Characteristics and Formation Conditions of Se-Bearing Metacinnabar in the Wanshan Mercury Ore Field, Eastern Guizhou
Abstract
:1. Introduction
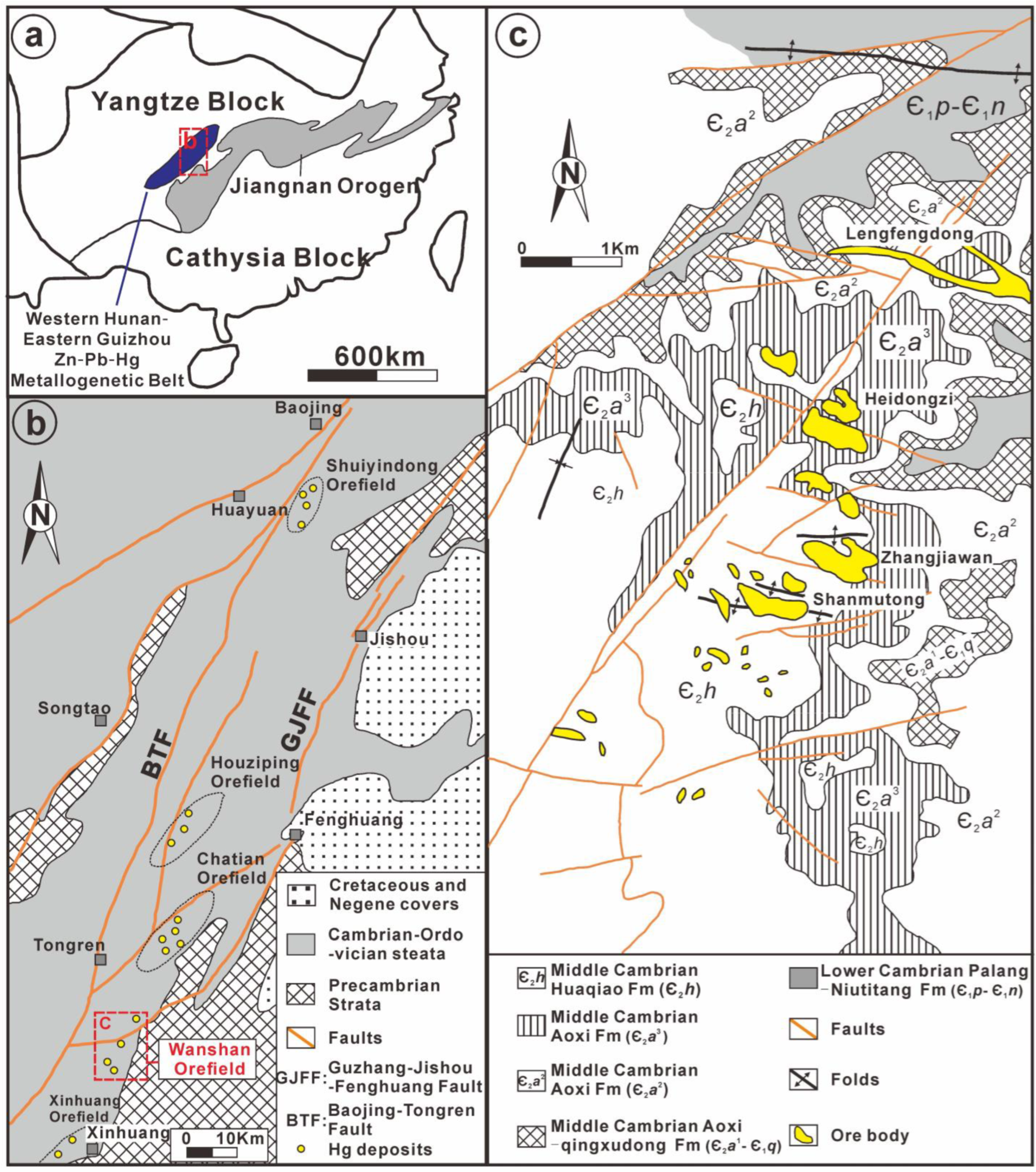
2. Regional Geology
3. Geological Characteristics of Deposit
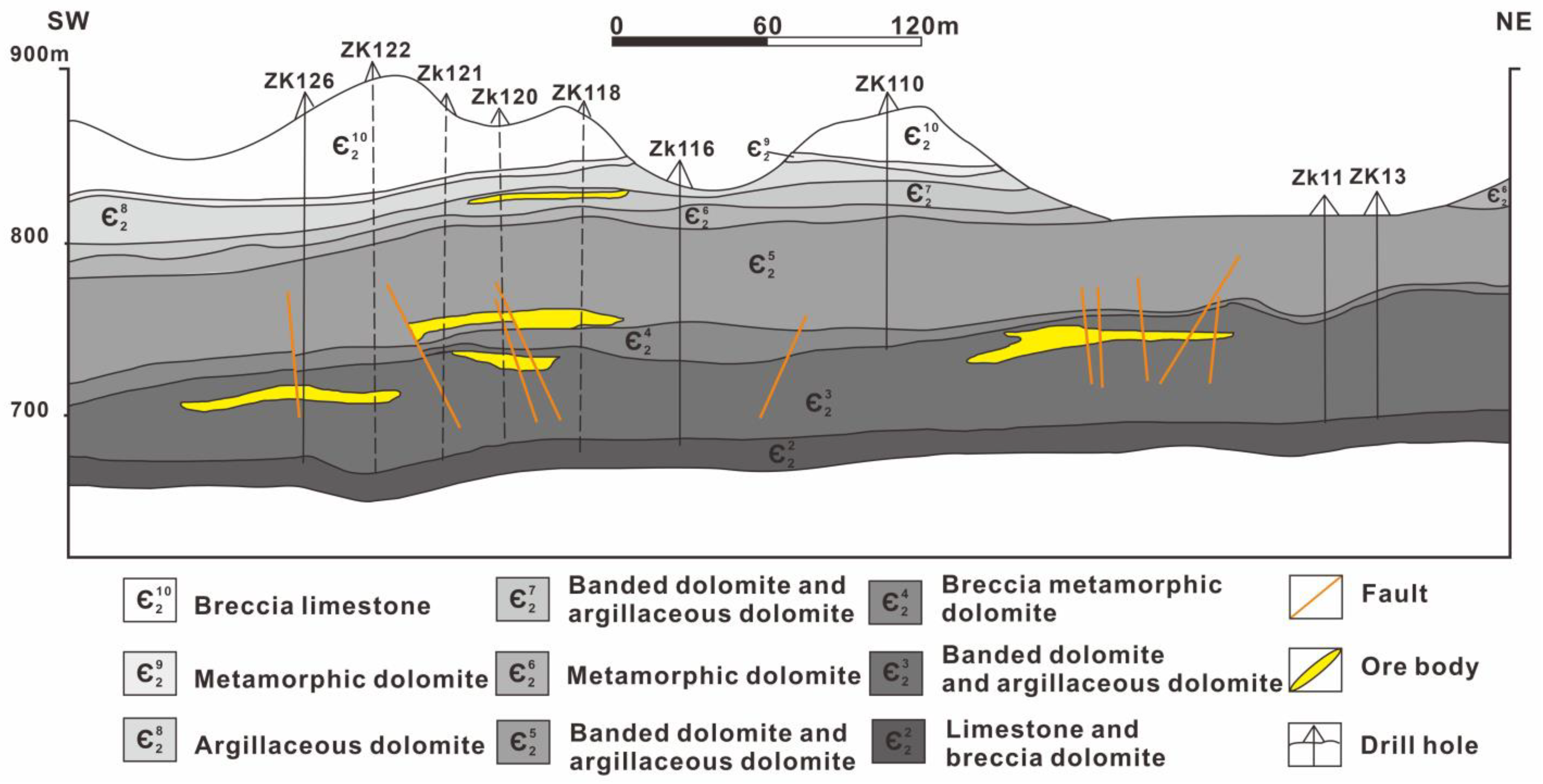
3.1. Ore Zones and Orebodies
3.2. Ore Mineralogy and Alteration
3.3. Mineralization Stages
4. Sampling and Analytical Methods
4.1. Sample Collection and Characteristics
4.2. XRD Analysis
4.3. EPMA
4.4. FESEM
5. Results
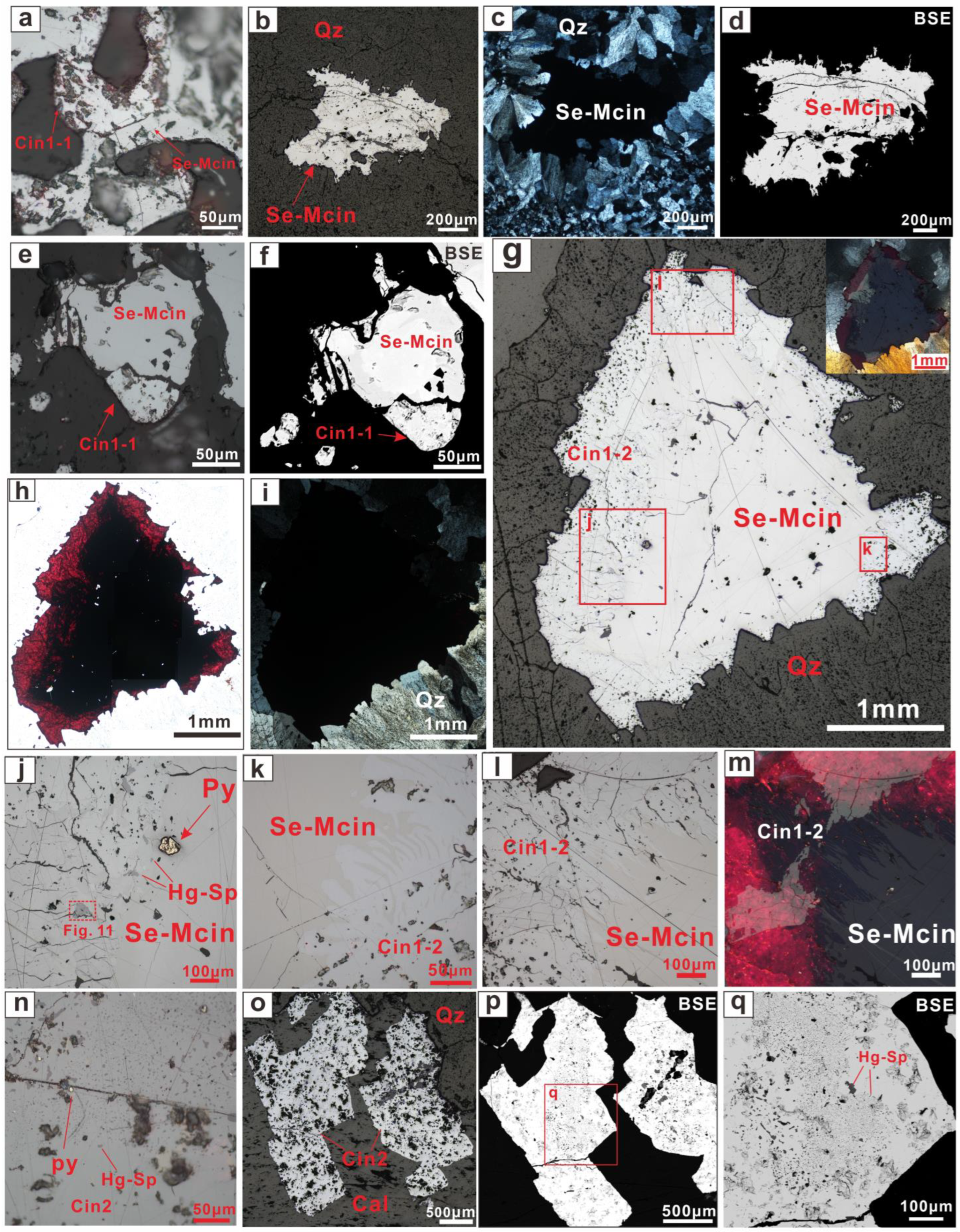
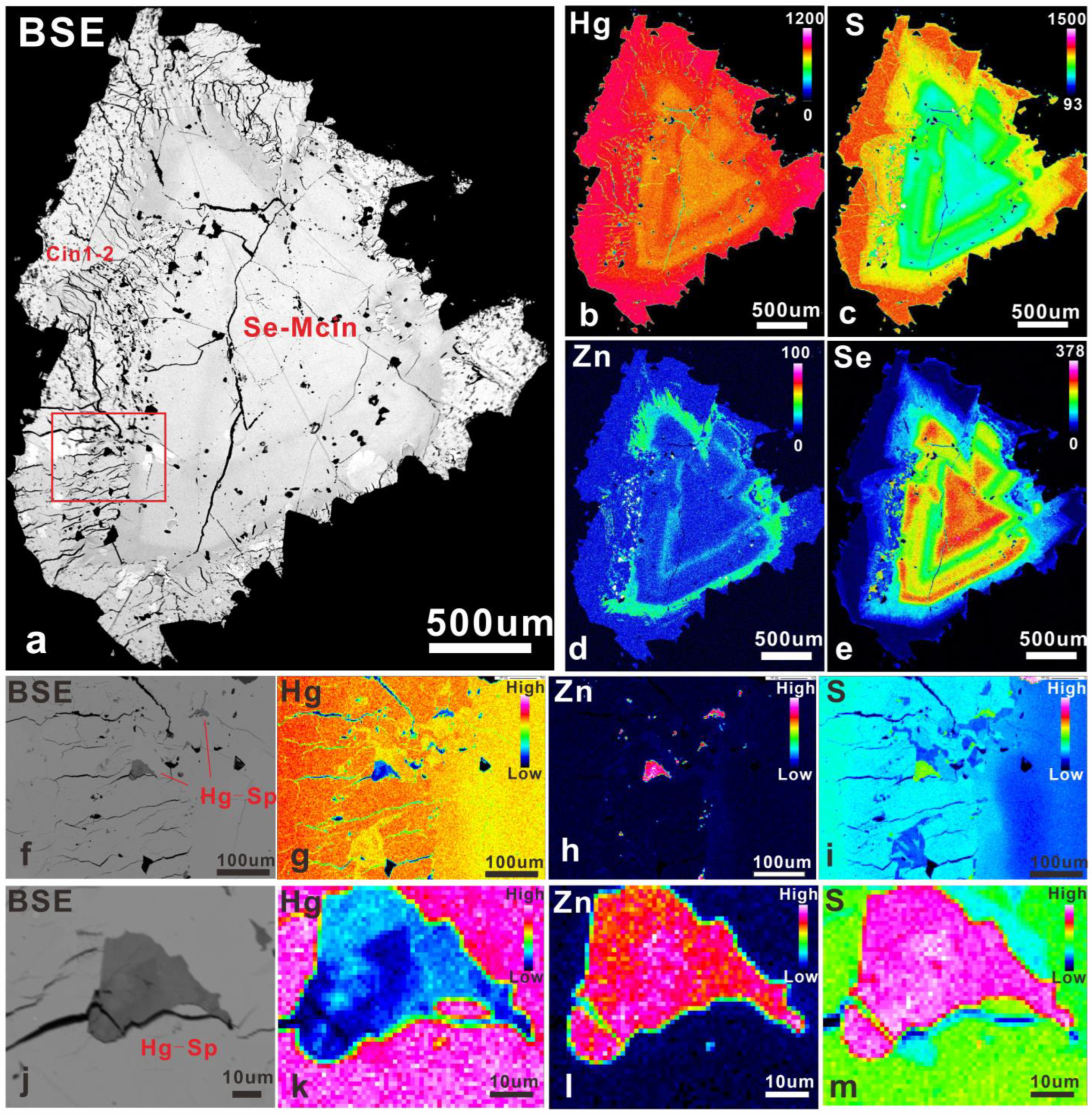
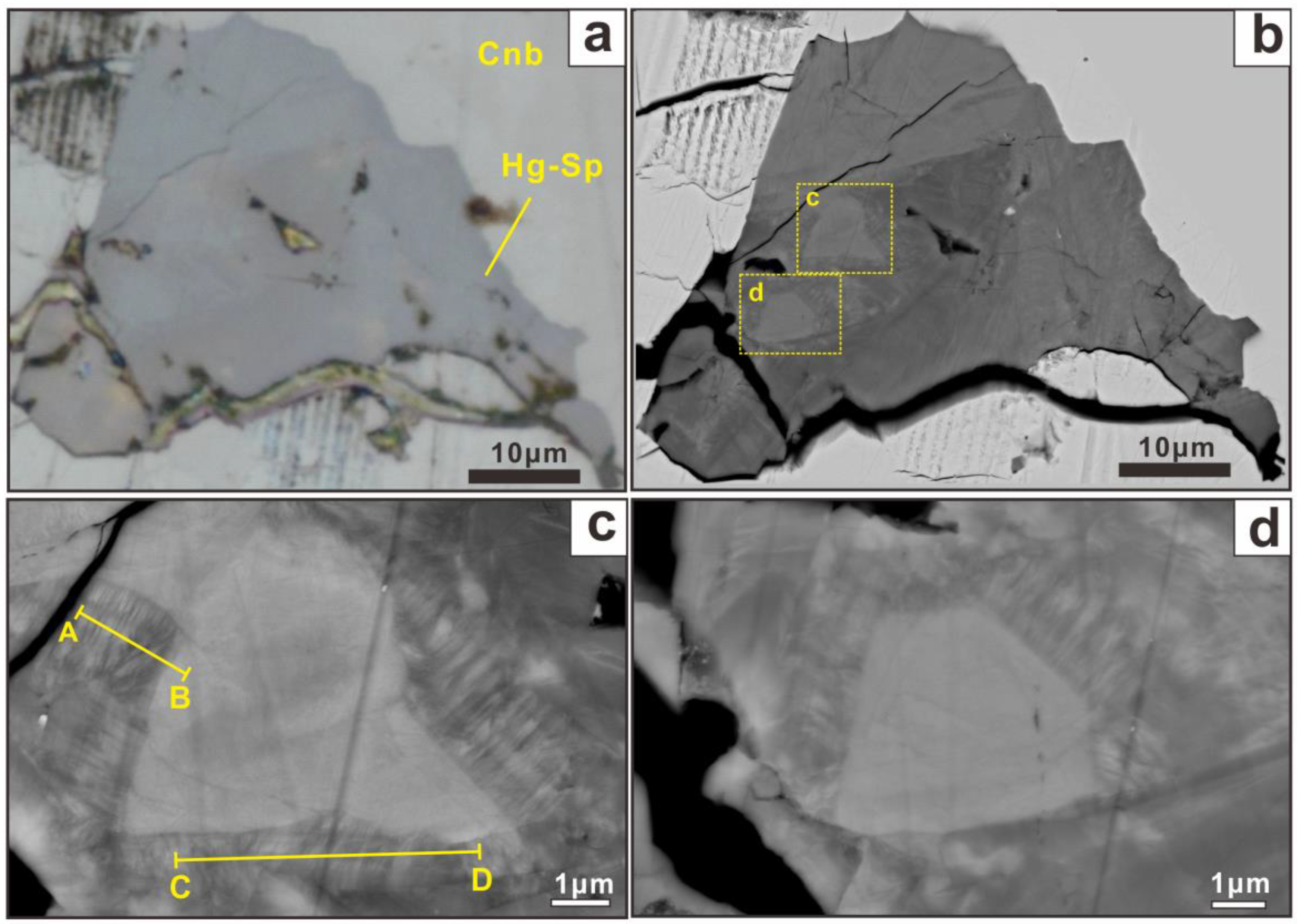
5.1. Microscopic Morphology
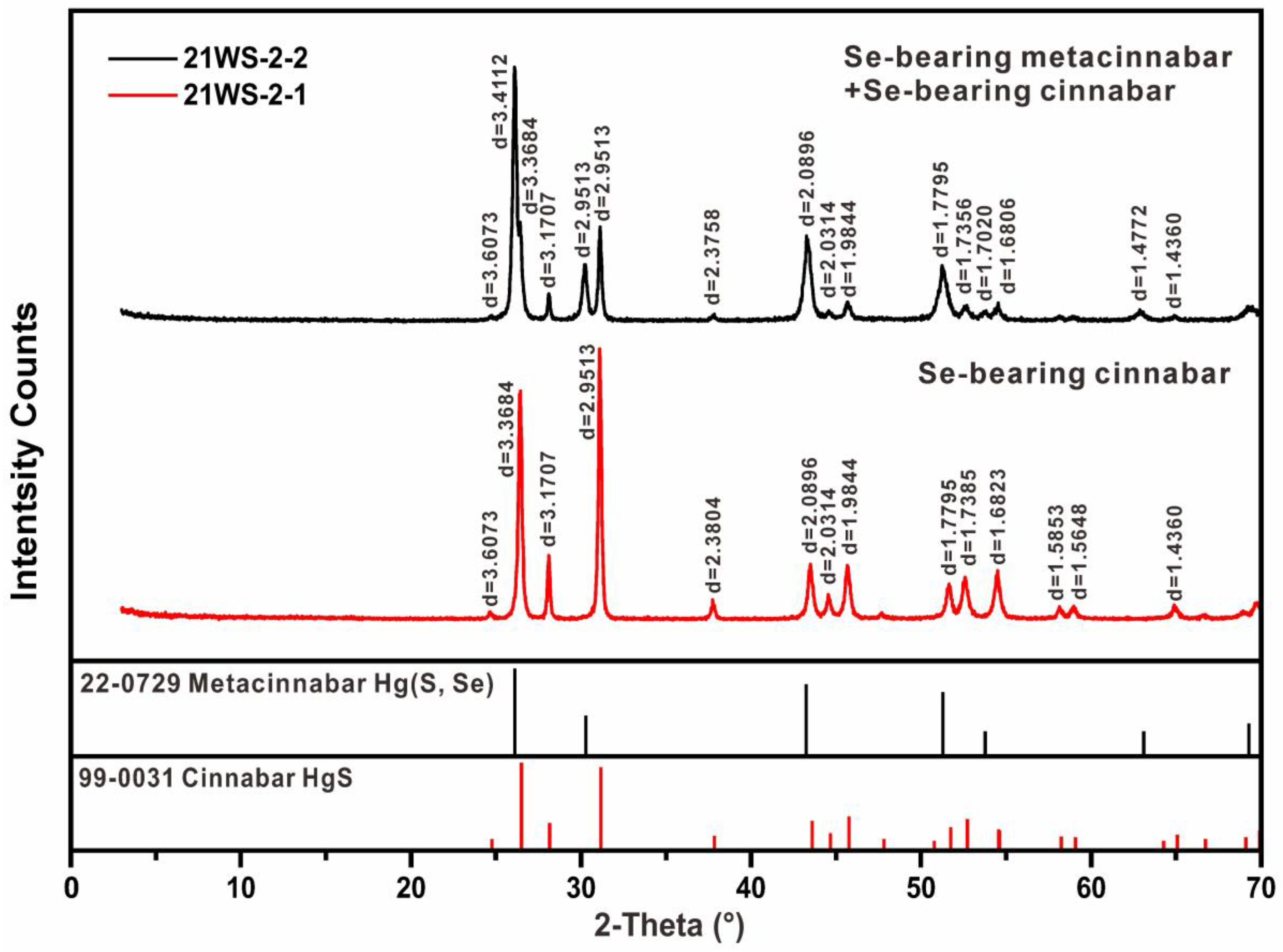
5.2. XRD Analysis
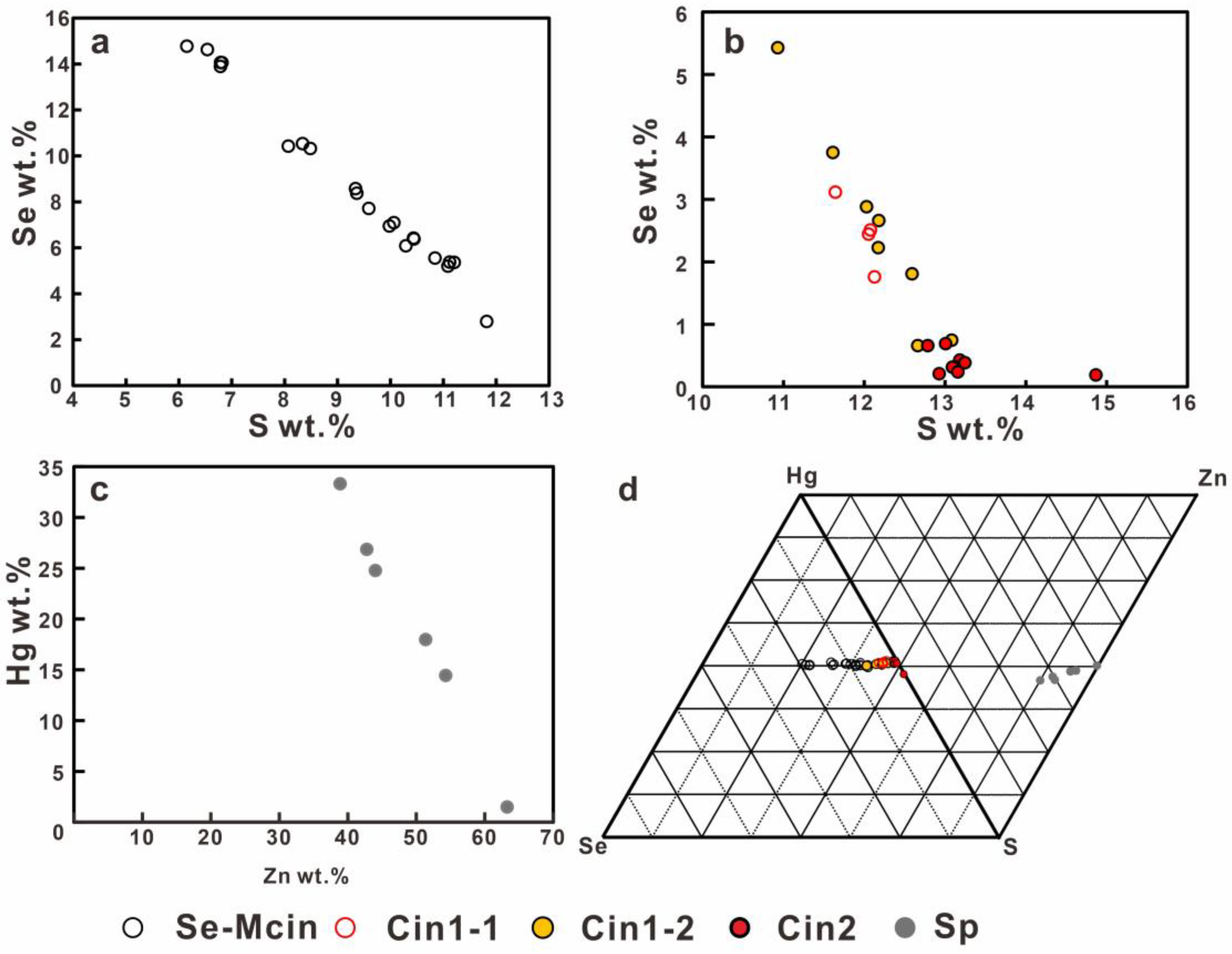
5.3. Chemical Compositions of Hg–Se–S and Zn–Hg–S Minerals
5.3.1. Se-Bearing Metacinnabar (Se-Mcin)
5.3.2. Se-Bearing Cinnabar (Cin1-1)
5.3.3. Cinnabar (Cin1-2)
5.3.4. Hg-Bearing Sphalerite (Hg-Sp) and the Cinnabar (Cin2)
5.3.5. Distribution of Elements in Partially Transformed Se-Bearing Metacinnabar
6. Discussion
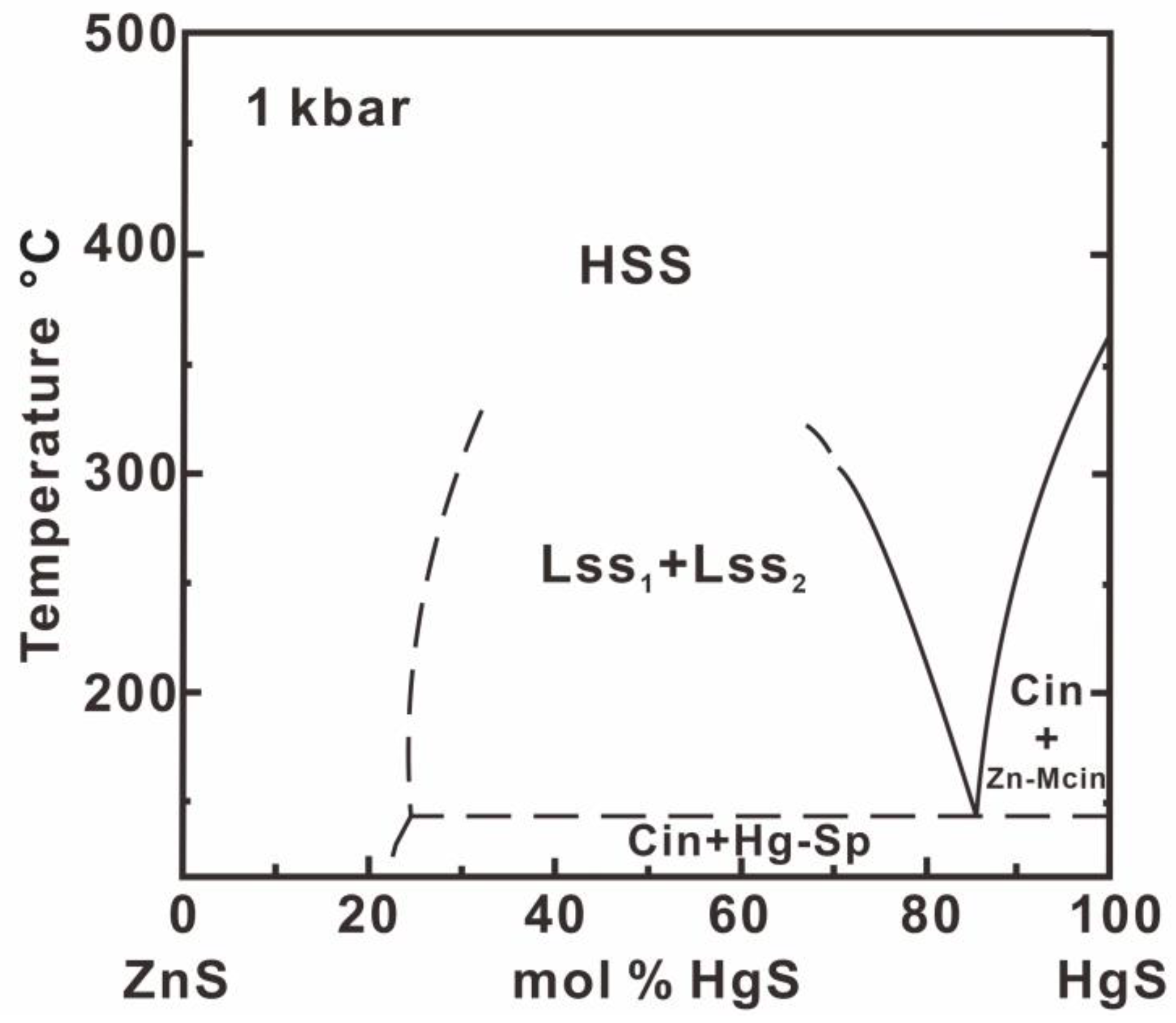
6.1. The Hg–Se–S and Zn–Hg–S System Minerals
6.2. Association of Se-Bearing Metacinnabar, Cinnabar and Sphalerite
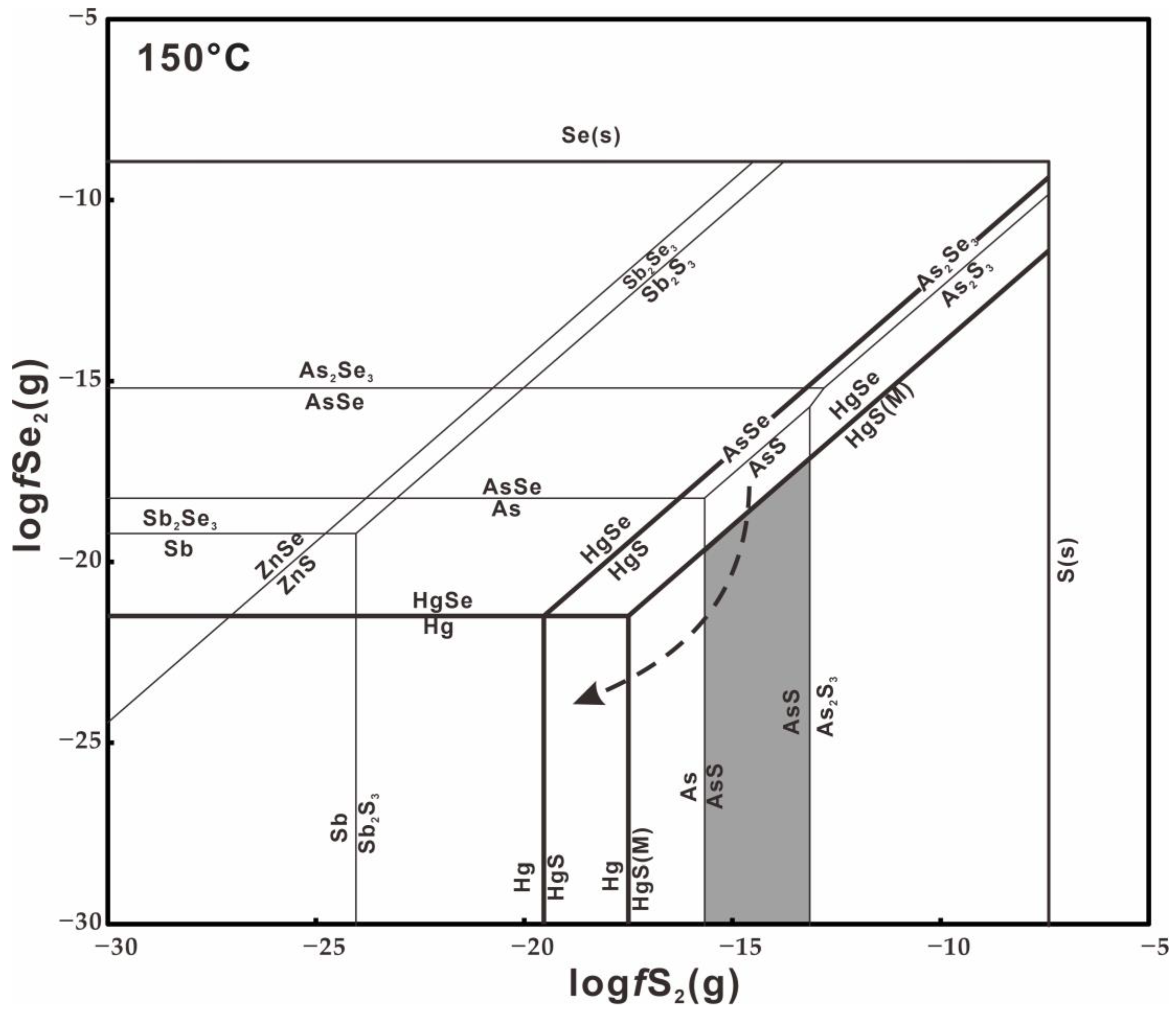
6.3. Physicochemical Conditions for the Formation of Se-Bearing Metacinnabar
| Hg + 0.5S2(g) = HgS | logk1 = 9.764 | (1) |
| Hg + 0.5Se2(g) = HgSe | logk2 = 10.746 | (2) |
| HgS + 0.5Se2(g) = 0.5S2(g) + HgSe | logk3 = 0.982 | (3) |
| Hg + 0.5S2(g) = HgS(M) | logk4 = 8.749 | (4) |
| HgS(M) + 0.5Se2(g) = 0.5S2(g) + HgSe | logk5 = 1.997 | (5) |
| 2Sb + 1.5S2(g) = Sb2S3 | logk6 = 36.058 | (6) |
| 2Sb + 1.5Se2(g) = Sb2Se3 | logk7 = 28.824 | (7) |
| Sb2S3 + 1.5Se2(g) = 1.5S2(g) + Sb2Se3 | logk8 = -7.34 | (8) |
| Zn + 0.5S2(g) = ZnS | logk9 = 27.942 | (9) |
| Zn + 0.5Se2(g) = ZnSe | logk10 = 25.161 | (10) |
| ZnS + 0.5Se2(g) = 0.5S2(g) + ZnSe | logk11 = −2.781 | (11) |
| As + 0.5S2(g) = AsS | logk12 = 7.833 | (12) |
| 2As + 1.5S2(g) = As2S3 | logk13 = 22.237 | (13) |
| As + 0.5Se2(g) = AsSe | logk14 = 9.188 | (14) |
| 2As + 1.5Se2(g) = As2Se3 | logk15 = 25.836 | (15) |
| 2AsS + 0.5S2(g) = As2S3 | logk16 = 6.527 | (16) |
| AsS + 0.5Se2(g) = 0.5S2(g) + AsSe | logk17 = 1.286 | (17) |
| 2AsS + 1.5Se2(g) = S2(g) + As2Se3 | logk18 = 10.170 | (18) |
| 0.5As2S3 + 0.5Se2(g) = 0.75S2(g) + AsSe | logk19 = −2.000 | (19) |
| As2S3 + 1.5Se2(g) = 1.5S2(g) + As2Se3 | logk20 = 3.599 | (20) |
| 2AsSe + 0.5Se2(g) = As2Se3 | logk21 = 7.599 | (21) |
| Se2(g) = 2Se(s) | logk22 = 8.935 | (22) |
| S2(g) = 2S(s) | logk23 = 7.403 | (23) |
7. Conclusions
- The Se-bearing metacinnabar contains 77.66–84.01 wt.% Hg, 0.18–1.17 wt.% Zn with extensive isomorphic substitution of Se and S (2.79–14.77 wt.% Se, 6.15–11.82 wt.% S), and the presence of impurity elements (Zn, Se) is considered to be the key factor for the stable existence of Se-bearing metacinnabar;
