paragenesis
5.4. Ore Mineral Chemistry and Sulfur Isotopes
5.4.1. Cassiterite
5.4.2. Sulfur Isotopic Compositions
5.5. Fluid Inclusion Petrography and Microthermometry



6. Discussion
6.1. Genetic Links
6.2. Mineral and Whole-Rock Chemistry Signature of the Granites
6.2.1. Fractionation Pattern of REE in the Granites
6.2.2. Physicochemical Evolution of the Magmatic-Hydrothermal System

6.2.3. Metal Partitioning
6.3. Source of Fluid and Ore Metals in the Gindi Akwati Region
6.3.1. Ore-Forming Conditions
6.3.2. Cassiterite Source and Growth Environment
6.3.3. Sulfur Sources in the Sulfides
6.4. Genetic Model

7. Conclusions
- (1)
- The forceful intrusion of Jurassic Younger granite porphyry sheared parts of the host rock (Precambrian Older Granite) in the Gindi Akwati region. Fluids that escaped during late-stage consolidation of a later intrusion (Jurassic biotite granite) greisenized marginal parts of the Older Granite. The compositional variations in the biotite granite show they are highly evolved in low oxygen fugacity conditions that favor tin mineralization.
- (2)
- The hydrothermal alteration that occurs with attendant mineralization is in three stages, i.e., an early one with a high-temperature stage characterized by feldspar and quartz replacements that induced greisenization; an intermediate-temperature stage, where volatile-rich fluids complexes partitioned metals, characterized by crystallization of lepidolite (Li-rich muscovite), fluorite, topaz, and apatite and accompanied by the introduction of oxide and sulfides in greisenized granites; and a depositional hydrothermal stage that formed quartz veins bearing sulfides and tin.
- (3)
- Constraints from stable isotope and fluid inclusion systematics show the hydrothermal history of greisens and veins began with hot, low to moderate saline fluids, which had an isotopic composition very close to that of typical magmatic fluids at or below the NNO buffer. This favors late magmatic to early post-magmatic models of mineralization related to the anorogenic Jurassic biotite granite intrusion.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehmann, B. Formation of tin ore deposits: A reassessment. Lithos 2020, 402–403, 105756. [Google Scholar] [CrossRef]
- René, M. Petrology, geochemistry and mineralogy of greisens associated with tin-tungsten mineralisation: Hub Stock deposit at Krásno-Horní Slavkov ore district, Czech Republic. In Contributions to Mineralization; Al-Juboury, A.I., Ed.; IntechOpen: London, UK, 2018; pp. 901–930. [Google Scholar] [CrossRef]
- Kinnaird, J. Hydrothermal alteration and mineralization of the alkaline anorogenic ring complexes of Nigeria. J. Afr. Earth Sci. 1983 1985, 3, 229–251. [Google Scholar] [CrossRef]
- Pastor, J.; Turaki, U. Primary mineralization in Nigerian ring complexes and its economic significance. J. Afr. Earth Sci. 1983 1985, 3, 223–227. [Google Scholar] [CrossRef]
- Goodenough, K.; Lusty, P.; Roberts, N.; Key, R.; Garba, A. Post-collisional Pan-African granitoids and rare metal pegmatites in western Nigeria: Age, petrogenesis, and the ‘pegmatite conundrum’. Lithos 2014, 200–201, 22–34. [Google Scholar] [CrossRef]
- Lehmann, B. Metallogeny of tin; magmatic differentiation versus geochemical heritage. Econ. Geol. 1982, 77, 50–59. [Google Scholar] [CrossRef]
- Melcher, F.; Graupner, T.; Gäbler, H.-E.; Sitnikova, M.; Henjes-Kunst, F.; Oberthür, T.; Gerdes, A.; Dewaele, S. Tantalum–(niobium–tin) mineralisation in African pegmatites and rare metal granites: Constraints from Ta–Nb oxide mineralogy, geochemistry and U–Pb geochronology. Ore Geol. Rev. 2013, 64, 667–719. [Google Scholar] [CrossRef]
- Benkhelil, J. The origin and evolution of the Cretaceous Benue Trough (Nigeria). J. Afr. Earth Sci. 1989, 8, 251–282. [Google Scholar] [CrossRef]
- Matheis, G. Nigerian rare-metal pegmatites and their lithological framework. Geol. J. 1987, 22, 271–291. [Google Scholar] [CrossRef]
- Abaa, S.I. The Geochemistry, Petrology and Mineralization at Ririwai, Gindi Akwati and Dutsen Wai in the Nigerian Younger Granite Province. Master’s Thesis, Ahmadu Bello University, Zaria, Nigeria, 1976; p. 16. Available online: http://kubanni.abu.edu.ng/jspui/handle/123456789/197 (accessed on 13 June 2022).
