Removal of Fluoride from Phosphogypsum Leaching Solution with Phosphate Tailing Based Layered Double Hydroxides: Kinetics and Equilibrium Isotherms
Abstract
1. Introduction
2. Experimental Plan
2.1. Materials
2.2. Synthesis of PTB-LDO3 and PTB-LDO4
2.3. Characterization
2.4. Adsorption Ability Assessment of PTB-LDO3 and PTB-LDO4
3. Results and Discussion
3.1. Characterization
3.1.1. SEM Analysis
3.1.2. BET Analysis
3.1.3. FTIR Analysis
3.1.4. XRD Analysis
3.2. Explanation of Absorption Experiment
3.2.1. Effect of pH
3.2.2. Effects of Dosage
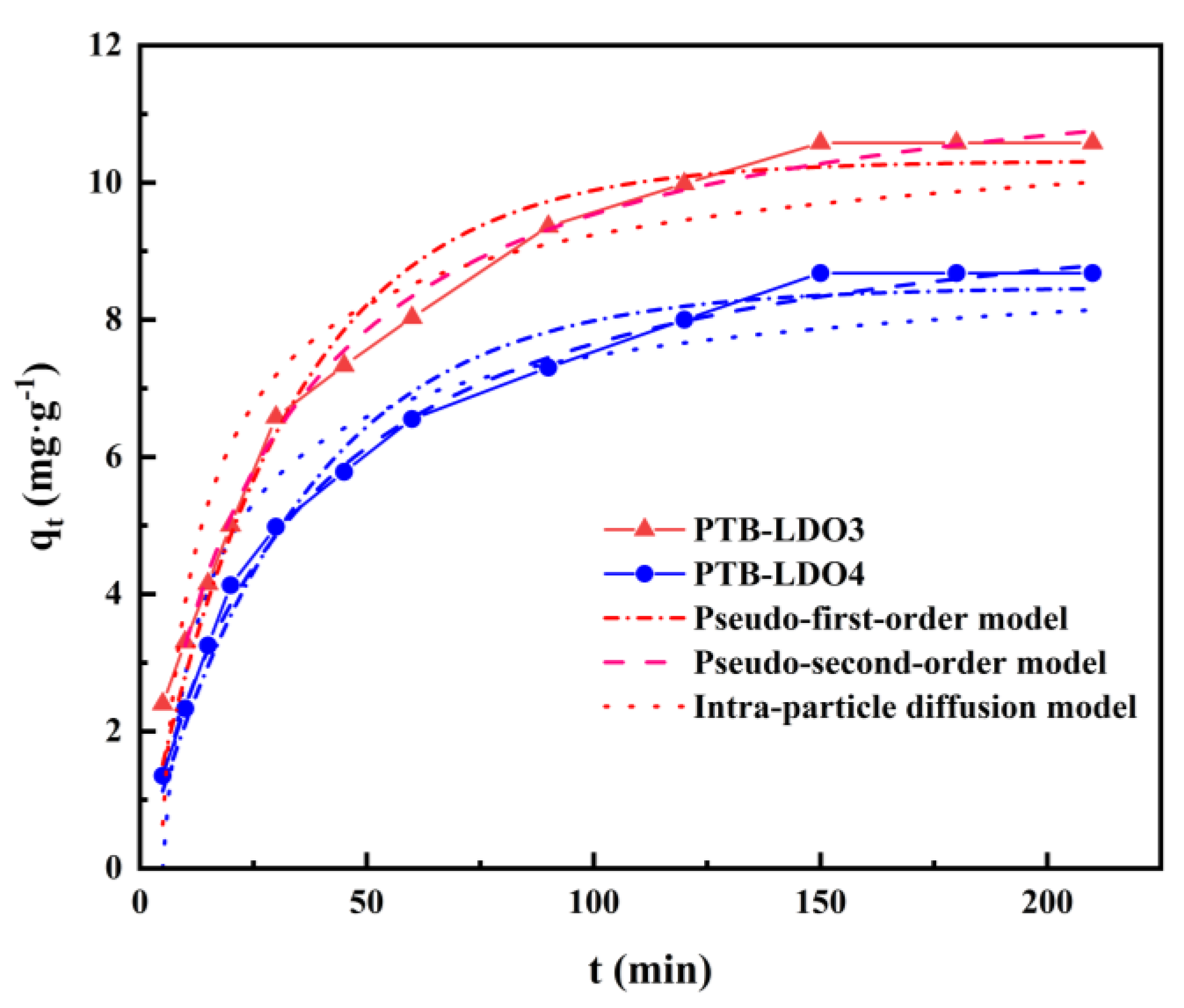
3.3. Adsorption Kinetics
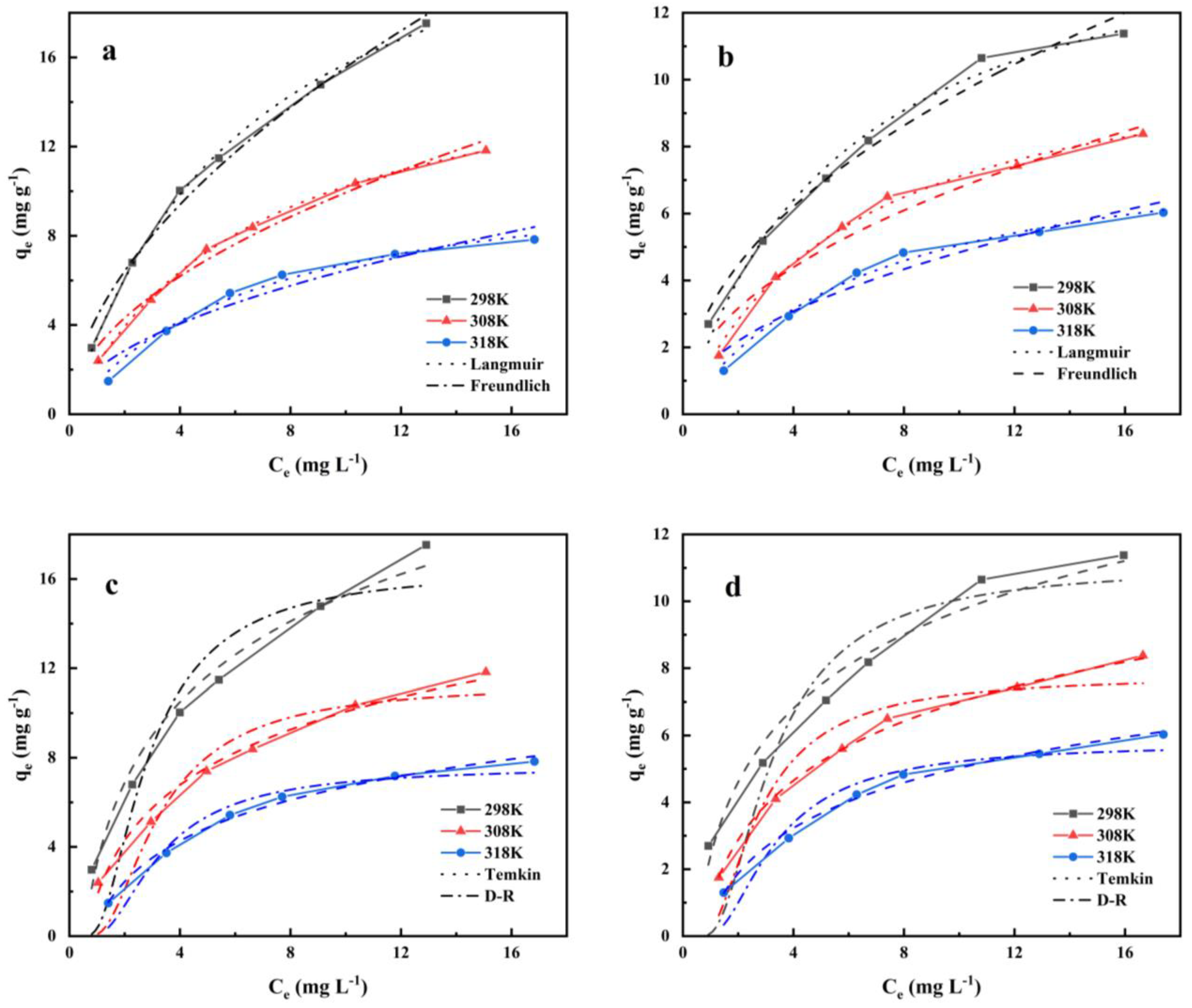
3.4. Adsorption Isotherm
3.5. Adsorption Thermodynamics
3.6. Comparison with Other Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Ren, Y.; Qu, G.; Liu, S.; Chen, B.; Liu, X.; Zhao, C.; Li, J. Utilization path of bulk industrial solid waste: A review on the multi-directional resource utilization path of phosphogypsum. J. Environ. Manag. 2022, 313, 114957. [Google Scholar] [CrossRef] [PubMed]
- Bisone, S.; Gautier, M.; Chatain, V.; Blanc, D. Spatial distribution and leaching behavior of pollutants from phosphogypsum stocked in a gypstack: Geochemical characterization and modeling. J. Environ. Manag. 2017, 193, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, Y.; Anderson, C.W.N.; Jeyakumar, P.; Yang, J. Effect of simulated acid rain on fluorine mobility and the bacterial community of phosphogypsum. Environ. Sci. Pollut. Res. 2018, 25, 15336–15348. [Google Scholar] [CrossRef] [PubMed]
- Fallahzadeh, R.A.; Miri, M.; Taghavi, M.; Gholizadeh, M.; Anbarani, A. Spatial variation and probabilistic risk assessment of exposure to fluoride in drinking water. Food Chem. Toxicol. 2018, 113, 314–321. [Google Scholar] [CrossRef]
