Adsorption Behaviors of Lanthanum (III) and Yttrium (III) Ions on Gibbsite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Samples
2.2. Adsorption Experiment
2.3. Adsorption Kinetics
2.4. Adsorption Isotherm and Thermodynamics
3. Results and Discussions
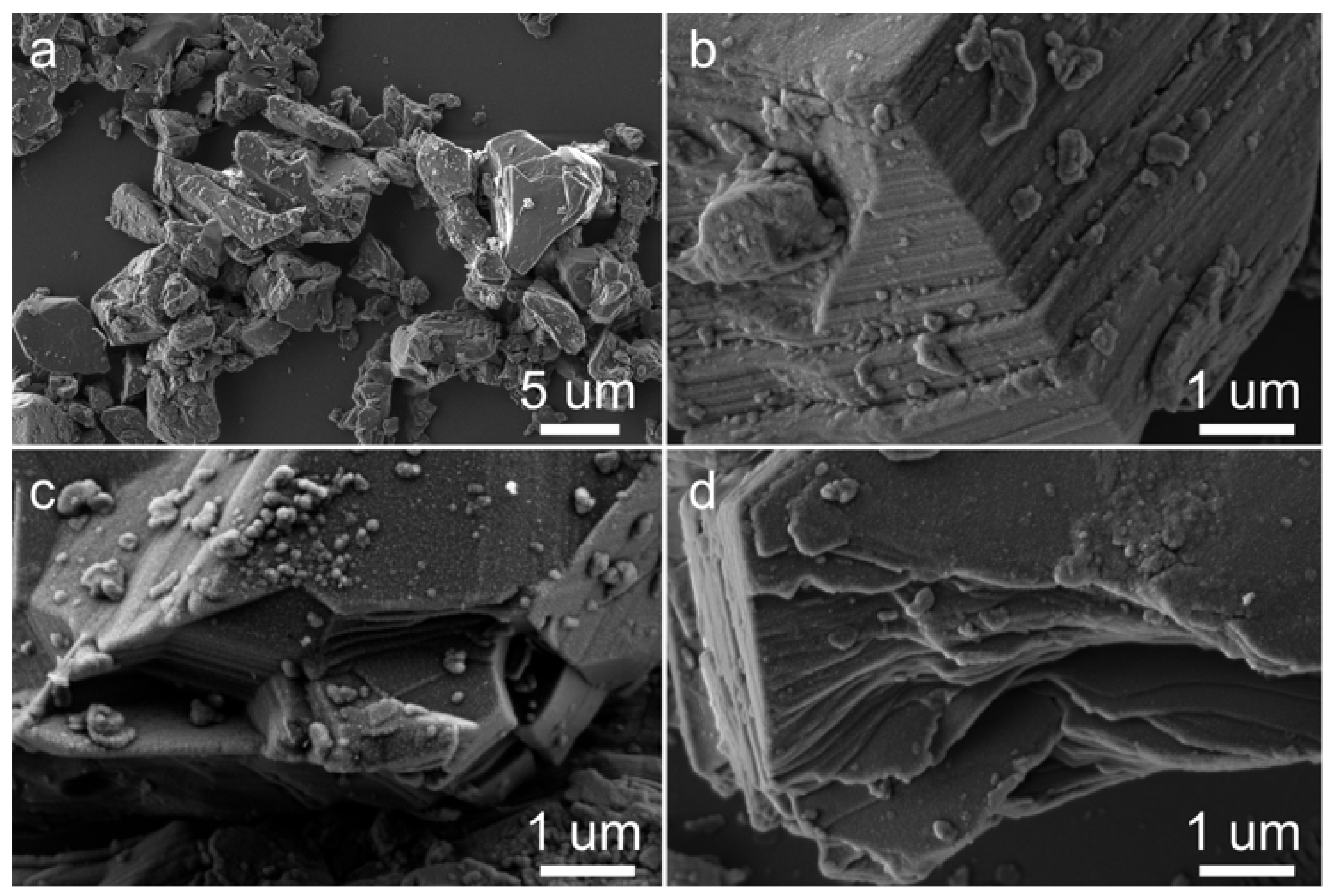
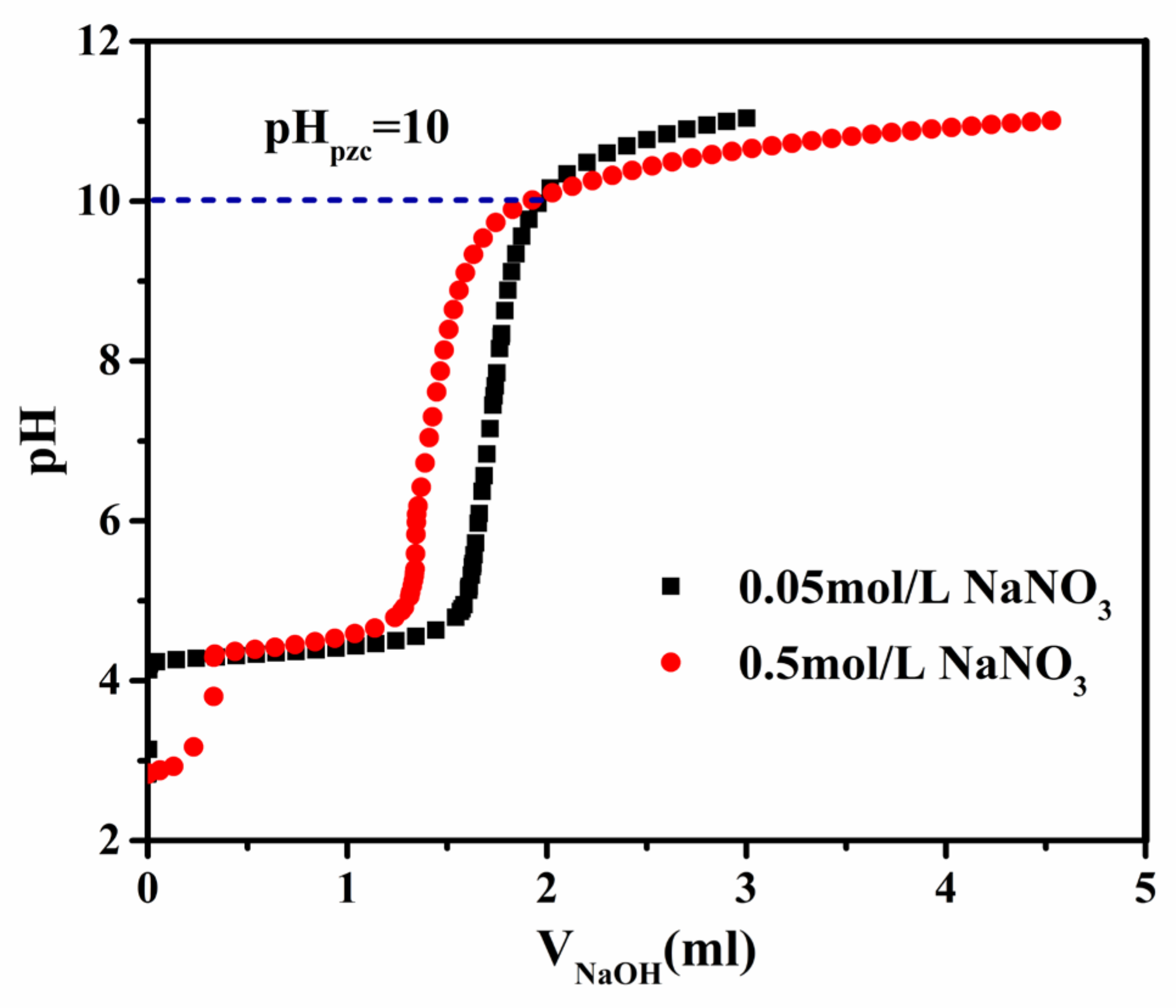
3.1. Characterization of Gibbsite Samples
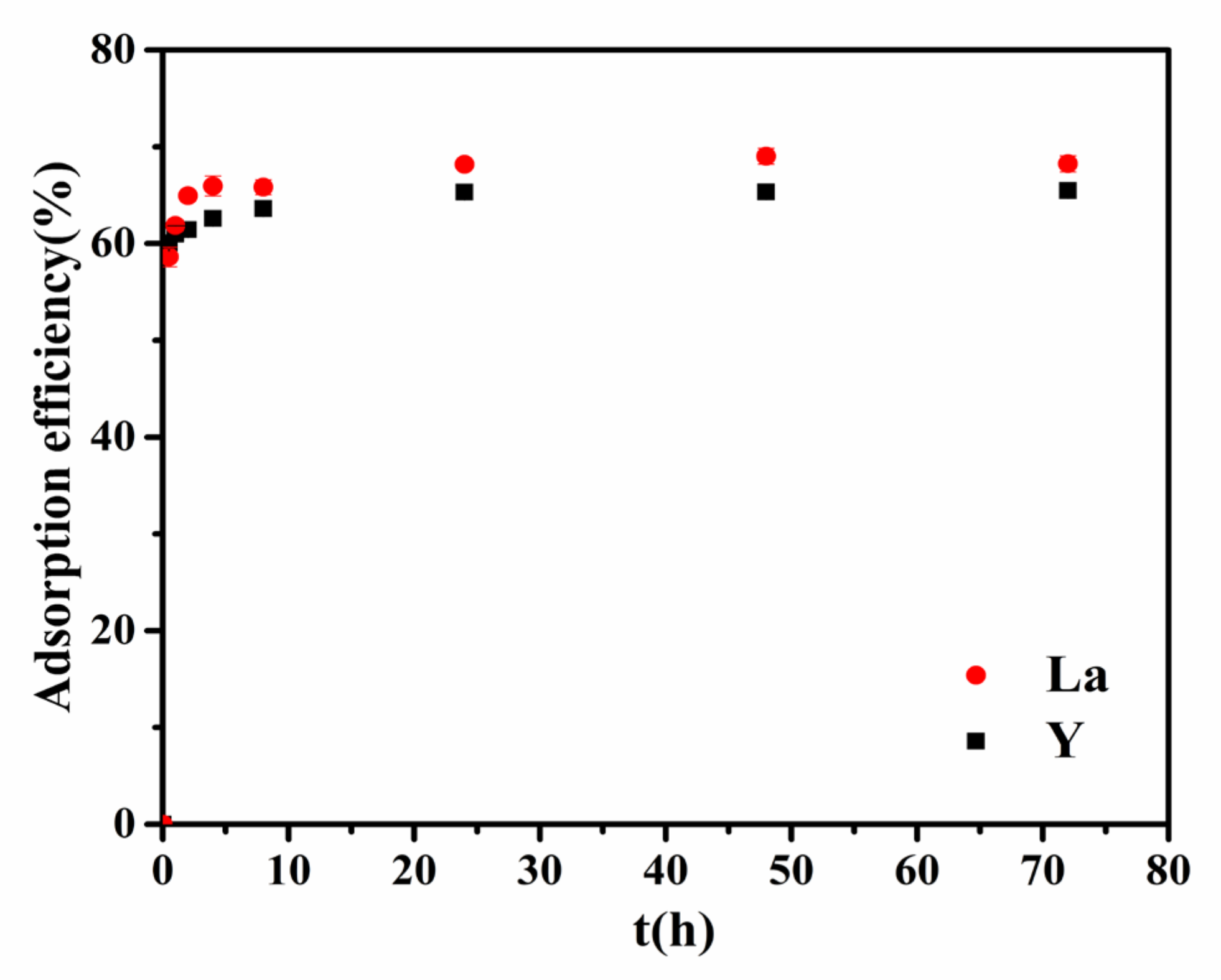
3.2. Effect of Adsorption Time
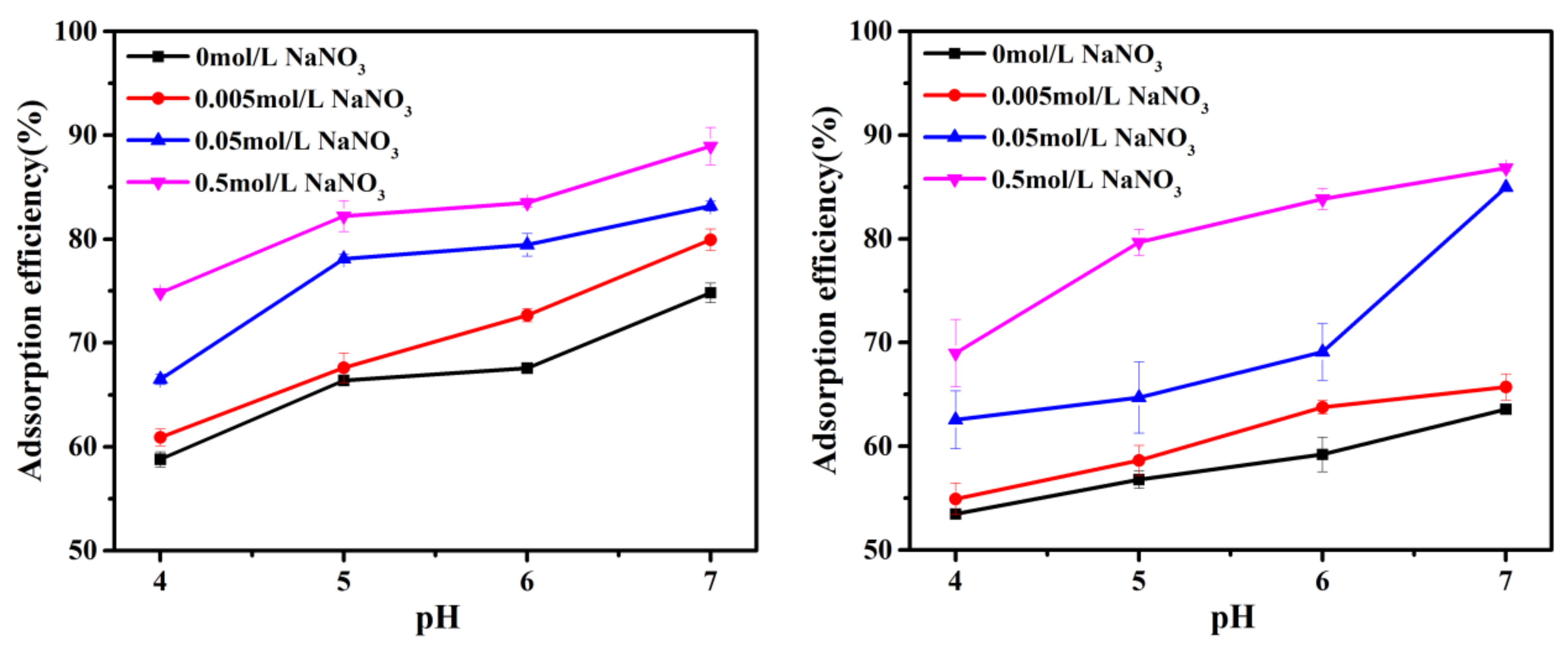
3.3. Effect of pH and Background Electrolyte Concentration
3.4. Adsorption Kinetics
3.5. Adsorption Isotherms and Thermodynamics
3.6. Comparison with Adsorption Behavior of Clay Minerals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, K.S.; Sharma, S.; Maity, J.P.; Martin-Ramos, P.; Fiket, Z.; Bhattacharya, P.; Zhu, Y.B. Occurrence of uranium, thorium and rare earth elements in the environment: A review. Front. Environ. Sci. 2023, 10, 1058053. [Google Scholar] [CrossRef]
- Balaram, V. Potential Future Alternative Resources for Rare Earth Elements: Opportunities and Challenges. Minerals 2023, 13, 425. [Google Scholar] [CrossRef]
- Swain, B. Red mud: An environmental challenge but overlooked treasure for critical rare earth metals. MRS Bull. 2022, 47, 289–302. [Google Scholar] [CrossRef]
