Proterozoic Deep Carbon—Characterisation, Origin and the Role of Fluids during High-Grade Metamorphism of Graphite (Lofoten–Vesterålen Complex, Norway)
Abstract
:1. Introduction
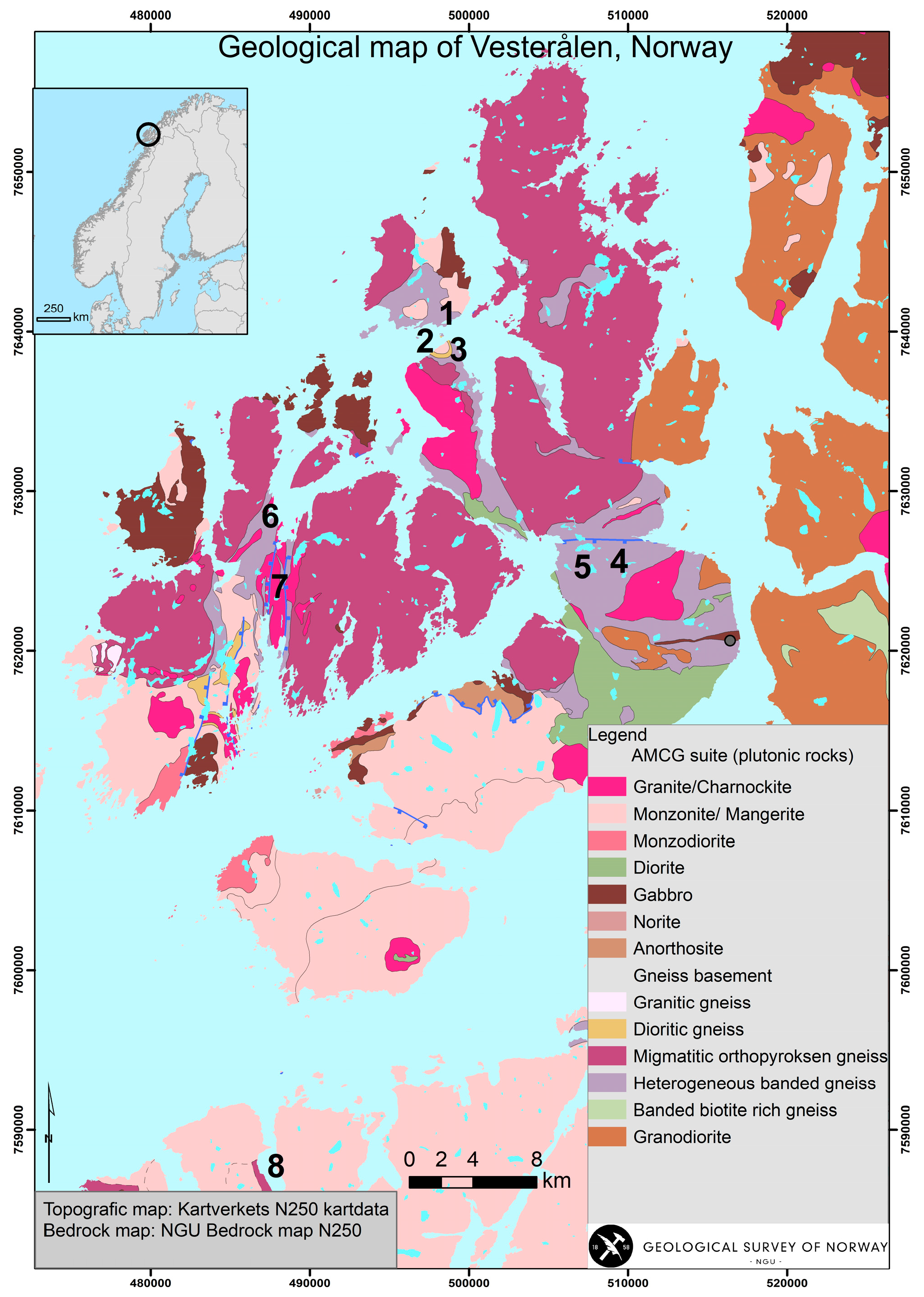
Geological Setting
2. Materials and Methods
2.1. Petrological Investigations
2.2. Whole Rock Chemistry, Total Carbon (TC), Total Organic Carbon (TOC) and Total Sulphur (TS)
2.3. Stable Isotopes
3. Results
3.1. Field Relations
3.2. Petrography and Mineral Chemistry
3.3. Petrology
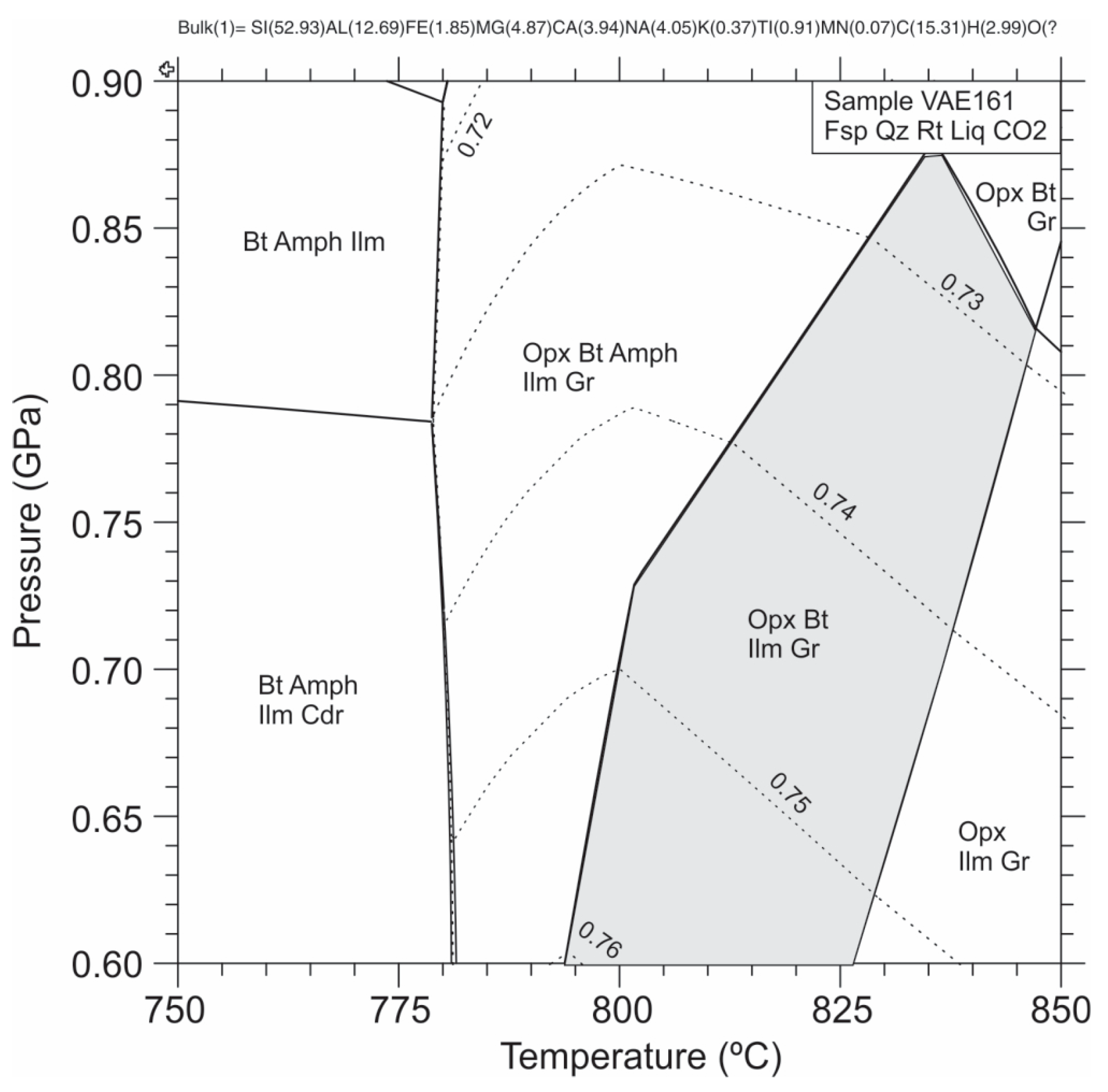
3.3.1. Pressure–Temperature Pseudosection Modelling
3.3.2. Zr-in-Rutile Thermometry
| Locality | Rock Type | Sample No. | Analyse | Zr ppm | P (kbar) | T (°C) | T (°C) | T (°C) |
|---|---|---|---|---|---|---|---|---|
| (assumed) | (57) | (56) | (55) | |||||
| Svinøy | Graphite schist | VAE159 | #18-19-20 | 991 | 7.5 | 751 | 742 | 737 |
| (Loc. 2) | #21-23 | 893 | 7.5 | 741 | 732 | 726 | ||
| Smines | Opx-gneis | VAE164 | #13 | 1855 | 7.5 | 819 | 806 | 813 |
| (Loc. 3) | (host rock) | #11-13-19 | 1369 | 7.5 | 785 | 774 | 775 | |
| Møkland | Opx-gneis | BH1BØ4-60 | #7-10 | 2476 | 7.5 | 854 | 838 | 851 |
| (Loc. 6) | (host rock) | #9 | 2046 | 7.5 | 831 | 817 | 825 |
3.4. Carbon and Oxygen Isotopes
| Sample | Rock Type | δ13C/δ12CVPDB Graphite | δ13C/δ12CVPDB Calcite | δ18O/δ16OVPDB | Δ13C Calcite and Graphite |
|---|---|---|---|---|---|
| SOM1701-59 AV (1) | Graphite schist/marble | −11.50 | −9.52 | −15.40 | 1.98 |
| SOM1701-60 AV (2) | Graphite schist/marble | −10.26 | −8.63 | −15.29 | 1.63 |
| SOM1701-61 AV (3) | Graphite schist/calc silicate | −8.88 | −8.56 | −15.59 | 0.32 |
| SOM1702-25 AV | Graphite schist | −30.18 | |||
| VAE146 | Graphite schist | −27.97 | |||
| VAE147 | Graphite schist | −28.01 | |||
