Recovery of Hematite from Banded Hematite Quartzite of Southern India by Magnetic Separation and Reverse Flotation
Abstract
:1. Introduction
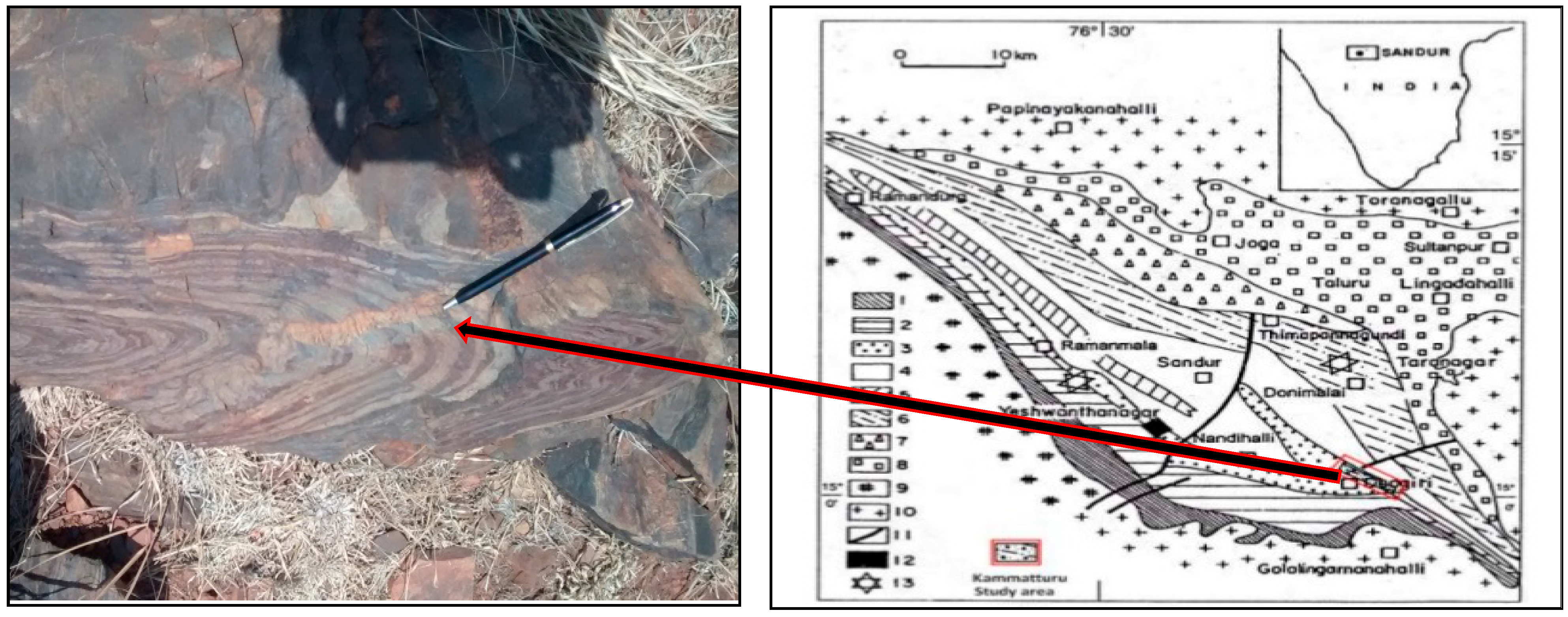
