Integration of Whole-Rock Geochemistry and Mineral Chemistry Data for the Petrogenesis of A-Type Ring Complex from Gebel El Bakriyah Area, Egypt
Abstract
:1. Introduction
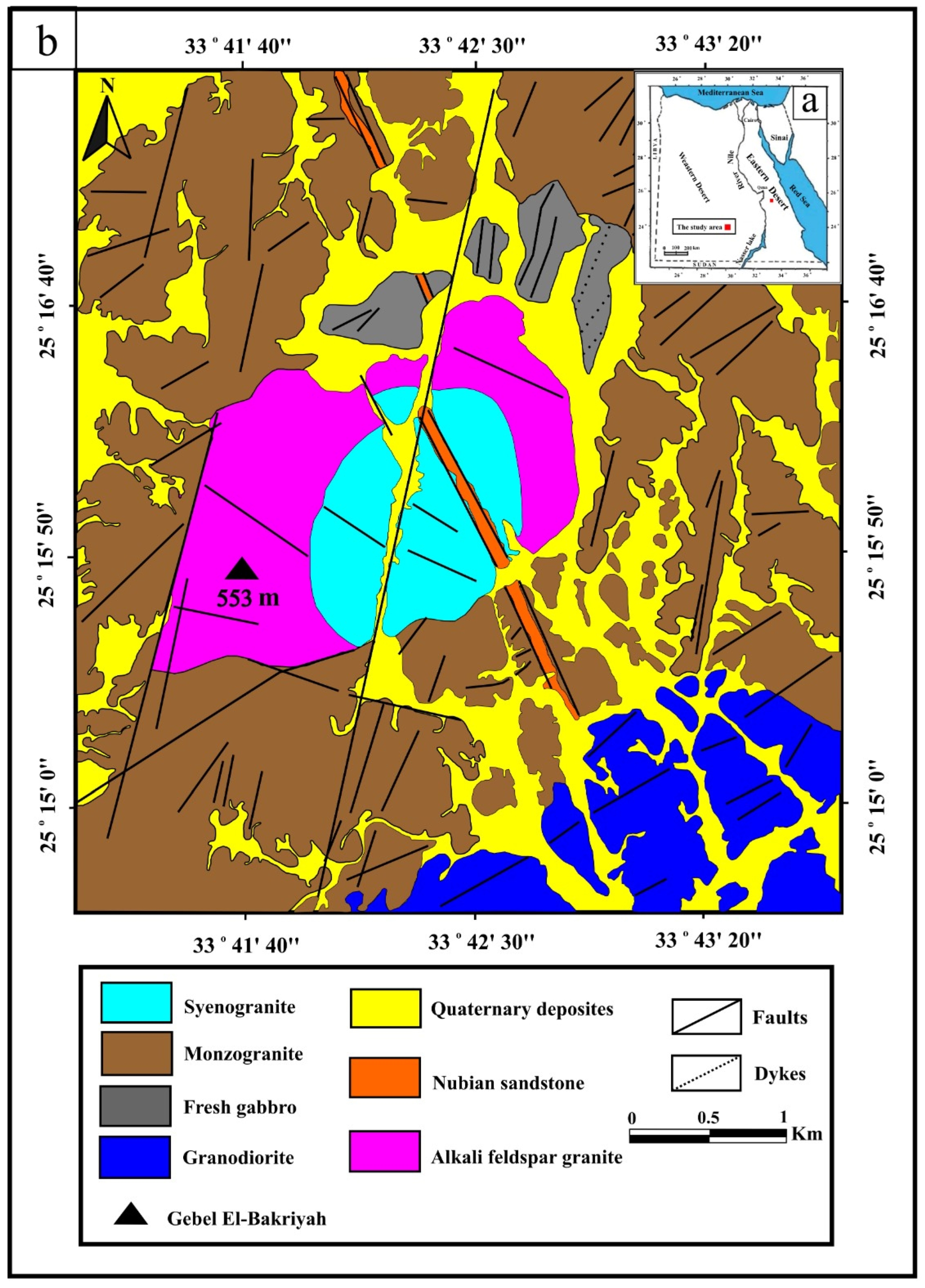
2. Geological Setting and Field Observation
3. Materials and Methods
4. Petrography
5. Whole-Rock Geochemistry
6. Mineral Chemistry
6.1. Feldspars
6.2. Biotite
6.3. Amphibole
6.4. Nb-Ta Oxides
6.5. Zircon and Apatite
6.6. Fe-Ti Oxide
6.7. Chlorite

7. Discussion
7.1. Conditions of Magmatic Crystallization
7.2. Magma Type and Tectonic Setting

7.3. Magma Source and Magmatic vs. Metasomtic Process

7.4. Mineralogical Constraints for Petrogenesis
7.4.1. Biotite and Apatite
7.4.2. Nb-Ta Oxides, Zircon, and REEs
7.4.3. Fe-Ti Oxides and Chlorite

7.5. Geodynamic Model
8. Conclusions
- (1)
- The Gebel El Bakriyah younger granites comprise a prominent ring complex (BRC) consisting of syenogranite core and alkali feldspar rim. These two granite varieties pertain to typical A-type characteristics. The ring complex was emplaced in a within-plate setting whereas the monzogranite represents a transition between the arc and anorogenic settings.
- (2)
- A calc-alkaline composition is assigned for the three younger granite varieties. All of them have the high-K signature of peraluminous melts that were emplaced in the form of their independent pulses, one produced the monzogranite country rocks and two successive ones formed the syenogranite and alkali feldspar granite, respectively.
- (3)
- The three varieties of younger granites of Ediacaran age in the Gebel El Bakriyah area show enrichment of rare metals (Mn-rich columbite-tantalite), F and Fe, i.e., ferroan granites, which are highly fractionated. This resulted in frequent interstitial fluorite in the granites as well as the formation of excavated fluorite-rich quartz veins.
- (4)
- From the geodynamic point of view, the Gebel El Bakriyah younger granites formed by high-T dehydration melting of a mixed crust-mantle source dominated by metasediments and amphibolite, i.e., delamination of the lithospheric crust. This is followed by high fractionation and upwelling of three independent felsic magma pulses along faults in an extensional tectonic regime.
- (5)
- The BRC and its monzogranite country rocks are peculiar examples of Neoproterozoic (Ediacaran) post-collisional magmatism comparable to those in other Precambrian Shields in the world.
