Study on the Phase Transition from Quartz to Coesite under High Temperature and High Pressure
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Starting Materials
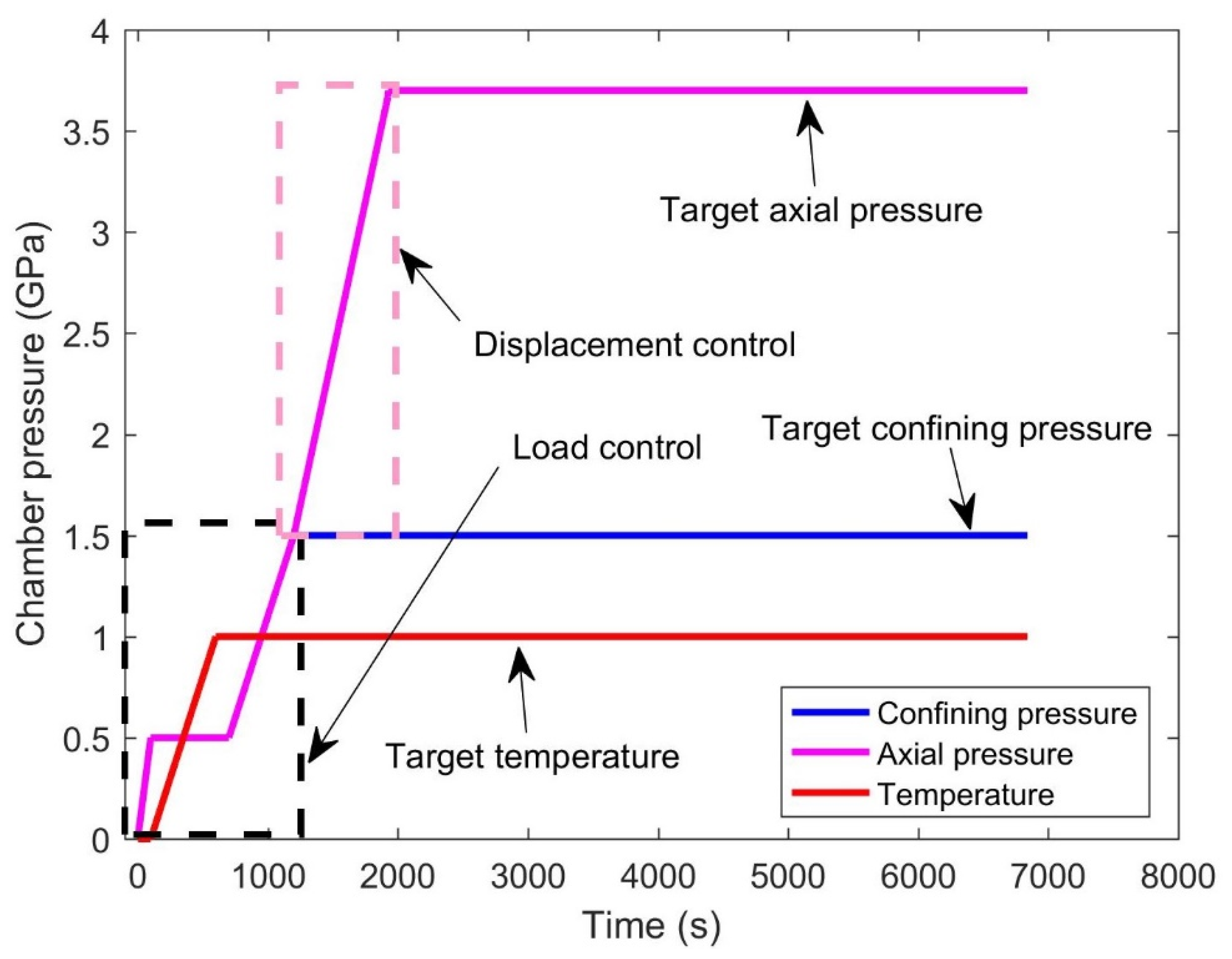
2.2. Experimental Design and Process
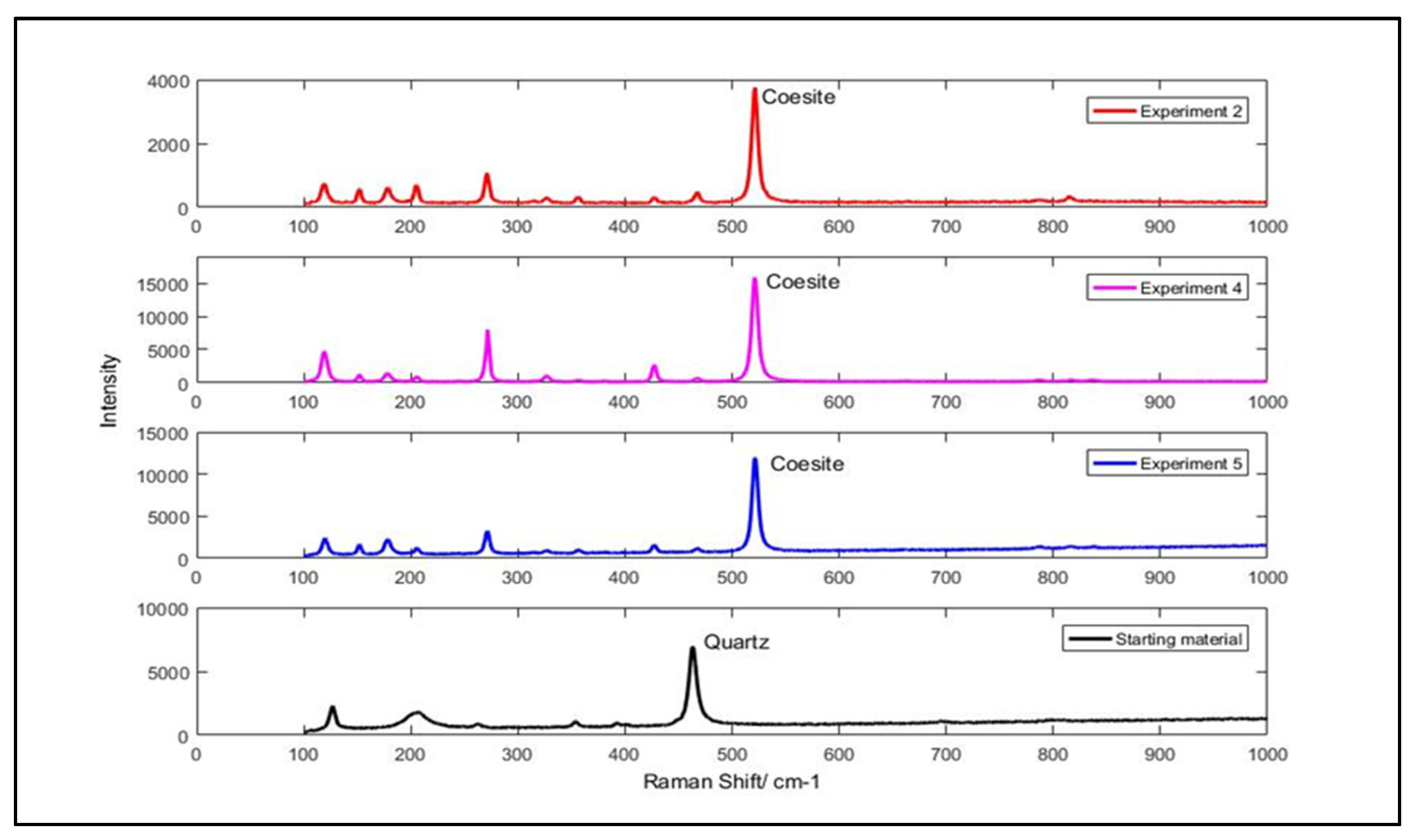
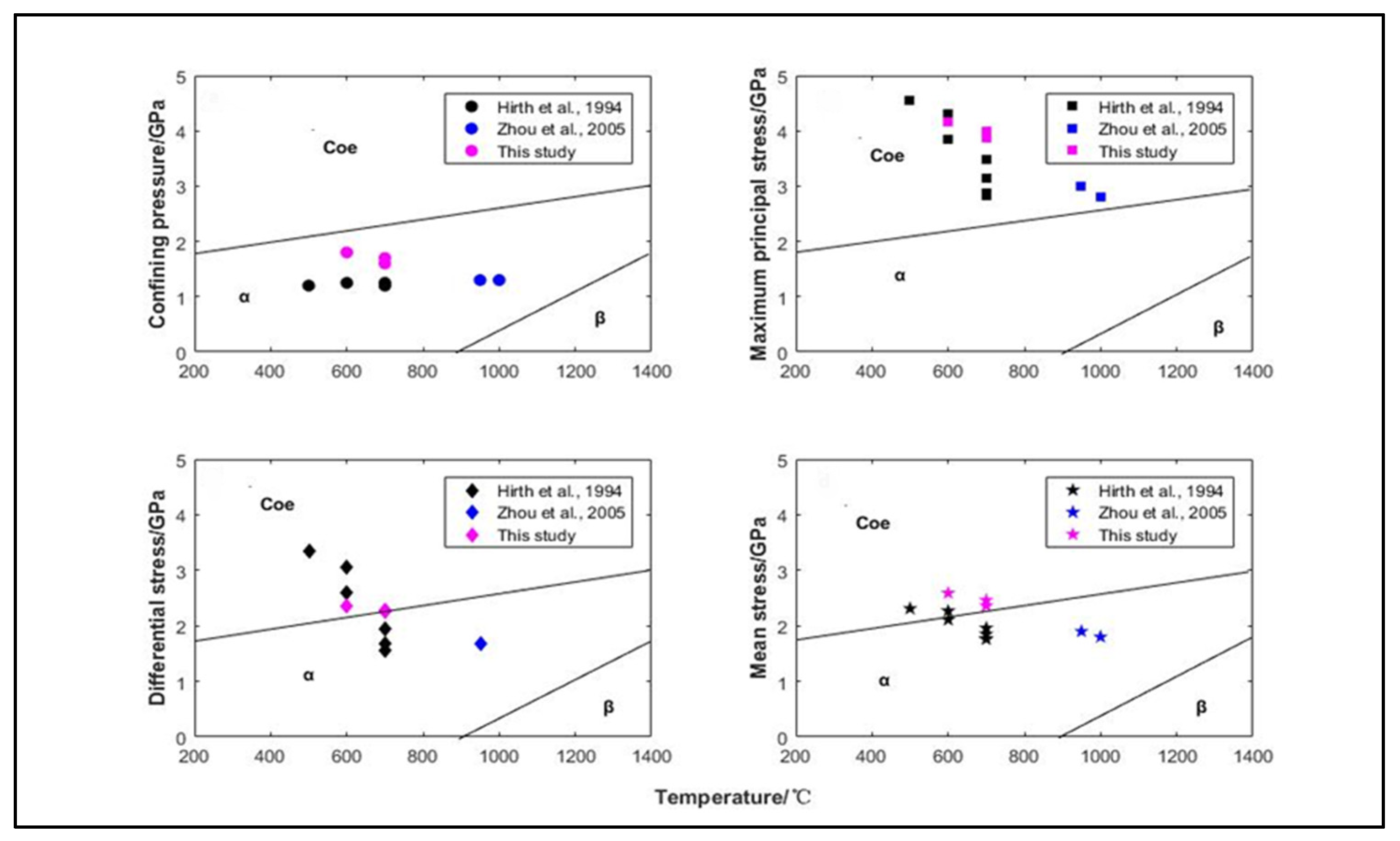
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; He, C.; Ma, S.; Song, J.; Ma, J. Effect of differential stress on quartz coesite conversion pressure. Sci. Bull. 2005, 50, 565–569. [Google Scholar] [CrossRef]
- Einicke, W.; Enke, D.; Dvoyashkin, M.; Valiullin, R.; Gläser, R. The Mechanism of pseudomorphic transformation of spherical silica gel into mcm-41 studied by PFG NMR diffusometry. Materials 2013, 6, 3688–3709. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.H.W.; Prieto, D.M.J.; Xiong, D.F.; Hassine, G.B.; Heyde, M.; Menzel, D.; Freund, H.J. A Silica Bilayer Supported on Ru (0001): Following the Crystalline-to Vitreous Transformation in Real Time with Spectro-microscopy. Angew. Chem. 2020, 63, 813–820. [Google Scholar]