- The partial transformation of Se-bearing metacinnabar is achieved by coupled dissolution–reprecipitation reactions, which occurred within the Zn-bearing area in Se-bearing metacinnabar, cause the expulsion of the Zn and the precipitation of large amounts of secondary Hg-bearing sphalerite within the replaced zones and the newly generated mineral fractures;
- The Se-bearing metacinnabar is closely associated with cinnabar, sphalerite, pyrite, realgar, stibnite, quartz and tiemannite and is accompanied by strong silicification, implying that it was formed in acidic conditions and originally precipitated at moderate fSe2 (logfS2 = − 15.663 to − 13.141 and logfSe2 < logfS2 − 3.994 at ~150 °C).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, A.; Gao, C.; Li, M.; He, C.; Huang, X.; Zhang, D.; Yu, C.; Liu, H.; Ma, Y.; Tian, Y. A study of the electrical properties of HgS under high pressure. J. Phys. Conden. Matter 2007, 19, 425222. [Google Scholar] [CrossRef]
- Biagioni, C.; Silvia, M.; Pasero, M. New data on metacinnabar from Tuscany (Italy). Atti della Soc. Toscana di Sci. Nat. 2017, 74, 13–19. [Google Scholar] [CrossRef]
- Biagioni, C.; Orlandi, P. Tiemannite e metacinabro della miniera Buca della Vena (Alpi Apuane). Atti della Soc. Toscana di Sci. Nat. Mem. 2009, 114, 13–17. [Google Scholar]
- Wang, P. Systematic Mineralogy; Geological Publishing House: Beijing, China, 1982; pp. 1–522, (In Chinese with English Abstract). [Google Scholar]
- Dickson, F.W.; Tunell, G. The stability relations of cinnabar and metacinnabar. Am. Miner. 1959, 44, 471–487. [Google Scholar]
- Potter, R. The Systematics of Polymorphism in Binary Sulfides, I. Phase Equilibria in the System Mercury Sulfur, II. Polymorphism in Binary Sulfides. Ph.D. Thesis, The Pennsylvania State University, State College, PA, USA, 1973. [Google Scholar]
- Potter, R.; Barnes, H. Phase relations in the binary Hg-S. Eur. J. Miner. 1978, 63, 1143–1152. [Google Scholar]
- Ballirano, P.; Botticelli, M.; Maras, A. Thermal behaviour of cinnabar, α-HgS, and the kinetics of the β-HgS(metacinnabar)→α-HgS conversion at room temperature. Eur. J. Miner. 2013, 25, 957–965. [Google Scholar] [CrossRef]
- Boctor, N. The Mercury-Selenium-Sulfur System and Its Geological Implications. Part 1: Phase Relations in the Mercury-Selenium-Sulfur System. Part 2: The Sulfoselenides and Sulfides of Mercury: Mineralogy and Geochemistry. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 1976. [Google Scholar]
- Ohmiya, T. Thermal expansion and the phase transformation in mercury sulphide. J. Appl. Crystallogr. 1974, 7, 396–397. [Google Scholar] [CrossRef]
- Vasil, V.I. New data on the composition of metacinnabar and Hg-sphalerite with an isomorphous Cd admixture. Russ. Geol. Geophys. 2011, 52, 701–708. [Google Scholar] [CrossRef]
- Bao, Z.; Bao, J. The occurrences of selenium in the western Hunan-eastern Guizhou mercury ore belt. Miner. Explor. 1995, 4, 30–34, (In Chinese with English abstract). [Google Scholar]
- Liu, J.P.; Rong, Y.A.; Zhang, S. Mineralogy of Zn–Hg–S and Hg–Se–S Series Minerals in Carbonate-Hosted Mercury Deposits in Western Hunan, South China. Minerals 2017, 7, 101. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z. Onofrite in the Dongping mercury deposit, Baojing, Hunan Province. Acta Miner. Sin. 1991, 11, 274–277, (In Chinese with English abstract). [Google Scholar]
