Effect of Al2O3 on the Structural Properties of Water-Quenched Copper Slag Related to Pozzolanic Activity
Abstract
1. Introduction
2. Materials and Methods
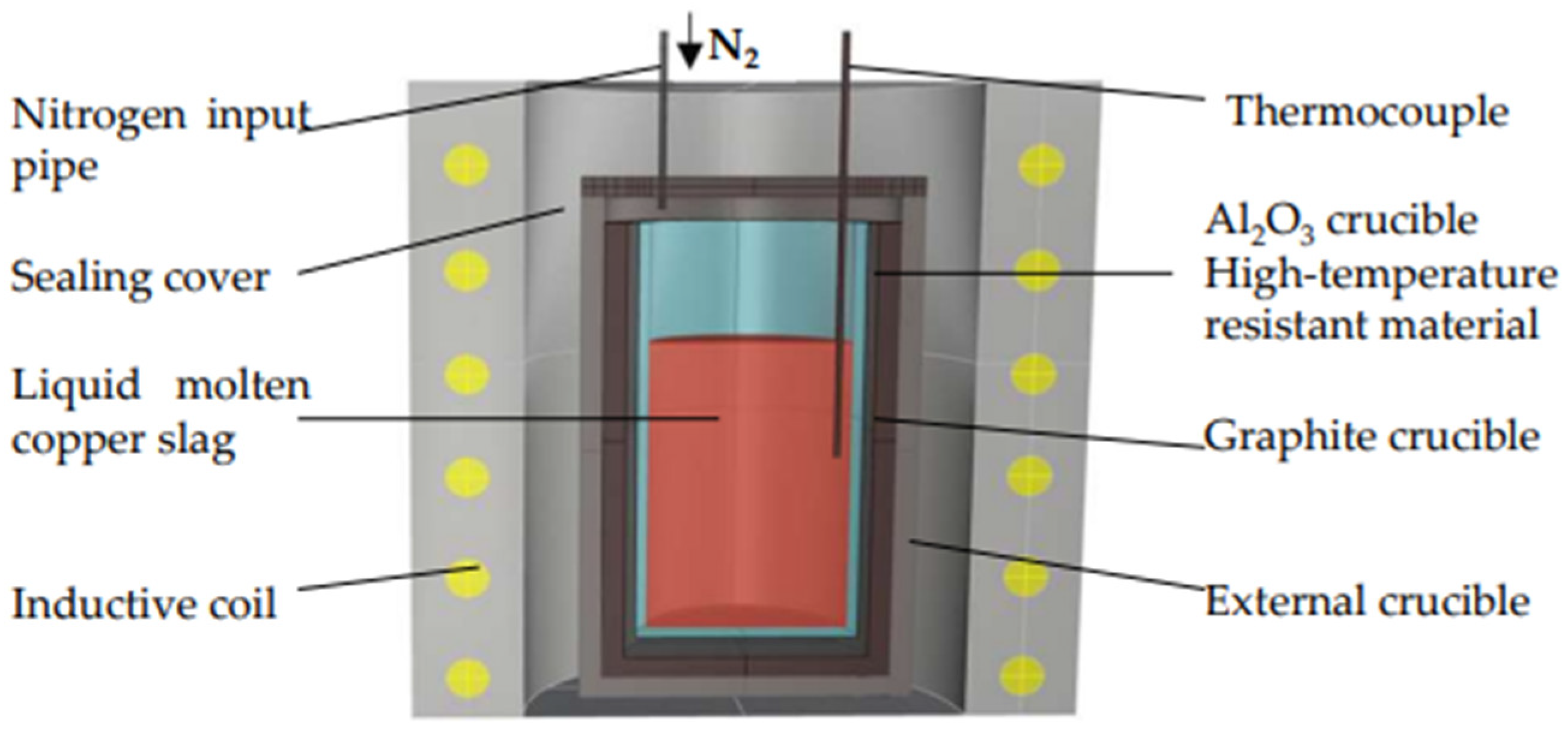
2.1. Preparation of WCS
2.2. Characterization and Tests
3. Experimental Results and Analysist
3.1. Amorphous Thermal Stability Analysis
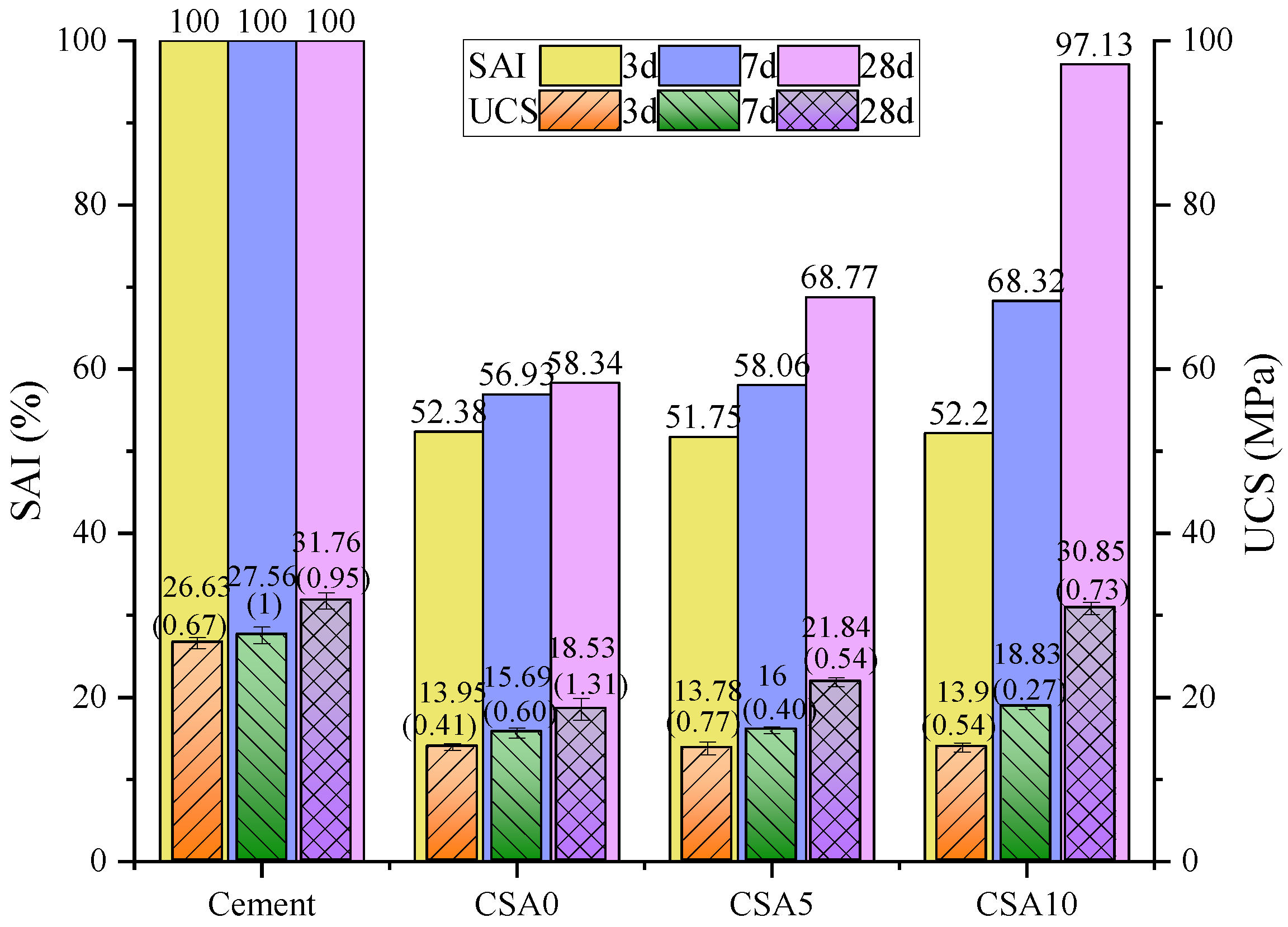
3.2. The Analysis of SAI and UCS of Blended Cement Paste
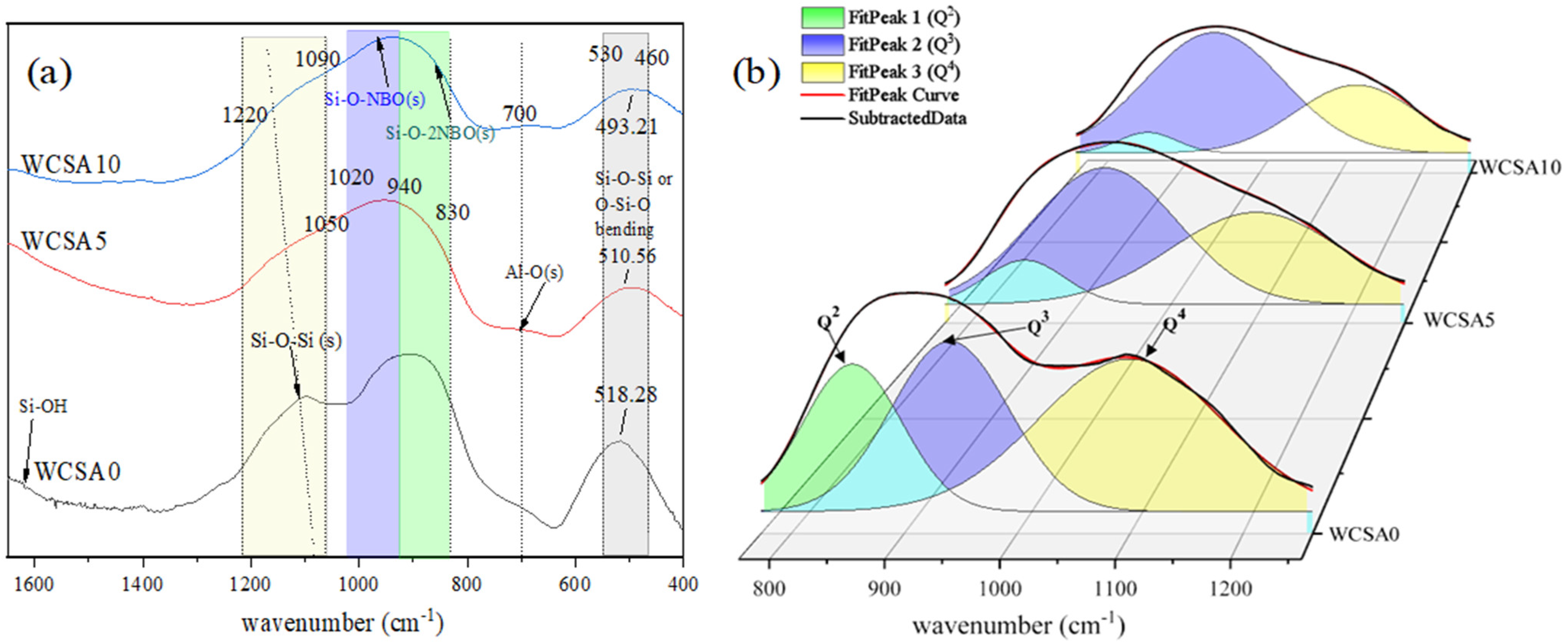
3.3. FTIR Spectroscopy
- (1)
- Changes in the chain structure
- (2)
- The changes in the percent of Qn
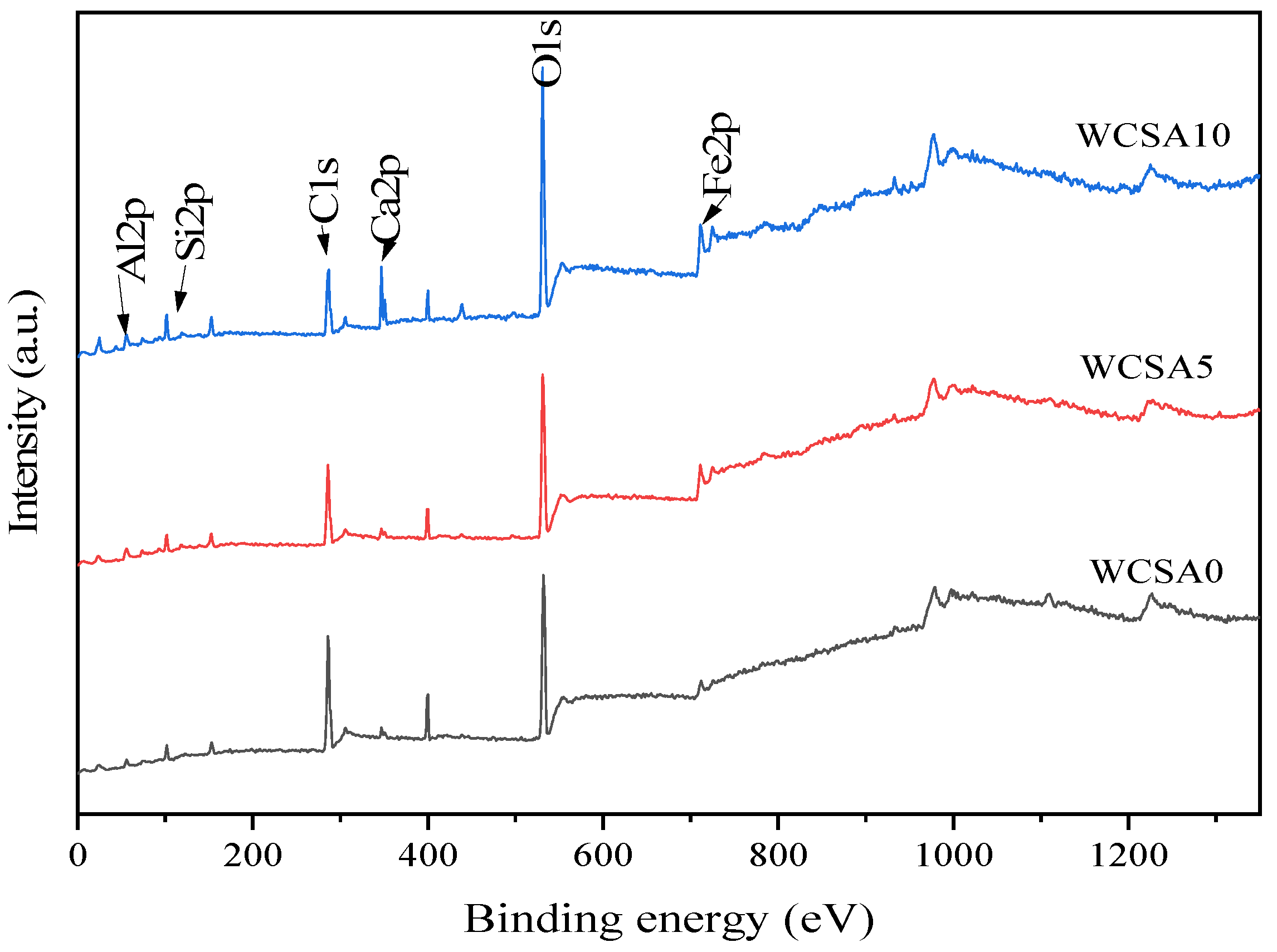
3.4. XPS Result
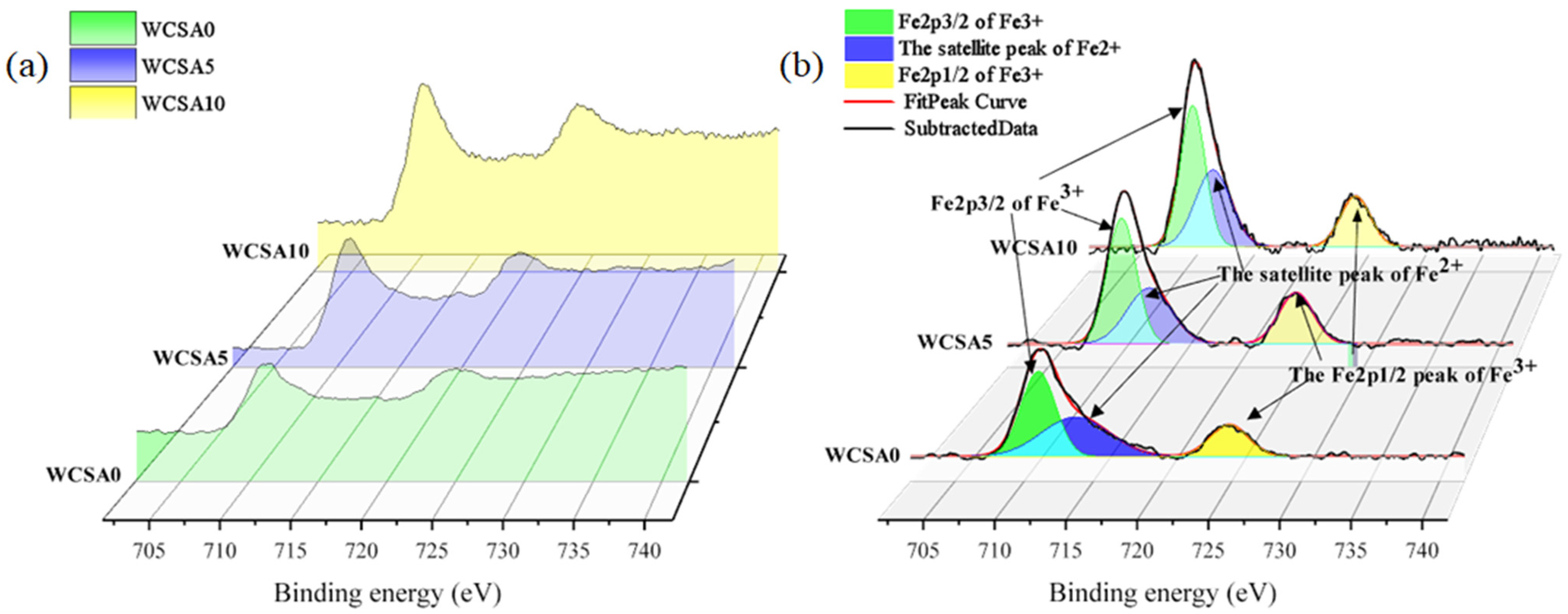
3.4.1. Fe2p
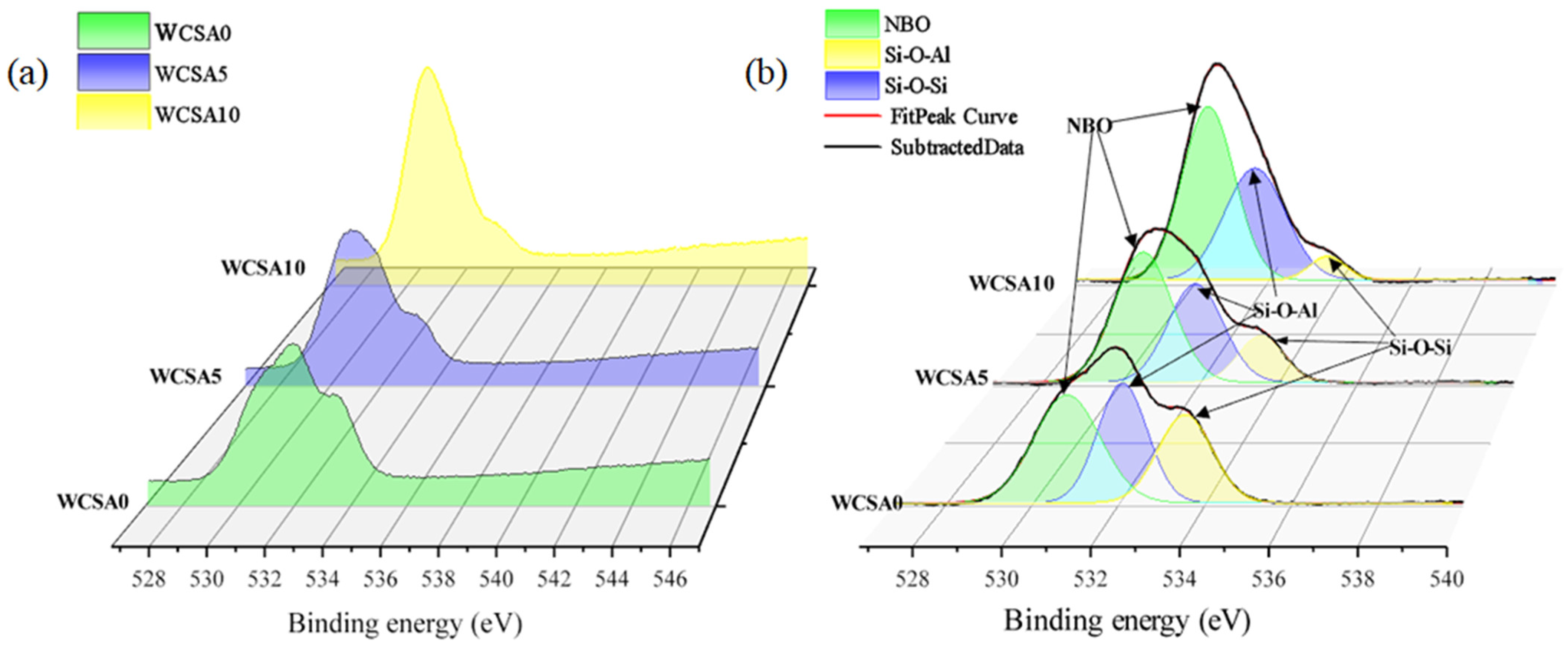
3.4.2. O1s
3.4.3. Si2p
4. Conclusions
- (1)
- The results of the XRD and DSC experiments illustrate that the WCS contained an abundant glass phase. In the DSC heating curve, the changes in the thermal stability parameter S and the enthalpy (ΔHc) show that the effect of alumina content on the thermal stability of the glass phase in the copper slag first decreases and then increases.
- (2)