- Wain, A. New evidence for coesite in eclogite and gneisses: Defining an ultrahigh-pressure province in the Western Gneiss region of Norway. Geology 1997, 25, 927–930. [Google Scholar] [CrossRef]
- Spektor, K.; Nylen, J.; Mathew, R.; Edén, M.; Stoyanov, E.; Navrotsky, A.; Häussermann, U. Formation of Hydrous Stishovite from Coesite in High-Pressure Hydrothermal Environments. Mineral. Soc. Am. 2016, 101, 2514–2524. [Google Scholar] [CrossRef]
- Zhou, W.G.; Xie, H.S.; Zhao, Z.D.; Zhou, H.; Guo, J. Calculation of the Strain, Stress and Elastic Energy for α–β Quartz Transition and Its Geological Significance. J. Geophys. 2002, 16, 241–248. [Google Scholar]
- Chen, X.Y.; Feng, W.J.; Zhang, G.Y.; Gao, Y. Raman Spectra of Quartz and Pb4+ SiO2 Crystals at Different Temperature and Pressure. Crystals 2019, 9, 569. [Google Scholar] [CrossRef] [Green Version]
- Griggs, D. Hydrolytic Weakening of Quartz and Other Silicates. Phys. Chem. Miner. 1967, 14, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Hülsmans, A.; Schmücker, M.; Mader, W.; Schneider, H. The Transformation of Andalusite to Mullite and Silica: Part I. Transformation Mechanism in [001] Direction. Am. Mineral. 2015, 85, 980–986. [Google Scholar] [CrossRef]