2. Materials
Methods
3. Results and Discussion
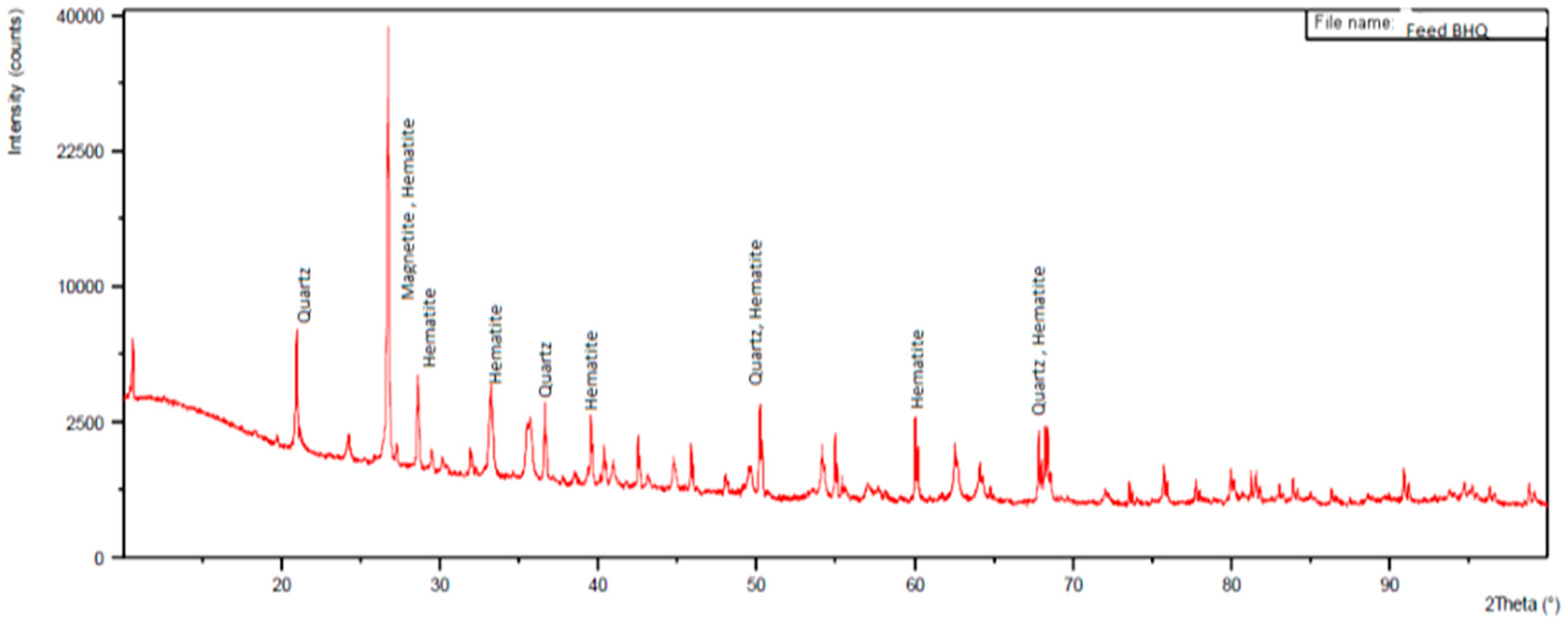
3.1. Feed Material Characterization
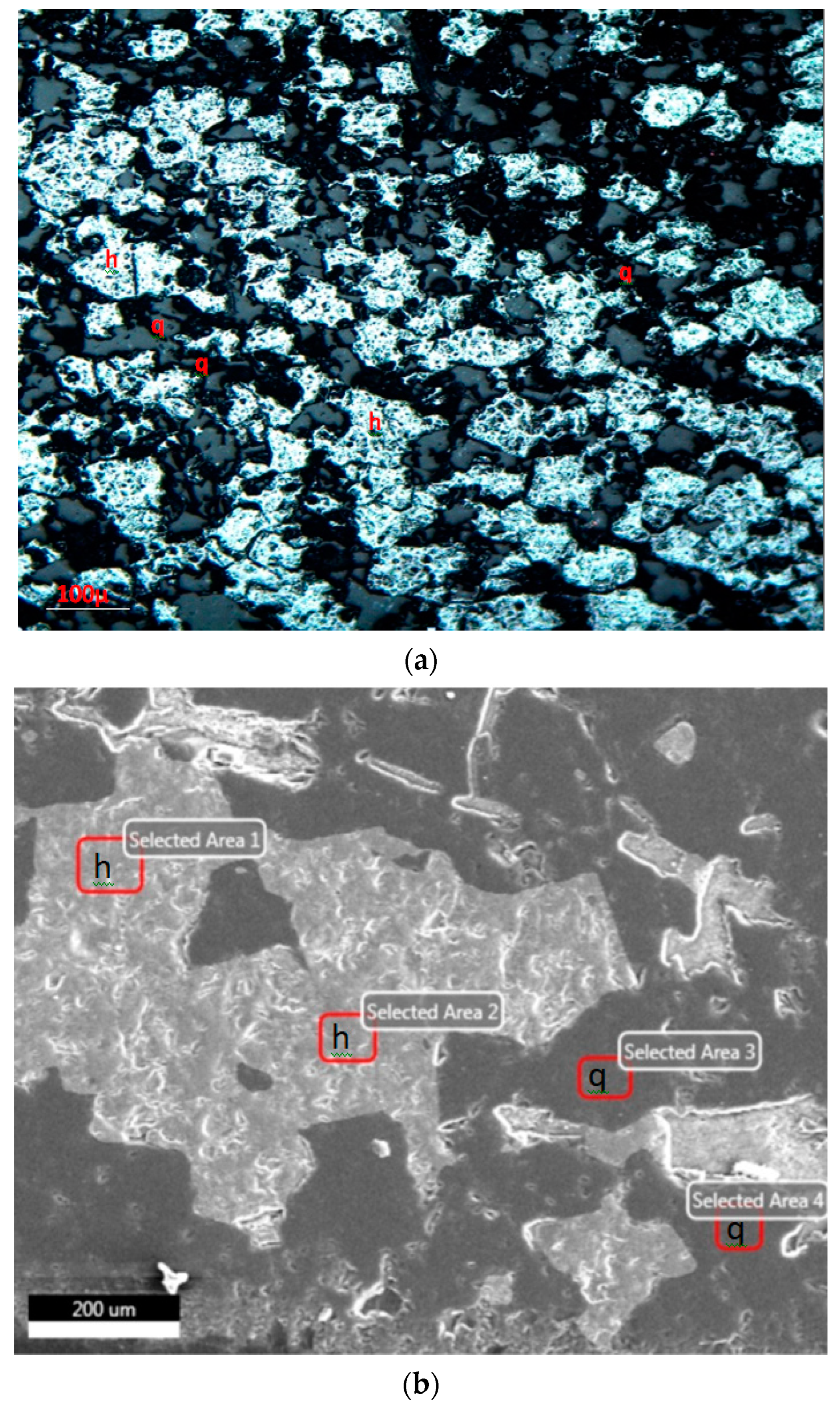