- Ye, L.; Cook, N.J.; Liu, T.; Ciobanu, C.L.; Gao, W.; Yang, Y. The Niujiaotang Cd-rich zinc deposit, Duyun, Guizhou province, southwest China: Ore genesis and mechanisms of cadmium concentration. Miner. Deposita 2012, 47, 683–700. [Google Scholar] [CrossRef]
- Bao, Y.; Wan, R.; Bao, Z. Discussion of the mercury mineralization related to the mercury metallogenic belt of Hunan Guizhou province. Beijing Geol. 1999, 2, 6–13, (In Chinese with English abstract). [Google Scholar]
- Wang, J. Mineralization, Geochronology and Geodynamics of the Low-Temperature Epithermal Metallogenic Domain in Southwestern China. Ph.D. Thesis, Graduate University of Chinese Academy of Sciences, Guiyang, China, 2012. [Google Scholar]
- Zhang, K. Geological characteristics, ore-controlling regularity and prospecting model of the Southern District of Wanshan mercury deposit. Miner. Resour. Geol. 2017, 31, 864–868, (In Chinese with English abstract). [Google Scholar]
- Zhang, L. Summary report of supplementary prospecting work in Yanwuping mining area, Tongren County, Guizhou Province; Guizhou Metallurgical Geology Team No. 1: Tongren, China, 1978. [Google Scholar] [CrossRef]
- Liang, H.; Guan, P.; Chen, M.; Bao, Z.; Bao, J. Metallogenic characteristics and prospecting significance of Hg-Se-Zn deposits at the depth and outside of Jiudiantang Hg deposit, west of Hunan. Miner. Resour. Geol. 2016, 30, 874–879, (In Chinese with English abstract). [Google Scholar]
- Zeng, R. The Mercury Deposits in China; Chengdu Geological and Mining Institute, Ministry of Geology and Mining: Chengdu, China, 1983; pp. 27–35, (In Chinese with English abstract). [Google Scholar]
- Hua, Y.; Cui, M. Geology of the Wanshan Mercury Deposits in Guizhou; Geological Publishing House: Beijing, China, 1995; pp. 1–143, (In Chinese with English abstract). [Google Scholar]
- Wu, S. Geological characteristics of the Fanjiashan Hg deposit with a research on its ore-forming rules. Miner. Resour. Geol. 2007, 21, 618–625, (In Chinese with English abstract). [Google Scholar]
- Chen, X.; Li, L. Geological characteristics and sulfur isotopic composition of wanshan mercury deposit in Guizhou and its significance. Sichuan Nonferrous Met. 2018, 4, 14–17, (In Chinese with English abstract). [Google Scholar]
- Wang, H.; Hu, K.; Wu, G.; Chen, H.; Liu, C. Genesis of Strata-Bound Mercury Deposits in the Border Area between Hunan and Guizhou; Geological Publishing House: Beijing, China, 1989; pp. 1–366, (In Chinese with English abstract). [Google Scholar]
- Liu, Y. Element Geochemistry; Sciences Publishing House: Beijing, China, 1984; pp. 1–548, (In Chinese with English abstract). [Google Scholar]
- Wen, H.; Xiao, H. A review of selenium minerals. Acta Petrol. Miner. 1998, 27, 69–74, (In Chinese with English abstract). [Google Scholar]
