Natural Magnetite Minerals Enhance 1,2-Dichloroethane Reductive Dechlorination
Abstract
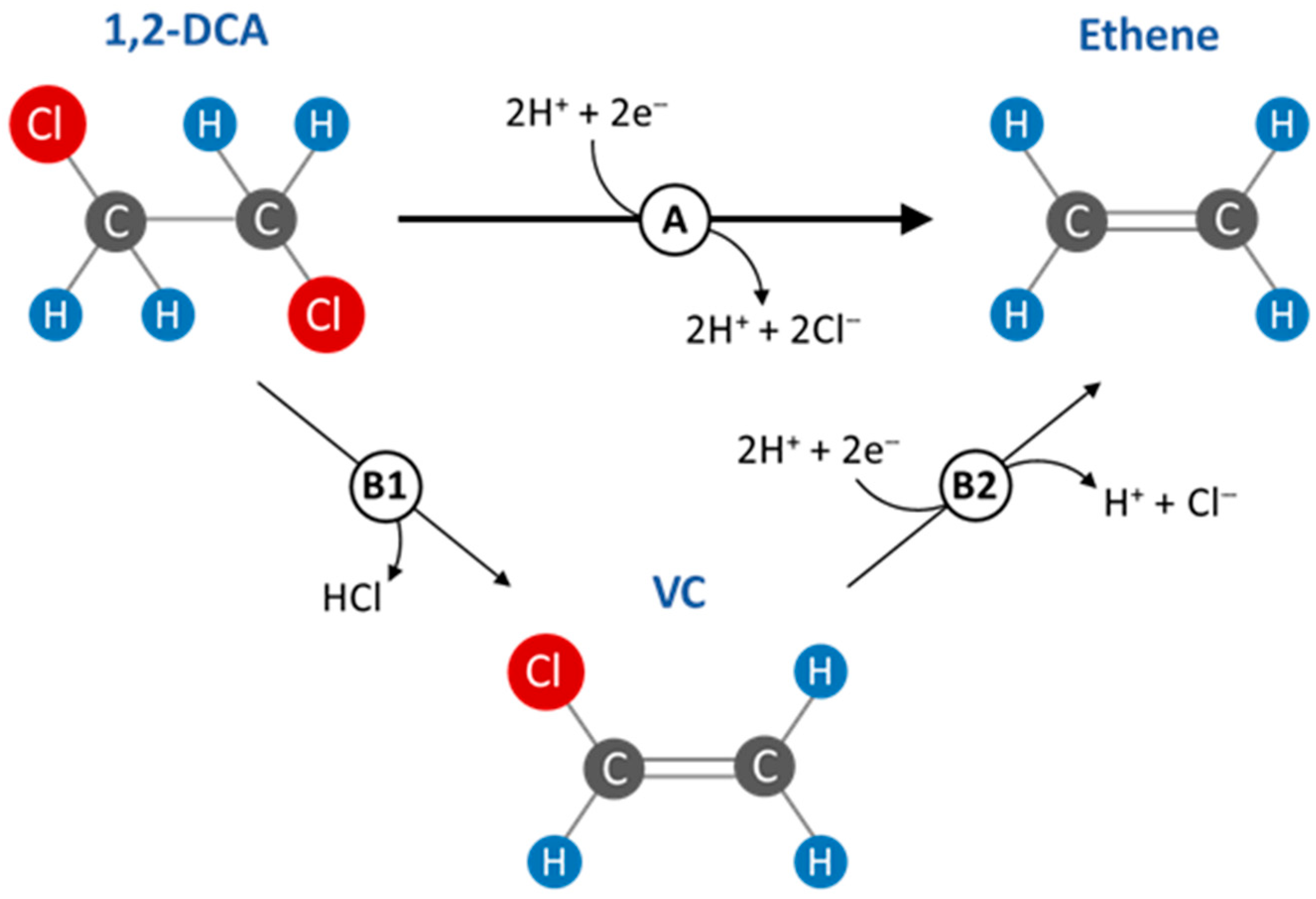
:1. Introduction
2. Materials and Methods
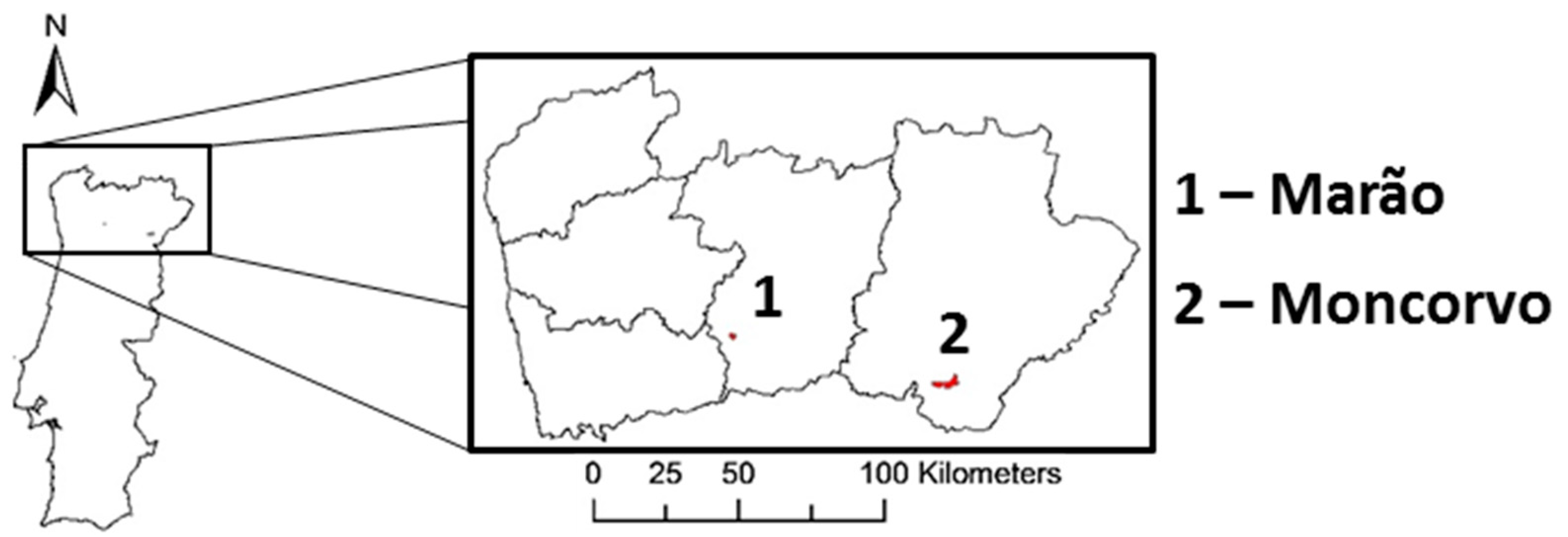
2.1. Magnetite and Hematite Minerals
2.2. 1,2-DCA-Dechlorinating Mixed Microbial Culture
2.3. Microcosm Setup
2.4. Analytical Methods
3. Results and Discussion
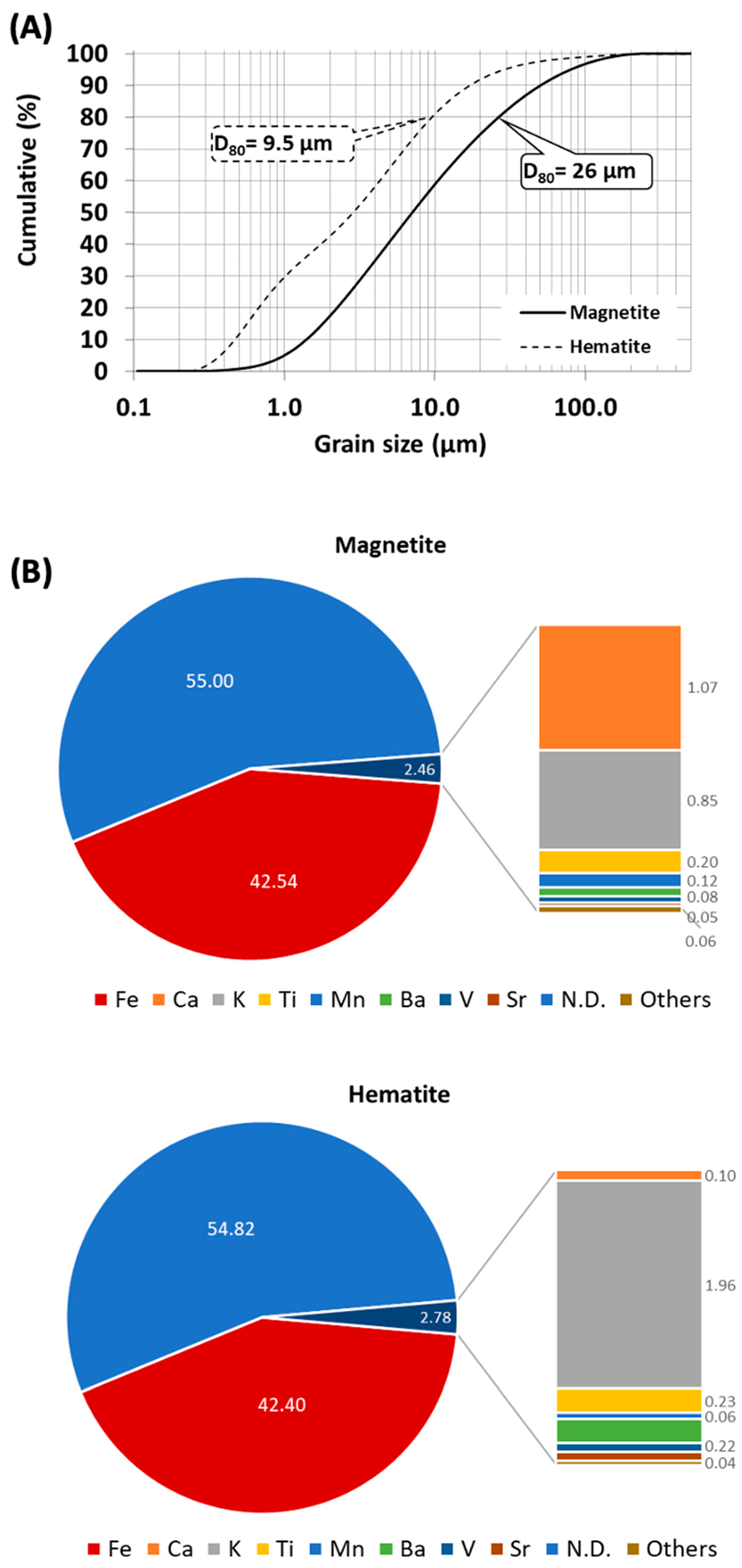
3.1. Natural Magnetite and Hematite Characterization
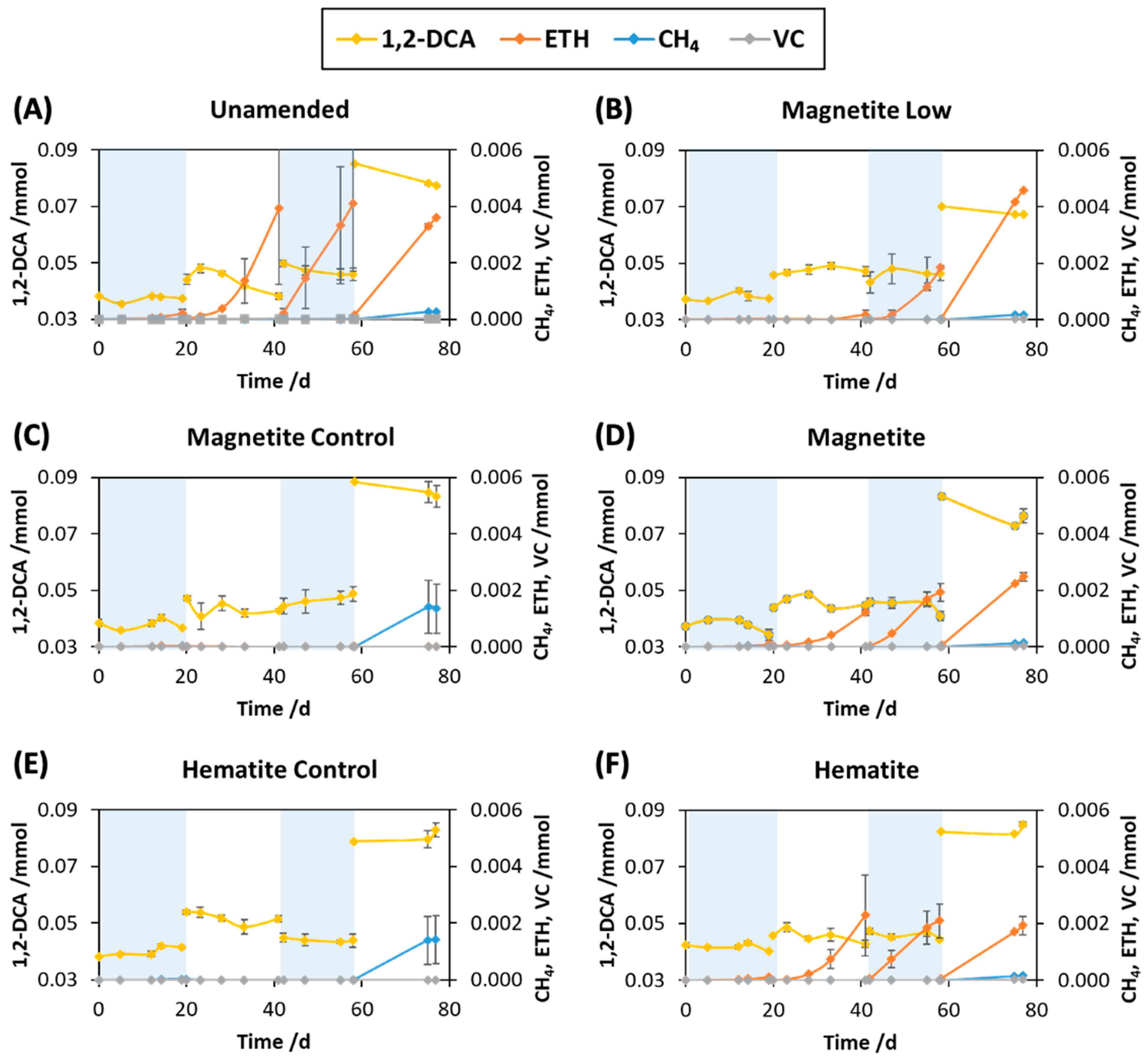
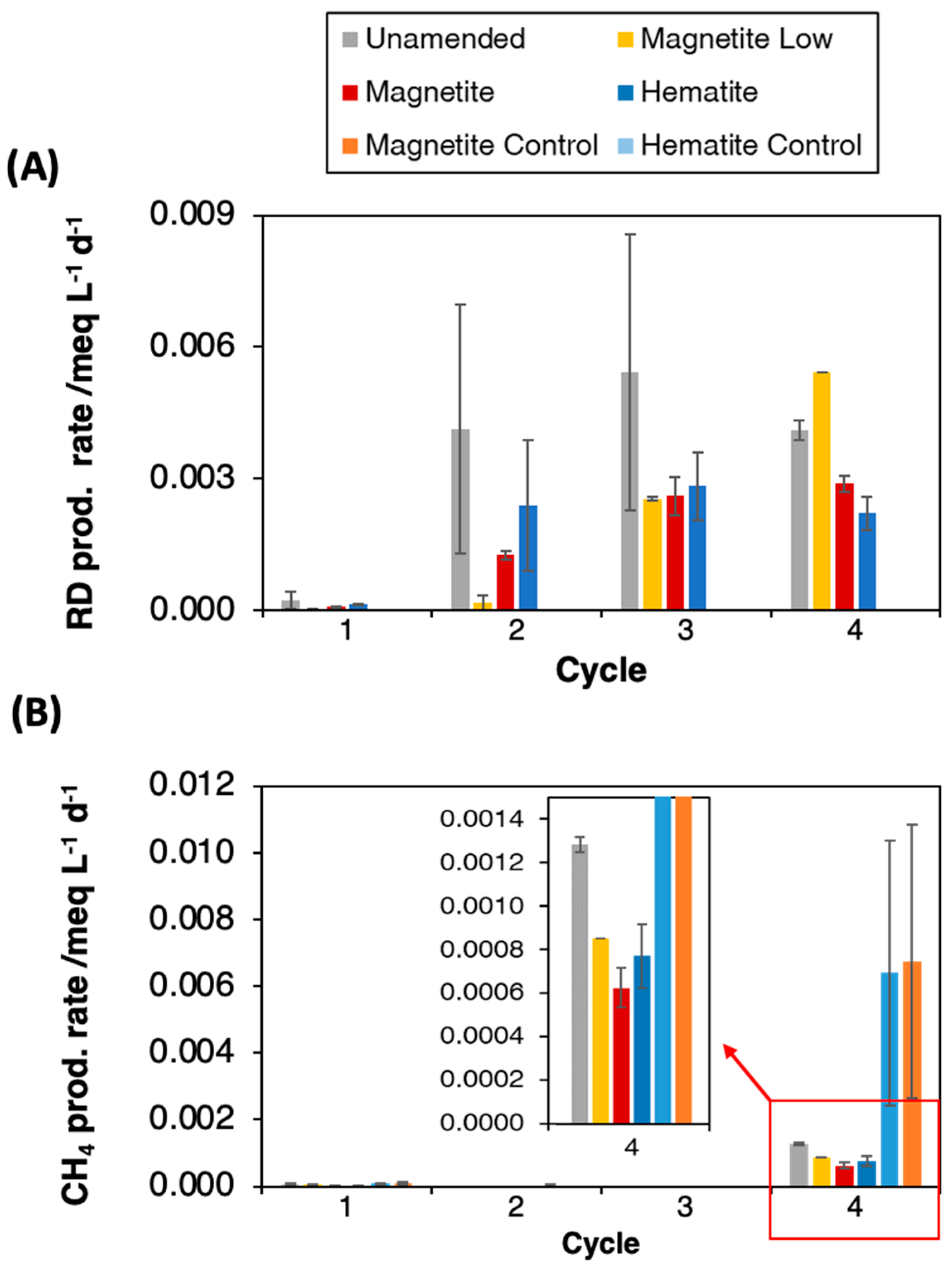
3.2. Influence of Magnetite and Hematite on Anaerobic Dechlorination
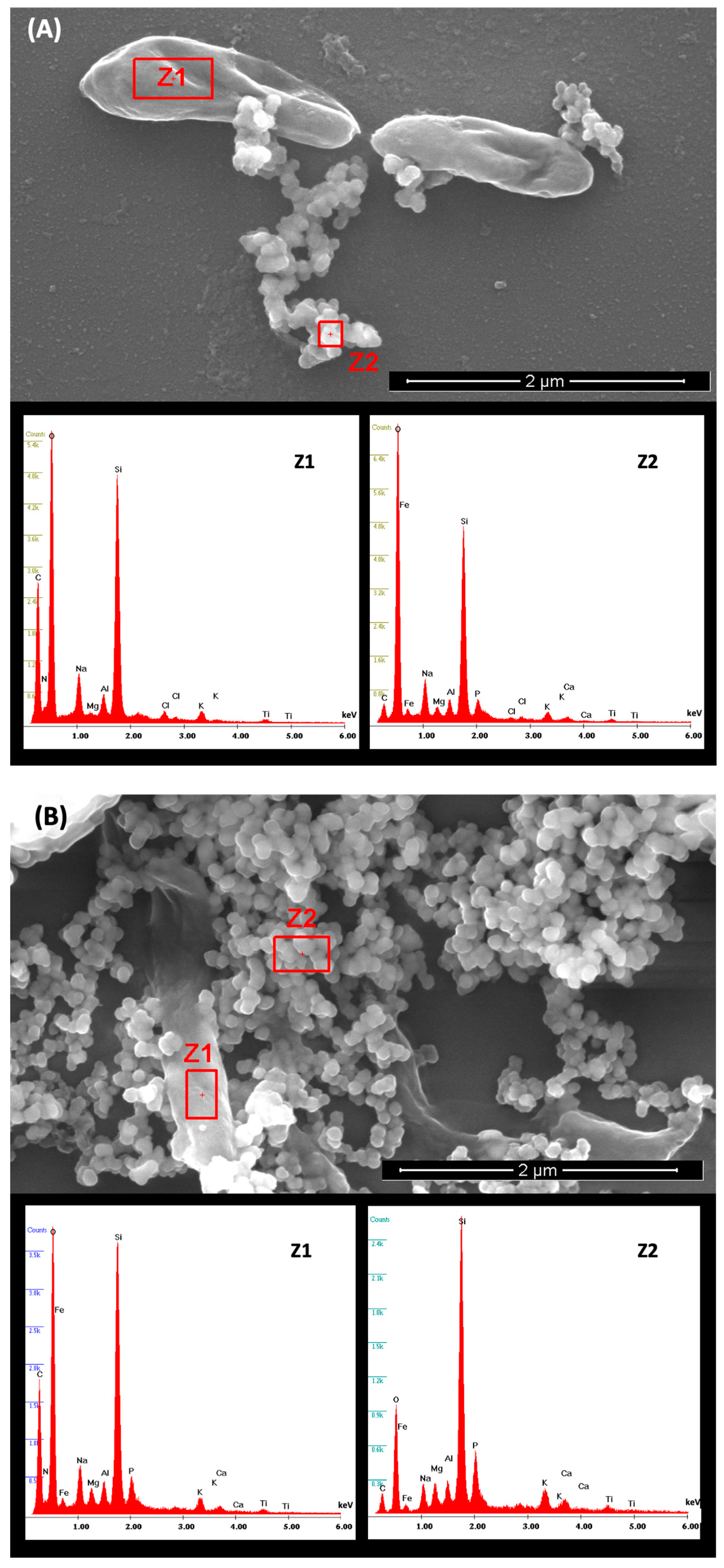
3.3. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dinglasan-Panlilio, M.J.; Dworatzek, S.; Mabury, S.; Edwards, E. Microbial Oxidation of 1,2-Dichloroethane under Anoxic Conditions with Nitrate as Electron Acceptor in Mixed and Pure Cultures. FEMS Microbiol. Ecol. 2006, 56, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovley, D.R. Bioremediation: Anaerobes to the Rescue. Science 2001, 293, 