Synergistic Effect of Frequently Found Ions in the Flotation of Pb-Zn Sulfide Ores on Air/Water Interface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dynamic Surface Tension Measurements
2.2. Bubble Coalescence Time Measurements
2.3. Measurements of Sauter Mean Diameter (SMD) and Bubbles Size Distribution (BSD)
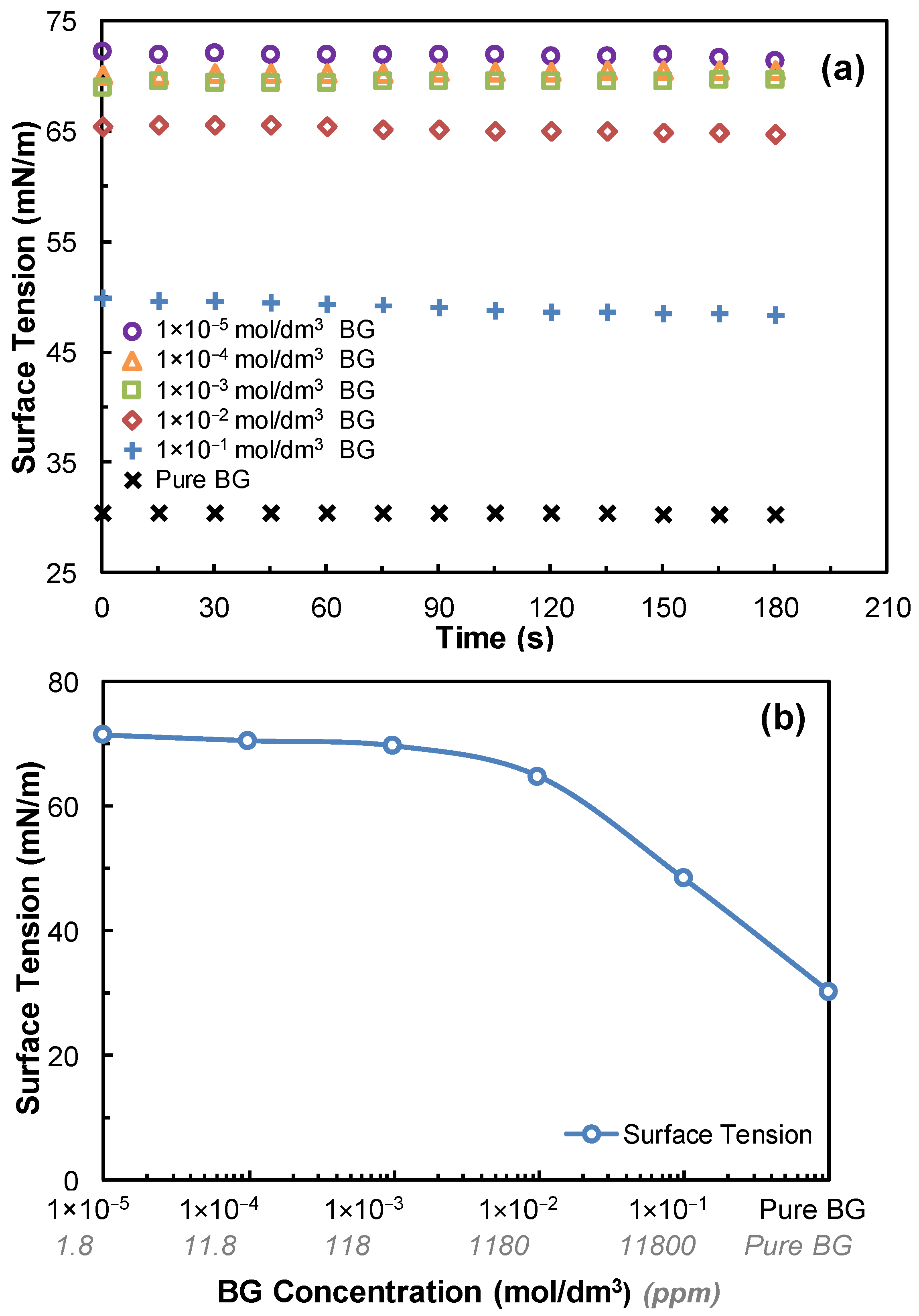
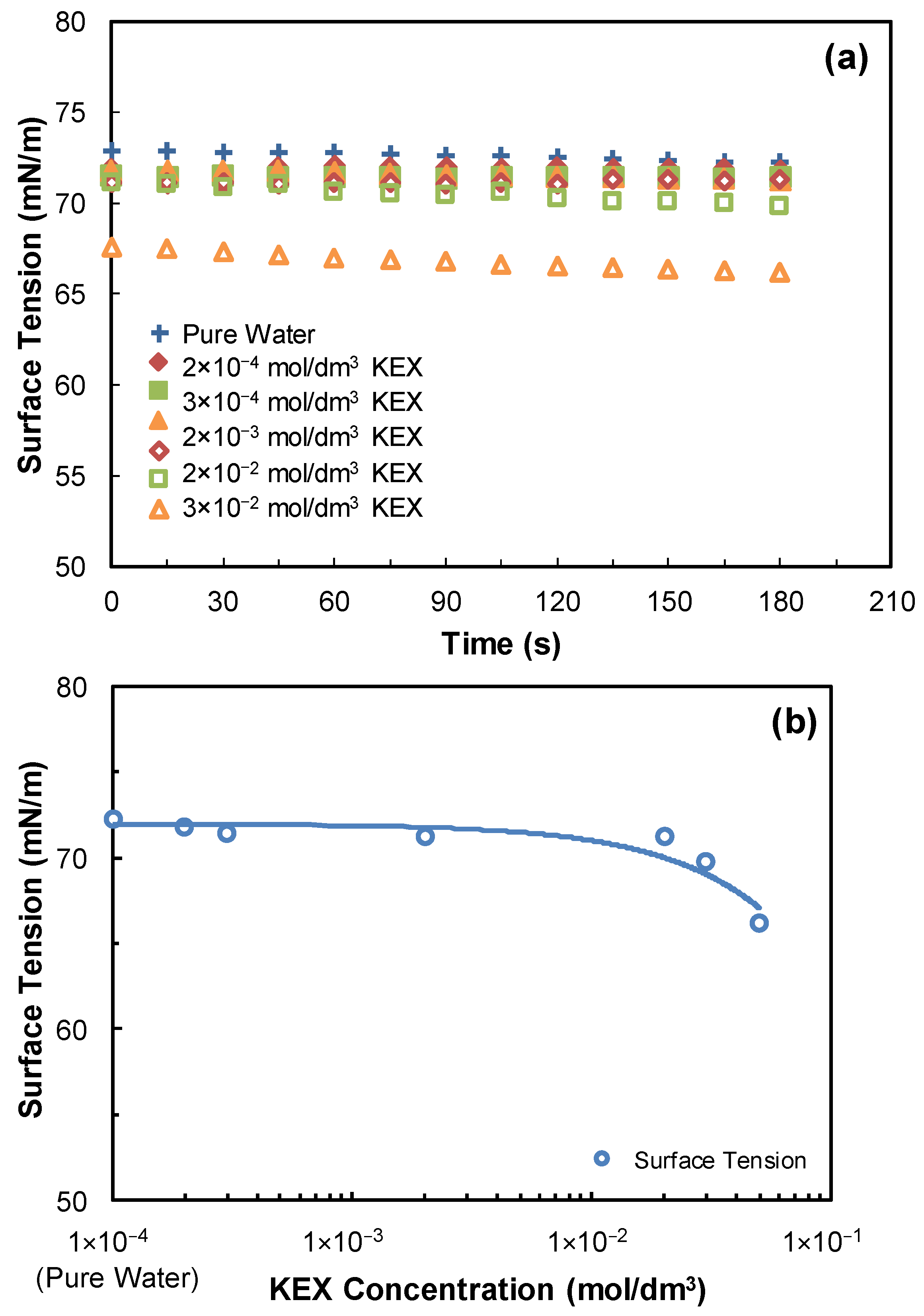
2.4. Determination of the Critical Coalescence Concentration (CCC) of Butyl Glycol
2.5. Dynamic Foam Stability (DFS) Measurements
3. Results and Discussion
3.1. Dynamic Surface Tension Measurements
3.2. Bubble Coalescence Time Measurements
3.3. SMD, BSD, and CCC Experiments
3.4. Dynamic Foam Stability (DFS) Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Laskowski, J.S.; Cho, Y.S.; Ding, K. Effect of frothers on bubble size and foam stability in potash ore flotation systems. Can. J. Chem. Eng. 2003, 81, 63–69. [Google Scholar] [CrossRef]
- Vazirizadeh, A.; Bouchard, J.; Chen, Y. Effect of particles on bubble size distribution and gas hold-up in column flotation. Int. J. Miner. Process. 2016, 157, 163–173. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Drzymala, J. Physical meaning of the Sauter mean diameter of spherical particulate matter. Part. Sci. Technol. 2016, 34, 645–647. [Google Scholar] [CrossRef]
- Tao, D. Role of bubble size in flotation of coarse and fine particles—A review. Sep. Sci. Technol. 2005, 39, 741–760. [Google Scholar] [CrossRef]
- Orvalho, S.; Ruzicka, M.C.; Olivieri, G.; Marzocchella, A. Bubble coalescence: Effect of bubble approach velocity and liquid viscosity. Chem. Eng. Sci. 2015, 134, 205–216. [Google Scholar] [CrossRef]
- Ata, S.; Jameson, G.J. The formation of bubble clusters in flotation cells. Int. J. Miner. Process. 2005, 76, 123–139. [Google Scholar] [CrossRef]
- Ata, S. Coalescence of bubbles covered by particles. Langmuir 2008, 24, 6085–6091. [Google Scholar] [CrossRef]
- Zhu, H.; Valdivieso, A.L.; Zhu, J.; Song, S.; Min, F.; Corona Arroyo, M.A. A study of bubble size evolution in Jameson flotation cell. Chem. Eng. Res. Des. 2018, 137, 461–466. [Google Scholar] [CrossRef]
