A CaH2-Assisted Reduction Method to Prepare Nanoscale Zero-Valent Iron (nZVI) from Fe2O3 for Water Remediation Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Preparation of Nanoscale Zero-Valent Iron from Fe2O3
3.2. Catalytic Tests with the Prepared Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasanbeigi, A.; Arens, M.; Price, L. Alternative emerging ironmaking technologies for energy-efficiency and carbon dioxide emissions reduction: A technical review. Renew. Sust. Energ. Rev. 2014, 33, 645–658. [Google Scholar] [CrossRef]
- Fan, Z.; Friedmann, S.J. Low-carbon production of iron and steel: Technology options, economic assessment, and policy. Joule 2021, 5, 829–862. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.; Li, F.; Feng, C.; Liu, Z.; Zhou, Y. Development and progress on hydrogen metallurgy. Int. J. Miner. Metall. Mater. 2020, 27, 713–723. [Google Scholar] [CrossRef]
- Bhaskar, A.; Assadi, M.; Somehsaraei, H.N. Decarbonization of the Iron and Steel Industry with Direct Reduction of Iron Ore with Green Hydrogen. Energies 2020, 13, 758. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, H.; Wang, J.; Xue, Q.; Ren, B.; Yang, F. The production and application of hydrogen in steel industry. Int. J. Hydrog. Energy 2021, 46, 10548–10569. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Nie, J.; Dewil, R.; Baeyens, J.; Deng, Y. The Direct Reduction of Iron Ore with Hydrogen. Sustainability 2021, 13, 8866. [Google Scholar] [CrossRef]
- Lan, C.; Hao, Y.; Shao, J.; Zhang, S.; Liu, R.; Lyu, Q. Effect of H2 on Blast Furnace Ironmaking: A Review. Metals 2022, 12, 1864. [Google Scholar] [CrossRef]
- Sastri, M.V.C.; Viswanath, R.P.; Viswanathan, B. Studies on the reduction of iron oxide with hydrogen. Int. J. Hydrog. Energy 1982, 7, 951–955. [Google Scholar] [CrossRef]
- Lin, H.; Chena, Y.; Li, C. The mechanism of reduction of iron oxide by hydrogen. Thermochim. Acta 2003, 400, 61–67. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2 Part I: Low temperature reduction of hematite. Thermochim. Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J. In-Situ Kinetic Study of Hydrogen Reduction of Fe2O3 for the Production of Fe Nanopowder. Mater. Trans. 2009, 50, 2270–2276. [Google Scholar] [CrossRef]
- Liang, M.; Kang, W.; Xie, K. Comparison of reduction behavior of Fe2O3, ZnO and ZnFe2O4 by TPR technique. J. Nat. Gas Chem. 2009, 18, 110–113. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, H.; Li, H.; Zhu, Q. Study on Kinetics of Iron Oxide Reduction by Hydrogen. Chin. J. Chem. Eng. 2012, 20, 10–17. [Google Scholar] [CrossRef]
- Lee, G.; Choi, J.; Song, J.; Jung, S.; Lee, J. The Kinetics of Isothermal Hydrogen Reduction of Nanocrystalline Fe2O3 Powder. Mater. Trans. 2014, 55, 1611–1617. [Google Scholar] [CrossRef]