| VAE159 | Graphite schist | −30.41 | |||
| VAE161 | Graphite schist | −32.02 | |||
| VAE163 | Graphite schist | −29.77 | |||
| VAE165 | Graphite schist | −38.67 | |||
| VAE166B | Graphite schist | −24.01 | |||
| VAE171 | Graphite schist | −21.01 | |||
| BH1BØ30.10 | Graphite schist | −17.52 | |||
| VAE206 | Graphite schist | −28.89 | |||
| VAE231 | Graphite schist | −23.19 | |||
| VAE232 | Graphite schist | −23.91 | |||
| VAE120 | Marble | 9.42 | −9.56 | ||
| VAE118 | Marble | 10.38 | −7.53 | ||
| VAE221 | Marble | 9.95 | −9.93 | ||
| VAE222 | Marble | 10.04 | −9.96 | ||
| VAE167 | Marble * | 6.14 | −13.60 | ||
| VAE172 | Marble * | 3.53 | −15.44 | ||
| VAE141 | Marble | 10.27 | −9.81 | ||
| VAE143C | Marble | 9.81 | −8.82 | ||
| VAE178 | Marble | 8.14 | −13.70 | ||
| VAE176 | Marble | 6.45 | −13.45 | ||
| VAE235 | Marble | 9.09 | −11.17 | ||
| VAE239 | Marble | 10.30 | −9.25 |
4. Discussion
4.1. Origin of C and Metamorphic Formation of High-Ordered Graphite
4.2. Granulite Facies Metamorphic Formation of High-Ordered Graphite
4.3. Role of Fluids
2 K(FeMg)3AlSi3O10(OH)2 + 6 SiO2 + CO2 => 3 (FeMg)2Si2O6 + 2 KAlSi3O8 + C + 2 H2O + O2
CaMg(CO3)2 + 2 SiO2 => CaMgSi2O6 + 2 CO2
2 CaMg(CO3)2 + SiO2 + O2 => Mg2SiO4 + 2 CaCO3 + 2 CO2
CaMg(CO3)2 + 2 SiO2 => CaMgSi2O6 + 2 C + 2 O2
2 CaMg(CO3)2 + SiO2 => Mg2SiO4 + 2 CaCO3 + 2 C + O2
5. Conclusions
- -
- High-ordered graphite occurs in assemblage with metamorphic orthopyroxene in granulite-facies gneisses. Pseudosection modelling of the graphite + orthopyroxene (Mg# = 0.74) + plagioclase + biotite + quartz + rutile + ilmenite assemblage constrains its stability field to pressure–temperature conditions of 810–835 °C and 0.73–0.77 GPa. Zr-in-rutile supports a temperature of formation of 726–854 °C, while thermometry based on the isotopic equilibrium between carbonate and graphitic carbon gives additional support to the high-grade metamorphic conditions ranging from 850 to 900 °C.
