Study of Using Quartz Powder as a Mineral Admixture to Produce Magnesium Oxysulfate Cement
Abstract
:1. Introduction
2. Materials and Methods
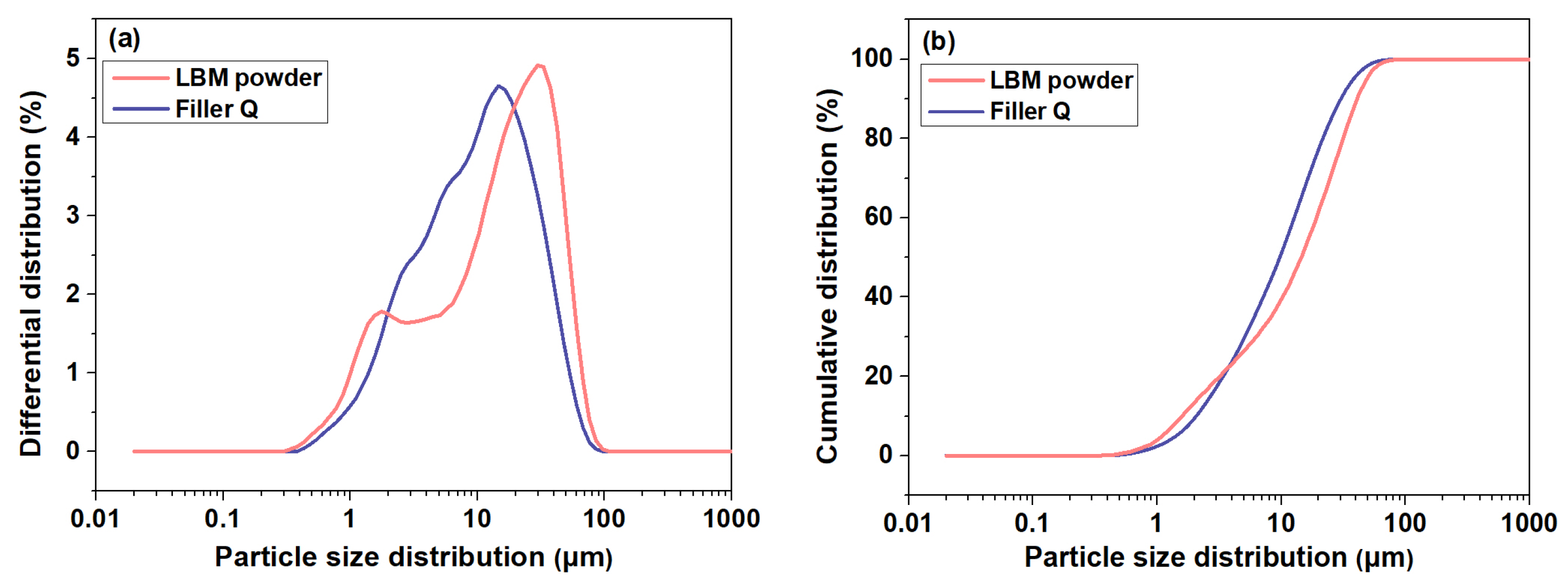
2.1. Raw Materials
2.2. Synthesis of MOS Cement Containing Filler Q
2.3. Characterization Methods
3. Results
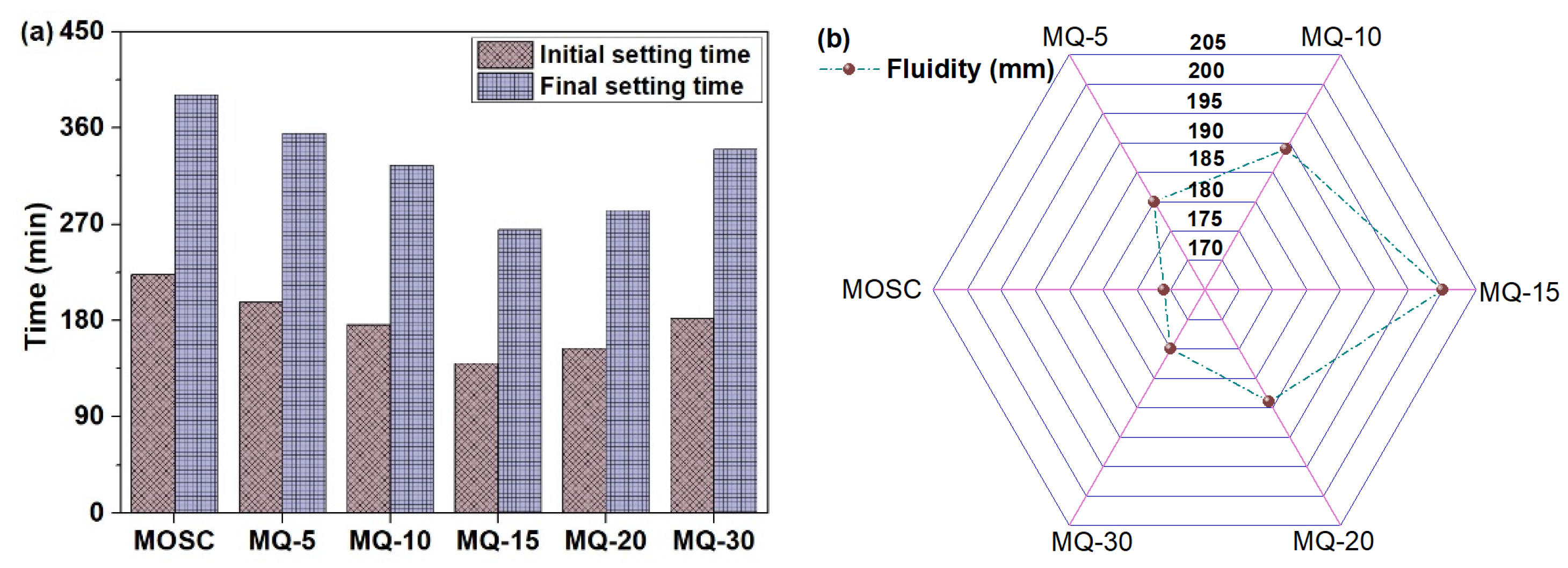
3.1. Setting Time and Fluidity of MOS Cement Containing Filler Q
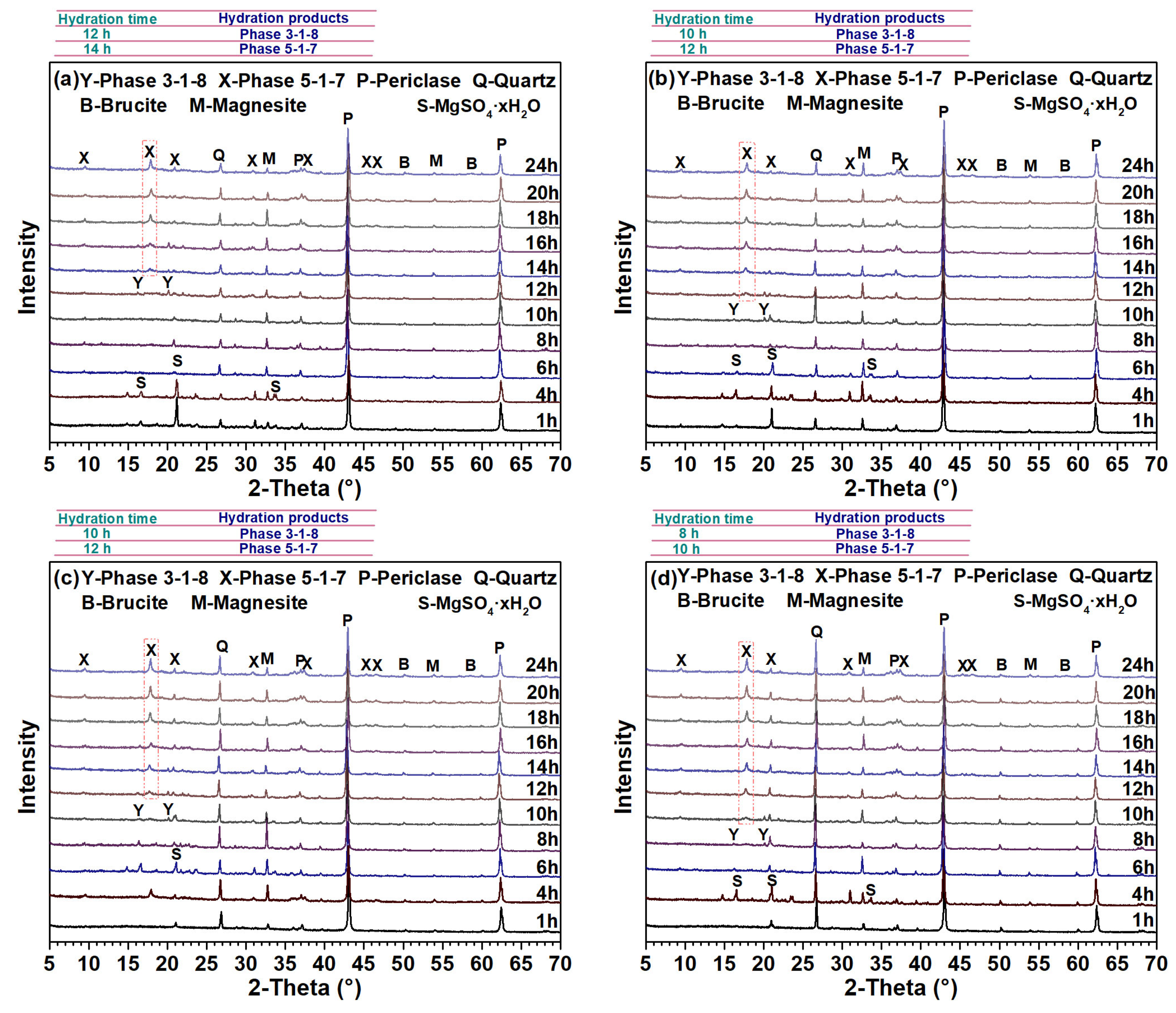
3.2. Early Hydration Process of MOS Cement Containing Filler Q
3.3. Mechanical Strength and Volume Stability of MOS Cement Containing Filler Q
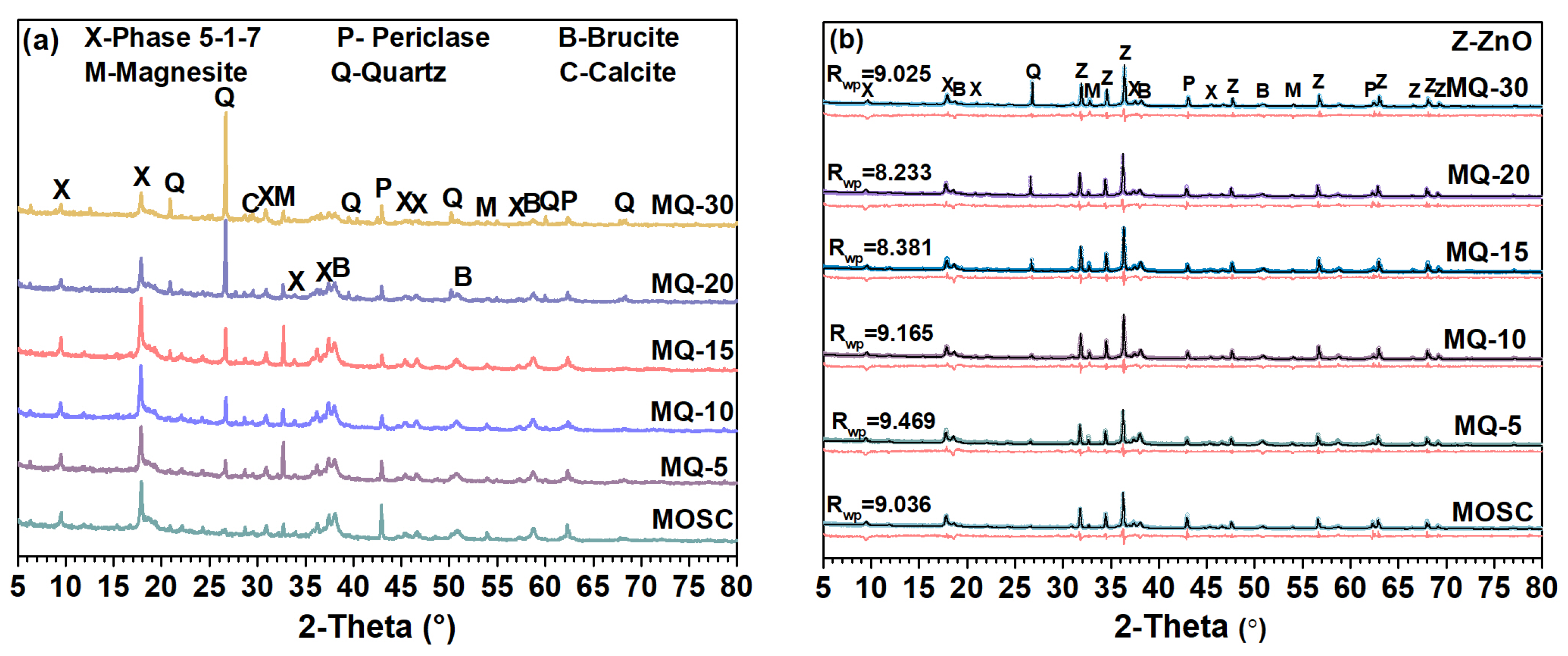
3.4. Phase Composition of MOS Cement Containing Filler Q
3.5. Microstructure of MOS Cement Containing Filler Q
4. Summary
5. Conclusions
- (1)
