Abstract
In this work, a complete beneficiation technical route combining physical and chemical methods, namely a roasting-flotation-leaching scheme, is proposed to produce a qualified grade Ti-concentrate from altered Vanadium titanomagnetite (VTM) ore. Based on the character of the ore sample, it is recommended to recover the Ti-bearing minerals, ilmenite and anatase, as composite mineral. Pretreatment experiments indicate that the oxidation roasting (800 °C) and acid washing methods increase the flotation indexes significantly. Flotation condition tests show that the optimal conditions are a grinding fineness of −0.045 mm 83%, sulfuric acid dosage of 2000 g/t, water glass dosage of 1500 g/t, oxalic acid dosage of 200 g/t, and EM328 dosage of 1500 g/t. An open flotation circuit test obtains a flotation concentrate with a TiO2 grade and recovery of 38.30% and 25.99%, respectively. A leaching exploration test shows that the TiO2 grade of the flotation concentrate can be improved to 53.90%. XRD analyses reveal that the ilmenite in the VTM ore is converted into anatase and rutile during the roasting process at 600–800 °C, but pseudobrookite begins to form at 900 °C. Compared to the flotation concentrate, it is confirmed that the content of Ti-bearing minerals is increased significantly in the leaching residue.
1. Introduction
Titanium (Ti) is widely used to make alloy materials with specific properties, which are used in the aerospace, defense and military, biomedical, and many other fields [1]. Vanadium (V) is also applied in many industrial processes, including the preparation of special steels and alloys, catalysts, materials, batteries, and so on, due to its outstanding mechanical and physical characteristics [2,3,4]. Vanadium titanomagnetite (VTM) is an important source of strategic metals Ti, V, and (Fe), with a high comprehensive utilization value.
VTM reserves are mainly distributed in China, South Africa, Russia, the USA, Canada, Norway, Finland, India, and Sweden, totally exceeding 40 Gt [4,5]. The VTM resources of the Panzhihua-Xichang area in China are very rich, with proven reserves of nearly 10 Gt [6]. The Ti and V reserves rank first and third on a global scale, respectively [7]. In the VTM ore, Ti mainly exists in the form of ilmenite, and Fe mainly exists in titanomagnetite. The gangue minerals usually are pyroxene, chlorite, feldspar, quartz, and calcite [8,9].
However, with over 50 years of exploitation, high-grade and easily processed VTM reserves continue to deplete, and low-grade and refractory reserves are necessary to utilize to ensure an adequate supply of concentrate of Ti, V, and Fe. Until now, mining areas have entered the middle and deep stage, resulting in changes in ore properties and difficulties in beneficiation [10]. The content of altered minerals has increased, and particle sizes have become extremely fine [9]. It is commonly found that valuable minerals such as titanomagnetite and ilmenite are gradually altered to anatase, chlorite, and titanite [11,12,13,14].
Altered Fe-Ti deposits have been reported all over the world, for example, Miocene fluvial deposits in northern Europe [13] and heavy mineral deposits in South Africa [15]. In China, VTM deposits in the Panzhihua-Xichang area, Shaanxi Ankang, and other regions exhibit varying degrees of alteration. The VTM ore found in the Renhe district of the Panzhihua-Xichang area is a typical example of altered VTM ore, with reserves exceeding 100 Mt. This ore is characterized by its low-grade of TiO2, consisting of ilmenite, anatase, trace titanomagnetite, and abundant chlorite. However, so far, rare studies on beneficiation techniques for altered VTM ores have been reported. Therefore, it is necessary to investigate the beneficiation route of altered VTM ore to realize the utilization of such resources.
For low-grade ore, pre-concentration is an effective method for enriching valuable minerals, removing gangue minerals, and promoting the grade [16,17]. The choice of pre-concentration method is based on the difference in the characteristics between valuable minerals and gangue minerals [18]. The common pre-concentration process for VTM ore is recovering titanium magnetite via low-intensity magnetic separation (LIMS) firstly, and then recovering ilmenite from its tailings. Due to the differences in specific gravity, magnetism, and electrical conductivity between Ti-bearing minerals and associated gangue minerals, which are mainly composed of silicate minerals, pre-concentration methods include gravity separation, magnetic separation, and electrostatic separation [19].
Flotation is an effective and popular method for recovering Ti-bearing minerals [20]. During the flotation process, reagents play conclusive roles in modifying the surface properties to promote their floatability. On an industrial production scale, the frequently used collectors in ilmenite flotation are fatty acids and their soaps, along with fuel oils [21,22]. Fatty acids and hydroxymates are usually used as collectors to float anatase, and the performance of hydroxymates is superior to fatty acids [23,24]. Styryl Phosphoric Acid (SPA) is a terrific collector for rutile flotation since it has a good selectivity and capability [25].
Due to the poor floatability of ilmenite, pretreatment methods were conducted by many researchers prior to the flotation of Ti-bearing minerals, including surface dissolution, ultrasonic pretreatment, microwave irradiation, oxidation roasting, and more [2,26,27]. It has been reported that the strengthening effect of oxidation roasting and microwave irradiation are similar to ilmenite flotation [28]. Furthermore, the strengthening effects of oxidation roasting on ilmenite are attributed to the conversion of Fe2+ into Fe3+, and the ilmenite phase to rutile, hematite, and pseudobrookite at temperatures over 750 °C [29]. However, it is unfortunate that these pretreatment methods have only been studied in relation to pure ilmenite minerals, and have not been tested on real ore.
In this work, altered VTM ore obtained from the Renhe district was selected as the research object. The character of the ore sample was studied and the separation of Ti was focused on. Magnetic separation and gravity separation were first used to conduct comparative experiments on pre-concentration processes. Then, the pre-concentrate was treated with pretreatment processes, i.e., oxidation roasting experiments and acid washing experiments. Next, flotation condition tests were carried out to determine the optimal grinding fineness and reagents dosage. After that, an open flotation circuit test was conducted to obtain the flotation concentrate. Finally, an acid leaching exploratory experiment on the flotation concentrate was performed to obtain a qualified Ti-concentrate. Furthermore, the strengthening mechanisms of the roasting pretreatment and acid leaching were analyzed. Based on the above research, a new route for Ti-concentrate production from altered VTM ore was proposed. The research will provide a technical basis for the development and utilization of such altered VTM resources around the world. Moreover, the TiO2 grade in the sample was low, even lower than in the VTM waste [30]. Therefore, the research will also provide guidance for upgrading the Ti grade of titaniferous waste and concentrate.
2. Materials and Experimental Methods
2.1. Materials
2.1.1. Ore Sample
The representative VTM sample was obtained from the Renhe district of the Panzhihua-Xichang area in China, with a high degree of alteration. The −3 mm (“−” denotes less than the given particle size, the same below [31]) of the VTM ore particle was used for the experiments. A chemical multi-element analysis of the raw ore was launched with acid dissolution, atomic absorption spectroscopy (AAS, Thermo Fisher Scientific Inc., Waltham, MA, USA), and inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer Inc., Waltham, MA, USA), and the results are shown in Table 1. As shown in Table 1, the contents of total Fe (TFe) and TiO2 in the sample were 14.40% and 7.15%, respectively, belonging to low-Fe and low-TiO2 VTM ore. The high contents of SiO2 and Al2O3 indicate that the ore contained high contents of silicate minerals.

Table 1.
Chemical multi-element analysis of the VTM raw ore (%).
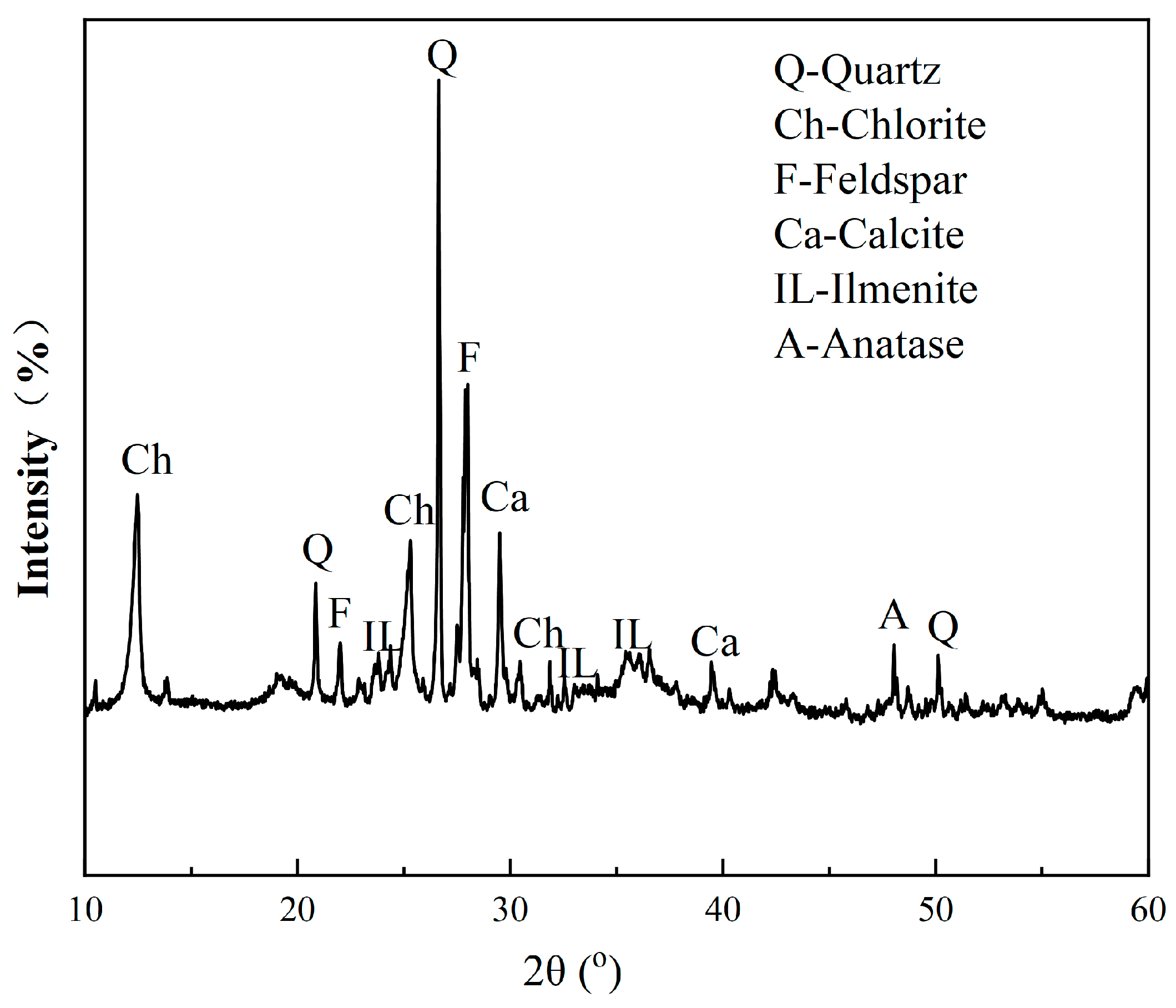
The XRD pattern of the raw ore is presented in Figure 1. A mineral content analysis was conducted using various methods, including microscopy, an electron microscope analysis, and an XRD analysis. Table 2 shows the mineral contents of the ore, indicating that the major industrial Fe- and Ti-bearing minerals’ contents were extremely low. The content of titanomagnetite was only 1%, which is the primary target mineral for Fe separation. The main Ti-bearing minerals were anatase and ilmenite, with concentrations of 4.21% and 2.71%, respectively. The main gangue minerals were chlorite, feldspar, quartz, and calcite, etc., with chlorite having a high content of 41.13%. The amounts of chlorite and transition phase from anatase to chlorite suggest severe alteration of the ore.
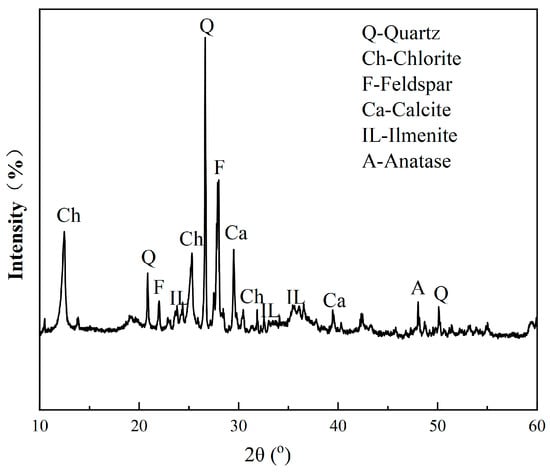
Figure 1.
XRD pattern of the VTM raw ore.

Table 2.
Mineral contents of the ore (%).
According to the sample characteristics, the recovery of Fe was not considered due to the 1% titanomagnetite content. Ti was mainly distributed in anatase and ilmenite, which tend to coexist closely at a fine particle size, making individual liberation difficult. Hence, Ti-bearing minerals are considered as composite minerals for Ti recovery.
2.1.2. Reagents
In the flotation tests, sulfuric acid (analytical grade, H2SO4, 98.0%) was used as a pH adjuster. Water glass (industrial grade, Na2SiO3) and oxalic acid (analytical grade, H2C2O4·H2O, 99.5%) were employed as depressants. The collector EM 328 is made of MOH (industrial grade, consisting of C5–8 fatty acid and their soap) and mixed hydroxamic acid (industrial grade), while EM 522 is composed of styrene phosphonic acid (industrial grade) and capryl octanol (analytical grade, C8H18O, 99.0%) with a mass ratio of 1:1. Tap water was used for all flotation tests.
2.2. Experimental Methods
2.2.1. Pre-Concentration
Magnetic Separation
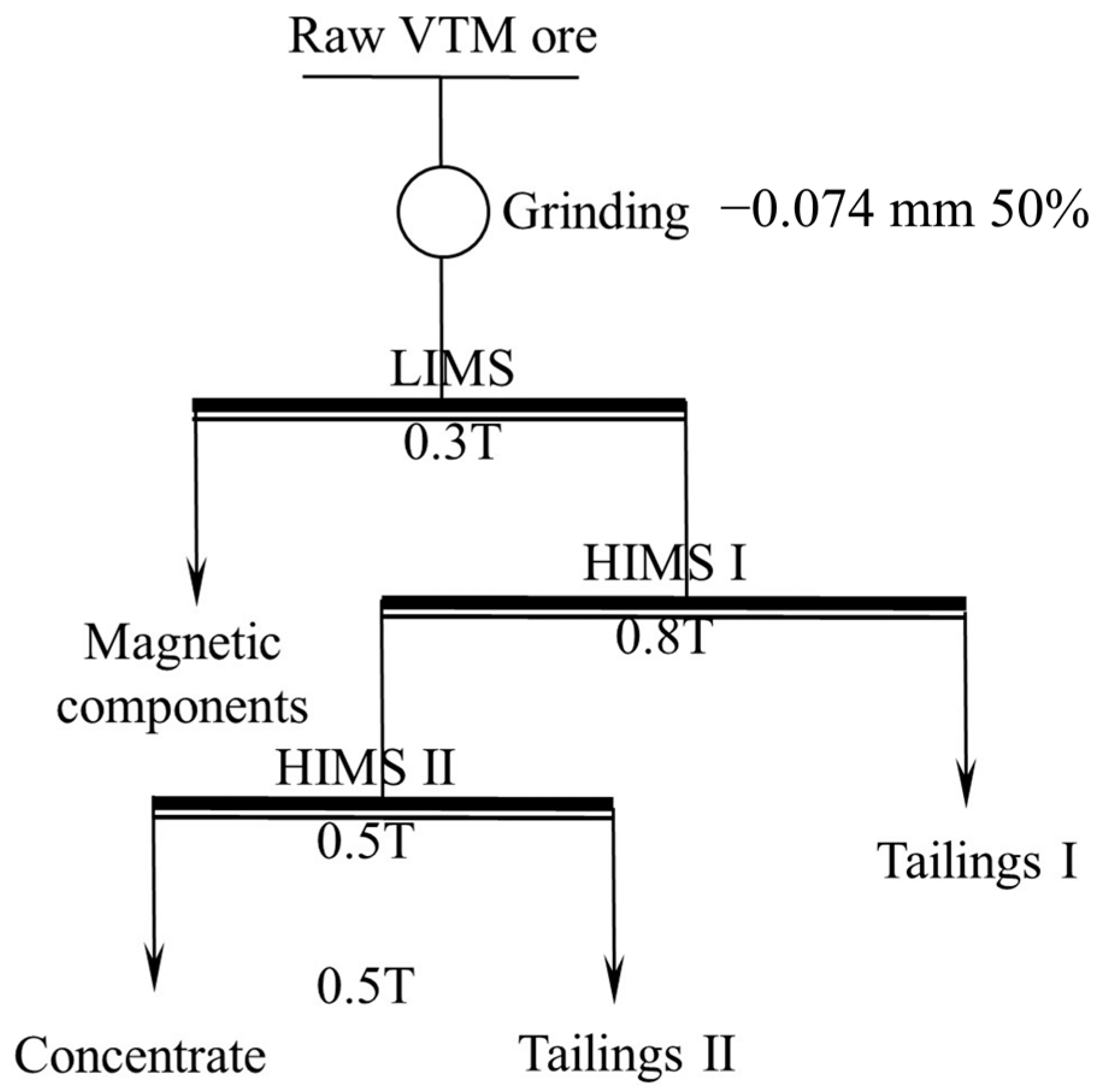
High-intensity magnetic separation (HIMS) was carried out using an SLon-500 high gradient magnetic separator(Slon Magnetic Separator Co., Ltd., Ganzhou, China). Magnetic components, including titanomagnetite and other substances in the ore, were removed via LIMS before HIMS. A flowsheet of the experiment is displayed in Figure 2.