- Liu, J.J.; Zhai, D.G.; Wang, D.Z.; Gao, S.; Yin, C.; Liu, Z.J.; Wang, J.P.; Wang, Y.H.; Zhang, F.F. Classification and mineralization of the Au-(Ag)-Te-Se deposits. Earth Sci. Front. 2020, 27, 79–98, (In Chinese with English abstract). [Google Scholar]
- Liu, J.J.; Zheng, M.H.; Liu, J.M.; Su, W.C. Geochemistry of the La’erma and Qiongmo Au–Se deposits in the western Qinling Mountains, China. Ore Geol. Rev. 2000, 17, 91–111. [Google Scholar] [CrossRef]
- Owen, N.D.; Ciobanu, C.L.; Cook, N.J.; Slattery, A.; Basak, A. Nanoscale study of clausthalite-bearing symplectites in Cu-Au-(U) ores: Implications for ore genesis. Miner. 2018, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Drábek, M.; Vymazalová, A.; Laufek, F. The system Hg-Pd-Se at 400 °C: Phase relations involving tischendorfite and other ternary phases. Can. Miner. 2014, 52, 763–768. [Google Scholar] [CrossRef]
- Števko, M.; Sejkora, J.; Dolníček, Z.; Škácha, P. Selenium-Rich Ag–Au Mineralization at the Kremnica Au–Ag Epithermal Deposit, Slovak Republic. Minerals 2018, 8, 572. [Google Scholar] [CrossRef] [Green Version]
- Cabral, A.R.; van den Kerkhof, A.; Sosa, G.; Nolte, N.; Ließmann, W.; Lehmann, B. Clausthalite (PbSe) and tiemannite (HgSe) from the type locality: New observations and implications for metallogenesis in the Harz Mountains, Germany. Ore Geol. Rev. 2018, 102, 728–739. [Google Scholar] [CrossRef]
- Marquez-Zavalia, M.F.; Galliski, M.A.; Škácha, P.; Macek, I.; Sejkora, J.; Dolníček, Z. Mineralogy of the Rincon Blanco selenide occurrence, La Rioja, Argentina. J. Geosci. 2021, 66, 1–14. [Google Scholar] [CrossRef]
- Bao, J. Occurrence characteristics and geological significance of selenium in Xiangxi Mercury Deposit. Hunan Geol. 1983, 2, 52–57, (In Chinese with English abstract). [Google Scholar]
- Chen, D.; Sun, S. Metacinnabar and tiemannite in Hunan-Guizhou (Xiangqian) mercury metallogenic belt. Acta Petrol. Miner. 1991, 10, 58–62, (In Chinese with English abstract). [Google Scholar]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Radosavljević, S.A.; Stojanović, J.N.; Pačevski, A.M. Hg-bearing sphalerite from the Rujevac polymetallic ore deposit, Podrinje Metallogenic District, Serbia: Compositional variations and zoning. Chem. Erde-Geochem. 2012, 72, 237–244. [Google Scholar] [CrossRef]
- Sokol, E.V.; Kokh, S.N.; Nekipelova, A.V.; Abersteiner, A.; Seryotkin, Y.V.; Ershov, V.V.; Nikitenko, O.A.; Deviatiiarova, A.S. Ge-Hg-Rich Sphalerite and Pb, Sb, As, Hg, and Ag Sulfide Assemblages in Mud Volcanoes of Sakhalin Island, Russia: An Insight into Possible Origin. Minerals 2021, 11, 1186. [Google Scholar] [CrossRef]
- Kremheller, A.; Levine, A.K.; Gashurov, G. Hydrothermal Preparation of Two-Component Solid Solutions from II–VI Compounds. J. Electrochem. Soc. 1960, 107, 1–27. [Google Scholar] [CrossRef]
- Tonkacheev, D.; Vikentyev, I.; Vymazalova, A.; Merkulova, M.; Trigub, A.; Kovalchuk, E.; Makeyev, A.; Osadchii, V. The chemical state of Hg in synthetic crystals of zinc and mercury sulfides studied by XAFS spectroscopy. J. Solid State Chem. 2022, 305, 122708. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhai, D.G.; Dai, H.Z.; de Fourestier, J.; Yu, C.; Gu, X.P.; Wang, Y.H.; Yu, H.; Wang, J.P.; Liu, Z.J. Nanoscale characterization of Au2Te grains from the Sandaowanzi gold deposit, northeast China. Can. Miner. 2017, 55, 181–194. [Google Scholar] [CrossRef]