- Hobbs, B.E. Recrystallization of single crystals of quartz. Tectonophysics 1968, 6, 353–401. [Google Scholar] [CrossRef]
- Green, H.W. Metastable growth of coesite in highly strained quartz. J. Geophys. Res. 1972, 77, 2478–2482. [Google Scholar] [CrossRef]
- Hirth, G.; Tullis, J. The brittle-plastic transition in experimentally deformed quartz aggregates. J. Geophys. Res. 1994, 99, 11731–11747. [Google Scholar] [CrossRef]
- Richter, B. Stresses and pressures at the quartz-to-coesite phase transition in shear deformation experiments. J. Geophys. Res.-Solid Earth 2016, 121, 8015–8033. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Li, H.; Cui, C.; Jiang, J. In situ surface-enhanced Raman spectroscopy study of thiocyanate ions adsorbed on silver nanoparticles under high pressure. Chem. Phys. 2018, 516, 1–5. [Google Scholar]

| Temperature/°C | Confining Pressure/GPa | Maximum Principal Stress/GPa | Differential Stress/GPa | Mean Stress/GPa | Strain | Holding Time/h | Coesite Present (Y/N) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 700 | 1.5 | 3.7 | 2.2 | 2.0 | 72.1 | 36 | N |
| 2 | 700 | 1.6 | 3.9 | 2.3 | 2.1 | 72.3 | 36 | Y |
| 3 | 600 | 1.8 | 4.1 | 2.3 | 2.4 | 73.8 | 24 | N |
| 4 | 600 | 1.8 | 4.2 | 2.4 | 2.4 | 75.6 | 36 | Y |
| 5 | 700 | 1.7 | 4.0 | 2.3 | 2.3 | 71.1 | 24 | Y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, D. Study on the Phase Transition from Quartz to Coesite under High Temperature and High Pressure. Minerals 2022, 12, 963. https://doi.org/10.3390/min12080963
Ren D. Study on the Phase Transition from Quartz to Coesite under High Temperature and High Pressure. Minerals. 2022; 12(8):963. https://doi.org/10.3390/min12080963
Chicago/Turabian StyleRen, Dongsheng. 2022. "Study on the Phase Transition from Quartz to Coesite under High Temperature and High Pressure" Minerals 12, no. 8: 963. https://doi.org/10.3390/min12080963
APA StyleRen, D. (2022). Study on the Phase Transition from Quartz to Coesite under High Temperature and High Pressure. Minerals, 12(8), 963. https://doi.org/10.3390/min12080963