- Hydration of reactive MgO to turn into phase 5-1-7 in MOS system was a multi-step process. Phase 3-1-8 was the first sediment and was converted to phase 5-1-7. The 5 wt.%–30 wt.% addition of filler Q promoted the generation of phase 3-1-8 and phase 5-1-7, shortened the setting time, and increased the fluidity of MOS slurry, while reduced the total heat release of MOS system due to its dilution and dispersion effects. After adding 5 wt.%–30 wt.% of filler Q to MOS paste, the fluidity of MOS slurry increased by 2.3%–16.5%; the initial and final setting times of MOS paste shortened by 11.7%–37.2% and 9.2%–32.2%, respectively.
- (2)
- The dilution and dispersion effects of filler Q increased the crystallite size and production of phase 5-1-7, meanwhile, the volume-filling effect of filler Q reduced the content of large pore and total pore volume and improved the pore structure of the MOS paste, enhancing the early and later mechanical strength of the MOS cement. MOS paste with 15 wt.% of filler Q showed the highest early and later mechanical capacities. The compressive and flexural strengths at 3-d can increase by 27.2% and 29.6%, and 28-d compressive and flexural strengths can reach to 94.1 MPa and 14.2 MPa, respectively, after adding 15 wt.% of filler Q to MOS paste.
- (3)
- A 5 wt.%–30 wt.% addition of filler Q inhibited the formation of brucite. Among them, a 5 wt.%–15 wt.% addition of filler Q increased the crystallite size and production of phase 5-1-7, while a 20 wt.%–30 wt.% one appeared to have the opposite effect. In addition, the crystallite size of phase 5-1-7 was larger than that of brucite in MOS cement.
