The Flotation Separation Mechanism of Smithsonite from Calcite and Dolomite with Combined Collectors
Abstract
:1. Introduction
2. Experimental
2.1. Minerals and Reagents
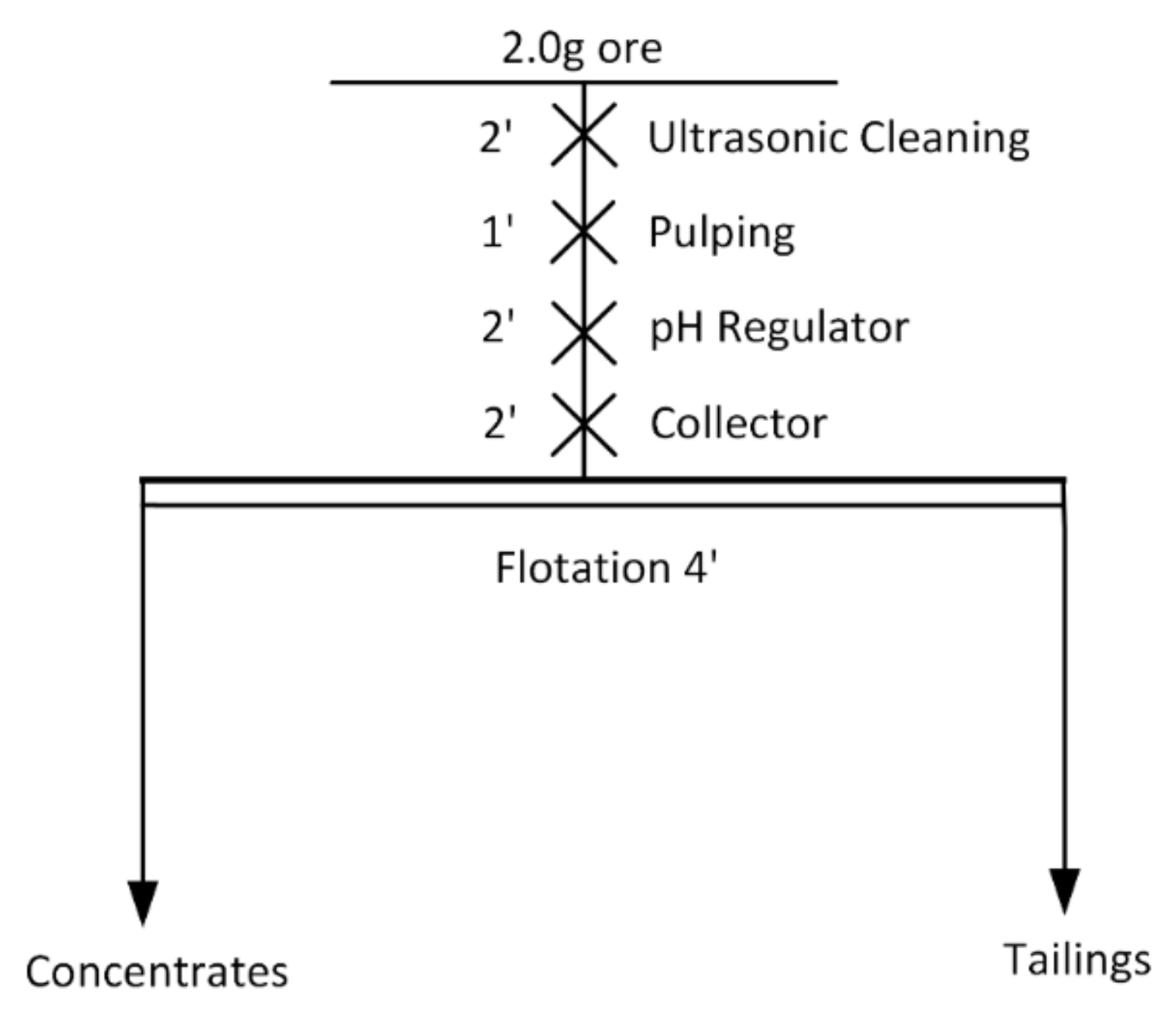
2.2. Flotation Tests
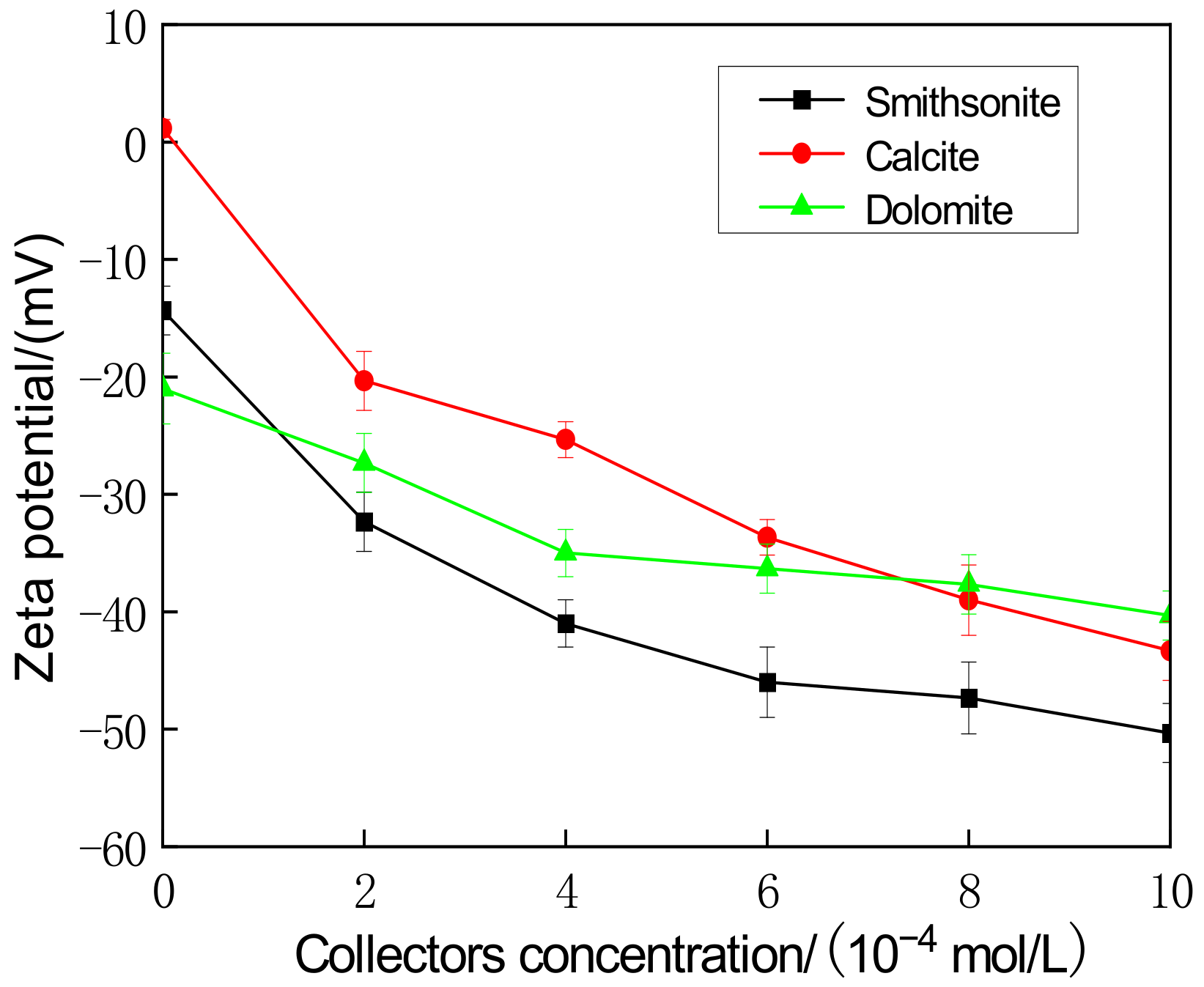
2.3. Zeta Potential Measurement
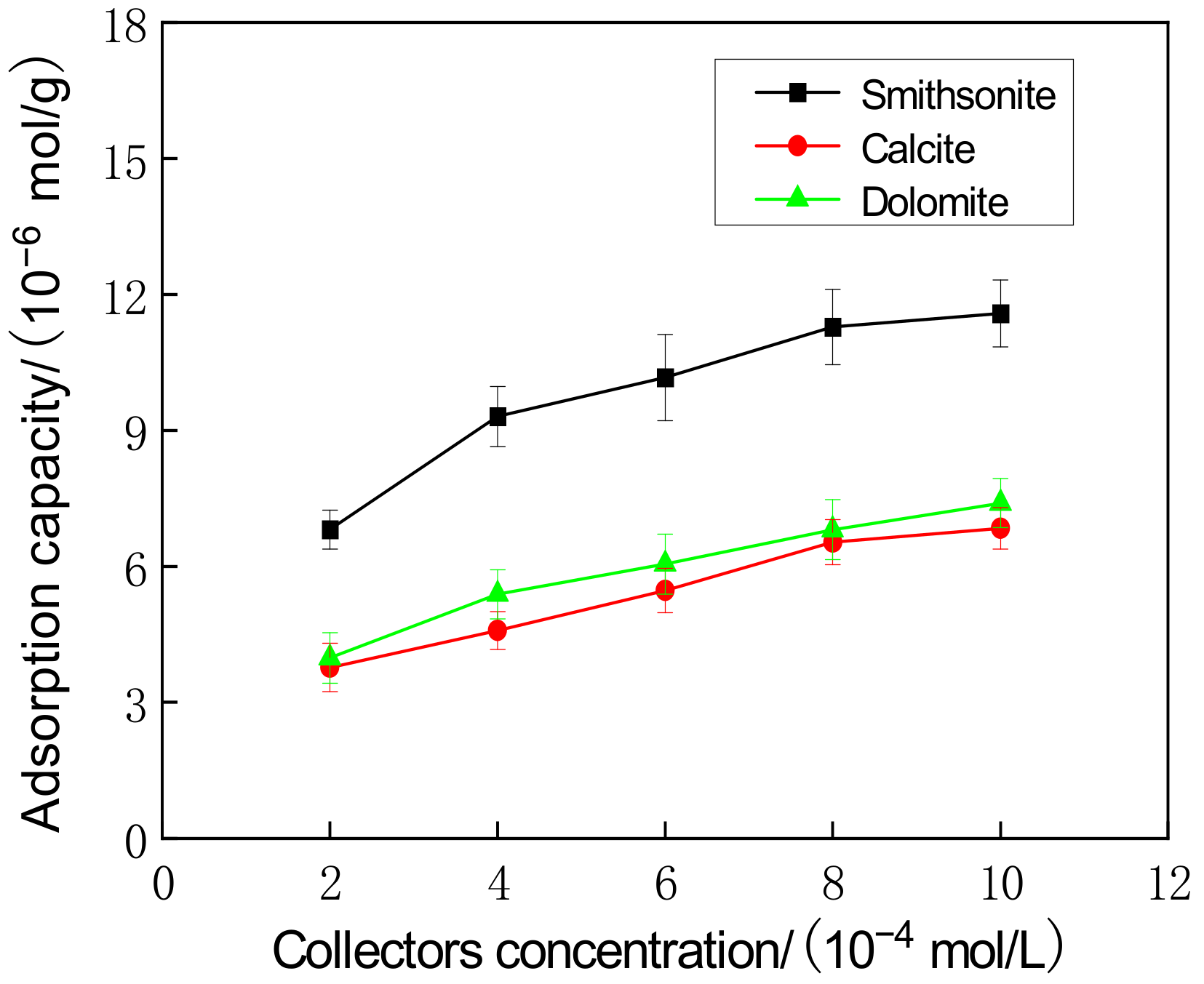
2.4. Collector Adsorption Test
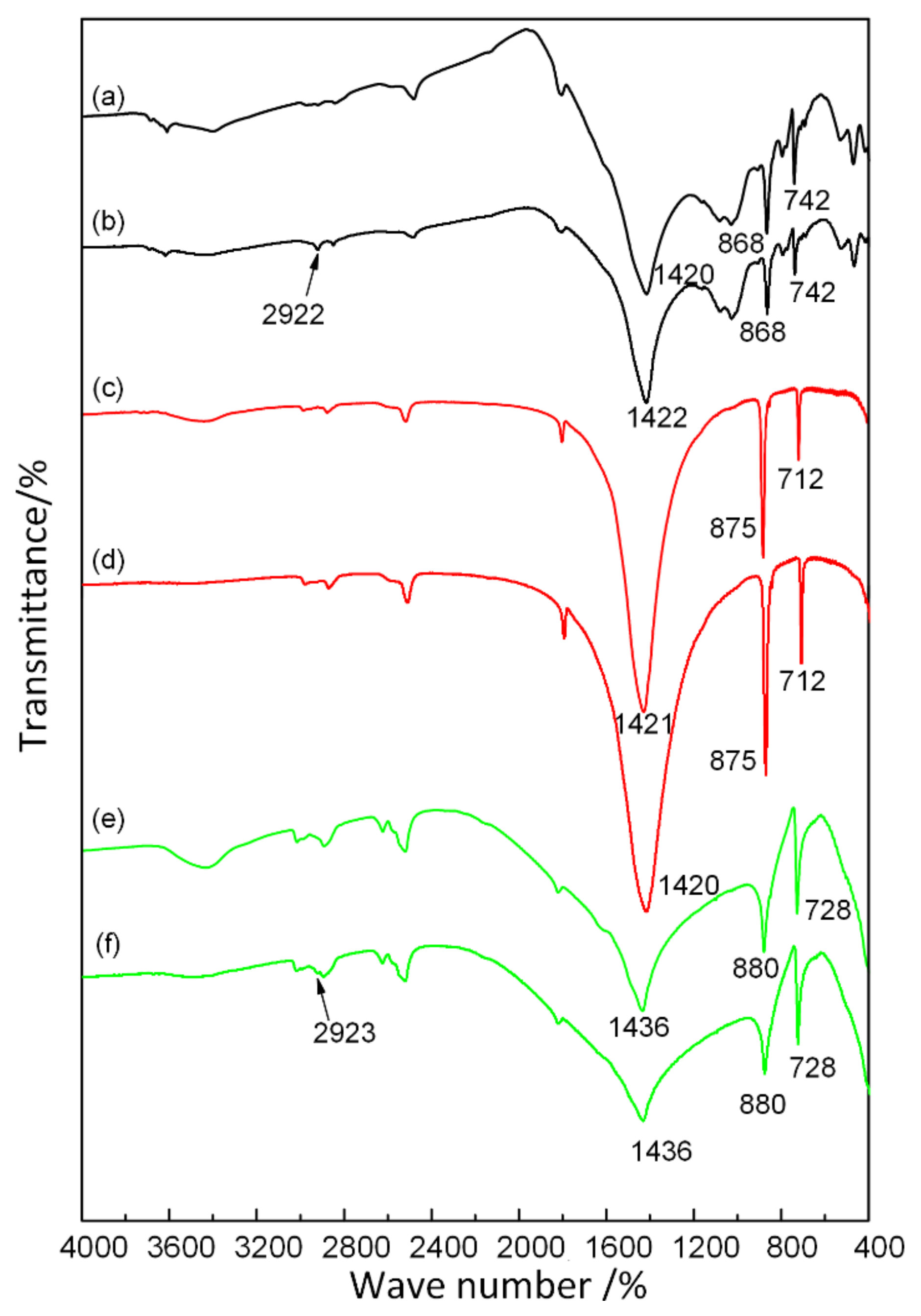
2.5. FTIR Measurement
2.6. XPS Measurement
3. Results and Discussion
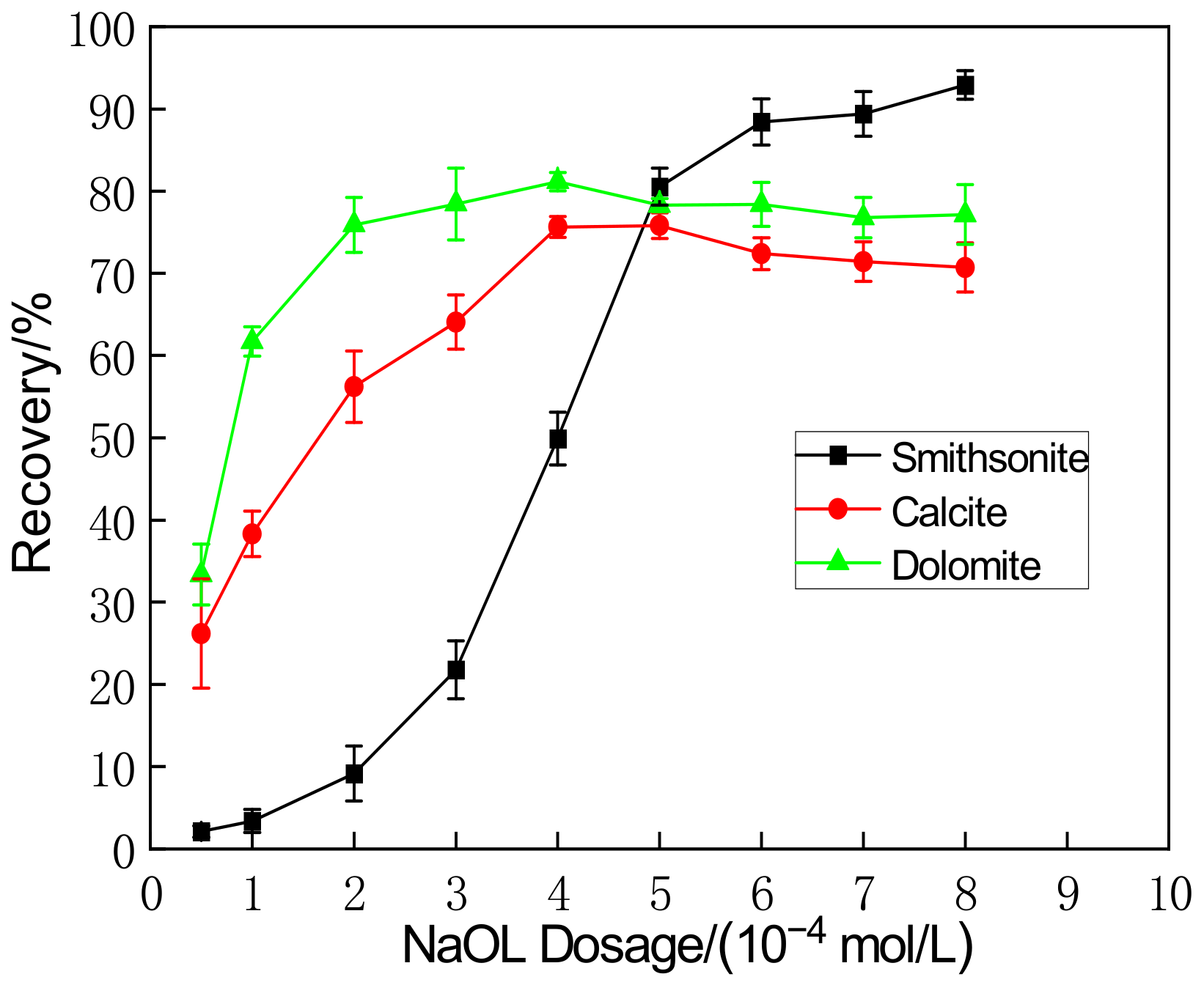
3.1. Floatability in Sodium Oleate
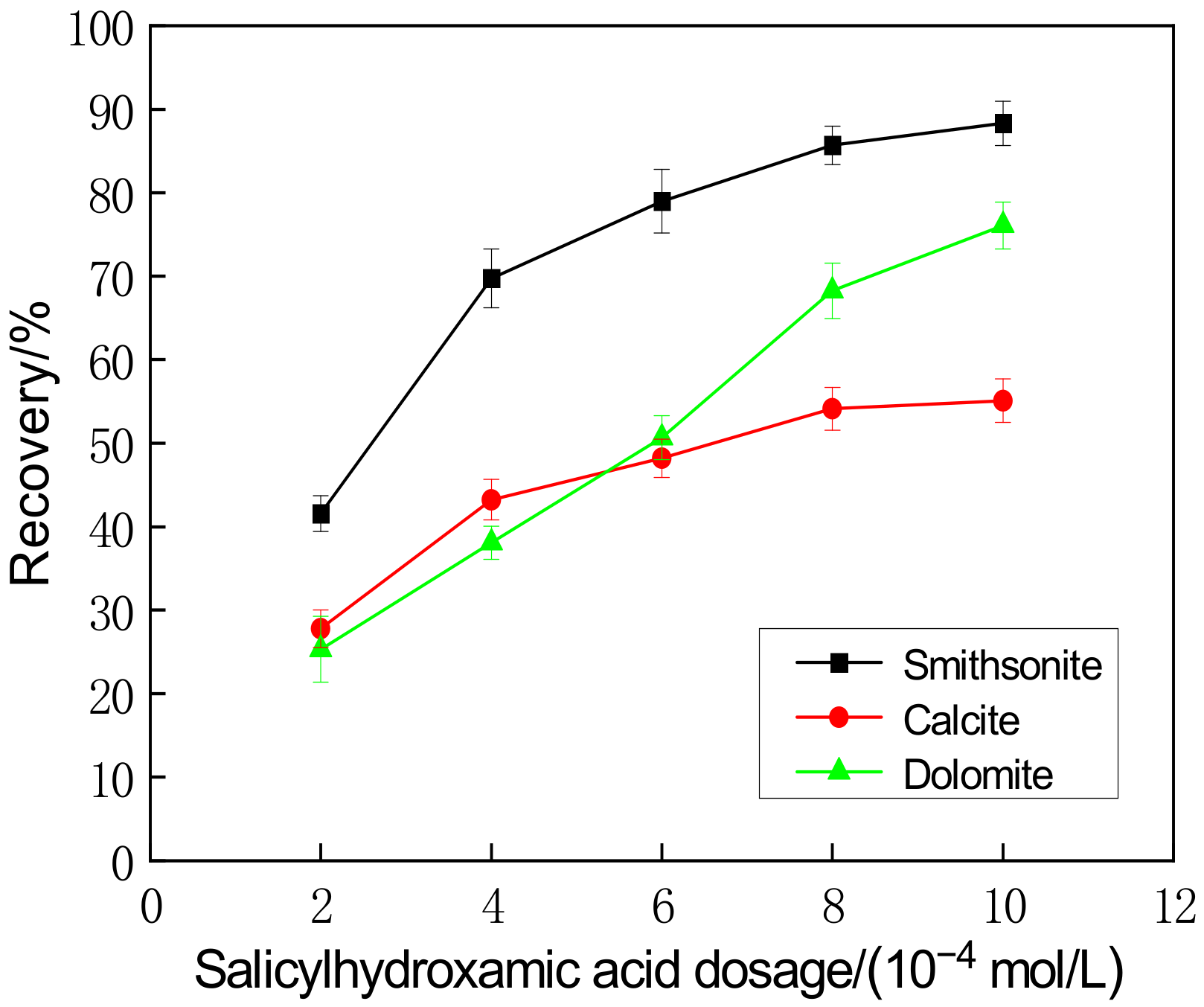
3.2. Floatability in Salicylhydroxamic Acid
3.3. Floatability in Salicylhydroxamic Acid and Sodium Oleate
3.4. Adsorption Mechanism of Combined Collectors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current status and perspectives of zinc-based absorbable alloys for biomedical applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zheng, X.; Liu, J.; Zhang, G.; Zhang, Y.; Wang, C.; Liu, Y. The levels, sources, and spatial distribution of heavy metals in soils from the drinking water sources of Beijing, China. Sustainability 2021, 13, 3719. [Google Scholar] [CrossRef]
- Wang, H.J.; Feng, Y.L.; Li, H.R.; Kang, J.X. Simultaneous extraction of gold and zinc from refractory carbonaceous gold ore by chlorination roasting process. Trans. Nonferrous Met. Soc. China 2020, 30, 1111–1123. [Google Scholar] [CrossRef]