1444–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.; Lege, S.; Nijenhuis, I. Comparison of 1,2-Dichloroethane, Dichloroethene and Vinyl Chloride Carbon Stable Isotope Fractionation during Dechlorination by Two Dehalococcoides Strains. Water Res. 2014, 52, 146–154. [Google Scholar] [CrossRef]
- Duhamel, M.; Edwards, E.A. Growth and Yields of Dechlorinators, Acetogens, and Methanogens during Reductive Dechlorination of Chlorinated Ethenes and Dihaloelimination of 1,2-Dichloroethane. Environ. Sci. Technol. 2007, 41, 2303–2310. [Google Scholar] [CrossRef]
- He, J.; Ritalahti, K.M.; Yang, K.-L.; Koenigsberg, S.S.; Löffler, F.E. Detoxification of Vinyl Chloride to Ethene Coupled to Growth of an Anaerobic Bacterium. Nature 2003, 424, 62–65. [Google Scholar] [CrossRef]
- Grostern, A.; Edwards, E.A. Characterization of a Dehalobacter Coculture That Dechlorinates 1,2-Dichloroethane to Ethene and Identification of the Putative Reductive Dehalogenase Gene. Appl. Environ. Microbiol. 2009, 75, 2684–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, A.; Van Raemdonck, H.; Smith, K.; Ossieur, W.; Lebbe, L.; Verstraete, W. Transport and Activity of Desulfitobacterium Dichloroeliminans Strain DCA1 during Bioaugmentation of 1,2-DCA-Contaminated Groundwater. Environ. Sci. Technol. 2006, 40, 5544–5552. [Google Scholar] [CrossRef]
- Marzorati, M.; De Ferra, F.; Van Raemdonck, H.; Borin, S.; Allifranchini, E.; Carpani, G.; Serbolisca, L.; Verstraete, W.; Boon, N.; Daffonchio, D. A Novel Reductive Dehalogenase, Identified in a Contaminated Groundwater Enrichment Culture and in Desulfitobacterium Dichloroeliminans Strain DCA1, Is Linked to Dehalogenation of 1,2-Dichloroethane. Appl. Environ. Microbiol. 2007, 73, 2990–2999. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Hou, Z.; Du, X.M.; Li, D.M.; Lu, X.X. Assessment of Biostimulation and Bioaugmentation for Removing Chlorinated Volatile Organic Compounds from Groundwater at a Former Manufacture Plant. Biodegradation 2016, 27, 223–236. [Google Scholar] [CrossRef]
- Chen, M.; Tong, H.; Li, F.; Liu, C.; Lan, Q.; Liu, C. The Effect of Electron Donors on the Dechlorination of Pentachlorophenol (PCP) and Prokaryotic Diversity in Paddy Soil. Eur. J. Soil Biol. 2018, 86, 8–15. [Google Scholar] [CrossRef]