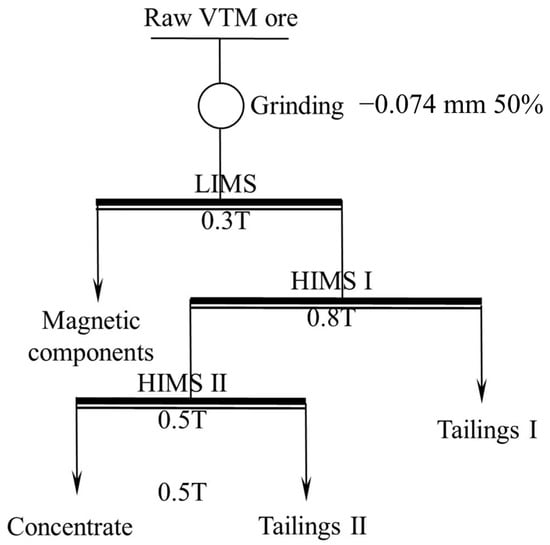
Figure 2.
Flowsheet of magnetic separation experiment.
Gravity Separation
Gravity separation was performed using an XZYK-1000 × 6000 shaking table (Sichuan 102 Machinery manufacturing Co., Ltd., Xichang, China). The raw VTM ore was first ground to different fineness, and then fed into gravity separation. The heavy minerals were recovered as a pre-concentrate, enriching the Ti-bearing minerals. A flowsheet of gravity separation experiment is presented in Figure 3.
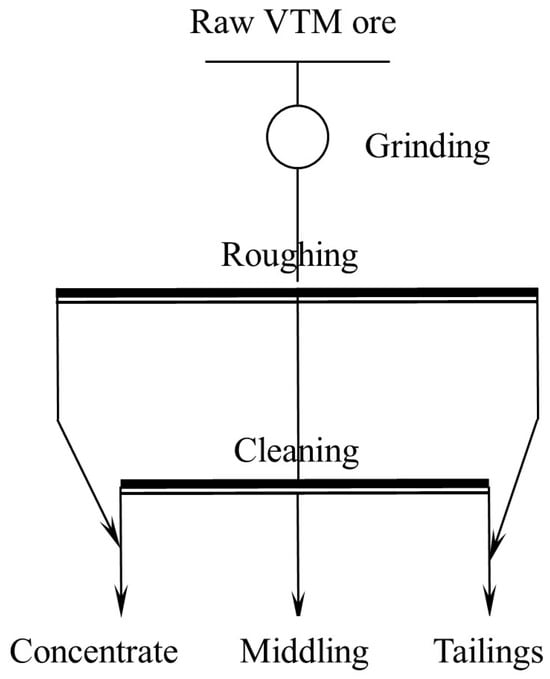
Figure 3.
Flowsheet of gravity separation experiment.
The pre-concentrates and tailings of the both separation methods were filtered, dried, weighed, and analyzed. The grade and recovery of TiO2 could then be obtained and calculated, respectively.
2.2.2. Pretreatment
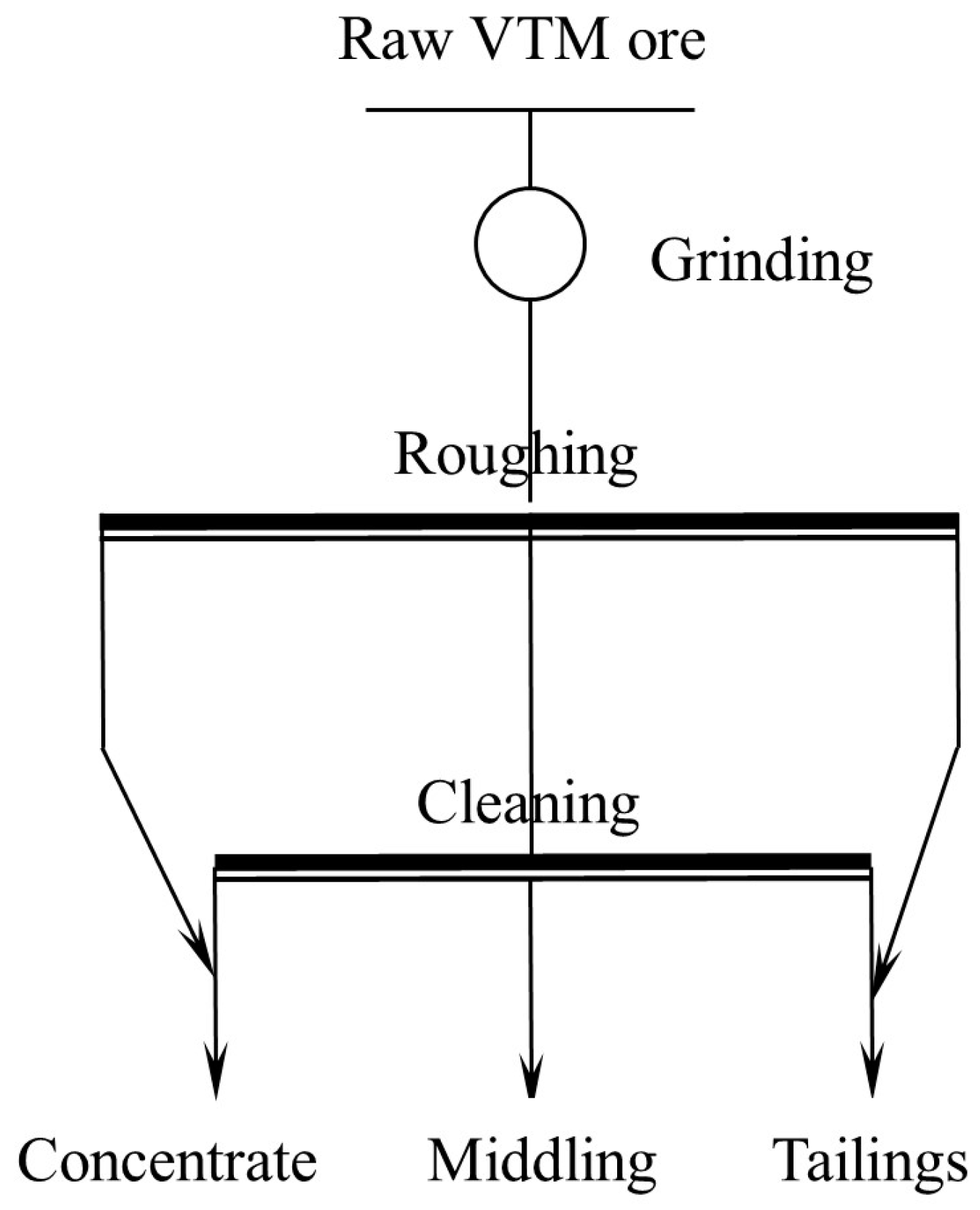
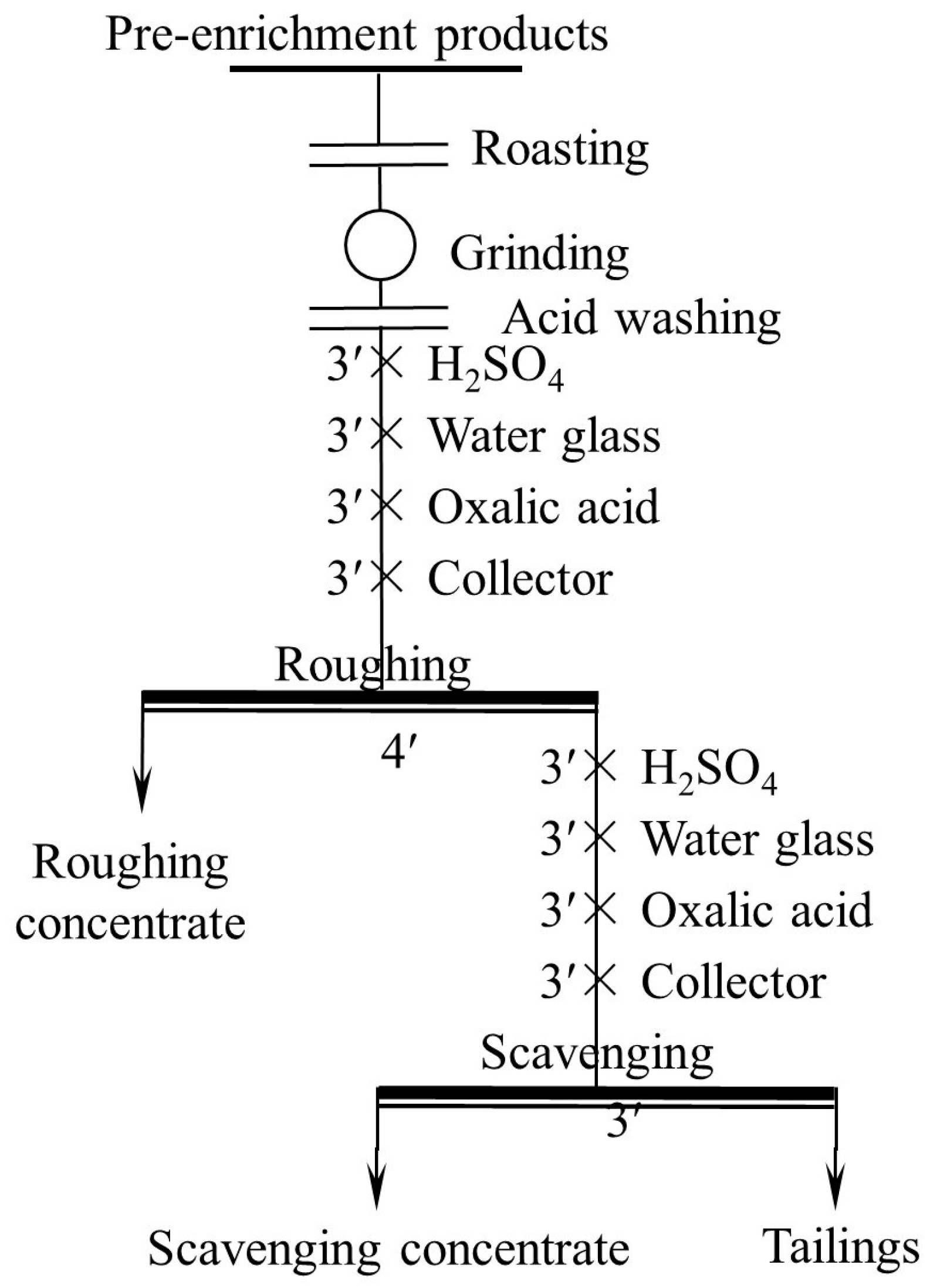
The roasting process was conducted in a laboratory muffle furnace. After the furnace was heated to the desired temperature, a crucible filled with 300 g of ore sample was placed in the furnace, injected with air through a tube in advance. The time of roasting was 2 h. When the furnace was cooled to room temperature, the crucible was removed from the furnace. The acid washing process was carried out using an XFG-type flotation machine with a 1.5 L cell. In each process, 300 g of ground ore sample and 1.5 L of 5 wt.% H2SO4 were placed in the cell and fully agitated for 1 h. The sample was then rinsed 3 times with water to remove residual acid, then filtered and dried. The effect of both pretreatment methods was tested by flotation, of which the flowsheet is one roughing (Figure 4).
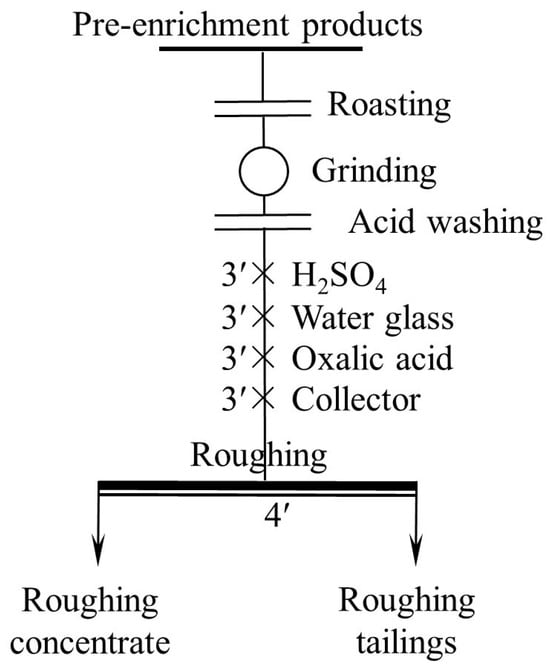
Figure 4.
Flowsheet of grinding fineness test. (“ ′ ”denotes minutes, the same below).