- Dushyantha, N.; Ratnayake, N.; Premasiri, R.; Batapola, N.; Panagoda, H.; Jayawardena, C.; Chandrajith, R.; Ilankoon, I.; Rohitha, S.; Ratnayake, A.S.; et al. Geochemical exploration for prospecting new rare earth elements (REEs) sources: REE potential in lake sediments around Eppawala Phosphate Deposit, Sri Lanka. J. Asian Earth Sci. 2023, 243, 105515. [Google Scholar] [CrossRef]
- Mancheri, N.A.; Sprecher, B.; Bailey, G.; Ge, J.; Tukker, A. Effect of Chinese policies on rare earth supply chain resilience. Resour. Conserv. Recycl. 2019, 142, 101–112. [Google Scholar] [CrossRef]
- Yang, M.J.; Liang, X.L.; Ma, L.Y.; Huang, J.; He, H.P.; Zhu, J.X. Adsorption of REEs on kaolinite and halloysite: A link to the REE distribution on clays in the weathering crust of granite. Chem. Geol. 2019, 525, 210–217. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.; Xin, C.; Zhu, J.; Xinghu, W.; Zhu, R.; Wang, H. Understanding the role of natural clay minerals as effective adsorbents and alternative source of rare earth elements: Adsorption operative parameters. Hydrometallurgy 2019, 185, 149–161. [Google Scholar] [CrossRef]
- Borst, A.M.; Smith, M.P.; Finch, A.A.; Estrade, G.; Villanova-de-Benavent, C.; Nason, P.; Marquis, E.; Horsburgh, N.J.; Goodenough, K.M.; Xu, C.; et al. Adsorption of rare earth elements in regolith-hosted clay deposits. Nat. Commun. 2020, 11, 4386. [Google Scholar] [CrossRef]
- Tertre, E.; Berger, G.; Simoni, E.; Castet, S.; Giffaut, E.; Loubet, M.; Catalette, H. Europium retention onto clay minerals from 25 to 150 °C: Experimental measurements, spectroscopic features and sorption modelling. Geochim. Cosmochim. Acta 2006, 70, 4563–4578. [Google Scholar] [CrossRef]