- Lovley, D.R. Syntrophy Goes Electric: Direct Interspecies Electron Transfer. Annu. Rev. Microbiol. 2017, 71, 643–664. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Tang, Z.; Ma, J.; Yu, Z.; Wang, Y.; Tang, J. Enhanced Anaerobic Biodegradation of Benzoate under Sulfate-Reducing Conditions with Conductive Iron-Oxides in Sediment of Pearl River Estuary. Front. Microbiol. 2019, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Tucci, M.; Cruz Viggi, C.; Dolfing, J.; Head, I.M.; Rotaru, A.E. An Underappreciated DIET for Anaerobic Petroleum Hydrocarbon-Degrading Microbial Communities. Microb. Biotechnol. 2020, 14, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Melton, E.D.; Swanner, E.D.; Behrens, S.; Schmidt, C.; Kappler, A. The Interplay of Microbially Mediated and Abiotic Reactions in the Biogeochemical Fe Cycle. Nat. Rev. Microbiol. 2014, 12, 797–808. [Google Scholar] [CrossRef]
- Byrne, J.M.; Klueglein, N.; Pearce, C.; Rosso, K.M.; Appel, E.; Kappler, A. Redox Cycling of Fe(II) and Fe(III) in Magnetite by Fe-Metabolizing Bacteria. Science 2015, 347, 1473–1476. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular Electron Transfer Mechanisms between Microorganisms and Minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Aulenta, F.; Fazi, S.; Majone, M.; Rossetti, S. Electrically Conductive Magnetite Particles Enhance the Kinetics and Steer the Composition of Anaerobic TCE-Dechlorinating Cultures. Process Biochem. 2014, 49, 2235–2240. [Google Scholar] [CrossRef]
- Aulenta, F.; Rossetti, S.; Amalfitano, S.; Majone, M.; Tandoi, V. Conductive Magnetite Nanoparticles Accelerate the Microbial Reductive Dechlorination of Trichloroethene by Promoting Interspecies Electron Transfer Processes. ChemSusChem 2013, 6, 433–436. [Google Scholar] [CrossRef]
- Leitão, P.; Aulenta, F.; Rossetti, S.; Nouws, H.P.A.; Danko, A.S. Impact of Magnetite Nanoparticles on the Syntrophic Dechlorination of 1,2-Dichloroethane. Sci. Total Environ. 2018, 624, 17–23. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Zheng, S.; Zang, H.; Liu, F.; Liu, F.; Liu, J. Stimulatory Effect of Magnetite on the Syntrophic Metabolism of Geobacter Co-Cultures: Influences of Surface Coating. Geochim. Cosmochim. Acta 2019, 256, 82–96. [Google Scholar] [CrossRef]
- Salazar-Camacho, C.; Villalobos, M.; de la Luz Rivas-Sánchez, M.; Arenas-Alatorre, J.; Alcaraz-Cienfuegos, J.; Gutiérrez-Ruiz, M.E. Characterization and Surface Reactivity of Natural and Synthetic Magnetites. Chem. Geol. 2013, 347, 233–245. [Google Scholar] [CrossRef]
- Leitão, P.; Rossetti, S.; Danko, A.S.; Nouws, H.; Aulenta, F. Enrichment of Dehalococcoides mccartyi Spp. from a Municipal Activated Sludge during AQDS-Mediated Bioelectrochemical Dechlorination of 1,2-Dichloroethane to Ethene. Bioresour. Technol. 2016, 214, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Zeikus, J.G. The Biology of Methanogenic Bacteria. Bacteriol. Rev. 1977, 41, 514–541. [Google Scholar] [CrossRef]
- Balch, W.E.; Fox, G.E.; Magrum, L.J.; Woese, C.R.; Wolfe, R.S. Methanogens: Reevaluation of a Unique Biological Group. Microbiol. Rev. 1979, 43, 260–296. [Google Scholar] [CrossRef]