2.2.3. Flotation Tests
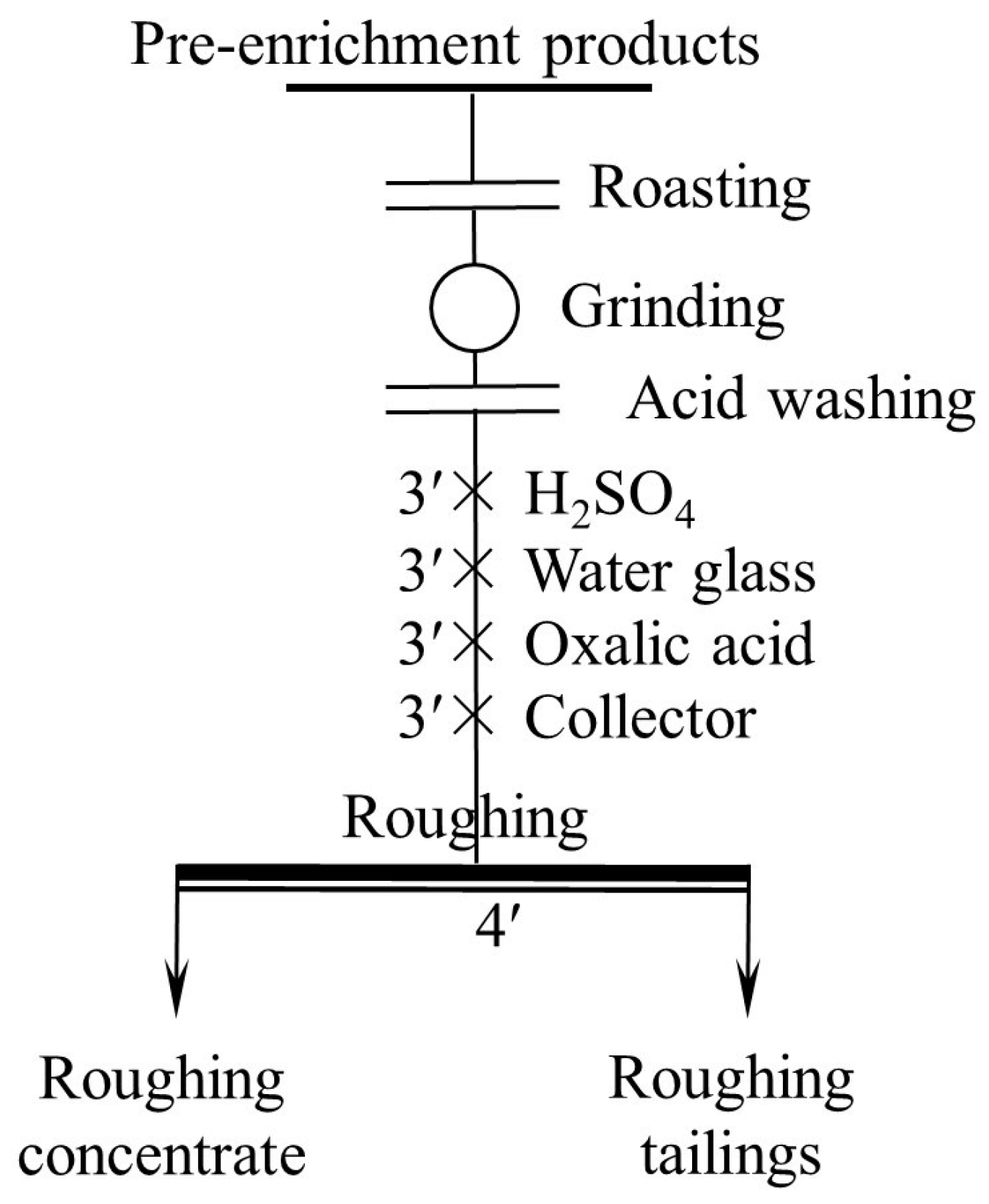
After the pre-concentration and pretreatment, ore flotation tests were conducted in an XFG-type flotation machine with various cells, including 3 L, 1.5 L, 1.0 L, 0.75 L, and 0.5 L. The 0.5 L cell was used in the flotation condition experiments, and all the cells were used in the open circuit flotation process. The flotation condition tests included the effect of the grinding fineness, pH condition, depressants dosage, collector species, and dosage tests. The process of the grinding fineness test is roughing, and that of other condition tests are roughing and scavenging. In each test, 100 g of unroasted or roasted sample of the pretreatment product was used. The two flotation flowsheets are shown in Figure 4 and Figure 5, respectively. It is worth noting that the dosage of reagents used in the scavenging was half of that in the roughing. The test was needed to be repeated if the error between the sum weight of the concentrate and tailings and the original feed weight, or that between the calculated grade and feed grade exceeded 3%.
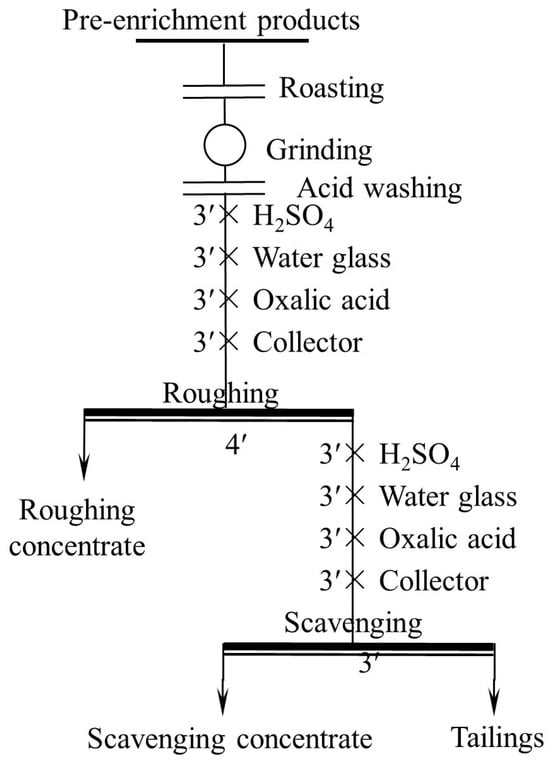
Figure 5.
Flowsheet of other flotation condition tests.
2.2.4. Acid Leaching Test
Acid leaching was carried out in a conical flask with 30 mL of a mass ratio of 1.5:1 mixture of hydrochloric acid and hydrofluoric acid. Leaching was conducted at 25 °C and 400 rpm for 7 h, with a liquid to solid ratio of 2:1. Subsequently, the slurry was filtered to obtain the leaching residue. The leaching residue was then washed, dried, and collected for the XRD analysis.
2.2.5. X-ray Diffraction (XRD)
The mineral phase of the ore samples was identified via X-ray diffraction (XRD) using Ultima IV diffractometer (Rigaku, Tokyo, Japan). The X-ray source was Cu Kα radiation, operating at 3 kW, 40 kV voltage, and 40 mA current. The scanning range was 5–65° at a scanning rate of 5°/min. The spectra were analyzed using the X’Pert HighScore (Plus) 2.0 software.
3. Results
3.1. Titanium Pre-Concentration Experiments
3.1.1. Magnetic Separation
The results of the magnetic experiment are presented in Table 3. As seen in Table 3, pre-concentrate with 10.43% TiO2 and a recovery of 26.94% was obtained, respectively, after two-stage HIMS. At the same time, the TiO2 grades of the two-stage HIMS tailings were 4.66% and 7.37%, respectively. Therefore, the HIMS had no obvious effect on Ti enrichment.

Table 3.
Results of high-intensity magnetic separation (HIMS).
3.1.2. Gravity Separation
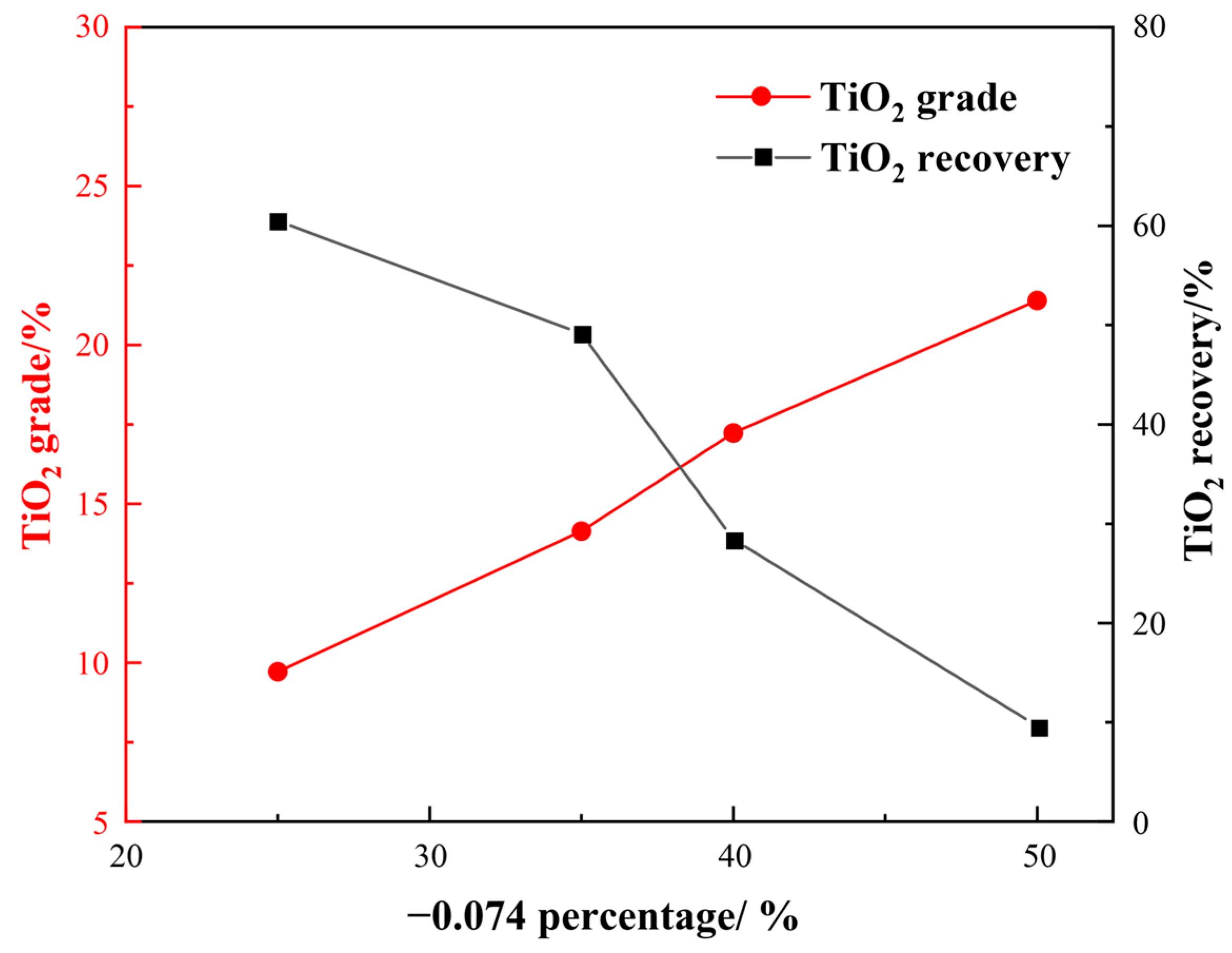
Gravity separation was conducted to remove part of the chlorite and other slime minerals and to improve the TiO2 grade. The −3 mm raw ore was ground to −0.074 mm, representing 25%, 35%, 40%, and 50%. The effect of a different grinding fineness on the separation indexes was studied. The results of the gravity separation experiment are presented in Figure 6.
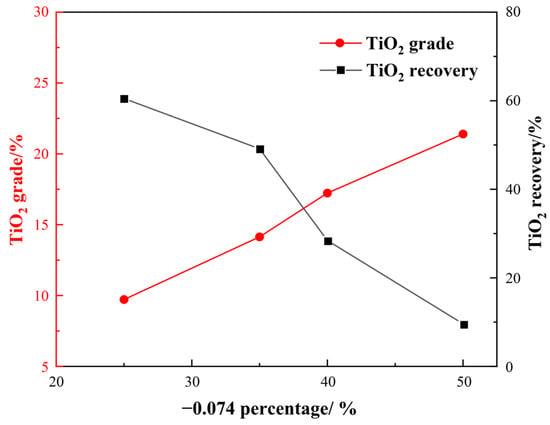
Figure 6.
Results of gravity separation.
From Figure 6, it is found that the TiO2 grade of the gravity concentrate increased, while the recovery decreased as the particle size of the sample became finer. Specifically, when the particle size was −0.074 mm, accounting for 35%, the TiO2 grade and recovery of the concentrate were determined to be 14.14% and 49.14%, respectively. However, when −0.074 mm accounted for 50%, only a TiO2 recovery of 9.63% was achieved. Therefore, it can be concluded that the gravity separation method could improve the TiO2 grade by almost 7% at the optimal grinding fineness of −0.074 mm, accounting for 35%.
3.2. Pretreatment Experiments
Due to the severe alteration of the ore sample, it was difficult to obtain concentrate directly via flotation. Therefore, a variety of pretreatment methods such as roasting and acid washing were investigated.
3.2.1. Oxidation Roasting Temperature Experiment
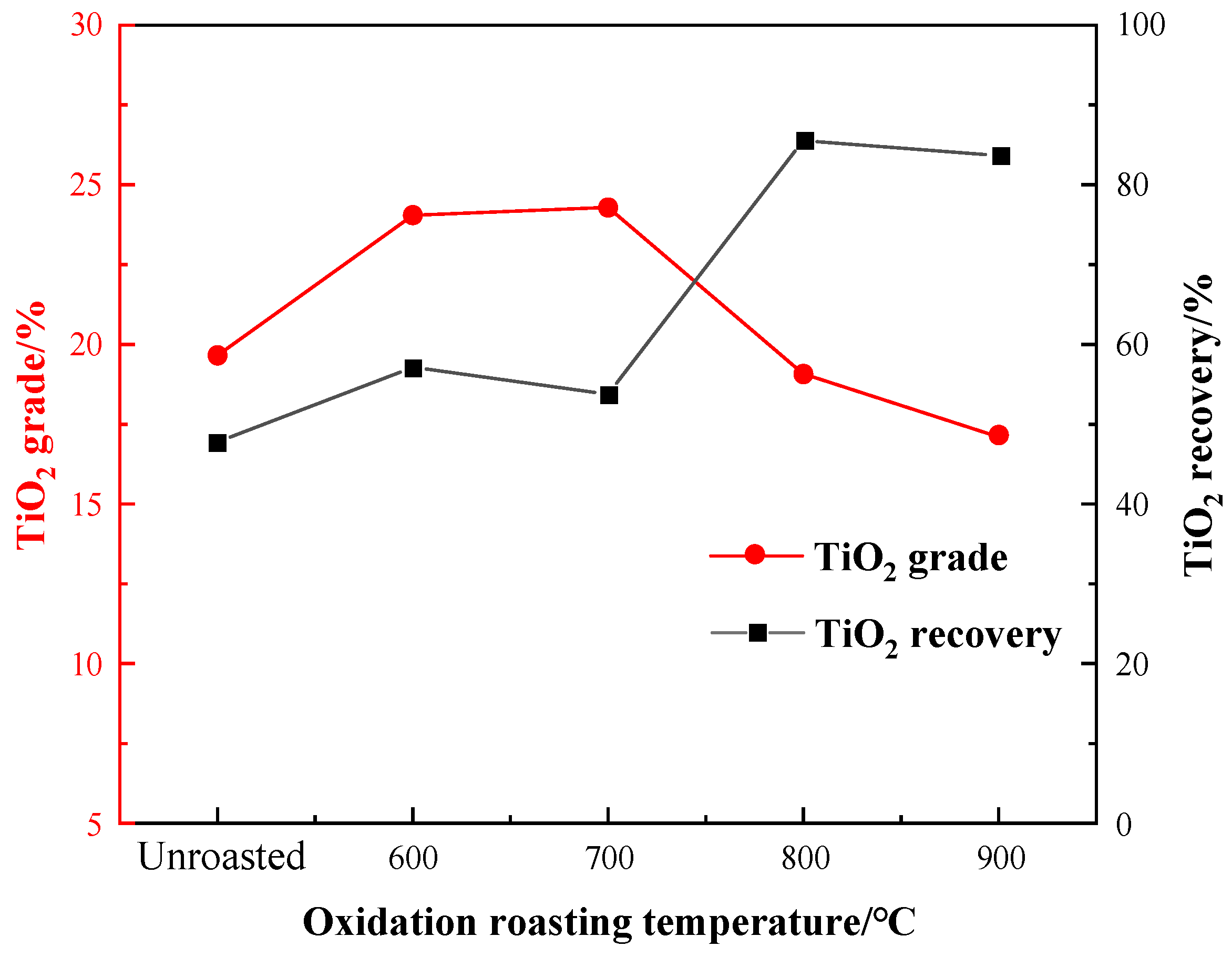
The oxidation roasting temperature is an important parameter affecting the flotation effect. To determine the optimal temperature of oxidation roasting for flotation separation, the roasting temperature experiment was conducted under the conditions of unroasted, 600 °C, 700 °C, 800 °C, and 900 °C. With a fixed grinding fineness of −0.045 mm 83%, a sulfuric acid dosage of 2000 g per ton (g/t), a water glass dosage of 1500 g/t, an oxalic acid dosage of 200 g/t, and EM328 dosage of 1500 g/t, the effect of the temperature on the flotation results is shown in Figure 7.