- (6)
- Although most of the fluorite and barite veins are excavated, there are still more exploration efforts that must be carried out to maximize the potentiality of critical materials in the area, including the Nb-Ta resources.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Bialy, M.Z.; Omar, M.M. Spatial association of Neoproterozoic continental arc I-type and post-collision A-type granitoids in the Arabian-Nubian Shield: The Wadi Al-Baroud older and younger granites, North Eastern Desert, Egypt. J. Afr. Earth Sci. 2015, 103, 1–29. [Google Scholar] [CrossRef]
- El-Bialy, M.Z. Precambrian Basement Complex of Egypt. In The Geology of Egypt; Regional Geology Reviews; Hamimi, Z., El-Barkooky, A., Martínez Frías, J., Fritz, H., Abd El-Rahman, Y., Eds.; Springer: Cham, Switzerland, 2020; pp. 37–79. [Google Scholar]
- Abd El-Naby, H.H. The Egyptian granitoids: An up-to-date synopsis. In Geology of the Egyptian Nubian Shield; Regional Geology Reviews; Hamimi, Z., Arai, S., Fowler, A.R., El-Bialy, M.Z., Eds.; Springer: Cham, Switzerland, 2021; pp. 239–265. [Google Scholar]
- Abuamarah, B.A. Genesis and petrology of post-collisional rare-metal-bearing granites in the Arabian Shield: A case study of Aja ring complex, northern Saudi Arabia. J. Geol. 2020, 128, 131–156. [Google Scholar] [CrossRef]
- Moussa, H.E.; Asimow, P.D.; Azer, M.K.; Maaty, M.A.A.; Adel, I.M.; Yanni, N.N.; Mubarak, H.S.; Wilner, M.J.; Elsagheer, M.A. Magmatic and hydrothermal evolution of highly fractionated rare-metal granites at Gabal Nuweibi, Eastern Desert, Egypt. Lithos 2021, 400–401, 106405. [Google Scholar] [CrossRef]
- Hassaan, M.M.; Desoky, E.H. Granites in the tectonic environs of the Nubian Shield, Egypt: Geochemical characterization and new contributions. Curr. Res. Earth Sci. 2016, 10, 59–103. [Google Scholar]
- Lundmark, A.M.; Andresen, A.; Hassan, A.; Augland, L.E.; Abu El-Rus, M.A.; Boghdady, G.Y. Repeated magmatic pulses in the East African Orogen of Central Eastern Desert, Egypt: An old idea supported by new evidence. Gondwana Res. 2012, 22, 227–237. [Google Scholar] [CrossRef]
- Hargrove, U.S.; Stern, R.J.; Griffin, W.R.; Johnson, P.R.; Abdelsalam, M.G. From island arc to craton: Timescales of crustal formation along the Neoproterozoic Bir Umq Suture zone, Kingdom of Saudi Arabia. In Saudi Geological Survey; Technical Report SGS-TR; Saudi Geological Survey: Jeddah, Saudi Arabia, 2006; 69p. [Google Scholar]
- Azer, M.K.; Abdelfadil, K.M.; Asimow, P.D.; Khalil, A.E. Tracking the transition from subduction-related to post-collisional magmatism in the north Arabian–Nubian Shield: A case study from the Homrit Waggat area of the Eastern Desert of Egypt. Geol. J. 2020, 55, 4426–4452. [Google Scholar] [CrossRef]
- Eby, G.N. Chemical subdivisions of the A-type granitoids: Petrogenesis and tectonic implications. Geology 1992, 20, 641–644. [Google Scholar] [CrossRef]
- Robinson, F.A.; Bonin, B.; Pease, V.; Anderson, J.L. A discussion on the tectonic implications of Ediacaran late- to post-orogenic A-type granite in the northeastern Arabian Shield, Saudi Arabia. Tectonics 2017, 36, 582–600. [Google Scholar] [CrossRef]
- Heikal, M.T.S.; Khedr, M.Z.; El-Monesf, M.A.; Gomaa, S.R. Petrogenesis and geodynamic evolution of Neoproterozoic Abu Dabbab albite Granite, Central Eastern Desert of Egypt: Petrological and geochemical constraints. J. Afr. Earth Sci. 2019, 158, 103518. [Google Scholar] [CrossRef]
- Abuamarah, B.A.; Azer, M.K.; Seddik, A.M.A.; Asimow, P.D.; Guzman, P.; Fultz, B.T.; Wilner, M.J.; Dalleska, N.; Darwish, M.H. Magmatic and post-magmatic evolution of post-collisional rare-metal bearing granite: The Neoproterozoic Homrit Akarem granitic intrusion, southeastern Desert of Egypt, Arabian-Nubian shield. Geochemistry 2022, 82, 125840. [Google Scholar] [CrossRef]
- Sami, M.; Ntaflos, T.; Farahat, E.S.; Mohamed, H.A.; Hauzenberger, C.; Ahmed, A.F. Petrogenesis and geodynamic implications of Ediacaran highly fractionated A-type granitoids in the north Arabian-Nubian Shield (Egypt): Constraints from whole-rock geochemistry and Sr-Nd isotopes. Lithos 2018, 304–307, 329–346. [Google Scholar] [CrossRef]
- Loiselle, M.; Wones, D. Characteristics and origin of anorogenic granites. In Abstracts with Programs; Geological Society of America: Boulder, CO, USA, 1979; Volume 11, p. 468. [Google Scholar]
- Moreno, J.A.; Montero, P.; Abu Anbar, M.; Molina, J.F.; Scarrow, J.H.; Talavera, C.; Cambeses, A.; Bea, F. SHRIMP U-Pb zircon dating of the Katerina ring complex: Insights into the temporal sequence of Ediacaran calc-alkaline to per-alkaline magmatism in southern Sinai, Egypt. Gond. Res. 2012, 12, 887–900. [Google Scholar] [CrossRef]
- Mahmoud, M.S. Geological and Geochemical Studies on the Rocks of Gebel El-Hisinat Area, Central Eastern Desert, Egypt. Master’s Thesis, Assuit University, Assuit, Egypt, 1984; 109p. [Google Scholar]
- El-Sayed, M.M.; Mohamed, F.H.; Furnes, H. Petrological and geochemical constraints on the evolution of late Pan-African Bakriya post-orogenic ring complex, Central Eastern Desert, Egypt. Neues Jahrb. Mineral. Ab-Handl. 2004, 180, 1–32. [Google Scholar] [CrossRef]
- Saleeb-Roufaiel, G.S.; Samuel, M.D.; Hilmy, M.E.; Moussa, H.E. Fluorite mineralization at El-Bakriya, Eastern Desert of Egypt, Egypt. J. Geol. 1982, 26, 9–18. [Google Scholar]
- Eyal, M.; Litvinovsky, B.; Jahn, B.M.; Zanvilevich, A.; Katzir, Y. Origin and evolution of post-collisional magmatism: Coeval Neoproterozoic calc-alkaline and alkaline suites of the Sinai Peninsula. Chem. Geol. 2010, 269, 153–179. [Google Scholar] [CrossRef]
- Be’eri-Shlevin, Y.; Samuel, M.D.; Azer, M.K.; Rämö, O.T.; Whitehouse, M.J.; Moussa, H.E. The late Neoproterozoic Ferani and Rutig volcano-sedimentary successions of the northernmost Arabian–Nubian Shield (ANS): New insights from zircon U-Pb geochronology, geochemistry and O–Nd isotope ratios. Precambrian Res. 2011, 188, 21–44. [Google Scholar] [CrossRef]
- Stern, R.J. Arc assembly and continental collision in the Neoproterozoic East African Orogen: Implications for the consolidation of Gondwanaland. Annu. Rev. Earth Planet. Sci. 1994, 22, 319–351. [Google Scholar] [CrossRef]
- Abdelnasser, A. Genesis of the Gold Mineralization at Atud Area, Central Eastern Desert of Egypt: Geological, Ore Mineralogical and Geochemical Approaches. Ph.D. Thesis, Istanbul Technical University, Istanbul, Turkey, 2016. [Google Scholar]
- Azer, M.K.; Surour, A.A.; Madani, A.A.; Ren, M.; Abd El-Fatah, A.A. Mineralogical and geochemical constraints on the post-collisional mafic magmatism in the Arabian-Nubian Shield: An example from the El-Bakriya Area, Central Eastern Desert, Egypt. J Geol. 2022, 130, 209–230. [Google Scholar] [CrossRef]
- Abd El-Fatah, A.A.; Surour, A.A.; Madani, A.A.; Azer, M.K. Integration of Landsat-8 and reflectance spectroscopy data for the mapping of Late Neoproterozoic igneous ring complexes in an arid environment: A case study of the Gebel El-Bakriyah area, Eastern Desert, Egypt. J. Min. Environ. 2023, 14, 13–31. [Google Scholar]
- El-Amin, H. Radiometric and Geological Investigations of El Bakriya Area, Eastern Desert, Egypt. Ph.D. Thesis, Cairo University, Cairo, Egypt, 1975; 224p. [Google Scholar]
- Bence, A.E.; Albee, A.L. Empirical correction factors for the electron microanalysis of silicates and oxides. J. Geol. 1968, 76, 382–403. [Google Scholar] [CrossRef]
- Middlemost, E.A.K. Magmas and magmatic rocks: An introduction to igneous petrology. Geol. Mag. 1985, 123, 87–88. [Google Scholar]
- Streckeisen, A. Each plutonic rock has its proper name. Earth-Sci. Rev. 1976, 12, 1–33. [Google Scholar] [CrossRef]
- Maniar, P.D.; Piccoli, P.M. Tectonic discrimination of granitoids. Geol. Soc. Am. Bull. 1989, 101, 635–643. [Google Scholar] [CrossRef]
- Evensen, N.M.; Hamilton, P.J.; Onions, R.K. Rare-earth abundances in chondritic meteorites. Geochim. Cosmochim. Acta 1978, 42, 1199–1212. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.E. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition processes. In Magmatism in the Ocean Basins. Geological Society; Saunders, A.D., Norry, M.J., Eds.; Special Publications: London, UK, 1989; pp. 313–345. [Google Scholar]
- Richard, L.R. MinPet: Mineralogical and Petrological Data Processing System, version 2.02; MinPet Geological Software: Québec, QC, Canada, 1995.