- -
- The graphite schist is hosted in sequences of banded orthopyroxene gneisses interlayered with horizons of marble, calcsilicate rocks and amphibolite. Graphite (modality < 39%) occurs in an assemblage with quartz, plagioclase (Ab47–93An5–52), orthopyroxene (En69–74Fs26–29; Mg# = 0.70–0.74), clinopyroxene (En33–53Fs1–14Wo44–53; Mg# = 0.70–0.97), biotite (Mg# = 0.67–0.91; Ti < 0.66 a.p.f.u.) and K-feldspar (Ab1–8Kfs92–99) or perthite (Ab35–64An3Kfs50–62), in addition to local epidote, clinozoisite, scapolite (Me36–37; S = 0.05–0.07 a.p.f.u.; Cl = 0.66 a.p.f.u), white mica and garnet (Alm16Prp47–48Grs5Sps31–32). Graphite schist is enriched in sulphides (pyrite, pyrrhotite and chalcopyrite) with additional accessories of apatite, rutile, titanite and ilmenite.
- -
- Stable C and O isotopes indicate an organic origin of graphite but with overprinting signatures of metamorphic and hydrothermal processes. Stable C isotopes support a source of organic carbon accumulated in sediments contemporaneous with the Early Proterozoic global Lomagundi–Jatuli isotopic excursion; the δ13Cgraphite of graphite schist is −38 to −17‰, while δ13Ccalcite values of marbles range from +3‰ to +10‰. Mixed graphitic and calcite carbon samples give lighter values for the calcite (δ13Ccalcite = −8.7‰ to −9.5‰) and heavier values for graphite (δ13Cgrapite = −11.5‰ to −8.9‰), indicating isotopic exchange between graphite and calcite during high-grade metamorphism. The δ18Ocalcite of marble shows relatively light values ranging from −15.4‰ to −7.5‰, possibly reflecting re-equilibration by metamorphic and fluid processes.