- Li, X.; Wu, L.; Zhou, J.; Luo, Y.; Zhou, T.; Li, Z.; Hu, P.J.; Christie, P. Potential environmental risk of natural particulate cadmium and zinc in sphalerite-and smithsonite-spiked soils. J. Hazard. Mater. 2022, 429, 128313. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, C.; Yang, G.; Zhang, G.; Fan, H.; Zhang, Y.; Wen, H. Primary study of germanium isotope composition in sphalerite from the Fule Pb–Zn deposit, Yunnan province. Ore Geol. Rev. 2020, 120, 103466. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, S.; Xian, Y.; Zhao, L.; Feng, Q.; Bai, S.; Lang, J. Lead ion modification and its enhancement for xanthate adsorption on smithsonite surface. Appl. Surf. Sci. 2019, 498, 143801. [Google Scholar] [CrossRef]
- Sun, H.; Niu, F.; Zhang, J. Investigation on the flotation separation of smithsonite from calcite using calcium lignosulphonate as a depressant. Colloid Surface. A 2021, 630, 127571. [Google Scholar] [CrossRef]
- Li, P.; Zhang, G.; Zhao, W.; Han, G.; Feng, Q. Interaction mechanism of Fe3+ with smithsonite surfaces and its response to flotation performance. Sep. Purif. Technol. 2022, 291, 121001. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, S.; Liang, G.; Xian, Y.; Chen, L. Ammonia pretreatment for enhancement of Pb ions adsorption on smithsonite surface and its flotation performance. Appl. Surf. Sci. 2022, 590, 153069. [Google Scholar] [CrossRef]
- Luo, B.; Liu, Q.; Deng, J.; Yu, L.; Lai, H.; Song, C.; Li, S. Characterization of sulfide film on smithsonite surface during sulfidation processing and its response to flotation performance. Powder Technol. 2019, 351, 144–152. [Google Scholar] [CrossRef]