3.2. Mineralogical Studies
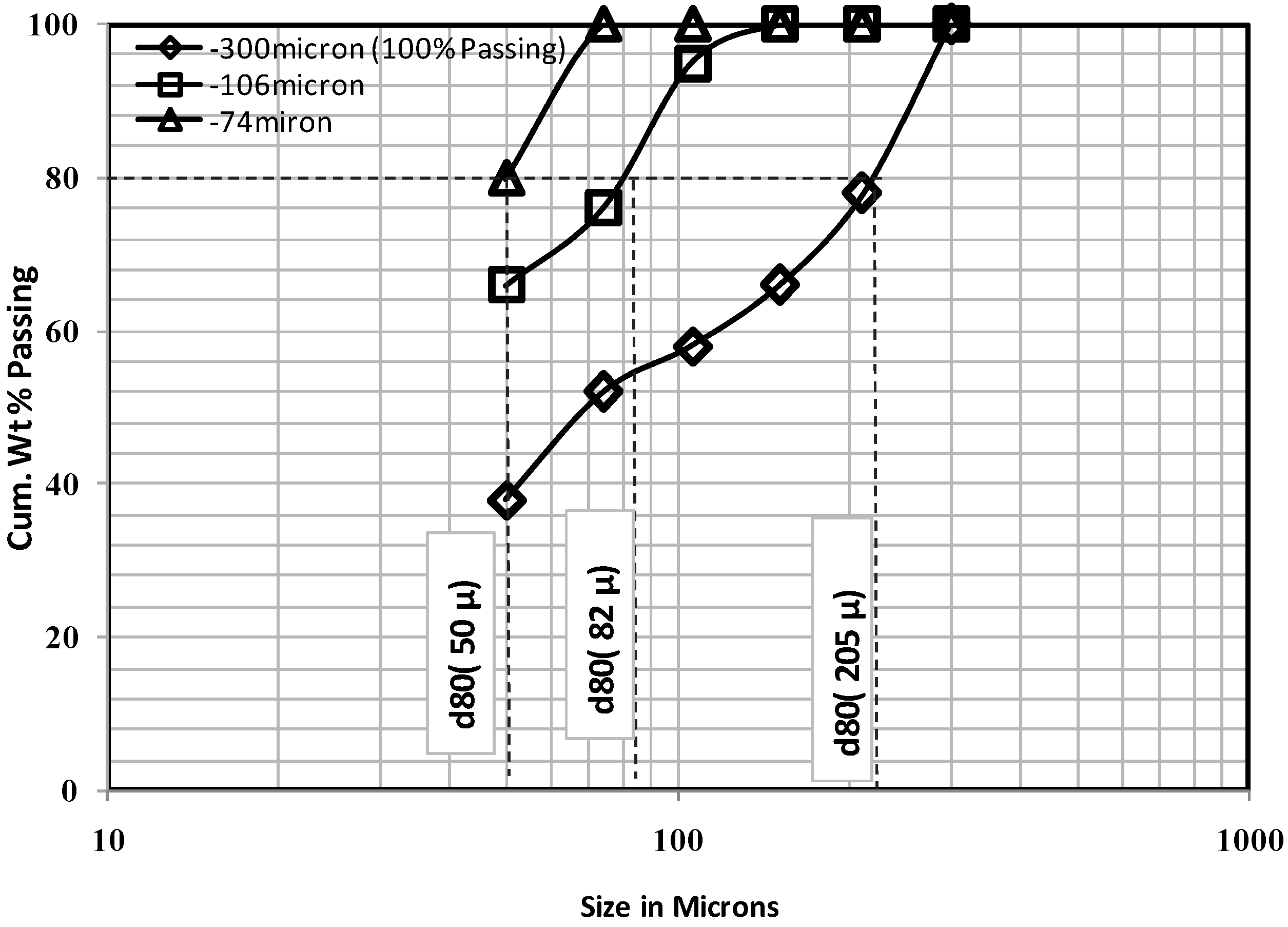
3.3. Process Diagnostic Amenability Tests
3.4. Wet High-Intensity Magnetic Separation Tests
3.5. Reverse Flotation Tests