- (4)
- Filler Q with low cost can be used as enhancer and coagulant in MOS cement and can also make MOS paste more suitable for application in building materials, e.g., preparing light weight, fireproof, or partition plates. However, its enhancement mechanisms are effective only when there is no excess filler Q, i.e., the content of filler Q should not be more than 20 wt.%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Runčevski, T.; Wu, C.; Yu, H.; Yang, B.; Dinnebier, R.E. Structural characterization of a new magnesium oxysulfate hydrate cement phase and its surface reactions with atmospheric carbon dioxide. J. Am. Ceram. Soc. 2013, 96, 3609–3616. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Cheeseman, C.; Bi, W.; Wang, S. Production of rapid-hardening magnesium oxysulfate (MOS) cement containing boric acid. J. Mater. Civ. Eng. 2022, 34, 04022045. [Google Scholar] [CrossRef]
- Chen, X.; Chen, B.; Chang, J.; Xu, X.; Sun, E.; Wang, S.; Guan, Y. Improved mechanical strength of magnesium oxysulfate cement using ferric sulfate. J. Build. Eng. 2023, 67, 106007. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.J.; Chen, T.F. Recycling of raw rice husk to manufacture magnesium oxysulfate cement based lightweight building materials. J. Clean. Prod. 2018, 191, 220–232. [Google Scholar] [CrossRef]
- Li, Q.Y.; Su, A.S.; Gao, X.J. Preparation of durable magnesium oxysulfate cement with the incorporation of mineral admixtures and sequestration of carbon dioxide. Sci. Total Environ. 2021, 809, 152127. [Google Scholar] [CrossRef]
- Jin, K.; Bi, W.; Guan, Y.; Chen, X.; Zhou, X.; Wang, D. Performance of magnesium oxysulfate (MOS) cement with granite powders after combined sulfate and chloride attacks. Construct. Build. Mater. 2023, 366, 130141. [Google Scholar] [CrossRef]
- Cui, P.; Wu, C.R.; Chen, J.; Luo, F.M.; Kou, S.C. Preparation of magnesium oxysulfate cement as a 3D printing material. Construct. Build. Mater. 2021, 282, 122677. [Google Scholar] [CrossRef]
- Wang, R.; Qin, L.; Gao, X.J. Mechanical strength and water resistance of magnesium oxysulfate cement based lightweight materials. Cement Concr. Compos. 2020, 109, 103554. [Google Scholar] [CrossRef]
- Qiu, Y.; Sun, Q.; Feng, K.; Luo, A.; Wu, X.; Ba, M. Experimental study on the preparation of magnesium oxysulfate cement-based composites using waste locust powders to replace non-renewable magnesium oxide. Construct. Build. Mater. 2023, 366, 130129. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesium-based cements: A journey of 150 years, and cements for the future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Zhang, R.; Arrigoni, A.; Panesar, D.K. Could reactive MgO cement be a green solution? The effect of CO2 mineralization and manufacturing route on the potential global warming impact. Cement Concr. Compos. 