- The uniaxial compressive test and SAI results show that the influence of Al2O3 content on the uniaxial compressive strength and the strength activity index (SAI) of blended cement pastes is related to the curing age. When the age is 3 days, the alumina’s addition hardly affects the pastes’ strength. When the age is 7 and 28 days, the uniaxial compressive strength and SAI of blended cement pastes are proportional to the Al2O3 content, and the strength growth of blended cement pastes showed a “the retardation of strength development”.
- (3)
- The FTIR spectrum showed that Q3 increases at the expense of Q2 and Q4 with the increase in the Al2O3 content. The O-Si-O or Si-O-Si bending vibration frequency moved to a low frequency and the number of Q4 decreased, indicating that the DP of the silicate network decreased with the increase in the Al2O3 content. The presence of the Al-O peak indicates that Al exists in the form of aluminum oxide tetrahedrons. The aluminum oxide tetrahedron replaces the silicon-oxygen tetrahedron in the silicate network to form aluminosilicate. It should be noted that, the results were obtained using FTIR, which may be more accurate using Raman spectroscopy.
- (4)
- The results of the XPS experiment show that aluminum-oxygen tetrahedrons substitute the silicon-oxygen tetrahedrons in the silicate network to form aluminosilicate. The addition of aluminum reduces the DP of the silicate network and enhances the pozzolanic activity of WCS. Fe act as a modifier in the silicate network.