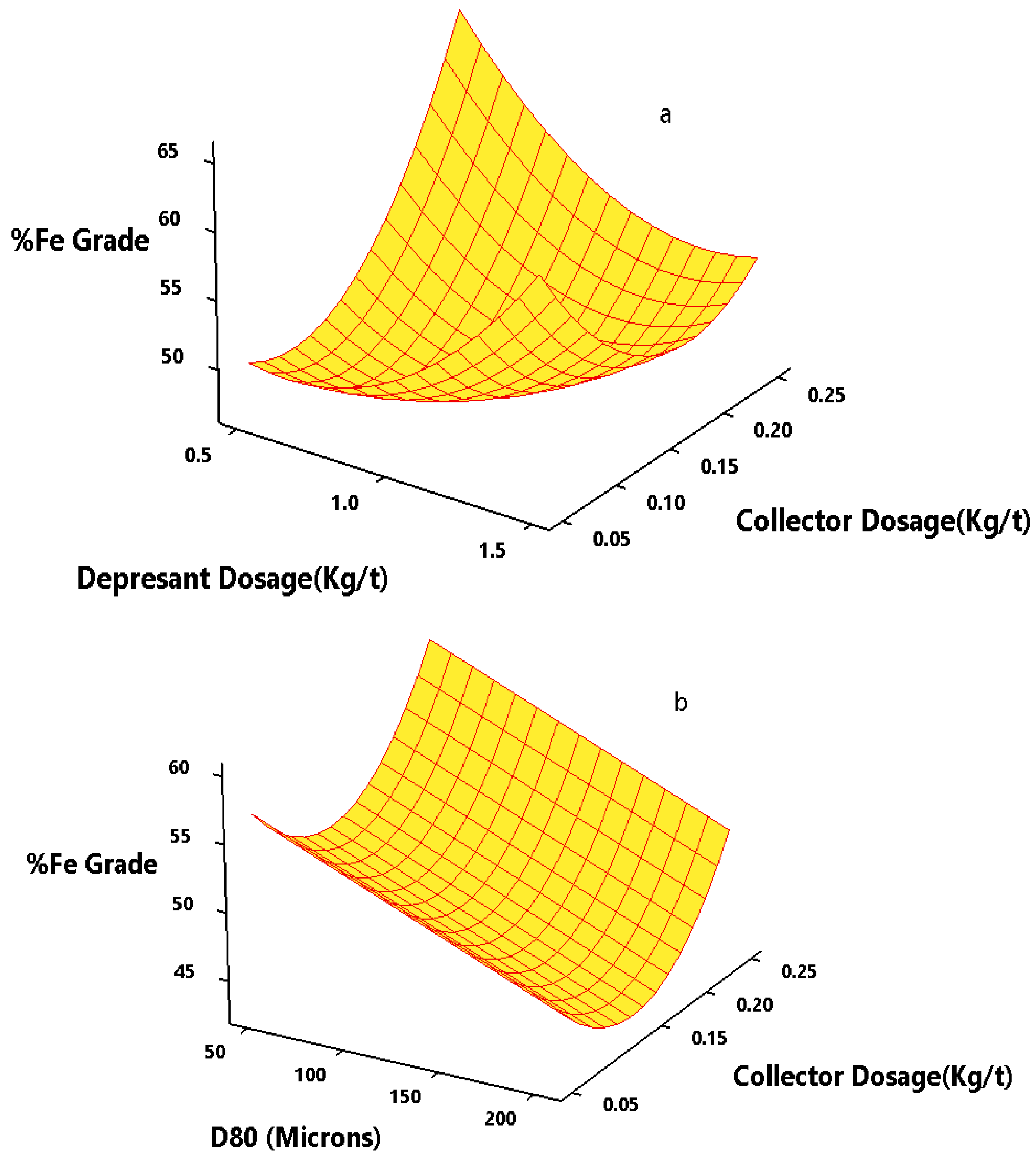
3.5.1. Effect of Flotation Variables on Grade (%Fe) of Concentrate
3.5.2. Effect of Flotation Variables on Recovery (%Fe) of Concentrate