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to Rock-Forming Minerals, 2nd ed.; Longman: Harlow, UK, 1992; 696p. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 1st ed.; Longman Scientific and Technical Publishing: Harlow, UK, 1966; 528p. [Google Scholar]
- Nachit, H.; Ibhi, A.; Ohoud, M.B. Discrimination between primary magmatic biotites, re-equilibrated biotites, and neoformed biotites. Comptes Rendus Geosci. 2005, 337, 1415–1420. [Google Scholar] [CrossRef]
- Leak, B.E.; Woolley, A.R.; Arps, C.E.S.; Birch, W.D.; Gilbert, M.C.; Grice, J.D.; Hawthorne, F.C.; Kato, A.; Kisch, H.J.; Krivovichev, V.G.; et al. Nomenclature of Amphiboles: Report of the sub-committee on amphiboles of the international mineralogical association, Commission on new minerals and mineral names. Mineral. Mag. 1997, 61, 295–310. [Google Scholar] [CrossRef]
- Hey, M.H. A new review of chlorites. Mineral. Mag. 1954, 30, 277–292. [Google Scholar] [CrossRef]
- Basak, A.; Goswami, B. The physico-chemical conditions of crystallization of the Grenvillian arfvedsonite granite of Dimra Pahar, Hazaribagh, India: Constraints on possible source regions. Mineral. Petrol. 2020, 114, 329–356. [Google Scholar] [CrossRef]
- Watson, E.B.; Harrison, T.M. Zircon saturation revisited: Temperature and composition effects in a variety of crustal magma types. Earth Planet. Sci. Lett. 1983, 64, 295–304. [Google Scholar] [CrossRef]
- Machev, P.; Klain, L.; Hecht, L. Mineralogy and geochemistry of biotites from the Belogradchik pluton—Some petrological implications for granitoid magmatism in north-west Bulgaria: Bulgarian Geological Society, Annual Scientific Conference of the Bulgarian Geological Society. Geology 2004, 16–17, 48–50. [Google Scholar]
- Jayasuriya, K.D.; O’Neill, H.S.C.; Berry, A.; Campbell, S.J. A Mössbauer study of the oxidation state of Fe in silicate melts. Am. Min. 2004, 89, 1597–1609. [Google Scholar] [CrossRef]
- Haggerty, S.E. Opaque mineral oxides in terrestrial igneous rocks. Mineral. Soc. Am.-Short Course Notes 1976, 3, 101–300. [Google Scholar]
- King, P.L.; Chappell, B.W.; Allen, C.M.; White, A.J.R. Are A-type granites the high-temperature felsic granites? Evidence from fractionated granites of the Wangrah Suite. Aust. J. Earth Sci. 2010, 48, 501–514. [Google Scholar] [CrossRef]
- Pearce, J.A.; Harris, N.B.W.; Tindle, A.G. Trace element discrimination diagrams for the tectonic interpretation of granitic rocks. J. Petrol. 1984, 25, 956–983. [Google Scholar] [CrossRef]
- Chappell, B.W.; Bryant, C.J.; Wyborn, D. Peraluminous I-type granites. Lithos 2012, 153, 142–153. [Google Scholar] [CrossRef]
- Whalen, J.B.; Frost, C. The Q-ANOR diagram: A tool for the petrogenetic and tectonomagmatic characterization of granitic suites. In Proceedings of the South-Central Section, 47th Annual Meeting, Austin, TX, USA, 4–5 April 2013; Geological Society of America: Boulder, CO, USA, 2013; Volume 7. [Google Scholar]
- Manning, D.A.C. The effect of fluorine on liquidus phase relationships in the system Qz-Ab-Or with excess water at 1 kb. Contrib. Mineral. Petrol. 1981, 76, 206–215. [Google Scholar] [CrossRef]
- Holtz, F.; Johannes, W.; Pichavant, M. Effect of excess aluminium on phase relations in the system Qz-Ab-Or. Experimental investigation at 2 Kbar and reduced H2O activity. Eur. J. Mineral. 1992, 4, 137–152. [Google Scholar] [CrossRef]
- Anderson, J.L.; Smith, D.R. The effects of temperature and fO2 on the Al-in-hornblende barometer. Am. Mineral. 1995, 80, 549–559. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.M. Nature of biotites from alkaline, calc-alkaline, and peraluminous magmas. J. Petrol. 1994, 35, 525–541. [Google Scholar] [CrossRef]
- Frost, B.R.; Barnes, C.G.; Collins, W.J.; Arculus, R.J.; Ellis, D.J.; Frost, C.D. A geochemical classification for granitic rocks. J. Petrol. 2001, 42, 2033–2048. [Google Scholar] [CrossRef]
- Seddik, A.M.A.; Darwish, M.H.; Azer, M.K.; Asimow, P.D. Assessment of magmatic versus post-magmatic processes in the Mueilha rare-metal granite, Eastern Desert of Egypt, Arabian-Nubian Shield. Lithos 2020, 366–367, 105542. [Google Scholar] [CrossRef]
- Kerr, A.; Fryer, B.J. Nd isotope evidence for crust-mantle interaction in the generation of A-type granitoid suites in Labrador, Canada. Chem. Geol. 1993, 104, 39–60. [Google Scholar] [CrossRef]
- Jarrar, G.H.; Manton, W.I.; Stern, R.J.; Zachmann, D. Late Neoproterozoic A-type granites in the northernmost Arabian-Nubian Shield formed by fractionation of basaltic melts. Chem. Erde Geochem. 2008, 68, 295–312. [Google Scholar] [CrossRef]
- Sylvester, P.J. Post-collisional alkaline granites. J. Geol. 1989, 97, 261–280. [Google Scholar] [CrossRef]
- Sisson, T.W.; Ratajeski, K.; Hankins, W.B.; Glazner, A.F. Voluminous granitic Desert, Egypt. Sci. J. Fac. Sci. Minufia Univ. 2005, 15, 107–129. [Google Scholar]
- Sami, M.; Ntaflos, T.; Farahat, E.S.; Mohamed, H.A.; Ahmed, A.F.; Hauzenberger, C. Mineralogical, geochemical and Sr-Nd isotopes characteristics of fluorite-bearing granites in the Northern Arabian-Nubian Shield, Egypt: Constraints on petrogenesis and evolution of their associated rare metal mineralization. Ore Geol. Rev. 2017, 88, 1–22. [Google Scholar] [CrossRef]