- -
- The proposed mineral reaction equations illustrate the production and consumption of COH fluids, leading to the stabilisation of graphite, orthopyroxene, carbonate and silicate minerals during high-grade metamorphism.
- -
- The high Mg# ratio of biotite and pyroxenes, together with a high Cl-F content of apatite (Cl < 2 a.p.f.u.; F < 1.44 a.p.f.u), supports the importance of fluid transport during the high-grade re-equilibration of graphite.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buseck, P.R.; Beyssac, O. From Organic Matter to Graphite: Graphitization. Elements 2014, 10, 421–426. [Google Scholar] [CrossRef]
- Case, G.N.D.; Karl, S.M.; Regan, S.P.; Johnson, C.A.; Ellison, E.T.; Caine, J.S.; Holm-Denoma, C.S.; Pianowski, L.S.; Marsh, J.H. Insights into the metamorphic history and origin of flake graphite mineralization at the Graphite Creek graphite deposit, Seward Peninsula, Alaska, USA. Miner. Depos. 2023, 58, 939–962. [Google Scholar] [CrossRef]
- Rumble, D. Hydrothermal graphitic carbon. Elements 2014, 10, 427–433. [Google Scholar] [CrossRef]
- Luque, F.; Huizenga, J.; Crespo-Feo, E.; Wada, H.; Ortega, L.; Barrenechea, J. Vein graphite deposits: Geological settings, origin, and economic significance. Miner. Depos. 2014, 49, 261–277. [Google Scholar] [CrossRef]
- Touret, J.L.R.; Huizenga, J.M.; Kehelpannala, K.V.W.; Piccoli, F. Vein-type graphite deposits in Sri Lanka: The ultimate fate of granulite fluids. Chem. Geol. 2019, 508, 167–181. [Google Scholar] [CrossRef]
- Santosh, M.; Wada, H. Microscale isotopic zonation in graphite crystals: Evidence for channelled CO influx in granulites. Earth Planet. Sci. Lett. 1993, 119, 19–26. [Google Scholar] [CrossRef]
- Boyd, R.; Nordgulen, Ø.; Thomas, R.J.; Bingen, B.; Bjerkgård, T.; Grenne, T.; Henderson, I.; Melezhik, V.A.; Often, M.; Sandstad, J.S.; et al. The geology and geochemistry of the East African orogen in North eastern Mosambique. S. Afr. J. Geol. 2010, 113, 87–129. [Google Scholar] [CrossRef]
- Collins, A.S.; Kinny, P.D.; Razakamanana, T. Depositional age, provenance and metamorphic age of metasedimentary rocks from southern Madagascar. Gondwana Res. 2012, 21, 353–361. [Google Scholar] [CrossRef]
- Rosing-Schow, N.; Bagas, L.; Kolb, J.; Balić-Žunić, T.; Korte, C.; Fiorentini, M.L. Hydrothermal flake graphite mineralisation in Paleoproterozoic rocks of south-east Greenland. Miner. Depos. 2017, 52, 769–789. [Google Scholar] [CrossRef]
- Gautneb, H.; Athola, T.; Torbjörn, B.; Gonzales, Y.; Aanders, H.; Schciptsov, V.; Voytekhovsky, Y. Industrial Mineral map of the Fennoscandian shield. In Proceedings of the 12th Biennal SGA Meeting, Uppsala, Sweden 2013; pp. 1767–1770. [Google Scholar]
- Palosaari, J.; Latonen, R.M.; Smått, J.H.; Raunio, S.; Eklund, O. The flake graphite prospect of Piippumäki—An example of a high-quality graphite occurrence in a retrograde metamorphic terrain in Finland. Miner. Depos. 2020, 55, 1647–1660. [Google Scholar] [CrossRef]
- Al-Ani, T.; Leinonen, S.; Ahtola, T.; Salvador, D. High-Grade Flake Graphite Deposits in Metamorphic Schist Belt, Central Finland—Mineralogy and Beneficiation of Graphite for Lithium-Ion Battery Applications. Minerals 2020, 10, 680. [Google Scholar] [CrossRef]
- Gautneb, H.; Tveten, E. The geology, exploration and characterisation of graphite deposits in the Jennestad area, Vesterålen, northern Norway. Nor. Geol. Undersøkelse Bull. 2000, 436, 67–74. [Google Scholar]