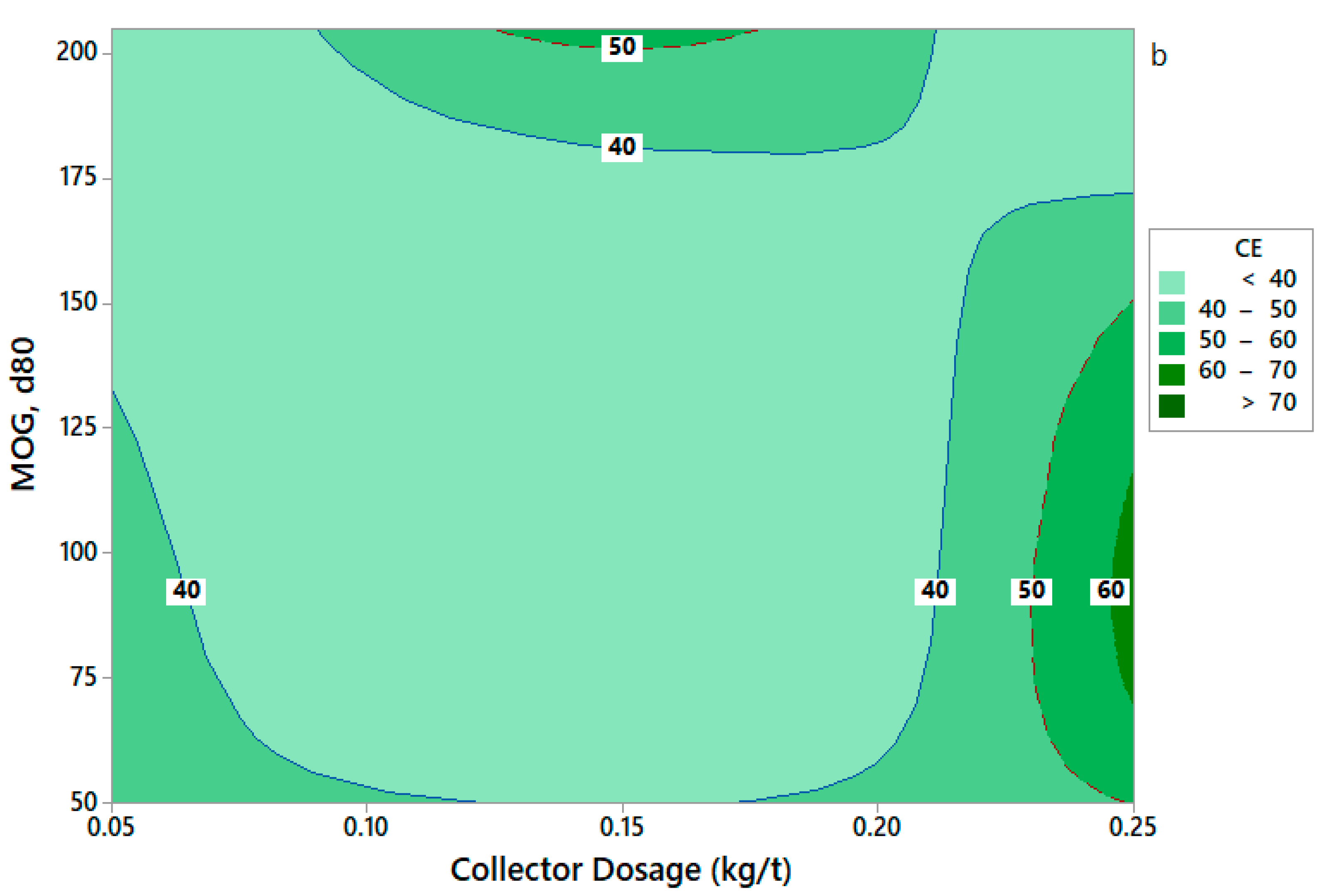
3.5.3. Effect of Flotation Variables on Concentration Efficiency (CE)
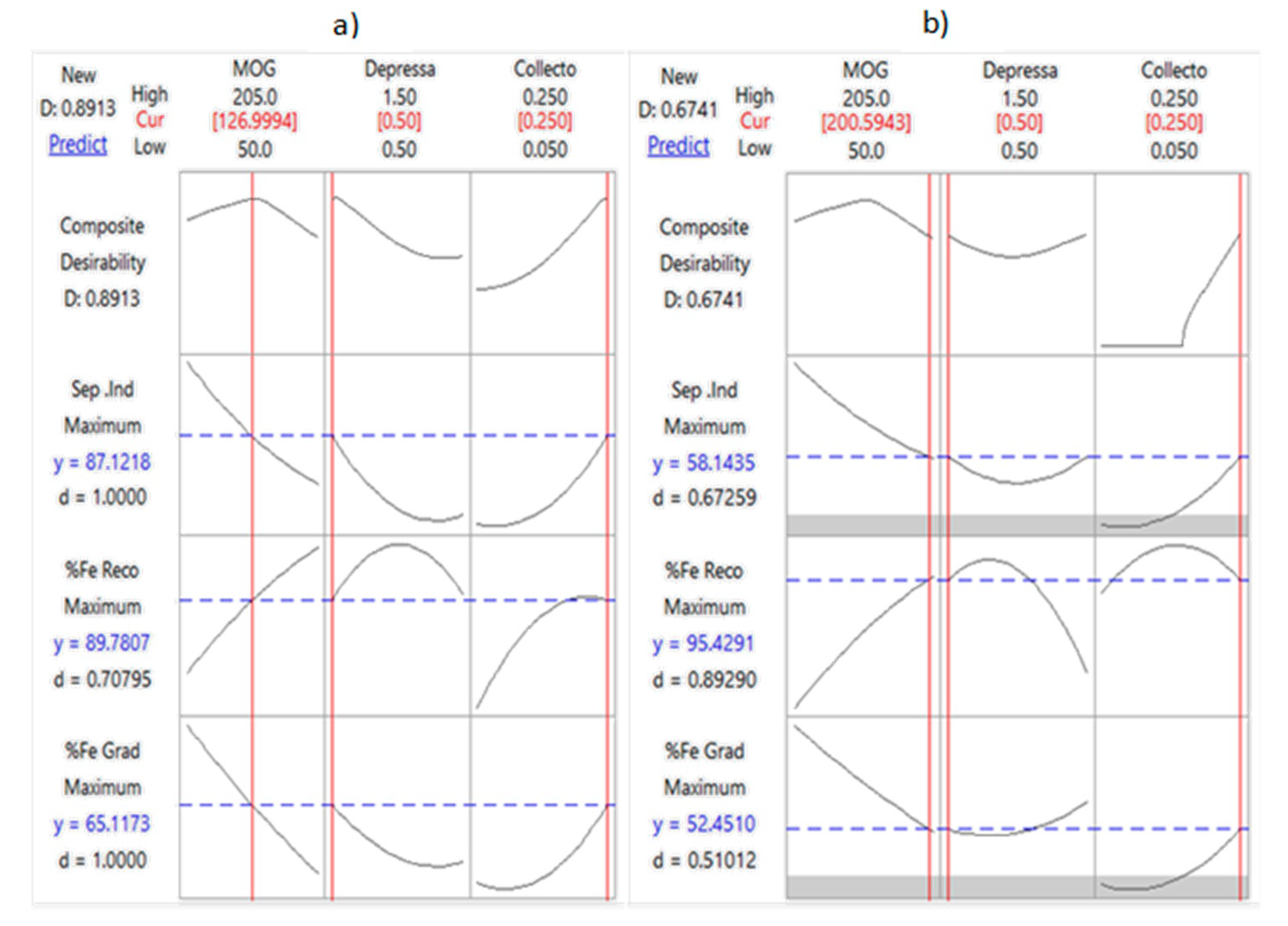
3.5.4. Optimization Studies
- a.