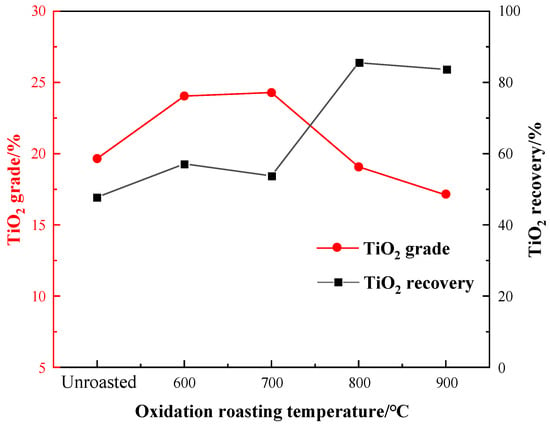
Figure 7.
Effect of oxidation roasting temperature on flotation.
It can be seen from Figure 7 that the TiO2 recovery of the flotation concentrate gradually increased, especially reaching 85.56% at 800 °C, while the TiO2 grade significantly improved after roasting at 600–700 °C, and gradually decreased over 800 °C. After the temperature reached 900 °C, the TiO2 grade had a downward trend, and the recovery decreased slightly. Therefore, the roasting pretreatment increased the flotation indexes obviously. Taking into account of the recovery and grade, an oxidation roasting temperature of 800 °C was selected.
3.2.2. Acid Washing Experiment
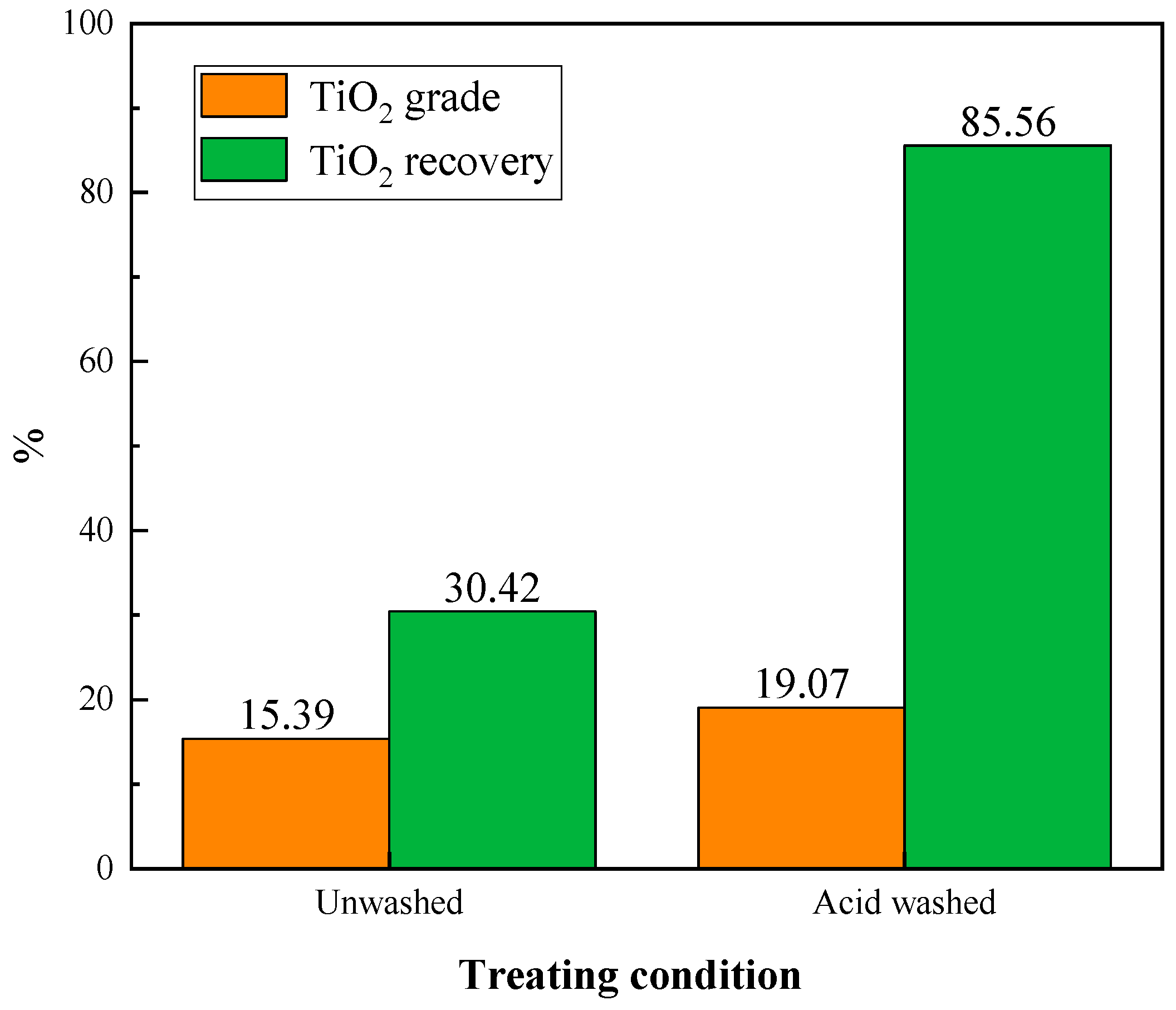
During the flotation process of the ore sample, we observed that the pH of the slurry continued to increase. This was attributed to the continuous dissolution of carbonate minerals such as calcite. To address this issue, the acid washing treatment was adopted. The influence of acid washing on the flotation of Ti-bearing minerals was investigated and compared. The ore samples, both acid-washed and untreated, were subjected to flotation tests using products obtained from roasting at 800 °C. The conditions were the same as those in oxidation roasting temperature experiment, and the results are shown in Figure 8.
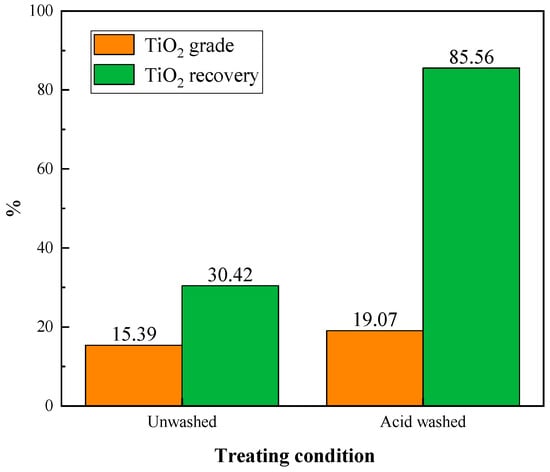
Figure 8.
Effect of acid washing on flotation.
From Figure 8, it can be seen that the acid washing pretreatment dramatically improved the grade and recovery of the TiO2 during flotation. The untreated sample obtained a TiO2 grade of 15.39% and recovery of 30.42% flotation concentrate. In comparison, the acid-washed sample yielded a TiO2 grade of 19.07% and recovery of 85.56% concentrate. Therefore, acid washing treatment prior to flotation could enhance the flotation efficiency of Ti-bearing minerals.
3.3. Flotation Tests
In this section, flotation condition and open circuit tests were conducted using the pretreatment procedure and flowsheet described in Section 2.2.3, respectively.
3.3.1. Effect of Grinding Fineness
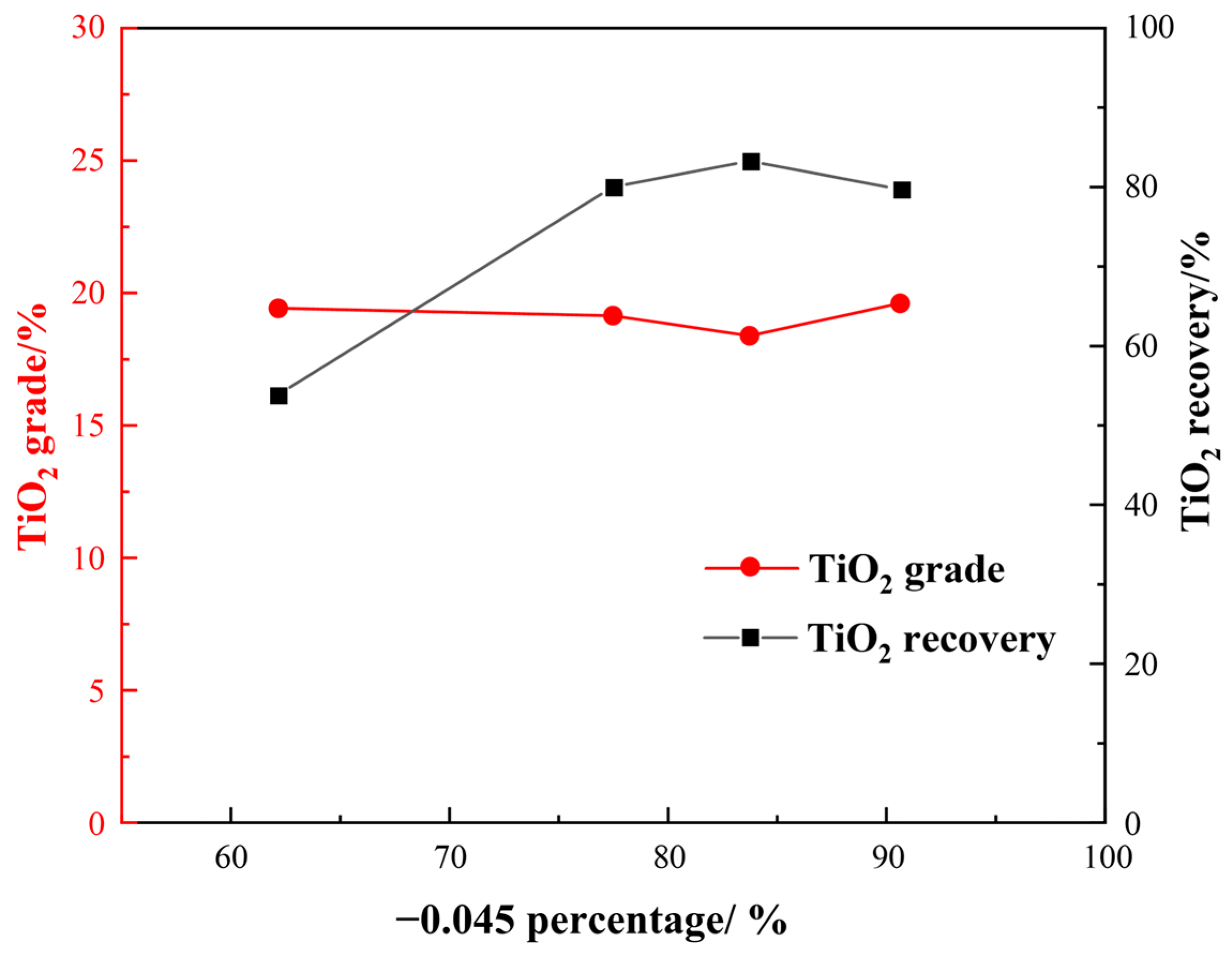
Grinding fineness plays a crucial role in determining beneficiation, especially considering that Ti-bearing minerals have very fine particle sizes. Hence, we first investigated the impact of grinding fineness on flotation indexes under the following conditions: a H2SO4 dosage of 2000 g/t, a water glass dosage of 1500 g/t, an oxalic acid dosage of 200 g/t, and an EM328 dosage of 1500 g/t. The effect of grinding fineness on flotation is shown in Figure 9.
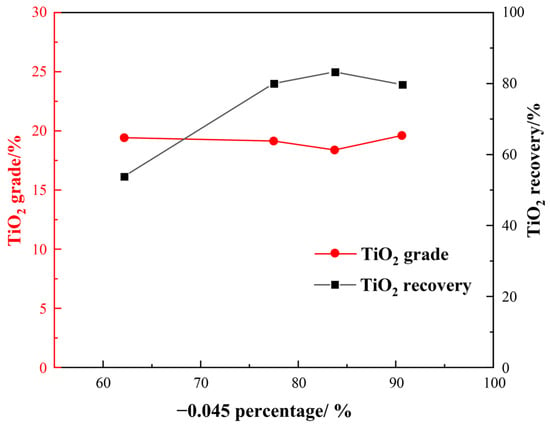
Figure 9.
Effect of grinding fineness on flotation.
Figure 9 demonstrates that, as the grinding size became finer, there was an initial increase followed by a decrease in TiO2 recovery for roughing, yet the overall change to the TiO2 grade was not significant. When the fraction of −0.045 mm was about 83%, the TiO2 recovery reached a maximum of 83.28%, with a TiO2 grade of 18.39%. For comprehensive consideration, a grinding fineness of −0.045 mm accounting for 83% was appropriate.
3.3.2. Effect of pH Condition
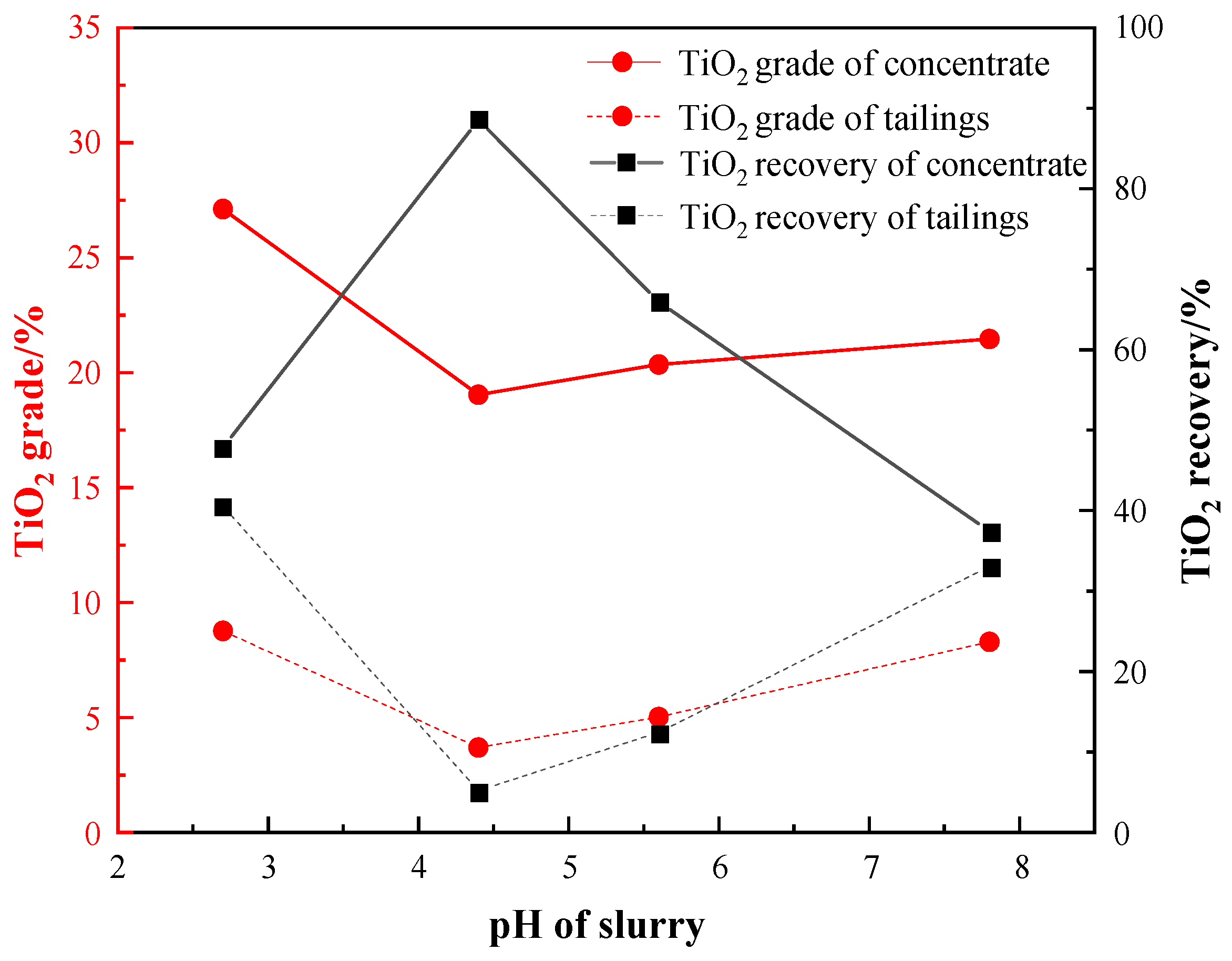
pH is an important factor influencing both the electrical properties of the mineral surface and the activity of the reagents during the flotation process. With a fixed grinding fineness of −0.045 mm 83%, a water glass dosage of 1500 g/t, an oxalic acid dosage of 200 g/t, and an EM328 dosage of 1500 g/t, the pH condition test was carried out, and the results are shown in Figure 10.