2021, 124, 104263. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, Z.; Zhang, Z.; Chang, J.; Bi, W.; Cheeseman, C.R.; Zhang, T. Effect of hydromagnesite addition on the properties and water resistance of magnesium oxysulfate (MOS) cement. Cement Concr. Res. 2021, 143, 106387. [Google Scholar] [CrossRef]
- Ruan, S.Q.; Unluer, C. Comparative life cycle assessment of reactive MgO and Portland cement production. J. Clean. Prod. 2016, 137, 258–273. [Google Scholar] [CrossRef]
- Guo, B.; Tan, Y.; Wang, L.; Chen, L.; Wu, Z.; Sasaki, K. High-efficiency and low-carbon remediation of zinc contaminated sludge by magnesium oxysulfate cement. J. Hazard. Mater. 2021, 408, 124486. [Google Scholar] [CrossRef]
- Li, X.; Qiu, R.; Xue, F.; Fang, L.; Cheng, F. Effects of unreactive MgO and impurities in light burned MgO on the hydration process and performance of base magnesium sulfate cement. Construct. Build. Mater. 2020, 240, 117854. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Zhou, Y.; Cheeseman, C.; Bi, W.; Zhang, T. Improved low-carbon magnesium oxysulfate cement pastes containing boric acid and citric acid. Cement Concr. Compos. 2022, 109, 104813. [Google Scholar] [CrossRef]
- Wu, C.; Yu, H.; Dong, J.; Zheng, L. Effects of material ratio, fly ash, and citric acid on magnesium oxysulfate cement. J. ACI Mater. 2014, 111, 291–297. [Google Scholar] [CrossRef]
- Barbieri, V.; Gualtieri, M.L.; Manfredini, T.; Siligardi, C. Hydration kinetics and microstructural development of a magnesium oxysulfate cement modified by macromolecules. Construct. Build. Mater. 2020, 248, 118624. [Google Scholar] [CrossRef]
- Kahle, K. The formation mechanism for magnesium oxysulfate cements. Silikatechnik 1972, 23, 148–151. [Google Scholar]
- Urwongse, L.; Sorrell, C.A. Phase relations in magnesium oxysulfate cements. J. Am. Ceram. Soc. 1980, 63, 523–526. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Pannach, M.; Freyer, D. 3Mg(OH)2·MgSO4·8H2O: A metastable phase in the system Mg(OH)2-MgSO4-H2O. Z. Anorg. Allg. Chem. 2013, 639, 1827–1833. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, H.; Ma, H.; Ma, H.; Ba, M. The phase composition of the MgO-MgSO4-H2O system and mechanisms of chemical additive. Compos. Part B Eng. 2022, 247, 110328. [Google Scholar] [CrossRef]
- Gu, K.; Chen, B.; Cui, Q. Experimental research on properties of magnesium oxysulfate cement during high temperature exposure. Compos. Part B Eng. 2022, 244, 110168. [Google Scholar] [CrossRef]