- Schlüter, T. Geological Atlas of Africa: With Notes on Stratigraphy, Tectonics, Economic Geology, Geohazards, Geosites and Geoscientific Education of Each Country; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–100. [Google Scholar] [CrossRef]
- Garba, I. Geochemical characteristics of mesothermal gold mineralisation in the Pan-African (600 ± 150 Ma) basement of Nigeria. Appl. Earth Sci. 2003, 112, 319–325. [Google Scholar] [CrossRef]
- Llorens, T.; Moro, M.C. Oxide minerals in the granitic cupola of the Jálama Batholith, Salamanca, Spain. Part I: Accessory Sn, Nb, Ta and Ti minerals in leucogranites, aplites and pegmatites. J. Geosci. 2012, 57, 25–43. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.C.; Li, X.H. Simultaneous and precise determination of 40 trace elements in rock samples using ICP-MS. Geochimica 1996, 25, 552–558, (In Chinese with English abstract). [Google Scholar]
- Qi, H.; Lu, S.; Yang, X.; Zhao, L.; Zhou, Y.; Deng, J.; Li, J. Genesis of Cretaceous igneous rocks and its related large scale porphyry Cu-Au mineralization in Chating, the Middle-Lower Yangtze River Metallogenic Belt: The geochemical constrains. Ore Geol. Rev. 2020, 127, 103793. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, L.; Zong, C.; Yuan, H.; Chen, K.; Dai, M. Development of pressed sulfide powder tablets for in situ sulfur and lead isotope measurement using LA-MC-ICP-MS. Int. J. Mass Spectrom. 2017, 421, 255–262. [Google Scholar] [CrossRef]
- Chen, L.; Chen, K.; Bao, Z.; Liang, P.; Sun, T.; Yuan, H. Preparation of standards for in situ sulfur isotope measurement in sulfides using femtosecond laser ablation MC-ICP-MS. J. Anal. At. Spectrom. 2016, 32, 107–116. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, X.; Chen, L.; Bao, Z.; Chen, K.; Zong, C.; Li, X.-C.; Qiu, J.W. Simultaneous measurement of sulfur and lead isotopes in sulfides using nanosecond laser ablation coupled with two multi-collector inductively coupled plasma mass spectrometers. J. Southeast Asian Earth Sci. 2017, 154, 386–396. [Google Scholar] [CrossRef]
- Bodnar, R. Revised equation and table for determining the freezing point depression of H2O-Nacl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Vry, V.H.; Wilkinson, J.J.; Seguel, J.; Millan, J. Multistage Intrusion, Brecciation, and Veining at El Teniente, Chile: Evolution of a Nested Porphyry System. Econ. Geol. 2010, 105, 119–153. [Google Scholar] [CrossRef]
- Winchester, J.; Floyd, P. Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem. Geol. 1977, 20, 325–343. [Google Scholar] [CrossRef]
- Lentz, D.R.; Lutes, G.; Hartee, R. Bi-Sn-Mo-W greisen mineralization associated with the True Hill granite, southwestern New Brunswick. Atl. Geol. 1988, 24, 321–380. [Google Scholar] [CrossRef][Green Version]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 3, pp. 1–64. [Google Scholar] [CrossRef]
- Babu, T.M. Comparative Studies of Tin Fertile Granitic Rocks in Space and Time. Resour. Geol. 1993, 43, 355–363. [Google Scholar] [CrossRef]
- El Bouseily, A.; El Sokkary, A. The relation between Rb, Ba and Sr in granitic rocks. Chem. Geol. 1975, 16, 207–219. [Google Scholar] [CrossRef]
- Lehmann, B.; Ishihara, S.; Michel, H.; Miller, J.; Rapela, C.W.; Sanchez, A.; Tistl, M.; Winkelmann, L. The Bolivian tin province and regional tin distribution in the Central Andes; a reassessment. Econ. Geol. 1990, 85, 1044–1058. [Google Scholar] [CrossRef]
