Pore Evolution Characteristics of Marine Organic-Rich Shale Based on a Pyrolysis Simulation Experiment
Abstract
:1. Introduction
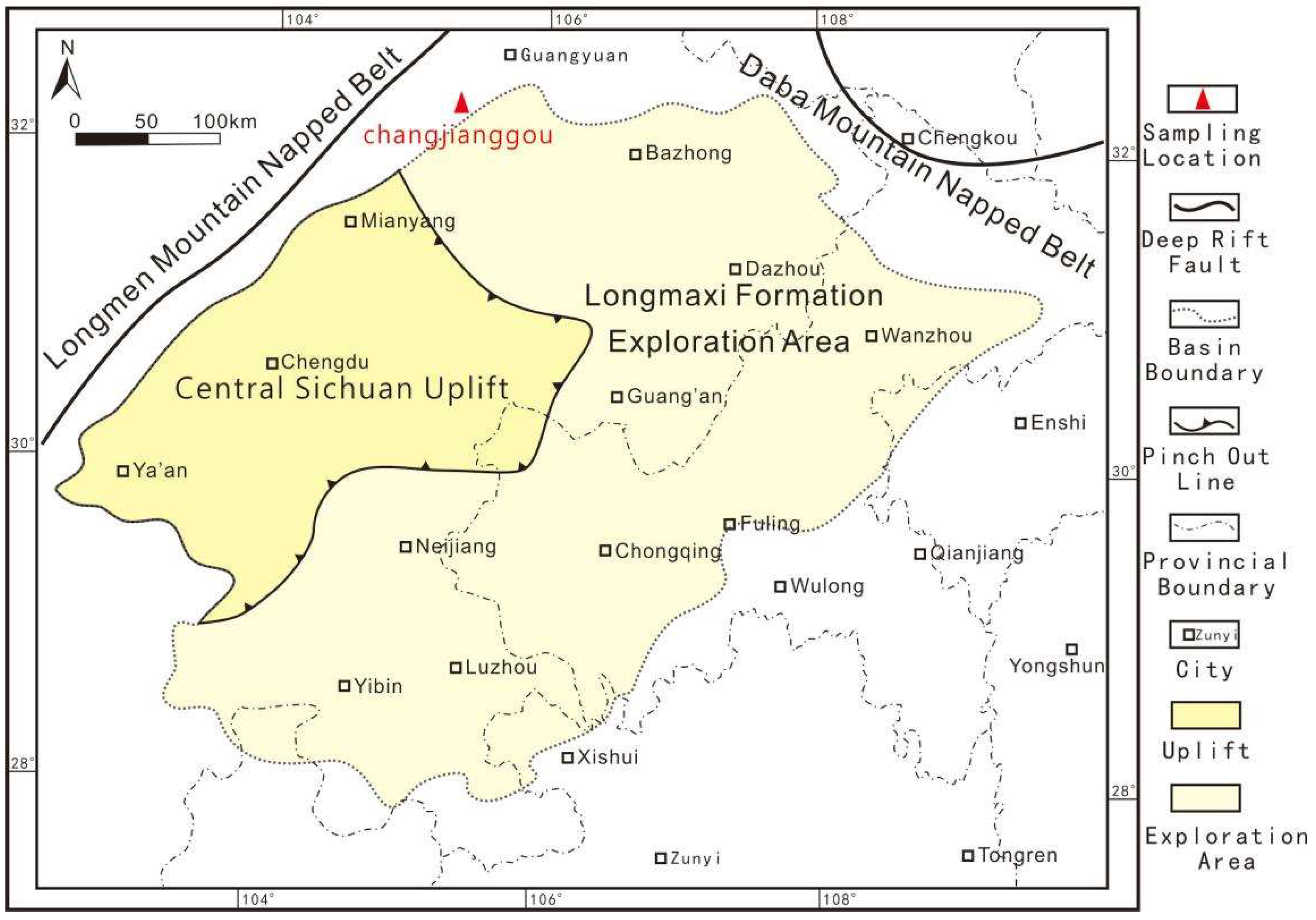
Samples and Experiment
- (1)
- Twelve column samples were divided into two groups (i.e., six for a group) and placed in the instrument. Brine with 40 mg/L salinity was used as the pore fluid.
- (2)