- Southwood, M.; Cairncross, B.; Rumsey, M.S. Minerals of the Kabwe (“Broken Hill”) mine, central province, Zambia. Rocks Miner. 2019, 94, 114–149. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, W.; Chen, C.; Chai, L.; Li, L.; Liu, S.; Zhang, T. Selective flotation of smithsonite, quartz and calcite using alkyl diamine ether as collector. Trans. Nonferrous Met. Soc. China 2018, 28, 163–168. [Google Scholar] [CrossRef]
- Cairncross, B. Minerals of Berg Aukas, Otavi Mountainland, Namibia. Rocks Miner. 2021, 96, 110–147. [Google Scholar] [CrossRef]
- Boni, M.; Gilg, H.A.; Balassone, G.; Mondillo, N.; Menschik, F.; Rumsey, M.; Struck, U. Stable isotopes of non sulphide Zn–Pb ores in Britain and Ireland: Fluid characteristics and palaeoclimatic variability. J. Geol. Soc. 2019, 176, 1107–1119. [Google Scholar] [CrossRef]
- Shang, Y.; Sun, C. Effect of Gangue Minerals on Pulp Rheology and Flotation Behavior of Smithsonite. Minerals 2023, 13, 66. [Google Scholar] [CrossRef]
- Khalil, A.; Argane, R.; Benzaazoua, M.; Bouzahzah, H.; Taha, Y.; Hakkou, R. Pb–Zn mine tailings reprocessing using centrifugal dense media separation. Miner. Eng. 2018, 131, 28–37. [Google Scholar] [CrossRef]
- Ebtedaei, A.; Farzanegan, A. A Detailed study of Applying Gravity Separation to Lead and Zinc Carbonate Ore for Smithsonite Concentration Using DMC. Miner. Process. Extr. Metall. Rev. 2020, 41, 381–395. [Google Scholar] [CrossRef]