- Olade, M.A. Geochemical characteristics of tin-bearing and tin-barren granites, northern Nigeria. Econ. Geol. 1980, 75, 71–82. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S. The geochemical evolution of the continental crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Baumgartner, L.P.; Olsen, S.N. A least-squares approach to mass transport calculations using the isocon method. Econ. Geol. 1995, 90, 1261–1270. [Google Scholar] [CrossRef]
- Štemprok, M.; Pivec, E.; Langrová, A. The petrogenesis of a wolframite-bearing greisen in the Vykmanov granite stock, Western Krušné hory pluton (Czech Republic). B. Geosci. 2005, 80, 163–184. [Google Scholar]
- Launay, G.; Sizaret, S.; Guillou-Frottier, L.; Fauguerolles, C.; Champallier, R.; Gloaguen, E. Dynamic permeability related to greisenization reactions in Sn-W ore deposits: Quantitative petrophysical and experi-mental evidence. Geofluids 2019, 2019, 5976545. [Google Scholar] [CrossRef]
- Peccerillo, A.; Taylor, S.R. Geochemistry of eocene calc-alkaline volcanic rocks from the Kastamonu area, Northern Turkey. Contrib. Miner. Petrol. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- Frost, B.R.; Barnes, C.G.; Collins, W.J.; Arculus, R.J.; Ellis, D.J.; Frost, C.D. A Geochemical Classification for Granitic Rocks. J. Pet. 2001, 42, 2033–2048. [Google Scholar] [CrossRef]
- Ballouard, C.; Poujol, M.; Boulvais, P.; Branquet, Y.; Tartèse, R.; Vigneresse, J.L. Nb-Ta fractionation in peraluminous granites: A marker of the magmatic-hydrothermal transition. Geology 2016, 44, 231–234. [Google Scholar] [CrossRef]
- Anders, E.; Grevesse, N. Abundances of the elements: Meteoritic and solar. Geochim. Cosmochim. Acta 1989, 53, 197–214. [Google Scholar] [CrossRef]
- Grant, J.A. Isocon analysis: A brief review of the method and applications. Phys. Chem. Earth, Parts A B C 2005, 30, 997–1004. [Google Scholar] [CrossRef]
- Kinnaird, J.; Bowden, P. African anorogenic alkaline magmatism and mineralization-a discussion with reference to the Niger-Nigerian province. Geol. J. 1987, 22, 297–340. [Google Scholar] [CrossRef]
- Eugster, H.P.; Wilson, G.A. Transport and deposition of ore-forming elements in hydrothermal systems associated with granites. In High Heat Production Granites, Hydrothermal Circulation and Ore Genesis; Hall, C., Ed.; Institution of Mining and Metallurgy: London, UK, 1985; pp. 87–98. [Google Scholar]
- Pirajno, F.; Bentley, P.N. Greisen-related scheelite, gold and sulphide mineralisation at Kirwans Hili and Bateman Creek, Reefton district, Westland, New Zealand. N. Z. J. Geol. Geophys. 1985, 28, 97–109. [Google Scholar] [CrossRef]
- Nambaje, C.; Eggins, S.M.; Yaxley, G.; Sajeev, K. Micro-characterisation of cassiterite by geology, texture and zonation: A case study of the Karagwe Ankole Belt, Rwanda. Ore Geol. Rev. 2020, 124, 103609. [Google Scholar] [CrossRef]
- Kinnaird, J.A. Hydrothermal Alteration and Mineralisation of the Nigerian Anorogenic Ring Complexes: With Special Reference to the Saiya Shokobo Complex. Ph.D. Thesis, University of St Andrews, St Andrews, Scotland, 1987. [Google Scholar]
- Kohút, M.; Recio, C. Sulphur isotope study of selected Hercynian granitic and surrounding rocks from the Western Carpathians (Slovakia). Geol. Carpathica 2002, 53, 3–13. [Google Scholar]