- Wang, N.; Yu, H.; Bi, W.; Tan, Y.; Zhang, N.; Wu, C.; Ma, H.; Hua, S. Effects of sodium citrate and citric acid on the properties of magnesium oxysulfate cement. Construct. Build. Mater. 2018, 169, 697–704. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Zhang, H.; Yu, H.; Zhang, W.; Jiang, N.; Liu, L. The hydration mechanism and performance of modified magnesium oxysulfate cement by tartaric acid. Construct. Build. Mater. 2017, 144, 516–524. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, H.; Ma, H.; Ba, M. Effects of different pH chemical additives on the hydration and hardening rules of basic magnesium sulfate cement. Construct. Build. Mater. 2021, 305, 124696. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Li, W.; Ye, H. Modification of magnesium oxysulfate cement by incorporating weak acids. J. Mater. Civ. Eng. 2018, 30, 4018209. [Google Scholar] [CrossRef]
- Sun, Q.; Ba, M.; Zhang, D.; Zhou, S.; Zhang, N.; Wang, F. Effects of citrate acid, solidum silicate and their compound on the properties of magnesium oxysulfate cement under carbonation condition. Construct. Build. Mater. 2022, 330, 127136. [Google Scholar] [CrossRef]
- Rahhal, V.; Bonavetti, V.; Trusilewicz, L.; Pedrajas, C.; Talero, R. Role of the filler on Portland cement hydration at early ages. Construct. Build. Mater. 2012, 27, 82–90. [Google Scholar] [CrossRef]
- Rahhal, V.; Talero, R. Early hydration of Portland cement with crystalline mineral additions. Cem. Concr. Res. 2005, 35, 1285–1291. [Google Scholar] [CrossRef]
- Chen, L.H.; Li, Q.; Jiang, T. Comprehensive utilization of tailings in quartz vein-hosted gold deposits. Minerals 2022, 12, 1481. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, B.; Su, Z.; Chen, X.; Lu, M.; Liu, S.; Liu, J.; Jiang, T. Effect of quartz on the preparation of sodium stannate from cassiterite concentrates by soda roasting process. Minerals 2019, 9, 605. [Google Scholar] [CrossRef]
- Xu, M.Z.; Chen, X.Y.; Han, L. Effect of tartaric acid on the early hydration process and water resistance of magnesium oxychloride cement. J. Build. Eng. 2023, 66, 105838. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, T.T.; Cheeseman, C.R. Effect of tartaric acid and phosphoric acid on the water resistance of magnesium oxychloride (MOC) cement. Construct. Build. Mater. 2019, 213, 528–536. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, J.; Cai, R.; Wei, C.; Chen, J.; Chen, E. In situ monitoring of hydration of magnesium oxysulfate cement paste: Effect of MgO/MgSO4 ratio. Construct. Build. Mater. 2020, 251, 119003. [Google Scholar] [CrossRef]