- Barde, A.A.; Klausner, J.F.; Mei, R. Solid state reaction kinetics of iron oxide reduction using hydrogen as a reducing agent. Int. J. Hydrog. Energy 2016, 41, 10103–10119. [Google Scholar] [CrossRef]
- Brandner, U.; Leuchtenmueller, M. Comparison of reduction kinetics of Fe2O3, ZnOFe2O3 and ZnO with hydrogen (H2) and carbon monoxide (CO). Int. J. Hydrog. Energy 2023, in press. [Google Scholar] [CrossRef]
- Wimmers, O.J.; Arnoldy, P.; Moulijn, J.A. Determination of the Reduction Mechanism by Temperature-Programmed Reduction: Application to Small Fe2O3 Particles. J. Phys. Chem. 1986, 90, 1331–1337. [Google Scholar] [CrossRef]
- Zielinski, J.; Zglinicka, I.; Znak, L.; Kaszkur, Z. Reduction of Fe2O3 with hydrogen. Appl. Catal. A Gen. 2010, 381, 191–196. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ruwan, G.; Tamada, Y.; Kohara, K.; Kusano, Y.; Sasano, T.; Ohno, K.; Tsujii, Y.; Kageyama, H.; Ono, T.; et al. Transformation of Nano- to Mesosized Iron Oxide Cores to r-Fe within Organic Shells Preserved Intact. Chem. Mater. 2011, 23, 1564–1569. [Google Scholar] [CrossRef]
- Kohara, K.; Yamamoto, S.; Seinberg, L.; Murakami, T.; Tsujimoto, M.; Ogawa, T.; Kurata, H.; Kageyama, H.; Takano, M. Carboxylated SiO2-coated a-Fe nanoparticles: Towards a versatile platform for biomedical applications. Chem. Commun. 2013, 49, 2563–2565. [Google Scholar] [CrossRef] [PubMed]
- Seinberg, L.; Yamamoto, S.; Tsujimoto, M.; Kobayashi, Y.; Takano, M.; Kageyama, H. CaH2-assisted low temperature synthesis of metallic magnetic nanoparticle-loaded multiwalled carbon nanotubes. Chem. Commun. 2014, 50, 6866–6868. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, S.; Tsujimoto, M. Well-defined SiO2-coated Fe2Co nanoparticles prepared by reduction with CaH2. RSC Adv. 2015, 5, 100084–100088. [Google Scholar] [CrossRef]
- Tsuchida, T.; Fukushima, J.; Tobise, M.; Hayashi, Y.; Takizawa, H. Low-temperature hydrogen reduction of iron oxide by controlling the water potential using a CaH2 drying agent. J. Solid State Chem. 2021, 302, 122441. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Tratnyek, P.G.; Sarathy, V.; Baer, D.R.; Amonette, J.E.; Pecher, K.; Wang, C.; Linehan, J.C.; Matson, D.W.; Penn, R.L.; et al. Characterization and Properties of Metallic Iron Nanoparticles: Spectroscopy, Electrochemistry, and Kinetics. Environ. Sci. Technol. 2005, 39, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Elliott, D.W.; Zhang, W. Zero-Valent Iron Nanoparticles for Abatement of Environmental Pollutants: Materials and Engineering Aspects. Crit. Rev. Solid State Mater. 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Mueller, N.C.; Braun, J.; Bruns, J.; Černík, M.; Rissing, P.; Rickerby, D.; Nowack, B. Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ. Sci. Pollut. Res. 2012, 19, 550–558. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211, 112–125. [Google Scholar] [CrossRef]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Tang, H.; Wang, J.; Zhang, S.; Pang, H.; Wang, X.; Chen, Z.; Li, M.; Song, G.; Qiu, M.; Yu, S. Recent advances in nanoscale zero-valent iron-based materials: Characteristics, environmental remediation and challenges. J. Clean. Prod. 2021, 319, 128. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Z.; Wang, H.; Yang, H.; Wen, T.; Wang, S.; Hu, B.; Wang, X. Removal of organic compounds by nanoscale zero-valent iron and its composites. Sci. Total Environ. 2021, 792, 148546. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef]
- Xue, W.; Huang, D.; Zeng, G.; Wan, J.; Cheng, M.; Zhang, C.; Hu, C.; Li, J. Performance and toxicity assessment of nanoscale zero valent iron particles in the remediation of contaminated soil: A review. Chemosphere 2018, 210, 1145–1156. [Google Scholar] [CrossRef]
- Tosco, T.; Papini, M.P.; Viggi, C.C.; Sethi, R. Nanoscale zerovalent iron particles for groundwater remediation: A review. J. Clean. Prod. 2014, 77, 10–21. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Raman, C.D.; Kanmani, S. Textile dye degradation using nano zero valent iron: A review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, J.; Ferguson, S.; Wang, T.; Bao, Y.; Hao, H. Mechanism, synthesis and modification of nano zerovalent iron in water treatment. Nanoscale 2016, 8, 9962–9975. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Kumar, R.; Sinha, A.; Lama, Y.; Saha, A.K. A review on synthesis, characterization, and applications of nano zero valent iron (nZVI) for environmental remediation. Crit. Rev. Environ. Sci. Technol. 2016, 46, 443–466. [Google Scholar] [CrossRef]
- Adusei-Gyamfia, J.; Acha, V. Carriers for nano zerovalent iron (nZVI): Synthesis, application and efficiency. RSC Adv. 2016, 6, 91025–91044. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Li, S.; Yan, W.; Zhang, W. Solvent-free production of nanoscale zero-valent iron (nZVI) with precision milling. Green Chem. 2009, 11, 1618–1626. [Google Scholar] [CrossRef]
- Wang, P.; Hu, J.; Liu, T.; Han, G.; Ma, W.; Li, J. New insights into ball-milled zero-valent iron composites for pollution remediation: An overview. J. Clean. Prod. 2023, 385, 135513. [Google Scholar] [CrossRef]
- Okazoe, S.; Yasaka, Y.; Kudo, M.; Maeno, H.; Murakami, Y.; Kimura, Y. Synthesis of zero-valent iron nanoparticles via laser ablation in a formate ionic liquid under atmospheric conditions. Chem. Commun. 2018, 54, 7834–7837. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W. Synthesizing Nanoscale Iron Particles for Rapid and Complete Dechlorination of TCE and PCBs. Environ. Sci. Technol. 1997, 31, 2154–2156. [Google Scholar] [CrossRef]