- Ishida, K.; Saito, T.; Aoyagi, N.; Kimura, T.; Nagaishi, R.; Nagasaki, S.; Tanaka, S. Surface speciation of Eu3+ adsorbed on kaolinite by time-resolved laser fluorescence spectroscopy (TRLFS) and parallel factor analysis (PARAFAC). J. Colloid Interface Sci. 2012, 374, 258–266. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and mineralogy of clay minerals. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 21–81. [Google Scholar]
- Abedini, A.; Mongelli, G.; Khosravi, M.; Sinisi, R. Geochemistry and secular trends in the middle-late Permian karst bauxite deposits, northwestern Iran. Ore Geol. Rev. 2020, 124, 103660. [Google Scholar] [CrossRef]
- Mondillo, N.; Di Nuzzo, M.; Kalaitzidis, S.; Boni, M.; Santoro, L.; Balassone, G. Petrographic and geochemical features of the B3 bauxite horizon (Cenomanian-Turonian) in the Parnassos-Ghiona area: A contribution towards the genesis of the Greek karst bauxites. Ore Geol. Rev. 2022, 143, 104759. [Google Scholar] [CrossRef]
- Abedini, A.; Khosravi, M. REE Geochemical Characteristics of the Huri Karst-Type Bauxite Deposit, Irano-Himalayan Belt, Northwestern Iran. Minerals 2023, 13, 926. [Google Scholar] [CrossRef]