- Ang, Z.; Bournival, G.; Ata, S. Influence of frothers on the detachment of galena particles from bubbles. Int. J. Miner. Process. 2013, 121, 59–64. [Google Scholar] [CrossRef]
- Ata, S. The detachment of particles from coalescing bubble pairs. J. Colloid Interface Sci. 2009, 338, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Chau, T.T.; Stevens, G.W.; Chan, D.Y.; Grieser, F.; Dagastine, R.R. Polymeric stabilized emulsions: Steric effects and deformation in soft systems. Langmuir 2012, 28, 4599–4604. [Google Scholar] [CrossRef] [PubMed]
- Bournival, G.; Pugh, R.J.; Ata, S. Examination of NaCl and MIBC as bubble coalescence inhibitor in relation to froth flotation. Miner. Eng. 2012, 25, 47–53. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y. The effect of saline water on mineral flotation–A critical review. Miner. Eng. 2014, 66–68, 13–24. [Google Scholar] [CrossRef]
- Wei, T.; Peng, Y.; Farrokhpay, S. Froth stability of coal flotation in saline water. Miner. Process. Extr. Metall. 2014, 123, 234–240. [Google Scholar] [CrossRef]
- Farrokhpay, S. The significance of froth stability in mineral flotation—A review. Adv. Colloid Interface Sci. 2011, 166, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, O. Specific ion effect of chloride salts on collectorless flotation of coal. Physicochem. Probl. Miner. Process. 2013, 49, 511–524. [Google Scholar] [CrossRef]
- Liu, W.; Moran, C.J.; Vink, S. A review of the effect of water quality on flotation. Miner. Eng. 2013, 53, 91–100. [Google Scholar] [CrossRef]
- Laskowski, J.; Castro, S. Flotation in concentrated electrolyte solutions. Int. J. Miner. Process. 2015, 144, 50–55. [Google Scholar] [CrossRef]
- October, L.; Corin, K.; Schreithofer, N.; Manono, M.; Wiese, J. Water quality effects on bubble-particle attachment of pyrrhotite. Miner. Eng. 2019, 131, 230–236. [Google Scholar] [CrossRef]