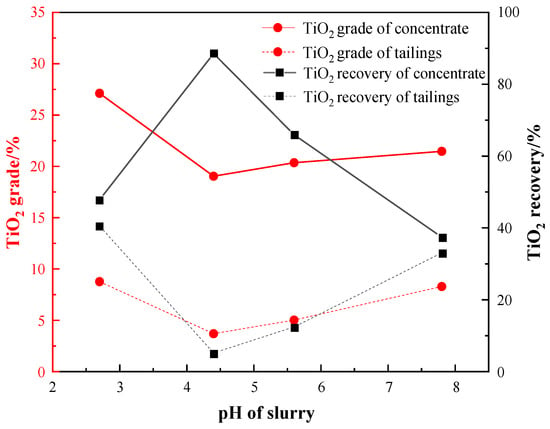
Figure 10.
Effect of pH condition on flotation.
The results presented in Figure 10 indicate that the TiO2 recovery of the roughing concentrate increased initially and then decreased with the pH decreasing after reaching a maximum value of 88.58% at pH 4.4. Under this condition, the grades of the TiO2 in the concentrate and tailings were 19.04% and 3.69%, respectively. This was due to the narrow optimal pH condition for Ti-bearing minerals’ flotation, usually around 4–5, which is consistent with other studies [32,33]. Therefore, a roughing pH of 4.4 was selected as the optimal condition when using a sulfuric acid dosage of 2000 g/t.
3.3.3. Effect of Depressants Dosage
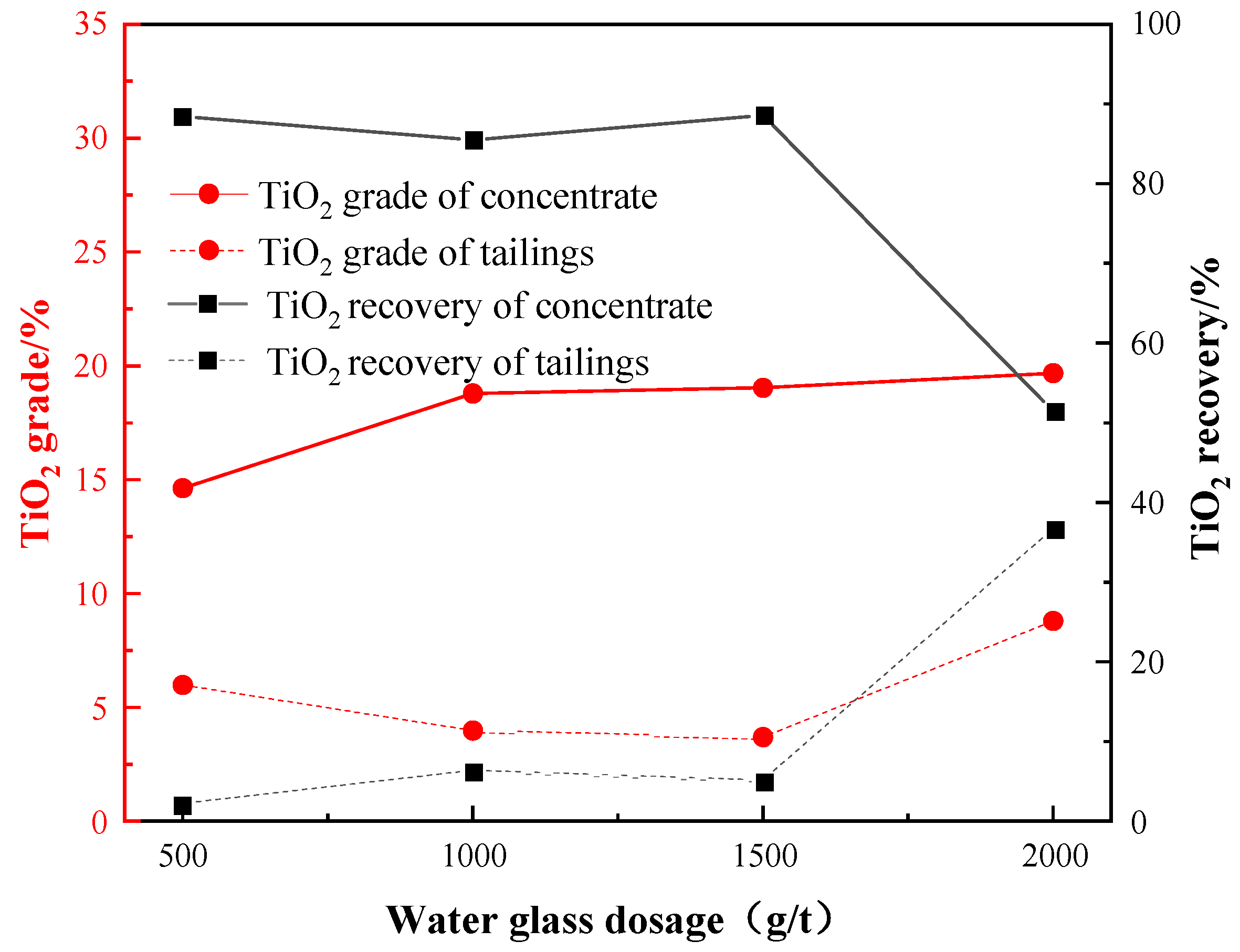
The previous tests showed that a combination of water glass and oxalic acid exhibited better depression effect on the gangue minerals. Under the conditions of: a grinding fineness of −0.045 mm 83%, a sulfuric acid dosage of 2000 g/t, an oxalic acid dosage of 200 g/t, and an EM328 dosage of 1500 g/t, the water glass dosage test was carried out to investigate the impact of the water glass dosage on the flotation performance, as shown in Figure 11.
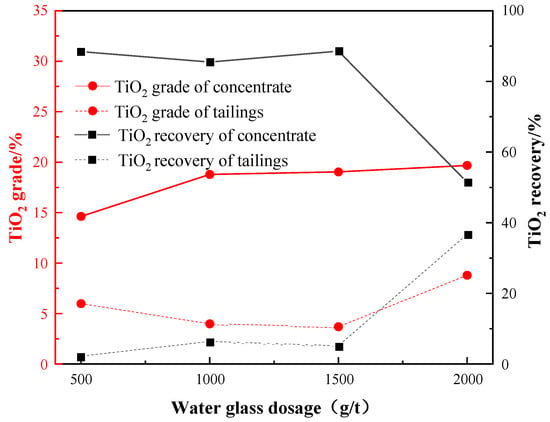
Figure 11.
Effect of water glass dosage on flotation.
According to the results, increasing the water glass dosage generally leads to a higher TiO2 grade but a lower recovery in concentrate. At a water glass dosage of 1500 g/t, a maximum recovery of 88.58% and grade of 19.04% were achieved. Additionally, this condition resulted in minimal loss of TiO2 in the tailings, thus confirming that a water glass dosage of 1500 g /t was suitable.
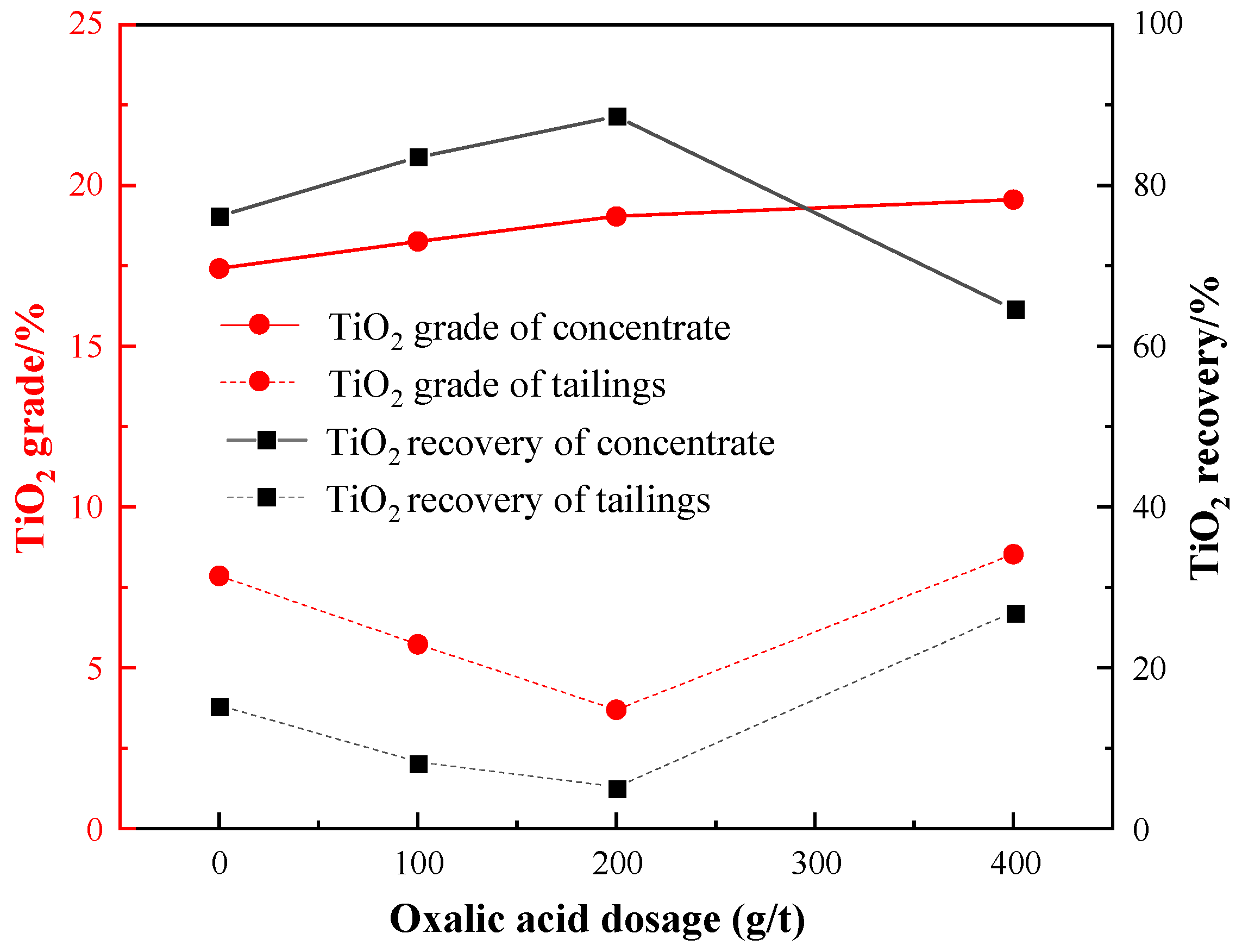
Under the conditions of: a grinding fineness of −0.045 mm 83%, a sulfuric acid dosage of 2000 g/t, a water glass dosage of 1500 g/t, and an EM328 dosage of 1500 g/t, the oxalic acid dosage test was carried out, and the results are shown in Figure 12.
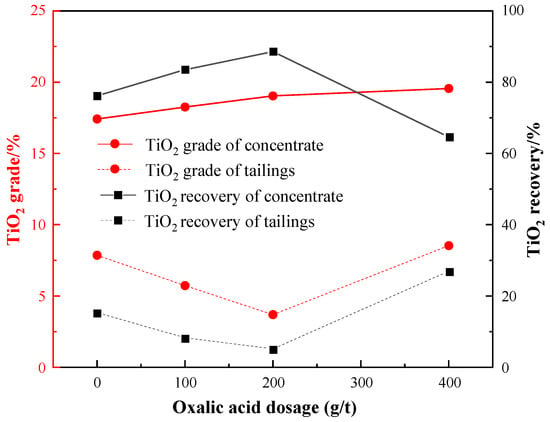
Figure 12.
Effect of oxalic acid dosage on flotation.
As depicted by Figure 12, when only water glass was used as a depressant, the grade of the roughing concentrate was solely 17.42%, with a recovery of 76.18%. However, when both oxalic acid and water glass were used as depressants, the grade of TiO2 in the concentrate rose gradually, and the recovery increased and then decreased, reaching a maximum value of 88.58% when the dosage of oxalic acid was 200 g/t. At this point, the grade and recovery of TiO2 in tailings were minimal. Thus, the optimal dosage of oxalic acid was determined to be 200 g/t.
3.3.4. Effect of Collector Species and Dosage
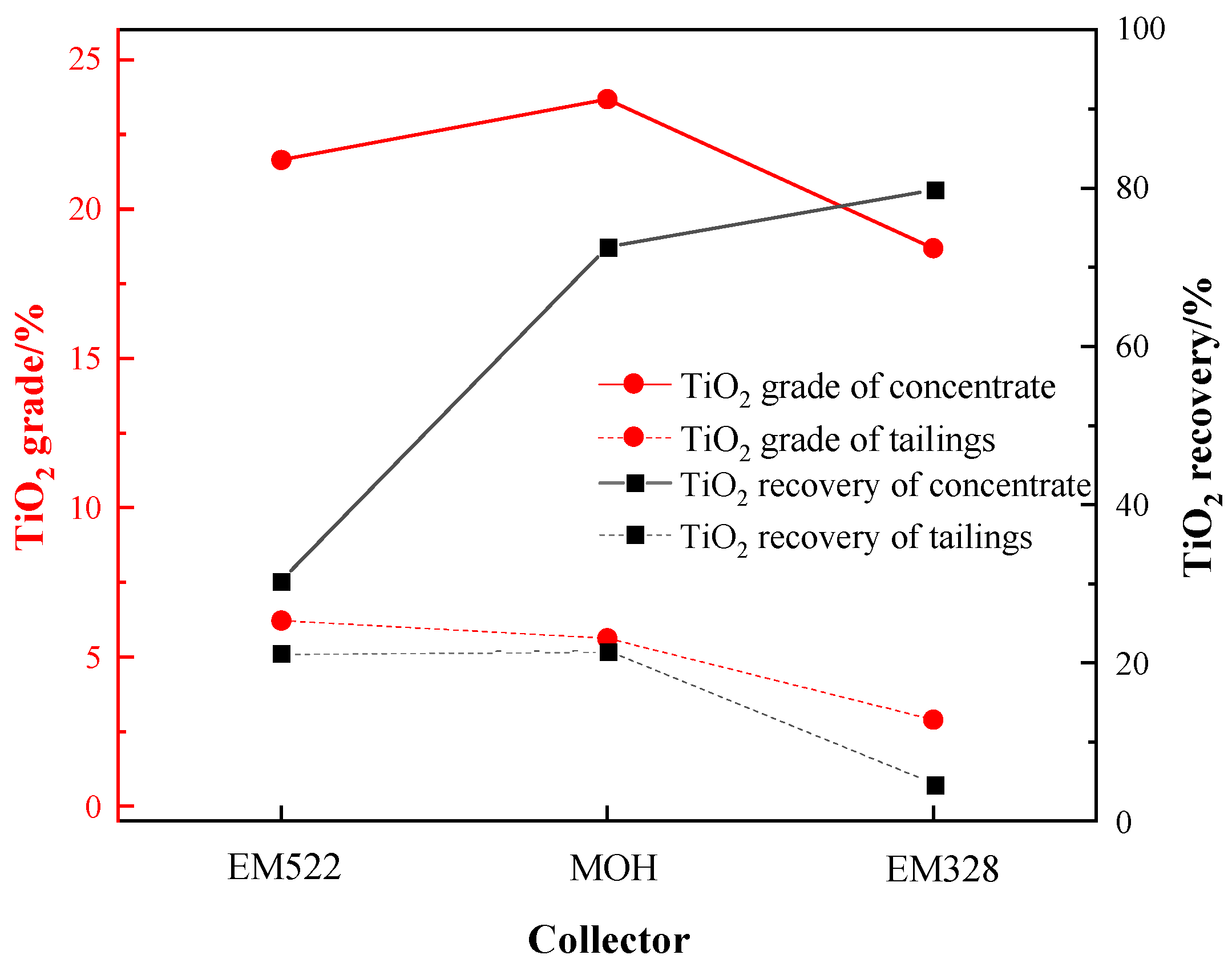
The EM 328 exhibit a superior catchability on fine-grained Ti-bearing minerals. So EM328, EM522, and the industrial collector MOH were selected to examine the flotation performance on Ti-bearing minerals. Under the conditions of: a grinding fineness of −0.045 mm 83%, a sulfuric acid dosage of 2000 g/t, a water glass dosage of 750 g/t, an oxalic acid dosage of 200 g/t, and a collector dosage of 1500 g/t, the flotation performance of the three collectors on the Ti-bearing minerals was carried out. The results are shown in Figure 13.