- Tang, S.; Wei, C.; Cai, R.; Huang, J.; Chen, E.; Yuan, J. In situ monitoring of pore structure of magnesium oxysulfate cement paste: Effect of MgSO4/H2O ratio. J. Ind. Eng. Chem. 2020, 83, 387–400. [Google Scholar] [CrossRef]
- GB/T-1346-2019; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. China Architecture & Building Press: Beijing, China, 2019.
- GB/T 8077-2012; Methods for Testing Uniformity of Concrete Admixture. Standards Press of China: Beijing, China, 2012.
- JC/T 313-2009; Test Method for Determining Expansive Ratio of Expansive Cement. China Building Materials Press: Beijing, China, 2009.
- Albertsson, J.; Abrahams, S.C.; Kvick, Å. Atomic displacement, anharmonic thermal vibration, expansivity and pyroelectric coefficient thermal dependences in ZnO. Acta Crys. Sec. B Struct. Sci. 1989, 45, 34–40. [Google Scholar] [CrossRef]
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louër, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Cryst. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Pyzalski, M.; Dąbek, J.; Adamczyk, A.; Brylewski, T. Physicochemical study of the self-disintegration of calcium orthosilicate (β→γ) in the presence of the C12A7 aluminate phase. Materials 2021, 14, 6459. [Google Scholar] [CrossRef]
- Guan, Y.; Chang, J.; Hu, Z.; Bi, W. Performance of magnesium hydroxide gel at different alkali concentrations and its effect on properties of magnesium oxysulfate cement. Construct. Build. Mater. 2022, 348, 128669. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, H.; Gong, W.; Liu, T.; Wang, N.; Tan, Y.; Wu, C. Effects of low- and high-calcium fly ash on the water resistance of magnesium oxysulfate cement. Construct. Build. Mater. 2020, 230, 116951. [Google Scholar] [CrossRef]
- Winnefeld, F.; Epifania, E.; Montagnaro, F.; Gartner, E.M. Further studies of the hydration of MgO-hydromagnesite blends. Cem. Concr. Res. 2019, 126, 105912. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Yu, B.; Zhu, B.; Liu, Q.; Hu, H.; Hu, Z.; Yu, B.; Wang, S.; Meng, J. A novelty method of multi-scale toughening cement-based materials: Reactive powder concrete with endogenous whiskers. J. Build. Eng. 2022, 50, 104142. [Google Scholar] [CrossRef]
- Gu, K.; Chen, B.; Yu, H.; Zhang, N.; Bi, W.; Guan, Y. Characterization of magnesium-calcium oxysulfate cement prepared by replacing MgSO4 in magnesium oxysulfate cement with untreated desulfurization gypsum. Cem. Concr. Compos. 2021, 121, 104091. [Google Scholar] [CrossRef]
- Ba, M.; Gao, Q.; Ma, Y.; Zhu, J.; Du, Y. Improved hydration and properties of magnesium oxysulfate (MOS) cement using sodium silicate as an additive. Construct. Build. Mater. 2021, 267, 120988. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, H.; Wang, N.; Gong, W.; Tan, Y.; Wu, C. Effects of low- and high-calcium fly ash on magnesium oxysulfate cement. Construct. Build. Mater. 2019, 215, 162–170. [Google Scholar] [CrossRef]