- (5)
- This study shows that WCS can partially replace cement and reduce the amount of cement. In order to further study the effect of alumina, the different hydration products of blended cement pastes with different alumina contents will be investigated in follow-up research.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Tao, Y.; Feng, Y.; Zhang, Q.; Liu, Y. Utilization of modified copper slag activated by Na2SO4 and CaO for unclassified lead/zinc mine tailings based cemented paste backfill. J. Environ. Manag. 2021, 290, 112608. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, Q.; Zhou, Y.; Yang, Q.; Zhang, Q.; Jiang, L.; Guo, H. Modification of glass structure via CaO addition in granulated copper slag to enhance its pozzolanic activity. Constr. Build. Mater. 2020, 240, 117970. [Google Scholar] [CrossRef]
- Kierczak, J.; Potysz, A.; Pietranik, A.; Tyszka, R.; Modelska, M.; Néel, C.; Ettler, V.; Mihaljevič, M. Environmental impact of the historical Cu smelting in the Rudawy Janowickie Mountains (south-western Poland). J. Geochem. Explor. 2013, 124, 183–194. [Google Scholar] [CrossRef]
- Song, J.; Feng, S.; Xiong, R.; Ouyang, Y.; Zeng, Q.; Zhu, J.; Zhang, C. Mechanical properties, pozzolanic activity and volume stability of copper slag-filled cementitious materials. Mater. Sci. Medzg. 2020, 26, 218–224. [Google Scholar] [CrossRef]