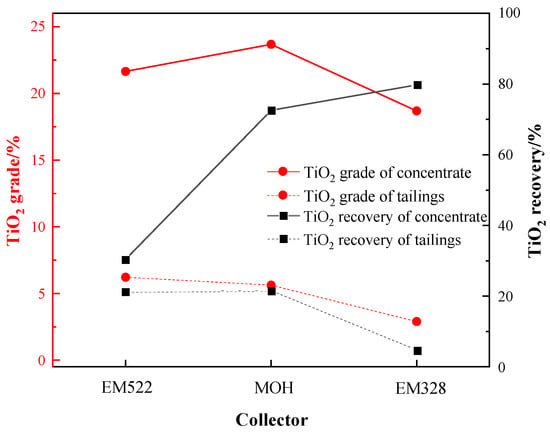
Figure 13.
Flotation performance of three collectors.
As shown in Figure 13, it can be observed that, at the same collector dosage, EM522 yielded a roughing concentrate with a TiO2 grade of 21.65%, while MOH produced a concentrate with a TiO2 grade of 23.68%. However, both these collectors resulted in more than 20% TiO2 in the tailings, i.e., remaining unrecovered. In comparison, when EM328 was used as a collector, a roughing concentrate with TiO2 grade of 18.69% and recovery of 79.80% could be obtained. Furthermore, the TiO2 grade in the tailings was significantly lower, measuring only 2.90%. In overall consideration, EM328 was selected as the preferred collector for the flotation of the sample.
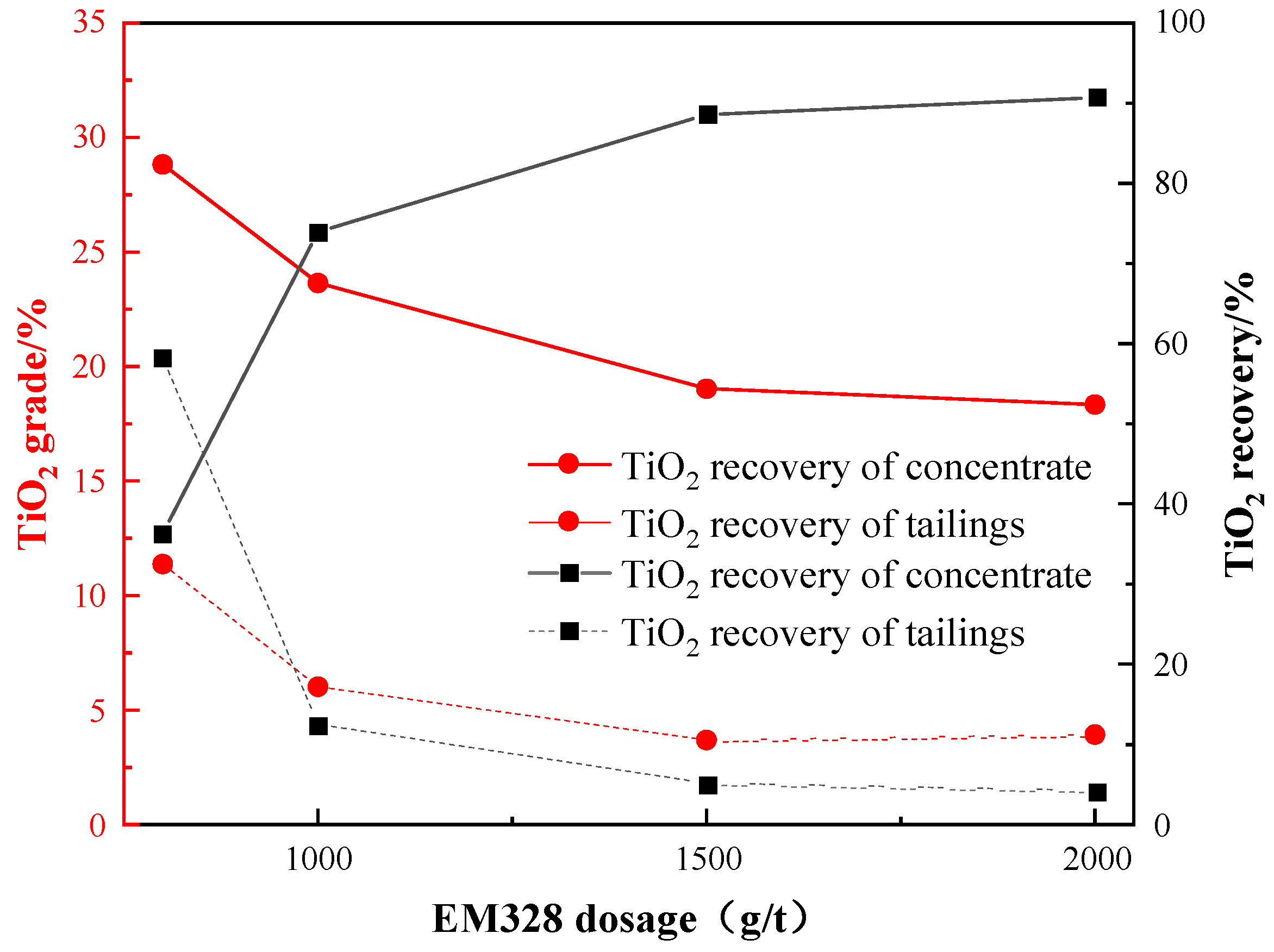
Then, under the given conditions of a grinding fineness of −0.045 mm 83%, a sulfuric acid dosage of 2000 g/t, a water glass dosage of 1500 g/t, and an oxalic acid dosage of 200 g/t, an investigation was conducted to examine the effect of EM328 dosage on the flotation of Ti-bearing minerals. The results are presented in Figure 14.
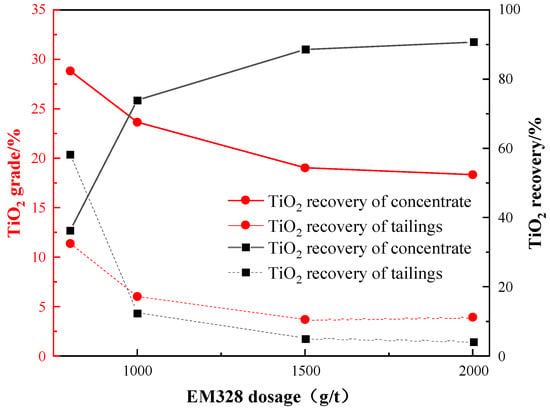
Figure 14.
Effect of EM328 dosage on flotation.
Figure 14 reveals that, as the dosage of EM328 increased, the TiO2 recovery of the roughing concentrate also increased, while the TiO2 grade decreased. Therefore, a trade-off between TiO2 grade and recovery needs to be considered. Based on the plot, the ideal dosage of EM328 to achieve a balance between TiO2 grade and recovery of the concentrate was determined to be 1500 g/t.
3.3.5. Open Flotation Circuit Test
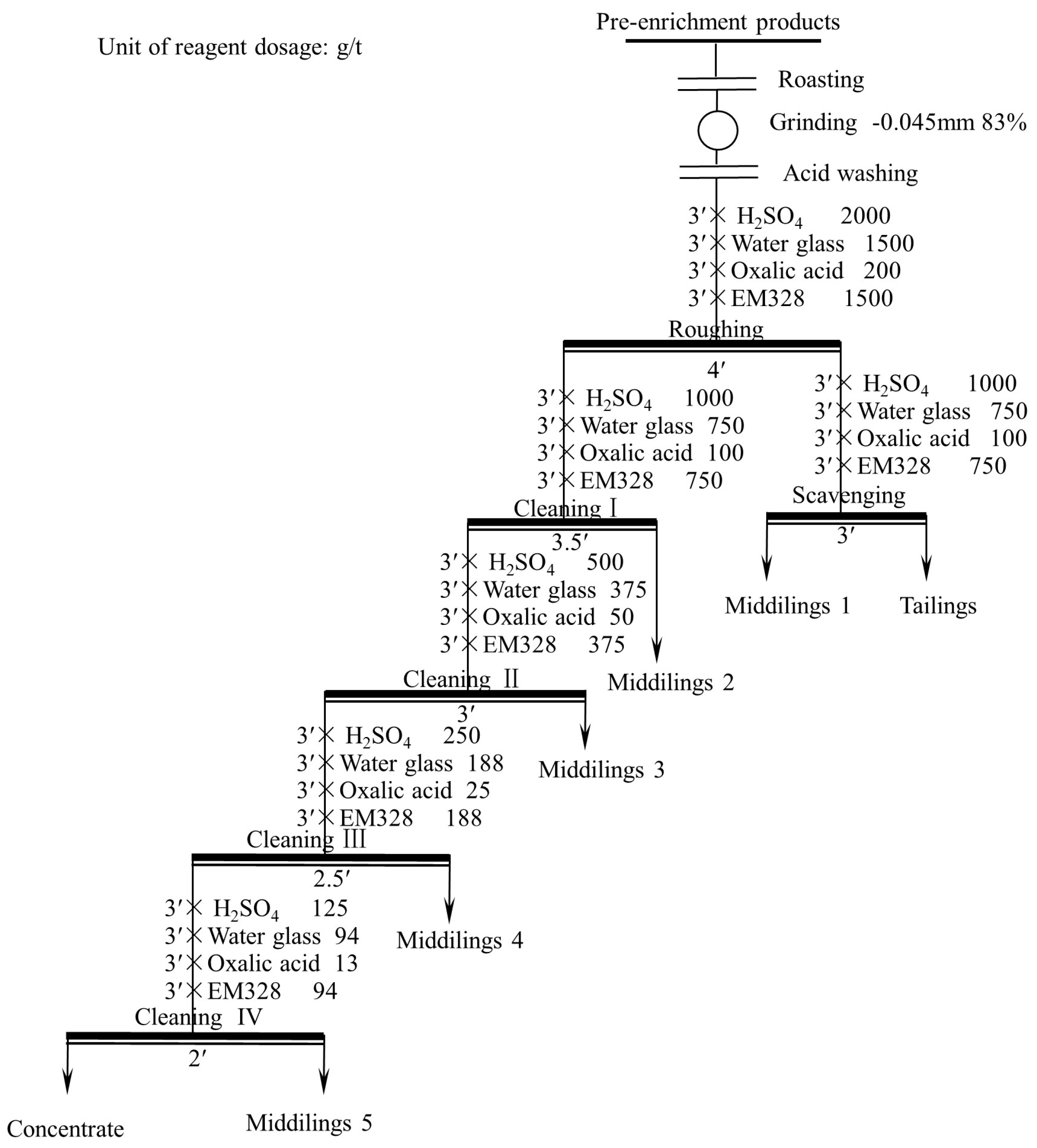
Based on the condition tests, am open flotation circuit test was conducted for one roughing, one scavenging, and four cleaning. The flotation flowsheet is shown in Figure 15, and the results are shown in Table 4. A flotation concentrate with a TiO2 grade of 38.30% and recovery of 25.99% was obtained through the circuit.
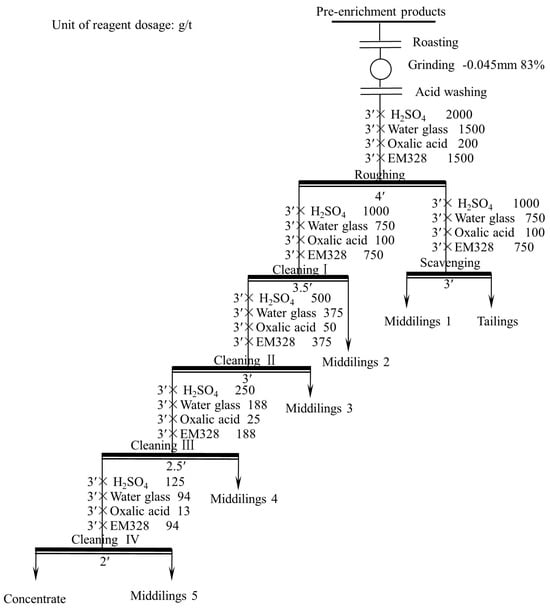
Figure 15.
Open flotation circuit test flowsheet.

Table 4.
Results of open flotation circuit test (%).
3.4. Leaching Exploration Test of Flotation Concentrate
A leaching exploration test of the flotation concentrate was conducted to improve the TiO2 grade. The results are presented in Table 5 and Table 6. It was found that a concentrate with a TiO2 grade and recovery of 53.90% and 91.05% (relative to the flotation concentrate) could be obtained. In addition, the contents of calcium, magnesium, and silicate gangue minerals decreased effectively, resulting in a qualified Ti-concentrate with a high grade.

Table 5.
Result of flotation concentrate leaching (%).

Table 6.
Analysis of leaching residue and flotation concentrate (%).