- Palosaari, J.; Latonen, R.-M.; Smått, J.-H.; Blomqvist, R.; Eklund, O. High-quality flake graphite occurrences in a high-grade metamorphic region in Sortland, Vesterålen, northern Norway. Nor. J. Geol. 2016, 96, 19–26. [Google Scholar] [CrossRef]
- Gautneb, H.; Rønning, J.S.; Engvik, A.K.; Henderson, I.H.C.; Larsen, B.E.; Solberg, J.K.; Ofstad, F.; Gellein, J.; Elvebakk, H.; Davidsen, B. The Graphite Occurrences of Northern Norway, a Review of Geology, Geophysics, and Resources. Minerals 2020, 10, 626. [Google Scholar] [CrossRef]
- Gautneb, H.; Rønning, J.S.; Larsen, B.E. A step towards meeting battery raw material demand: The geology and exploration of graphite deposits, examples from northern Norway. Geol. Soc. Lond. Spec. Publ. 2022, 526, 251–265. [Google Scholar] [CrossRef]
- Al-Ani, T.; Ahtola, T.; Cutts, K.; Torppa, A. Metamorphic evolution of graphite in the Paleoproterozoic Savo Schist Belt (SSB), Central Finland: Constraints from geothermetric modeling. Ore Geol. Rev. 2022, 141, 104672. [Google Scholar] [CrossRef]
- Touret, J. Le facies granulite en Norvege Meridionale: II. Les inclusions fluides. Lithos 1971, 4, 423–436. [Google Scholar] [CrossRef]
- Strauss, H.; Melezhik, V.A.; Lepland, A.; Fallick, A.E.; Hanski, E.J.; Filippov, M.M.; Deines, Y.E.; Illing, C.J.; Črne, A.E.; Brasier, A.T. 7.6 Enhanced Accumulation of Organic Matter: The Shunga Event. In Reading the Archive of Earth’s Oxygenation; Melezhik, V., Prave, A.R., Hanski, E.J., Fallick, A.E., Lepland, A., Kump, L.R., Strauss, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1195–1273. [Google Scholar]
- Newton, R.; Smith, J.; Windley, B. Carbonic metamorphism, granulites and crustal growth. Nature 1980, 288, 45–50. [Google Scholar] [CrossRef]
- Lamb, W.; Valley, J. Metamorphism of reduced granulites in low-CO2 vapour-free environment. Nature 1984, 312, 56–58. [Google Scholar] [CrossRef]
- Newton, R.C. Charnockitic alteration: Evidence for CO2 infiltration in granulite facies metamorphism. J. Metamorph. Geol. 1992, 10, 383–400. [Google Scholar] [CrossRef]
- Santosh, M.; Omori, S. CO2 flushing: A plate tectonic perspective. Gondwana Res. 2008, 13, 86–102. [Google Scholar] [CrossRef]
- Touret, J.L.R.; Huizenga, J.M. Fluid-assisted granulite metamorphism: A continental journey. Gondwana Res. 2012, 21, 224–235. [Google Scholar] [CrossRef]
- Luque, d.V.F.J.; Pasteris, J.D.; Wopenka, B.; Rodas, M.; Fernández Barrenechea, J.M. Natural fluid-deposited graphite: Mineralogical characteristics and mechanisms of formation. Am. J. Sci. 1998, 298, 471–498. [Google Scholar] [CrossRef]
- Galvez, M.E.; Beyssac, O.; Martinez, I.; Benzerara, K.; Chaduteau, C.; Malvoisin, B.; Malavieille, J. Graphite formation by carbonate reduction during subduction. Nat. Geosci. 2013, 6, 473–477. [Google Scholar] [CrossRef]
- Galvez, M.E.; Pubellier, M. How Do Subduction Zones Regulate the Carbon Cycle? In Deep Carbon: Past to Present; Orcutt, B.N., Daniel, I., Dasgupta, R., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 276–312. [Google Scholar]
- Luque, F.J.; Crespo-Feo, E.; Barrenechea, J.F.; Ortega, L. Carbon isotopes of graphite: Implications on fluid history. Geosci. Front. 2012, 3, 197–207. [Google Scholar] [CrossRef]
- Tveten, E. Svolvær. Berggrunnskart Svolvær M 1:250 000. Geological Survey of Norway. 1978. Available online: https://openarchive.ngu.no/ngu-xmlui/handle/11250/2663386 (accessed on 3 May 2023).