- Augustsson, A.; Berger, T. Assessing the risk of an excess fluoride intake among Swedish children in households with private wells-Expanding static single-source methods to a probabilistic multi-exposure-pathway approach. Environ. Int. 2014, 68, 192–199. [Google Scholar] [CrossRef]
- Wambu, E.W.; Onindo, C.O.; Ambusso, W.; Muthakia, G.K. Removal of Fluoride from Aqueous Solutions by Adsorption Using a Siliceous Mineral of a Kenyan Origin. Acta Hydroch. Hydrob. 2013, 41, 340–348. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. An empirical model for defluoridation by batch monopolar electrocoagulation/flotation (ECF) process. J. Hazard. Mater. 2006, 131, 118–125. [Google Scholar] [CrossRef]
- Lhassani, A.; Rumeau, M.; Benjelloun, D.; Pontie, M. Selective demineralization of water by nanofiltration application to the defluofination ofbrackish water. Water Res. 2001, 35, 3260–3264. [Google Scholar] [CrossRef]
- Qureshi, S.Z.; Khan, M.A.; Rahman, N. Removal of fluoride ion by zirconium(IV) arseniate vanadate using ion-selective electrode. Water Treat. 1995, 10, 307–312. [Google Scholar]
- Annouar, S.; Mountadar, M.; Soufiane, A.; Elmidaoui, A.; Sahli, M.A.M. Defluoridation of underground water by adsorption on the chitosan and by electrodialysis. Desalination 2004, 165, 437. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.-X.; Qu, J.-H.; Liu, R.-P.; Lan, H.-C. Adsorption of fluoride onto different types of aluminas. Chem. Eng. J. 2012, 189, 126–133. [Google Scholar] [CrossRef]