4. Discussions
4.1. Strengthening Mechanism of Roasting Pretreatment
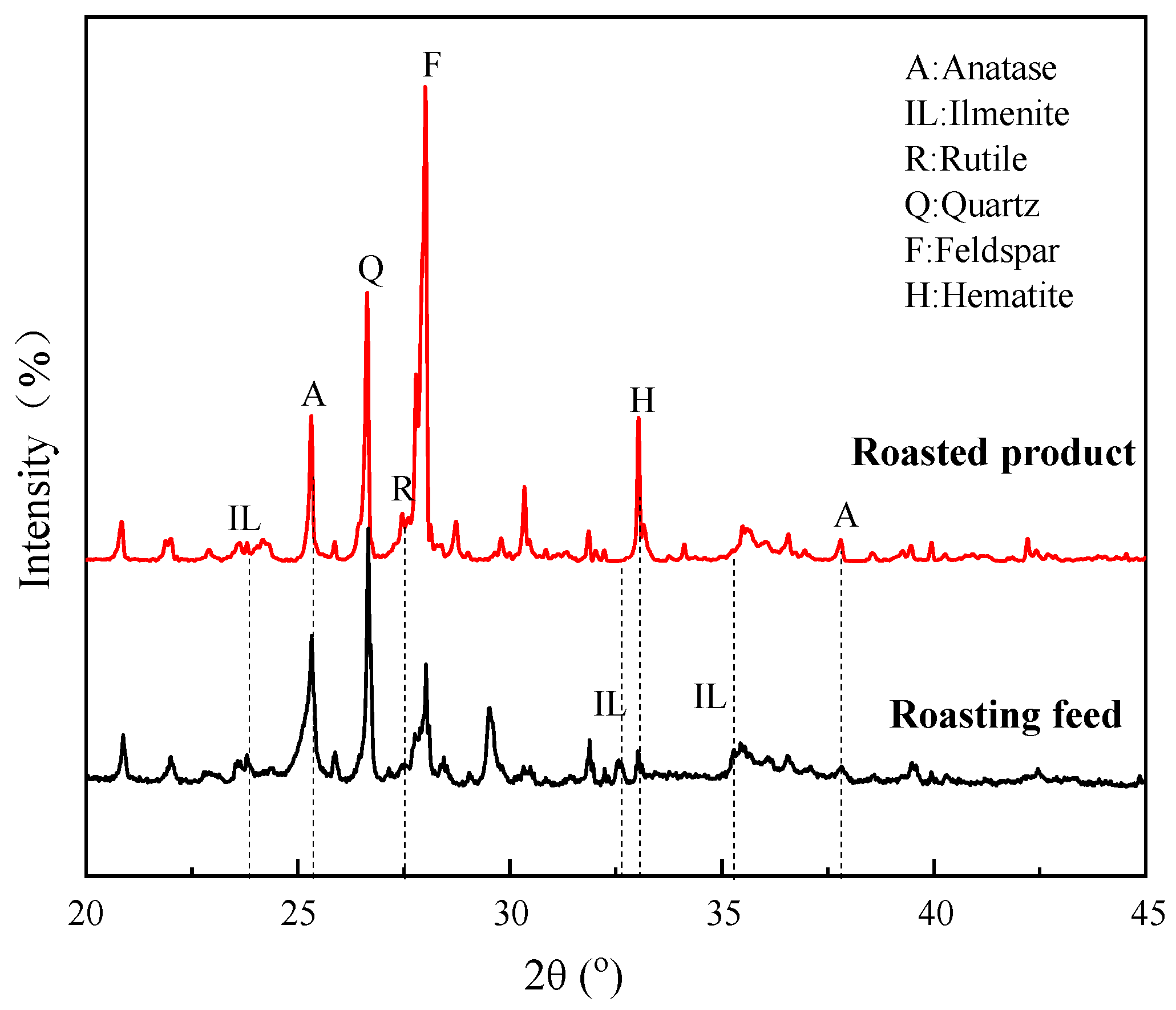
Figure 16 shows the XRD patterns of the roasting feed and roasted product. The most intense diffraction peak of anatase at 2θ = 25.31° was significantly enhanced when the sample was roasted at 800 °C. There were also increases in the peaks at 2θ = 27.44°, 36.09°, and 37.80°, which are attributed to rutile and anatase, respectively. The peaks at 2θ = 32.59°, 35.29°, and 23.83° all weakened or disappeared to varying degrees. The diffraction peak of hematite at 2θ = 33.12° showed an enhancement. It can be concluded that the ilmenite in the ore was converted to anatase and rutile during the roasting process, while hematite was formed simultaneously [28,29]. It is also consistent with the fact that flotation indexes are better after roasting, because anatase and rutile possess a better floatability than ilmenite [34].
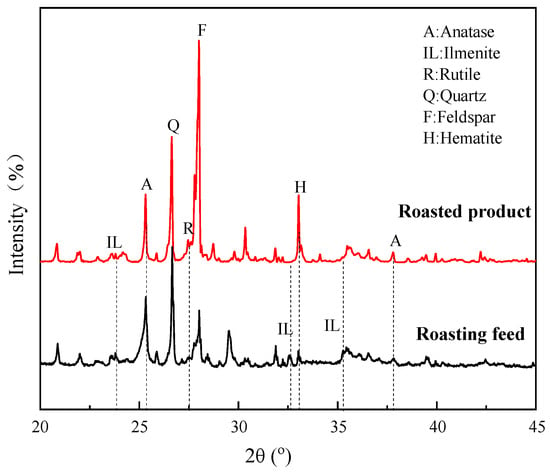
Figure 16.
XRD patterns of roasting feed and roasted product.
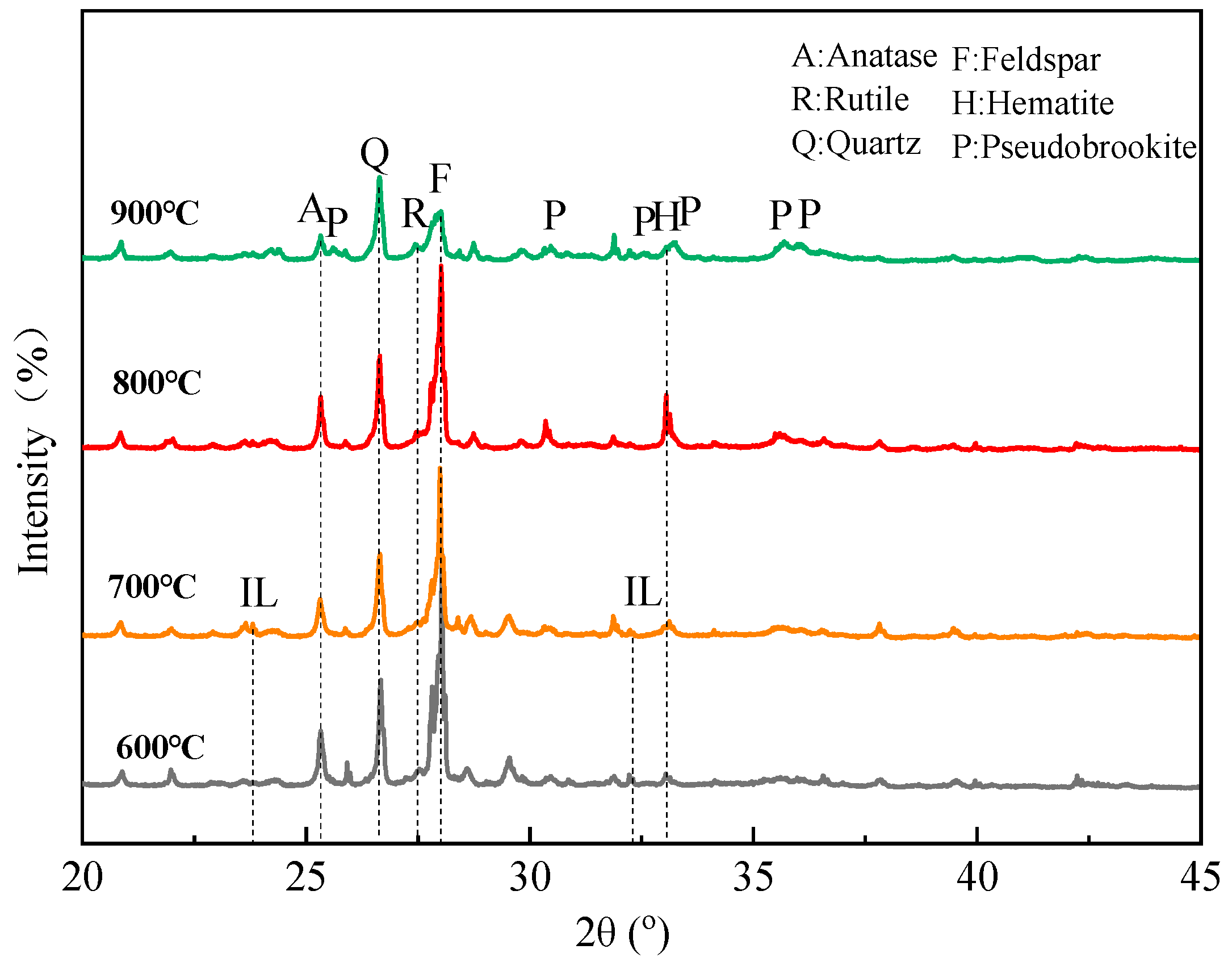
The XRD patterns of the roasted product at different temperatures are shown in Figure 17. It is observed that weak diffraction peaks of ilmenite appear at 600–700 °C, but they almost disappear at 800–900 °C. With the temperature increasing, the peaks of anatase, rutile, and hematite become more prominent. It is noteworthy that the contents of anatase and hematite reach a maximum at 800 °C, followed by a decline at 900 °C. Meanwhile, the formation of pseudobrookite (Fe2TiO5) begins at 900 °C. That is, ilmenite converts into anatase and rutile at 600–800 °C, but pseudobrookite begins to form by combining hematite, anatase, and rutile at 900 °C, which is harmful to the flotation. These phase changes are in good agreement with the other literature [29]. The changes also explain the flotation phenomenon that 800 °C is the optimal roasting temperature.
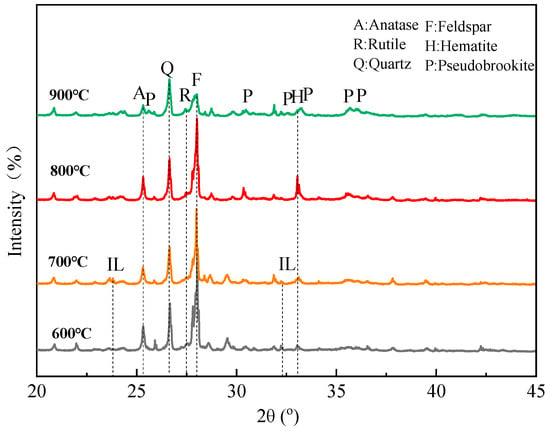
Figure 17.
XRD patterns of roasted product at different temperatures (600 °C, 700 °C, 800 °C, and 900 °C).
4.2. Mechanism of Acid Leaching
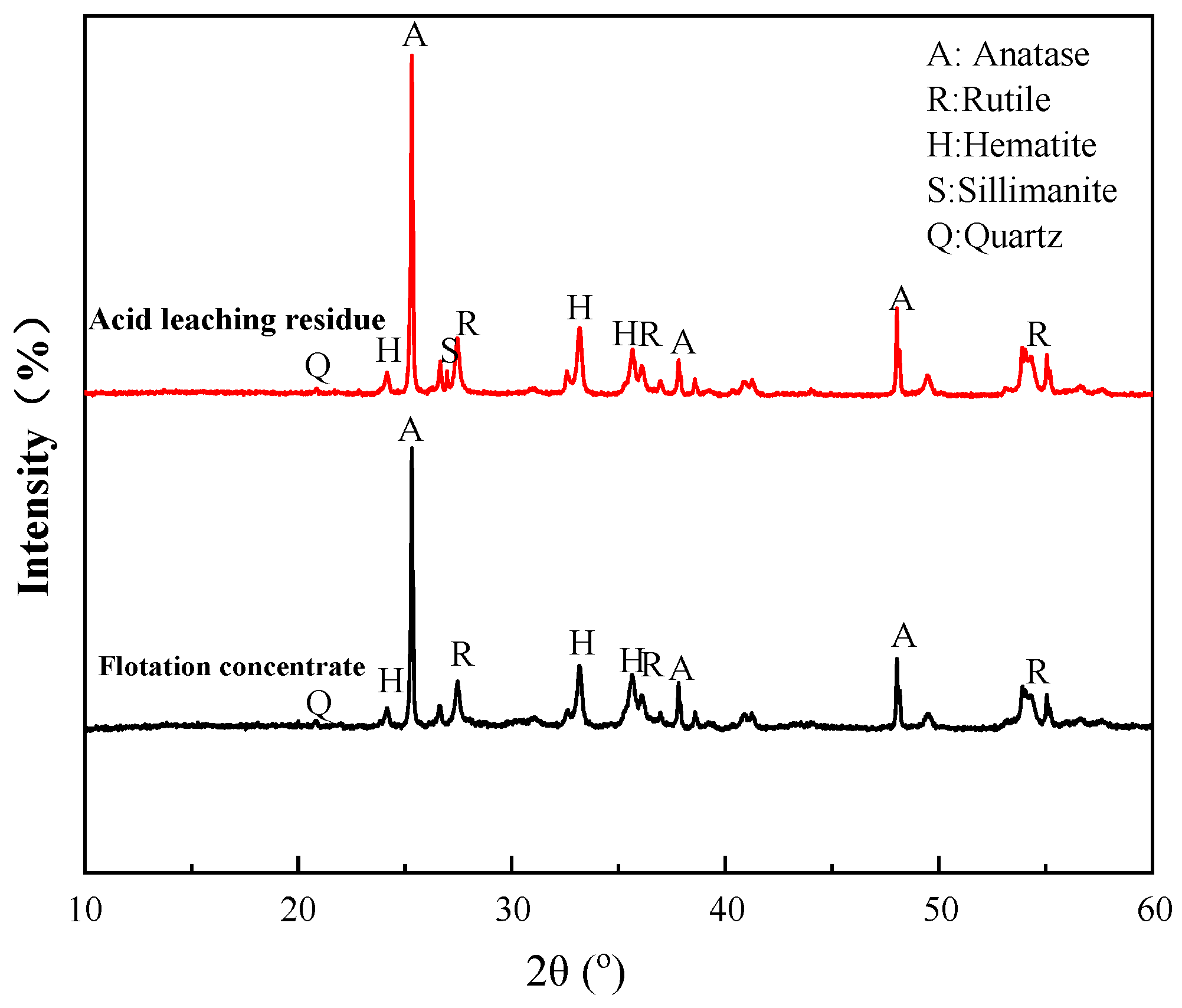
The flotation concentrate and acid leaching residue were subjected to XRD to determine the mineral contents. As seen in Figure 18, the main contents of the both products were anatase, followed by rutile and hematite. However, compared to the flotation concentrate, the contents of Ti-bearing minerals, anatase, and rutile were significantly increased in the acid leaching residue. It was observed that a small amount of sillimanite was generated in the leaching process, together with a decrease in quartz, probably due to the reaction between acid and quartz in the concentrate. These results agree with the chemical analysis in Section 3.4.
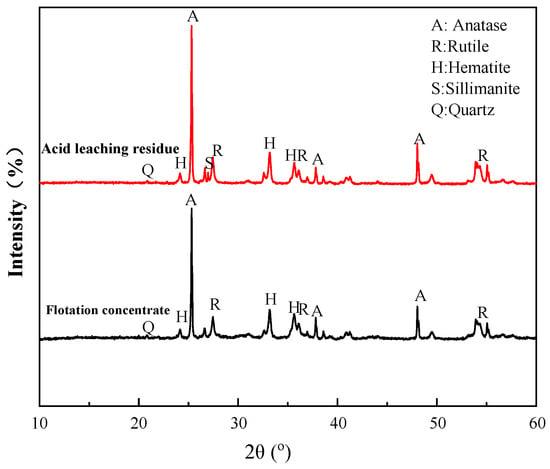
Figure 18.
XRD patterns of acid leaching residue and flotation concentrate.