- Jiao, H.; Chen, W.; Wu, A.; Yu, Y.; Ruan, Z.; Honaker, R.; Chen, X.; Yu, J. Flocculated unclassified tailings settling efficiency improvement by particle collision optimization in the feedwell. Int. J. Miner. Metall. Mater. 2022, 29, 2126–2135. [Google Scholar] [CrossRef]
- Nazer, A.; Payá, J.; Borrachero, M.V.; Monzó, J. Use of ancient copper slags in Portland cement and alkali activated cement matrices. J. Environ. Manag. 2016, 167, 115–123. [Google Scholar] [CrossRef]
- Mirhosseini, S.R.; Fadaee, M.; Tabatabaei, R.; Fadaee, M.J. Mechanical properties of concrete with Sarcheshmeh mineral complex copper slag as a part of cementitious materials. Constr. Build. Mater. 2017, 134, 44–49. [Google Scholar] [CrossRef]
- He, R.X.; Zhang, S.Y.; Zhang, X.L.; Zhang, Z.H.; Zhao, Y.L.; Ding, H.X. Copper slag: The leaching behavior of heavy metals and its applicability as a supplementary cementitious material. J. Environ. Chem. Eng. 2021, 9, 105132. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Chen, Q.; Kero, J.; Andersson, A.; Ahmed, H.; Engström, F.; Samuelsson, C. Characterization and evaluation of the pozzolanic activity of granulated copper slag modified with CaO. J. Clean. Prod. 2019, 232, 1112–1120. [Google Scholar] [CrossRef]