- Na, C.-K.; Park, H.-J. Defluoridation from aqueous solution by lanthanum hydroxide. J. Hazard. Mater. 2010, 183, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Ravulapalli, S.; Ravindhranath, K. Novel adsorbents possessing cumulative sorption nature evoked from Al2O3 nanoflakes, C.urens seeds active carbon and calcium alginate beads for defluoridation studies. J. Taiwan Inst. Chem. Eng. 2019, 101, 50–63. [Google Scholar] [CrossRef]
- Nogueira, K.A.B.; Cecilia, J.A.; Santos, S.O.; Aguiar, J.E.; Vilarrasa-García, E.; Rodríguez-Castellón, E.; Azevedo, D.C.S.; Silva, I.J. Adsorption behavior of bovine serum albumin on Zn–Al and Mg–Al layered double hydroxides. J. Sol-Gel Sci. Technol. 2016, 80, 748–758. [Google Scholar] [CrossRef]
- Reichle, W.T. Catalytic reactions by thermally activated, synthetic, anionic clay minerals. J. Catal. 1985, 94, 547–557. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Theiss, F.L.; Couperthwaite, S.J.; Ayoko, G.A.; Frost, R.L. A review of the removal of anions and oxyanions of the halogen elements from aqueous solution by layered double hydroxides. J. Colloid Interface Sci. 2014, 417, 356–368. [Google Scholar] [CrossRef]
- Lv, L.; He, J.; Wei, M.; Evans, D.G.; Duan, X. Factors influencing the removal of fluoride from aqueous solution by calcined Mg–Al–CO3 layered double hydroxides. J. Hazard. Mater. 2006, 133, 119–128. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Xiao, H.; Lu, H.; Zhou, Y. Synthesis of Li–Al Layered Double Hydroxides (LDHs) for Efficient Fluoride Removal. Ind. Eng. Chem. Res. 2012, 51, 11490–11498. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Xiong, P.; Ma, W.; Qian, N.; Lu, W. A novel method for synthesis of Co–Al layered double hydroxides and their conversions to mesoporous CoAl2O4 nanostructures for applications in adsorption removal of fluoride ions. Microporous Mesoporous Mater. 