- Gossett, J.M. Measurement of Henry’s Law Constants for Hydrocarbons. Environ. Sci. Technol. 1987, 21, 202–208. [Google Scholar] [CrossRef]
- Kielhorn, J.; Melber, C.; Wahnschaffe, U.; Aitio, A.; Mangelsdorf, I. Vinyl Chloride: Still a Cause for Concern. Environ. Health Perspect. 2000, 108, 579–588. [Google Scholar] [CrossRef]
- Zaa, C.L.Y.; McLean, J.E.; Dupont, R.R.; Norton, J.M.; Sorensen, D.L. Dechlorinating and Iron Reducing Bacteria Distribution in a TCE-Contaminated Aquifer. Groundw. Monit. Remediat. 2010, 30, 46–57. [Google Scholar] [CrossRef]
- Löffler, F.E.; Yan, J.; Ritalahti, K.M.; Adrian, L.; Edwards, E.A.; Konstantinidis, K.T.; Müller, J.A.; Fullerton, H.; Zinder, S.H.; Spormann, A.M. Dehalococcoides mccartyi Gen. Nov., Sp. Nov., Obligately Organohalide-Respiring Anaerobic Bacteria Relevant to Halogen Cycling and Bioremediation, Belong to a Novel Bacterial Class, Dehalococcoidia Classis Nov., Order Dehalococcoidales Ord. Nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2013, 63, 625–635. [Google Scholar] [CrossRef] [Green Version]