- Obtain the individual desirability (d) for each response;
- b.
- Combine the individual desirability to obtain the combined or composite desirability (D);
- c.
- Maximize the composite desirability and identify the optimal input variable settings.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Swamy, K.; Suresh, N.; Kumar, P.S. Resource Management and Utilization of Lean Grade Iron Ore Resources of Karnataka, India. J. Mines Met. Fuels 2022, 70, 233–241. [Google Scholar] [CrossRef]
- IBM. Iron and Steel Vision 2020; Indian Bureau of Mines: Nagpur, India, 2011. [Google Scholar]
- IBM. Monograph on Iron Ore; Indian Bureau of Mines: Nagpur, India, 1997. [Google Scholar]
- Raj, B. Development of a Process flow sheet for Beneficiation of Indian Banded Hematite Quartz (BHQ) Iron Ore. In Proceedings of the Iron Ore 2007 Conference Perth: AusIMM, Perth, Australia, 20–22 August 2007; pp. 375–380. [Google Scholar]
- Anupam, A.; Singh, G.; Raghav, P.K.; Suresh, N. Studies on beneficiation of BHQ samples by different methods. In Proceedings of the XI International Seminar on Mineral Processing Technology (MPT-2010), Jamshedpur, India, 15–17 December 2010; p. 572. [Google Scholar]
- Das, B.; Rath, S.S.; Reddy, P.S.; Das, S.K.; Mishra, B.K. The flotation response of Banded Iron Ore of Karnataka Region, India. In Proceedings of the 26th International Mineral Processing Congress (IMPC 2012), New Delhi, India, 24–28 September 2012; pp. 1030–1040. [Google Scholar]
- Mohan Rao, A.; Rudramuniyappa, M.V.; Hiremath, M.S.; Naganoor, P.C. Flotation studies on banded hematite quartzite from Sandur Schist belt, Bellary district, Karnataka. In Proceedings of the 26th International Mineral Processing Congress (IMPC 2012), New Delhi, India, 24–28 September 2012; pp. 34–52. [Google Scholar]
- Gurulakshmi, S.N.; Ghosh, T.K.; Mukherjee, A.K. State of Art Characterization Study of Banded Hematite Jasper for Beneficiation. In Proceedings of the XI International Seminar on Mineral Processing Technology (MPT-2010), Jamshedpur, India, 15–17 December 2010; p. 440. [Google Scholar]
- Gurulakshmi, S.N.; John, S.T.; Dixit, T. Flotation studies on Banded Hematite Jasper iron ores. In Proceedings of the 26th International Mineral Processing Congress (IMPC 2012), New Delhi, India, 24–28 September 2012; p. 5181. [Google Scholar]
- Nanda, N.K.; Gopalkrishna, S.J. Development of Process for Beneficiation of low-grade iron ore fins from Karnataka. J. Mines Met. Fuels 2015, 5–9. [Google Scholar]
- IBM. Chemical Analysis of Ores Samples; Indian Bureau of Mines Nagpur: Nagpur, India, 2011. [Google Scholar]
- Menasinakai, V.; Pujar, G.S.; Swami, M.; Asode, A.N. Mineralogy of Banded Iron Formations in and around Kammatturu, Sandur Schist Belt, Karnataka. 2014. Available online: www.semanticscholar.org (accessed on 25 March 2021).
- Krishna, S.J.; Patil, M.R.; Rudrappa, C.; Kumar, S.P.; Ravi, B.P. Characterization and processing of some Iron ores of Indium. J. Inst. Eng. Sec D 2013, 113–120. [Google Scholar] [CrossRef]
- Mishra, B.K.; Reddy, P.S.; Das, B.; Biswal, S.K.; Prakash, S.; Das, S.K. Issues Relating to Characterization and Beneficiation of Low-Grade Iron Ore Fines. December 2007. Available online: www.worldsteel.org (accessed on 15 April 2022).