- Wang, Q.; Snyder, S.; Kim, J.; Choi, H. Aqueous Ethanol modified Nanoscale Zerovalent Iron in Bromate Reduction: Synthesis, Characterization, and Reactivity. Environ. Sci. Technol. 2009, 43, 3292–3299. [Google Scholar] [CrossRef]
- Wang, Q.; Kanel, S.R.; Park, H.; Ryu, A.; Choi, H. Controllable synthesis, characterization, and magnetic properties of nanoscale zerovalent iron with specific high Brunauer–Emmett–Teller surface area. J. Nanopart. Res. 2009, 11, 749–755. [Google Scholar] [CrossRef]
- Hwang, Y.; Kim, D.; Shin, H. Effects of synthesis conditions on the characteristics and reactivity of nano scale zero valent iron. Appl. Catal. B Environ. 2011, 105, 144–150. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N. Preparation and characterization of zero valent iron nanoparticles. Dig. J. Nanomater. Biostructures 2011, 6, 1771–1776. [Google Scholar]
- Jamei, M.R.; Khosravi, M.R.; Anvaripour, B. A novel ultrasound assisted method in synthesis of NZVI particles. Ultrason. Sonochem. 2014, 21, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Hoch, L.B.; Mack, E.J.; Hydutsky, B.W.; Hershman, J.M.; Skluzacek, J.M.; Mallouk, T.E. Carbothermal Synthesis of Carbon-supported Nanoscale Zero-valent Iron Particles for the Remediation of Hexavalent Chromium. Environ. Sci. Technol. 2008, 42, 2600–2605. [Google Scholar] [CrossRef]
- Choi, C.J.; Tolochko, O.; Kim, B.K. Preparation of iron nanoparticles by chemical vapor condensation. Mater. Lett. 2002, 56, 289–294. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, G.; Liu, S.; Ang, H.M.; Tade, M.O.; Wang, S. Nano-Fe0 Encapsulated in Microcarbon Spheres: Synthesis, Characterization, and Environmental Applications. ACS Appl. Mater. Interfaces 2012, 4, 6235–6241. [Google Scholar] [CrossRef] [PubMed]
- Joseyphus, R.J.; Kodama, D.; Matsumoto, T.; Sato, Y.; Jeyadevan, B.; Tohji, K. Role of polyol in the synthesis of Fe particles. J. Magn. Magn. Mater. 2007, 310, 2393–2395. [Google Scholar] [CrossRef]
- Joseyphus, R.J.; Shinoda, K.; Kodama, D.; Jeyadevan, B. Size controlled Fe nanoparticles through polyol process and their magnetic properties. Mater. Chem. Phys. 2010, 123, 487–493. [Google Scholar] [CrossRef]
- Chen, S.; Hsu, H.; Li, C. A new method to produce nanoscale iron for nitrate removal. J. Nanopart. Res. 2004, 6, 639–647. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Yu, X.; Hu, J.; Cheng, J.; Jing, H. Research progress in the preparation of iron by electrochemical reduction route without CO2 emissions. J. Appl. Electrochem. 2023, 53, 1521–1536. [Google Scholar] [CrossRef]
