Magmatic Processes of the Upper Cretaceous Susuma–Nagaho Plutonic Complex, Southwest Japan: Its Role on Crustal Growth and Recycling in Active Continental Margins
Abstract
:1. Introduction
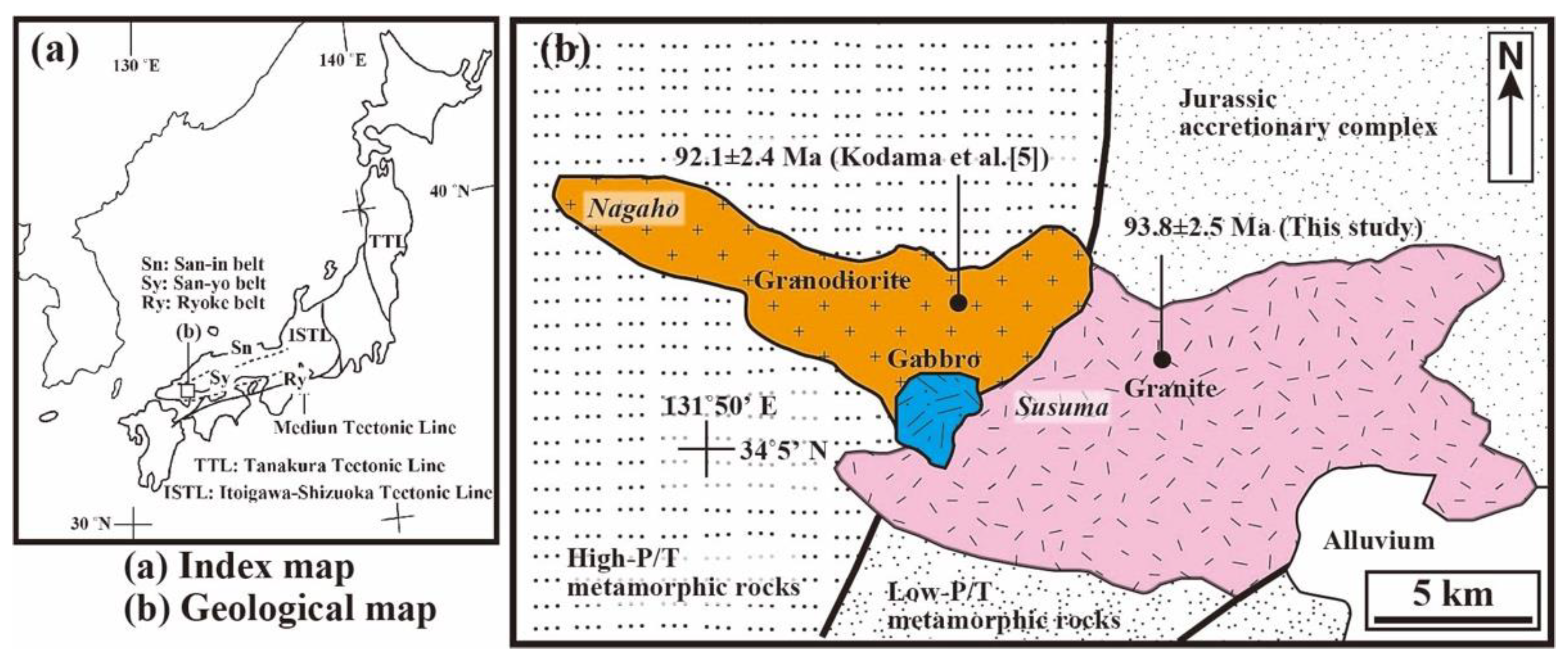
2. General Geology
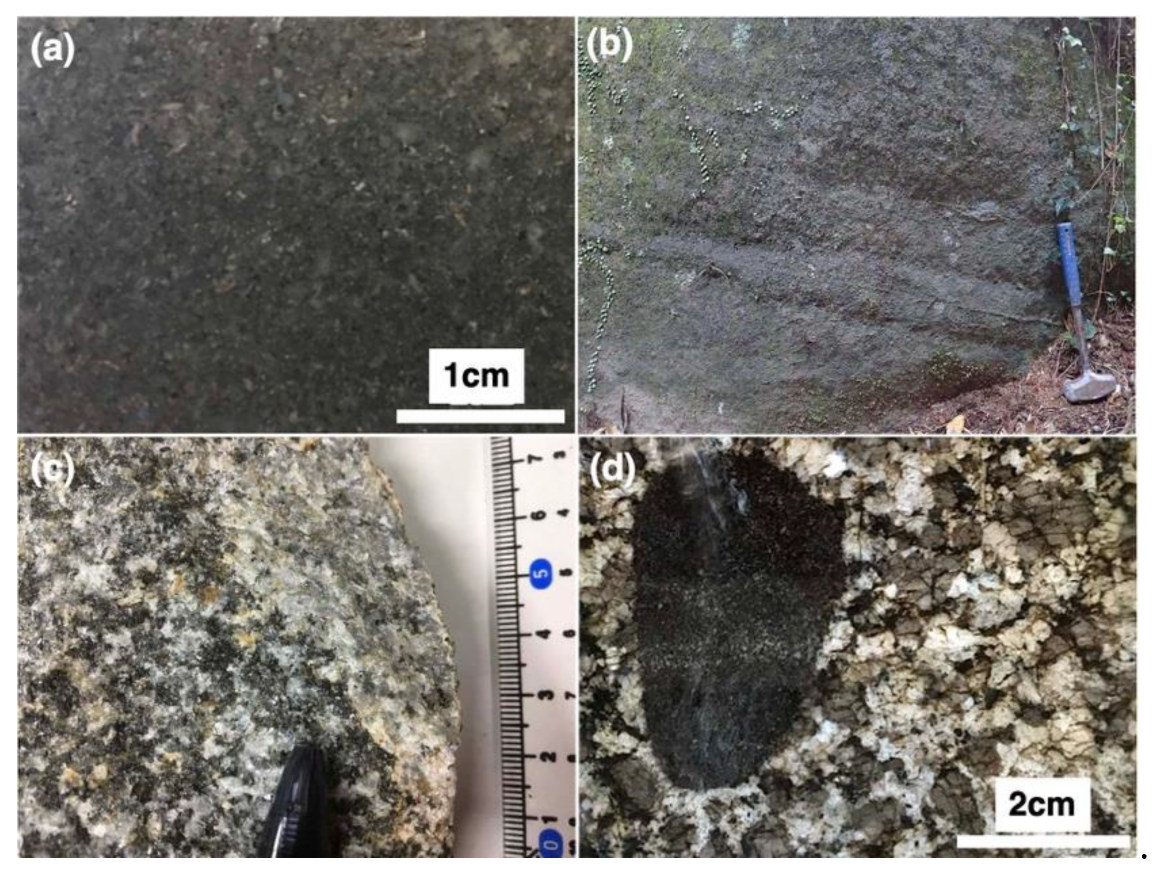
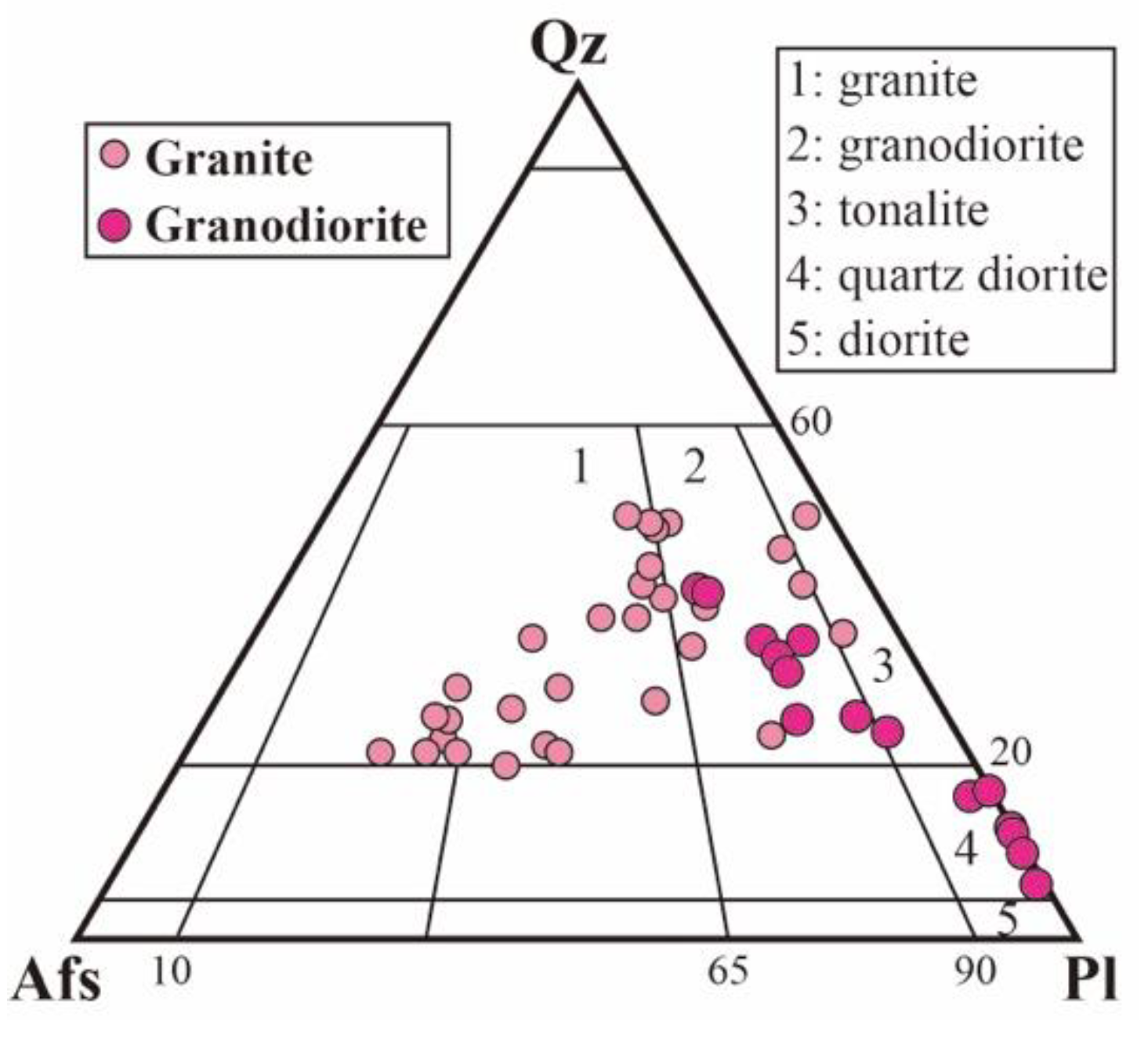
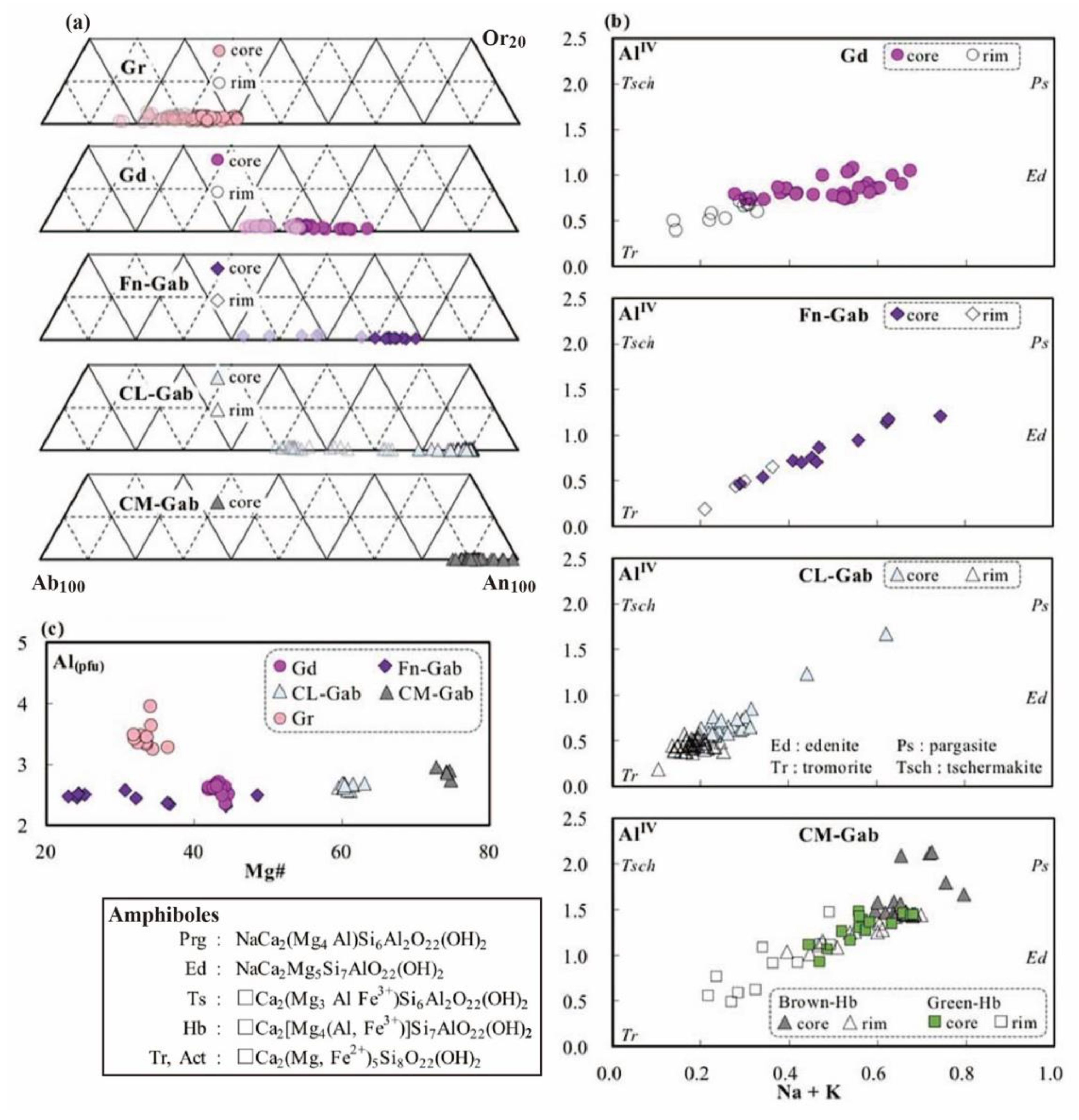
3. Description and Mineral Chemistry of Analyzed Samples
4. Whole-Rock Geochemistry
4.1. Analytical Procedure
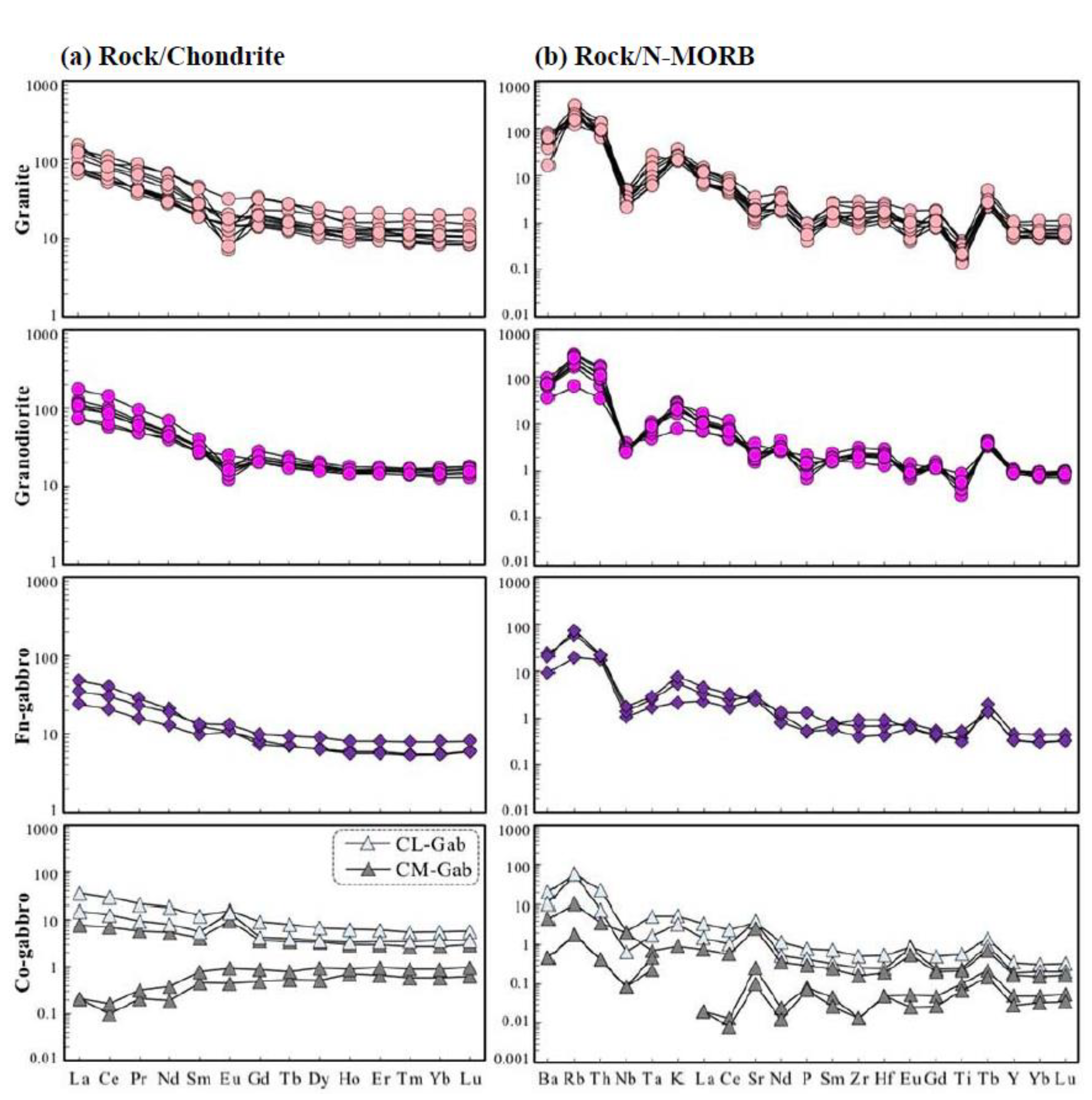
4.2. Results
5. Discussion
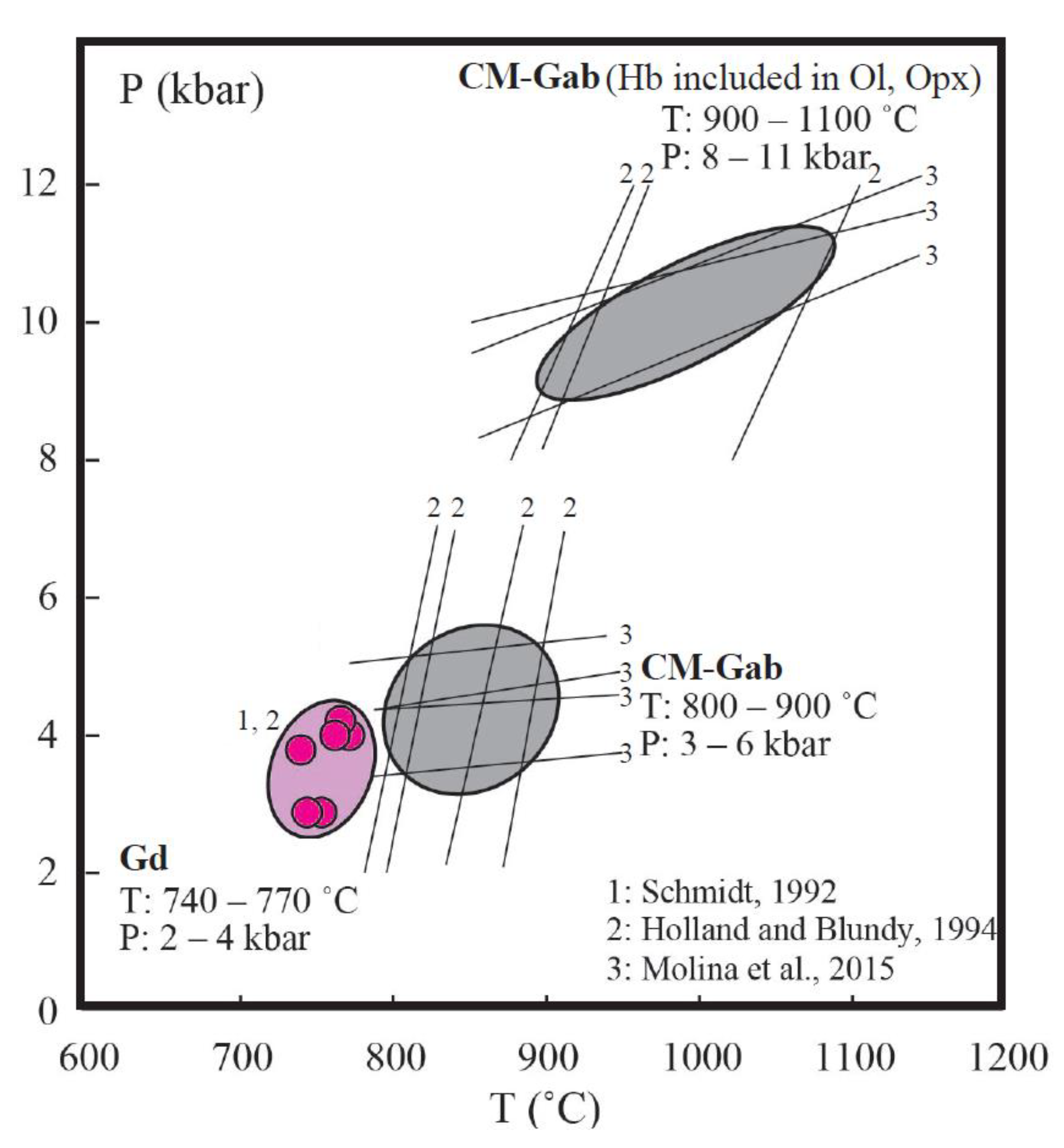
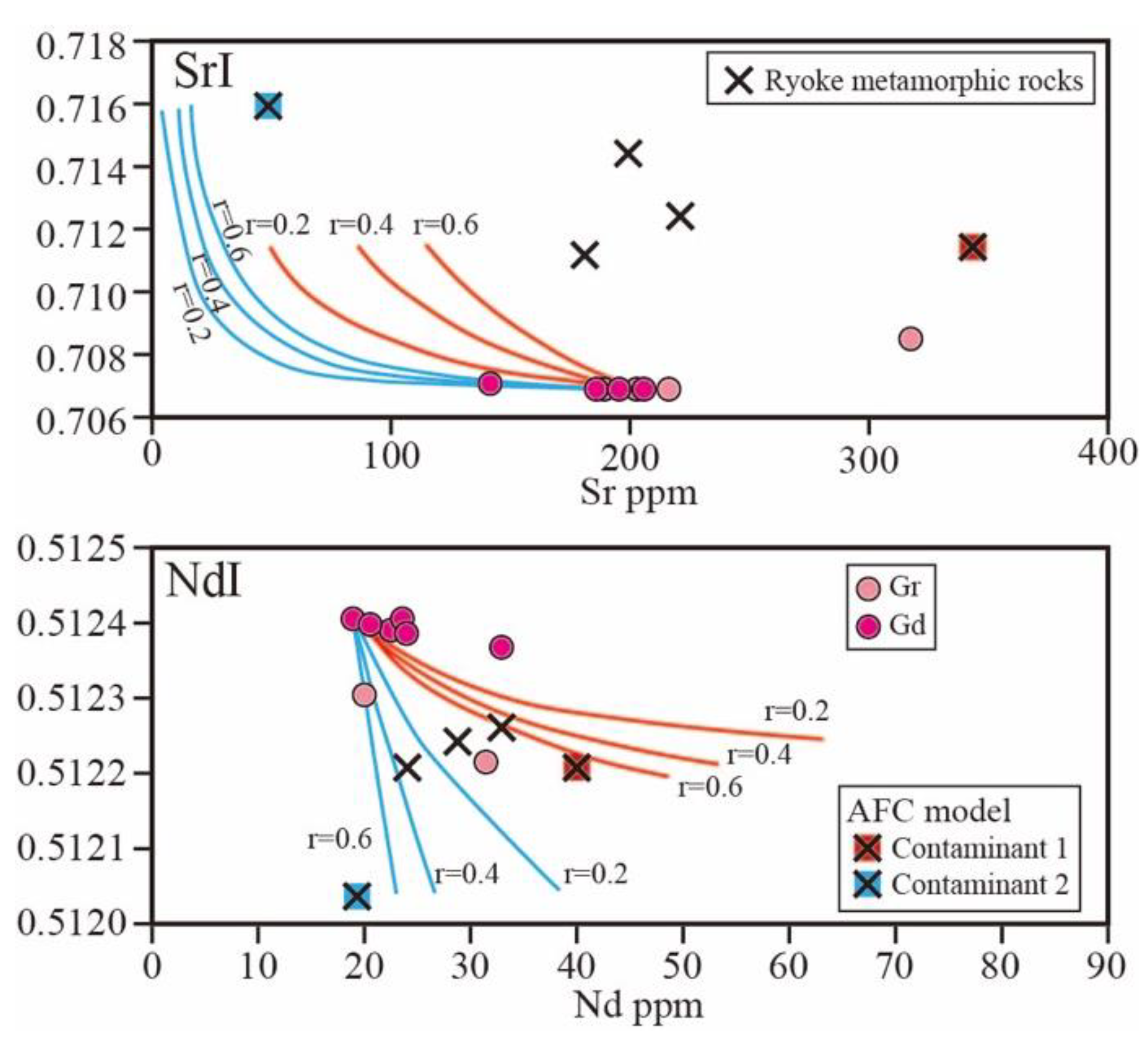
5.1. Genetic Relationships between Gabbro and Granodiorite
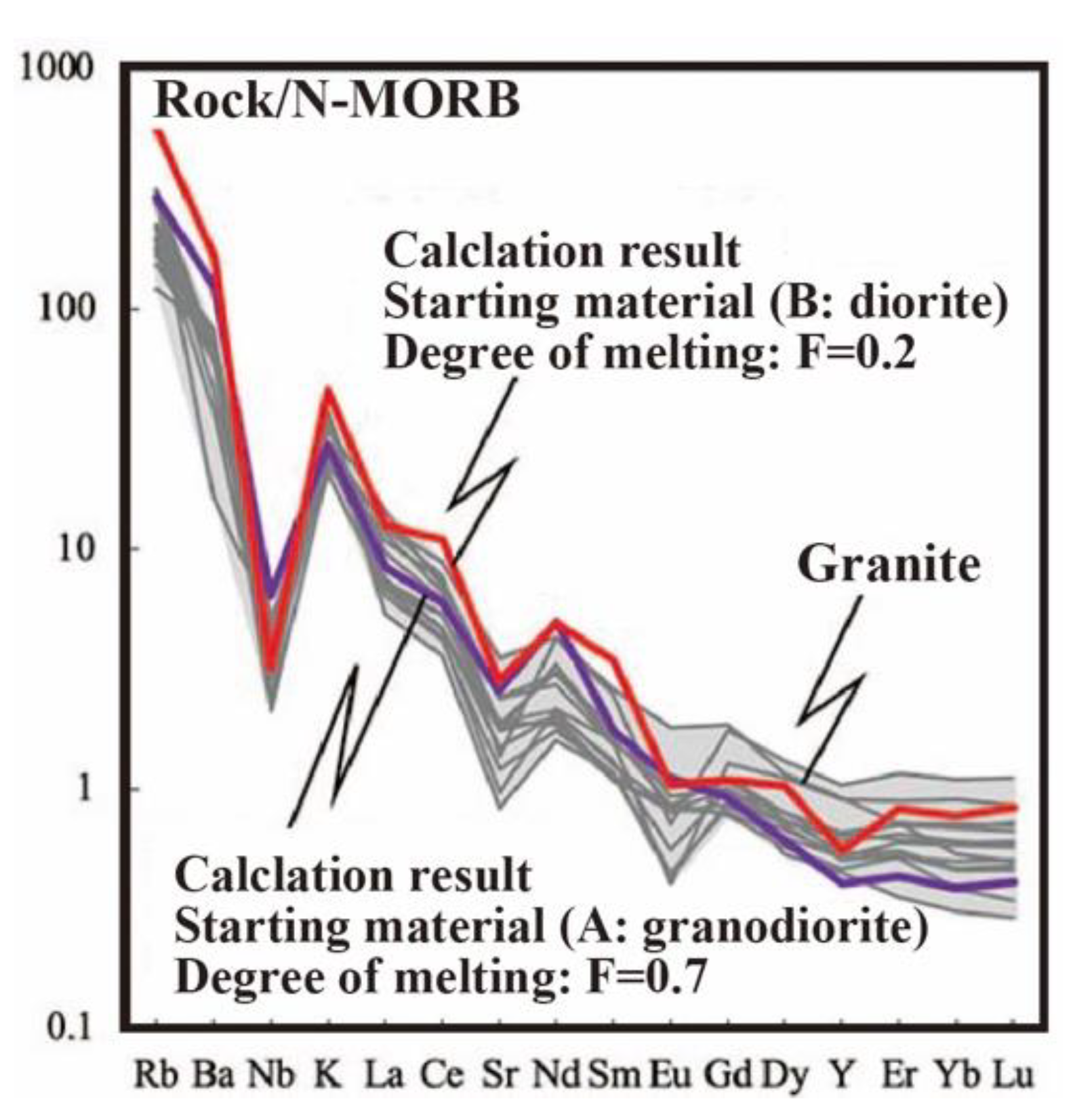
5.2. Petrogenesis of Granite Magma
5.3. Formation of Basaltic Magma and Cretaceous Mantle Dynamics
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rudnick, R.L. Making continental crust. Nature 1995, 378, 571–578. [Google Scholar] [CrossRef]
- Moyen, J.-F.; Laurent, Q.; Chelle-Michou, C.; Couzinié, S.; Vanderhaeghe, O.; Zeh, A.; Villaros, A.; Gardien, V. Collision vs. subduction-related magmatism: Two contrasting ways of granite formation and implications for crustal growth. Lithos 2017, 277, 154–177. [Google Scholar] [CrossRef] [Green Version]
- Moyen, J.-F.; Janousek, V.; Laurent, O.; Vachmann, O.; Jacob, J.-B.; Farian, F.; Fiannacca, P.; Villaros, A. Crustal melting vs. fractionation of basaltic magmas: Part I, granites and paradigms. Lithos 2021, 402–403, 106291. [Google Scholar] [CrossRef]
- Ishihara, S. Modal and chemical compositions of the granitic rocks related to the major molybdenum and tungsten deposits in the Inner Zone of southwest Japan. J. Geol. Soc. Jpn. 1971, 77, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Kodama, S.; Owada, M.; Imaoka, T.; Kamei, A. Sr–Nd isotopic compositions of the Susuma–Nagaho Plutonic Complex in the San-yo Belt, Southwest Japan: Implications for the Cretaceous enriched mantle. J. Mineral. Petrol. Sci. 2019, 114, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Eshima, K.; Owada, M. Whole-rock geochemistry of diorite and dikes from Mt. Shaku-dake area, Fukuoka, Kyushu. J. Geol. Soc. Jpn. 2018, 124, 857–862. [Google Scholar] [CrossRef] [Green Version]
- Kagami, H.; Iwata, M.; Sano, S.; Honma, H. Sr and Nd isotopic compositions and Rb, Sr, Sm and nd concentrations of standard samples. Tech. Rep. ISEI Okayama Univ. 1987, 4, 1–16. [Google Scholar]
- Iizumi, S. Sr and Nd isotopic analyses, using a thermal ionization mass spectrometer MAT262. Geosci. Rept. Shimane Univ. 1996, 15, 153–159. [Google Scholar]
- Kagami, H.; Yokose, H.; Honma, H. 87Sr/86Sr and 143Nd/144Nd ratios of GSJ rock reference samples; JB-1a, JA-1 and JG-1a. Geochem. J. 1989, 23, 204–209. [Google Scholar] [CrossRef]