- Somasundaran, P. Adsorption of starch and oleate and interaction between them on calcite in aqueous solutions. J. Colloid Interface Sci. 1969, 31, 557–565. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippov, I.V.; Severov, V.V. New technologies for producing hematite concentrate from wastes generated in the processing of iron quartzite. Metallurgist 2010, 54, 268–272. [Google Scholar] [CrossRef]
- Somasundaran, P.; Cleverdon, J. A study of polymer/surfactant interaction at Mineral solution interface. Colloids Surf. 1985, 13, 73–85. [Google Scholar] [CrossRef]
- Houot, R. Beneficiation of iron ore by flotation- review of industrial and potential applications. Int. J. Miner. Processing 1983, 10, 183–204. [Google Scholar] [CrossRef]
- Nummela, W.; Iwasaki, I. Iron ore Flotation. In Advances in Mineral Processing; Somasundaran, P., Ed.; AIME: Mumbai, India, 1986; pp. 308–342. [Google Scholar]
- Wills, B.A.; Napier Munn, T.J. Mineral Processing Technology—An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2006; p. 18. [Google Scholar]
- Pinto, C.L.L.; Araujo, A.C.; Peres, A.E.C. The effect of starch, amylose, and amylopectin on the depression of oxi-minerals. Miner. Eng. 1992, 5, 469–478. [Google Scholar] [CrossRef]
- Pradip. Beneficiation of alumina rich Indian iron ores limes-Scientific challenges and techno-economic considerations. Met. Mater. Processes. 1994, 6, 179–194. [Google Scholar]
- Su, F.; Rao, K.H.; Forssberg KS, E.; Samskog, P.O. Influence of temperature on the kinetics of apatite flotation from magnetite fines. Int. J. Miner. Processing 1998, 54, 131–145. [Google Scholar] [CrossRef]
- Bhaskar Raju, G.; Prabhakar, S.; Subba Rao, S. Development of indigenous column flotation technology at NML to beneficiate low-grade ores. Met. Mater. Processes 2004, 16, 143–156. [Google Scholar]
- Kumar, P.S.; Ravi, B.P.; Sreedhar, G.E.; Naganoor, P.C. Modeling and Optimization of Vertical Pulsating High Gradient Magnetic Separator for Iron ore Slime Processing Using Response Surface Methodology. Int. J. Miner. Processing Extr. Metallurgy. 2016, 1, 56–63. [Google Scholar] [CrossRef]
- Araujo, A.C.; Amarante, S.C.; Souza, C.C.; Silva, R.R.R. Ore mineralogy and its relevance for selection of concentration methods in processing of Brazilian iron ores. Trans. IMM 2003, 112, C54–C64. [Google Scholar] [CrossRef]
- Lima, N.P.; Valadão, G.E.; Peres, A.E. Effect of amine and starch dosages on the reverse cationic flotation of iron ore. Miner. Eng. 2013, 45, 180–184. [Google Scholar] [CrossRef]
- Nanda, S.K.; Pani, S.; Beura, D. Recovery of iron values through conventional beneficiation techniques fromBHJ of eastern India with reference to mineralogical and chemical characterization. Ind. J. Sci. Tech. 2020, 13, 3960–3969. [Google Scholar] [CrossRef]
- Ravi, B.P.; Kumar, G.S.; Ananth, B.D.; Hussain, M.I.; Kristappa, M.G. Process Characterization of some Iron ores of India. Indian Mineral. 2013, 25–134. [Google Scholar]
- Nayak, N.; Das, A.; Paul, B.K. Feasibility of beneficiation of BHJ of eastern India. IJERT 2012, 1, 1–11. [Google Scholar]
- Ravishankar, S.A.; Sankar, T.A.P.; Khosla, N.K. Beneficiation studies on alumina–rich Indian iron ore slimes using selective dispersants, flocculants and flotation collectors. In Proceedings of the XVIII International Mineral Processing Congress, Sydney, Australia, 23–28 May 1993; AusIMM: Carlton, Australia; pp. 1289–1294. [Google Scholar]




| Ingredient | Specification in Percentage | |