- Hoag, G.E.; Collins, J.B.; Holcomb, J.L.; Hoag, J.R.; Nadagouda, M.N.; Varma, R.S. Degradation of bromothymol blue by ‘greener’ nano-scale zero-valent iron synthesized using tea polyphenols. J. Mater. Chem. 2009, 19, 8671–8677. [Google Scholar] [CrossRef]
- Machado, S.; Pinto, S.L.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci. Total Environ. 2013, 445, 1–8. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesis of iron nanoparticles by various tea extracts: Comparative study of the reactivity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 295–301. [Google Scholar] [CrossRef]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Chemistry of Borohydride Reduction of Iron(II) and Iron(III) Ions in Aqueous and Nonaqueous Media. Formation of Nanoscale Fe, FeB, and Fe2B Powders. Inorg. Chem. 1995, 34, 28–35. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Lien, H.; Koel, B.E.; Zhang, W. Iron nanoparticles for environmental clean-up: Recent developments and future outlook. Environ. Sci. Process. Impacts 2013, 15, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Jia, F.; Ai, Z.; Zhang, L. Iron oxide shell mediated environmental remediation properties of nano zero-valent iron. Environ. Sci. Nano 2017, 4, 27–45. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Sun, Y.; Liang, L.; Pan, B.; Zhang, W.; Guan, X. Advances in Sulfidation of Zerovalent Iron for Water Decontamination. Environ. Sci. Technol. 2017, 51, 13533–13544. [Google Scholar] [CrossRef]
- Bae, S.; Collins, R.N.; Waite, T.D.; Hanna, K. Advances in Surface Passivation of Nanoscale Zerovalent Iron: A Critical Review. Environ. Sci. Technol. 2018, 52, 12010–12025. [Google Scholar] [CrossRef]
- Ken, D.S.; Sinha, A. Recent developments in surface modification of nano zero-valent iron (nZVI): Remediation, toxicity and environmental impacts. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100344. [Google Scholar] [CrossRef]
- Ezzatahmadi, N.; Ayoko, G.A.; Millar, G.J.; Speight, R.; Yan, C.; Li, J.; Li, S.; Zhu, J.; Xi, Y. Clay-supported nanoscale zero-valent iron composite materials for the remediation of contaminated aqueous solutions: A review. Chem. Eng. J. 2017, 312, 336–350. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, M.; Zhou, M.; Lia, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.; Igalavithana, A.D.; et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Peng, C.; Tan, J.; Wan, J.; Sun, P.; Li, Q.; Ji, X.; Zhang, Q.; Wu, Y.; et al. Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: A critical review. J. Hazard. Mater. 2022, 426, 127993. [Google Scholar] [CrossRef]