- Tauson, V.L.; Ab Ramovih, M.G. A Study of the System ZnS-HgS by the Hydrothermal Method. Geochem.Int. 1980, 17, 117–128. [Google Scholar]
- Din, A.; Benvenuti, M.; Lattanzi, P.; Tanelli, G. Mineral assemblages in the Hg-Zn-(Fe)-S system at Levigliani, Tuscany, Italy. Eur. J. Miner. 1995, 7, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Boctor, N.; Shieh, Y.; Kullerud, G. Mercury ores from the New Idria Mining District, California: Geochemical and stable isotope studies. Geochim. Cosmochim. Acta 1987, 51, 1705–1715. [Google Scholar] [CrossRef]
- Velebil, D.; Zacharias, J. Fluid inclusion study of the Horní Luby cinnabar deposit, Saxothuringian Zone, Bohemian Massif: Clues for the metamorphic remobilization of mercury. J. Geosci. 2013, 58, 283–298. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Liu, J.J.; Li, Y.S.; de Fourestier, J.; Wang, D.Z.; Zhai, D.G.; Yu, X.F.; Lü, X.; Li, X.F. Mineral paragenesis of the Anfangba gold deposit, western Qinling Orogen, China: Implication for coupled dissolution-reprecipitation reactions and the liquid bismuth collector model. Ore Geol. Rev. 2021, 139, 104502. [Google Scholar] [CrossRef]
- Sung, Y.-H.; Brugger, J.; Ciobanu, C.; Pring, A.; Skinner, W.; Nugus, M. Invisible gold in arsenian pyrite and arsenopyrite from a multistage Archaean gold deposit: Sunrise Dam, Eastern Goldfields Province, Western Australia. Miner. Depos. 2009, 44, 765–791. [Google Scholar] [CrossRef]
- Brown, W.; Fyfe, W.; Turner, F. Aragonite in California glaucophane schists, and the kinetics of the aragonite—Calcite transformation. Can. J. Chem. 1962, 3, 566–582. [Google Scholar] [CrossRef]
- Putnis, A. Mineral Replacement Reactions. Rev. Miner. Geochem. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Liu, J.J.; Wang, D.Z.; Zhai, D.G.; Xia, Q.; Zheng, B.; Gao, S.; Zhong, R.C.; Zhao, S.J. Super-enrichment mechanisms of precious metals by low-melting point copper-philic element (LMCE) melts. Acta Petrol. Sin. 2021, 37, 2629–2656, (In Chinese with English abstract). [Google Scholar]
- Boctor, N.; Kullerud, G. Mercury selenide stoichiometry and phase relations in the mercury-selenium system. J. Solid State Chem. 1986, 62, 177–183. [Google Scholar] [CrossRef]
- Sharma, R.C.; Chang, Y.A. Thermodynamic analysis and phase equilibria calculations for the Zn-Te, Zn-Se and Zn-S systems. J. Cryst. Growth. 1988, 88, 193–204. [Google Scholar] [CrossRef]
- Beaudoin, G. Acicular sphalerite enriched in Ag, Sb, and Cu embedded within color-banded sphalerite from the Kokanee Range, British Columbia, Canada. Can. Miner. 2000, 38, 1387–1398. [Google Scholar] [CrossRef]