- Griffin, W.L.; Taylor, P.N.; Hakkinen, J.W.; Heier, K.S.; Iden, I.K.; Krogh, E.J.; Malm, O.; Olsen, K.I.; Ormaasen, D.E.; Tveten, E. Archaean and Proterozoic crustal evolution in Lofoten–Vesterålen, N Norway. J. Geol. Soc. 1978, 135, 629–647. [Google Scholar] [CrossRef]
- Corfu, F. U–Pb Age, Setting and Tectonic Significance of the Anorthosite–Mangerite–Charnockite–Granite Suite, Lofoten–Vesterålen, Norway. J. Petrol. 2004, 45, 1799–1819. [Google Scholar] [CrossRef]
- Corfu, F. Multistage metamorphic evolution and nature of the amphibolite–granulite facies transition in Lofoten–Vesterålen, Norway, revealed by U–Pb in accessory minerals. Chem. Geol. 2007, 241, 108–128. [Google Scholar] [CrossRef]
- Davidsen, B.; Skår, Ø. Lofoten and Vesterålen: A Precambrian puzzle. In Proceedings of the Geologiska Föreningens Förhandlingar, Nordic Geological Winter Meeting Sweden, Uppsala, Sweden, 6–9 January 2004; pp. 20–21. [Google Scholar]
- Corfu, F.; Andersen, T.; Gasser, D. The Scandinavian Caledonides: Main features, conceptual advances and critical questions. Geol. Soc. Lond. Spec. Publ. 2014, 390, 9–43. [Google Scholar] [CrossRef]
- Markl, G.; Frost, B.R.; Bucher, K. The Origin of Anorthosites and Related Rocks from the Lofoten Islands, Northern Norway: I. Field Relations and Estimation of Intrinsic Variables. J. Petrol. 1998, 39, 1425–1452. [Google Scholar] [CrossRef]
- Keilhau, B.M. Beretning om en geonostisk reise i Norlandene i 1855. Nyt Mag. Naturvidenskaberne 1855, 11, 1–34. [Google Scholar]
- Baker, A.J.; Fallick, A.E. Heavy carbon in two-billion-year-old marbles from Lofoten-Vesterȧlen, Norway: Implications for the Precambrian carbon cycle. Geochim. Et Cosmochim. Acta 1989, 53, 1111–1115. [Google Scholar] [CrossRef]
- Baker, A.J.; Fallick, A.E. Evidence for CO2 infiltration in granulite facies marbles from Lofoten-Vesteralen, Norway. Earth Planet. Sci. Lett. 1988, 91, 132–140. [Google Scholar] [CrossRef]
- Rønning, J.S.; Gautneb, H.; Larsen, B.E.; Baranval, V.C.; Davidsen, B.; Engvik, A.; Gellein, J.; Knežević, J.; Ofstad, F.; Ren, X.; et al. Geophysical and Geological Investigations of Graphite Occurrences in Vesterålen, Northern Norway, in 2018 and 2019; Geological Survey of Norway Report 2019.031; Geological Survey of Norway: Trondheim, Norway, 2019; pp. 1–212. [Google Scholar]
- Pouchou, J.P.; Pichoir, F. Cameca PAP program. La Rech. Aerosp. 1984, 3, 167–192. [Google Scholar]
- Luvizotto, G.L.; Zack, T.; Meyer, H.P.; Ludwig, T.; Triebold, S.; Kronz, A.; Münker, C.; Stockli, D.F.; Prowatke, S.; Klemme, S.; et al. Rutile crystals as potential trace element and isotope mineral standards for microanalysis. Chem. Geol. 2009, 261, 346–369. [Google Scholar] [CrossRef]
- de Capitani, C.; Petrakakis, K. The computation of equilibrium assemblage diagrams with Theriak/Domino software. Am. Mineral. 2010, 95, 1006–1016. [Google Scholar] [CrossRef]
- Holland, T.; Powell, R. An internally consistent thermodynamic data set for phases of petrological interest. J. Metamorph. Geol. 1998, 16, 309–343. [Google Scholar] [CrossRef]
- Newton, R.; Charlu, T.; Kleppa, O. Thermochemistry of the high structural state plagioclases. Geochim. Et Cosmochim. Acta 1980, 44, 933–941. [Google Scholar] [CrossRef]
- Baldwin, J.; Powell, R.; Brown, M.; Moraes, R.; Fuck, R. Modelling of mineral equilibria in ultrahigh-temperature metamorphic rocks from the Anápolis–Itauçu Complex, central Brazil. J. Metamorph. Geol. 2005, 23, 511–531. [Google Scholar] [CrossRef]
- White, R.W.; Powell, R.; Holland, T.J.B. Progress relating to calculation of partial melting equilibria for metapelites. J. Metamorph. Geol. 2007, 25, 511–527. [Google Scholar] [CrossRef]
- Holland, T.; Powell, R. Thermodynamics of order-disorder in minerals; I, Symmetric formalism applied to minerals of fixed composition. Am. Mineral. 1996, 81, 1413–1424. [Google Scholar] [CrossRef]