2015, 201, 91–98. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, X.; Zhang, Y.; Zhang, Q.; Pan, B. Enhanced fluoride removal by La-doped Li/Al layered double hydroxides. J. Colloid Interface Sci. 2018, 509, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, M.; Asadpour, S.; Sarmast, N.; Dinari, M. A review of the synthesis methods, properties, and applications of layered double hydroxides/carbon nanocomposites. J. Mol. Liq. 2022, 348, 118399. [Google Scholar] [CrossRef]
- Kusrini, E.; Sofyan, N.; Suwartha, N.; Yesya, G.; Priadi, C.R. Chitosan-praseodymium complex for adsorption of fluoride ions from water. J. Rare Earths 2015, 33, 1104–1113. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, B.; You, W.; Yu, J.; Ho, W. 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to Congo red, methyl orange and Cr(VI) ions. J. Hazard. Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, K.; Zhao, Q.; Xie, C.; Qiu, W.; Jia, T. Kinetics and equilibrium of adsorption of dissolved organic matter fractions from secondary effluent by fly ash. J. Environ. Sci. 2011, 23, 1057–1065. [Google Scholar] [CrossRef]
- Velu, S.; Ramkumar, V.; Narayanan, A.; Swamy, C.S. Effect of interlayer anions on the physicochemical properties of zinc–aluminium hydrotalcite-like compounds. J. Mater. Sci. 1997, 32, 957–964. [Google Scholar] [CrossRef]