- Gamaletsos, P.N.; Godelitsas, A.; Filippidis, A.; Pontikes, Y. The Rare Earth Elements Potential of Greek Bauxite Active Mines in the Light of a Sustainable REE Demand. J. Sustain. Metall. 2019, 5, 20–47. [Google Scholar] [CrossRef]
- Deady, E.A.; Mouchos, E.; Goodenough, K.; Williamson, B.J.; Wall, F. A review of the potential for rare-earth element resources from European red muds: Examples from Seydisehir, Turkey and Parnassus-Giona, Greece. Mineral. Mag. 2016, 80, 43–61. [Google Scholar] [CrossRef]
- Gibaga, C.R.L.; Samaniego, J.O.; Tanciongco, A.M.; Quierrez, R.N.M.; Montano, M.O.; Gervasio, J.H.C.; Reyes, R.C.G.; Peralta, M.J.V. The rare earth element (REE) potential of the Philippines. J. Geochem. Explor. 2022, 242, 107082. [Google Scholar] [CrossRef]
- Long, K.; Fu, Y.; Long, Z.; Tian, J.; Zheng, J. Resource potential analysis of REE and Sc in global bauxite. Acta Geol. Sin. 2019, 93, 1279–1295. [Google Scholar]
- Chen, J.; Wang, Q.; Zhang, Q.; Carranza, E.J.M.; Wang, J. Mineralogical and geochemical investigations on the iron-rich gibbsitic bauxite in Yongjiang basin, SW China. J. Geochem. Explor. 2018, 188, 413–426. [Google Scholar] [CrossRef]
- Torró, L.; Proenza, J.A.; Aiglsperger, T.; Bover-Arnal, T.; Villanova-de-Benavent, C.; Rodríguez-García, D.; Ramírez, A.; Rodríguez, J.; Mosquea, L.A.; Salas, R. Geological, geochemical and mineralogical characteristics of REE-bearing Las Mercedes bauxite deposit, Dominican Republic. Ore Geol. Rev. 2017, 89, 114–131. [Google Scholar] [CrossRef]