- Green, E.; Holland, T.; Powell, R. An order-disorder model for omphacitic pyroxenes in the system jadeite-diopside-hedenbergite-acmite, with applications to eclogitic rocks. Am. Mineral. 2007, 92, 1181–1189. [Google Scholar] [CrossRef]
- Rønning, J.S.; Larsen, B.E.; Elvebakk, H.; Gautneb, H.; Ofstad, F.; Knežević, J. Geophysical Investigations of Graphite Occurrences in Bø and Øksnes Municipalities, Vesterålen, Nordland County, Northern Norway 2015–2016; NGU Report 2017.014; Geological Survey of Norway: Trondheim, Norway, 2017; pp. 1–50. [Google Scholar]
- Gautneb, H.; Knežević, J.; Johannesen, N.E.; Wanvik, J.E.; Engvik, A.; Davidsen, B.; Rønning, J.S. Geological and ore Dressing Investigations of Graphite Occurrences in Bø, Sortland, Hadsel and Øksnes Municipalities, Vesterålen, Nordland County, Northern Norway 2015–2016; NGU Report 2017.015; Geological Survey of Norway: Trondheim, Norway, 2017; pp. 1–70. [Google Scholar]
- Rodionov, A.; Ofstad, F.; Stampolidis, A.; Tassis, G. Helicopter-Borne Magnetic, Electromagnetic and Radiometric Geophysical Survey at Langøya in Vesterålen, Nordland; NGU Report 2013.044; Geological Survey of Norway: Trondheim, Norway, 2013; pp. 1–26. [Google Scholar]
- Engvik, A.K.; Gautneb, H.; Baranwal, V.C.; Rønning, J.-S.; Solberg, K.J.; Liu, Y.; Austrheim, H. The control of shear-zone development and electric conductivity by graphite in granulite: An example from the Proterozoic Lofoten-Vesterålen Complex of northern Norway. Terra Nova 2021, 33, 529–539. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Rønning, J.S.; Gautneb, H.; Larsen, B.E.; Knežević, S.J.; Baranval, V.C.; Elvebakk, H.; Gellein, J.; Ofstad, F.; Brönner, M. Geophysical and Geological Investigations of Graphite Occurrences in Vesterålen and Lofoten, Northern Norway 2017; Geological Survey of Norway: Trondheim, Norway, 2018; pp. 1–180. [Google Scholar]
- Kohn, M.J. A refined zirconium-in-rutile thermometer. Am. Mineral. 2020, 105, 963–971. [Google Scholar] [CrossRef]
- Tomkins, H.S.; Powell, R.; Ellis, D.J. The pressure dependence of the zirconium-in-rutile thermometer. J. Metamorph. Geol. 2007, 25, 703–713. [Google Scholar] [CrossRef]
- Watson, E.B.; Wark, D.A.; Thomas, J.B. Crystallization thermometers for zircon and rutile. Contrib. Mineral. Petrol. 2006, 151, 413–433. [Google Scholar] [CrossRef]
- Dunn, S.; Valley, J. Calcite–graphite isotope thermometry: A test for polymetamorphism in marble, Tudor gabbro aureole, Ontario, Canada. J. Metamorph. Geol. 1992, 10, 487–501. [Google Scholar] [CrossRef]
- Wada, H.; Suzuki, K. Carbon isotopic thermometry calibrated by dolomite-calcite solvus temperatures. Geochim. Et Cosmochim. Acta 1983, 47, 697–706. [Google Scholar] [CrossRef]
- Schidlowski, M. Organic matter in sedimentary rocks: «The dust we tread upon was once alive». Terra Cogn. 1983, 4, 45–49. [Google Scholar]
- Schidlowski, M. Carbon isotopes as biogeochemical recorders of life over 3.8 Ga of Earth history: Evolution of a concept. Precambrian Res. 2001, 106, 117–134. [Google Scholar] [CrossRef]
- Paiste, K.; Lepland, A.; Zerkle, A.L.; Kirsimäe, K.; Kreitsmann, T.; Mänd, K.; Romashkin, A.E.; Rychanchik, D.V.; Prave, A.R. Identifying global vs. basinal controls on Paleoproterozoic organic carbon and sulfur isotope records. Earth-Sci. Rev. 2020, 207, 103230. [Google Scholar] [CrossRef]
- Parnell, J.; Brolly, C.; Boyce, A.J. Graphite from Palaeoproterozoic enhanced carbon burial, and its metallogenic legacy. Geol. Mag. 2021, 158, 1711–1718. [Google Scholar] [CrossRef]
- Melezhik, V.A.; Fallick, A.E. A widespread positive δ13Ccarb anomaly at around 2.33–2.06 Ga on the Fennoscandian Shield: A paradox? Terra Nova 1996, 8, 141–157. [Google Scholar] [CrossRef]
- Melezhik, V.A.; Huhma, H.; Condon, D.J.; Fallick, A.E.; Whitehouse, M.J. Temporal constraints on the Paleoproterozoic Lomagundi-Jatuli carbon isotopic event. Geology 2007, 35, 655–658. [Google Scholar] [CrossRef]
- Melezhik, V.A.; Filippov, M.M.; Romashkin, A.E. A giant Palaeoproterozoic deposit of shungite in NW Russia: Genesis and practical applications. Ore Geol. Rev. 2004, 24, 135–154. [Google Scholar] [CrossRef]