- Dhengare, S.; Sharma, R.L.; Sobti, J.; Gajbhiye, A.R.; Bhagat, R. Binary concrete expansion by means of copper slag, fly ash, sugarcane bagasse ash, and rice husk ash as partial replacement of cement. Int. J. Adv. Sci. Technol. 2020, 29, 9973–9993. [Google Scholar]
- Black, L.; Stumm, A.; Garbev, K.; Stemmermann, P.; Hallam, K.R.; Allen, G.C. X-ray photoelectron spectroscopy of aluminium-substituted tobermorite. Cem. Concr. Res. 2005, 35, 51–55. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Q.; Feng, Y.; Chen, Q.; Xiao, C.; Li, H.; Xiang, Y.; Qi, C. Hydration and mechanical properties of blended cement with copper slag pretreated by thermochemical modification. Materials 2022, 15, 3477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, B.; Feng, Y.; Qi, C.; Chen, Q.; Xiao, C. Hydration development of blended cement paste with granulated copper slag modified with CaO and Al2O3. J. Mater. Res. Technol. 2022, 18, 909–920. [Google Scholar] [CrossRef]
- Erdemoğlu, M.; Birinci, M.; Uysal, T. Alumina Production from Clay Minerals: Current Reviews. J. Polytech. 2018, 21, 387–396. [Google Scholar] [CrossRef]
- Kashcheev, I.D.; Zemlyanoi, K.G.; Stepanova, K.O. Acidic Methods of Alumina Production (Review). Refract. Ind. Ceram. 2019, 60, 237–242. [Google Scholar] [CrossRef]