- Tanaka, T.; Togashi, S.; Kamioka, H.; Amakawa, H.; Kagami, H.; Hamamoto, T.; Yuhara, M.; Orihashi, Y.; Yoneda, S.; Shimizu, H.; et al. JNdi-1; a neodymium isotopic reference in consistency with La Jolla neodymium. Chem. Geol. 2000, 168, 279–281. [Google Scholar] [CrossRef]
- Steiger, R.H.; Jäger, E. Subcommission on geochronology: Convention on the use of decay constants in geo- and cosmochronology. Earth Planet Sci. Lett. 1977, 36, 359–362. [Google Scholar] [CrossRef]
- Lugmair, G.W.; Marti, K. Lunar initial 143Nd/144Nd: Differential evolution of the lunar crust and mantle. Earth Planet Sci. Lett. 1978, 39, 349–357. [Google Scholar] [CrossRef]
- DePaolo, D.J.; Wasserburg, G.J. Petrogenetic mixing models and Nd–Sr isotopic patterns. Geochem. Cosmochem. Acta 1979, 43, 615–627. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. In Magmatism in the Oceanic Basins; Saunders, A.D., Norry, M.J., Eds.; Geological Society of London, Special Publications: London, UK, 1989; Volume 42, pp. 313–345. [Google Scholar]
- Akasaki, E.; Owada, M.; Kamei, A. Crustal differentiation due to partial melting of granitic rocks in an active continental margin, the Ryoke Belt, Southwest Japan. Lithos 2015, 230, 82–91. [Google Scholar] [CrossRef]
- Ikeda, Y.; Owada, M.; Nishizuka, D.; Kamei, A. Magma process of Gamano granodiorite in Ryoke belt, Yanai region, Yamaguchi, Southwest Japan. Jpn. Mag. Mineral. Petrol. Sci. 2019, 125, 167–182, (In Japanese with English Abstract). [Google Scholar] [CrossRef] [Green Version]
- Molina, J.F.; Moreno, J.A.; Castro, A.; Rodriguez, C.; Fershtater, G.B. Calcic amphibole thermobarometry in metamorphic and igneous rocks: New calibrations based on plagioclase/amphibole Al–Si partitioning and amphibole/liquid Mg partitioning. Lithos 2015, 232, 286–305. [Google Scholar] [CrossRef]
- Holland, T.; Blundy, J. Non-ideal interactions on calcic amphiboles and their bearing on amphibole-plagioclase thermometry. Contrib. Mineral Petrol. 1994, 116, 433–447. [Google Scholar] [CrossRef]
- Schmidt, M.W. Amphibole composition in tonalite as a function of pressure: An experimental calibration of the Al-in-hornblende barometer. Contrib. Mineral Petrol. 1992, 110, 304–310. [Google Scholar] [CrossRef]
- White, A.J.R.; Chappell, B.W. Ultrametamorphism and granitoid genesis. Techtonophysics 1977, 43, 7–22. [Google Scholar] [CrossRef]
- DePaolo, D.J. Trace element and isotopic effects of combined wall-rock assimilation and fractional crystallization. Earth Planet Sci. Lett. 1981, 53, 189–202. [Google Scholar] [CrossRef]
- Conrad, W.K.; Nicholls, I.A.; Wall, V.J. Water-saturated and -undersaturated melting of metaluminous and peraluminous crustal compositions at 10 kb: Evidence for the origin of silicic magmas in the Taupo volcanic Zone, New Zealand, and other occurrences. J. Petrol. 1988, 29, 765–803. [Google Scholar] [CrossRef]