4.3. Route of Ti-Concentrate Production from Altered VTM Ore
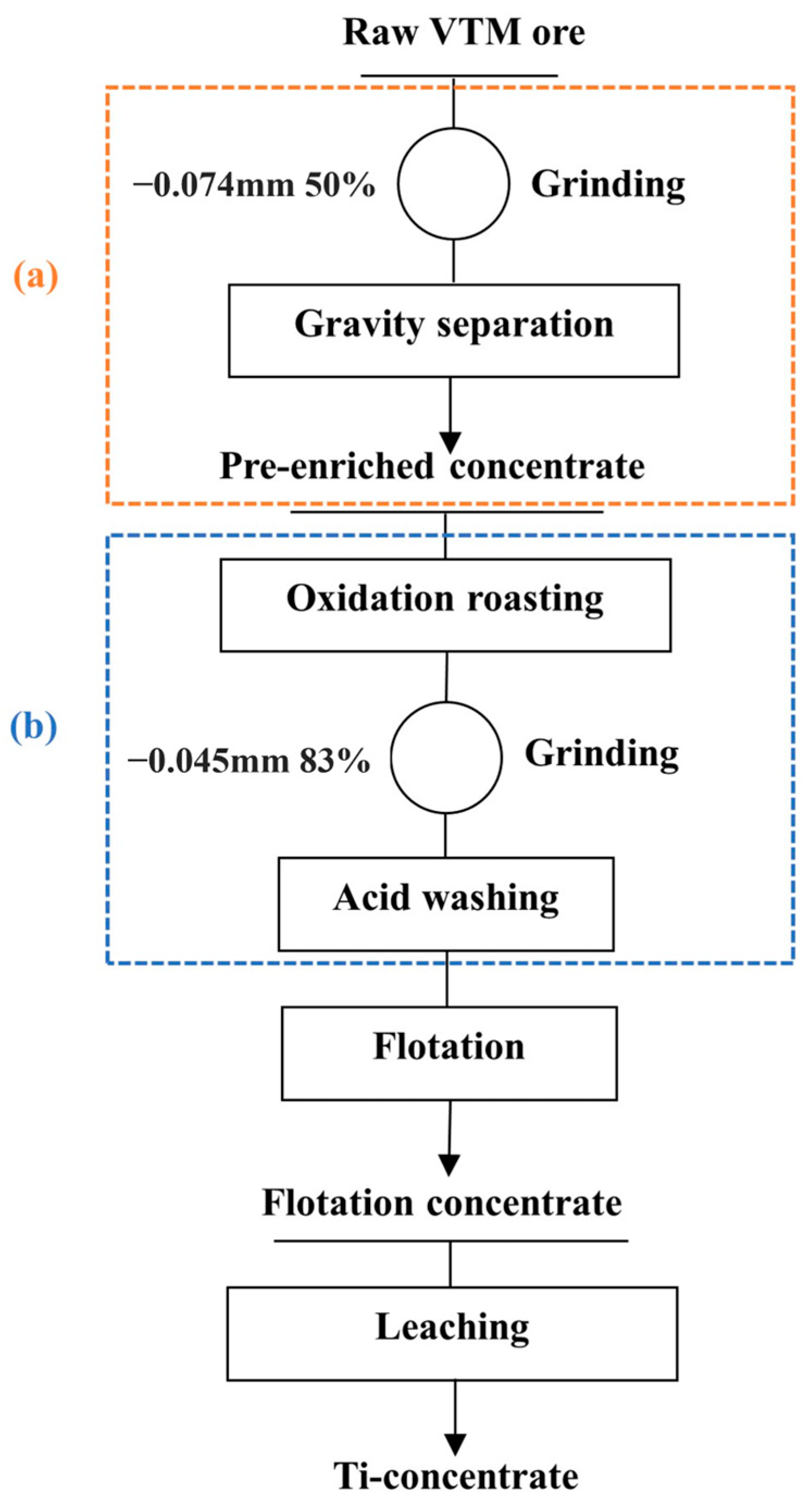
Based on the above research, a new route of Ti-concentrate production from this altered VTM ore sample is proposed. As shown in Figure 19, the route comprises four main parts: pre-concentration, pretreatment, flotation, and leaching.
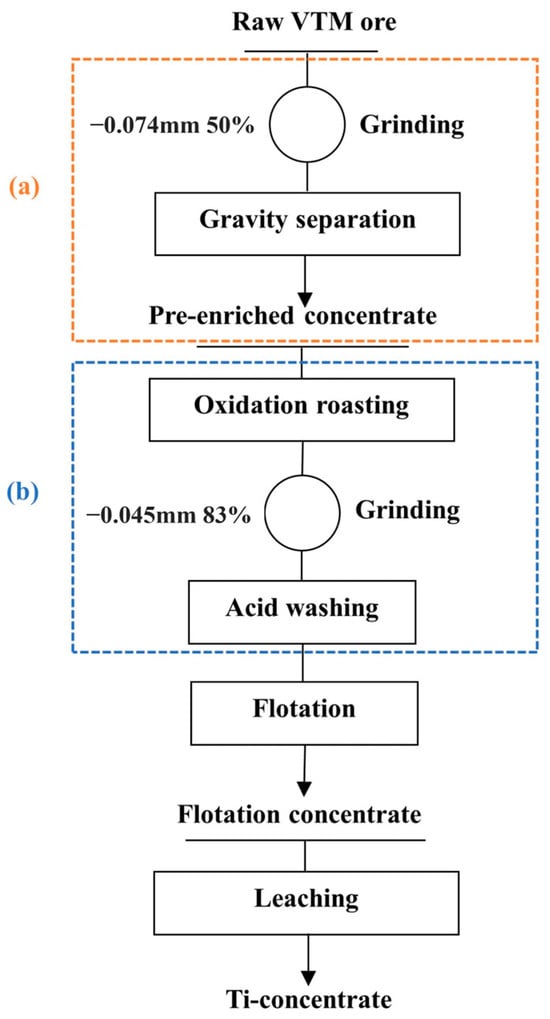
Figure 19.
Principle flowsheet of Ti-concentrate production process from the altered VTM ore ((a): pre-concentration and (b): pretreatment).
The route has been proven to be effective for low-grade, altered VTM ore consisting of anatase and ilmenite, which tend to coexist closely at a fine particle size in a laboratory-scale study. From the perspective of environmental protection, it is promising to replace the acid used during acid washing and leaching processes with the waste acid generated during the production of titanium dioxide [35,36]. In future, more efforts will be made to investigate the feasibility of waste acid and optimize the acid leaching parameters to reduce the cost and optimize the process. Extended experimentation should be followed to test the efficiency and applicability on a semi-industrial scale.
5. Conclusions
In this work, we found a new route combining physical and chemical beneficiation methods, namely a roasting-flotation-leaching scheme, for the separation of Ti-bearing minerals from altered VTM ore, which increased the grade of TiO2 from 7.15% to 53.90%. The character of the ore sample was figured out and the main optimal conditions of the route were then proposed. The mechanisms of roasting pretreatment and acid leaching were also investigated. Based on the results, the primary conclusions are presented below:
- The study of VTM ore sample’s character showed that the TFe and TiO2 contents were 14.40% and 7.15%, respectively, with a high degree of alteration. Ti-bearing minerals, ilmenite and anatase, were considered to recover as composite mineral.
- Comparative titanium pre-concentration experiments showed that the gravity separation method achieved a better result, improving the TiO2 grade by approximately 7% at a grinding fineness of −0.074 mm, accounting for 35%.
- The oxidation roasting and acid washing pretreatments increased the flotation indexes obviously. Compared to the unroasted sample, the TiO2 recovery of the flotation concentrate rose from 47.78% to 85.56% at 800 °C roasting. Compared to the flotation concentrate obtained from the untreated sample, the TiO2 grade and recovery of that from acid-washed sample were enhanced to 3.68% and 55.14%, respectively.
- Flotation condition tests showed that the optimal conditions were a grinding fineness of −0.045 mm 83%, a sulfuric acid dosage of 2000 g/t, a water glass dosage of 1500 g/t, an oxalic acid dosage of 200 g/t, and an EM328 dosage of 1500 g/t. Open flotation circuit tests resulted in a TiO2 grade and recovery of 38.30% and 25.99%, respectively, in the flotation concentrate. A leaching exploration test showed that the TiO2 grade of the flotation concentrate could be improved to 53.90%, and the recovery was 91.05% (relative to flotation concentrate).
- XRD revealed that ilmenite in the VTM ore was converted into anatase and rutile during the roasting process at 600–800 °C, but pseudobrookite began to form by combining hematite, anatase, and rutile at 900 °C, which is harmful to flotation. Compared to the flotation concentrate, the content of Ti-bearing minerals was significantly increased in the acid leaching residue.
Author Contributions
Y.X.—data curation, formal analysis, investigation, writing—original draft; C.C.—formal analysis, methodology, writing—review and editing; Y.Y.—conceptualization, funding acquisition, project administration, supervision; W.D.—validation, visualization; F.L.—investigation, software. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (Grant No. 2021YFC2900800), China Geological Survey, Ministry of Natural Resources (Grant No. DD20230039), the Major Scientific and Technological Research Program in Panxi Pilot Zone, Key Research and Development Program of Science and Technology Department of Sichuan Province (Grant No. 2022YFS0455), and Economy and Information Department of Sichuan Province.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feng, E.; Gao, D.; Wang, Y.; Yu, F.; Wang, C.; Wen, J.; Gao, Y.; Huang, G.; Xu, S. Sustainable recovery of titanium from secondary resources: A review. J. Environ. Manag. 2023, 339, 117818. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.-Y.; Yang, J.H.; Kim, H.-T. A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Wierzbicki, T. Determination of vanadium species in environmental samples. Talanta 2004, 64, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, R.; Nikoloski, A.N. The extraction of vanadium from titanomagnetites and other sources. Miner. Eng. 2020, 146, 106106. [Google Scholar] [CrossRef]
- Maphutha, M.; Ramaili, M.; Sitefane, M.; Goso, X. The effect of magnesia and alumina crucible wear on the smelting characteristics of titaniferous magnetite. J. South. Afr. Inst. Min. Metall. 2017, 117, 649–655. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Zhang, L.-N.; Zhang, L.; Sui, Z.-T.; Tu, G.-F. Selective enrichment of TiO2 and precipitation behavior of perovskite phase in titania bearing slag. Trans. Nonferrous Met. Soc. China 2006, 16, 421–425. [Google Scholar] [CrossRef]
- Lv, C.; Bai, S. Upgrading of raw vanadium titanomagnetite concentrate. J. South. Afr. Inst. Min. Metall. 2019, 119, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Kou, J.; Sun, T.-C.; Wu, S.-C.; Zhao, Y.-Q. Effects of calcium compounds on the carbothermic reduction of vanadium titanomagnetite concentrate. Int. J. Miner. Metall. Mater. 2020, 27, 301–309. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, Z.; Gao, G.; Qiu, K.; Peng, W. Process Mineralogy of Vanadium Titanomagnetite Ore in Panzhihua, China. Separations 2023, 10, 147. [Google Scholar] [CrossRef]
- Teng, Y.; Yang, J.; Zuo, R.; Wang, J. Impact of urbanization and industrialization upon surface water quality: A pilot study of Panzhihua mining town. J. Earth Sci. 2011, 22, 658–668. [Google Scholar] [CrossRef]
- Katsura, T.; Ikuo, K. Titanomaghemite in igneous rocks. Am. Mineral. 1961, 46, 134–145. [Google Scholar]
- Shau, Y.H.; Torii, M.; Horng, C.S.; Peacor, D. Subsolidus evolution and alteration of titanomagnetite in ocean ridge basalts from Deep Sea Drilling Project/Ocean Drilling Program Hole 504B9 Leg 83: Implications for the timing of magnetization. J. Geophys. Res. Solid Earth 2000, 105, 23635–23649. [Google Scholar] [CrossRef]
- Weibel, R. Alteration of detrital Fe-Ti oxides in Miocene fluvial deposits, central Jutland, Denmark. Bull. Geol. Soc. Den. 2003, 50, 141–208. [Google Scholar] [CrossRef]
- Von Gruenewaldt, G.; Klemm, D.D.; Henckel, J.; Dehm, R.M. Exsolution features in titanomagnetites from massive magnetite layers and their host rocks of the upper zone, eastern Bushveld Complex. Econ. Geol. 1985, 80, 1049–1061. [Google Scholar] [CrossRef]
- Hugo, V.E. A Study of Titanium-Bearing Oxides in Heavy Mineral Deposits Along the East Coast of South Africa. Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 1993. [Google Scholar]
- Park, I.; Kanazawa, Y.; Sato, N.; Galtchandmani, P.; Jha, M.K.; Tabelin, C.B.; Jeon, S.; Ito, M.; Hiroyoshi, N. Beneficiation of Low-Grade Rare Earth Ore from Khalzan Buregtei Deposit (Mongolia) by Magnetic Separation. Minerals 2021, 11, 1432. [Google Scholar] [CrossRef]
- Özbayoğlu, G.; Ümit Atalay, M. Beneficiation of bastnaesite by a multi-gravity separator. J. Alloys Compd. 2000, 303–304, 520–523. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wang, W.P.; Yang, J.; You, J.G. Beneficiation of a low grade titanomagnetite ore in mining engineering. Adv. Mater. Res. 2012, 577, 187–190. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Liu, T.; Huang, J. Characterization and pre-concentration of low-grade vanadium-titanium magnetite ore. Minerals 2017, 7, 137. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Liu, Y.; Han, Y. Flotation separation of ilmenite from titanaugite using mixed collectors. Sep. Sci. Technol. 2016, 51, 1840–1846. [Google Scholar] [CrossRef]
- Wu, H.; Fang, S.; Shu, K.; Xu, Y.; Wang, Z.; Luo, L.; Yang, J.; Xu, L. Selective flotation and adsorption of ilmenite from titanaugite by a novel method: Ultrasonic treatment. Powder Technol. 2020, 363, 38–47. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, P.; Wang, H.; Hu, Y.; Sun, W. Flotability Improvement of Ilmenite Using Attrition-Scrubbing as a Pretreatment Method. Minerals 2017, 7, 13. [Google Scholar] [CrossRef]
- Mathur, S. Kaolin Flotation. J. Colloid Interface Sci. 2002, 256, 153–158. [Google Scholar] [CrossRef]
- Barnard, K.R.; McDonald, R.G.; Pownceby, M.I.; Sparrow, G.J.; Zhang, W. Processing anatase ores for pigment production. Hydrometallurgy 2019, 185, 226–237. [Google Scholar] [CrossRef]
- Kasomo, R.M.; Li, H.; Zheng, H.; Chen, Q.; Weng, X.; Mwangi, A.D.; Kiamba, E.; Song, S. Depression of the selective separation of rutile from almandine by Sodium Hexametaphosphate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124631. [Google Scholar] [CrossRef]
- Parapari, P.S.; Irannajad, M.; Mehdilo, A. Modification of ilmenite surface properties by superficial dissolution method. Miner. Eng. 2016, 92, 160–167. [Google Scholar] [CrossRef]
- Fan, X.; Waters, K.E.; Rowson, N.A.; Parker, D.J. Modification of ilmenite surface chemistry for enhancing surfactants adsorption and bubble attachment. J. Colloid Interface Sci. 2009, 329, 167–172. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M. Comparison of microwave irradiation and oxidation roasting as pretreatment methods for modification of ilmenite physicochemical properties. J. Ind. Eng. Chem. 2016, 33, 59–72. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Rezai, B. Effect of oxidation roasting on ilmenite flotation. Physicochem. Probl. Miner. Process. 2014, 50, 493–505. [Google Scholar] [CrossRef]
- Silva, A.M.; Souza, R.F.M.; Aguilera, L.S.; de Campos, J.B.; Brocchi, E.A. Upgrade of titanium content in a vanadiferous titanomagnetite waste: Design of a roast-leach route based on thermodynamics simulations. Miner. Eng. 2022, 179, 107460. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Liu, S.; Deng, J. Influence of particle size on flotation separation of ilmenite, olivine, and pyroxene. Physicochem. Probl. Miner. Process. 2021, 57, 106–117. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.H.; Yang, Y.H.; Liu, J.; Zeng, X.B.; Deng, W. Selective flotation separation of ilmenite from titanaugite using mixed anionic/cationic collectors. Int. J. Miner. Process. 2017, 166, 102–107. [Google Scholar] [CrossRef]
- Yoon, R.H.; Nagaraj, D.R.; Wang, S.S.; Hildebrand, T.M. Beneficiation of kaolin clay by froth flotation using hydroxamate collectors. Miner. Eng. 1992, 5, 457–467. [Google Scholar] [CrossRef]
- Luo, L.P.; Wu, H.Q.; Yang, J.; Tang, Z.; Shu, K.Q.; Xu, Y.B.; Yan, W.P.; Xu, L.H. Effects of microwave pre-treatment on the flotation of ilmenite and titanaugite. Miner. Eng. 2020, 155, 106452. [Google Scholar] [CrossRef]
- Tang, C.F.; Zhang, R.L.; Zhang, W.; Yang, R.X.; Li, C.; Zeng, J. Kinetics Study on the Leaching of Copper from Calcification Roasting Copper Refining Slag Using Waste Acid. Min. Metall. Explor. 2023, 40, 171–179. [Google Scholar] [CrossRef]
- Ma, G.Q.; Cheng, M. Experimental study on preparation of titanium-rich material by pressure leaching of titanium concentrate from titanium dioxide waste acid. Ferroelectrics 2021, 581, 281–286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).