- The static rock pressure was set to maintain a certain pressure difference between the two groups of static rock pressure and formation pressure (Table 2).
- (3)
- The target temperature was set to 350 °C, and the heating rate was 20 °C/h. The temperature was sustained for 2 days after reaching the target temperature. The fluid pressure was then checked and naturally cooled to room temperature. The samples after the pyrolysis simulation experiment were taken out for a scanning electron microscope observation and a series of follow-up experiments, including TOC determination, gas composition analysis, X-ray diffraction whole rock analysis, and clay mineral determination analysis.
- (4)
- The second sample was replaced, the target temperature was set to 400 °C. Step (3) was then repeated.
- (5)
- The temperature was set to 450 °C, 500 °C, 550 °C, and 600 °C. Step (3) and (4) were then repeated.
2. Experiment Results
2.1. Pore Morphological Characteristics
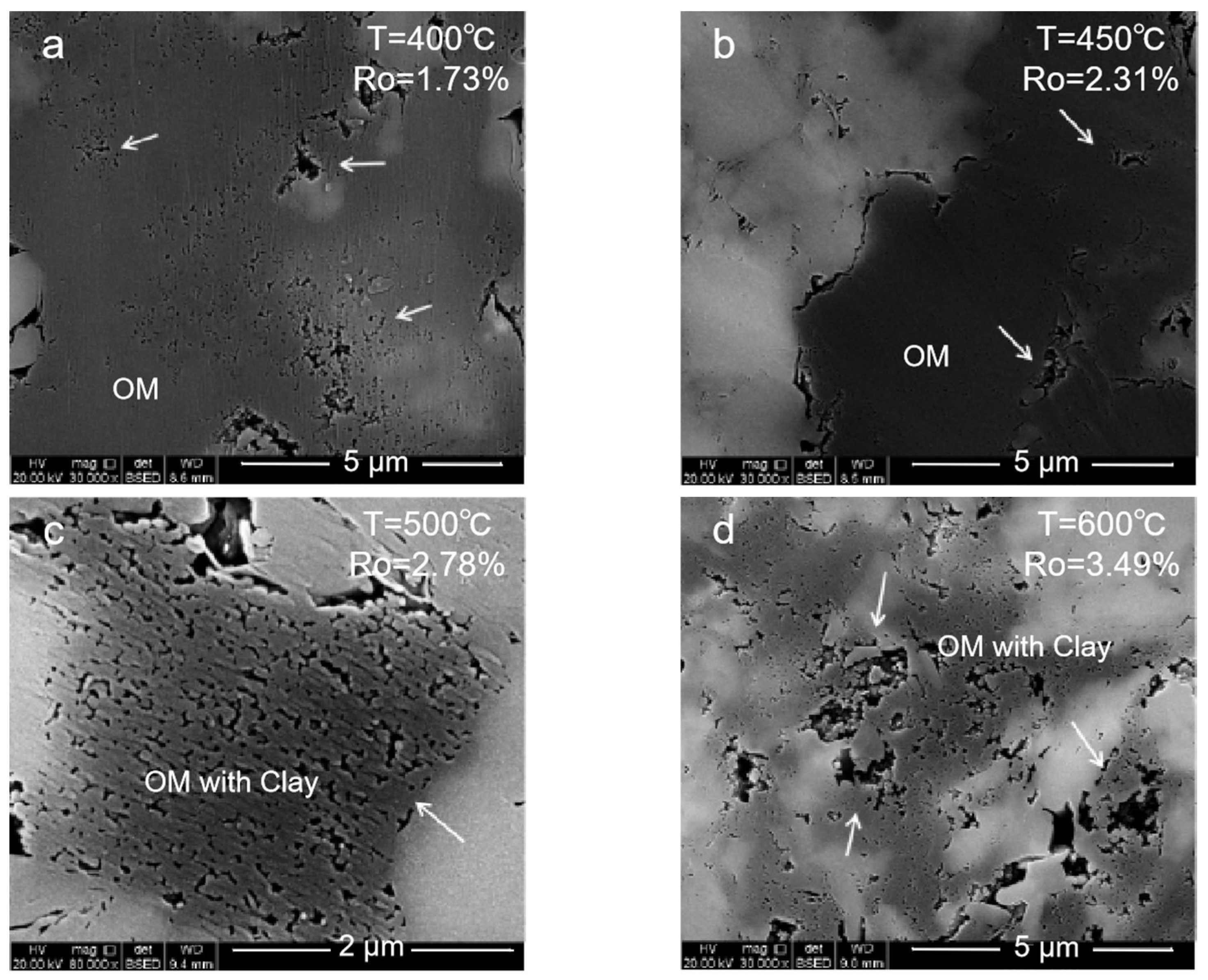
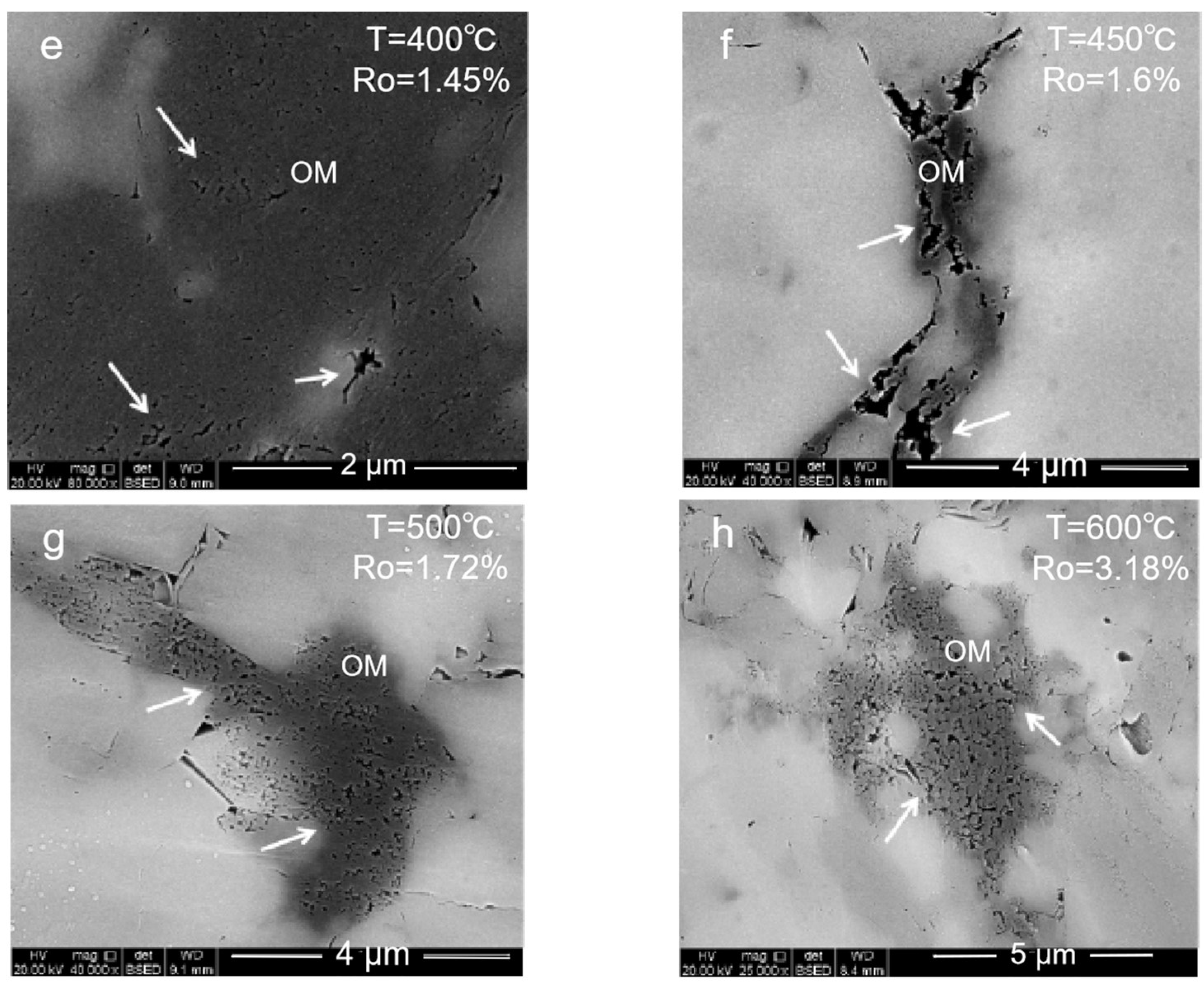
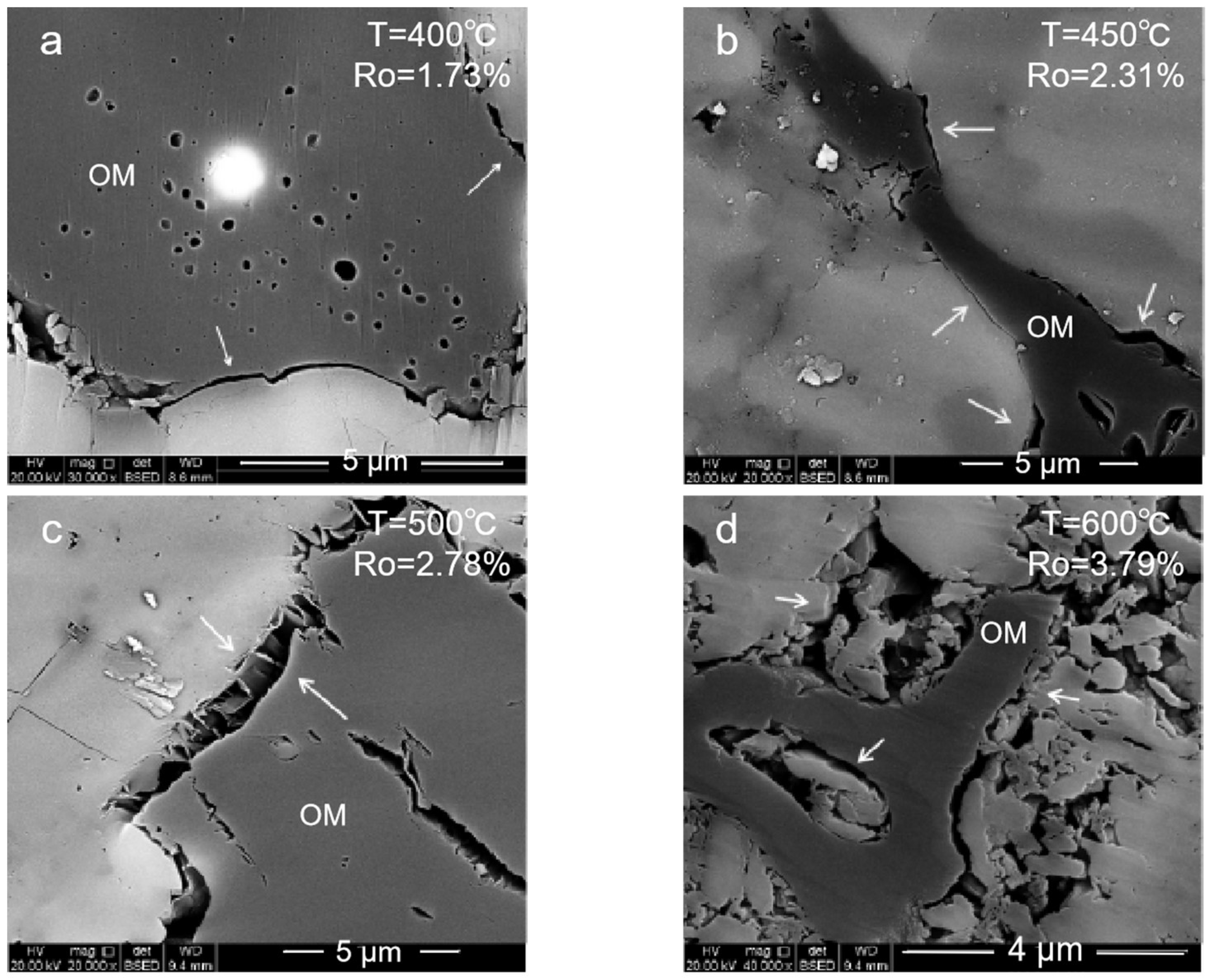
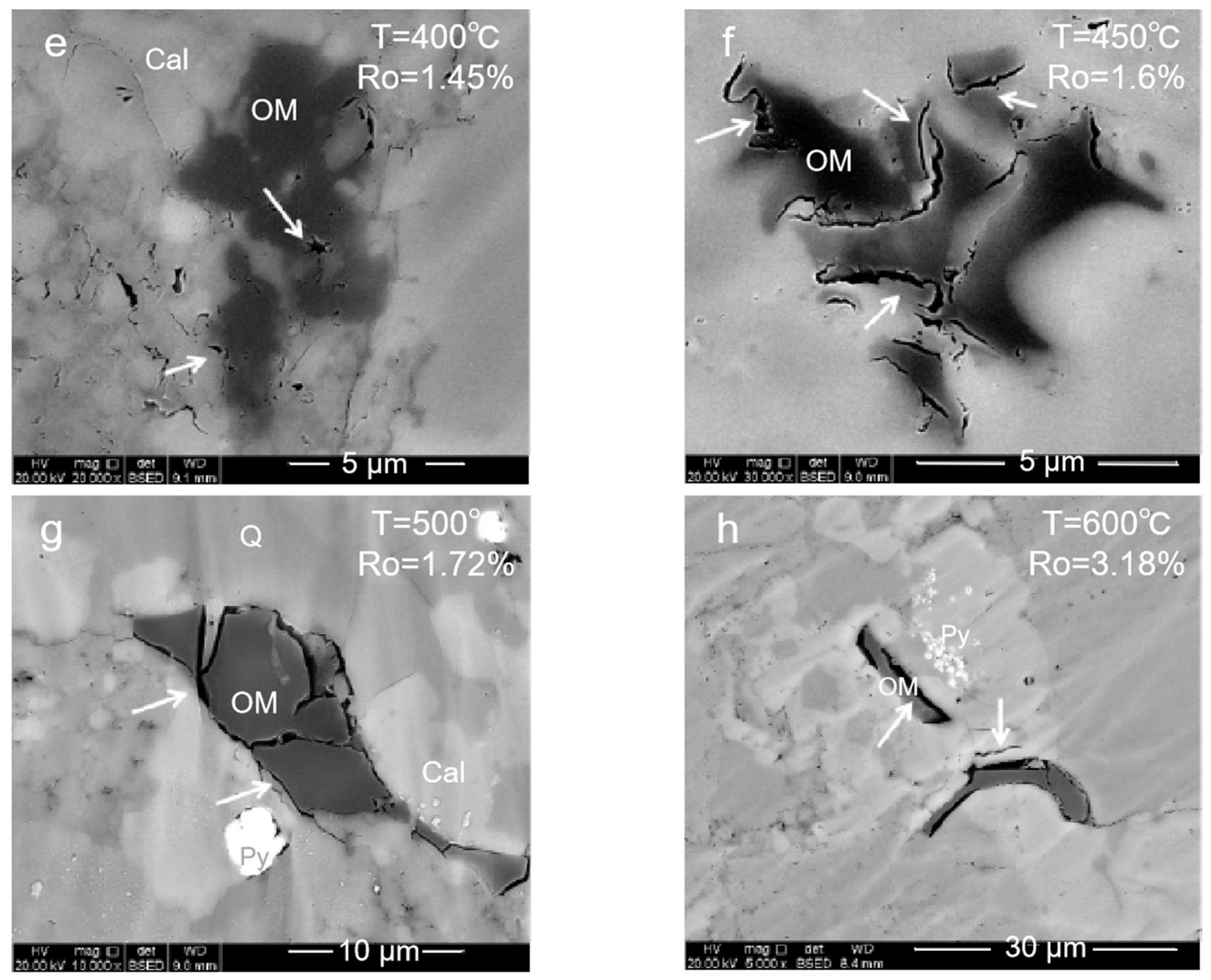
2.1.1. Organic Pore
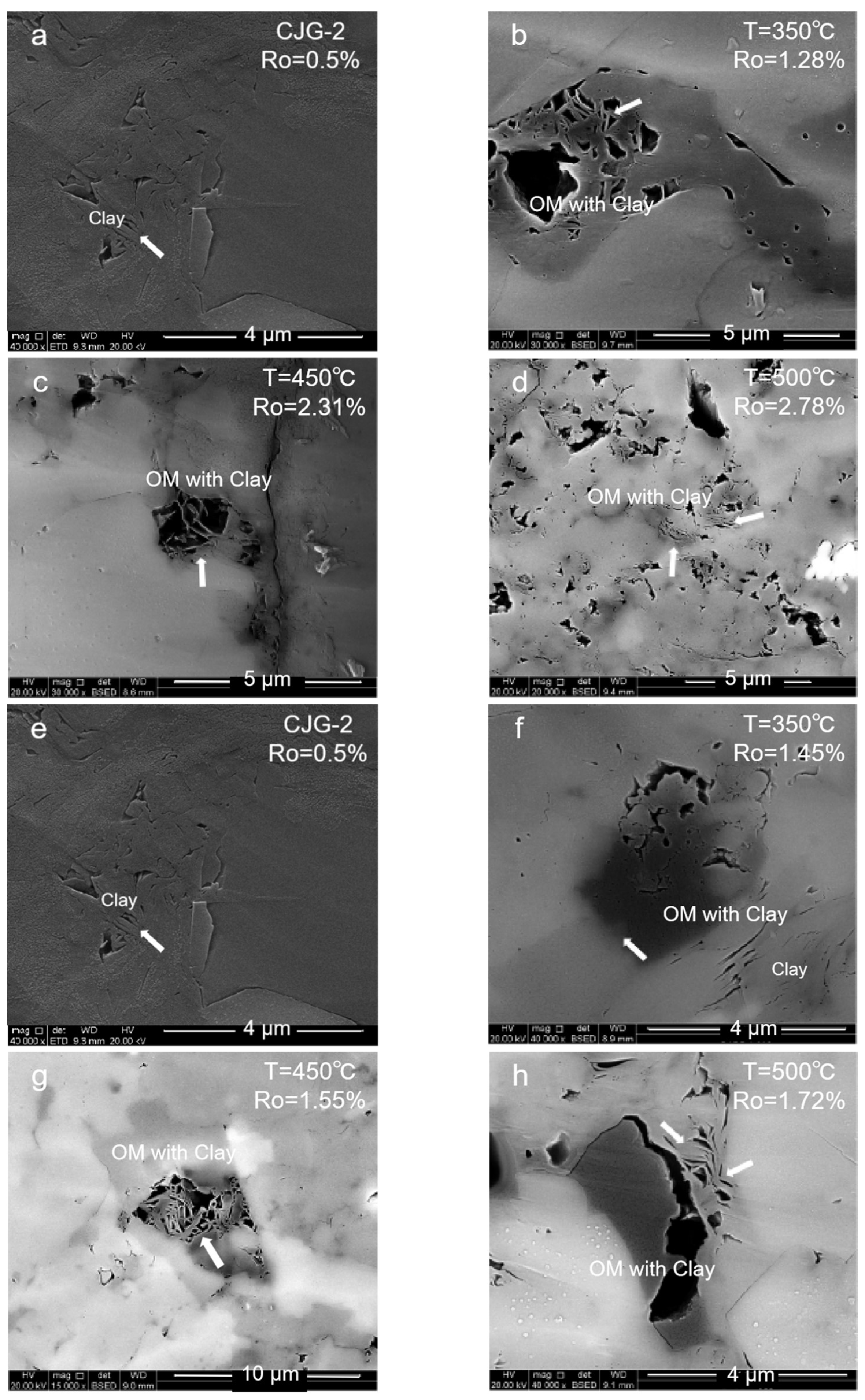