- Ballouard, C.; Poujol, M.; Boulvais, P.; Branquet, Y.; Tartèse, R.; Vigneresse, J.L. Nb-Ta fractionation in per-aluminous granites: A marker of the magmatic-hydrothermal transition. J. Geol. 2016, 44, 231–234. [Google Scholar] [CrossRef]
- Dingwell, D.B. The structures and properties of fluorine-rich magmas: A review of experimental studies. Can. Inst. Min. Metall. Pet. 1988, 39, 1–12. [Google Scholar]
- Wang, R.C.; Wu, F.Y.; Xie, L.; Liu, X.C.; Wang, J.M.; Yang, L.; Lai, W.; Liu, C. A preliminary study of rare-metal mineralization in the Himalayan leucogranite belts, South Tibet. Sci. China Earth Sci. 2017, 60, 1655–1663. [Google Scholar] [CrossRef]
- Chappell, B.W. Aluminium saturation in I-and S-type granites and the characterization of fractionated haplogranites. Lithos 1999, 46, 535–551. [Google Scholar] [CrossRef]
- Khalil, A.E.S.; Obeid, M.A.; Azer, M.K.; Asimow, P.D. Geochemistry and petrogenesis of post-collisional alkaline and peralkaline granites of the Arabian-Nubian Shield: A case study from the southern tip of the Sinai Peninsula, Egypt. Int. Geol. Rev. 2018, 60, 998–1018. [Google Scholar] [CrossRef]
- Irber, W.; Förster, H.J.; Hecht, L.; Möller, P.; Morteani, G. Experimental, geochemical, mineralogical and O-isotope constraints on the late-magmatic history of the Fichtelgebirge granites (Germany). Int. J. Earth Sci. 1997, 86, 110–124. [Google Scholar] [CrossRef]
- Nicolae, I.; Saccani, E. Petrology and geochemistry of the Late Jurassic calc-alkaline series associated to Middle Jurassic ophiolites in the South Apuseni Mountains (Romania). Swiss J. Geosci. 2003, 83, 81–96. [Google Scholar]
- De Souza, Z.S.; Martin, H.; Peucat, J.J.; Jardim de Sá, E.F.; de Freitas Macedo, M.H. Calc Alkaline Magmatism at the Archean- Proterozoic Transition: The Caicoó Complex Basement (NE Brazil). J. Petrol. 2007, 48, 2149–2185. [Google Scholar] [CrossRef]
- Schiano, P.; Monzier, M.; Eissen, J.P. Simple mixing as the major control of the evolution of volcanic suites in the Ecuadorian Andes. Contrib. Mineral. Petrol. 2010, 160, 297–312. [Google Scholar] [CrossRef]
- Henry, D.J.; Guidotti, C.V.; Thomson, J.A. The Ti-saturation surface for low- to medium-pressure metapelitic biotites: Implications for geothermometry and Ti-substitution mechanisms. Am. Min. 2005, 90, 316–328. [Google Scholar] [CrossRef]
- Piccoli, P.M.; Candela, P.A. Apatite in igneous systems. In Phosphates: Geochemical, Geobiological, and Materials Importance; Reviews in Mineralogy and Geochemistry; GeoScienceWorld: McLean, VA, USA, 2002; Volume 48, pp. 255–292. [Google Scholar]
- Webster, J.D.; Piccoli, P.M. Magmatic apatite: A powerful, yet deceptive, mineral. Elements 2015, 11, 177–182. [Google Scholar] [CrossRef]
- Zeng, L.P.; Li, X.F.; Hu, H.; McFarlane, C. In situ elemental and isotopic analysis of fluorapatite from the Taocun magnetite-apatite deposit, Eastern China: Constraints on fluid metasomatism. Am. Min. 2016, 101, 2468–2483. [Google Scholar] [CrossRef]
- Chen, L.; Yan, Z.; Wang, Z.Q.; Wang, K. Characteristics of Apatite from 160–140 Ma Cu (Mo) and Mo (W) Deposits in East Qinling. Geol. Acta 2017, 91, 1925–1941. [Google Scholar]
- Miles, A.J.; Graham, C.M.; Hawkesworth, C.J.; Gillespie, M.R.; Hinton, R.W.; Bromiley, G.D. Apatite: A new redox proxy for silicic magmas. Geochim. Cosmochim. Acta 2014, 132, 101–119. [Google Scholar] [CrossRef]
- Nathwani, C.L.; Loader, M.A.; Wilkinson, J.J.; Buret, Y.; Sievwright, R.H.; Hollings, P. Multi-stage arc magma evolution recorded by apatite in volcanic rocks. Geology 2020, 48, 323–327. [Google Scholar] [CrossRef]
- Prowatke, S.; Klemme, S. Trace element partitioning between apatite and silicate melts. Geochim. Cosmochim. Acta 2006, 70, 4513–4527. [Google Scholar] [CrossRef]
- Hoskin, P.W.O.; Schaltegger, U. The composition of zircon and igneous and metamorphic petrogenesis. Rev. Mineral. Geochem. 2003, 53, 27–62. [Google Scholar] [CrossRef]
- Erdmann, S.; Wodicka, N.; Jackson, S.E.; Corrigan, D. Zircon textures and composition refractory recorders of magmatic volatile evolution. Contrib. Mineral. Petrol. 2013, 165, 45–71. [Google Scholar] [CrossRef]
- Abuamarah, B.A.; Azer, M.K.; Asimow, P.D.; Shi, Q. Petrogenesis of the post-collisional rare-metal-bearing Ad-Dayheen granite intrusion, Central Arabian Shield. Lithos 2019, 384–385, 105956. [Google Scholar] [CrossRef]
- McKay, G.A. Partitioning of rare earth elements between major silicate minerals and basaltic melts. In Geochemistry and Mineralogy of Rare Earth Elements; Lipin, B.R., McKay, G.A., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 1989; pp. 45–78. [Google Scholar]
- Lee, S.G.; Asahara, Y.; Tanaka, T.; Lee, S.R.; Lee, T. Geochemical significance of the Rb-Sr, La-Ce, and Sm-Nd isotope systems in A-type rocks with REE tetrad patterns and negative Eu and Ce anomalies: The Cretaceous Muamsa and Weolaksan granites, South Korea. Geochemistry 2013, 73, 75–88. [Google Scholar] [CrossRef]
- London, D. The application of experimental petrology to the genesis and crystallization of granitic pegmatites. Can. Mineral. 1992, 30, 499–540. [Google Scholar]