- Beard, J.S.; Lofgren, G.E. Dehydration melting and water-saturated melting of basaltic and andesitic greenstones and amphibolite at 1, 3 and 6.9 kb. J. Petrol. 1991, 32, 365–401. [Google Scholar] [CrossRef]
- Wedepohl, K.H. Chemical composition and fractionation of the continental crust. Geol. Rundsch. 1991, 80, 207–223. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochem. Cosmochem. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Kamei, A. Petrogenesis of Cretaceous peraluminous granite suites with low initial Sr isotopic ratios, Kyushu Island, Southwest Japan Arc. Gondwana Res. 2002, 5, 813–822. [Google Scholar] [CrossRef]
- Skrzypek, E.; Kawakami, T.; Hirajima, T.; Sakata, S.; Hirata, T.; Ikeda, T. Revisiting the high temperature metamorphic field gradient of the Ryoke Belt (SW Japan): New constraints from the Iwakuni–Yanai area. Lithos 2016, 260, 9–27. [Google Scholar] [CrossRef]
- Mateen, T.; Okamoto, K.; Chung, S.-L.; Lee, H.-Y.; Abe, S.; Mita, Y.; Rehman, H.U.; Terabayashi, M.; Yamamoto, H. LA-ICP-MS zircon U–Pb age and Hf isotope data from the granitic rocks in the Iwakuni area, Southwest Japan: Re-evaluation of emplacement order and the source magma. Geosci. J. 2019, 23, 917–931. [Google Scholar] [CrossRef]
- Imaoka, T.; Nakashima, K.; Kamei, A.; Itaya, T.; Ohira, T.; Nagashima, M.; Kono, N.; Kiji, M. Episodic magmatism at 105 Ma in the Kinki district, SW Japan: Petrogenesis of Nb-rich lamprophyres and adakites, and geodynamic implications. Lithos 2014, 184–187, 105–131. [Google Scholar] [CrossRef]
- Imaoka, T.; Kiminami, K.; Nishida, M.; Takemoto, T.; Ikawa, T.; Kagami, T.; Iizumi, S. K–Ar age and geochemistry of the SW Japan Paleogene cauldron cluster: Implications for Eocene–Oligocene thermo-tectonic reactivation. J. Asian Earth Sci. 2011, 40, 509–533. [Google Scholar] [CrossRef]
- Sission, T.W.; Grove, T.L. Experimental investigations of the role of H2O in calc-alkaline differentiation and subduction zone magmatism. Contrib. Mineral Petrol. 1993, 113, 143–166. [Google Scholar] [CrossRef]
- Takagi, D.; Sato, H.; Nakagawa, M. Experimental study of a low-alkali tholeiite at 1–5 kbar: Optimal condition for the crystallization of high-An plagioclase in hydrous arc tholeiite. Contrib. Mineral. Petrol. 2005, 149, 527–540. [Google Scholar] [CrossRef]
- Hamada, M.; Fujii, T. H2O-rich island arc low-K tholeiite magma inferred from Ca-rich plagioclase-melt inclusion equilibria. Geochem. J. 2007, 41, 437–461. [Google Scholar] [CrossRef] [Green Version]