- Farag, M.Z.; Abdel-Khalek, N.A.; Hassan, M.S.; El Aref, M.M.; El Manawi, A.W. Upgrading of Egyptian Nonsulfide Zinc Ore by Gravity Separation Techniques. J. Metall. Eng. 2012, 1, 6–13. [Google Scholar]
- Yang, J.; Huo, X.; Li, Z.; Ma, S. Study on Hydrometallurgical Treatment of Oxide Ores Bearing Zinc. Minerals 2022, 12, 1264. [Google Scholar] [CrossRef]
- Bishop, J.L.; King, S.J.; Lane, M.D.; Brown, A.J.; Lafuente, B.; Hiroi, T.; Roberts, R.; Swayze, G.A.; Lin, J.-F.; Sánchez Román, M. Spectral properties of anhydrous carbonates and nitrates. Earth Space Sci. 2021, 8, e2021EA001844. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.; Huang, K.; Yang, S.; Liang, Z. Studies of benzyl hydroxamic acid/calcium lignosulphonate addition order in the flotation separation of smithsonite from calcite. Int. J. Min. Sci. Technol. 2021, 31, 1153–1158. [Google Scholar] [CrossRef]
- Yang, D.; Li, B.; Feng, D.; Xie, X.; Mo, F.; Tong, X.; Song, Q. Flotation separation of smithsonite from calcite with guar gum as a depressant. Colloid Surf. A 2022, 650, 129562. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Ji, B.; Fan, R.; Liu, R.; Chen, P.; Sun, W.; Hu, Y. Selective Flotation of Cassiterite from Calcite with Salicylhydroxamic Acid Collector and Carboxymethyl Cellulose Depressant. Minerals 2018, 8, 316. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Adsorption studies of smithsonite flotation using dodecylamine and oleic acid. Miner. Metall. Process. 2006, 2, 87–96. [Google Scholar] [CrossRef]