- Sun, K.; Nguyen, C.V.; Nguyen, N.N.; Ma, X.; Nguyen, A.V. Crucial roles of ion-specific effects in the flotation of water-soluble KCl and NaCl crystals with fatty acid salts. J. Colloid Interface Sci. 2023, 636, 413–424. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Liu, H.; Zhang, H.; Miller, J.D. Some physicochemical aspects of water-soluble mineral flotation. Adv. Colloid Interface Sci. 2016, 235, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Nguyen, C.V.; Nguyen, N.N.; Nguyen, A.V. Flotation surface chemistry of water-soluble salt minerals: From experimental results to new perspectives. Adv. Colloid Interface Sci. 2022, 309, 102775. [Google Scholar] [CrossRef] [PubMed]
- Weedon, D.; Grano, S.; Akroyd, T.; Goncalves, K.; Moura, R. Effects of high magnesium ion concentration on KCl flotation: Part II—Plant research. Miner. Eng. 2007, 20, 716–721. [Google Scholar] [CrossRef]
- Weedon, D.; Grano, S.; Akroyd, T.; Goncalves, K.; Moura, R. Effects of high Mg2+ concentration on KCl flotation: Part I–Laboratory research. Miner. Eng. 2007, 20, 675–683. [Google Scholar] [CrossRef]
- Choi, J.; Choi, S.Q.; Park, K.; Han, Y.; Kim, H. Flotation behaviour of malachite in mono- and di-valent salt solutions using sodium oleate as a collector. Int. J. Miner. Process. 2016, 146, 38–45. [Google Scholar] [CrossRef]
- Jung, M.; Tadesse, B.; Dick, C.; Logan, A.; Dyer, L.; Albijanic, B. Influence of monovalent and divalent cations on monazite flotation. Colloids Surf. A Physicochem. Eng. 2022, 653, 129975. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, W.; Han, G.; Feng, Q. Utilization of lead ions to improve surface hydrophobicity and flotation recovery of sulfidized smithsonite. Colloids Surf. A Physicochem. Eng. 2023, 663, 131126. [Google Scholar] [CrossRef]
- Manono, M.S.; Corin, K.C.; Wiese, J.G. An investigation into the effect of various ions and their ionic strength on the flotation performance of a platinum bearing ore from the Merensky reef. Miner. Eng. 2012, 36–38, 231–236. [Google Scholar] [CrossRef]
- Wan, H.; Yi, P.; Song, X.; Luukkanen, S.; Qu, J.; Yang, W.; Li, H.; Bu, X. Role of improving molybdenite flotation by using aromatic hydrocarbon collector in high-calcium water: A multiscale investigation. Miner. Eng. 2023, 191, 107984. [Google Scholar] [CrossRef]
- Gungoren, C. An investigation of air/water interface in mixed aqueous solutions of KCl, NaCl, and DAH. Physicochem. Probl. Miner. Process. 2019, 55, 1259–1270. [Google Scholar] [CrossRef]