2.1.2. Clay Mineral Pores
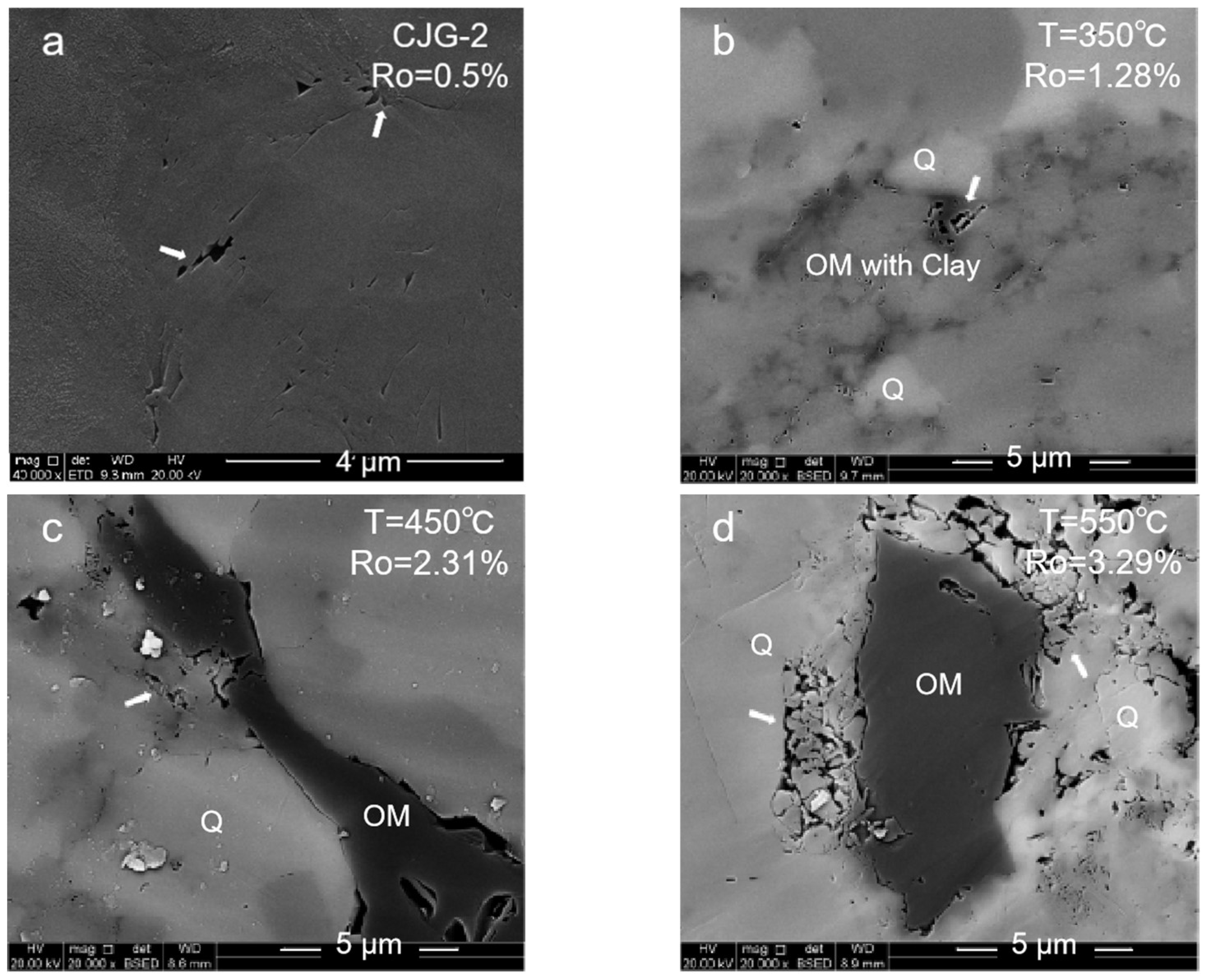
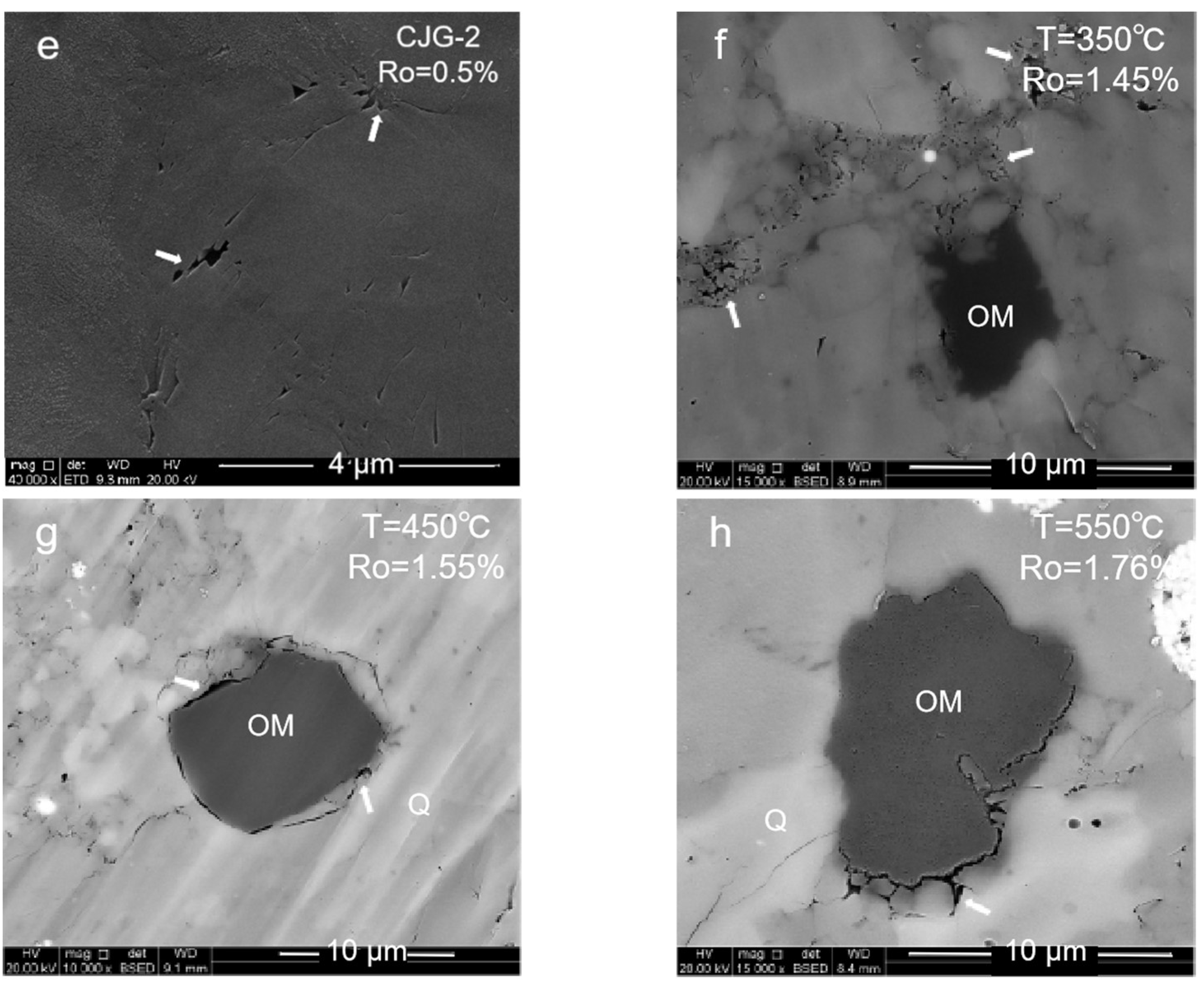
2.1.3. Brittle Mineral Pores
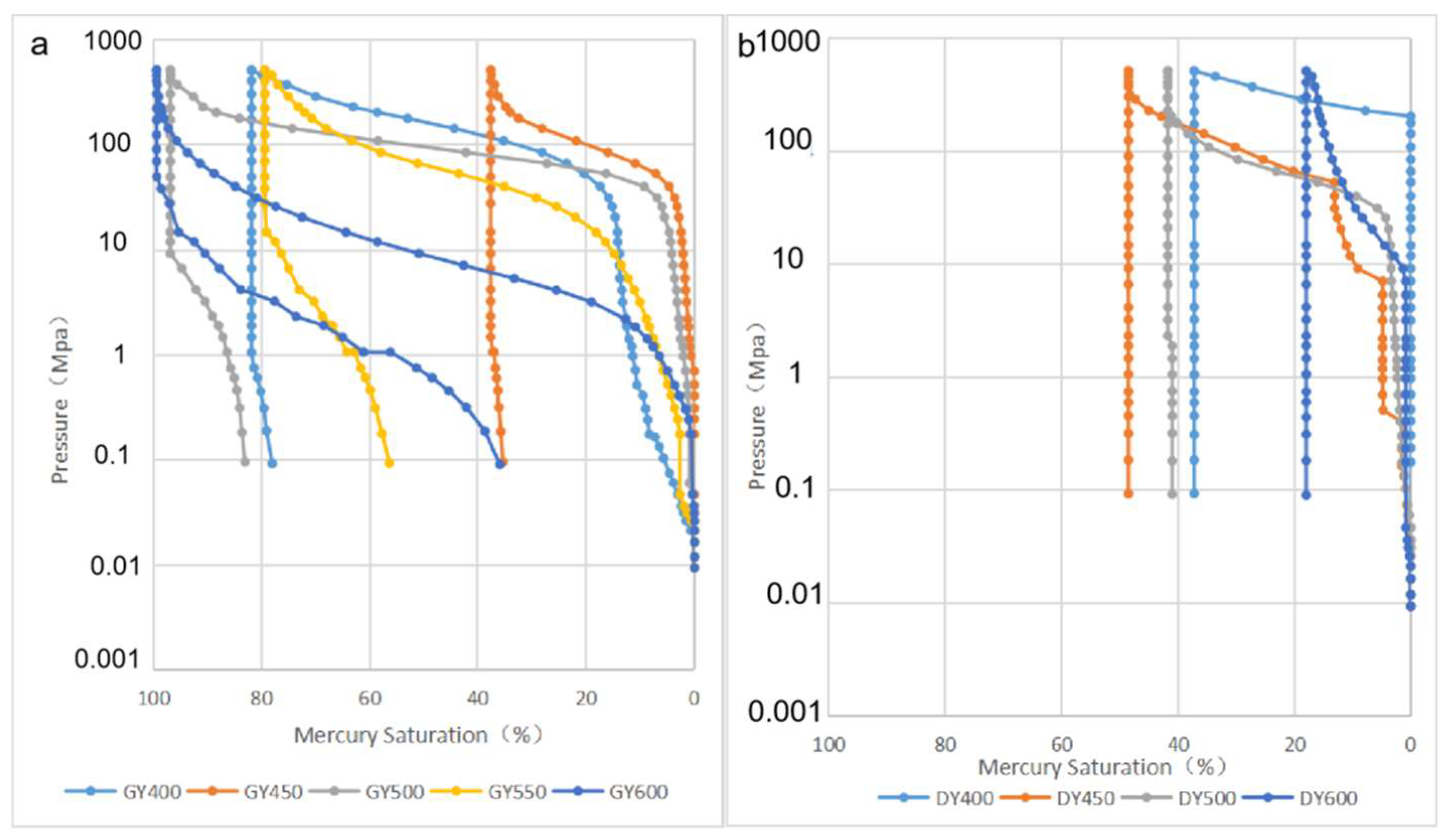
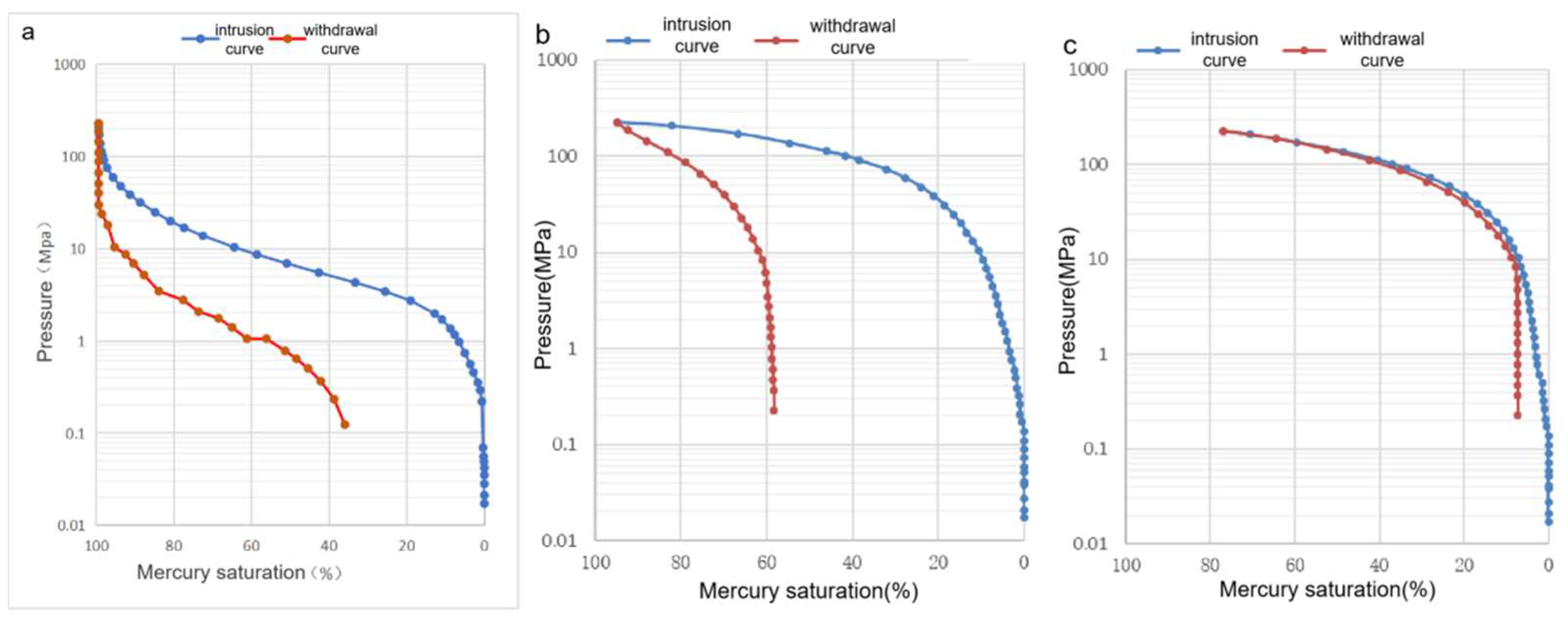
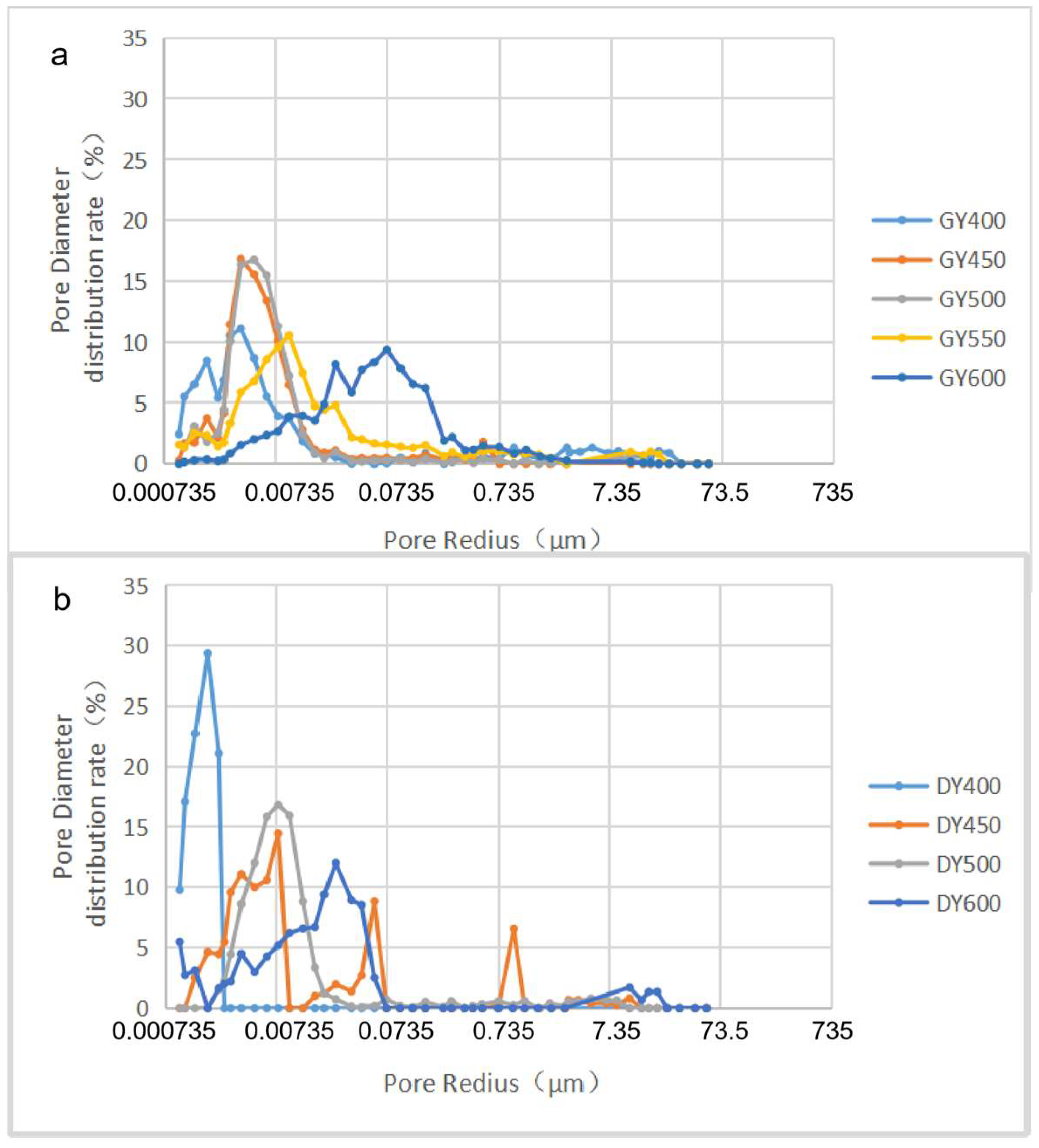
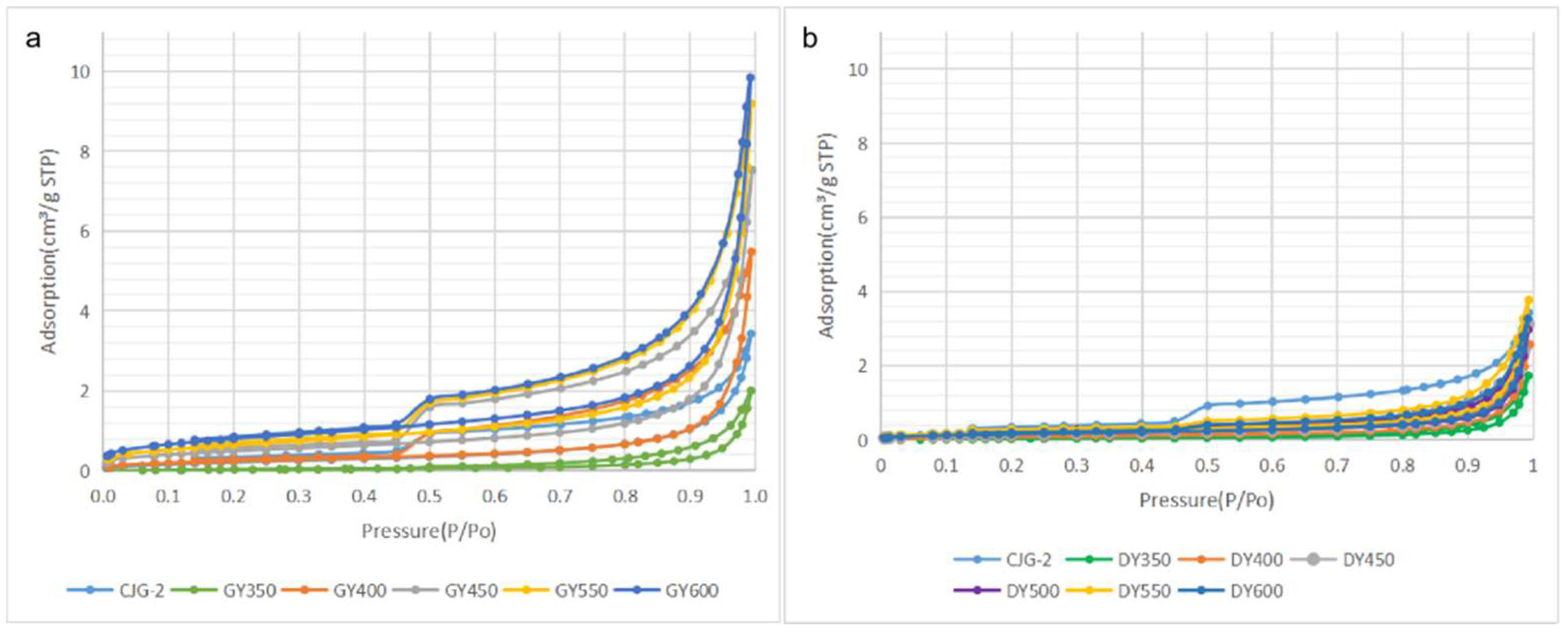
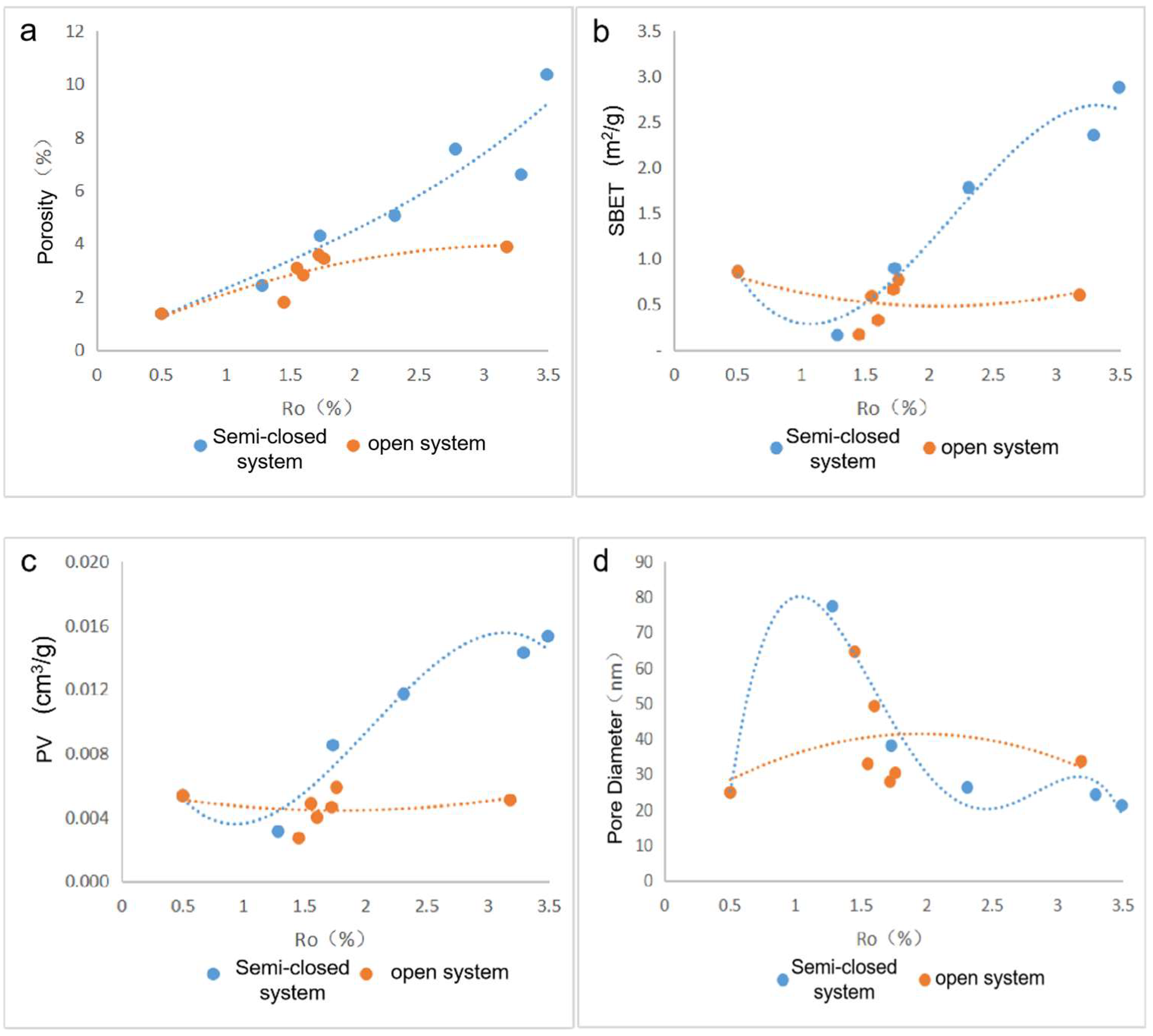
2.2. Pore Structure
2.3. Process of Pore Evolution
- (1)
- Mature stage: At 350–400 °C, the organic matter enters the mature and high-mature stages. The generated hydrocarbon produces a large number of organic pores with a large pore size, and the clay minerals begin to transform into illite. However, in this period, the pores could be filled with liquid hydrocarbons, which will result in pore size reduction and a poor connectivity.