- Cook, N.J.; Etschmann, B.; Ciobanu, C.L.; Geraki, K.; Howard, D.L.; Williams, T.; Rae, N.; Pring, A.; Chen, G.; Johannessen, B. Distribution and substitution mechanism of Ge in a Ge-(Fe)-bearing sphalerite. Minerals 2015, 5, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.; Barnes, H. Sphalerite-wurtzite equilibria and stoichiometry. Geochim. Cosmochim. Acta 1972, 36, 1275–1295. [Google Scholar] [CrossRef]
- Shore, M.; Fowler, A.D. Oscillatory zoning in minerals: A common phenomenon. Can Miner. 1996, 34, 1111–1126. [Google Scholar]
- Elardo, S.M.; Shearer, C.K. Magma chamber dynamics recorded by oscillatory zoning in pyroxene and olivine phenocrysts in basaltic lunar meteorite Northwest Africa 032. Am. Miner. 2014, 99, 355–368. [Google Scholar] [CrossRef]
- Boctor, N.; Brady, J.B. Interdiffusion of S and Se in tiemannite. Am. Miner. 1980, 65, 797–799. [Google Scholar]
- Li, W.; Li, B.; Gu, X.; Huang, Z.; Xiao, D.; Cheng, W.; Chen, C.; Dong, S. Fluid inclusion evidences for the mineralization of the organic fluid in the Yanwuping mercury deposit, Guizhou. Earth Sci. Front. 2013, 20, 72–81, (In Chinese with English abstract). [Google Scholar]
- Simon, G.; Essene, E.J. Phase relations among selenides, sulfides, tellurides, and oxides; I, Thermodynamic properties and calculated equilibria. Econ. Geol. 1996, 91, 1183–1208. [Google Scholar] [CrossRef]
- Xu, W.; Fan, H.; Hu, F.; Santosh, M.; Yang, K.; Lan, T.; Wen, B. Gold mineralization in the Guilaizhuang deposit, southwestern Shandong Province, China: Insights from phase relations among sulfides, tellurides, selenides and oxides. Ore Geol. Rev. 2014, 56, 276–291. [Google Scholar] [CrossRef]




| No. | Hg | Fe | Zn | Cd | Se | S | Total | Ore Occurrence Deposit | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 85.54 | 0.04 | 12.67 | 98.25 | Zhifang, Guizhou, China | Chen [15] | |||
| 85.58 | 0.06 | 12.41 | 98.05 | ||||||
| 2 | 84.06 | 0.01 | 2.00 | 12.51 | 98.58 | Dadongla, Guizhou, China | Chen [15] | ||
| 82.67 | 1.07 | 1.00 | 14.80 | 99.54 | |||||
| 3 | 77.96 | 0.06 | 5.70 | 14.92 | 98.64 | Chatian, Guizhou, China | Chen [15] | ||
| 76.57 | 6.13 | 15.50 | 98.2 | ||||||
| 77.24 | 0.04 | 6.57 | 14.96 | 98.81 | |||||
| 4 | 4.64 | Zhifang, Guizhou, China | Zeng [16] | ||||||
| 5 | 83.64 | 6.52 | 90.16 | Jiudiantang, Hunan, China | Bao [17] | ||||
| 6 | 84.04 | 4.23 | 11.02 | 99.29 | Baojing, Hunan, China | Huang [14] | |||
| 7 | 77.42 | 0.02 | 19.04 | 4.20 | 100.68 | Dongping, Hunan, China | Liu [13] | ||
| 76.88 | 0.01 | 15.25 | 6.35 | 98.49 | |||||
| 76.81 | 0.07 | 17.60 | 4.14 | 98.62 | |||||
| 80.18 | 0.02 | 0.17 | 14.63 | 6.44 | 101.44 | ||||
| 78.92 | 0.05 | 0.05 | 14.66 | 6.62 | 100.3 | ||||
| 78.58 | 0.02 | 13.24 | 7.20 | 99.04 | |||||
| 78.30 | 0.01 | 0.19 | 14.69 | 6.58 | 99.77 | ||||
| 78.43 | 0.02 | 0.09 | 0.02 | 15.26 | 6.32 | 100.14 | |||
| 79.33 | 0.04 | 0.04 | 14.64 | 6.71 | 100.76 | ||||
| 78.37 | 0.05 | 0.04 | 13.76 | 7.04 | 99.26 | ||||
| 80.31 | 0.02 | 0.19 | 13.29 | 7.19 | 101 | ||||
| 77.95 | 0.02 | 0.05 | 14.86 | 6.47 | 99.35 | ||||
| 79.38 | 0.03 | 0.13 | 13.54 | 7.16 | 100.24 | ||||
| 79.18 | 0.02 | 0.19 | 0.01 | 13.87 | 6.81 | 100.08 | |||
| 79.73 | 13.91 | 6.64 | 100.28 | ||||||
| 80.1 | 0.02 | 0.13 | 0.01 | 13.24 | 7.13 | 100.63 | |||