- Salau, L.S. Geology, Geochemistry and Genesis of Gold Mineralization in the Anka Schist Belt, North-Western Nigeria. Ph.D. Thesis, Ahmadu Bello University, Zaria, Nigeria, 2020. [Google Scholar]
- Garba, I. Gold prospect of the Nigerian Pan-African terrain of West Africa. J. Min. Geol. 2000, 36, 123–156. [Google Scholar]
- El-Nafaty, J.M. Rare earth element and stable sulphur (δ 34S) isotope study of baryte–Copper mineralization in Gulani area, Upper Benue Trough, NE Nigeria. J. Afr. Earth Sci. 2015, 106, 147–157. [Google Scholar] [CrossRef]
- Crawford, M.L. Phase equilibria in aqueous fluid inclusions. In Fluid Inclusions: Applications to Petrology; Hollister, L.S., Crawford, M.L., Eds.; The Mineralogical Association of Canada: Quebec City, QC, Canada, 1981; pp. 75–100. [Google Scholar]
- Zarasvandi, A.; Liaghat, S.; Lentz, D.; Hossaini, M. Characteristics of Mineralizing Fluids of the Darreh-Zerreshk and Ali-Abad Porphyry Copper Deposits, Central Iran, Determined by Fluid Inclusion Microthermometry. Resour. Geol. 2013, 63, 188–209. [Google Scholar] [CrossRef]
- Hennigh, Q.; Hutchinson, R.W. Cassiterite at Kidd Creek: An example of volcanogenic massive sulfide-hosted tin mineraliza-tion. Econ. Geol. Monogr. 1999, 10, 431–440. [Google Scholar] [CrossRef]
- Tindle, A.G.; Breaks, F.W. Oxide minerals of the separation rapids rare-element granitic pegmatite group, northwestern Ontario. Can. Mineral. 1998, 36, 609–635. [Google Scholar]
- Hoefs, J. Stable Isotope Geochemistry, 6th ed.; Springer: Berlin, Germany, 2009; ISBN 978-3-540-70703-5. [Google Scholar]
- Roedder, E. Fluid Inclusions: An Introduction to Studies of All Types of Fluid Inclusions, Gas, Liquid, or Melt, Trapped in Materials from Earth and Space, and Their Application to the Understanding of Geologic Processes; Reviews in Mineralogy; Mineral Society of America: Washington, DC, USA, 1984; ISBN 978-0-939950-16-4. [Google Scholar]
- Wilkinson, J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Dewaele, S.; De Clercq, F.; Muchez, P.; Schneider, J.; Burgess, R.; Boyce, A.; Fernandez Alonso, M. Geology of the cassiterite mineralisation in the Rutongo area, Rwanda (Central Africa): Current state of knowledge. Geol. Belgica 2010, 13, 91–112. [Google Scholar]
- Černý, P.; Blevin, P.L.; Cuney, M.; London, D. Granite-related ore deposits. In Economic Geology One Hundredth Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 337–370. [Google Scholar] [CrossRef]
- Hulsbosch, N. Nb-Ta-Sn-W distribution in granite-related ore systems: Fractionation mechanisms and examples from the Karagwe-Ankole belt of Central Africa. In Ore Deposits: Origin, Exploration, and Exploitation, Geophysical Monograph Series; Chapter 4; Decrée, S., Robb, L., Eds.; John Wiley & Sons: New York, NY, USA, 2019; pp. 75–107. [Google Scholar] [CrossRef]
- Tillberg, M.; Maskenskaya, O.M.; Drake, H.; Hogmalm, J.K.; Broman, C.; Fallick, A.E.; Åström, M.E. Fractionation of Rare Earth Elements in Greisen and Hydrothermal Veins Related to A-Type Magmatism. Geofluids 2019, 2019, 4523214. [Google Scholar] [CrossRef]
- Rossi, J.N.; Toselli, A.J.; Basei, M.-A.; Sial, A.N.; Baez, M. Geochemical indicators of metalliferous fertility in the Carboniferous San Blas pluton, Sierra de Velasco, Argentina. Geol. Soc. Lond. Spec. Publ. 2011, 350, 175–186. [Google Scholar] [CrossRef]