- Klym, H.; Karbovnyk, I.; Piskunov, S.; Popov, A.I. Positron Annihilation Lifetime Spectroscopy Insight on Free Volume Conversion of Nanostructured MgAl2O4 Ceramics. Nanomaterials 2021, 11, 3373. [Google Scholar] [CrossRef]
- Bechar, S.; Zerrouki, D. Effect of natural pozzolan on the fresh and hardened cement slurry properties for cementing oil well. World J. Eng. 2018, 15, 513–519. [Google Scholar] [CrossRef]
- Feng, Y.; Kero, J.; Yang, Q.; Chen, Q.; Engström, F.; Samuelsson, C.; Qi, C. Mechanical activation of granulated copper slag and its influence on hydration heat and compressive strength of blended cement. Materials 2019, 12, 772. [Google Scholar] [CrossRef]
- de Rojas, M.I.S.; Rivera, J.; Frías, M.; Marín, F. Use of recycled copper slag for blended cements. J. Chem. Technol. Biotechnol. 2008, 83, 209–217. [Google Scholar] [CrossRef]
- Mucsi, G.; Kumar, S.; Csőke, B.; Kumar, R.; Molnár, Z.; Rácz, Á.; Mádai, F.; Debreczeni, Á. Control of geopolymer properties by grinding of land filled fly ash. Int. J. Miner. Process. 2015, 143, 50–58. [Google Scholar] [CrossRef]
- GB/T 12957-2005; Test Method for Activity of Industrial Waste Slag Used as Addition to Cement. China National Intellectual Property Administration: Beijng, China, 2005.