- Kuritani, T. Water and magma. J. Geogr. 2007, 116, 133–153. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Sakuyama, M.; Fukuyama, H.; Kushiro, I. Generation of arc basalt magmas and thermal structure of the mantle wedge in subduction zones (Japan arc). J. Geophys. Res. 1983, 88, 5815–5825. [Google Scholar] [CrossRef]
- Imaoka, T.; Kawabata, H.; Nagashima, M.; Nakashima, K.; Kamei, A.; Yagi, K.; Itaya, T.; Kiji, M. Petrogenesis of an Early Cretaceous lamprophyre dike from Kyoto Prefecture, Japan; Implications for the generation of high-Nb basalt magmas in subduction zones. Lithos 2017, 290–291, 18–33. [Google Scholar] [CrossRef]
- Hildreth, W.; Moorbath, S. Crustal contributions to arc magmatism in the Andes of central Chile. Contrib. Mineral. Petrol. 1988, 98, 455–489. [Google Scholar] [CrossRef]
- Annen, C.; Blundy, J.D.; Sparks, R.S.J. The genesis of intermediate and silicic magmas in deep crustal hot zones. J. Petrol. 2006, 47, 505–539. [Google Scholar] [CrossRef] [Green Version]
- Collins, W.J.; Murphy, J.B.; Johnson, T.E.; Huang, H.Q. Critical role of water in the formation of continental crust. Nat. Geosci. 2020, 13, 331–338. [Google Scholar] [CrossRef]
- Nabelek, P.I. Petrogenesis of leucogranites in collisional orogens. In Post-Archean Granitic Rocks: Contrasting Petrogenetic Processes and Tectonic Environments; Janoúsek, V., Bonin, B., Collins, W.J., Farina, F., Bowden, P., Eds.; Geological Society of London: London, UK, 2020; Volume 491, pp. 179–207. [Google Scholar]
- Kim, S.W.; Kwon, S.; Park, S.-I.; Lee, C.; Cho, D.-L.; Lee, H.-J.; Ko, K.; Kim, S.J. SHRIMP U–Pb dating and geochemistry of the Cretaceous plutonic rocks in the Korean Peninsula: A new tectonic model of the Cretaceous Korean Peninsula. Lithos 2016, 262, 88–106. [Google Scholar] [CrossRef]
- Aignertorres, M.; Blundy, J.; Ulmer, P.; Pettke, T. Laser Ablation ICPMS study of trace element partitioning between plagioclase and basaltic melts: An experimental approach. Contrib. Mineral. Petrol. 2007, 153, 647–667. [Google Scholar] [CrossRef] [Green Version]
- Bacon, C.R.; Druitt, T.H. Compositional evolution of the zoned calcalkaline magma chamber of Mount-Mazama, Crater Lake, Oregon. Contrib. Mineral. Petrol. 1988, 98, 224–256. [Google Scholar] [CrossRef]
- Beattie, P. The effect of partial melting of spinel peridotite on uranium series disequilibria: Constraints from partitioning studies. Earth Planet Sci. Lett. 1993, 177, 379–391. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Davis, A.M.; Drake, M.J. Ion microprobe study of plagioclase-basalt partition experiments at natural concentration levels of trace elements. Geochem. Cosmochem. Acta 1998, 62, 1175–1193. [Google Scholar] [CrossRef]
- Brenan, J.M.; Shaw, H.F.; Ryerson, F.J.; Phinney, D.L. Experimental determination of trace element partitioning between pargasite and synthetic hydrous andesitic melt. Earth Planet Sci. Lett. 1995, 135, 1–11. [Google Scholar] [CrossRef]