- Kobayashi, Y. Synthesis of Porous Ni3Al Intermetallic Nano-compounds in a Molten LiCl with Assistance of CaH2 as a Structure-controlling Agent. Chem. Lett. 2019, 48, 1496–1499. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Suzuki, D.; Yokoyama, S.; Shoji, R. Molten salt synthesis of high-entropy alloy AlCoCrFeNiV nanoparticles for the catalytic hydrogenation of p-nitrophenol by NaBH4. Int. J. Hydrog. Energy 2022, 47, 3722–3732. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yokoyama, S.; Shoji, R. Molten salt synthesis of CrMnFeNi alloy nanopowder passivated by TiOx–ZrOy shell used as a superior catalyst support in liquid-phase hydrogenation. RSC Adv. 2023, 13, 10790–10799. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, R.D.; Kamm, G.E.; McDermott, M.J.; Hermann, R.P.; Vasquez-Garcia, N.; Sacci, R.L.; Persson, K.A.; Chapman, K.W.; Veith, G.M. Direct Mechanochemical Synthesis, Phase Stability, and Electrochemical Performance of α-NaFeO2. Inorg. Chem. 2023, 62, 3358–3367. [Google Scholar] [CrossRef]
- Gomez-García, J.F.; Pfeiffer, H. Effect of Chemical Composition and Crystal Phase of (Li,Na)FeO2 Ferrites on CO2 Capture Properties at High Temperatures. J. Phys. Chem. C 2018, 122, 21162–21171. [Google Scholar] [CrossRef]
- Yanase, I.; Onozawa, S.; Ogasawara, K.; Kobayashi, H. A novel application of α- and β-sodium ferrite as a CO2-capturing solid in air with water vapor. J. CO2 Util. 2018, 24, 200–209. [Google Scholar] [CrossRef]
- Yanase, I.; Onozawa, S.; Ohashi, Y.; Takeuchi, T. CO2 capture fromambient air by β-NaFeO2 in the presence ofwater vapor at 25–100 °C. Powder Technol. 2019, 348, 43–50. [Google Scholar] [CrossRef]
- Durai, L.; Gopalakrishnan, A.; Badhulika, S. One-step solid-state reaction synthesis of b-NaFeO2 nanopebble as high capacity cathode material for sodium ion batteries. Mater. Lett. 2020, 270, 127739. [Google Scholar] [CrossRef]
- Viret, M.; Rubi, D.; Colson, D.; Lebeugle, D.; Forget, A.; Bonville, P.; Dhalenne, G.; Saint-Martin, R.; Andre, G.; Ott, F. b-NaFeO2, a new room-temperature multiferroic material. Mater. Res. Bull. 2012, 47, 2294–2298. [Google Scholar] [CrossRef]
- Singh, S.; Tovstolytkin, A.; Lotey, G.S. Heating loss mechanism in β-NaFeO2 nanoparticles for cancer treatment under alternating field. Materialia 2021, 18, 101152. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Teah, H.Y.; Hanada, N. Environmentally friendly chemical synthesis of intermetallic iron aluminide submicrometer particles. J. Clean. Prod. 2021, 316, 128264. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Teah, H.Y.; Hanada, N. Chemical synthesis of unique intermetallic TiFe nanostructures originating from the morphology of oxide precursors. Nanoscale Adv. 2021, 3, 5284–5291. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Teah, H.Y.; Yokoyama, S.; Shoji, R.; Hanada, N. Environmentally friendly molten salt synthesis of high-entropy AlCoCrFeNi alloy powder with high catalytic hydrogenation activity. Int. J. Hydrog. Energy 2023, 48, 30963–30973. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Teah, H.Y.; Yokoyama, S.; Shoji, R.; Hanada, N. A Molten Salt Synthesis Method of the High-Entropy Alloy CrMnFeCoNi for High Catalytic Performance and Low Life Cycle GHG Emissions. ACS Sustain. Chem. Eng. 2022, 10, 15046–15057. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yokoyama, S.; Shoji, R. Core-Shell Multicomponent Alloys with High Specific Surface Areas Prepared by Molten Salt Synthesis for Catalytic Hydrogenation of p-Nitrophenol by NaBH4. ACS Appl. Eng. Mater. 2023, 1, 152–164. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yokoyama, S.; Shoji, R. Molten Salt Synthesis of Intermetallic Compound TiNi Nanopowder Prepared from NiTiO3 for Catalytic Hydrogenation. Materials 2022, 15, 8536. [Google Scholar] [CrossRef]
- Sravanthi, K.; Ayodhya, D.; Swamy, P.Y. Green synthesis, characterization and catalytic activity of 4-nitrophenol reduction and formation of benzimidazoles using bentonite supported zero valent iron nanoparticles. Mater. Sci. Energy Technol. 2019, 2, 298–307. [Google Scholar] [CrossRef]
- Bae, S.; Gim, S.; Kim, H.; Hanna, K. Effect of NaBH4 on properties of nanoscale zero-valent iron and its catalytic activity for reduction of p-nitrophenol. Appl. Catal. B Enviro. 2016, 182, 541–549. [Google Scholar] [CrossRef]
- Lu, H.; Qiao, X.; Wang, W.; Tan, F.; Xiao, Z.; Chen, J. Chitosan stabilised nanozero-valent iron for the catalytic reduction of p-nitrophenol. Micro Nano Lett. 2014, 9, 446–450. [Google Scholar] [CrossRef]
- Malik, M.A.; Alshehri, A.A.; Patel, R. Facile one-pot green synthesis of Ag-Fe bimetallic nanoparticles and their catalytic capability for 4-nitrophenol reduction. J. Mater. Res. Technol. 2021, 12, 455–470. [Google Scholar] [CrossRef]
- Wu, K.; Yu, R.; Wei, X. Monodispersed FeNi2 alloy nanostructures: Solvothermal synthesis, magnetic properties and size-dependent catalytic activity. CrystEngComm 2012, 14, 7626–7632. [Google Scholar] [CrossRef]
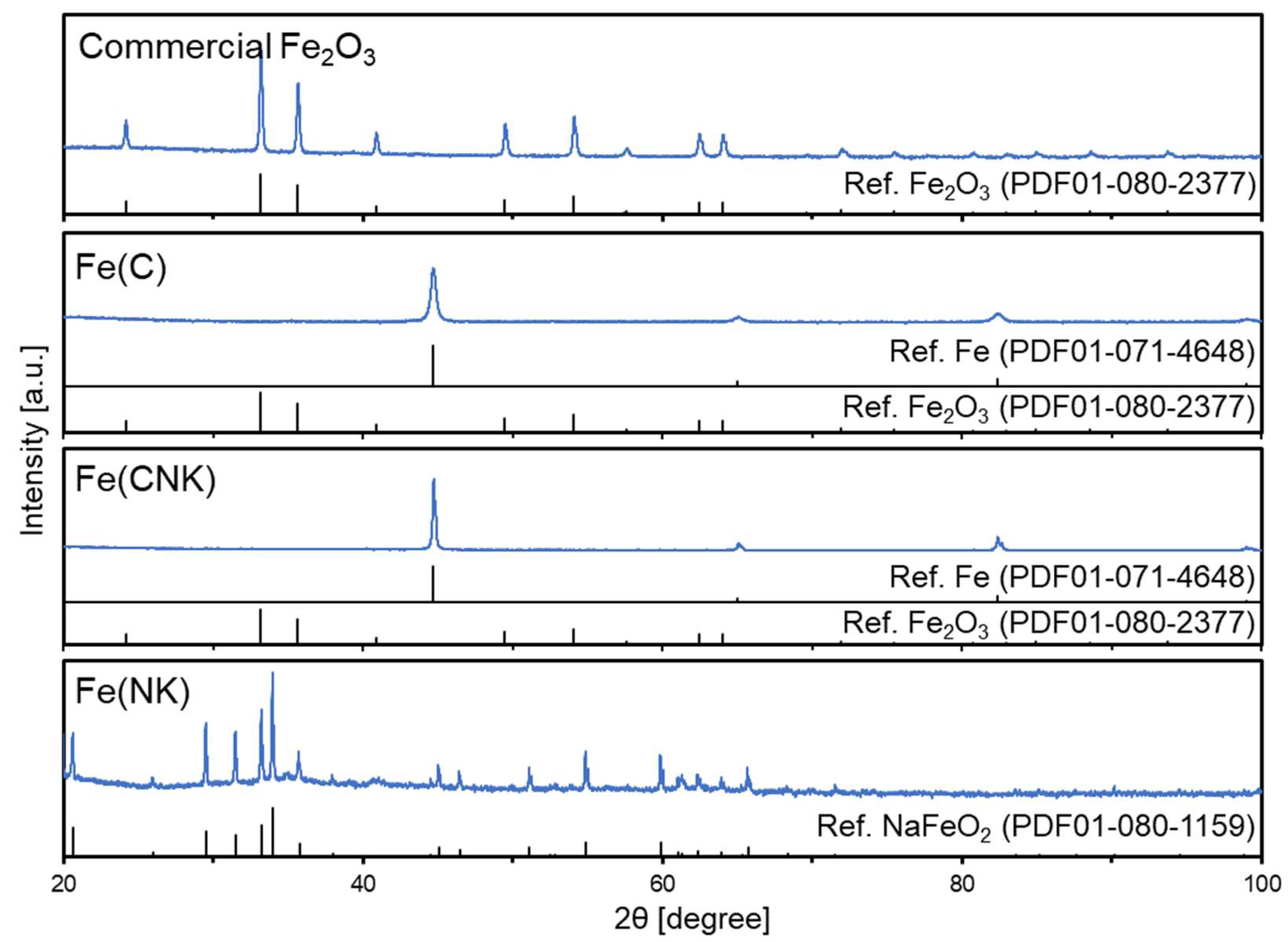
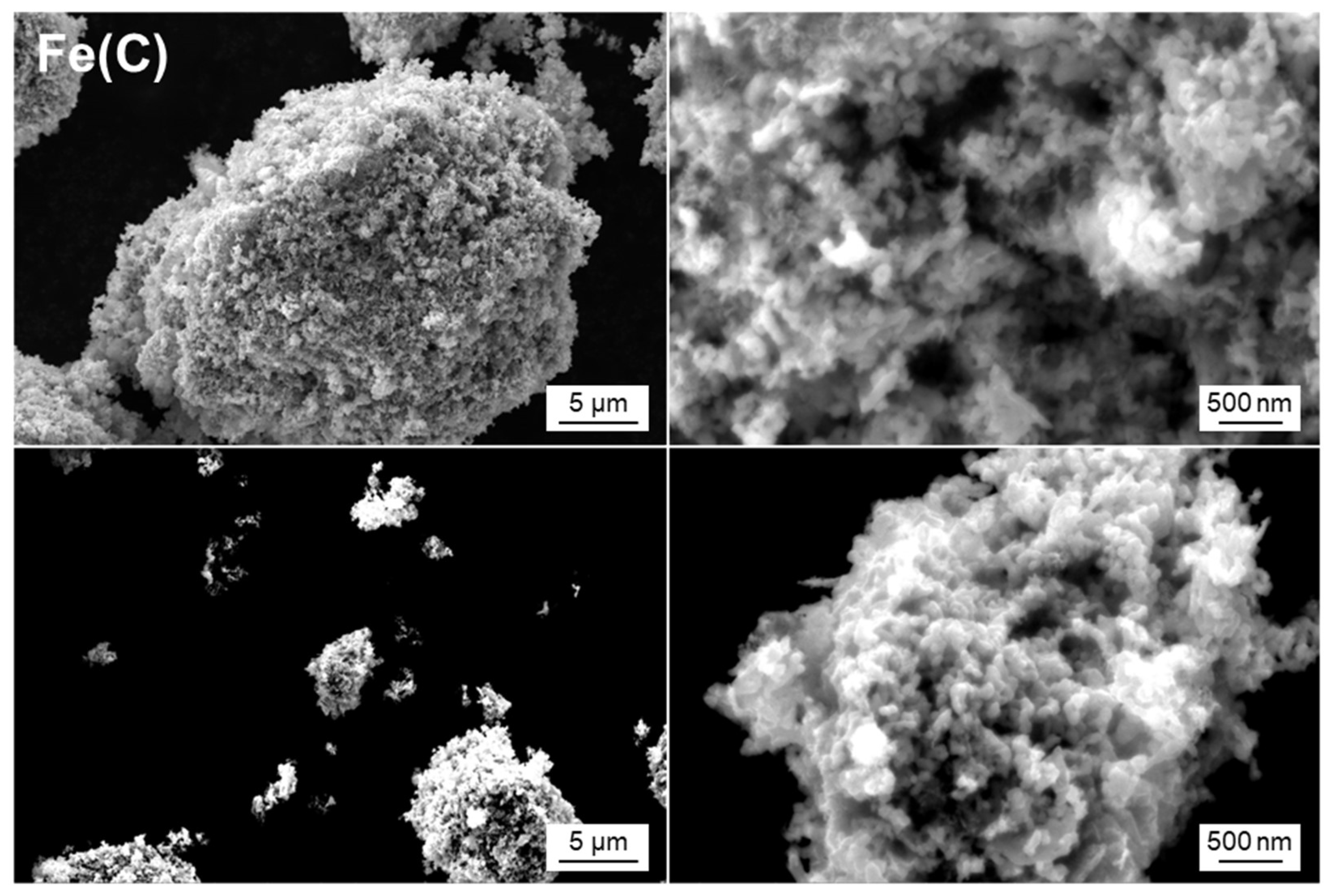


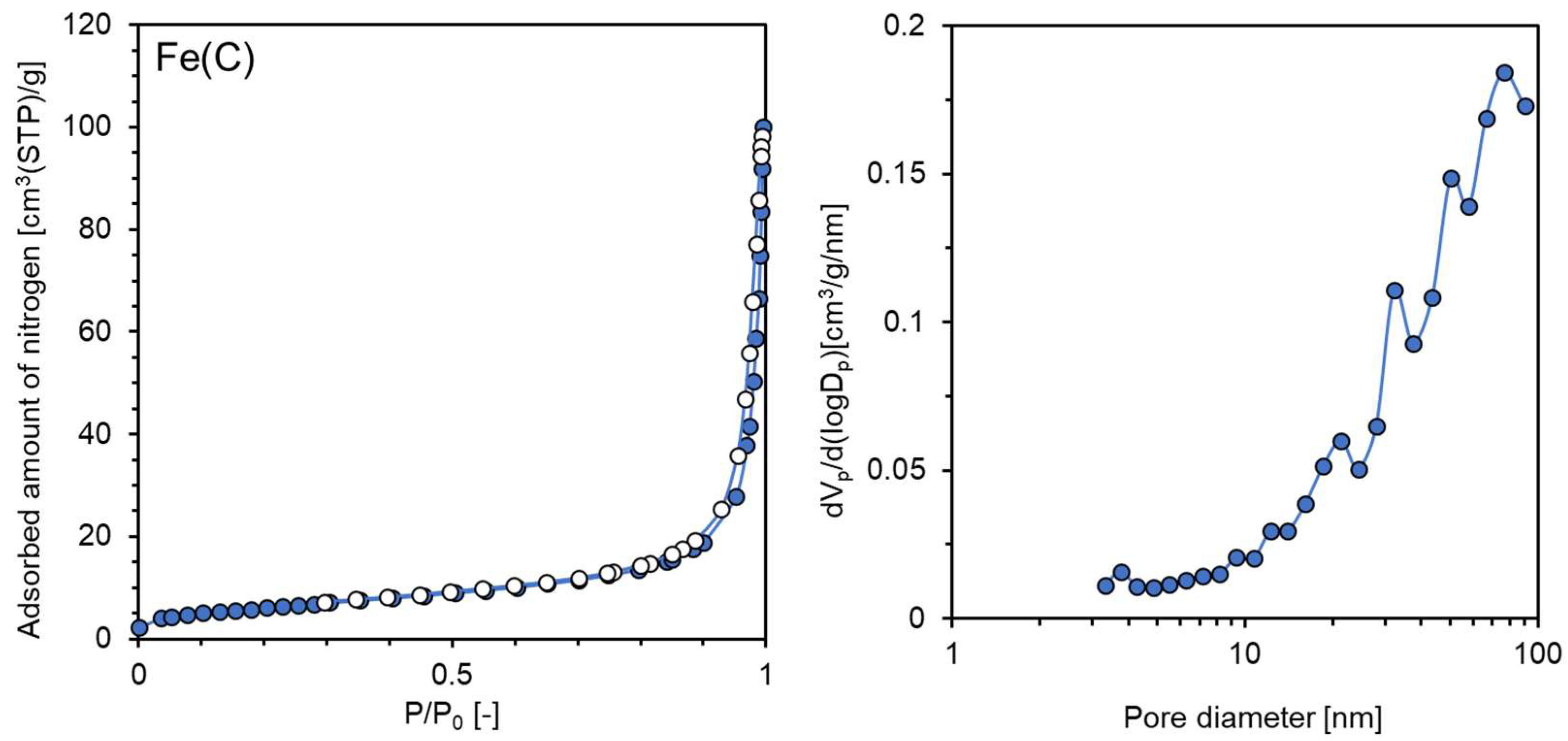
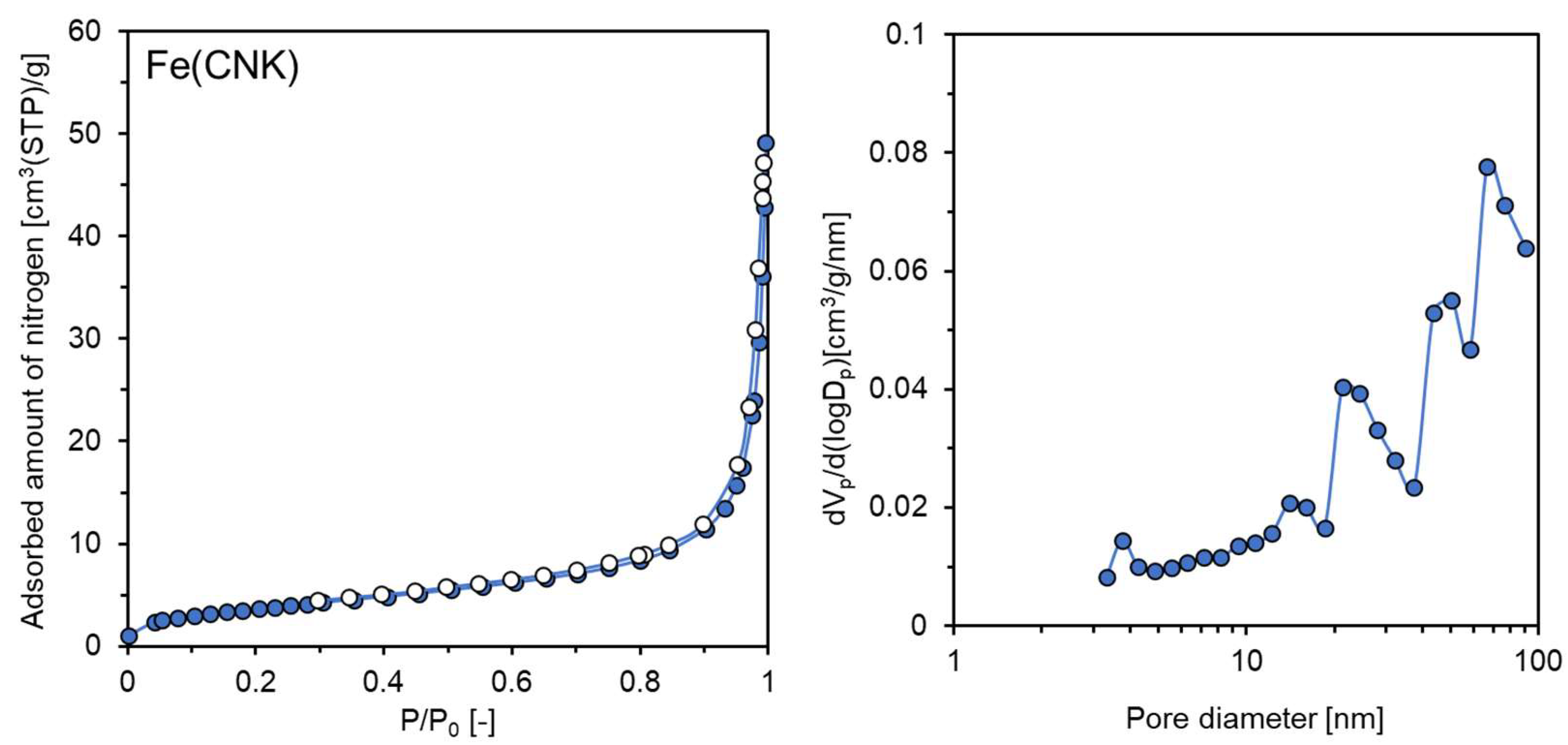
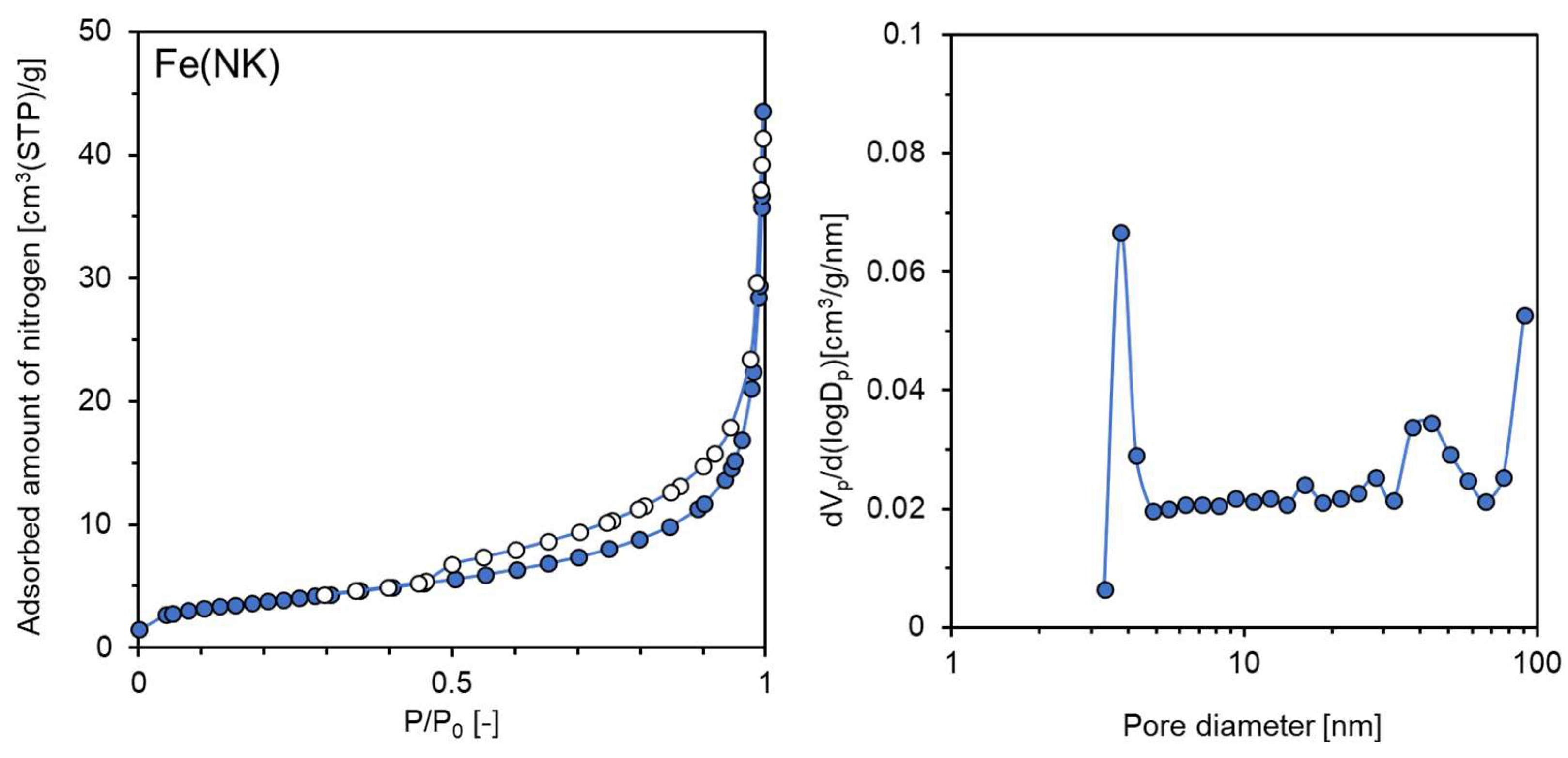
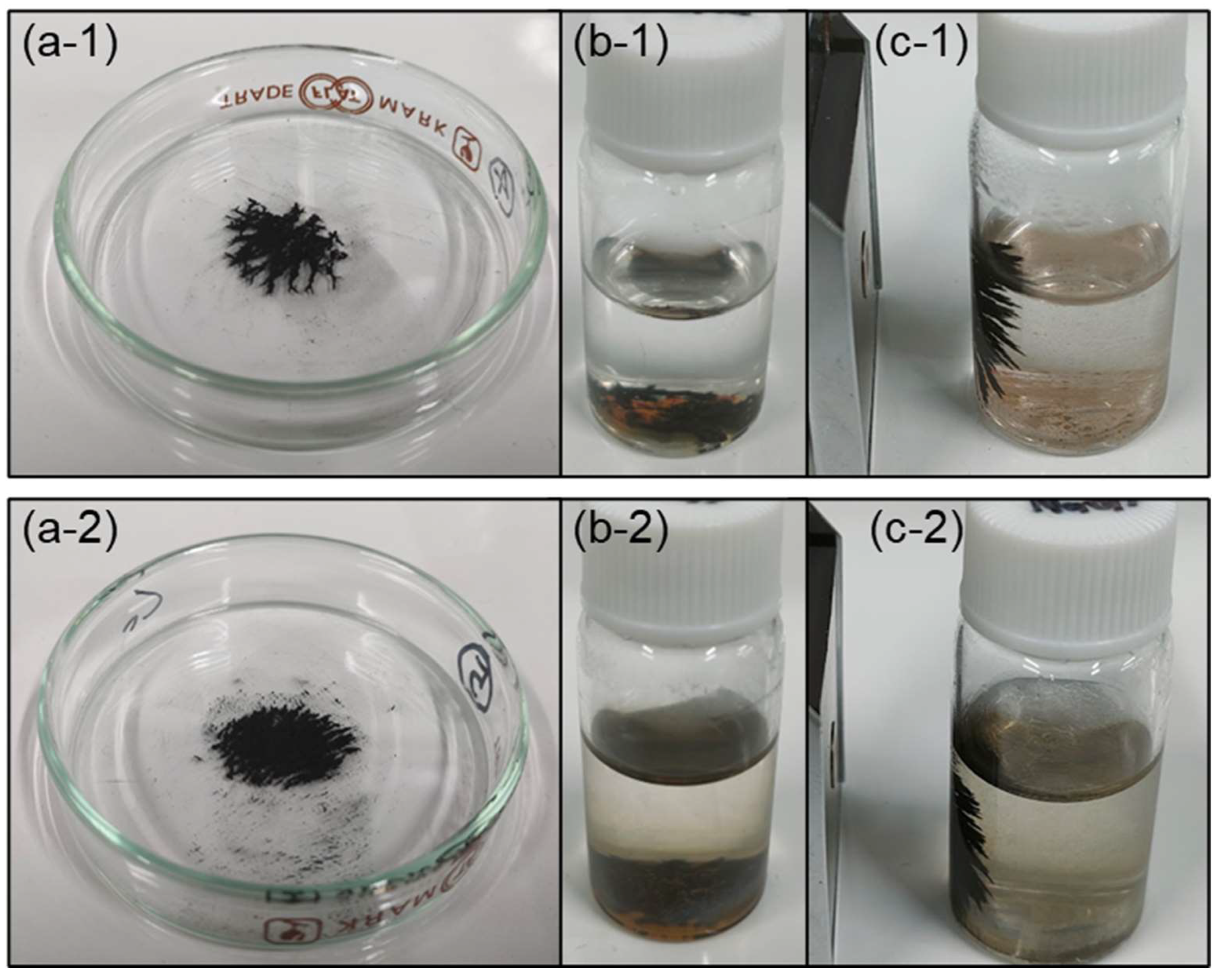
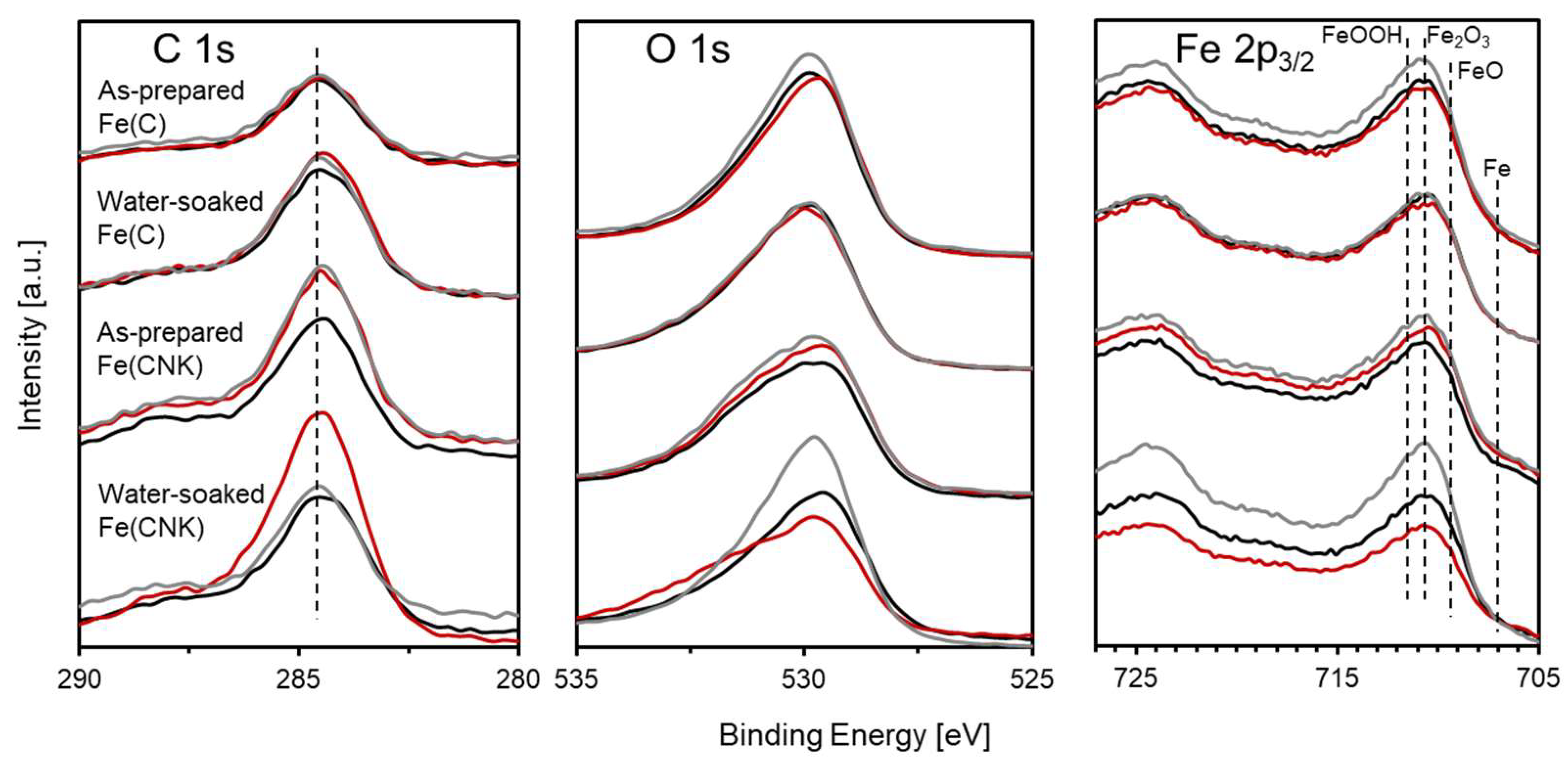


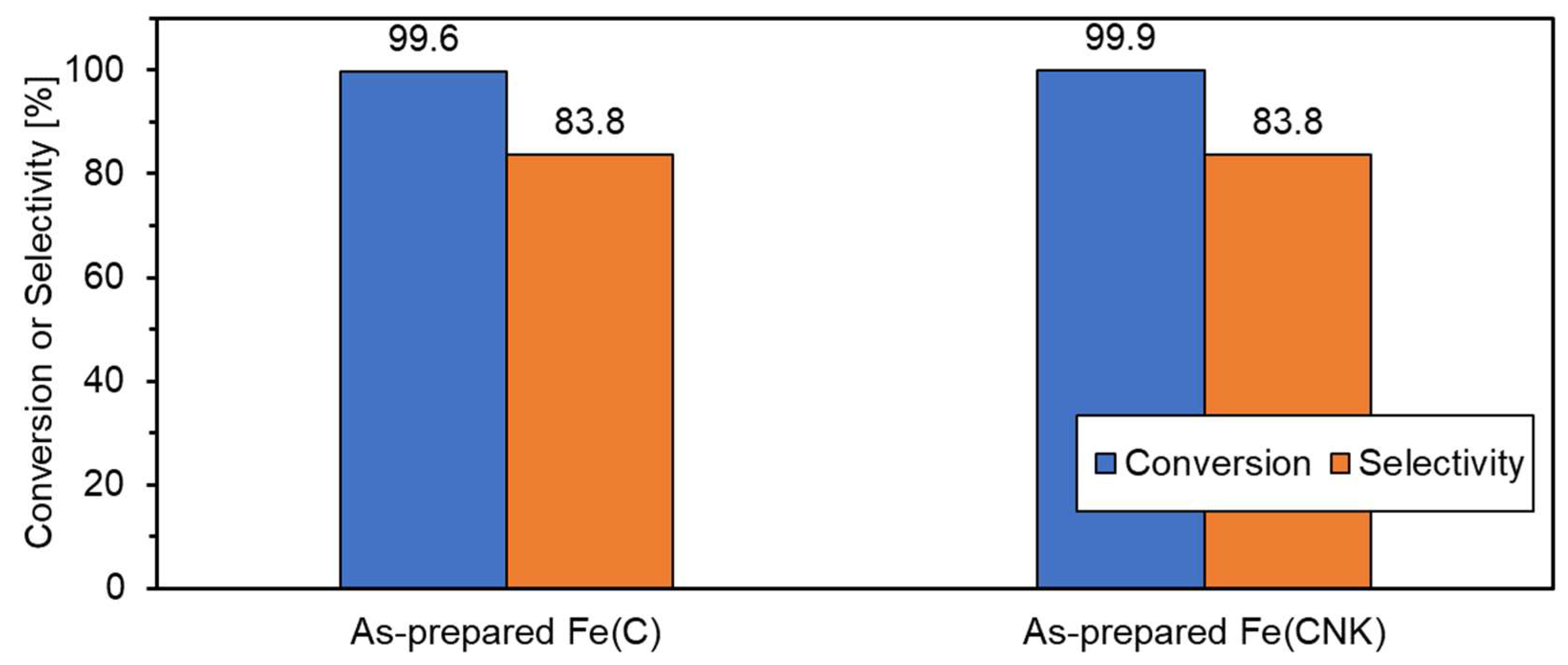
| Sample Name | Preparation Conditions | XRD | N2 Adsorption | ||||
|---|---|---|---|---|---|---|---|
| Reducing Agent | Molten Salt | Reduction Temp. [°C] | Identified Fe Compound | Crystallite Size [nm] * | BET Surface Area [m2/g] | Pore Volume [cm3/g] | |
| Commercial Fe2O3 | None | None | None | Fe2O3 | 52 | 10.5 | 0.118 |
| Fe(C) | CaH2 | None | 220 | Fe | 24 | 22.5 | 0.116 |