- Monecke, T.; Kempe, U.; Monecke, J.; Sala, M.; Wolf, D. Tetrad effect in rare earth element distribution patterns: A method of quantification with application to rock and mineral samples from granite-related rare metal deposits. Geochim. Cosmochim. Acta 2002, 66, 1185–1196. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.M.; El-Kibbi, M.M. Anorogenic magmatism: Chemical evolution of the Mount El-Sibai A-type complex (Egypt), and implications for the origin of within-plate felsic magmas. Geol. Mag. 2001, 138, 67–85. [Google Scholar] [CrossRef]
- Agangi, A.; Kamenetsky, V.S.; McPhie, J. The role of fluorine in the concentration and transport of lithophile trace elements in felsic magmas: Insights from the Gawler Range Volcanics, South Australia. Chem. Geol. 2010, 273, 314–325. [Google Scholar] [CrossRef]
- Surour, A.A.; Ahmed, A.H.; Harbi, H.M. Mineral chemistry as a tool for understanding the petrogenesis of Cryo-genian (arc-related) Ediacaran (post-collisional) gabbros in the western Arabian Shield of Saudi Arabia. Int. J. Earth Sci. 2017, 106, 1597–1617. [Google Scholar] [CrossRef]
- Laird, J. Chlorites: Metamorphic petrology. In Hydrous Phyllosilicates. (Exclusive of Micas); Reviews in Mineralogy; Bailey, S.W., Ed.; Walter de Gruyter GmbH & Co KG.: Berlin, Germany, 1988; pp. 405–453. [Google Scholar]
- Inoue, A.; Kurokawa, K.; Hatta, T. Application of chlorite geothermometry to hydrothermal alteration in Toyoha geothermal system, Southwestern Hokkaido, Japan. Resour. Geol. 2010, 160, 52–70. [Google Scholar] [CrossRef]
- Jowett, E.C. Fitting iron and magnesium into the hydrothermal chlorite geothermometer. In Proceedings of the GAC/MAC/SEG Joint Annual Meeting, Program with Abstracts, Toronto, ON, Canada, 27–29 May 1991; Volume 16, p. 62. [Google Scholar]
- Patiño Douce, A.E. What do experiments tell us about relative contributions of crust and mantle to the origin of granitic magma? In Understanding Granites: Integrating New and Classical Techniques; Castro, A., Fernandez, C., Vigneress, J.L., Eds.; Geological Society, London, Special Publications: London, UK, 1999; Volume 168, pp. 55–75. [Google Scholar]
- Xie, Y.W.; Zhang, Y.Q. Peculiarities and genetic significance of hornblende from granite in the Hengduansan region. Acta Mineral. Sin. 1990, 10, 35–45. [Google Scholar]
- Zhou, Z.X. The origin of intrusive mass in Fengshandong, Hubei province. Acta Petrol. Sin. 1986, 2, 59–70. [Google Scholar]
- Ali, K.A.; Kröner, A.; Hegner, E.; Wong, J.; Li, S.-Q.; Gahlan, H.A.; Abu El Ela, F.F. U-Pb zircon geochronology and Hf–Nd isotopic systematics of Wadi Beitan granitoid gneisses, Southeastern Desert, Egypt. Gondwana Res. 2015, 27, 811–824. [Google Scholar] [CrossRef]
- Stern, R.J.; Hedge, C.E. Geochronologic constraints on late Precambrian crustal evolution in the Eastern Desert of Egypt. Am. J. Sci. 1985, 285, 97–127. [Google Scholar] [CrossRef]
- Ali, K.A.; Andresen, A.; Stern, R.J.; Manton, W.I.; Omar, S.A.; Maurice, A.E. U-Pb zircon and Sr-Nd-Hf isotopic evidence for a juvenile origin of the El-Shalul Granite, Central Eastern Desert, Egypt. Geol. Mag. 2012, 149, 783–797. [Google Scholar] [CrossRef]
- Zoheir, B.; Goldfarb, R.; Holzheid, A.; Helmy, H.; El Sheikh, A. Geochemical and geochronological characteristics of the Um Rus granite intrusion and associated gold deposit, Eastern Desert, Egypt. Geosci. Front. 2019, 11, 325–345. [Google Scholar] [CrossRef]
- El Bahariya, G.A.; Abu anbar, M.M.; El Galy, M.M. Petrology and geochemistry of Um Rus and Samadi granites, central Eastern Desert, Egypt: Implications for I-type granites of variable magma sources. Ann. Geol. Surv. Egypt 2008, 39, 1–15. [Google Scholar]
- Anderson, P.V.; Kerr, B.J.; Weber, T.E.; Ziemer, C.J.; Shurson, G.C. Determination, and prediction of digestible and metabolizable energy from chemical analysis of corn coproducts fed to finishing pigs. J. Anim. Sci. 2012, 90, 1242–1254. [Google Scholar] [CrossRef]
- Ali, K.A.; Zoheir, B.A.; Stern, R.J.; Andresen, A.; Whitehouse, M.J.; Bishara, W.W. Lu-Hf and O isotopic compositions on single zircons from the Northeastern Desert of Egypt, Arabian- Nubian shield: Implications for crustal evolution. Gondwana Res. 2016, 32, 181–192. [Google Scholar] [CrossRef]
- Bentor, Y.K. The crustal evolution of the Arabo-Nubian massif with special reference to the Sinai Peninsula. Precambrian Res. 1985, 28, 1–74. [Google Scholar] [CrossRef]
- Andresen, A.; El-Rus, M.M.A.; Myhre, P.I.; Boghdady, G.Y.; Corfu, F. U-Pb TIMS age constraints on the evolution of the Neoproterozoic Meatiq Gneiss Dome, Eastern Desert, Egypt. Int. J. Earth Sci. 2009, 98, 481–497. [Google Scholar] [CrossRef]
- Andresen, A.; Abu El-Enen, M.M.; Stern, R.J.; Wilde, S.A.; Ali, K.A. The Wadi Zaghra metaconglomerates of Sinai, Egypt: New constraints on the Ediacaran tectonic evolution of the northernmost Arabian-Nubian Shield. Int. Geol. Rev. 2014, 56, 1020–1038. [Google Scholar] [CrossRef]
- Morag, N.; Avigada, D.; Gerdesb, A.; Belousovac, E.; Harlavand, Y. Crustal evolution and recycling in the northern Arabian-Nubian Shield: New perspectives from zircon Lu–Hf and U-Pb systematics. Precambrian Res. 2011, 186, 101–116. [Google Scholar] [CrossRef]
- El-Manharawy, S.M. Geochronological Investigations of Some Basement Rocks in the Central Eastern Desert, Egypt, between Latitudes 25”-26” N. Ph.D. Thesis, Cairo University, Cairo, Egypt, 1977; 220p. [Google Scholar]