- Ozdemir, O.; Karakashev, S.I.; Nguyen, A.V.; Miller, J.D. Adsorption and surface tension analysis of concentrated alkali halide brine solutions. Miner. Eng. 2009, 22, 263–271. [Google Scholar] [CrossRef]
- Manono, M.S.; Corin, K.C.; Wiese, J.G. The effect of ionic strength of plant water on foam stability: A 2-phase flotation study. Miner. Eng. 2013, 40, 42–47. [Google Scholar] [CrossRef]
- October, L.L.; Manono, M.S.; Wiese, J.G.; Schreithofer, N.; Corin, K.C. Fundamental and flotation techniques assessing the effect of water quality on bubble-particle attachment of chalcopyrite and galena. Miner. Eng. 2021, 167, 1–7. [Google Scholar] [CrossRef]
- Ornelas Tabares, J.; Madrid Ortega, I.; Reyes Bahena, J.L.; Sánchez López, A.A.; Valdez Pérez, D.; López Valdivieso, A. Surface properties and flotability of molybdenite. In Proceedings of the 2006 China-Mexico Workshop on Minerals Particle Technology, San Luis Potosí, Mexico, 5–7 December 2006; pp. 115–124. [Google Scholar]
- Xue, J.; Fuchida, S.; Ishida, S.; Tokoro, C. Insight on Exogenous Calcium/Magnesium in Weakening Pyrite Floatability with Prolonged Pre-Oxidation: Localized and Concomitant Secondary Minerals and Their Depression Characteristics. Minerals 2022, 12, 115. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, H.; Yang, B.; Jia, F.; Yan, H.; Zeng, M.; Qu, H. Effect of Pb2+ on the flotation of molybdenite in the presence of sulfide ion. Results Phys. 2019, 14, 102361. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, H.; Li, X.; Khoso, S.A.; Xiang, G.; Han, W. Different Insights into Silicate Rectorite Modification and Its Role in Removal of Heavy Metal Ions from Wastewater. Minerals 2020, 10, 176. [Google Scholar] [CrossRef]
- Poliseno, V.; Chaves, S.; Brunetti, L.; Loiodice, F.; Carrieri, A.; Laghezza, A.; Tortorella, P.; Magalhaes, J.D.; Cardoso, S.M.; Santos, M.A.; et al. Derivatives of Tenuazonic Acid as Potential New Multi-Target Anti-Alzheimer’s Disease Agents. Biomolecules 2021, 11, 111. [Google Scholar] [CrossRef]