| Magnetite Control | Hematite Control | Unamended | Magnetite Low | Magnetite | Hematite | |
|---|---|---|---|---|---|---|
| Inoculum (mL) | / | / | 4 | 4 | 4 | 4 |
| Magnetite (mg) | 5.3 ± 0.3 | / | / | 1.2 ± 0.3 | 5.3 ± 0.3 | / |
| Hematite (mg) | / | 5.3 ± 0.3 | / | / | / | 5.3 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitão, P.; Tucci, M.; Cruz Viggi, C.; Nouws, H.; Danko, A.S.; Aulenta, F. Natural Magnetite Minerals Enhance 1,2-Dichloroethane Reductive Dechlorination. Minerals 2022, 12, 816. https://doi.org/10.3390/min12070816
Leitão P, Tucci M, Cruz Viggi C, Nouws H, Danko AS, Aulenta F. Natural Magnetite Minerals Enhance 1,2-Dichloroethane Reductive Dechlorination. Minerals. 2022; 12(7):816. https://doi.org/10.3390/min12070816
Chicago/Turabian StyleLeitão, Patrícia, Matteo Tucci, Carolina Cruz Viggi, Henri Nouws, Anthony S. Danko, and Federico Aulenta. 2022. "Natural Magnetite Minerals Enhance 1,2-Dichloroethane Reductive Dechlorination" Minerals 12, no. 7: 816. https://doi.org/10.3390/min12070816