- Xu, L.; Liu, D.; Sun, R.; Wang, Y.; Liu, Z.; Shao, P.; Wang, C.; Wen, S. Flotation separation of smithsonite and calcite in sodium oleate system using soluble starch as depressant. Miner. Eng. 2024, 205, 108490. [Google Scholar] [CrossRef]
- Araújo, A.C.; Lima, R.M. Influence of cations Ca2+, Mg2+ and Zn2+ on the flotation and surface charge of smithsonite and dolomite with sodium oleate and sodium silicate. Int. J. Miner. Process. 2017, 167, 35–41. [Google Scholar] [CrossRef]
- Nie, S.; Guo, Z.; Tian, M.; Sun, W. Selective Flotation Separation of Cassiterite and Calcite Through Using Cinnamohydroxamic Acid as the Collector and Pb2+ as the Collector. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131262. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Han, H.; Sun, W.; Hu, Y. Improved Flotation Separation of Cassiterite from Calcite Using a Mixture of Lead (II) Ion/Benzohydroxamic Acid as Collector and Carboxymethyl Cellulose as Depressant. Miner. Eng. 2017, 113, 68–70. [Google Scholar] [CrossRef]
- Song, Z.; Wen, S.; Han, G.; Feng, Q. Recent Progress on Chelating Reagents in Flotation of Zinc Oxide Ores: A Review. Minerals 2023, 13, 1278. [Google Scholar] [CrossRef]
- Sun, K.; Nguyen, C.V.; Nguyen, N.N.; Nguyen, A.V. Flotation surface chemistry of water-soluble salt minerals: From experimental results to new perspectives. Adv. Colloid Interface Sci. 2022, 16, 102775. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, C.; Sun, W.; Gao, Y.; Kowalczuk, P.B. Froth flotation of fluorite: A review. Adv. Colloid Interface Sci. 2021, 290, 102382. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, G.