- Drake, M.J.; Weill, D.F. Partition of Sr, Ba, Ca, Y, Eu2+, Eu3+, and other REE between plagioclase feldspar and magmatic liquid-experimental study. Geochem. Cosmochem. Acta 1975, 39, 689–712. [Google Scholar] [CrossRef]
- Dostal, J.; Dupuy, C.; Carron, J.P.; Dekerneizon, M.L.; Maury, R.C. Partition-coefficients of trace elements application to volcanic rocks of St. Vincent, West Indies. Geochem. Cosmochem. Acta 1983, 47, 525–533. [Google Scholar] [CrossRef]
- Dudas, M.J.; Schmitt, R.A.; Harward, M.E. Trace element partitioning between volcanic plagioclase and dacitic pyroclastic matrix. Earth Planet Sci. Lett. 1971, 11, 440–446. [Google Scholar] [CrossRef]
- Dunn, T.; Sen, C. Mineral/matrix partition-coefficients for orthopyroxene, plagioclase, and olivine in basaltic to andesitic systems—A combined analytical and experimental study. Geochem. Cosmochem. Acta 1994, 58, 717–733. [Google Scholar] [CrossRef]
- Ewart, A.; Bryan, W.B.; Gill, J.B. Mineralogy and geochemistry of the younger volcanic islands of Tonga, S.W. Pacific. J. Petrol. 1973, 14, 429–465. [Google Scholar] [CrossRef]
- Ewart, A.; Griffin, W.L. Application of proton-microprobe data to trace element partitioning in volcanic rocks. Chem. Geol. 1994, 117, 251–284. [Google Scholar] [CrossRef]
- Forsythe, L.M.; Nielsen, R.L.; Fisk, M.R. High-Field-Strength Element partitioning between pyroxene and basaltic to dacitic magmas. Chem. Geol. 1994, 117, 107–125. [Google Scholar] [CrossRef]
- Frey, F.A. Rare earth abundances in a high-temperature peridotite inclusion. Geochem. Cosmochem. Acta 1969, 33, 1429–1447. [Google Scholar] [CrossRef]
- Fujimaki, H.; Tatsumoto, M.; Aoki, K. Partitioning coefficients of Hf, Zr, and REE between phenocrysts and groundmasses. J. Geophys. Res. 1984, 89, 662–672. [Google Scholar] [CrossRef]
- Green, T.H.; Pearson, N.J. An experimental study of Nb and Ta partitioning between Ti-rich minerals and silicate liquids at high pressure and temperature. Geochem. Cosmochem. Acta 1987, 51, 55–62. [Google Scholar] [CrossRef]
- Green, T.H.; Adam, J.; Site, S.H. Proton microprobe determined trace element partition coefficients between pargasite, augite and silicate or carbonatitic melts. EOS 1993, 74, 340. [Google Scholar]
- Higuchi, H.; Nagasawa, H. Partition of trace elements between rock-forming minerals and the host volcanic rocks. Earth Planet Sci. Lett. 1969, 7, 281–287. [Google Scholar] [CrossRef]
- Irving, A.J.; Frey, F.A. Trace-element abundances in megacrysts and their host basalts-constraints on partition-coefficients and megacryst genesis. Geochem. Cosmochem. Acta 1984, 48, 1201–1221. [Google Scholar] [CrossRef]
- Keleman, P.B.; Dunn, J.T. Depletion of Nb relative to other highly incompatible elements by melt/rock reaction in the upper mantle. EOS 1992, 73, 656–657. [Google Scholar]