| Fe(CNK) | CaH2 | NaOH-KOH | 220 | Fe | 64 | 13.6 | 0.056 |
| Fe(NK) | None | NaOH-KOH | 220 | β-NaFeO2 | 105 | 13.7 | 0.046 |
| BET Surface Area [m2/g] | Preparation Method | Reference |
|---|---|---|
| 39 | Precision milling | [43] |
| 33.5 | Liquid reduction with NaBH4 | [46] |
| 26.58 | [50] | |
| 45.4 | [49] | |
| 62.48 | [48] | |
| 67.51 | [47] | |
| 42 | Ultrasound assisted reduction with NaBH4 | [51] |
| 25.4 | Electrochemical | [57] |
| 5.8 | Green synthesis | [61] |
| Sample Name | Molar Ratio [mol%] | |
|---|---|---|
| O | Fe | |
| As-prepared Fe(C) | 78.5 | 21.5 |
| Water-soaked Fe(C) | 81.9 | 18.1 |
| As-prepared Fe(CNK) | 84.4 | 15.6 |
| Water-soaked Fe(CNK) | 79.9 | 20.1 |
| Sample | BET Surface Area [m2/g] | Reaction Conditions | k [min−1] | Reference |
|---|---|---|---|---|
| As-prepared Fe(C) | 22.5 | 25 °C 4−NP (1.6 mM) NaBH4 (47 mM) 10 mg−cat/9 mL | 0.048 | This study |
| Water-soaked Fe(C) | − | 0.023 | ||
| As-prepared Fe(CNK) | 13.6 | 0.040 | ||
| Water-soaked Fe(CNK) | − | 0.026 | ||
| Fe40Mn15Cr15Ni25Al5 | 11.3 | 50 °C 4−NP (1.6 mM) NaBH4 (47 mM) 10 mg−cat/9 mL | 0.01 | [86] |
| Fe35Mn10Cr20Ni35 | 2.2 | 0.02 | ||
| Fe50Mn27Cr13Ni10 | 2.4 | 0.04 | ||
| TiNi | 6.0 | 0.14–0.31 | [87] | |
| Bentonite clay-supported Fe nanoparticles | 62.47 | R.T. 4−NP (0.2 mM) NaBH4 (200 mM) 10 mg−cat/L | 0.141 | [88] |
| Nanoscale zero-valent iron | − | R.T. 4−NP (0.1 mM) NaBH4 (50 mM) 3.5 mg−cat/L | 0.31 | [89] |
| Chitosan-stabilised Nanozero-valent iron | − | 25 °C 4−NP (0.2 mM) NaBH4 (20 mM) 1000 mg−cat/L | 0.147 | [90] |
| FeAg bimetallic nanoparticles | − | 25 °C 4−NP (0.07 mM) NaBH4 (3 mM) 5 mg−cat/3 mL | 0.065 | [91] |
| Monodispersed FeNi2 alloy nanostructures | 27–43 | 20 °C 4−NP (0.03 mM) NaBH4 (20 mM) 33 mg−cat/L | 0.057 | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, Y.; Yamamoto, K.; Shoji, R. A CaH2-Assisted Reduction Method to Prepare Nanoscale Zero-Valent Iron (nZVI) from Fe2O3 for Water Remediation Application. Minerals 2023, 13, 1385. https://doi.org/10.3390/min13111385
Kobayashi Y, Yamamoto K, Shoji R. A CaH2-Assisted Reduction Method to Prepare Nanoscale Zero-Valent Iron (nZVI) from Fe2O3 for Water Remediation Application. Minerals. 2023; 13(11):1385. https://doi.org/10.3390/min13111385
Chicago/Turabian StyleKobayashi, Yasukazu, Koharu Yamamoto, and Ryo Shoji. 2023. "A CaH2-Assisted Reduction Method to Prepare Nanoscale Zero-Valent Iron (nZVI) from Fe2O3 for Water Remediation Application" Minerals 13, no. 11: 1385. https://doi.org/10.3390/min13111385
APA StyleKobayashi, Y., Yamamoto, K., & Shoji, R. (2023). A CaH2-Assisted Reduction Method to Prepare Nanoscale Zero-Valent Iron (nZVI) from Fe2O3 for Water Remediation Application. Minerals, 13(11), 1385. https://doi.org/10.3390/min13111385