- (2)
- High-mature stage: At 450–500 °C, the organic matter and the liquid hydrocarbons begin to crack to generate gaseous hydrocarbons. A large number of organic pores are generated inside the organic matter. At this stage, the liquid hydrocarbons filled in the pores are cracked, and the blocked pores are reopened. The mineral dissolution pores in the brittleness around the organic matter begin to develop, and the porosity and the connectivity increase.
- (3)
- Over mature stage: At 550–600 °C enters gas generation. The macropores are almost invisible. Mesopores and micropores are developed. These pores are important storage space for the hydrocarbon gases generated in the high-mature stage. Influenced by the strong compaction, the pore closure phenomenon will also occur. All these make the pore types of organic matter in the high-mature stage diversified and the pore structure more complex.
- (1)
- High-mature stage: At 350–550 °C, the organic matter is in a high-maturity stage with Ro ≤ 1.8% due to the insufficient formation pressure in the system. Many primary inorganic pores, such as brittle mineral intergranular pores and clay mineral pores, are observed. With the temperature increase and compaction strengthening, the pore evolution was limited. Only a small number of organic pores and edge cracks was developed.
- (2)
- Overmature stage: At 600 °C, the organic matter maturity enters the overmature stage. The organic matter generates hydrocarbon gas, and a certain formation pressure in the shale promotes the development of the organic matter pores. However, due to excessive compaction, the pore–throat closure is serious, and more ink bottle-shaped pores are formed. The overall pore structure of the organic matter is also poorly developed.
3. Conclusions
- (1)
- The porosity increases nonlinearly with the increase in maturity. The porosity growth rate of the semiclosed system is higher than that of the open system. The semiclosed system mainly develops mesopores, while the open system develops micropores. The macropore development is restricted by the influence of compaction. The key factor influencing the shale pore system evolution is the generation and adjustment of organic pores. The inorganic mineral conversion (kaolinite into illite) contributes less to the pore evolution.
- (2)
- The semiclosed system develops wedge- or V-shaped pores that are open at one or both ends, while the open system develops ink bottle-shaped and closed pores with small throats. The pore openness and the connectivity of the semiclosed system are better than those of the open system.
- (3)
- The semiclosed system is more conducive to the organic pore development than the open system due to the formation pressure. The semiclosed system can reach the overmature stage earlier. The overmature stage is the most important stage for the evolution and development of organic pores.
- (4)
- Using the thermal evolution experiment of the low-maturity shale in Dalong Formation, the evolution process of the high-maturity shale in Longmaxi Formation is forward modeled, guiding the pore evolution of the marine high-maturity shale reservoir in the Sichuan Basin.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chengzao, J.; Min, Z.; Yongfeng, Z. Four important theoretical issue of unconventional petroleum geology. Acta Pet. Sin. 2014, 35, 1–10. [Google Scholar]
- Qun, Z.; Shen, Y.; Hongyan, W.; Xinchun, J.; Dexun, L.; Qinping, S.; Huanrong, Z. China’s Shale Gas Development and its Prospects. Environ. Impact Assess. 2019, 41, 6–10. [Google Scholar]
- Panpan, Z.; Xiaoping, L.; Yajie, W.; Xuejiao, S. Research progress in shale nanopores. Adv. Earth Sci. 2014, 29, 1242–1249. [Google Scholar]
- Qingyan, T.; Mingjie, Z.; Tongwei, Z.; Hui, S.; Yao, L. A Review on Pyrolysis Experimentation on Hydrocarbon Generation. J. Southwest Pet. Univ. 2013, 35, 52–62. [Google Scholar]
- Weilong, P.; Guoyi, H.; Quanyou, L.; Nan, J.; Chenchen, F.; Deyu, G.; Cong, Y.; Yue, L.; Pengwei, W.; Ziqi, F. Research status of thermal simulation experiment and several issues for concerns. Nat. Gas Geosci. 2018, 29, 1252–1263. [Google Scholar]
- Li, W.; Rongze, Y.; Xiaowei, Z.; Wei, G.; Danfeng, L.; Shaoyuan, S. Shale gas development in China and US: Comparison and thinking. Sci. Technol. Rev. 2016, 34, 28–31. [Google Scholar]
- Jianhua, H.; Wenlong, D.; Jinglong, F.; Ang, L.; Peng, D. Study on genetic type of micropore in shale reservoir. Lithol. Reserv. 2014, 26, 30–35. [Google Scholar]
- Chuxiong, L.; Baojian, S.; Anyang, P.; Wentao, Z.; Ang, L.; Jianghui, D. Therma-pressure simulation experiment of pore evolution of Upper Ordovician shale in Baltic Basin. Pet. Geol. Exp. 2020, 42, 434–442. [Google Scholar]
- Chuxiong, L.; Qilin, X.; Qi, C.; Xingchao, J. Evolution characteristics and controls of shale nanopores during thermal maturation of organic matter. Pet. Geol. Exp. 2019, 41, 901–909. [Google Scholar]
- Ming, M.; Guojun, C.; Yong, X.; Shijun, H.; Chengfu, L.; Lianhua, X. Fractal characteristics of pore structure of continental shale in the process of thermal evolution. Coal Geol. Explor. 2017, 45, 41–47. [Google Scholar]
- Dongjun, S.; Jincai, T.; Yetong, W.; Chenjun, W.; Mingfeng, Z. Research Advances on Characteristics of Nanopore Structure of Organic-rich Shales. Acta Sedimentol. Sin. 2019, 37, 1309–1324. [Google Scholar]
- Liang, W.; Haihong, C. A possible mechanism of organic pores evolution in shale: A case from Dalong Formation, Lower Yangtze area. Nat. Gas Geosci. 2016, 39, 520–523. [Google Scholar]
- Chunmei, D.; Cunfei, M.; Guoqiang, L.; Chengyan, L.; Xianguo, Z.; Lihua, R. Pyrolysis Simulation Experiment and Diagenesis Evolution Pattern of Shale. Acta Sedimentol. Sin. 2015, 33, 1053–1061. [Google Scholar]
- Hongjing, L.; Xinong, X.; Zhengliang, L.; Jiaxin, Y.; Lian, Z.; Xiang, X.; Ming, S. Organic matter enrichment of Dalong Formation in Guangyuan area of the Sichuan Basin. Geol. Sci. Technol. Inf. 2009, 28, 98–103. [Google Scholar]
- Xiaodong, F.; Jianzhong, Q.; Tenger; Xiaofang, W. Evaluation on Dalong Formation source rock in the north Sichuan Basin. Pet. Geol. Exp. 2010, 32, 566–571. [Google Scholar]
- Yigang, W.; Yingchu, W.; Haitao, H.; Maolong, X.; Shuyun, S. Dalong Formation found in Kaijiang-Liangping Oceanic through in Sichuan Basin. Nat. Gas Ind. 2006, 26, 32–36. [Google Scholar]
- Maolong, X.; Long, W.; Yigang, W.; Yi, F.; Yingchu, W. High quality source rocks in through facies of Upper Permian Dalong Formation of Sichuan Basin. Pet. Explor. Dev. 2010, 37, 654–662. [Google Scholar]
- Bin, C.; XiongFei, C.; QingLai, F.; Songzhu, G. The Dalong Formation section in Changjianggou, Guangyuan, Sichuan:Sedimentary characteristics and their bearings on the source rocks. Sediment. Geol. Tethyan Geol. 2015, 35, 35–43. [Google Scholar]
- Kanglin, L.; Xichun, W.; Shugen, L.; Jie, L.; Jinxi, L. Sedimentary characteristics of Upper Permian Changxing and Dalong Formation in northwestern Sichuan Basin. Lithol. Reserv. 2011, 23, 30–34. [Google Scholar]