- Rahhal, V.; Talero, R. Very early age detection of ettringite from pozzolan origin. Construct. Build. Mater. 2014, 53, 674–679. [Google Scholar] [CrossRef]
- Rahhal, V.; Bonavetti, V.; Delgado, A.; Pedrajas, C.; Talero, R. Scheme of the Portland cement hydration with crystalline mineral admixtures and other aspects. Silic. Ind. 2009, 74, 347–352. [Google Scholar]
- Talero, R.; Pedrajas, C.; González, M.; Aramburo, C.; Blázquez, A.; Rahhal, V. Role of the filler on Portland cement hydration at very early ages: Rheological behaviour of their fresh cement pastes. Construct. Build. Mater. 2017, 151, 939–949. [Google Scholar] [CrossRef]
- Dionisio, A.K.; Gomes, C.M. Behaviour of autoclaved magnesium oxysulfate cement pastes. J. Build. Eng. 2023, 76, 107263. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.; Li, L.; Fan, W.; Dong, Y.; Liang, H.; Yang, H. Effect of fly ash and metakaolin on properties and microstructure of magnesium oxysulfate cement. Materials 2022, 15, 1334. [Google Scholar] [CrossRef] [PubMed]



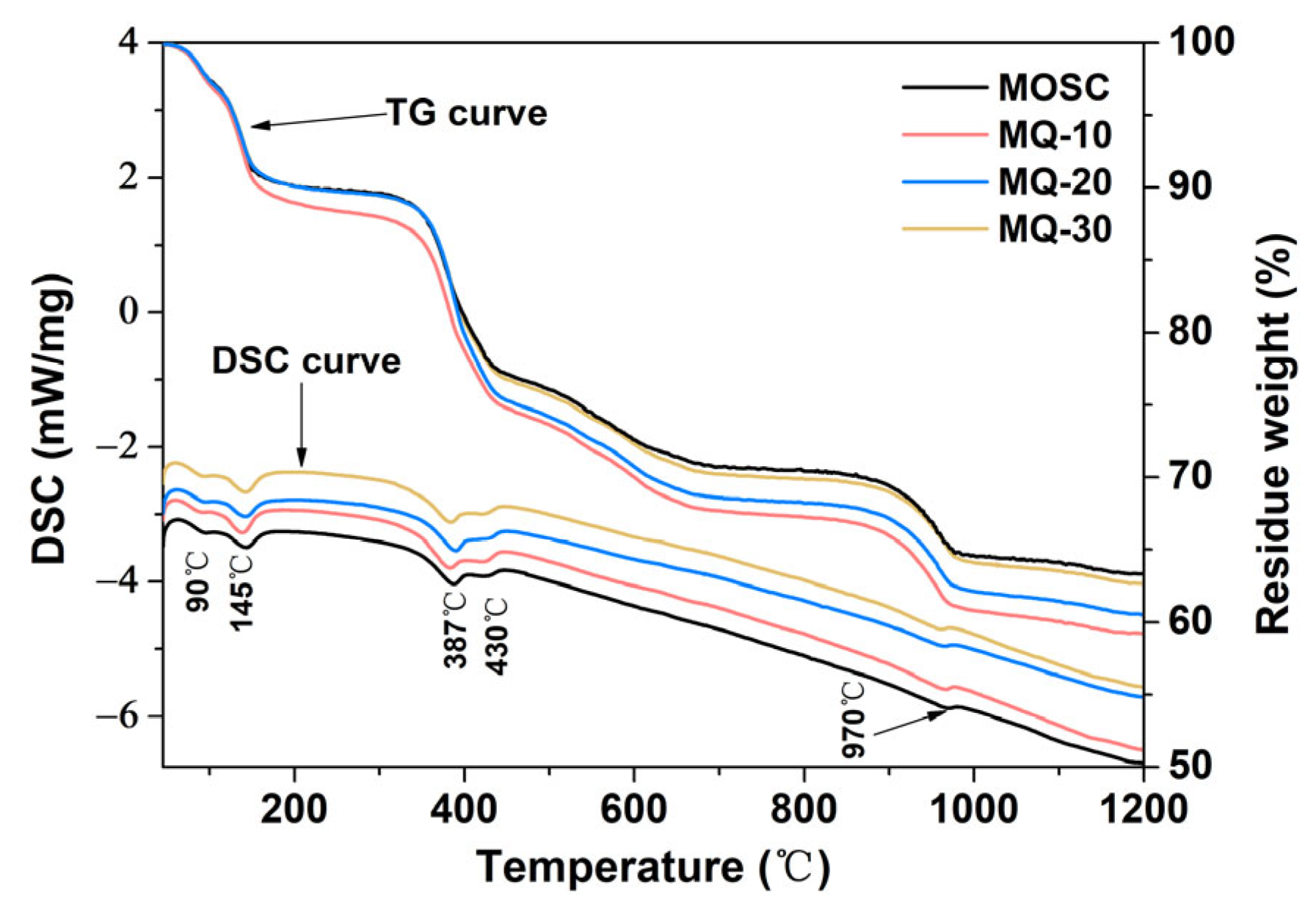
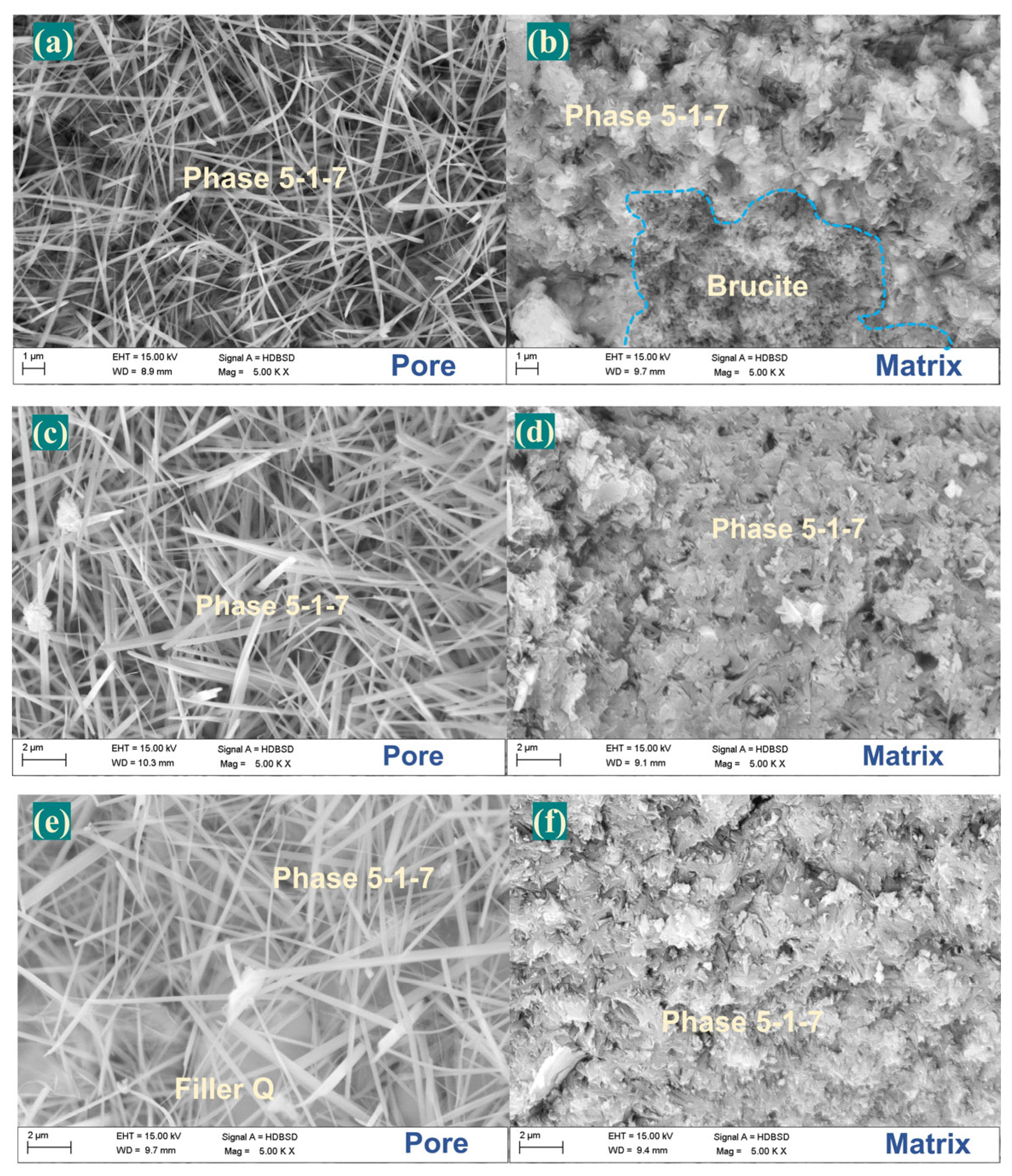
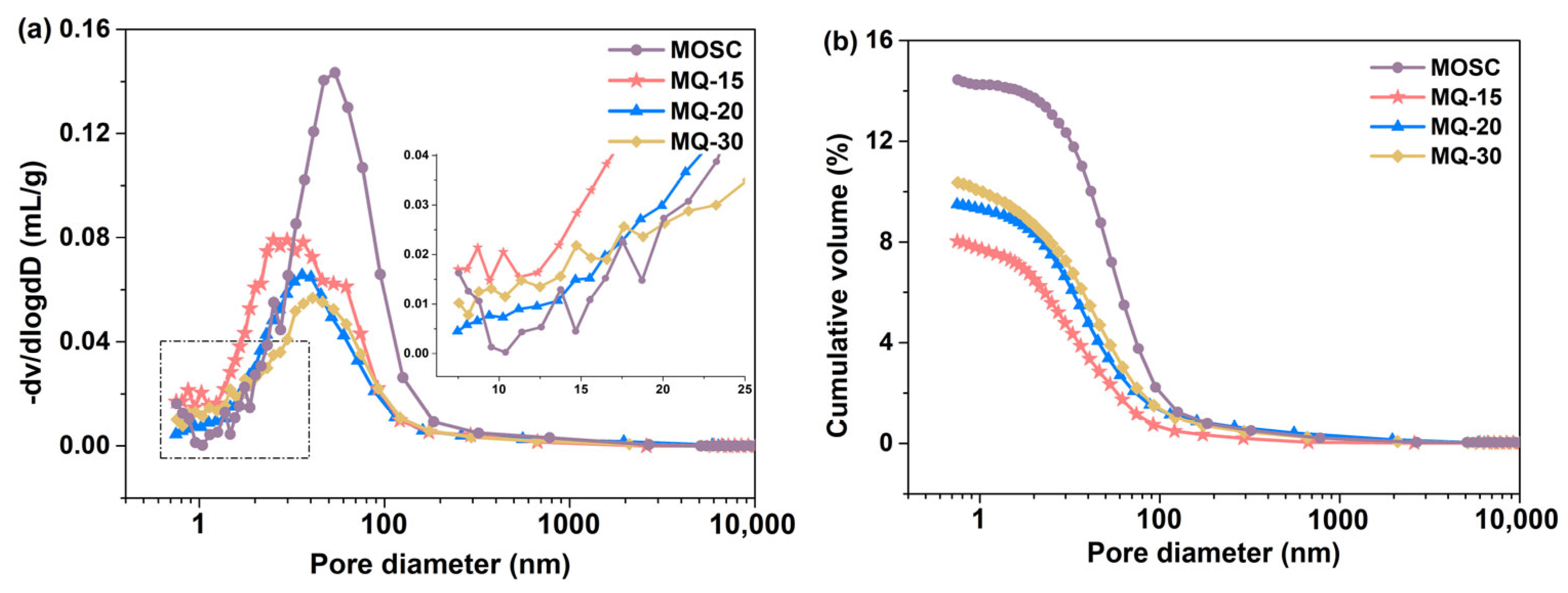
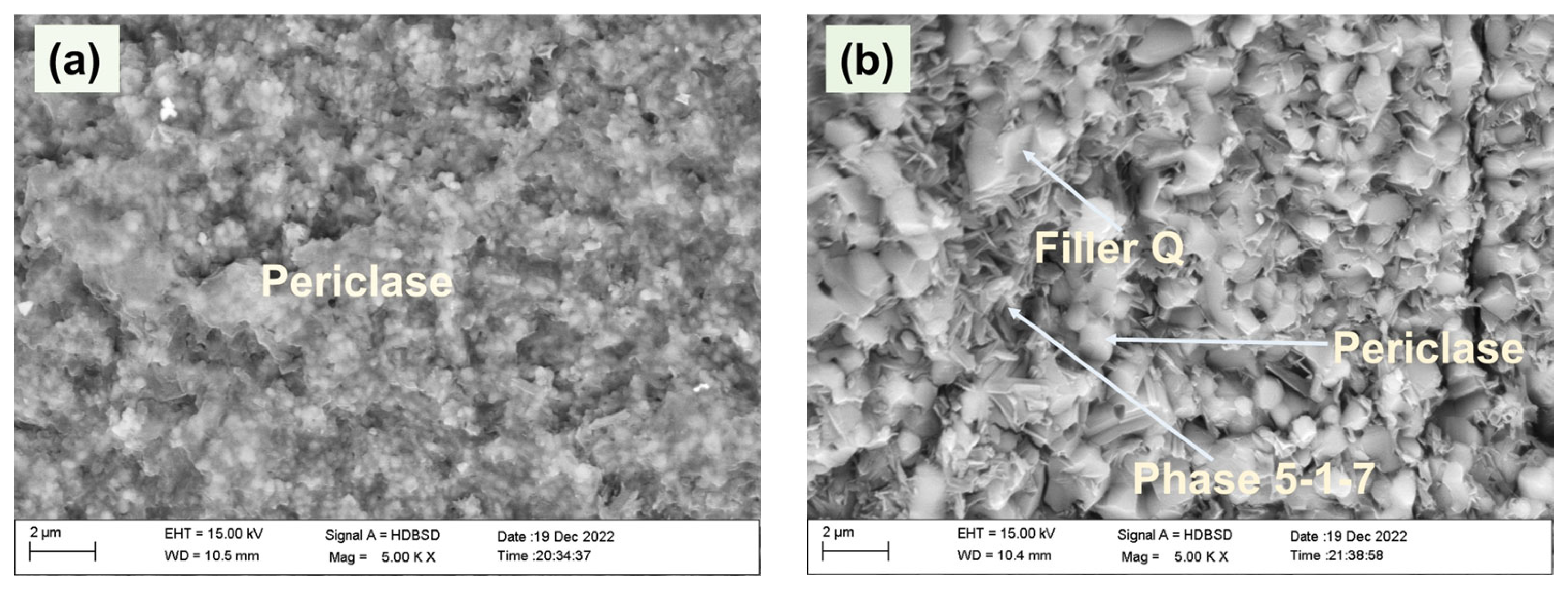
| Samples | LBM/g | MgSO4·7H2O/g | H2O/g | CA/g | Filler Q/g |
|---|---|---|---|---|---|
| MOSC | 100 | 48.4 | 45.8 | 0.5 | - |
| MQ-5 | 100 | 48.4 | 45.8 | 0.5 | 5 |
| MQ-10 | 100 | 48.4 | 45.8 | 0.5 | 10 |
| MQ-15 | 100 | 48.4 | 45.8 | 0.5 | 15 |
| MQ-20 | 100 | 48.4 | 45.8 | 0.5 | 20 |
| MQ-30 | 100 | 48.4 | 45.8 | 0.5 | 30 |
| Samples | Total Pore Volume/% | Pore Volume Distribution/% | ||
|---|---|---|---|---|
| <10 nm | 10–100 nm | >100 nm | ||
| MOSC | 14.45 | 6.09 | 74.28 | 19.63 |
| MQ-15 | 7.54 | 7.23 | 81.61 | 11.16 |
| MQ-20 | 8.03 | 7.86 | 81.56 | 10.58 |
| MQ-30 | 10.37 | 8.27 | 79.19 | 12.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Pang, D.; Chen, S.; Zhang, T.; Bi, W.; Chen, X. Study of Using Quartz Powder as a Mineral Admixture to Produce Magnesium Oxysulfate Cement. Minerals 2023, 13, 1240. https://doi.org/10.3390/min13101240
Wang S, Pang D, Chen S, Zhang T, Bi W, Chen X. Study of Using Quartz Powder as a Mineral Admixture to Produce Magnesium Oxysulfate Cement. Minerals. 2023; 13(10):1240. https://doi.org/10.3390/min13101240
Chicago/Turabian StyleWang, Shaoyan, Daijun Pang, Shengyang Chen, Tongqing Zhang, Wanli Bi, and Xiaoyang Chen. 2023. "Study of Using Quartz Powder as a Mineral Admixture to Produce Magnesium Oxysulfate Cement" Minerals 13, no. 10: 1240. https://doi.org/10.3390/min13101240