- Ekwere, S.J. Li, F and Rb contents and Ba/Rb and Rb/Sr ratios as indicators of postmagmatic alteration and mineralization in the granitic rocks of the Banke and Ririwai Younger Granite complexes, Northern Nigeria. Miner. Depos. 1985, 20, 89–93. [Google Scholar] [CrossRef]
- Girei, M.B.; Li, H.; Algeo, T.J.; Bonin, B.; Ogunleye, P.O.; Bute, S.I.; Ahmed, H.A. Petrogenesis of A-type granites associated with Sn–Nb–Zn mineralization in Ririwai complex, north-Central Nigeria: Constraints from whole-rock Sm Nd and zircon Lu Hf isotope systematics. Lithos 2019, 340–341, 49–70. [Google Scholar] [CrossRef]
- Imeokparia, E.G. Ore-bearing potential of granitic rocks from the Jos-Bukuru Complex, northern Nigeria. Chem. Geol. 1980, 28, 69–77. [Google Scholar] [CrossRef]
- Neiva, A. Geochemistry of tin-bearing granitic rocks. Chem. Geol. 1984, 43, 241–256. [Google Scholar] [CrossRef]
- Lehmann, B.; Dietrich, A.; Wallianos, A. From rocks to ore. Geol. Rundsch. 2000, 89, 284–294. [Google Scholar] [CrossRef]
- Imeokparia, E.G. Fluorine in biotites from the Afu Younger Granite Complex (central Nigeria). Chem. Geol. 1981, 32, 247–254. [Google Scholar] [CrossRef]
- Ishihara, S. The magnetite-series and ilmenite-series granitic rocks. Min. Geol. 1977, 27, 293–305. [Google Scholar] [CrossRef]
- Manning, D. The effect of fluorine on liquidus phase relationships in the system Qz-Ab-Or with excess water at 1 kb. Contrib. Miner. Pet. 1981, 76, 206–215. [Google Scholar] [CrossRef]
- Watson, E.B.; Harrison, T.M. Zircon saturation revisited: Temperature and composition effects in a variety of crustal magma types. Earth Planet. Sci. Lett. 1983, 64, 295–304. [Google Scholar] [CrossRef]
- Boehnke, P.; Watson, E.B.; Trail, D.; Harrison, T.M.; Schmitt, A.K. Zircon saturation re-revisited. Chem. Geol. 2013, 351, 324–334. [Google Scholar] [CrossRef]
- Ishihara, S.; Hashimoto, M.; Machida, M. Magnetite/Ilmenite-series Classification and Magnetic Susceptibility of the Mesozoic-Cenozoic Batholiths in Peru. Resour. Geol. 2000, 50, 123–129. [Google Scholar] [CrossRef]
- Ishihara, S. The redox state of granitoids relative to tectonic setting and earth history: The magnetite–ilmenite series 30 years later. Trans. R. Soc. Edinburgh: Earth Sci. 2004, 95, 23–33. [Google Scholar] [CrossRef]
- Yang, X.-M.; Lentz, D.R.; Chi, G. Ferric-ferrous iron oxide ratios: Effect on crystallization pressure of granites estimated by Qtz-geobarometry. Lithos 2020, 380–381, 105920. [Google Scholar] [CrossRef]
- Schmidt, C.; Romer, R.L.; Wohlgemuth-Ueberwasser, C.C.; Appelt, O. Partitioning of Sn and W between granitic melt and aqueous fluid. Ore Geol. Rev. 2019, 117, 103263. [Google Scholar] [CrossRef]
- Seward, T.M.; Williams-Jones, A.E.; Migdisov, A.A. The Chemistry of Metal Transport and Deposition by Ore-Forming Hydrothermal Fluids. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; Volume 13, pp. 29–57. ISBN 978-0-08-098300-4. [Google Scholar]
- Pirajno, F. Halogens in hydrothermal fluids and their role in the formation and evolution of hydrothermal mineral systems. In The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes; Springer Geo-chemistry; Harlov, D.E., Aranovich, L., Eds.; Springer: Cham, Germany, 2018; pp. 759–804. [Google Scholar] [CrossRef]
- Zajacz, Z.; Halter, W.E.; Pettke, T.; Guillong, M. Determination of fluid/melt partition coefficients by LA-ICPMS analysis of co-existing fluid and silicate melt inclusions: Controls on element partitioning. Geochim. Cosmochim. Acta 2008, 72, 2169–2197. [Google Scholar] [CrossRef]