- Zarasvandi, A.; Charchi, A.; Carranza, E.J.M.; Alizadeh, B. Karst bauxite deposits in the Zagros Mountain Belt, Iran. Ore Geol. Rev. 2008, 34, 521–532. [Google Scholar] [CrossRef]
- Baumer, T.; Kay, P.; Hixon, A.E. Comparison of europium and neptunium adsorption to aluminum (hydr)oxide minerals. Chem. Geol. 2017, 464, 84–90. [Google Scholar] [CrossRef]
- Tokoro, C.; Sakakibara, T.; Suzuki, S. Mechanism investigation and surface complexation modeling of zinc sorption on aluminum hydroxide in adsorption/coprecipitation processes. Chem. Eng. J. 2015, 279, 86–92. [Google Scholar] [CrossRef]
- Ogata, F.; Tominaga, H.; Yabutani, H.; Taga, A.; Kawasaki, N. Granulation of gibbsite with inorganic binder and its ability to adsorb Mo(VI) from aqueous solution. Toxicol. Environ. Chem. 2012, 94, 650–659. [Google Scholar] [CrossRef]
- Guo, X.M.; Yan, Q.C.; Meng, X.T.; Ma, R.X. UV-Visible Spectrophotometry with Arsenazo III for the Determination of Samarium. J. Appl. Spectrosc. 2019, 86, 542–548. [Google Scholar] [CrossRef]
- Savvin, S.B. Analytical use of arsenazo III: Determination of thorium, zirconium, uranium and rare earth elements. Talanta 1961, 8, 673–685. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Pechenyuk, S.I. The use of the pH at the point of zero charge for characterizing the properties of oxide hydroxides. Russ. Chem. Bull. 1999, 48, 1017–1023. [Google Scholar] [CrossRef]