| 81.34 | 0.03 | 1.08 | 0.05 | 8.59 | 9.28 | 100.37 | |||
| 83.97 | 0.59 | 0.01 | 6.81 | 10.32 | 101.7 | ||||
| 77.2 | 0.02 | 0.22 | 19.21 | 4.34 | 100.99 | ||||
| 81.13 | 11.07 | 8.01 | 100.21 | ||||||
| 80.75 | 0.06 | 0.61 | 0.04 | 10.30 | 8.33 | 100.09 | |||
| 79.65 | 0.01 | 0.25 | 13.18 | 6.89 | 99.98 | ||||
| 79.65 | 0.04 | 0.26 | 0.06 | 11.22 | 7.98 | 99.21 | |||
| 77.76 | 0.02 | 0.08 | 13.95 | 6.58 | 98.39 | ||||
| 80.95 | 0.03 | 0.23 | 0.03 | 10.4 | 8.69 | 100.33 | |||
| 81 | 0.06 | 0.6 | 0.02 | 8.91 | 9.34 | 99.93 | |||
| 76.57 | 0.01 | 0.04 | 18.39 | 4.65 | 99.66 | ||||
| 78.92 | 0.13 | 16.35 | 5.47 | 100.87 | |||||
| 8 | 79.05 | 4.8 | 1.07 | 15.51 | 100.43 | Chauvai, Kyrgyzstan | Vasil’ev [11] | ||
| 79.3 | 4.46 | 0.75 | 15.29 | 99.8 | |||||
| 78.95 | 4.31 | 1.27 | 15.29 | 99.82 | |||||
| 9 | 56.43 | 10.35 | 13.98 | 0.26 | 17.8 | 98.82 | Murzinskoe | Vasil’ev [11] | |
| 56.47 | 10.7 | 14.1 | 0.27 | 18.19 | 99.73 | ||||
| 56.25 | 10.83 | 14.1 | 0.19 | 18.3 | 99.67 | ||||
| 56.42 | 10.24 | 13.74 | 0.3 | 18.04 | 98.74 | ||||
| 55.47 | 10.51 | 13.35 | 0.24 | 18.36 | 97.93 | ||||
| 10 * | 77.68 | 5.36 | 14.97 | 98.01 | Felsöbánya, Transylvania | Dickson [5] | |||
| 11 * | 79.73 | 4.23 | 1.08 | 14.58 | 99.62 | Guadalcazar, Mexico | Dickson [5] | ||
| 12 * | 79.69 | 1.04 | 3.32 | 14.97 | 99.02 | Pola de Lena, Spain | Dickson [5] | ||
| 13 * | 83.38 | 2.17 | 14.24 | 99.79 | Levigliani, Italy | Dickson [5] | |||
| 14 * | 81.83 | 6.49 | 10.3 | 98.62 | San Onofre, Mexico | Dickson [5] | |||
| 15 | 80.95 | 0.44 | 1.82 | 0.22 | 15.99 | 99.42 | Levigliani, Italy | Din [18] | |
| 82.06 | 0.3 | 2.13 | 0.12 | 16.13 | 100.74 | ||||
| 16 | 83.61 | 1.88 | 0.07 | 14.03 | 99.59 | Diablo Mountain, CA, USA | Boctor [19] | ||
| 83.14 | 2.15 | 14.34 | 99.63 | ||||||
| 84.67 | 0.96 | 0.15 | 14.29 | 100.07 | |||||
| 82.98 | 2.49 | 0.15 | 14.52 | 100.14 | |||||
| 82.12 | 0.13 | 0.23 | 6.15 | 10.18 | 98.81 | ||||
| 83.28 | 0.27 | 0.29 | 4.05 | 11.88 | 99.77 | ||||
| 84.28 | 0.38 | 0.25 | 2.91 | 12.11 | 99.93 | ||||
| 84.45 | 0.74 | 0.1 | 1.17 | 12.96 | 99.42 | ||||
| 85.39 | 0.66 | 0.2 | 0.5 | 13.81 | 100.56 |
| No. | 1-1 | 1-2 | 1-3 | 1-4 | 1-5 | 1-6 | 1-7 | 1-8 | 1-9 | 1-10 | 1-11 | 1-12 | 2-1 | 2-2 | 2-3 | 3-1 | 4-1 | 5-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin | Se-Mcin |
| Hg | 83.40 | 81.76 | 80.09 | 78.36 | 78.85 | 78.30 | 77.66 | 79.42 | 80.73 | 80.69 | 79.42 | 81.93 | 81.95 | 82.66 | 81.08 | 81.20 | 82.08 | 82.46 |
| S | 11.09 | 11.12 | 8.07 | 6.79 | 6.54 | 6.79 | 6.15 | 8.34 | 8.49 | 10.07 | 6.82 | 9.37 | 10.44 | 10.29 | 9.98 | 9.59 | 9.34 | 11.21 |
| Se | 5.20 | 5.37 | 10.42 | 14.06 | 14.63 | 13.89 | 14.77 | 10.53 | 10.32 | 7.09 | 14.05 | 8.37 | 6.42 | 6.08 | 6.94 | 7.71 | 8.57 | 5.36 |
| Zn | 1.17 | 1.00 | 0.25 | 0.17 | 0.21 | 0.08 | 0.21 | 0.25 | 0.28 | 0.67 | 0.19 | 0.41 | 0.85 | 0.26 | 0.66 | 0.50 | 0.41 | 1.05 |
| Total | 100.85 | 99.26 | 98.82 | 99.38 | 100.22 | 99.05 | 98.79 | 98.54 | 99.82 | 98.52 | 100.48 | 100.07 | 99.66 | 99.30 | 98.67 | 98.99 | 100.40 | 100.08 |