- Lunju, Z.; Jianzhong, Q.; Sheng, H.; Guangyou, L.; Zhiming, L. Preliminary study of formation porosity thermocompression simulation experiment of hydrocarbon generation and expulsion. Pet. Geol. Exp. 2009, 31, 296–302. [Google Scholar]
- Songtao, W.; Rukai, Z.; Jinggang, C.; Jingwei, C.; Bin, B.; Xiangxiang, Z.; Xu, J.; Desheng, Z.; Jianchang, Y.; Xiaohong, L. Characteristics of lacustrine shale porosity evolution, Triassic Chang 7 Member, Ordos Basin, NW China. Pet. Expluration Dev. 2015, 42, 167–176. [Google Scholar]
- Qun, W.; Jinning, P. Burial and thermal histories of northeastern Sichuan Basin: A case study of well Puguang 2. Pet. Geol. Exp. 2013, 35, 133–138. [Google Scholar]
- Chuan-Qing, Z.; Song, R.; Yu-Song, Y. Thermal evolution of the main Paleozoic shale rocks in the Southeastern Sichuan Basin. J. China Coal Soc. 2013, 38, 834–839. [Google Scholar]
- Liu, B.; Schieber, J.; Mastalerz, M. Combined SEM and reflected light petrography of organic matter in the New Albany Shale (Devonian-Mississippian) in the Illinois Basin: A perspective on organic pore development with thermal maturation. Int. J. Coal Geol. 2017, 184, 57–72. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, X. Evolution of nanoporosity in organic-rich shales during thermal maturation. Fuel 2014, 129, 173–181. [Google Scholar] [CrossRef]
- Löhr, S.C.; Baruch, E.T.; Hall, P.A. Is organic pore development in gas shales influenced by the primary porosity and structure of thermally immature organic matter? Org. Geochem. 2015, 87, 119–132. [Google Scholar] [CrossRef]
- Zhuoheng, C.; Shuai, Y.; Wang, N.; Stright, L. A Reassessmeng of Gas Resources in Selected Upper Cretaceous Biogenic Gas Accumulations in Southeastern Alberta and Southwestern Saskatchewan, Canada. Bull. Can. Pet. Geol. 2015, 63, 5–19. [Google Scholar]
- Wenping, L.; Chenglin, Z.; Guidong, G.; Chao, L.; Wei, W.; Xuewen, S.; Jian, Z.; Wuguang, L.; Xiaohang, D.; Xiaohua, H. Controlling factors and evolution laws of shale porosity in Longmaxi Formation, Sichuan Basin. Acta Pet. Sin. 2017, 38, 175–184. [Google Scholar]
- Bingsong, Y. Classification and characterization of gas shale pore system. Earth Sci. Front. 2013, 20, 211–220. [Google Scholar]
- Ko, L.T.; Loucks, R.G.; Zhang, t.; Ruppel, S.C.; Shao, d. Pore and pore network evolution of Upper Cretaceous Boquillas (Eagle Ford–equivalent) mudrocks: Results from gold tube pyrolysis experiments. AAPG Bull. 2016, 100, 1693–1722. [Google Scholar] [CrossRef]
- Ko, L.T.; Ruppel, S.C.; Loucks, R.G.; Hackley, P.C.; Zhang, t. Pore-types and pore-network evolution in Upper Devonian-Lower Mississippian Woodford and Mississippian Barnett mudstones: Insights from laboratory thermal maturation and organic petrology. Int. J. Coal Geol. 2018, 190, 3–28. [Google Scholar] [CrossRef]
- Wenbin, L.; Zhongliang, M.; Lunju, Z.; Jingqiang, T.; Zhanghu, W.; Chuanxiang, N. Evolution of pore structure during diagenesis and htdrocarbon generation of marine shale: The inspiration from thermal simulation experiment. Acta Pet. Sin. 2020, 41, 540–552. [Google Scholar]
- Lei, X.; Zhuo, L.; Youdong, Y.; Ling, T.; Chengxiang, W.; Zhikai, L.; Hailong, Y.; Yufei, H.; Liwei, W. Pore Evolution Model and Influencing Factors of Continental Shale in Yanchang Formation, Ordos Basin. Sci. Technol. Eng. 2020, 20, 8122–8132. [Google Scholar]
- Jianfa, W.; Juan, W.; Wenping, L.; Zheng, Z.; Chao, L.; Wei, W.; Xiaojia, L.; Bin, D. Stage division of shale gas accumulation process: An example from the Wufeng Formation-Longmaxi Formation shale gas reservoir in the Ningxi area of the Sichuan Basin. Nat. Gas Ind. 2021, 41, 83–92. [Google Scholar]
- Haiyan, H. Porosity evolution of the organic-rich shale with thermal maturity increasing. Acta Pet. Sin. 2013, 34, 820–825. [Google Scholar]
- Han, Y.; Horsfield, B.; Wirth, R. Oil retention and porosity evolution in organic-rich shales. AAPG Bull. 2017, 101, 807–827. [Google Scholar] [CrossRef]
- Mathia, E.J.; Bowen, L.; Thomas, K.M.; Aplin, A.C. Evolution of porosity and pore types in organic-rich, calcareous, Lower Toarcian Posidonia Shale. Mar. Pet. Geol. 2016, 75, 117–139. [Google Scholar] [CrossRef] [Green Version]
- Yuwen, C.; Shuichang, Z.; Kun, H.E.; Jingkui, M.; Wenlong, Z.; Xiaomei, W.; Huajian, W. Effects of inorganic minerals in source rocks on hydrocarbon generation from organic matter. Pet. Geol. Exp. 2017, 39, 253–260. [Google Scholar]
- Jingang, H.; Yong, H.; Shu-Heng, T. The Pore Structure Evolution of Black Shale in Western Hunan-Hubei Provinces Based on Pyrolysis Simulation Experiment. Sci. Technol. Eng. 2020, 20, 4306–4315. [Google Scholar]
- Jian-Bing, X.; Yi, Z.; Xin, Z.; Hu, L.; Zhiwei, W.; Bin, C.; Shouzhi, H.; Zewen, L. Evolution characteristics of the residual kerogens in shales during thermal evolution. Geochimica 2019, 48, 521–532. [Google Scholar]
- Hao, X.; Wen, Z.; Rui, Z.; Shimin, L.; Qiumei, Z. Characterizations of pore, mineral and petrographic properties of marine shale using multiple techniques and their implications on gas storage capability for Sichuan Longmaxi gas shale field in China. Fuel 2019, 241, 360–371. [Google Scholar]
- Hao, X.; Wen, Z.; Qinhong, H.; Xianghua, X.; Cong, Z.; Haotian, Z. Fluid distribution and gas adsorption behaviors in over-mature shales insouthern China. Mar. Pet. Geol. 2019, 109, 223–232. [Google Scholar]

| Number | Mineral Content (%) | Ro (%) | TOC (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartz (Q) | Plagioclase | Calcite (Cal) | Dolomite | Pyrite (Py) | Clay | Imon Mixed Layer | Illite | |||
| CJG-2 | 34 | 4 | 24 | 4 | 4 | 30 | 69 | 31 | 0.5 | 9.14 |
| Number | Temperature | Formation Pressure | Static Rock Pressure | Ro | TOC | |
|---|---|---|---|---|---|---|
| °C | MPa | MPa | % | % | ||
| Group 1 | CJGGY-1-350 | 350 | 37 | 89 | 1.28 | 1.41 |
| CJGGY-1-400 | 400 | 50 | 125 | 1.73 | 1.4 | |
| CJGGY-1-450 | 450 | 59 | 148 | 2.31 | 1.24 | |
| CJGGY-1-500 | 500 | 61 | 153 | 2.78 | 1.11 | |
| CJGGY-1-550 | 550 | 71 | 176 | 3.29 | 1.15 | |
| CJGGY-1-600 | 600 | 79 | 196 | 3.49 | 1.08 | |
| Group 2 | DYDB-1-350 | 350 | - | 89 | 1.45 | 0.99 |
| DYDB-1-400 | 400 | - | 125 | 1.6 | 0.95 | |
| DYDB-1-450 | 450 | - | 148 | 1.55 | 0.94 | |
| DYDB-1-500 | 500 | - | 153 | 1.72 | 1.01 | |
| DYDB-1-550 | 550 | - | 176 | 1.76 | 0.98 | |
| DYDB-1-600 | 600 | - | 196 | 3.18 | 1.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhou, W.; Xu, H.; Chen, W.; Jiang, K. Pore Evolution Characteristics of Marine Organic-Rich Shale Based on a Pyrolysis Simulation Experiment. Minerals 2022, 12, 1098. https://doi.org/10.3390/min12091098
Zhao X, Zhou W, Xu H, Chen W, Jiang K. Pore Evolution Characteristics of Marine Organic-Rich Shale Based on a Pyrolysis Simulation Experiment. Minerals. 2022; 12(9):1098. https://doi.org/10.3390/min12091098
Chicago/Turabian StyleZhao, Xin, Wen Zhou, Hao Xu, Wenling Chen, and Ke Jiang. 2022. "Pore Evolution Characteristics of Marine Organic-Rich Shale Based on a Pyrolysis Simulation Experiment" Minerals 12, no. 9: 1098. https://doi.org/10.3390/min12091098