- Eliwa, H.A.; Breitkreuz, C.; Murata, M.; Khalaf, I.M.; Bühler, B.; Itaya, T.; Takahashi, T.; Hirahara, Y.; Miyazaki, T.; Kimura, J.I.; et al. SIMS zircon U-Pb and mica K–Ar geochronology, and Sr-Nd isotope geochemistry of Neoproterozoic granitoids and their bearing on the evolution of the northeastern Desert, Egypt. Gondwana Res. 2014, 25, 1570–1598. [Google Scholar] [CrossRef]
- Johnson, P.R.; Andresen, A.; Collins, A.S.; Fowler, A.R.; Fritz, H.; Ghebreab, W.; Kusky, T.; Stern, R.J. Late Cryoge-nian-Ediacaran history of the Arabian-Nubian Shield: A review of depositional, plutonic, structural, and tectonic events in the closing stages of the northern East African Orogen. J. Afr. Earth Sci. 2011, 61, 167–232. [Google Scholar] [CrossRef]
- Moghazi, A.M.; Harbi, H.M.; Ali, K.A. Geochemistry of the Late Neoproterozoic Hadb adh Dayheen ring complex, Central Arabian Shield: Implications for the origin of rare-metal-bearing post-orogenic A-type granites. J. Asian Earth Sci. 2011, 42, 1324–1340. [Google Scholar] [CrossRef]
- Gahlan, H.A.; Azer, M.K.; Al-Hashim, M.H.; Heikal, M.T.S. Highly evolved rare-metal bearing granite overprinted by alkali metasomatism in the Arabian Shield: A case study from the Jabal Tawlah granites. J. Afr. Earth Sci. 2022, 192, 104556. [Google Scholar] [CrossRef]
- Yang, W.-B.; Niu, H.-C.; Hollings, P.; Zurevinski, S.E.; Bo Li, N. The role of recycled oceanic crust in the generation of alkaline A-type granites. J. Geophys. Res. Solid Earth 2017, 122, 7975–7983. [Google Scholar] [CrossRef]
- Mushkin, A.; Navon, O.; Halicz, L.; Hartmann, G.; Stein, M. The petrogenesis of A-type magmas from the Amram Massif, southern Israel. J. Petrol. 2003, 44, 815–832. [Google Scholar] [CrossRef]
- Kessel, R.; Stein, M.; Navon, O. Petrogenesis of late Neoproterozoic dikes in the northern Arabian-Nubian Shield Implication for the origin of A-type granites. Precambrian Res. 1998, 92, 195–213. [Google Scholar] [CrossRef]
- Weissman, A.; Kessel, R.; Oded, N.; Mordechai, S. The petrogenesis of calc-alkaline granites from the Elat massif, Northern Arabian–Nubian shield. Precambrian Res. 2013, 236, 252–264. [Google Scholar] [CrossRef]
- Tindle, A.G.; Webb, P.C. Estimation of lithium contents in trioctahedral micas using microprobe data: Application to micas from granitic rocks. Eur. J. Mineral. 1990, 2, 595–610. [Google Scholar] [CrossRef]





| Sample # | FS1 | FS11 | FS2 | FS5 | FS6d | FS7 | S26 | S28 | S29 | S30 | S31 | S33 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major oxides (wt.%) | ||||||||||||
| SiO2 | 74.33 | 74.74 | 76.32 | 73.91 | 77.88 | 74.84 | 77.18 | 73.56 | 75.65 | 73.41 | 75.28 | 75.08 |
| TiO2 | 0.05 | 0.05 | 0.05 | 0.09 | 0.05 | 0.07 | 0.02 | 0.06 | 0.05 | 0.06 | 0.06 | 0.03 |
| Al2O3 | 13.61 | 13.05 | 12.42 | 13.08 | 12.57 | 13.37 | 12.6 | 13.8 | 12.96 | 13.53 | 13.28 | 12.96 |
| Fe2O3 | 0.91 | 1.31 | 0.34 | 0.78 | 0.36 | 0.57 | 0.56 | 1.18 | 0.76 | 1.47 | 0.67 | 0.79 |
| MnO | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.03 | 0.01 | 0.01 |
| MgO | 0.14 | 0.14 | 0.1 | 0.15 | 0.08 | 0.16 | 0.07 | 0.16 | 0.15 | 0.17 | 0.13 | 0.14 |
| CaO | 0.73 | 0.76 | 0.75 | 1.19 | 0.61 | 0.83 | 0.56 | 0.94 | 0.64 | 0.88 | 0.84 | 0.77 |
| Na2O | 4.05 | 2.87 | 2.66 | 3.46 | 1.04 | 3.92 | 3.84 | 4.09 | 3.44 | 3.89 | 3.74 | 3.96 |
| K2O | 4.69 | 5.08 | 4.48 | 4.24 | 4.27 | 4.23 | 4.31 | 4.77 | 4.44 | 4.72 | 4.74 | 4.69 |
| P2O5 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| LOI | 1.11 | 1.42 | 1.74 | 2.13 | 2.33 | 2.04 | 1.06 | 1.87 | 1.68 | 1.73 | 1.43 | 1.38 |
| Total | 99.65 | 99.45 | 98.88 | 99.07 | 99.22 | 100.05 | 100.23 | 100.45 | 99.79 | 99.9 | 100.19 | 99.82 |
| Trace elements (ppm) | ||||||||||||
| Rb | 218.94 | 205.71 | 247.81 | 201.62 | 272.77 | 212.06 | 224.59 | 191.59 | 212.44 | 206.48 | 237.84 | 215.57 |
| Ba | 256.83 | 326.31 | 394.28 | 309.47 | 278.8 | 177.01 | 154.51 | 371.04 | 152.34 | 170.52 | 156.51 | 115.23 |
| Sr | 47.61 | 41.79 | 33.59 | 51.97 | 30.75 | 39.74 | 30.92 | 52.09 | 37.8 | 50.73 | 38.58 | 39.96 |
| Nb | 153.67 | 155.18 | 171.82 | 141.83 | 246.72 | 164.96 | 171.76 | 156.52 | 170.69 | 147.39 | 172.34 | 168.27 |
| Zr | 284.54 | 285.62 | 260.56 | 232.17 | 320.55 | 311.42 | 250.38 | 285.32 | 285.19 | 271.93 | 314.08 | 302.31 |
| Y | 87.21 | 87.6 | 92.42 | 81.55 | 106.17 | 82.68 | 98.98 | 79.61 | 96.54 | 78.41 | 90.93 | 98.17 |
| Zn | 9.98 | 11.83 | 26.71 | 17.66 | 8.35 | 7.91 | 9.19 | 10.73 | 11.22 | 24.93 | 8.89 | 10.47 |
| Cu | <d.l. | 1.55 | 1.83 | 2.06 | 1.38 | <d.l. | 1.31 | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. |
| Ni | 12.32 | 11.89 | 7.89 | 14.67 | 3.96 | 10.17 | 7.98 | 16.41 | 8.87 | 15.23 | 8.22 | 10.05 |
| Co | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. |
| Cr | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. |
| V | 16.07 | 14.6 | 12.18 | 15.76 | 9.95 | 12.86 | 10.68 | 15.33 | 13.15 | 16.03 | 13.53 | 12.13 |
| Pb | 12.8 | 11.22 | 9.1 | 22.36 | 7.77 | 10.74 | 9.99 | 17.69 | 3.93 | 18.42 | 13.39 | 10.25 |
| Ga | 40.47 | 35.31 | 43.16 | 33.47 | 44.84 | 35.78 | 40.12 | 31.82 | 40.38 | 34.03 | 39.29 | 37.65 |
| Sc | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. |
| Mo | 1.99 | 1.89 | 2.27 | 1.08 | 4.18 | 1.58 | 2.19 | 1.49 | 3.24 | 1.59 | 1.79 | 1.77 |
| Cs | 0.5 | 0.64 | 0.73 | 0.48 | 1.09 | 0.76 | 0.73 | 0.27 | 0.71 | 0.46 | 0.78 | 0.66 |