- Tochiyama, O.; Yamazaki, H.; Li, N. Effect of the concentration of metal ions on their adsorption on various hydrous iron and aluminum oxides. J. Nucl. Sci. Technol. 1996, 33, 846–851. [Google Scholar] [CrossRef]
- Ahmed, A.; Yujun, W.; Johannes, L.; Marcelo Eduardo, A. Calcium Uptake on Kaolinite and Gibbsite: Effects of Sulfate, pH, and Salt Concentration with Additional Insight from Second Harmonic Generation on Temperature Dependencies with Sapphire-Basal Planes and the Potential Relevance to Ice Nucleation. In Advanced Sorption Process Applications; Serpil, E., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 1–22. [Google Scholar]
- Saeki, K. Adsorption of Fe2+ and Mn2+ on silica, gibbsite, and humic acids. Soil Sci. 2004, 169, 832–840. [Google Scholar] [CrossRef]
- Persson, I.; D’Angelo, P.; De Panfilis, S.; Sandström, M.; Eriksson, L. Hydration of Lanthanoid(III) Ions in Aqueous Solution and Crystalline Hydrates Studied by EXAFS Spectroscopy and Crystallography: The Myth of the “Gadolinium Break”. Chem. Eur. J. 2008, 14, 3056–3066. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.A.; Djanashvili, K.; Geraldes, C.F.G.C.; Platas-Iglesias, C. The chemical consequences of the gradual decrease of the ionic radius along the Ln-series. Coord. Chem. Rev. 2020, 406, 213146. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Coppin, F.; Berger, G.; Bauer, A.; Castet, S.; Loubet, M. Sorption of lanthanides on smectite and kaolinite. Chem. Geol. 2002, 182, 57–68. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. X. Update. Adv. Colloid Interface Sci. 2023, 319, 102973. [Google Scholar] [CrossRef]