- Domínguez, S.; Torres, J.; Morales, P.; Heinzen, H.; Bertucci, A.; Kremer, C. Complexation and antioxidant activity of flavonoids with biologically relevant metal ions. In Coordination Chemistry Research Progress; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2008; pp. 305–328. [Google Scholar]
- Yang, B.; Tong, X.; Deng, Z.; Lv, X. The Adsorption of Cu Species onto Pyrite Surface and Its Effect on Pyrite Flotation. J. Chem. 2016, 2016, 4627929. [Google Scholar] [CrossRef]
- Gungoren, C.; Islek, E.; Baktarhan, Y.; Kurşun Unver, I.; Ozdemir, O. A novel technique to investigate the bubble coalescence in the presence of surfactant (MIBC) and electrolytes (NaCl and CaCl2). Physicochem. Probl. Miner. Process. 2018, 54, 1215–1222. [Google Scholar] [CrossRef]
- Cho, Y.S.; Laskowski, J.S. Bubble coalescence and its effect on dynamic foam stability. Can. J. Chem. Eng. 2002, 80, 299–305. [Google Scholar] [CrossRef]
- Szyszka, D. Critical Coalescence Concentration (CCC) for Surfactants in Aqueous Solutions. Minerals 2018, 8, 431. [Google Scholar] [CrossRef]
- Smallwood, I.M. Ethylene glycol monobutyl ether. In Handbook of Organic Solvent Properties; Smallwood, I.M., Ed.; Butterworth-Heinemann: Oxford, UK, 1996; pp. 131–133. [Google Scholar]
- Velasco-Medina, A.A.; Gracia-Fadrique, J. Surface Tension and Density of Ethylene Glycol Monobutyl Ether–Water Mixtures from 277.15 to 308.15 K. J. Chem. Eng. Data 2020, 65, 4547–4555. [Google Scholar] [CrossRef]
- Miller, D.J.; Lisy, J.M. Hydrated Alkali-Metal Cations: Infrared Spectroscopy and ab Initio Calculations of M+(H2O)x=2-5Ar cluster ions for M ) Li, Na, K, and Cs. J. Am. Chem. Soc. 2008, 130, 15381–15392. [Google Scholar] [CrossRef]
- Huang, B.; Muy, S.; Feng, S.; Katayama, Y.; Lu, Y.C.; Chen, G.; Shao-Horn, Y. Non-covalent interactions in electrochemical reactions and implications in clean energy applications. Phys. Chem. Chem. Phys. 2018, 20, 15680–15686. [Google Scholar] [CrossRef] [PubMed]
- Franks, G.V.; Johnson, S.B.; Scales, P.J.; Boger, D.V.; Healy, T.W. Ion-Specific Strength of Attractive Particle Networks. Lungmuir 1999, 15, 4411–4420. [Google Scholar] [CrossRef]