- LaTourrette, T.Z.; Burnett, D.S. Experimental determination of U–partitioning and Th–partitioning between clinopyroxene and natural and synthetic basaltic liquid. Earth Planet Sci. Lett. 1992, 110, 227–244. [Google Scholar] [CrossRef]
- Matsui, Y.; Onuma, N.; Nagasawa, H.; Higuch, H.; Banno, S. Crystal structure control in trace element partition between crystal and magma. Bull. Soc. Fr. Minér. Cristallogr. 1977, 100, 315–324. [Google Scholar] [CrossRef]
- McCallum, I.S.; Charette, M.P. Zr and Nb partition coefficients: Implications for the genesis of mare basalts, KREEP and sea floor basalts. Geochem. Cosmochem. Acta 1978, 42, 859–869. [Google Scholar] [CrossRef]
- McKenzie, D.; O’Nions, R.K. Partial melt distributions from inversion of rare earth element concentrations. J. Petrol. 1991, 32, 1021–1091. [Google Scholar] [CrossRef]
- Nagasawa, H.; Schnetzler, C.C. Partitioning of rare earth, alkali, and alkaline earth elements between phenocrysts and acidic igneous magmas. Geochem. Cosmochem. Acta 1971, 35, 953–968. [Google Scholar] [CrossRef]
- Nash, W.P.; Crecraft, H.R. Partition coefficients for trace elements in silicic magmas. Geochem. Cosmochem. Acta 1985, 49, 2309–2322. [Google Scholar] [CrossRef]
- Nielsen, R.L.; Gallahan, W.E.; Newberger, F. Experimentally determined mineral-melt partition coefficients for Sc, Y and REE for olivine, orthopyroxene, pigeonite, magnetite and ilmenite. Contrib. Mineral. Petrol. 1992, 110, 488–499. [Google Scholar] [CrossRef]
- Okamoto, K. Geochemical study on magmatic differentiation of Asama Volcano, central Japan. J. Geol. Soc. Jpn. 1979, 85, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Paster, T.P.; Schauwecker, D.S.; Haskin, L.A. The behavior of some trace elements during solidification of the Skaergard layered series. Geochem. Cosmochem. Acta 1974, 38, 1549–1577. [Google Scholar] [CrossRef]
- Philpotts, J.A.; Schnetzler, C.C. Phenocryst-matrix partition and basalt genesis. Geochem. Cosmochem. Acta 1970, 34, 307–322. [Google Scholar] [CrossRef]
- Reid, F. Origine of the rhyolitic rocks of the Taupo Volcanic Zone, New Zealand. J. Volcanol. Geothem. Res. 1983, 15, 315–338. [Google Scholar] [CrossRef]
- Rollinson, H.R. Using Geochemical Data: Evaluation, Presentation, Interpretation; Routledge Publishing Ltd.: Singapore, 1993; 352p. [Google Scholar]
- Ronov, A.B.; Yaroshevskiy, A.A. A new model for the chemical structure of the Earth’s crust. Geochem. Int. 1976, 13, 89–121. [Google Scholar]
- Schnetzler, C.C.; Philpotts, J.A. Partition coefficients of rare-earth elements between igneous matrix material and rock-forming mineral phenocrysts; II. Geochem. Cosmochem. Acta 1970, 34, 331–340. [Google Scholar] [CrossRef]