-Y.; Sun, W.; Khoso, S.A.; Liu, R.-Q.; Zhang, X.-F. Selective flotation of smithsonite from dolomite by using novel mixed collector system. Trans. Nonferrous Met. Soc. China 2019, 5, 1082–1089. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Wang, J.; Wang, L.; Xiao, J. A comparison study of adsorption of benzohydroxamic acid and amyl xanthate on smithsonite with dodecylamine as co-collector. Appl. Surf. Sci. 2017, 426, 1141–1147. [Google Scholar] [CrossRef]
- Irannajad, M.; Ejtemaei, M.; Gharabaghi, M. The effect of reagents on selective flotation of smithsonite–calcite–quartz. Miner. Eng. 2009, 9, 766–771. [Google Scholar] [CrossRef]
- Zeng, Y.; Yao, X.; Liu, G.; He, G.; Yu, X.; He, G.; Huang, Z.; Zhang, R.; Cheng, C. Flotation behavior and mechanism of phenylpropenyl hydroxamic acid for the separation of smithsonite and calcite. J. Mol. Liq. 2021, 339, 116893. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. FT-Raman and FT-IR spectroscopic study of synthetic Mg/Zn/Al-hydrotalcites. J. Raman Spectrosc. 2004, 35, 967–974. [Google Scholar] [CrossRef]
- Shukla, N.; Liu, C.; Jones, P.M.; Weller, D. FTIR study of surfactant bonding to FePt nanoparticles. J. Magn. Magn. Mater. 2003, 266, 178–184. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wharton, D.; Hickey, L.; Frost, R.L. Infrared and Raman study of interlayer anions CO32−, NO3−, SO42− and ClO4− in Mg/Al-hydrotalcite. Am. Mineral. 2002, 87, 623–629. [Google Scholar] [CrossRef]