- Chen, Q.; Sun, S.; Wang, Y.; Zhang, Q.; Zhu, L.; Liu, Y. In-situ remediation of phosphogypsum in a cement-free pathway: Utilization of ground granulated blast furnace slag and NaOH pretreatment. Chemosphere 2023, 313, 137412. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, L.; Wang, Y.; Chen, J.; Qi, C. The carbon uptake and mechanical property of cemented paste backfill carbonation curing for low concentration of CO2. Sci. Total Environ. 2022, 852, 158516. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, Q.; Wang, Y.; Zhang, Q.; Liu, Y. Highly-efficient fluoride retention in on-site solidification/stabilization of phosphogypsum: Cemented paste backfill synergizes with poly-aluminum chloride activation. Chemosphere 2022, 309, 136652. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dai, S.; Zhou, L.; Zhang, J.; Tian, W.; Xu, J. Viscosity and structure of MgO–SiO2-based slag melt with varying B2O3 content. Ceram. Int. 2020, 46, 3631–3636. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Chai, L.Y.; Peng, B.; Liang, Y.J.; He, Y.; Yan, Z.H. Arsenic vitrification by copper slag based glass: Mechanism and stability studies. J. Non-Cryst. Solids 2017, 466–467, 21–28. [Google Scholar] [CrossRef]
- Madhuri, J.H.; Chanakya, N.; Satyavardhan, D.; Ramesh, C.; Upender, G. XPS, FTIR, DSC and optical absorption investigations on 55B2O3–20ZnO–(25-x)Li2O–xBi2O3 (0≤x≤25 mol%) glass system. J. Non-Cryst. Solids 2023, 600, 82–98. [Google Scholar] [CrossRef]
- Liao, M.; Sun, H.; Wen, L.; Fang, Y.; Hu, L. Effect of alkali and alkaline earth fluoride introduction on thermal stability and structure of fluorophosphate glasses. Mater. Chem. Phys. 2006, 98, 154–158. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Zhang, L.; Liu, F.; Peng, B.; Chai, L.; Liu, D.; Liu, D.; Wang, T.; Liu, H.; et al. Formation mechanism of zinc-doped fayalite (Fe2-xZnxSiO4) slag during copper smelting. J. Hazard. Mater. 2019, 364, 488–498. [Google Scholar] [CrossRef]
- Imran, M.M.; Bhandari, D.; Saxena, N.S. Glass transition phenomena, crystallization kinetics and thermodynamic properties of ternary Se80Te20−xInx (x = 2, 4, 6, 8 and 10) semiconducting glasses: Theoretical and experimental aspects. Mater. Sci. Eng. 2000, 292, 56–65. [Google Scholar] [CrossRef]
- Yao, G.; Cui, T.; Zhang, J.; Wang, J.; Lyu, X. Effects of mechanical grinding on pozzolanic activity and hydration properties of quartz. Adv. Powder Technol. 2020, 31, 4500–4509. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Su, Y.; Tan, H.; Yang, J.; Lan, M.; Ma, M.; Strnadel, B. Self-hydration characteristics of ground granulated blast-furnace slag (GGBFS) by wet-grinding treatment. Constr. Build. Mater. 2018, 167, 96–105. [Google Scholar] [CrossRef]
- Liu, R.; Han, F.; Yan, P. Characteristics of two types of C-S-H gel in hardened complex binder pastes blended with slag. Sci. China Technol. Sci. 2013, 56, 1395–1402. [Google Scholar] [CrossRef]
- Zhao, Z.; Song, Y.; Min, X.; Liang, Y.; Chai, L.; Shi, M. XPS and FTIR studies of sodium arsenate vitrification by cullet. J. Non-Cryst. Solids 2016, 452, 238–244. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; A.Fanny, M.; Hassaan, M.Y.; ElBatal, H.A. Optical, FTIR and DC Conductivity of Soda Lime Silicate Glass Containing Cement Dust and Transition Metal Ions. Silicon 2015, 8, 443–453. [Google Scholar] [CrossRef]
- Hassaan, M.Y.; Saudi, H.A.; Saad, H.M.H.; Mostafa, A.G.; Ahmed, M.A.; Iida, Y.; Kubuki, S.; Nishida, T. Structural study of glass and glass ceramics prepared with Egyptian Basalt. Silicon 2015, 7, 383–391. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Li, N.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Rózycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Mansour, E. Semi-quantitative analysis for FTIR spectra of Al2O3-PbO-B2O3-SiO2 glasses. J. Non-Cryst. Solids 2012, 358, 454–460. [Google Scholar] [CrossRef]
- Nesbitt, H.; Bancroft, G.; Henderson, G.; Ho, R.; Dalby, K.; Huang, Y.; Yan, Z. Bridging, non-bridging and free (O2–) oxygen in Na2O-SiO2 glasses: An X-ray Photoelectron Spectroscopic (XPS) and Nuclear Magnetic Resonance (NMR) study. J. Non-Cryst. Solids 2011, 357, 170–180. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Shaharyar, Y.; Cheng, J.Y.; Han, E.; Maron, A.; Weaver, J.; Marcial, J.; McCloy, J.S.; Goel, A. Elucidating the effect of Iron speciation (Fe2+/Fe3+) on crystallization Kinetics of sodium aluminosilicate glasses. J. Am. Ceram. Soc. 2016, 99, 2306–2315. [Google Scholar] [CrossRef]