| Hf | 7.11 | 7.68 | 9.54 | 9.54 | 11.22 | 9.09 | 8.31 | 6.85 | 7.46 | 9.48 | 7.85 | 11.02 |
| Ta | 17.94 | 18.56 | 26.13 | 14.15 | 42.28 | 20.37 | 33.54 | 17.93 | 21.57 | 19.93 | 20.31 | 19.67 |
| Th | 34.36 | 33.6 | 44.12 | 28.13 | 48.45 | 36.93 | 43.49 | 28.71 | 36.45 | 26.95 | 33.56 | 35.82 |
| U | 9.33 | 11.25 | 11.82 | 5.75 | 12.13 | 11.76 | 12.02 | 8.04 | 11.15 | 9.65 | 10.82 | 9.88 |
| Rare-earth elements (ppm) | ||||||||||||
| La | 47.42 | 49.33 | 50.94 | 24.93 | 32.51 | 33.56 | 29.69 | 43.86 | 38.88 | 45.19 | 35.73 | 32.74 |
| Ce | 100.95 | 108.81 | 110.64 | 57.33 | 77.4 | 78.11 | 66.13 | 94 | 84.15 | 100.92 | 85.58 | 75.65 |
| Pr | 12.4 | 12.59 | 12.87 | 7.54 | 9.7 | 9.71 | 8.41 | 10.6 | 9.54 | 11.37 | 10.94 | 9.33 |
| Nd | 42.8 | 42.34 | 47.48 | 29.12 | 35.28 | 36.48 | 31.47 | 38.32 | 32.64 | 40.61 | 40.42 | 35.1 |
| Sm | 8.71 | 8.74 | 10.09 | 6.61 | 7.92 | 7.43 | 6.92 | 8.17 | 7.14 | 9.02 | 8.87 | 7.93 |
| Eu | 0.93 | 0.91 | 1.02 | 0.79 | 1.19 | 0.59 | 0.62 | 1.03 | 0.74 | 0.7 | 0.78 | 0.61 |
| Gd | 11.15 | 10.61 | 12.24 | 7.67 | 8.81 | 7.96 | 7.95 | 9.48 | 9.44 | 10.31 | 10.22 | 9.21 |
| Tb | 1.99 | 1.75 | 1.95 | 1.32 | 1.56 | 1.42 | 1.35 | 1.62 | 1.59 | 1.78 | 1.8 | 1.56 |
| Dy | 12.35 | 11.9 | 12.45 | 8.57 | 11.35 | 10.05 | 8.78 | 10.95 | 10.21 | 12.07 | 12.53 | 11.01 |
| Ho | 2.76 | 2.62 | 2.86 | 1.91 | 2.49 | 2.27 | 2.16 | 2.37 | 2.33 | 2.66 | 2.83 | 2.52 |
| Er | 8.94 | 8.54 | 9.17 | 5.36 | 8.91 | 7.79 | 6.43 | 7.57 | 7.27 | 8.59 | 9.91 | 9.02 |
| Tm | 1.52 | 1.46 | 1.55 | 0.89 | 1.61 | 1.25 | 1.11 | 1.25 | 1.22 | 1.48 | 1.78 | 1.55 |
| Yb | 10.31 | 10.4 | 10.78 | 6.08 | 11.16 | 9.01 | 7.96 | 8.81 | 9.01 | 10.12 | 12.52 | 11.38 |
| Lu | 1.42 | 1.43 | 1.53 | 1.04 | 1.71 | 1.25 | 1.23 | 1.26 | 1.29 | 1.47 | 1.83 | 1.72 |
| Eu* | 10.39 | 10.49 | 11.4 | 7.06 | 8.76 | 8.49 | 7.63 | 9.31 | 8.25 | 10.13 | 9.85 | 8.6 |
| Eu/Eu* | 4.12 | 4.04 | 4.17 | 4.12 | 4.03 | 4.29 | 4.13 | 4.12 | 3.95 | 4.01 | 4.1 | 4.08 |
| (La/Yb)n | 3.84 | 3.93 | 4.81 | 5.25 | 5.44 | 4.74 | 6.76 | 3.79 | 5.01 | 3.14 | 3.39 | 3.97 |
| ((La/Sm)n | 2.77 | 2.67 | 3.43 | 3.73 | 4.99 | 3.8 | 5.17 | 2.71 | 3.82 | 2.37 | 3.07 | 3.84 |
| (Gd/Lu)n | 5.73 | 5.99 | 6.51 | 7.43 | 4.92 | 5.94 | 6.23 | 6.54 | 5.85 | 6.09 | 4.98 | 5.12 |
| (La/Lu)n | 22.61 | 23.01 | 28.46 | 31.61 | 30.09 | 29.54 | 39.18 | 22.97 | 29.88 | 18.21 | 18.85 | 23.11 |
| T1.3 | 1.04 | 1.04 | 0.99 | 1.02 | 1.06 | 1.04 | 0.99 | 1.02 | 1.02 | 1.04 | 1.04 | 1.02 |
| ΣREEs | 263.65 | 271.43 | 285.57 | 159.16 | 211.6 | 206.88 | 180.21 | 239.29 | 215.45 | 256.29 | 235.74 | 209.33 |
| Y/Ho | 31.6 | 33.44 | 32.31 | 42.7 | 42.64 | 36.42 | 45.82 | 33.59 | 41.43 | 29.48 | 32.13 | 38.96 |
| T (°C) Zr | 842 | 852 | 850 | 824 | 905 | 854 | 834 | 837 | 852 | 835 | 851 | 843 |
| Alkali Feldspar Granite | Monzogranite | Granodiorite | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample # | FS13 | FS13A | S7b | S35A | S8 | S13 | S18 | S20 | FS23 | S39 | FS12 | FS15 | S35b | S1 |
| Major oxides (wt.%) | ||||||||||||||
| SiO2 | 72.78 | 69.66 | 66.64 | 71.19 | 73.68 | 74.37 | 74.48 | 74.12 | 74.53 | 75.42 | 74.37 | 75.52 | 64.9 | 63.92 |
| TiO2 | 0.07 | 0.18 | 0.33 | 0.13 | 0.31 | 0.29 | 0.36 | 0.31 | 0.26 | 0.21 | 0.29 | 0.29 | 0.66 | 0.69 |
| Al2O3 | 13.78 | 14.12 | 15.48 | 14.34 | 13.45 | 13.09 | 13.77 | 12.76 | 13.17 | 12.87 | 13.09 | 12.61 | 16.31 | 15.86 |
| Fe2O3 | 1.82 | 2.93 | 3.21 | 2.28 | 1.79 | 1.45 | 1.28 | 1.56 | 1.58 | 1.3 | 1.45 | 1.21 | 4.72 | 4.81 |
| MnO | 0.03 | 0.06 | 0.09 | 0.03 | 0.03 | 0.01 | 0.02 | 0.04 | 0.05 | 0.03 | 0.01 | 0.04 | 0.08 | 0.07 |
| MgO | 0.19 | 0.21 | 0.24 | 0.2 | 0.33 | 0.32 | 0.38 | 0.35 | 0.3 | 0.27 | 0.32 | 0.31 | 1.72 | 1.69 |
| CaO | 1.02 | 1.11 | 1.29 | 1.08 | 0.56 | 0.54 | 0.55 | 0.56 | 0.52 | 0.46 | 0.54 | 0.49 | 3.56 | 3.58 |
| Na2O | 4.56 | 4.64 | 4.48 | 4.59 | 3.49 | 3.44 | 3.37 | 3.43 | 3.53 | 3.45 | 3.44 | 3.28 | 4.16 | 4.41 |
| K2O | 4.88 | 5.04 | 4.37 | 5.01 | 4.58 | 4.78 | 4.54 | 4.44 | 4.64 | 4.75 | 4.78 | 4.67 | 2.74 | 2.28 |
| P2O5 | 0.02 | 0.04 | 0.12 | 0.03 | 0.06 | 0.05 | 0.07 | 0.04 | 0.04 | 0.04 | 0.05 | 0.04 | 0.15 | 0.14 |
| LOI | 1.22 | 1.36 | 2.91 | 1.23 | 1.25 | 1.52 | 1.12 | 0.92 | 0.83 | 1.02 | 1.52 | 1.33 | 1.14 | 1.95 |
| Total | 100.37 | 99.35 | 99.16 | 100.1 | 99.53 | 99.86 | 99.94 | 98.53 | 99.45 | 99.82 | 99.86 | 99.79 | 100.1 | 99.4 |
| Trace elements (ppm) | ||||||||||||||
| Rb | 179.66 | 170.87 | 167.2 | 172.4 | 56.26 | 79.86 | 96.66 | 84.48 | 81.84 | 90.86 | 60.48 | 122.49 | 80.77 | 60.48 |
| Ba | 300.73 | 409.67 | 468.4 | 402.5 | 1012.6 | 765.5 | 812.5 | 768.0 | 551.9 | 977.3 | 404.1 | 741.54 | 378.2 | 404.1 |
| Sr | 57.14 | 66.7 | 69.84 | 61.42 | 189.64 | 175.8 | 153.1 | 139.7 | 126.3 | 123.5 | 272.6 | 133.71 | 267.9 | 272.6 |
| Nb | 132.41 | 124.59 | 112.6 | 119.6 | 9.89 | 9.05 | 11.71 | 11.53 | 13.03 | 15.21 | 7.65 | 19.27 | 8.38 | 7.65 |
| Zr | 372.31 | 512.18 | 507.4 | 439.5 | 257.33 | 256.4 | 243.6 | 240.3 | 236.9 | 224.8 | 282.6 | 217.75 | 290.2 | 282.6 |
| Y | 88.55 | 75.33 | 56.89 | 76.89 | 47.68 | 46.79 | 48.82 | 46.28 | 51.51 | 54.58 | 29.97 | 57.69 | 30.78 | 29.97 |
| Zn | 57.24 | 96.41 | 60.88 | 92.11 | 52.79 | 59.03 | 203.9 | 226.3 | 60.04 | 51.28 | 56.46 | 75.51 | 57.78 | 56.46 |
| Cu | <d.l. | <d.l. | <d.l. | <d.l. | 5.97 | 4.79 | 4.43 | 3.76 | 3.68 | 2.71 | 10.76 | 2.33 | 11.71 | 10.76 |