- Hishida, M.; Kaneko, Y.; Okuno, M.; Yamamura, Y.; Ishibashi, T.A.; Saito, K. Communication: Salt-induced water orientation at a surface of non-ionic surfactant in relation to a mechanism of Hofmeister effect. J. Chem. Phys. 2015, 142, 171101. [Google Scholar] [CrossRef]
- Hervo-Hansen, S.; Heyda, J.; Lund, M.; Matubayasi, N. Anion-cation contrast of small molecule solvation in salt solutions. Phys. Chem. Chem. Phys. 2022, 24, 3238–3249. [Google Scholar] [CrossRef]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Path. Pharm. 1888, 24, 247–260. [Google Scholar] [CrossRef]
- Marcus, Y. Specific ion effects on the surface tension and surface potential of aqueous electrolytes. Curr. Opin. Colloid Interface Sci. 2016, 23, 94–99. [Google Scholar] [CrossRef]
- Witecki, K.; Polowczyk, I.; Kowalczuk, P.B. Chemistry of wastewater circuits in mineral processing industry—A review. J. Water Process. Eng. 2022, 45, 102509. [Google Scholar] [CrossRef]
- Marcus, Y. Individual ionic surface tension increments in aqueous solutions. Langmuir 2013, 29, 2881–2888. [Google Scholar] [CrossRef]
- Roberto, P.-G.; Arturo, B.-T. Coalescence of air bubbles: Effect of the electrical double layer. Miner. Eng. 2020, 150, 106301. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Zaleska, E.; Danczak, O. Flotation of carbonaceous copper shale–quartz mixture with poly(ethylene glycol) alkyl ethers. Trans. Nonferrous Met. Soc. China 2015, 25, 314–318. [Google Scholar] [CrossRef]
- Zhang, W.; Nesset, J.E.; Rao, R.; Finch, J.A. Characterizing frothers through critical coalescence concentration (CCC) 95-hydrophile-lipophile balance (HLB) relationship. Minerals 2012, 2, 208–227. [Google Scholar] [CrossRef]
- Góralczyk, D.; Ha̧c, K.; Wydro, P. Surface properties of the binary mixed systems of alkylpyridinium halides and sodium alkylsulfonates. Colloids Surf. A Physicochem. Eng. 2003, 220, 55–60. [Google Scholar] [CrossRef]
- Naik, P.K.; Reddy, P.S.R.; Misra, V.N. Interpretation of interaction effects and optimization of reagent dosages for fine coal flotation. Int. J. Miner. Process. 2005, 75, 83–90. [Google Scholar] [CrossRef]