- Hirst, C.; Andersson, P.S.; Shaw, S.; Burke, I.T.; Kutscher, L.; Murphy, M.J.; Maximov, T.; Pokrovsky, O.S.; Morth, C.-M.; Porcelli, D. Characterisation of Fe-bearing particles and colloids in the Lena River basin, NE Russia. Geochim. Cosmochim. Acta 2017, 213, 553–573. [Google Scholar] [CrossRef]
- Wainipee, W.; Cuadros, J.; Sephton, M.A.; Unsworth, C.; Gill, M.G.; Strekopytov, S.; Weiss, D.J. The effects of oil on As(V) adsorption on illite, kaolinite, montmorillonite and chlorite. Geochim. Cosmochim. Acta 2013, 121, 487–502. [Google Scholar] [CrossRef]
- Yusoff, Z.M.; Ngwenya, B.T.; Parsons, I. Mobility and fractionation of REEs during deep weathering of geochemically contrasting granites in a tropical setting, Malaysia. Chem. Geol. 2013, 349, 71–86. [Google Scholar] [CrossRef]
- Granados-Correa, F.; Vilchis-Granados, J.; Jimenez-Reyes, M.; Quiroz-Granados, L.A. Adsorption Behaviour of La(III) and Eu(III) Ions from Aqueous Solutions by Hydroxyapatite: Kinetic, Isotherm, and Thermodynamic Studies. J. Chem. 2013, 2013, 751696. [Google Scholar] [CrossRef]

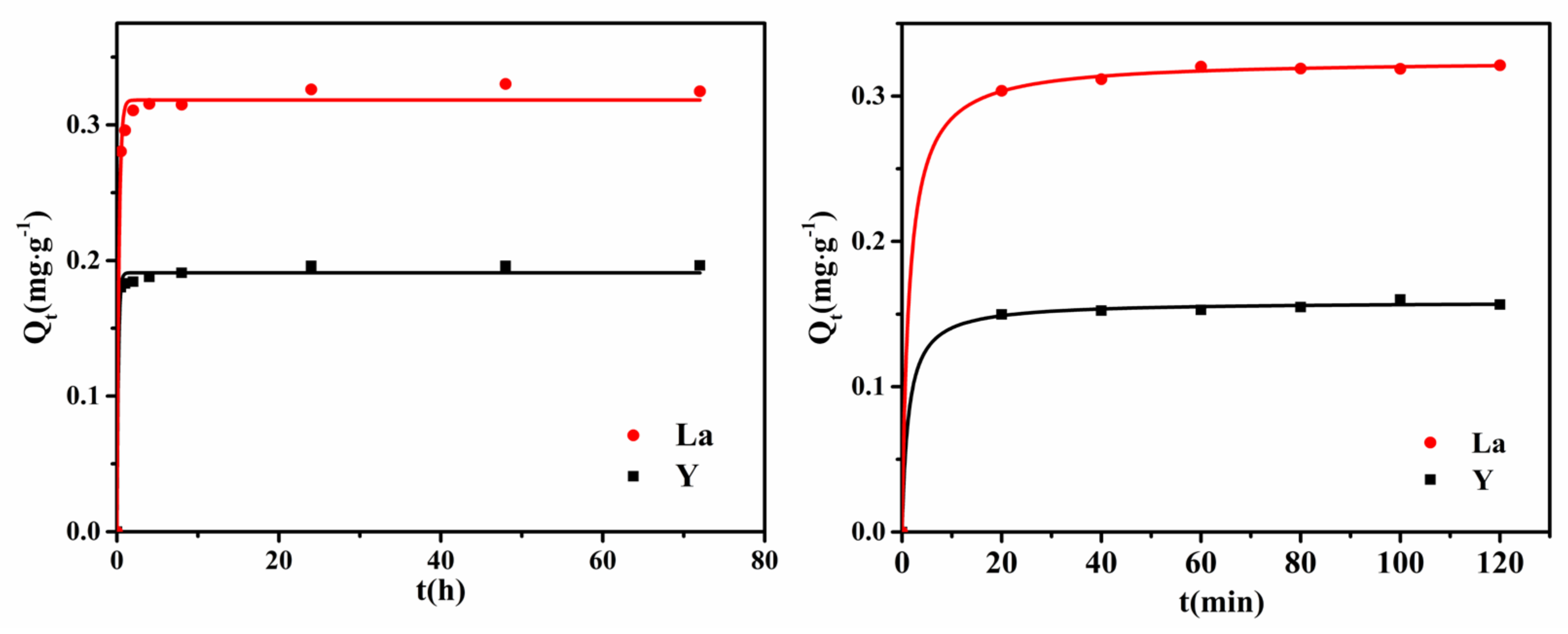
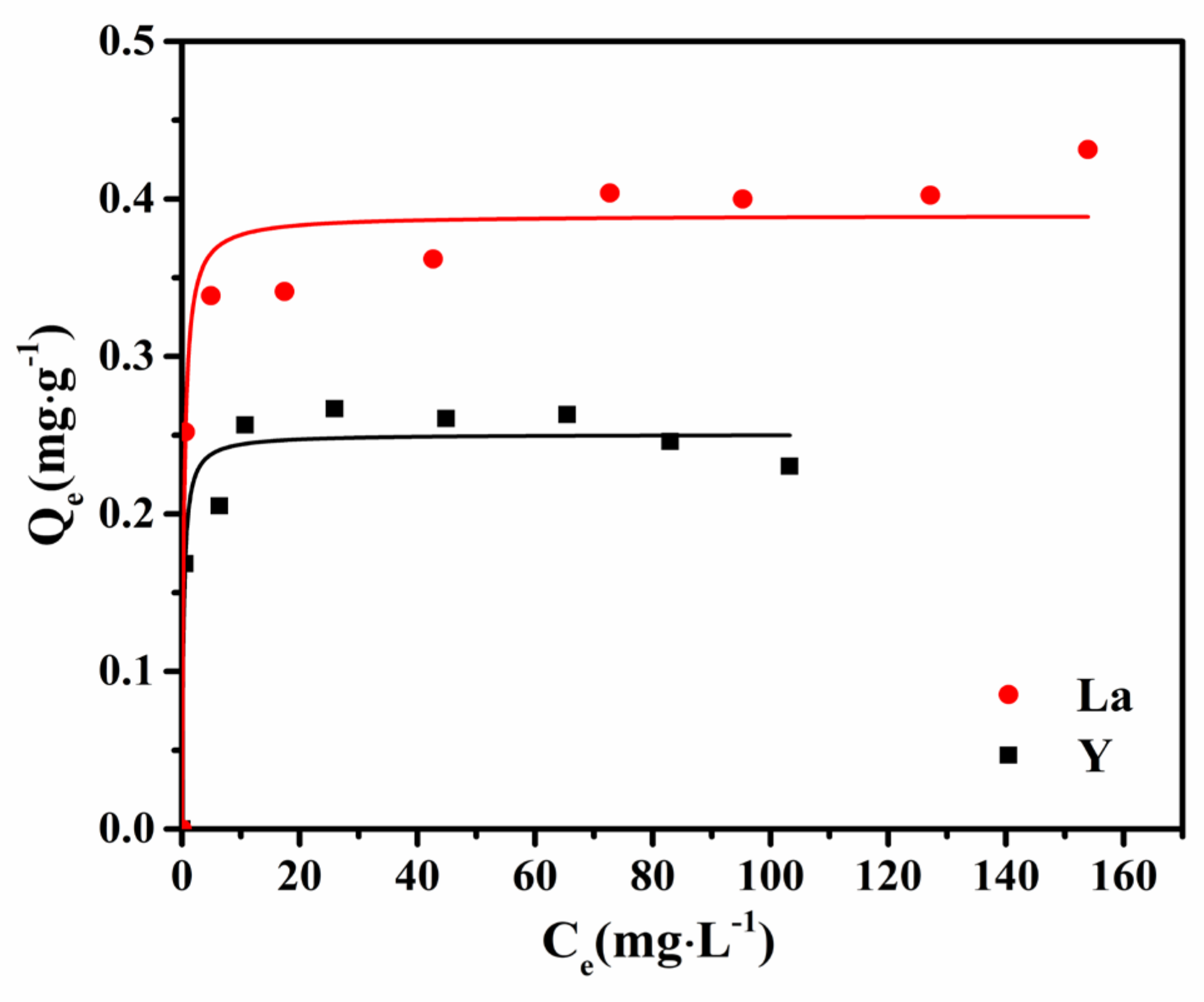
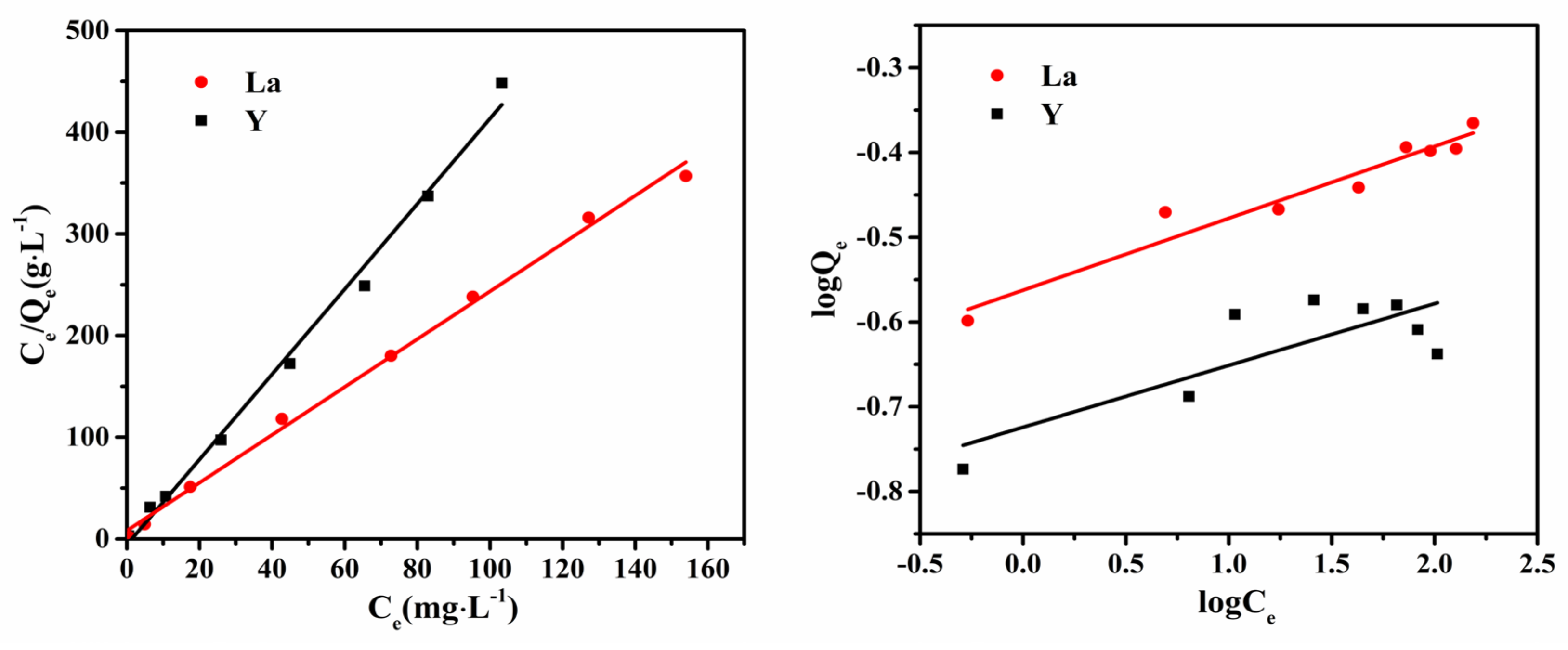
| Kinetics Model | Pseudo-First-Order Kinetics Model | Pseudo-Second-Order Kinetics Model | ||||