- Sisson, T.W. Hornblende-melt trace element partitioning measured by Ion-microprobe. Chem. Geol. 1994, 117, 331–334. [Google Scholar] [CrossRef]
- Skulski, T.; Minarik, W.; Watson, E.B. High-pressure experimental trace element partitioning between clinopyroxene and basaltic melts. Chem. Geol. 1994, 117, 127–147. [Google Scholar] [CrossRef]
- Sobolev, A.V.; Migdisov, A.A.; Portnyagin, M.V. Incompatible element partitioning between clinopyroxene and basaltic liquid revealed by the study of melt inclutions in minerals from Troods lavas, Cyprus. Petrology 1996, 4, 307–317. [Google Scholar]
- Villemant, B.; Jaffrezic, H.; Joron, J.L.; Treuil, M. Distribution coefficients of major and trace elements fractional crystallization on the Alkali Basalt Series of Chaine-Des-Puys (Massif Central, France). Geochem. Cosmochem. Acta 1981, 45, 1997–2016. [Google Scholar] [CrossRef]




| Sample 17101203 | Mineral | K (%) | 40Ar rad (nl/g) | 40Ar air (%) | Age (Ma) | Error (1s) |
|---|---|---|---|---|---|---|
| Granite | Biotite | 7.11 | 26.11 | 6.2 | 93.8 | 2.5 |
| Stage 1 | Parent 17032305 | Daughter 16042904 | Fractionated Minerals | |||||
| Pl | Opx | Cpx | Ol | calc. | diff. | |||
| fraction | 1.0000 | 0.3752 | 0.3044 | 0.1539 | 0.1245 | 0.0421 | ||
| SiO2 | 49.26 | 53.24 | 45.18 | 53.01 | 52.88 | 35.92 | 49.26 | −0.06 |
| TiO2 | 0.47 | 1.12 | 0.01 | 0.23 | 0.30 | 0.01 | 0.47 | −0.03 |
| Al2O3 | 17.32 | 18.05 | 34.88 | 1.16 | 1.32 | 0.01 | 17.32 | −0.06 |
| Fe2O3 | 8.54 | 8.95 | 0.18 | 19.16 | 7.37 | 33.91 | 8.54 | −0.07 |
| MgO | 8.08 | 3.76 | 0.03 | 23.81 | 15.51 | 28.89 | 8.08 | −0.05 |
| CaO | 11.12 | 7.65 | 18.31 | 1.19 | 21.81 | 0.03 | 11.12 | −0.05 |
| Na2O | 1.38 | 2.86 | 1.00 | 0.02 | 0.14 | 0.01 | 1.38 | −0.01 |
| K2O | 0.28 | 1.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.28 | −0.10 |
| Sum of squares of residuals = | 0.03 | |||||||
| Stage 2 | Parent 16042904 | Daughter 16081401A | Fractionated Minerals | |||||
| Pl(core) | Hb*(rim) | Hb(core) | Ilm | calc. | diff. | |||
| fraction | 1.0000 | 0.3446 | 0.4037 | 0.1506 | 0.0886 | 0.0126 | ||
| SiO2 | 53.24 | 58.92 | 52.48 | 51.12 | 45.70 | 0.03 | 53.24 | 0.00 |
| TiO2 | 1.12 | 0.72 | 0.01 | 0.08 | 1.45 | 51.73 | 1.12 | 0.06 |
| Al2O3 | 18.05 | 15.69 | 29.71 | 0.54 | 6.90 | 0.01 | 18.05 | 0.12 |
| Fe2O3 | 8.95 | 6.50 | 0.14 | 30.30 | 18.53 | 42.14 | 8.95 | −0.03 |
| MgO | 3.76 | 2.62 | 0.01 | 11.18 | 10.48 | 0.10 | 3.76 | 0.28 |
| CaO | 7.65 | 5.54 | 11.85 | 1.57 | 10.40 | 0.13 | 7.65 | −0.12 |
| Na2O | 2.68 | 2.75 | 4.59 | 0.08 | 1.21 | 0.02 | 2.68 | −0.04 |
| K2O | 1.00 | 2.17 | 0.15 | 0.02 | 0.66 | 0.01 | 1.00 | 0.12 |
| Sum of squares of residuals = | 0.13 | |||||||
| Stage 3 | Parent 16081401A | Daughter 16042001 | Fractionated Minerals | |||||