- Xu, Q.; Nakajima, M.; Ichikawa, S.; Nakamura, N.; Roy, P.; Okadome, H.; Shiina, T. Effects of surfactant and electrolyte concentrations on bubble formation and stabilization. J. Colloid Interface Sci. 2009, 332, 208–214. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Samin, A.M. Influence of surfactant and electrolyte concentrations on surfactant adsorption and foaming characteristics. J. Pet. Sci. Eng. 2017, 149, 612–622. [Google Scholar] [CrossRef]
- Corin, K.C.; Tetlow, S.; Manono, M.S. Considering the action of frothers under degrading water quality. Miner. Eng. 2022, 181, 107546. [Google Scholar] [CrossRef]
- Corin, K.C.; Reddy, A.; Miyen, L.; Wiese, J.G.; Harris, P.J. The effect of ionic strength of plant water on valuable mineral and gangue recovery in a platinum bearing ore from the Merensky reef. Miner. Eng. 2011, 24, 131–137. [Google Scholar] [CrossRef]
- Manev, E.D.; Pugh, R.J. Drainage and equilibrium thickness of aqueous films containing nonionic frothers and xanthate flotation collector. J. Colloid Interface Sci. 1992, 151, 505–516. [Google Scholar] [CrossRef]
- Pan, Y.; Bournival, G.; Ata, S. The role of non-frothing reagents on bubble size and bubble stability. Miner. Eng. 2021, 161, 106652. [Google Scholar] [CrossRef]
- Pan, Y.; Bournival, G.; Karakashev, S.I.; Yang, B.; Wang, L.; Ata, S. Effect of electrolyte and flotation collector on foam films in the presence of frothers. Chem. Eng. Sci. 2022, 264, 118176. [Google Scholar] [CrossRef]
- Pan, Y.; Gresham, I.; Bournival, G.; Prescott, S.; Ata, S. Synergistic effects of frothers, collector and salt on bubble stability. Powder Technol. 2022, 397, 117028. [Google Scholar] [CrossRef]
- Manono, M.; Corin, K. Considering Specific Ion Effects on Froth Stability in Sulfidic Cu-Ni-PGM Ore Flotation. Minerals 2022, 12, 321. [Google Scholar] [CrossRef]
- Wang, J.; Forbes, G.; Forbes, E. Frother Characterization Using a Novel Bubble Size Measurement Technique. Appl. Sci. 2022, 12, 750. [Google Scholar] [CrossRef]
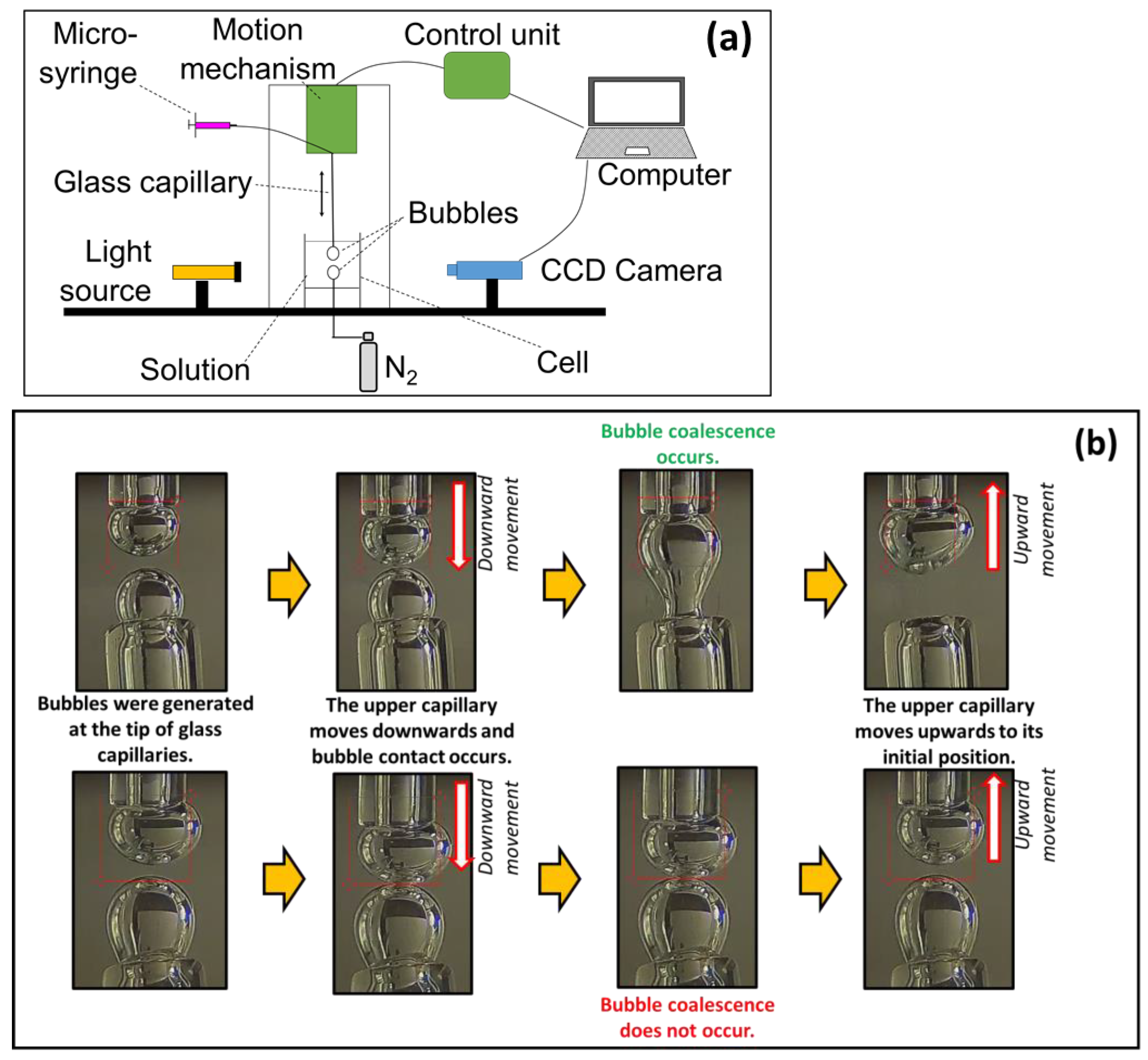
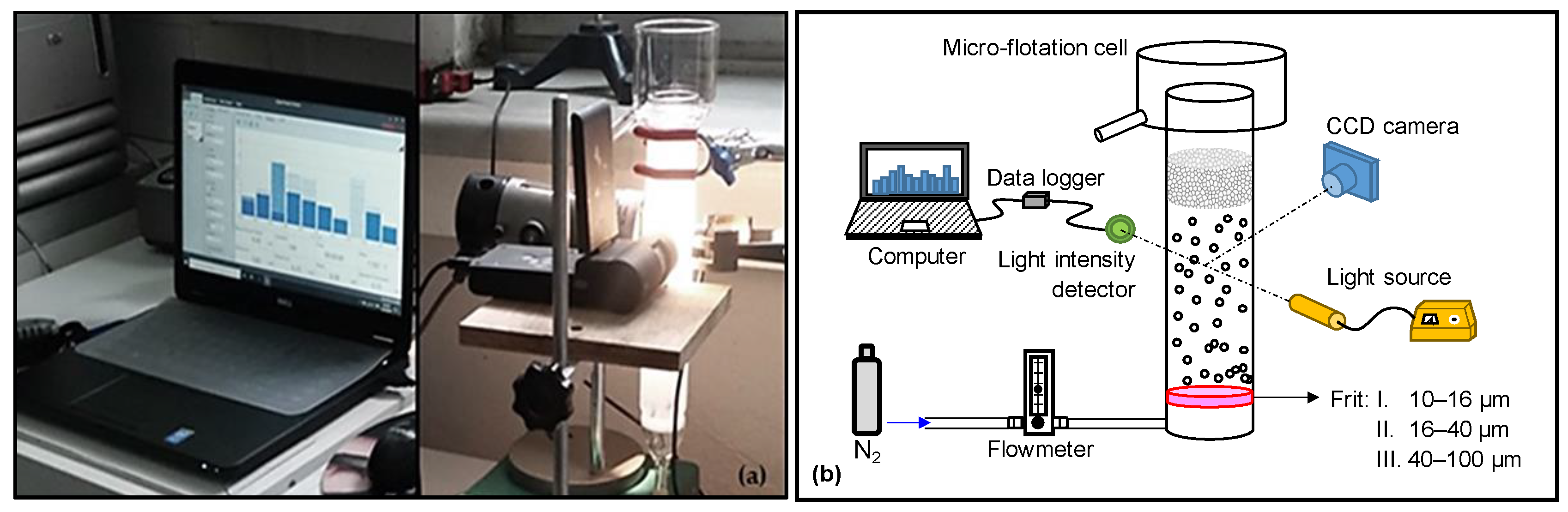


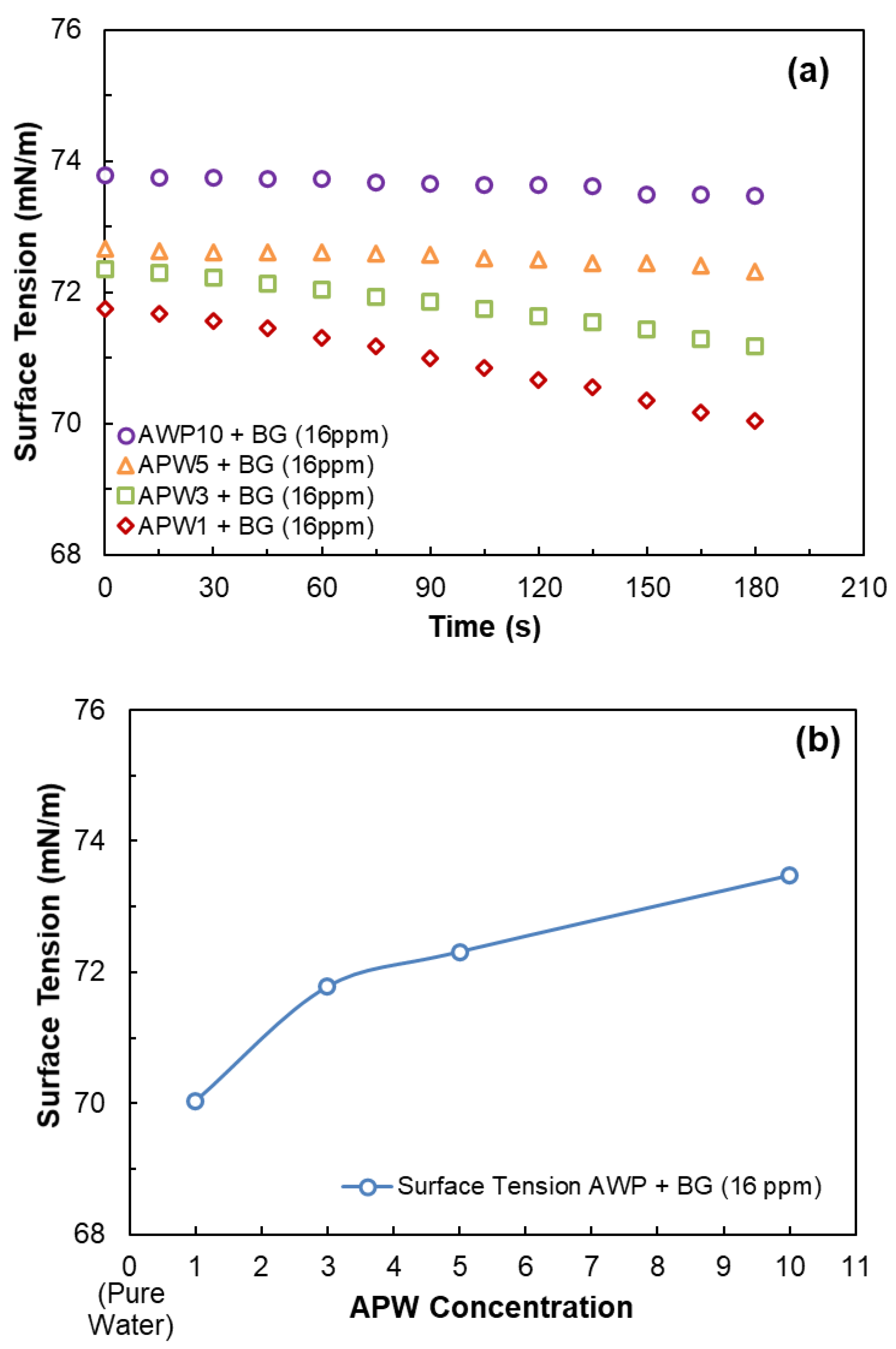
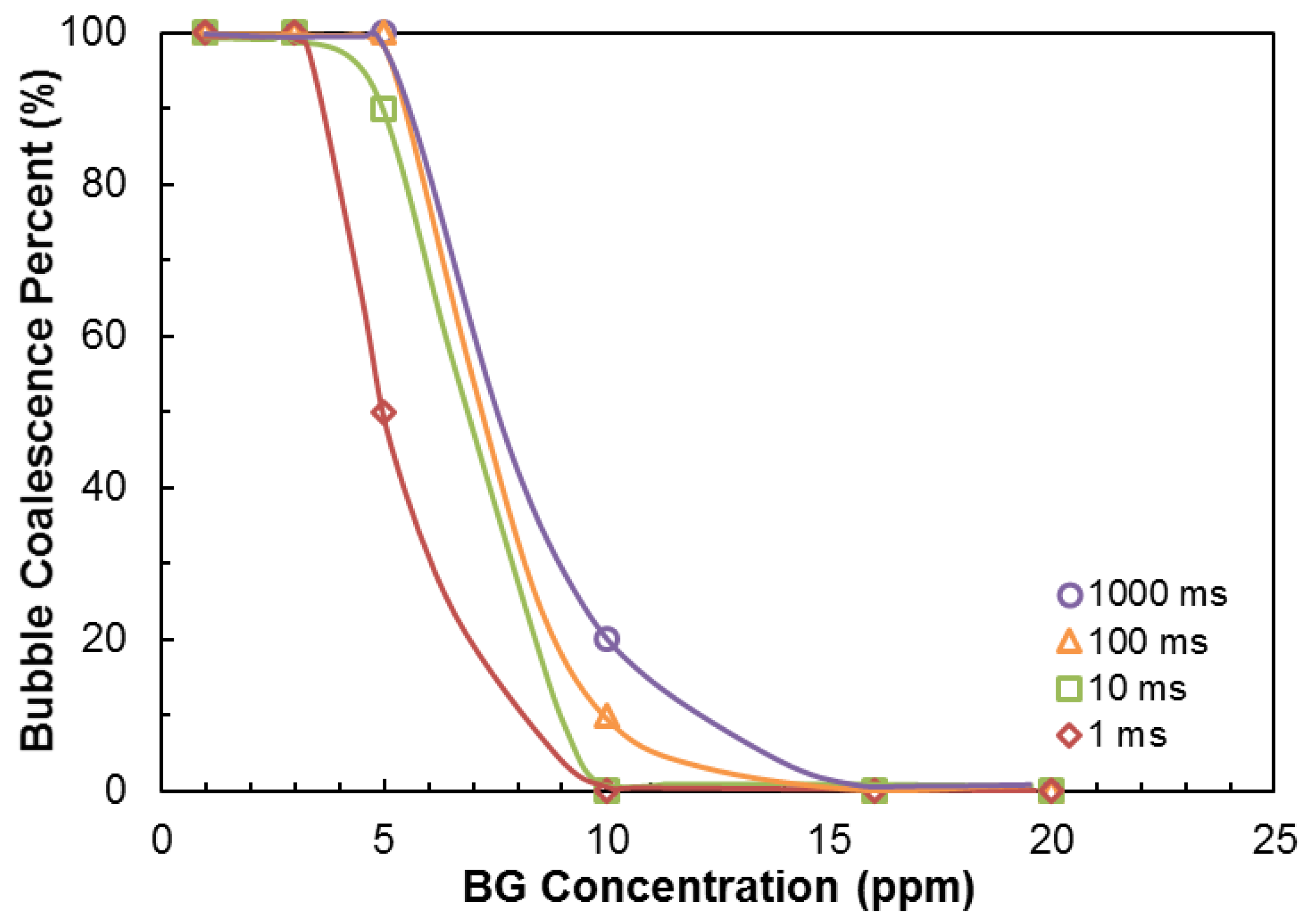
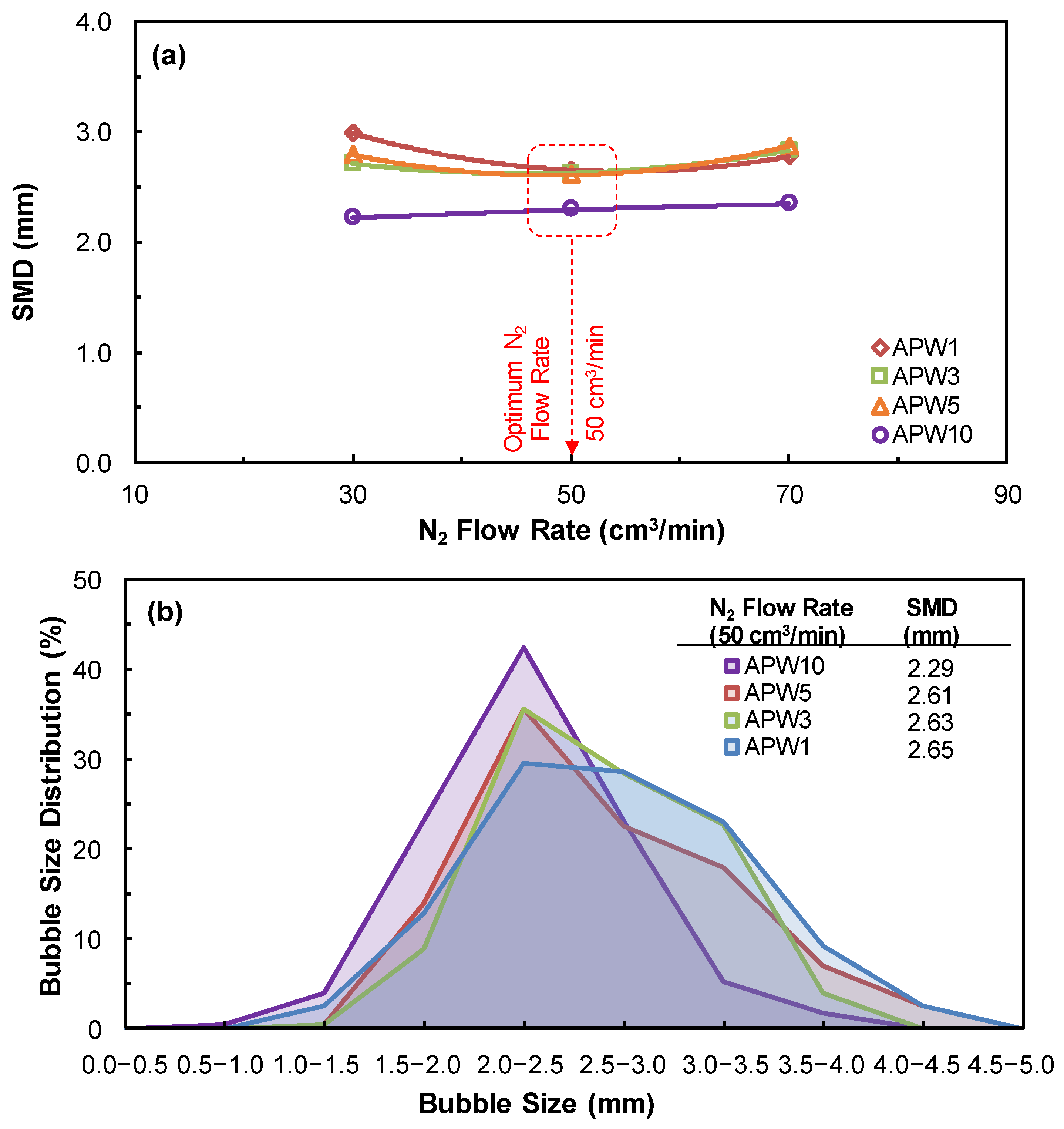