- Zaghloul, A.; Benhiti, R.; Ait Ichou, A.; Carja, G.; Soudani, A.; Zerbet, M.; Sinan, F.; Chiban, M. Characterization and application of MgAl layered double hydroxide for methyl orange removal from aqueous solution. Mater. Today Proc. 2021, 37, 3793–3797. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Lin, S. Efficient antimicrobial properties of layered double hydroxide assembled with transition metals via a facile preparation method. Chin. Chem. Lett. 2020, 31, 1511–1515. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, R.; Ebina, Y.; Iyi, N.; Takada, K.; Sasaki, T. General Synthesis and Delamination of Highly Crystalline Transition-Metal-Bearing Layered Double Hydroxides. Langmuir 2007, 23, 861–867. [Google Scholar] [CrossRef]
- Sarma, G.K.; Rashid, M.H. Synthesis of Mg/Al Layered Double Hydroxides for Adsorptive Removal of Fluoride from Water: A Mechanistic and Kinetic Study. J. Chem. Eng. Data 2018, 63, 2957–2965. [Google Scholar] [CrossRef]
- Hu, C.Y.; Lo, S.L.; Kuan, W.H. Effects of the molar ratio of hydroxide and fluoride to Al(III) on fluoride removal by coagulation and electrocoagulation. J. Colloid Interface Sci. 2005, 283, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S. Chemistry of the solid-water interface: Processes at the mineral-water and particle-water interface in natural systems. Geochim. Cosmochim. Ac. 1992, 57, 205. [Google Scholar] [CrossRef][Green Version]
- Panday, K.K.; Prasad, G.; Singh, V.N. Copper(II) removal from aqueous solutions by fly ash. Water Res. 1985, 19, 869–873. [Google Scholar] [CrossRef]
- Rahman, M.T.; Kameda, T.; Miura, T.; Kumagai, S.; Yoshioka, T. Removal of sulfate from wastewater via synthetic Mg–Al layered double hydroxide: An adsorption, kinetics, and thermodynamic study. J. Indian Chem. Soc. 2021, 98, 100185. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption onto carbon from solutions. J. Sanit. Eng. Div. ASCE 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Jiang, S.; Yu, T.; Xia, R.; Wang, X.; Gao, M. Realization of super high adsorption capability of 2D δ-MnO2/GO through intra-particle diffusion. Mater. Chem. Phys. 2019, 232, 374–381. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surface of glass, mica, and platinum. J. Am. Chem. Soc. 1916, 40, 1361–1368. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, T.; Cheng, H.; Wu, H.; Wu, C.; Hu, A.; Wang, D. Preparation of composite sludge carbon-based materials by LDHs conditioning and carbonization and its application in the simultaneous removal of dissolved organic matter and phosphate in sewage. Chemosphere 2021, 270, 129485. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Yıldırım, E.; Aydın, S.; Emik, S.; Ağun, T.; Osra, F.; Wasswa, J. A facile polymerisation of magnetic coal to enhanced phosphate removal from solution. J. Environ. Manag. 2019, 24, 256–362. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gao, H.; Yang, Q.; Zhang, H.; Wang, D.; Zhang, W.; Yang, X. Removal of Typical Organic Contaminants with a Recyclable Calcined Chitosan-Supported Layered Double Hydroxide Adsorbent: Kinetics and Equilibrium Isotherms. J. Chem. Eng. Data 2018, 63, 159–168. [Google Scholar] [CrossRef]
- Yang, G.; Wang, R.; Ma, W.; Tian, L.; Cheng, Z. Layered Double Hydroxides for the Adsorption of Fluoride from Aqueous Solution. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; pp. 1–4. [Google Scholar]