- Wang, R.C.; Yu, A.-P.; Chen, J.; Xie, L.; Lu, J.-J.; Zhu, J.-C. Cassiterite exsolution with ilmenite lamellae in magnetite from the Huashan metaluminous tin granite in southern China. Miner. Pet. 2012, 105, 71–84. [Google Scholar] [CrossRef]
- Webster, J.; Thomas, R.; Seltmann, R.; Tappen, C. Geochemical evolution of halogen-enriched granite magmas and mineralizing fluids of the Zinnwald tin-tungsten mining district, Erzgebirge, Germany. Miner. Depos. 2004, 39, 452–472. [Google Scholar] [CrossRef]
- Abaa, S.I. Hydrothermal fluids responsible for the formation of precious minerals in the Nigerian Younger Granite Province. Miner. Depos. 1991, 26, 34–39. [Google Scholar] [CrossRef]
- Bachinski, D.J. Bond strength and sulfur isotopic fractionation in coexisting sulfides. Econ. Geol. 1969, 64, 56–65. [Google Scholar] [CrossRef]
- Seal, I.R.R. Sulfur Isotope Geochemistry of Sulfide Minerals. Rev. Miner. Geochem. 2006, 61, 633–677. [Google Scholar] [CrossRef]
- Driesner, T.; Geiger, S. Numerical Simulation of Multiphase Fluid Flow in Hydrothermal Systems. Rev. Miner. Geochem. 2007, 65, 187–212. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, S.-F.; Li, H.-L.; Jiang, S.-Y. Origin and Evolution of the Ore-Forming Fluids in the Liyuan Gold Deposit, Central North China Craton: Constraints from Fluid Inclusions and H-O-C Isotopic Compositions. Geofluids 2017, 2017, 3107280. [Google Scholar] [CrossRef]
- Bodnar, R.J. Introduction to aqueous fluid systems. In Fluid Inclusions: Analysis and Interpretation; Sam-son, I., Anderson, A., Marshall, D., Eds.; The Mineralogical Association of Canada: Quebec City, QC, Canada, 2003; ISBN 0-921294-32-8. [Google Scholar]
- Borisenko, A.S. Study of the salt composition of solutions in gas-liquid inclusions in minerals by the cryometric method. Soviet Geol. Geophys. 1978, 18, 11–19. [Google Scholar]
- Choi, S.H. Geochemical Evolution of Hydrothermal Fluids at the Daejang Cu-Zn-Pb Vein Deposit, Korea. Resour. Geol. 1998, 48, 171–182. [Google Scholar] [CrossRef]
- Urubek, T.; Dolníček, Z.; Kropáč, K. Genesis of Syntectonic Hydrothermal Veins in the Igneous Rock of Teschenite Association (Outer Western Carpathians, Czech Republic): Growth Mechanism and Origin of Fluids. Geol. Carpathica 2015, 65, 419–431. [Google Scholar] [CrossRef]
- Chen, L.-L.; Ni, P.; Dai, B.-Z.; Li, W.-S.; Chi, Z.; Pan, J.-Y. The Genetic Association between Quartz Vein- and Greisen-Type Mineralization at the Maoping W–Sn Deposit, Southern Jiangxi, China: Insights from Zircon and Cassiterite U–Pb Ages and Cassiterite Trace Element Composition. Minerals 2019, 9, 411. [Google Scholar] [CrossRef]
- Murciego, A.; Sanchez, A.G.; Dusausoy, Y.; Pozas, J.M.M.; Ruck, R. Geochemistry and EPR of cassiterites from the Iberian Hercynian Massif. Miner. Mag. 1997, 61, 357–365. [Google Scholar] [CrossRef]
- Zoheir, B.A. Microchemistry and stable isotope systematics of gold mineralization in a gabbro–diorite complex, SE Egypt. Microchem. J. 2012, 103, 148–157. [Google Scholar] [CrossRef]