- Tao, F.F. Design of an in-house ambient pressure AP-XPS using a bench-top X-ray source and the surface chemistry of ceria under reaction conditions. Chem. Commun. 2012, 48, 3812–3814. [Google Scholar] [CrossRef]
- Nikitin, A.; Näslund, L.Å.; Zhang, Z.; Nilsson, A. C–H bond formation at the graphite surface studied with core level spectroscopy. Surf. Sci. 2008, 602, 2575–2580. [Google Scholar] [CrossRef]
- Nesov, S.N.; Korusenko, P.M.; Bolotov, V.V.; Povoroznyuk, S.N.; Smirnov, D.A. Electronic structure of nitrogen-containing carbon nanotubes irradiated with argon ions: XPS and XANES studies. Phys. Solid State 2017, 59, 2030–2035. [Google Scholar] [CrossRef]
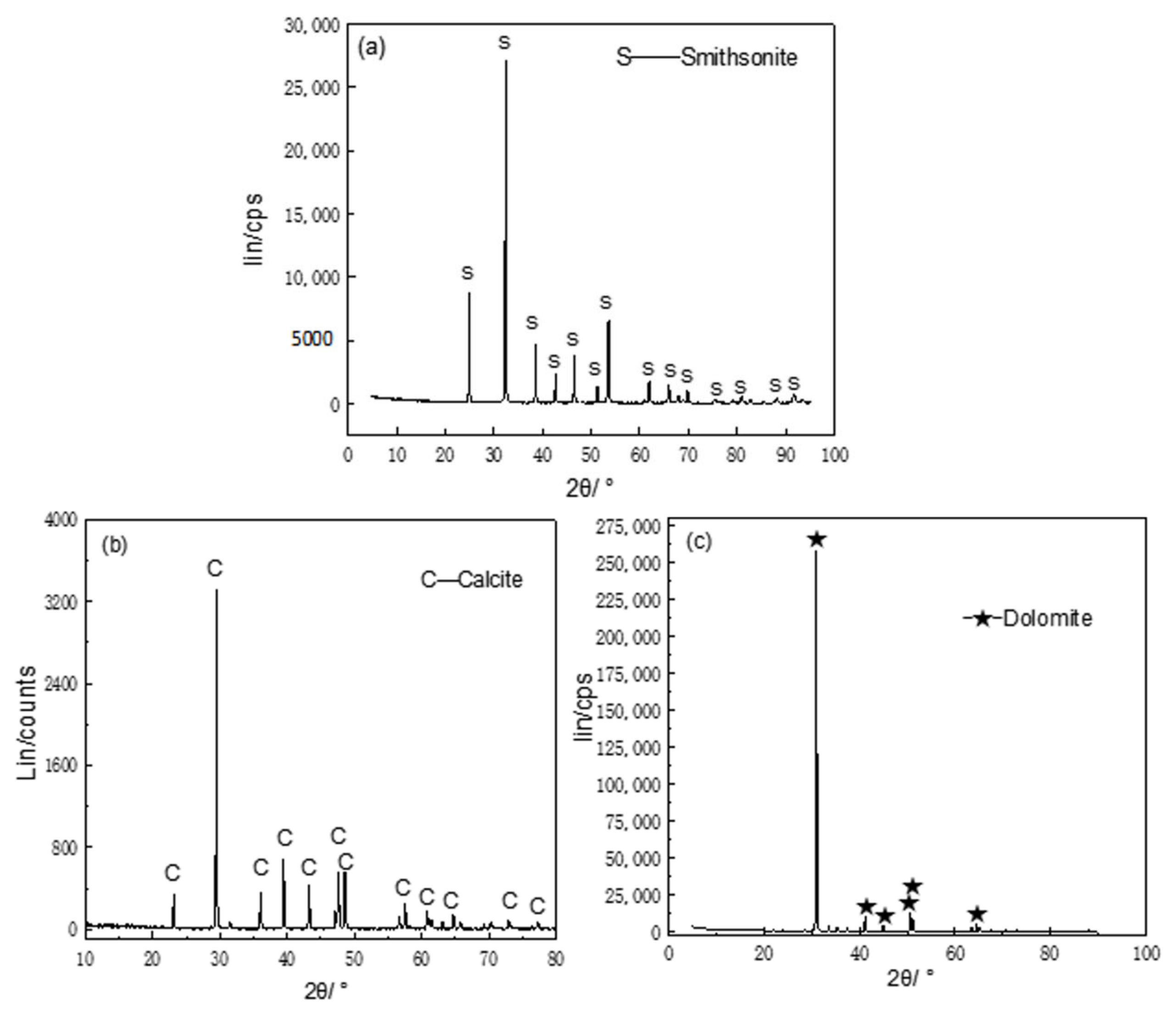



| Minerals | MgO/% | CaO/% | SiO2/% | Zn/% | Al2O3/% | FeO/% | Purity/% |
|---|---|---|---|---|---|---|---|
| Calcite | - | 55.47 | 0.52 | - | 0.22 | 0.21 | 99.05 |
| Dolomite | 21.6 | 30.85 | 0.05 | - | - | - | 99.41 |
| Smithsonite | - | 0.29 | 0.63 | 50.88 | 0.21 | 0.35 | 97.84 |
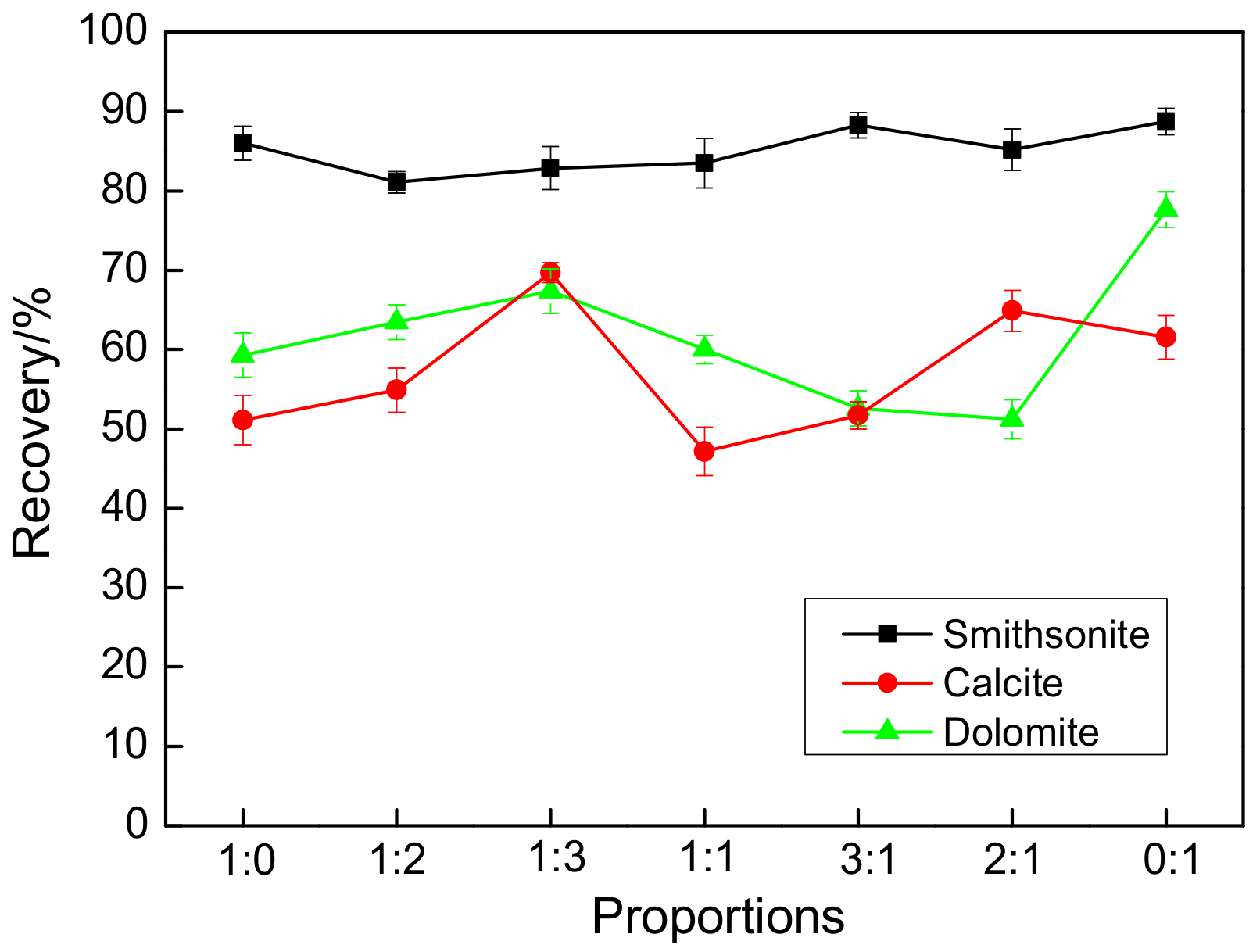
| Salicylhydroxamic Acid/Sodium Oleate Molar Ratio | 1:0 | 1:2 | 1:3 | 1:1 | 3:1 | 2:1 | 0:1 |
|---|---|---|---|---|---|---|---|
| The recovery difference between smithsonite and calcite/% | 36.27 | 26.60 | 12.81 | 35.80 | 37.18 | 20.16 | 28.10 |
| The recovery difference between smithsonite and dolomite/% | 26.60 | 17.92 | 14.70 | 23.48 | 35.90 | 34.16 | 11.68 |
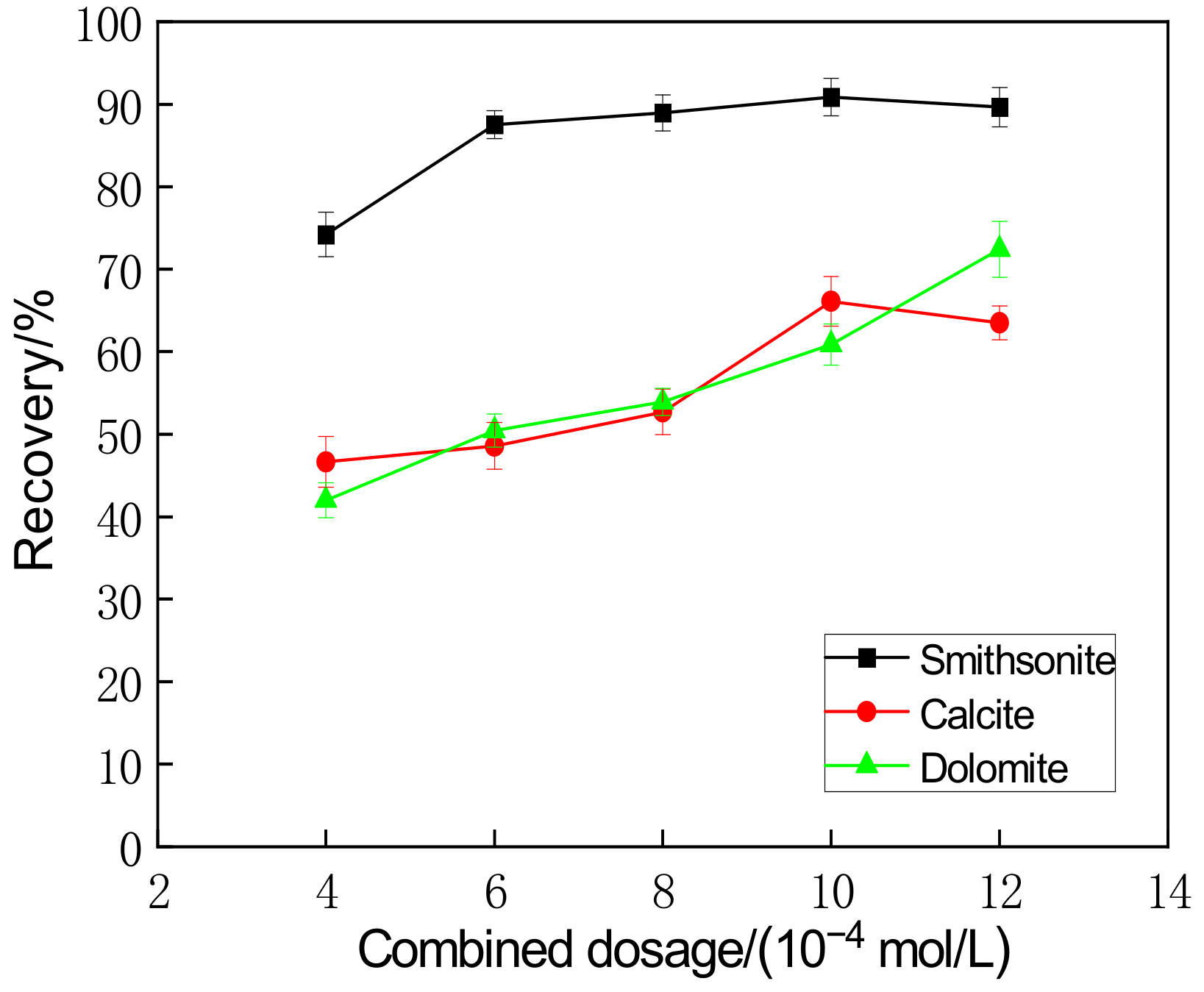
| Salicylhydroxamic Acid + Sodium Oleate Dosage (mol/L) | 4 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|
| The recovery difference between smithsonite and calcite/% | 27.92 | 39.13 | 36.78 | 24.54 | 25.33 |