- Biswas, K.; Gupta, K.; Ghosh, U.C. Adsorption of fluoride by hydrous iron(III)–tin(IV) bimetal mixed oxide from the aqueous solutions. Chem. Eng. J. 2009, 149, 196–206. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Yakout, S.M.; Elreefy, S.A. Adsorption of fluoride in aqueous solutions using KMnO4-modified activated carbon derived from steam pyrolysis of rice straw. J. Hazard. Mater. 2007, 147, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Saha, S.K.; Ghosh, U.C. Adsorption of Fluoride from Aqueous Solution by a Synthetic Iron(III)−Aluminum(III) Mixed Oxide. Ind. Eng. Chem. Res. 2007, 46, 5346–5356. [Google Scholar] [CrossRef]
- Yao, R.; Meng, F.; Zhang, L.; Ma, D.; Wang, M. Defluoridation of water using neodymium-modified chitosan. J. Hazard. Mater. 2009, 165, 454–460. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, Z.; Feng, C.; Sugiura, N.; Li, M.; Chen, R. Fluoride removal from water by granular ceramic adsorption. J. Colloid Interface Sci. 2010, 348, 579–584. [Google Scholar] [CrossRef]
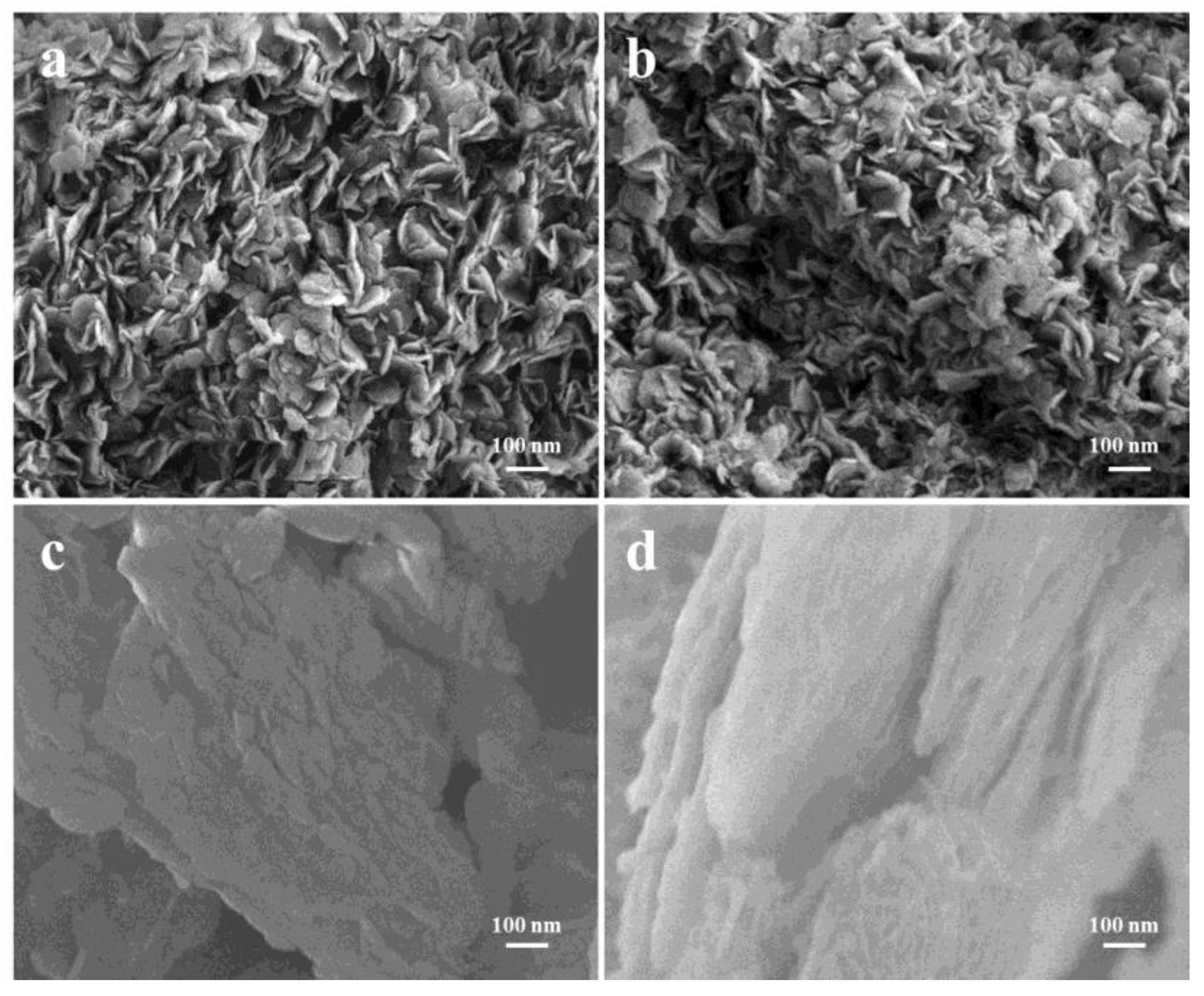


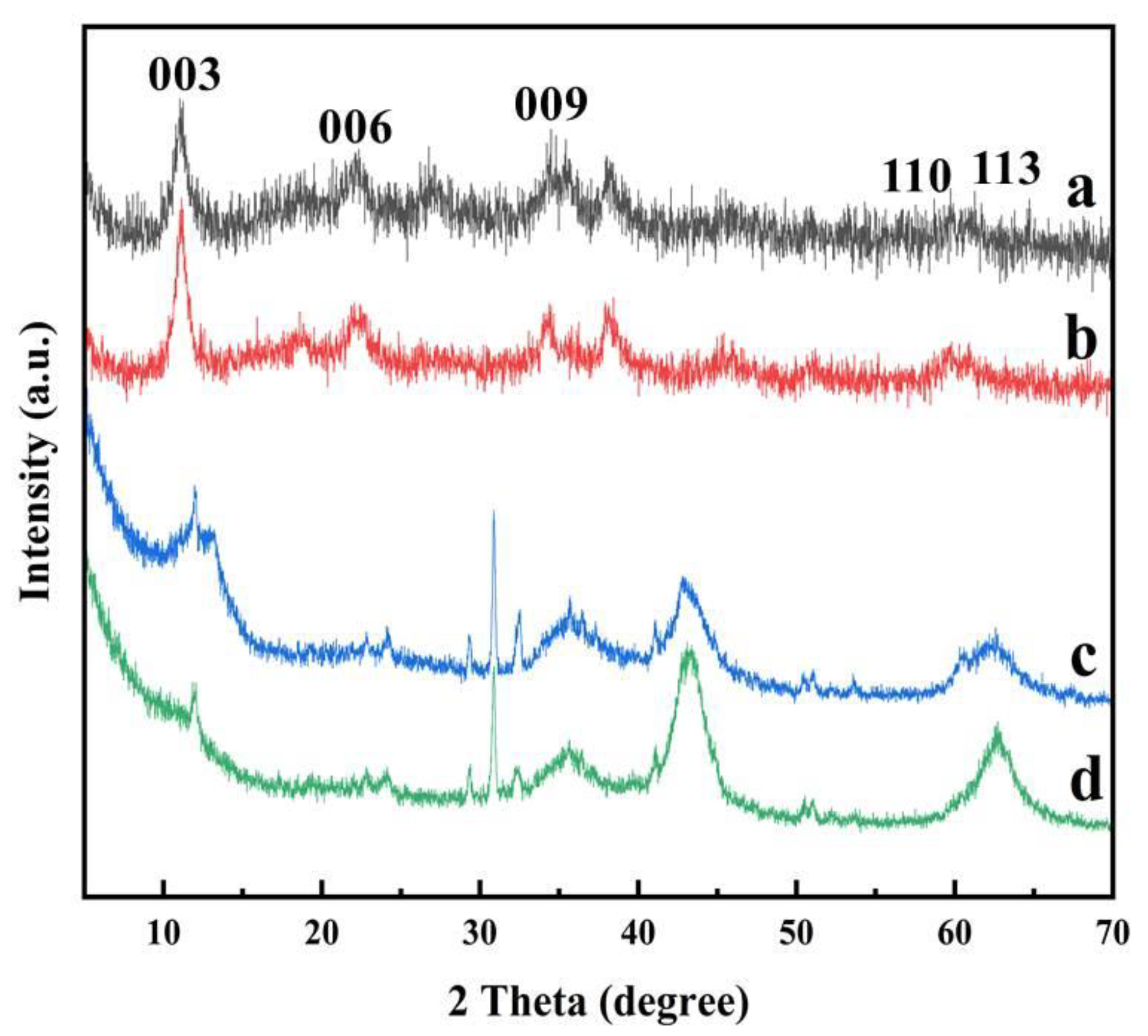
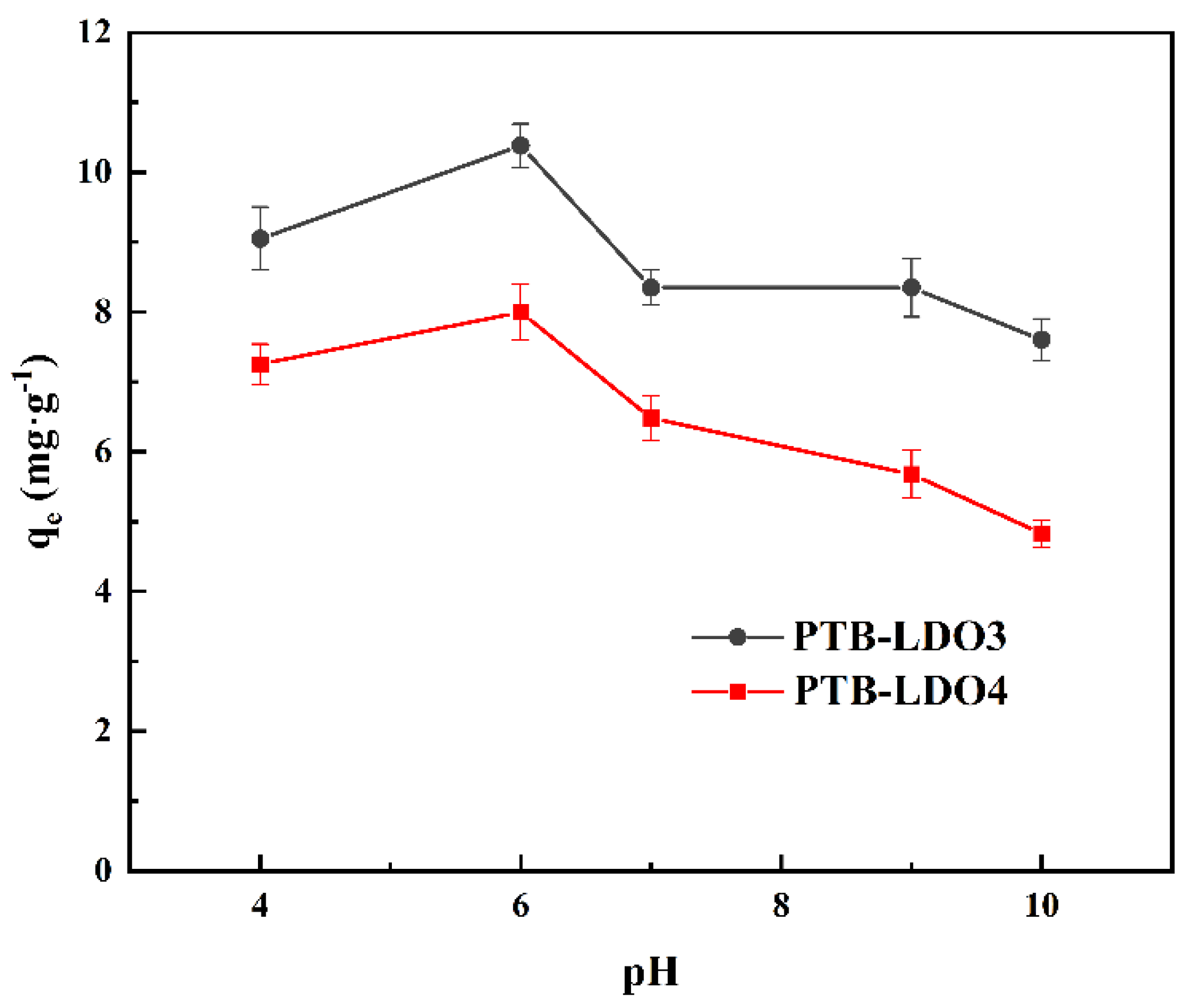
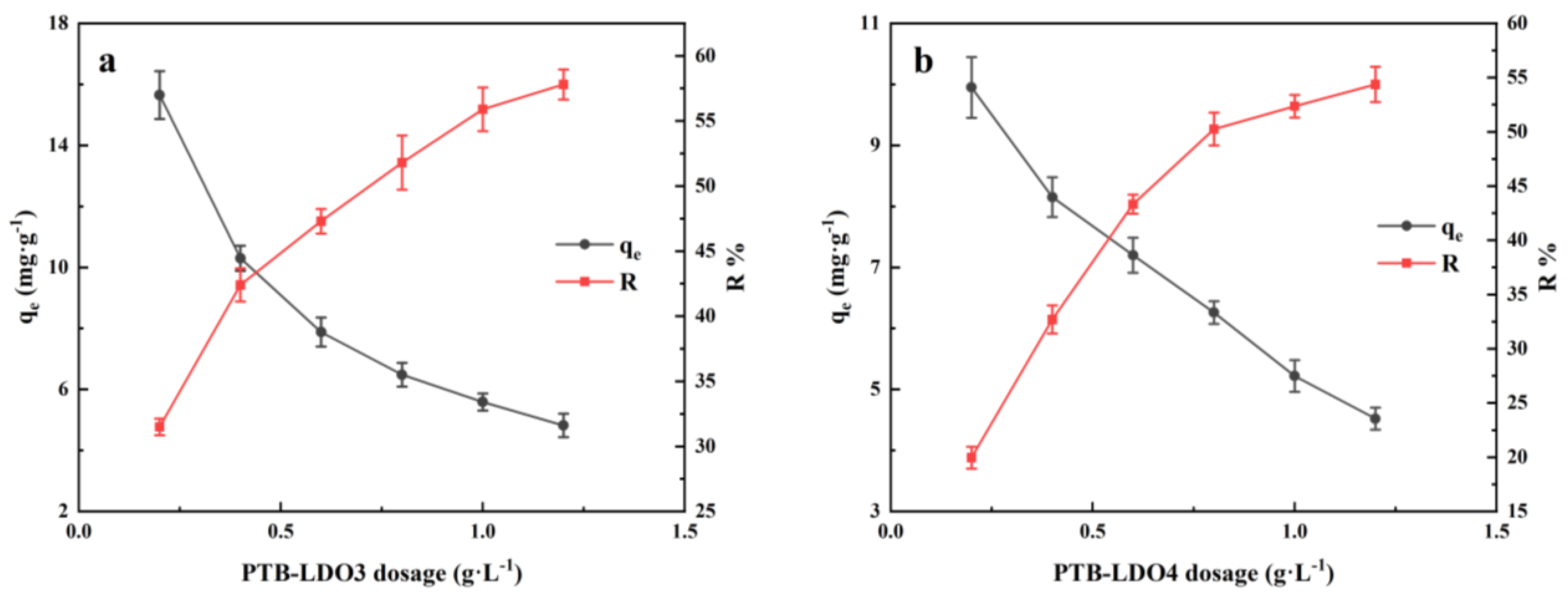
| Materials | SBET a/m2 g−1 | SSPA b/ m2 g−1 | VSPA c/cm3 g−1 | Dp d/ nm |
|---|---|---|---|---|
| PTB-LDH3 | 71.6387 | 70.4108 | 0.3421 | 19.1017 |
| PTB-LDO3 | 372.3441 | 363.9801 | 0.6145 | 6.6017 |
| PTB-LDH4 | 11.4616 | 11.0509 | 0.0406 | 14.1789 |
| PTB-LDO4 | 72.4138 | 70.6970 | 0.3468 | 19.1575 |
| T(K)/Absorbent | ∆S (J mol−1 K−1) | ∆H (kJ mol−1) | ∆G (kJ mol−1) | R2 |
|---|---|---|---|---|
| 298/PTB-LDO3 | 36.12 | −1.686 | −12.45 | 0.9367 |
| 308/PTB-LDO3 | −12.81 | |||
| 318/PTB-LDO3 | −13.17 | |||
| 298/PTB-LDO4 | 21.65 | −6.322 | −12.77 | 0.9492 |
| 308/PTB-LDO4 | −12.99 | |||
| 318/PTB-LDO4 | −13.21 |
| Adsorbents | qmax (mg g−1) | pH | References |
|---|---|---|---|