- Yang, F.; Zhai, W.; Sun, X.; Klemd, R.; Sun, Y.; Wu, Y.; Hua, R.; Zheng, S. Fluid Inclusions and Stable Isotopic Characteristics of the Yaoling Tungsten Deposit in South China: Metallogenetic Constraints. Resour. Geol. 2018, 69, 107–122. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y. Sulfur and lead isotope composition on tracing ore-forming materials of the Xihuashan tungsten deposit in Southern Jiangxi. Bull. Miner. Pet. Geochem. 2014, 33, 342–347, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Cai, Y.; Ma, D.S.; Lu, J.J.; Huang, H.; Zhang, R.Q.; Qu, W.J. Re-Os geochronology and S isotope geochem-istry of Dengfuxian tungsten deposit, Hunan Province, China. Acta Pet. Sin. 2012, 28, 3798–3808, (In Chinese with English abstract). [Google Scholar]
- Reed, M.H. Sulfide Mineral Precipitation from Hydrothermal Fluids. Rev. Miner. Geochem. 2006, 61, 609–631. [Google Scholar] [CrossRef]
- Plümper, O.; Putnis, A. The Complex Hydrothermal History of Granitic Rocks: Multiple Feldspar Replacement Reactions under Subsolidus Conditions. J. Pet. 2009, 50, 967–987. [Google Scholar] [CrossRef]
- Stevenson, C. The relationship between forceful and passive emplacement: The interplay between tectonic strain and magma supply in the Rosses Granitic Complex, NW Ireland. J. Struct. Geol. 2009, 31, 270–287. [Google Scholar] [CrossRef]
- Chicharro, E.; Boiron, M.-C.; López-García, J.; Barfod, D.N.; Villaseca, C. Origin, ore forming fluid evolution and timing of the Logrosán Sn–(W) ore deposits (Central Iberian Zone, Spain). Ore Geol. Rev. 2016, 72, 896–913. [Google Scholar] [CrossRef]
- Du, Y.; Qin, X.; Barnes, C.G.; Cao, Y.; Dong, Q.; Du, Y. Sulphide melt evolution in upper mantle to upper crust magmas, Tongling, China. Geosci. Front. 2014, 5, 237–248. [Google Scholar] [CrossRef]
- Giaccherini, A.; Montegrossi, G.; Di Benedetto, F. Stability of Naturally Relevant Ternary Phases in the Cu–Sn–S System in Contact with an Aqueous Solution. Minerals 2016, 6, 79. [Google Scholar] [CrossRef]
- Kinnaird, J.A.; Nex, P.A.; Milani, L. Tin in Africa. Episodes 2016, 39, 361–380. [Google Scholar] [CrossRef]
- Amuda, A.K.; Yang, X.; Deng, J.; Faisal, M.; Cao, J.; Bute, S.I.; Girei, M.B.; Elatikpo, S.M. Petrogenesis of the peralkaline Dutsen Wai and Ropp complexes in the Nigerian younger granites: Implications for crucial metal enrichments. Int. Geol. Rev. 2020, 63, 2057–2081. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amuda, A.K.; Li, S.; Yang, X.; Cao, J.; Faisal, M. Genetic Association between Granites and Mineralization at the Gindi Akwati Cassiterite–Sulfide Deposit, North-Central Nigeria: Insights from Mineralogy, Fluid Inclusions, and Sulfur Isotopes. Minerals 2022, 12, 761. https://doi.org/10.3390/min12060761
Amuda AK, Li S, Yang X, Cao J, Faisal M. Genetic Association between Granites and Mineralization at the Gindi Akwati Cassiterite–Sulfide Deposit, North-Central Nigeria: Insights from Mineralogy, Fluid Inclusions, and Sulfur Isotopes. Minerals. 2022; 12(6):761. https://doi.org/10.3390/min12060761
Chicago/Turabian StyleAmuda, Abdulgafar Kayode, Shuang Li, Xiaoyong Yang, Jingya Cao, and Mohamed Faisal. 2022. "Genetic Association between Granites and Mineralization at the Gindi Akwati Cassiterite–Sulfide Deposit, North-Central Nigeria: Insights from Mineralogy, Fluid Inclusions, and Sulfur Isotopes" Minerals 12, no. 6: 761. https://doi.org/10.3390/min12060761
APA StyleAmuda, A. K., Li, S., Yang, X., Cao, J., & Faisal, M. (2022). Genetic Association between Granites and Mineralization at the Gindi Akwati Cassiterite–Sulfide Deposit, North-Central Nigeria: Insights from Mineralogy, Fluid Inclusions, and Sulfur Isotopes. Minerals, 12(6), 761. https://doi.org/10.3390/min12060761