- Griffin, W.L. ‘On the eclogites of Norway’—65 years later. Mineral. Mag. 1987, 51, 333–343. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Chopin, C.; Rouzaud, J. Raman spectra of carbonaceous material in metasediments: A new geothermometer. J. Metamorph. Geol. 2002, 20, 859–871. [Google Scholar] [CrossRef]
- Rantitsch, G.; Lämmerer, W.; Fisslthaler, E.; Mitsche, S.; Kaltenböck, H. On the discrimination of semi-graphite and graphite by Raman spectroscopy. Int. J. Coal Geol. 2016, 159, 48–56. [Google Scholar] [CrossRef]
- Pasteris, J.D. In Situ Analysis in Geological Thin-Sections by Laser Raman Microprobe Spectroscopy: A Cautionary Note. Appl. Spectrosc. 1989, 43, 567–570. [Google Scholar] [CrossRef]
- Rantitsch, G.; Linner, M. Graphitization during high-grade metamorphism in the southern Bohemian Massif. Int. J. Coal Geol. 2021, 248, 103864. [Google Scholar] [CrossRef]
- Satish-Kumar, M. Graphite-bearing CO2-fluid inclusions in granulites: Insights on graphite precipitation and carbon isotope evolution. Geochim. Et Cosmochim. Acta 2005, 69, 3841–3856. [Google Scholar] [CrossRef]
- Huizenga, J.M.; Touret, J.L. Granulites, CO2 and graphite. Gondwana Res. 2012, 22, 799–809. [Google Scholar] [CrossRef]
- Harley, S.L. Extending our understanding of ultrahigh temperature crustal metamorphism. J. Mineral. Petrol. Sci. 2004, 99, 140–158. [Google Scholar] [CrossRef]
- Parnell, J.; Brolly, C.; Boyce, A. Mixed metamorphic and fluid graphite deposition in Palaeoproterozoic supracrustal rocks of the Lewisian Complex, NW Scotland. Terra Nova 2021, 33, 541–550. [Google Scholar] [CrossRef]
- Kirilova, M.; Toy, V.G.; Timms, N.; Halfpenny, A.; Menzies, C.; Craw, D.; Beyssac, O.; Sutherland, R.; Townend, J.; Boulton, C. Textural changes of graphitic carbon by tectonic and hydrothermal processes in an active plate boundary fault zone, Alpine Fault, New Zealand. Geol. Soc. Lond. Spec. Publ. 2018, 453, 205–223. [Google Scholar] [CrossRef]
- Engvik, A.K.; Ihlen, P.M.; Austrheim, H. Characterisation of Na-metasomatism in the Sveconorwegian Bamble Sector of South Norway. Geosci. Front. 2014, 5, 659–672. [Google Scholar] [CrossRef]
- Engvik, A.K.; Mezger, K.; Wortelkamp, S.; Bast, R.; Corfu, F.; Korneliussen, A.; Ihlen, P.; Bingen, B.; Austrheim, H. Metasomatism of gabbro—Mineral replacement and element mobilization during the Sveconorwegian metamorphic event. J. Metamorph. Geol. 2011, 29, 399–423. [Google Scholar] [CrossRef]
- Kullerud, K. Chlorine-rich amphiboles: Interplay between amphibole composition and an evolving fluid. Eur. J. Mineral. 1996, 8, 355–370. [Google Scholar] [CrossRef]
- Engvik, A.K.; Golla-Schindler, U.; Berndt, J.; Austrheim, H.; Putnis, A. Intragranular replacement of chlorapatite by hydroxy-fluor-apatite during metasomatism. Lithos 2009, 112, 236–246. [Google Scholar] [CrossRef]
- Liebscher, A. Experimental Studies in Model Fluid Systems. Rev. Mineral. Geochem. 2007, 65, 15–47. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engvik, A.K.; Gautneb, H.; Mørkved, P.T.; Solberg, J.K.; Erambert, M. Proterozoic Deep Carbon—Characterisation, Origin and the Role of Fluids during High-Grade Metamorphism of Graphite (Lofoten–Vesterålen Complex, Norway). Minerals 2023, 13, 1279. https://doi.org/10.3390/min13101279
Engvik AK, Gautneb H, Mørkved PT, Solberg JK, Erambert M. Proterozoic Deep Carbon—Characterisation, Origin and the Role of Fluids during High-Grade Metamorphism of Graphite (Lofoten–Vesterålen Complex, Norway). Minerals. 2023; 13(10):1279. https://doi.org/10.3390/min13101279
Chicago/Turabian StyleEngvik, Ane K., Håvard Gautneb, Pål Tore Mørkved, Janja Knežević Solberg, and Muriel Erambert. 2023. "Proterozoic Deep Carbon—Characterisation, Origin and the Role of Fluids during High-Grade Metamorphism of Graphite (Lofoten–Vesterålen Complex, Norway)" Minerals 13, no. 10: 1279. https://doi.org/10.3390/min13101279