| The recovery difference between smithsonite and dolomite/% | 27.23 | 37.34 | 35.23 | 29.33 | 16.9 |
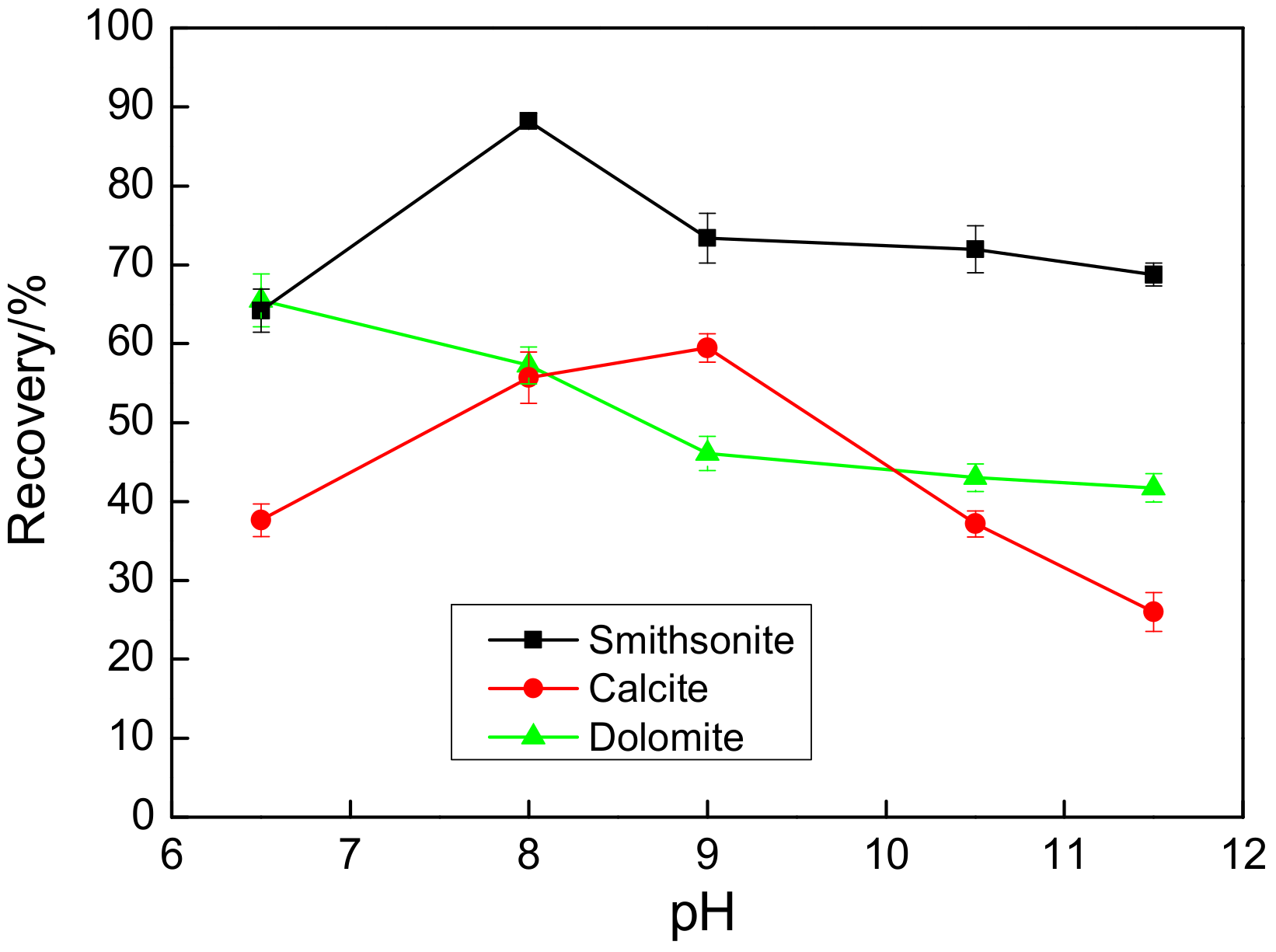
| pH | 6.5 | 8.0 | 9.0 | 10.5 | 11.5 |
|---|---|---|---|---|---|
| The recovery difference between smithsonite and calcite/% | 3.01 | 38.46 | 28.33 | 25.99 | 30.22 |
| The recovery difference between smithsonite and dolomite/% | −2.49 | 37.98 | 31.5 | 31.8 | 33.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Bai, J.; Zhang, Z.; Qiang, W.; Huang, S.; Ouyang, Y.; Liu, T.; Yin, W. The Flotation Separation Mechanism of Smithsonite from Calcite and Dolomite with Combined Collectors. Minerals 2023, 13, 1527. https://doi.org/10.3390/min13121527
Chen X, Bai J, Zhang Z, Qiang W, Huang S, Ouyang Y, Liu T, Yin W. The Flotation Separation Mechanism of Smithsonite from Calcite and Dolomite with Combined Collectors. Minerals. 2023; 13(12):1527. https://doi.org/10.3390/min13121527
Chicago/Turabian StyleChen, Xiangxiang, Junzhi Bai, Zhaoyang Zhang, Wen Qiang, Shiyi Huang, Yunfei Ouyang, Tianhao Liu, and Wanzhong Yin. 2023. "The Flotation Separation Mechanism of Smithsonite from Calcite and Dolomite with Combined Collectors" Minerals 13, no. 12: 1527. https://doi.org/10.3390/min13121527
APA StyleChen, X., Bai, J., Zhang, Z., Qiang, W., Huang, S., Ouyang, Y., Liu, T., & Yin, W. (2023). The Flotation Separation Mechanism of Smithsonite from Calcite and Dolomite with Combined Collectors. Minerals, 13(12), 1527. https://doi.org/10.3390/min13121527