- Rada, S.; Dehelean, A.; Stan, M.; Chelcea, R.; Culea, E. Structural studies on iron–tellurite glasses prepared by sol–gel method. J. Alloy. Compd. 2011, 509, 147–151. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Sønderby, C.; Li, Z.; Søgaard, E.G. XPS and FT-IR investigation of silicate polymers. J. Mater. Sci. 2009, 44, 2079–2088. [Google Scholar] [CrossRef]
- Qing, M.; Yang, Y.; Wu, B.; Xu, J.; Zhang, C.; Gao, P.; Li, Y. Modification of Fe–SiO2 interaction with zirconia for iron-based Fischer–Tropsch catalysts. J. Catal. 2011, 279, 111–122. [Google Scholar] [CrossRef]
- Li, K.; Xue, D. Estimation of electronegativity values of elements in different valence states. J. Phys. Chem. A 2006, 110, 11332–11337. [Google Scholar] [CrossRef]
- Tognonvi, M.T.; Zidol, A.; Aïtcin, P.-C.; Tagnit-Hamou, A. Aging of glass powder surface. J. Non-Cryst. Solids 2015, 427, 175–183. [Google Scholar] [CrossRef]


| Sample | The Main Chemical Composition (wt.%) | Physical Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FeO | SiO2 | CaO | Fe2O3 | Al2O3 | MgO | ZnO | Cu2O | K2O | BET Surface Area (m2·g−1) | Density (g·cm−3) | |
| WCSA0 | 35.89 | 33.40 | 4.00 | 7.14 | 3.50 | 1.39 | 1.35 | 1.65 | - | 0.67 | 3.56 |
| WCSA5 | 33.91 | 32.7 | 3.9 | 9.1 | 6.6 | 0.82 | 1.02 | 0.68 | - | 0.67 | 3.49 |
| WCSA10 | 32.14 | 32.7 | 3.7 | 9.47 | 9.8 | 0.82 | 0.95 | 0.64 | - | 0.69 | 3.46 |
| PC | - | 18.1 | 62.1 | 2.8 | 4.9 | 1.2 | - | - | 2.3 | 0.47 | 3.08 |
| Sample | Tg (°C) | Tc (°C) | Tp (°C) | ΔT = Tc − Tg (°C) | S | ΔHc (J/g) |
|---|---|---|---|---|---|---|
| WCSA0 | 652.17 | 975.26 | 1026.17 | 323.09 | 25.22 | 869.24 |
| WCSA5 | 691.50 | 1073.18 | 1089.50 | 381.68 | 9.01 | 5782.6 |
| WCSA10 | — | 1081.50 | 1090.17 | — | — | 267.13 |
| Sample | Q2 | Q3 | Q4 | |||
|---|---|---|---|---|---|---|
| C | A | C | A | C | A | |
| WCSA0 | 854.4 | 23.0 | 940.6 | 32.6 | 1100.0 | 44.5 |
| WCSA5 | 858.1 | 9.1 | 943.0 | 51.0 | 1104.0 | 39.6 |
| WCSA10 | 861.8 | 4.7 | 944.1 | 63.7 | 1120.3 | 31.6 |
| Sample | Fe2p3/2 of Fe3+ | The Satellite Peak of Fe2+ | Fe2p1/2 of Fe3+ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C (eV) | FWHM (eV) | A (%) | C (eV) | FWHM (eV) | A (%) | C (eV) | FWHM (eV) | A (%) | |
| WCSA0 | 711.0 | 2.94 | 42.90 | 713.72 | 5.77 | 39.10 | 724.82 | 3.30 | 18.00 |
| WCSA5 | 710.84 | 2.70 | 46.37 | 713.03 | 4.12 | 31.43 | 724.64 | 3.19 | 22.20 |
| WCSA10 | 710.74 | 2.52 | 45.19 | 712.49 | 3.62 | 35.21 | 724.74 | 3.04 | 19.60 |
| Sample | NBO | Si-O-Al | Si-O-Si | BESi-O-Si-BENBO (eV) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C (eV) | FWHM (eV) | A (%) | C (eV) | FWHM (eV) | A (%) | C (eV) | FWHM (eV) | A (%) | ||
| WCSA0 | 530.82 | 1.82 | 40.14 | 532.11 | 1.35 | 33.13 | 533.56 | 1.48 | 26.73 | 2.74 |
| WCSA5 | 530.61 | 1.74 | 50.12 | 531.93 | 1.64 | 35.85 | 533.59 | 1.36 | 14.03 | 2.98 |
| WCSA10 | 530.42 | 1.70 | 54.38 | 531.71 | 1.97 | 40.52 | 533.71 | 1.17 | 5.10 | 3.29 |
| Sample | C (eV) | FWHM (eV) |
|---|---|---|
| WCSA0 | 101.8832 | 1.68478 |
| WCSA5 | 101.6424 | 1.69039 |
| WCSA10 | 101.5011 | 1.70077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Deng, D.; Feng, Y.; Wang, D.; Liu, B.; Chen, Q. Effect of Al2O3 on the Structural Properties of Water-Quenched Copper Slag Related to Pozzolanic Activity. Minerals 2023, 13, 174. https://doi.org/10.3390/min13020174
Zhang Q, Deng D, Feng Y, Wang D, Liu B, Chen Q. Effect of Al2O3 on the Structural Properties of Water-Quenched Copper Slag Related to Pozzolanic Activity. Minerals. 2023; 13(2):174. https://doi.org/10.3390/min13020174
Chicago/Turabian StyleZhang, Qinli, Dengwen Deng, Yan Feng, Daolin Wang, Bin Liu, and Qiusong Chen. 2023. "Effect of Al2O3 on the Structural Properties of Water-Quenched Copper Slag Related to Pozzolanic Activity" Minerals 13, no. 2: 174. https://doi.org/10.3390/min13020174
APA StyleZhang, Q., Deng, D., Feng, Y., Wang, D., Liu, B., & Chen, Q. (2023). Effect of Al2O3 on the Structural Properties of Water-Quenched Copper Slag Related to Pozzolanic Activity. Minerals, 13(2), 174. https://doi.org/10.3390/min13020174