| No. | 5-2 | 6-1 | 6-2 | 7-1 | 8-1 | 9-1 | 10-1 | 11-1 | 11-2 | 11-3 | 11-4 | 11-5 | 11-6 | 11-7 | 11-8 | |||
| Type | Se-Mcin | Se-Mcin | Se-Mcin | n = 21 | Cin1-1 | Cin1-1 | Cin1-1 | Cin1-1 | n = 4 | Cin1-2 | Cin1-2 | Cin1-2 | Cin1-2 | Cin1-2 | Cin1-2 | Cin1-2 | Cin1-2 | n = 8 |
| Hg | 82.35 | 84.01 | 81.49 | 80.95 | 85.07 | 83.68 | 85.13 | 84.32 | 84.55 | 83.77 | 85.73 | 85.32 | 83.55 | 85.67 | 86.34 | 82.13 | 84.84 | 84.67 |
| S | 10.45 | 11.82 | 10.85 | 9.22 | 12.13 | 11.65 | 12.06 | 12.08 | 11.98 | 11.61 | 12.18 | 12.67 | 12.03 | 12.59 | 13.08 | 10.93 | 12.18 | 12.16 |
| Se | 6.38 | 2.79 | 5.55 | 8.78 | 1.76 | 3.11 | 2.44 | 2.51 | 2.46 | 3.75 | 2.23 | 0.66 | 2.88 | 1.81 | 0.74 | 5.43 | 2.66 | 2.52 |
| Zn | 0.73 | 0.19 | 0.76 | 0.49 | 0.30 | 0.04 | 0.28 | 0.08 | 0.18 | 0.16 | 0.27 | 0.23 | 0.07 | 0.08 | 0.08 | 1.05 | 0.04 | 0.25 |
| Total | 99.90 | 98.81 | 98.65 | 99.44 | 99.25 | 98.48 | 99.91 | 98.99 | 99.16 | 99.29 | 100.40 | 98.87 | 98.53 | 100.15 | 100.24 | 99.54 | 99.71 | 99.59 |
| No. | 12-1 | 13-1 | 14-1 | 15-1 | 16-1 | 16-2 | 17-1 | 18-1 | 19-1 | 20-1 | 21-1 | 22-1 | 23-1 | 24-1 | 25-1 | |||
| Type | Cin2 | Cin2 | Cin2 | Cin2 | Cin2 | Cin2 | Cin2 | Cin2 | Cin2 | n = 9 | Hg-Sp | Hg-Sp | Hg-Sp | Hg-Sp | Hg-Sp | Hg-Sp | n = 6 | |
| Hg | 87.22 | 86.52 | 86.13 | 86.48 | 85.61 | 85.55 | 84.68 | 86.36 | 85.33 | 85.99 | 26.85 | 33.31 | 1.50 | 14.43 | 17.98 | 24.77 | 19.80 | |
| S | 13.18 | 12.79 | 13.16 | 13.25 | 13.09 | 13.01 | 14.87 | 12.93 | 13.16 | 13.27 | 29.42 | 29.41 | 32.22 | 31.79 | 31.09 | 30.60 | 30.76 | |
| Se | 0.43 | 0.66 | 0.31 | 0.38 | 0.31 | 0.69 | 0.19 | 0.21 | 0.24 | 0.38 | 0.38 | 0.31 | bd | bd | 0.01 | bd | 0.23 | |
| Zn | 0.26 | 0.20 | 0.20 | 0.24 | 0.18 | 0.28 | 0.23 | 0.30 | 0.03 | 0.21 | 42.78 | 38.88 | 63.28 | 54.29 | 51.36 | 44.03 | 49.10 | |
| Total | 101.08 | 100.16 | 99.81 | 100.35 | 99.20 | 99.52 | 99.97 | 99.80 | 98.76 | 99.85 | 99.42 | 101.91 | 97.00 | 100.52 | 100.44 | 99.41 | 99.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, J.; Carranza, E.J.M.; Zhai, D.; Zhao, Q.; Weng, G.; Zhang, B. Characteristics and Formation Conditions of Se-Bearing Metacinnabar in the Wanshan Mercury Ore Field, Eastern Guizhou. Minerals 2023, 13, 173. https://doi.org/10.3390/min13020173
Wang X, Liu J, Carranza EJM, Zhai D, Zhao Q, Weng G, Zhang B. Characteristics and Formation Conditions of Se-Bearing Metacinnabar in the Wanshan Mercury Ore Field, Eastern Guizhou. Minerals. 2023; 13(2):173. https://doi.org/10.3390/min13020173
Chicago/Turabian StyleWang, Xiao, Jiajun Liu, Emmanuel John M. Carranza, Degao Zhai, Qingqing Zhao, Guoming Weng, and Bin Zhang. 2023. "Characteristics and Formation Conditions of Se-Bearing Metacinnabar in the Wanshan Mercury Ore Field, Eastern Guizhou" Minerals 13, no. 2: 173. https://doi.org/10.3390/min13020173
APA StyleWang, X., Liu, J., Carranza, E. J. M., Zhai, D., Zhao, Q., Weng, G., & Zhang, B. (2023). Characteristics and Formation Conditions of Se-Bearing Metacinnabar in the Wanshan Mercury Ore Field, Eastern Guizhou. Minerals, 13(2), 173. https://doi.org/10.3390/min13020173