|---|---|---|
| BF Grade | DRI Grade | |
| Fe | >63.0 | >65.0 |
| SiO2 + Al2O3 | <6.00 | <2.00 |
| S and P | <0.05 (each) | <0.05 |
| (Al2O3/SiO2) Ratio | <0.5 | <0.5 |
| MOG/# D80 µm | Products | Wt% | %Fe | |
|---|---|---|---|---|
| Assay | Distribution | |||
| −50# 205 µm | Mag Conc. | 34.7 | 44.07 | 36.4 |
| Non-Mag tails | 65.3 | 27.10 | 63.6 | |
| Head Cal | 100.0 | 42.01 | 100.0 | |
| 150# 82 µm | Mag Conc. | 56.0 | 45.00 | 63.6 |
| Non-Mag tails | 44.0 | 32.79 | 36.4 | |
| Head Cal | 100.0 | 40.00 | 100.0 | |
| 200# 50 µm | Mag Conc. | 41.0 | 47.30 | 47.3 |
| Non-Mag tails | 59.0 | 36.60 | 52.7 | |
| Head Cal | 100.0 | 41.05 | 100.0 | |
| Variables Varied | Units | Real Values of Coded Levels | ||
|---|---|---|---|---|
| Low (−1) | Centre (0) | High (+1) | ||
| D80, D100 % passing feed sizes | µm | 50, −74 | 82, −106 | 205, –300 |
| Collector dosage | kg/t | 0.05 | 0.15 | 0.25 |
| Depressant dosage | kg/t | 0.5 | 1.0 | 1.5 |
| Run Order | MOG | Depressant Dosage (kg/t) | Collector Dosage (kg/t) | Wt% | %Fe Grade | %Fe Recovery wrt Mag conc | % Concentration EfficiencywrtFeed | |||
|---|---|---|---|---|---|---|---|---|---|---|
| No | A | B | C | Y | Observed | Predicted | Observed | Predicted | Observed | Predicted |
| 1 | 50 | 0.5 | 0.15 | 49.65 | 63.6 | 65.55 | 74.49 | 72.3 | 63.35 | 72.06 |
| 2 | 205 | 0.5 | 0.15 | 83.68 | 49.8 | 37.15 | 98.7 | 97.7 | 37.86 | 24.62 |
| 3 | 50 | 1.5 | 0.15 | 79.24 | 45.5 | 47.23 | 85.4 | 82.4 | 15.51 | 28.75 |
| 4 | 205 | 1.5 | 0.15 | 60.89 | 60.6 | 57.67 | 87.4 | 85.6 | 66.79 | 58.09 |
| 5 | 50 | 1 | 0.05 | 48.69 | 59.1 | 56.95 | 68.16 | 71.8 | 49.05 | 34.47 |
| 6 | 205 | 1 | 0.05 | 87.77 | 45.7 | 47.97 | 95 | 97.4 | 18.23 | 25.59 |
| 7 | 50 | 1 | 0.25 | 39.97 | 63.3 | 59.93 | 92.6 | 86 | 50.29 | 42.93 |
| 8 | 205 | 1 | 0.25 | 43.39 | 49.6 | 50.98 | 96.8 | 89 | 19.11 | 33.70 |
| 9 | 82 | 0.5 | 0.05 | 65.11 | 50.5 | 50.31 | 77.88 | 80.1 | 32.18 | 38.05 |
| 10 | 82 | 1.5 | 0.05 | 49.45 | 63.6 | 63.64 | 74.49 | 79.2 | 63.10 | 64.45 |
| 11 | 82 | 0.5 | 0.25 | 59.15 | 64.6 | 65.49 | 90.5 | 83.1 | 79.01 | 77.66 |
| 12 | 82 | 1.5 | 0.25 | 71.51 | 53.3 | 54.41 | 90.28 | 82.2 | 47.29 | 41.41 |
| 13 | 82 | 1 | 0.15 | 84.53 | 46.8 | 47.42 | 93.7 | 91.6 | 23.11 | 23.11 |
| 14 | 82 | 1 | 0.15 | 84.53 | 46.8 | 47.42 | 93.7 | 91.6 | 23.11 | 23.11 |
| 15 | 82 | 1 | 0.15 | 84.53 | 46.8 | 47.42 | 93.7 | 91.6 | 23.11 | 23.11 |
| Products | Wt% | %Fe | |
|---|---|---|---|
| Assay | Distn. | ||
| Reverse Flotation Conc | 37.4 | 64.60 | 60.4 |
| Silica float | 18.6 | 6.90 | 3.2 |
| WHIMS non-mag | 44.0 | 32.79 | 36.4 |
| Head-cal | 100.0 | 40.00 | 100.0 |
| Fe | 64.60% | SiO2 + Al2O3 | 4.12% |
| SiO2 | 3.12% | Al2O3 to SiO2 ratio | 0.32 |
| Al2O3 | 1.00% | LOI | 1.06% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swamy, A.K.; Nikkam, S.; Palthur, S.K. Recovery of Hematite from Banded Hematite Quartzite of Southern India by Magnetic Separation and Reverse Flotation. Minerals 2022, 12, 1095. https://doi.org/10.3390/min12091095
Swamy AK, Nikkam S, Palthur SK. Recovery of Hematite from Banded Hematite Quartzite of Southern India by Magnetic Separation and Reverse Flotation. Minerals. 2022; 12(9):1095. https://doi.org/10.3390/min12091095
Chicago/Turabian StyleSwamy, Aspari Kumara, Suresh Nikkam, and Sharath Kumar Palthur. 2022. "Recovery of Hematite from Banded Hematite Quartzite of Southern India by Magnetic Separation and Reverse Flotation" Minerals 12, no. 9: 1095. https://doi.org/10.3390/min12091095