| MgAlFe-HTlc500 | 25.4 | 6 | [44] |
| Iron-tin mixed oxide | 10.47 | 6.4 | [45] |
| KMnO4-modified carbon | 15.9 | 2 | [46] |
| Iron-aluminum mixed oxide | 17.73 | 6.9 | [47] |
| Nd-modified chitosan | 22.38 | 7 | [48] |
| CSLDH-75 | 12.12 | 5-8 | [49] |
| PTB-LDO3 | 26.03 | 5-6 | Present study |
| PTB-LDO4 | 15.66 | 5-6 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, H.; Xiao, D.; Wu, H.; Zhang, Z.; Xu, L.; Cheng, Q.; Zhou, H.; Yu, J.; Pan, Z.; et al. Removal of Fluoride from Phosphogypsum Leaching Solution with Phosphate Tailing Based Layered Double Hydroxides: Kinetics and Equilibrium Isotherms. Minerals 2022, 12, 858. https://doi.org/10.3390/min12070858
Liu Y, Zhang H, Xiao D, Wu H, Zhang Z, Xu L, Cheng Q, Zhou H, Yu J, Pan Z, et al. Removal of Fluoride from Phosphogypsum Leaching Solution with Phosphate Tailing Based Layered Double Hydroxides: Kinetics and Equilibrium Isotherms. Minerals. 2022; 12(7):858. https://doi.org/10.3390/min12070858
Chicago/Turabian StyleLiu, Yanming, Han Zhang, Dunquan Xiao, Hanjun Wu, Zhenyue Zhang, Lulu Xu, Qingrong Cheng, Hong Zhou, Junxia Yu, Zhiquan Pan, and et al. 2022. "Removal of Fluoride from Phosphogypsum Leaching Solution with Phosphate Tailing Based Layered Double Hydroxides: Kinetics and Equilibrium Isotherms" Minerals 12, no. 7: 858. https://doi.org/10.3390/min12070858
APA StyleLiu, Y., Zhang, H., Xiao, D., Wu, H., Zhang, Z., Xu, L., Cheng, Q., Zhou, H., Yu, J., Pan, Z., & Wang, D. (2022). Removal of Fluoride from Phosphogypsum Leaching Solution with Phosphate Tailing Based Layered Double Hydroxides: Kinetics and Equilibrium Isotherms. Minerals, 12(7), 858. https://doi.org/10.3390/min12070858