| Ni | 16.42 | 18.17 | 21.05 | 17.22 | 5.49 | 3.91 | 2.04 | <d.l. | <d.l. | <d.l. | 5.03 | <d.l. | 4.15 | 5.03 |
| Co | <d.l. | 2.24 | 3.1 | <d.l. | 4.46 | 3.07 | <d.l. | 2.14 | 2.72 | 1.5 | 9.52 | 2.42 | 10.28 | 9.52 |
| Cr | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | <d.l. | 24.33 | <d.l. | 16.47 | 24.33 |
| V | 17.03 | 18.37 | 19.62 | 16.98 | 34.97 | 28.74 | 29.26 | 21.61 | 22.3 | 12.14 | 47.76 | 9.18 | 45.54 | 47.76 |
| Pb | 12.79 | 16.37 | 18.66 | 17.61 | 27.9 | 11.66 | 27.01 | 37.93 | 16.03 | 14.66 | 18.56 | 15.02 | 17.66 | 18.56 |
| Ga | 36.11 | 31.05 | 27.29 | 35.77 | 20.04 | 19.23 | 20.59 | 21.93 | 24.04 | 22.23 | 21.36 | 25.13 | 21.59 | 21.36 |
| Sc | <d.l. | <d.l. | <d.l. | <d.l. | 0.57 | 0.53 | 0.46 | 0.48 | 0.31 | 0.24 | 7.01 | 0.19 | 6.86 | 7.01 |
| Mo | 5.05 | 5.12 | 4.75 | 4.03 | 0.94 | 1.44 | 1.88 | 2.32 | 2.38 | 2.04 | 2.25 | 3.37 | 1.57 | 2.25 |
| Cs | 0.83 | 0.43 | 0.51 | 0.79 | 0.38 | 0.48 | 0.41 | 0.52 | 0.48 | 0.59 | 0.69 | 0.65 | 1.05 | 0.69 |
| Hf | 12.68 | 13.18 | 12.96 | 10.53 | 6.67 | 6.27 | 7.09 | 6.76 | 6.66 | 7.11 | 4.72 | 7.37 | 5.57 | 4.72 |
| Ta | 16.55 | 9.74 | 8.05 | 12.23 | 0.25 | 0.22 | 0.28 | 0.44 | 0.35 | 0.47 | 0.05 | 0.59 | 0.09 | 0.05 |
| Th | 26.25 | 18.71 | 16.44 | 20.44 | 5.27 | 4.83 | 5.35 | 4.74 | 5.73 | 5.24 | 3.96 | 6.34 | 4.12 | 3.96 |
| U | 8.75 | 7.21 | 5.61 | 7.66 | 2.22 | 2.16 | 2.48 | 2.76 | 2.42 | 3.21 | 1.6 | 3.78 | 1.25 | 1.6 |
| Rare-earth elements (ppm) | ||||||||||||||
| La | 88.63 | 57.59 | 44.24 | 69.85 | 27.84 | 24.03 | 36.13 | 31.34 | 43.27 | 39.91 | 16.88 | 41.65 | 19.68 | 16.88 |
| Ce | 257.77 | 166.45 | 142.7 | 212.9 | 73.07 | 63.12 | 91.23 | 81.61 | 101.8 | 96.42 | 38.66 | 101.95 | 45.21 | 38.66 |
| Pr | 26.29 | 17.63 | 15.35 | 21.45 | 8.98 | 7.27 | 12.24 | 10.21 | 11.6 | 13.84 | 4.97 | 13.99 | 5.7 | 4.97 |
| Nd | 90.14 | 60.51 | 52.3 | 68.63 | 34.48 | 27.01 | 45.41 | 40.3 | 41.86 | 50.34 | 20.28 | 52.59 | 22.97 | 20.28 |
| Sm | 18.25 | 12.02 | 9.66 | 13.49 | 7.03 | 5.99 | 8.44 | 7.98 | 9.51 | 10.8 | 4.59 | 11.02 | 4.95 | 4.59 |
| Eu | 1.52 | 1.42 | 0.88 | 1.28 | 1.16 | 1.47 | 1.58 | 1.44 | 1.82 | 1.74 | 1.41 | 1.91 | 1.45 | 1.41 |
| Gd | 19.9 | 13.06 | 8.64 | 15.46 | 8.04 | 6.36 | 9.78 | 9.12 | 11.17 | 10.94 | 4.39 | 13.81 | 5.17 | 4.39 |
| Tb | 2.81 | 1.81 | 1.29 | 2.37 | 1.17 | 0.95 | 1.53 | 1.29 | 1.72 | 1.48 | 0.71 | 2.04 | 0.82 | 0.71 |
| Dy | 16.16 | 10.88 | 7.97 | 14.01 | 6.73 | 5.79 | 8.72 | 7.37 | 9.56 | 8.46 | 4.41 | 11.59 | 5.04 | 4.41 |
| Ho | 3.31 | 2.18 | 1.61 | 2.77 | 1.28 | 1.15 | 1.6 | 1.38 | 1.79 | 1.6 | 0.93 | 2.2 | 1.08 | 0.93 |
| Er | 9.66 | 6.16 | 4.69 | 7.95 | 3.42 | 3.12 | 4.44 | 3.67 | 4.79 | 4.26 | 2.63 | 5.53 | 3.05 | 2.63 |
| Tm | 1.46 | 0.99 | 0.74 | 1.18 | 0.51 | 0.45 | 0.64 | 0.55 | 0.69 | 0.57 | 0.41 | 0.79 | 0.46 | 0.41 |
| Yb | 9.82 | 6.76 | 5.17 | 8.22 | 3.38 | 3.06 | 4.13 | 3.59 | 4.63 | 3.86 | 2.74 | 5.34 | 3.07 | 2.74 |
| Lu | 1.6 | 1.03 | 0.81 | 1.35 | 0.51 | 0.45 | 0.64 | 0.55 | 0.68 | 0.56 | 0.44 | 0.8 | 0.48 | 0.44 |
| Eu* | 21.9 | 14.56 | 12.18 | 17.01 | 7.95 | 6.6 | 10.16 | 9.03 | 10.5 | 12.23 | 4.78 | 12.42 | 28.54 | 81.48 |
| Eu/Eu* | 4.12 | 4.16 | 4.29 | 4.03 | 4.34 | 4.09 | 4.47 | 4.46 | 3.99 | 4.12 | 4.25 | 4.24 | 0.88 | 0.27 |
| (La/Yb)n | 2.72 | 3.04 | 3.51 | 2.57 | 1.54 | 1.55 | 1.2 | 1.29 | 1.2 | 1.23 | 1.51 | 1.15 | 4.33 | 5.75 |
| ((La/Sm)n | 1 | 1.06 | 1.07 | 0.95 | 0.59 | 0.66 | 0.44 | 0.46 | 0.49 | 0.38 | 0.8 | 0.45 | 2.51 | 3.27 |
| (Gd/Lu)n | 12.5 | 12.14 | 13.05 | 11.43 | 13.78 | 13.31 | 13.19 | 14.51 | 13.78 | 18.95 | 11.2 | 13.95 | 1.32 | 1.4 |
| (La/Lu)n | 17.98 | 18.9 | 22.22 | 17.32 | 10.33 | 10.73 | 8.36 | 8.62 | 8.3 | 9.19 | 9.66 | 8.03 | 4.2 | 5.3 |
| T1.3 | 1.08 | 1.08 | 1.13 | 1.15 | 1.05 | 1.06 | 1.08 | 1.04 | 1.06 | 1.03 | 1 | 1.04 | 1 | 1.15 |
| ΣREEs | 547.32 | 358.49 | 296.0 | 441 | 177.6 | 150.2 | 226.5 | 200.4 | 244.9 | 244.7 | 103.4 | 265.21 | 119.1 | 103.4 |
| Y/Ho | 26.75 | 34.56 | 35.34 | 27.76 | 37.25 | 40.69 | 30.51 | 33.54 | 28.78 | 34.11 | 32.23 | 26.22 | 28.5 | 32.23 |
| T (°C) Zr | 851 | 878 | 892 | 858 | 842 | 839 | 842 | 833 | 832 | 827 | 848 | 825 | 821 | 815 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Fatah, A.A.; Surour, A.A.; Azer, M.K.; Madani, A.A. Integration of Whole-Rock Geochemistry and Mineral Chemistry Data for the Petrogenesis of A-Type Ring Complex from Gebel El Bakriyah Area, Egypt. Minerals 2023, 13, 1273. https://doi.org/10.3390/min13101273
Abd El-Fatah AA, Surour AA, Azer MK, Madani AA. Integration of Whole-Rock Geochemistry and Mineral Chemistry Data for the Petrogenesis of A-Type Ring Complex from Gebel El Bakriyah Area, Egypt. Minerals. 2023; 13(10):1273. https://doi.org/10.3390/min13101273
Chicago/Turabian StyleAbd El-Fatah, Ahmed A., Adel A. Surour, Mokhles K. Azer, and Ahmed A. Madani. 2023. "Integration of Whole-Rock Geochemistry and Mineral Chemistry Data for the Petrogenesis of A-Type Ring Complex from Gebel El Bakriyah Area, Egypt" Minerals 13, no. 10: 1273. https://doi.org/10.3390/min13101273
APA StyleAbd El-Fatah, A. A., Surour, A. A., Azer, M. K., & Madani, A. A. (2023). Integration of Whole-Rock Geochemistry and Mineral Chemistry Data for the Petrogenesis of A-Type Ring Complex from Gebel El Bakriyah Area, Egypt. Minerals, 13(10), 1273. https://doi.org/10.3390/min13101273