| Pl(rim) | Hb(rim) | Bt | Qz | calc. | diff. | |||
| fraction | 1.0000 | 0.3251 | 0.3225 | 0.1264 | 0.1262 | 0.0998 | ||
| SiO2 | 58.92 | 65.08 | 54.89 | 48.09 | 34.58 | 100.00 | 58.92 | −0.04 |
| TiO2 | 0.72 | 0.38 | 0.02 | 0.70 | 4.89 | 0.00 | 0.72 | −0.12 |
| Al2O3 | 15.69 | 13.82 | 28.15 | 5.52 | 14.04 | 0.00 | 15.69 | −0.05 |
| Fe2O3 | 6.50 | 3.34 | 0.15 | 18.79 | 22.72 | 0.00 | 6.50 | 0.14 |
| MgO | 2.62 | 1.18 | 0.01 | 10.79 | 9.23 | 0.00 | 2.62 | −0.31 |
| CaO | 5.54 | 3.09 | 10.24 | 10.60 | 0.69 | 0.00 | 5.54 | −0.08 |
| Na2O | 2.75 | 2.62 | 5.54 | 0.73 | 0.13 | 0.00 | 2.75 | 0.07 |
| K2O | 2.17 | 3.69 | 0.18 | 0.35 | 8.05 | 0.00 | 2.17 | −0.17 |
| Sum of squares of residuals = | 0.17 | |||||||
| (wt%) | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 |
| A | 67.58 | 0.56 | 15.30 | 3.97 | 0.06 | 0.95 | 3.81 | 2.50 | 2.55 | 0.08 |
| B | 51.22 | 0.91 | 17.63 | 9.14 | 0.17 | 5.28 | 8.55 | 3.10 | 1.49 | 0.13 |
| (ppm) | Rb | Ba | Nb | Sr | Y | |||||
| A | 115 | 562 | 13 | 238 | 11 | |||||
| 68 | B | 262 | 5.3 | 240 | 18 | |||||
| (ppm) | La | Ce | Nd | Sm | Eu | Gd | Dy | Er | Yb | Lu |
| A | 17 | 34 | 16 | 3.4 | 1.1 | 3 | 2.4 | 1.2 | 1.1 | 0.2 |
| B | 13 | 26 | 13 | 3.1 | 0.9 | 3.1 | 3.5 | 2.0 | 1.9 | 0.3 |
| SiO2 | TiO2 | Al2O3 | FeO* | MnO | MgO | CaO | Na2O | K2O | P2O5 | Total | Mg# | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fn-Gab | 51.41 | 0.49 | 18.08 | 8.02 | 0.19 | 8.43 | 11.61 | 1.44 | 0.29 | 0.04 | 100.00 | 69 |
| Prim. Comp. | 50.42 | 0.45 | 16.44 | 8.35 | 0.17 | 12.01 | 10.55 | 1.31 | 0.27 | 0.04 | 100.00 | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodama, S.; Owada, M.; Nagashima, M.; Kamei, A. Magmatic Processes of the Upper Cretaceous Susuma–Nagaho Plutonic Complex, Southwest Japan: Its Role on Crustal Growth and Recycling in Active Continental Margins. Minerals 2022, 12, 762. https://doi.org/10.3390/min12060762
Kodama S, Owada M, Nagashima M, Kamei A. Magmatic Processes of the Upper Cretaceous Susuma–Nagaho Plutonic Complex, Southwest Japan: Its Role on Crustal Growth and Recycling in Active Continental Margins. Minerals. 2022; 12(6):762. https://doi.org/10.3390/min12060762
Chicago/Turabian StyleKodama, Shogo, Masaaki Owada, Mariko Nagashima, and Atsushi Kamei. 2022. "Magmatic Processes of the Upper Cretaceous Susuma–Nagaho Plutonic Complex, Southwest Japan: Its Role on Crustal Growth and Recycling in Active Continental Margins" Minerals 12, no. 6: 762. https://doi.org/10.3390/min12060762
APA StyleKodama, S., Owada, M., Nagashima, M., & Kamei, A. (2022). Magmatic Processes of the Upper Cretaceous Susuma–Nagaho Plutonic Complex, Southwest Japan: Its Role on Crustal Growth and Recycling in Active Continental Margins. Minerals, 12(6), 762. https://doi.org/10.3390/min12060762