|---|---|---|---|---|---|---|
| Qe/(mg∙g−1) | k1/(1/h) | R2 | Qe/(mg∙g−1) | k2/(g/mg·min−1) | R2 | |
| La | 0.3183 | 4.0353 | 0.9930 | 0.3247 | 2.1907 | 0.9999 |
| Y | 0.1909 | 5.6090 | 0.9942 | 0.1584 | 4.9150 | 0.9987 |
| Thermodynamic Model | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qmax/(mg∙g−1) | K/(L∙mg−1) | R2 | KF | 1/n | R2 | |
| La | 0.4249 | 0.0517 | 0.9964 | 0.2739 | 0.0848 | 0.9456 |
| Y | 0.2614 | 1.0821 | 0.9936 | 0.2207 | 0.0388 | 0.6537 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Wan, Q.; Yu, W.; Nie, X.; Yang, S.; Yang, S.; Qin, Z. Adsorption Behaviors of Lanthanum (III) and Yttrium (III) Ions on Gibbsite. Minerals 2023, 13, 1530. https://doi.org/10.3390/min13121530
Zhou Z, Wan Q, Yu W, Nie X, Yang S, Yang S, Qin Z. Adsorption Behaviors of Lanthanum (III) and Yttrium (III) Ions on Gibbsite. Minerals. 2023; 13(12):1530. https://doi.org/10.3390/min13121530
Chicago/Turabian StyleZhou, Zongke, Quan Wan, Wenbin Yu, Xin Nie, Shuguang Yang, Shuqin Yang, and Zonghua Qin. 2023. "Adsorption Behaviors of Lanthanum (III) and Yttrium (III) Ions on Gibbsite" Minerals 13, no. 12: 1530. https://doi.org/10.3390/min13121530
APA StyleZhou, Z., Wan, Q., Yu, W., Nie, X., Yang, S., Yang, S., & Qin, Z. (2023). Adsorption Behaviors of Lanthanum (III) and Yttrium (III) Ions on Gibbsite. Minerals, 13(12), 1530. https://doi.org/10.3390/min13121530