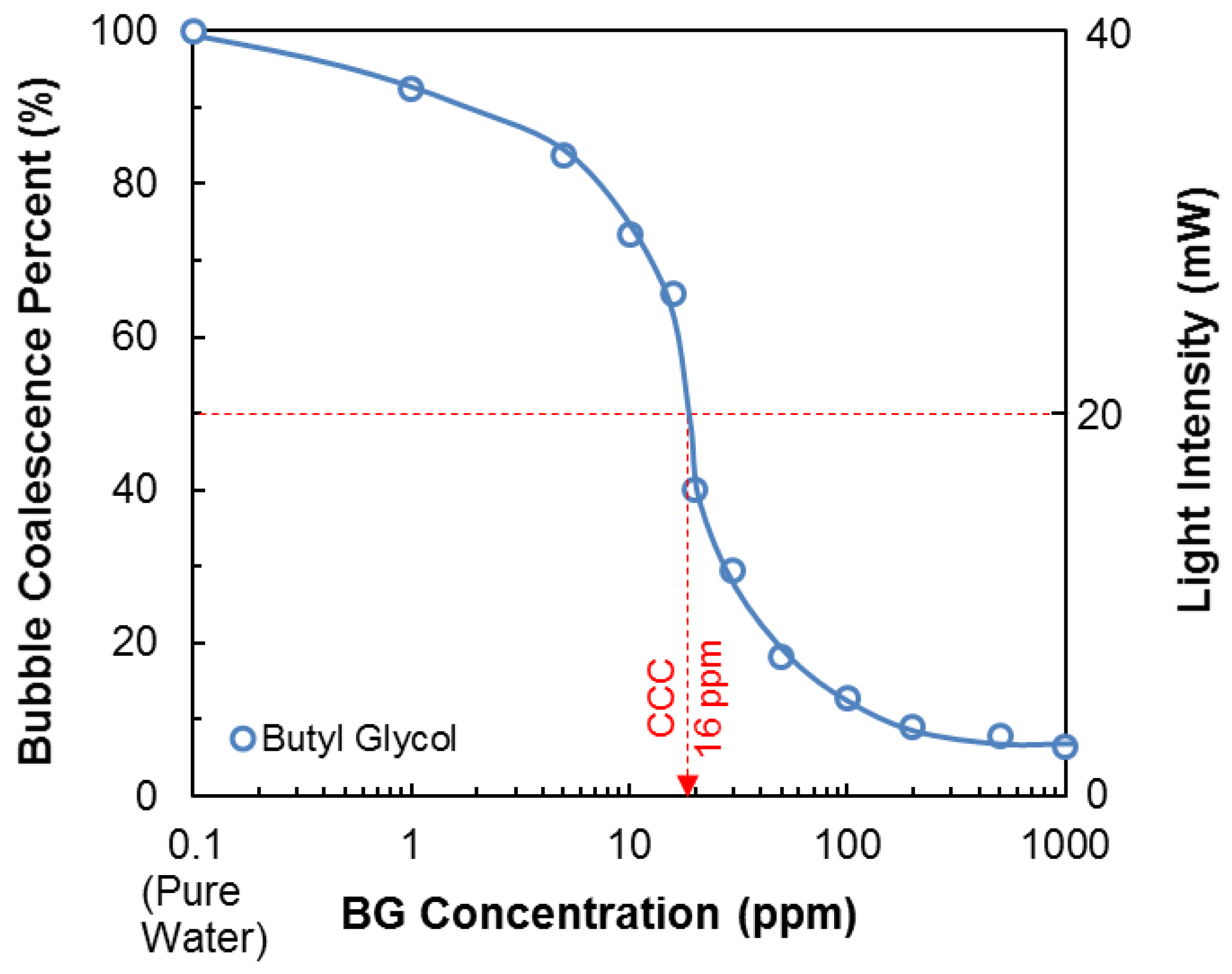

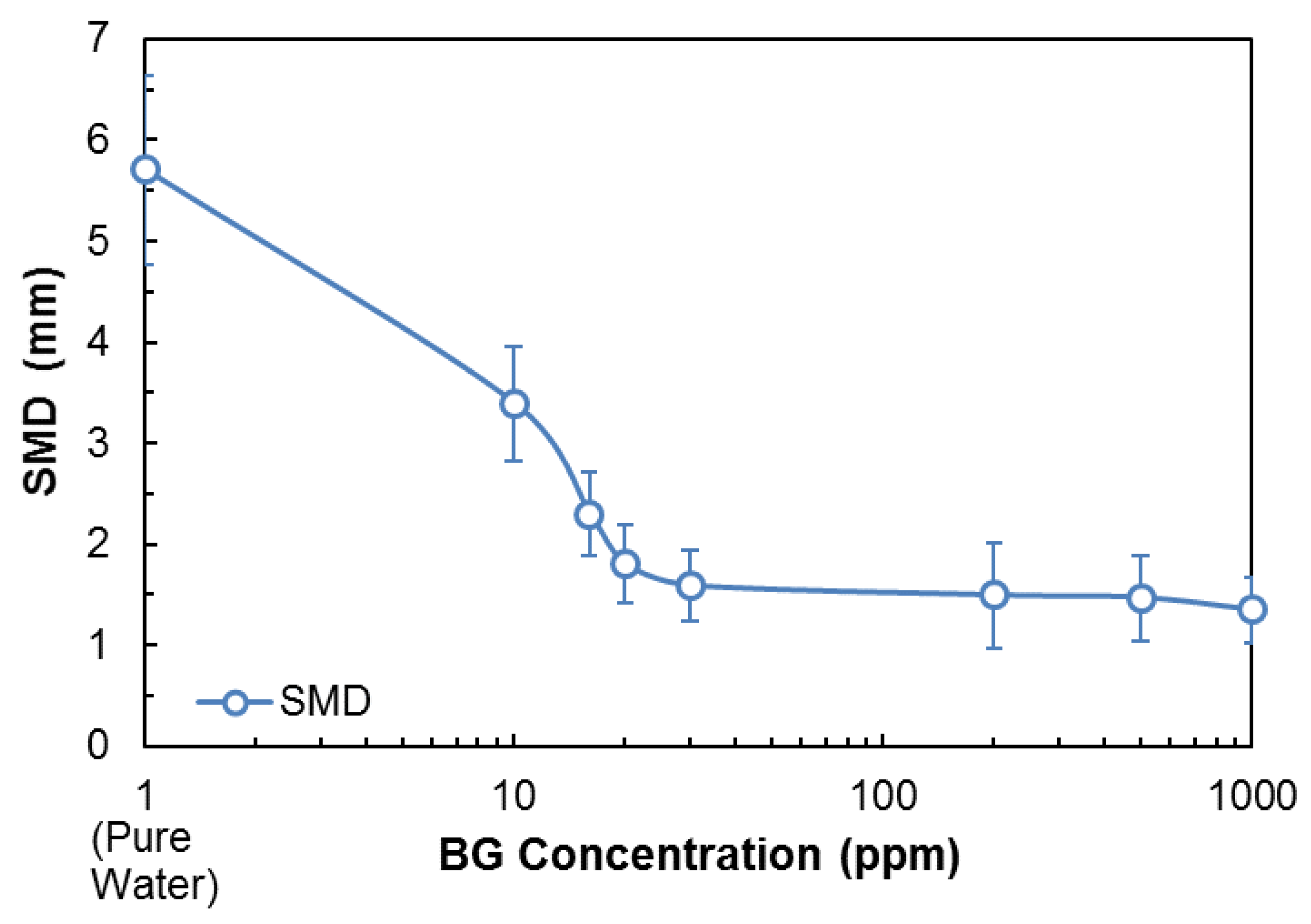
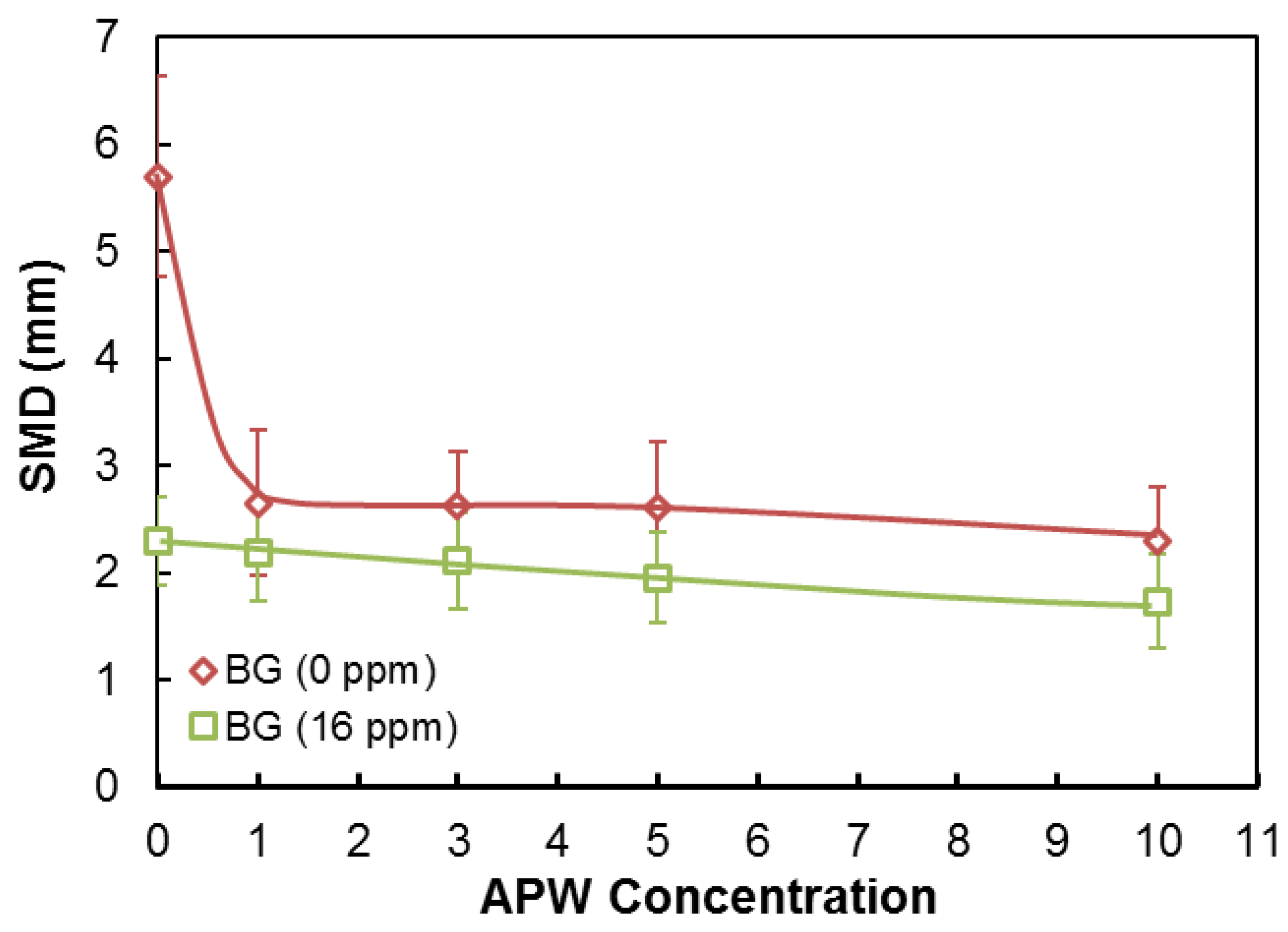
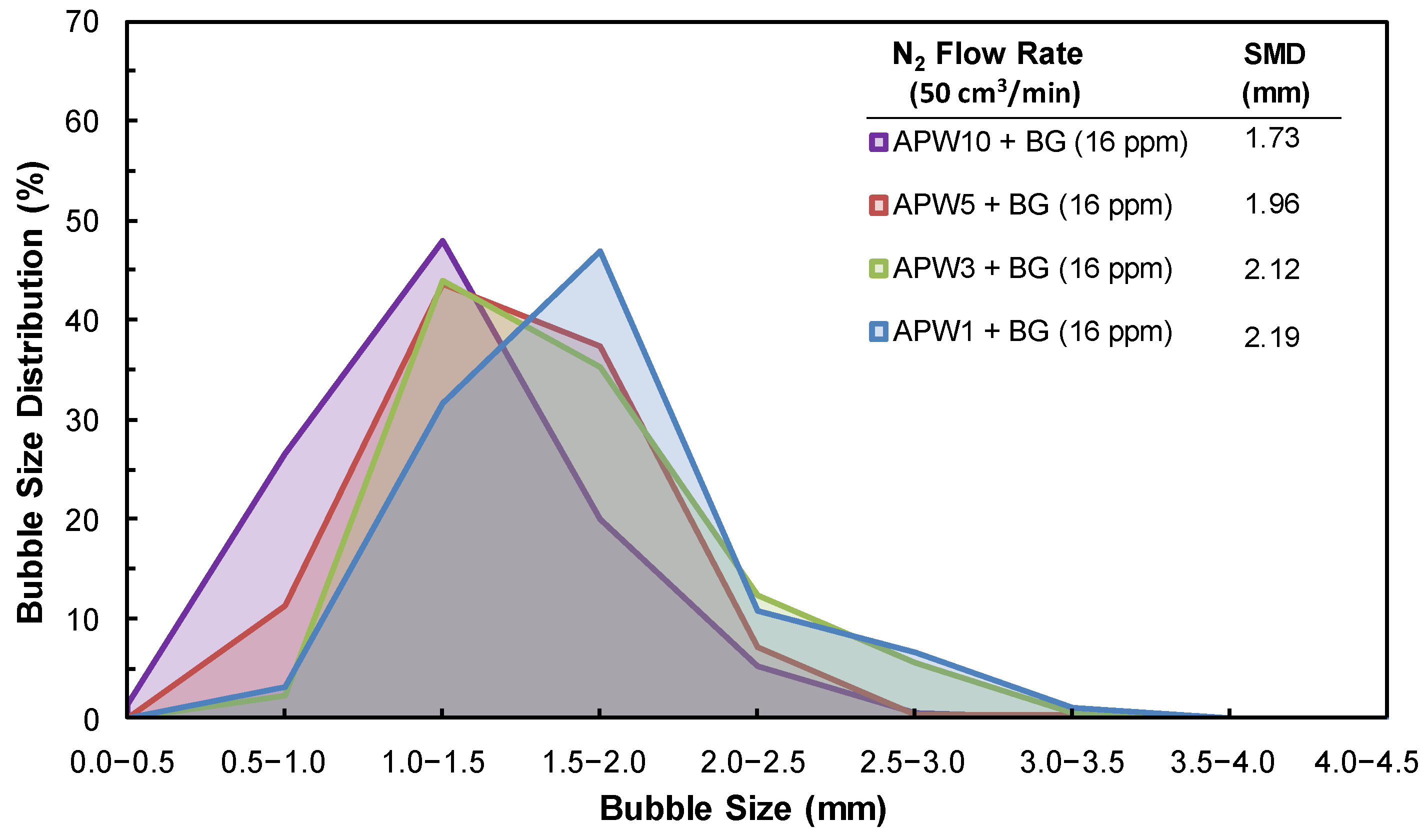

| Na+ (ppm) | Ca2+ (ppm) | Mg2+ (ppm) | Pb2+ (ppm) | Zn2+ (ppm) | Cu2+ (ppm) | Fe3+ (ppm) | SO42− (ppm) | Cl− (ppm) | CO32− (ppm) | NO3− (ppm) | TDS (mg/dm3) | I (mol/dm3) | pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APW1 | 153 | 80 | 70 | 1 | 1 | 1 | 1 | 276.9 | 238.2 | 120 | 3.4 | 945.5 | 0.03 | 6.72 |
| APW3 | 459 | 240 | 210 | 3 | 3 | 3 | 3 | 830.8 | 7146 | 360 | 10.1 | 2836.5 | 0.08 | 6.80 |
| APW5 | 765 | 400 | 350 | 5 | 5 | 5 | 5 | 1384.7 | 1191 | 600 | 16.8 | 4727.5 | 0.13 | 6.80 |
| APW10 | 1530 | 800 | 700 | 10 | 10 | 10 | 10 | 2769.4 | 2382 | 1200 | 33.0 | 9454.9 | 0.26 | 7.03 |
| Condition | Coalescence Result |
|---|---|
| APW1 | No coalescence |
| APW3 | No coalescence |
| APW5 | No coalescence |
| APW10 | No coalescence |
| APW1 + BG (16 ppm) | No coalescence |
| APW3 + BG (16 ppm) | No coalescence |
| APW5 + BG (16 ppm) | No coalescence |
| APW10 + BG (16 ppm) | No coalescence |
| APW1 + BG (16 ppm) + KEX (50 g/t) | No coalescence |
| APW3 + BG (16 ppm) + KEX (50 g/t) | No coalescence |
| APW5 + BG (16 ppm) + KEX (50 g/t) | No coalescence |
| APW10 + BG (16 ppm) + KEX (50 g/t) | No coalescence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gungoren, C.; Muse, S.M.; Terzi, M.; Eskibalci, M.F.; Kursun Unver, I.; Ozdemir, O. Synergistic Effect of Frequently Found Ions in the Flotation of Pb-Zn Sulfide Ores on Air/Water Interface. Minerals 2023, 13, 1236. https://doi.org/10.3390/min13101236
Gungoren C, Muse SM, Terzi M, Eskibalci MF, Kursun Unver I, Ozdemir O. Synergistic Effect of Frequently Found Ions in the Flotation of Pb-Zn Sulfide Ores on Air/Water Interface. Minerals. 2023; 13(10):1236. https://doi.org/10.3390/min13101236
Chicago/Turabian StyleGungoren, Can, Saleban Mohamed Muse, Mert Terzi, Mehmet Faruk Eskibalci, Ilgin Kursun Unver, and Orhan Ozdemir. 2023. "Synergistic Effect of Frequently Found Ions in the Flotation of Pb-Zn Sulfide Ores on Air/Water Interface" Minerals 13, no. 10: 1236. https://doi.org/10.3390/min13101236








