Studies on the Enrichment Feasibility of Rare Earth-Bearing Minerals in Mine Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tailings and Model Minerals
2.2. Flotation Reagents
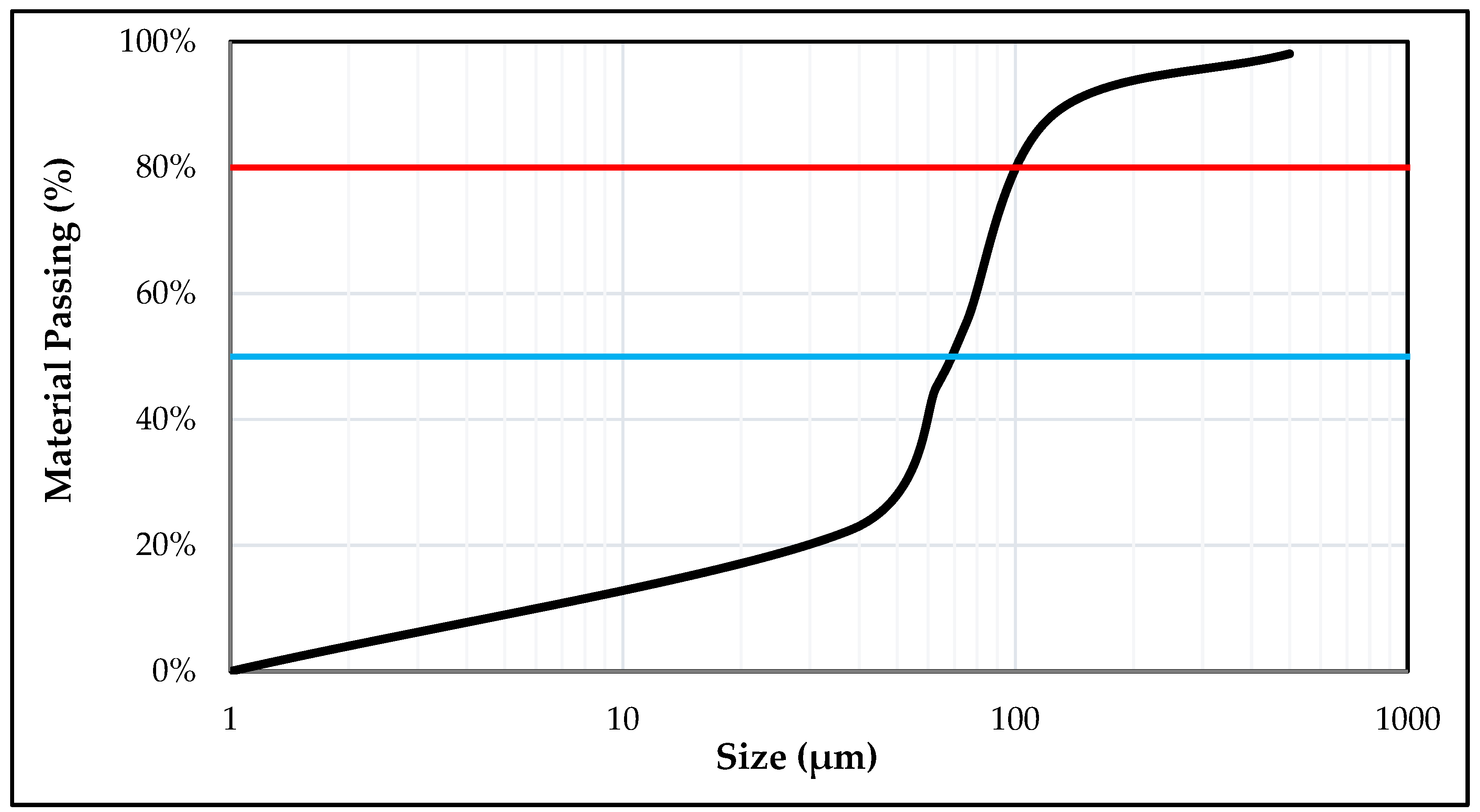
2.3. Grain Size Analysis/Sieve Analysis
2.4. Induced Coupled Plasma–Mass Spectrometry Analyses
2.5. X-ray Diffraction Studies
2.6. X-ray-Fluorescence Studies
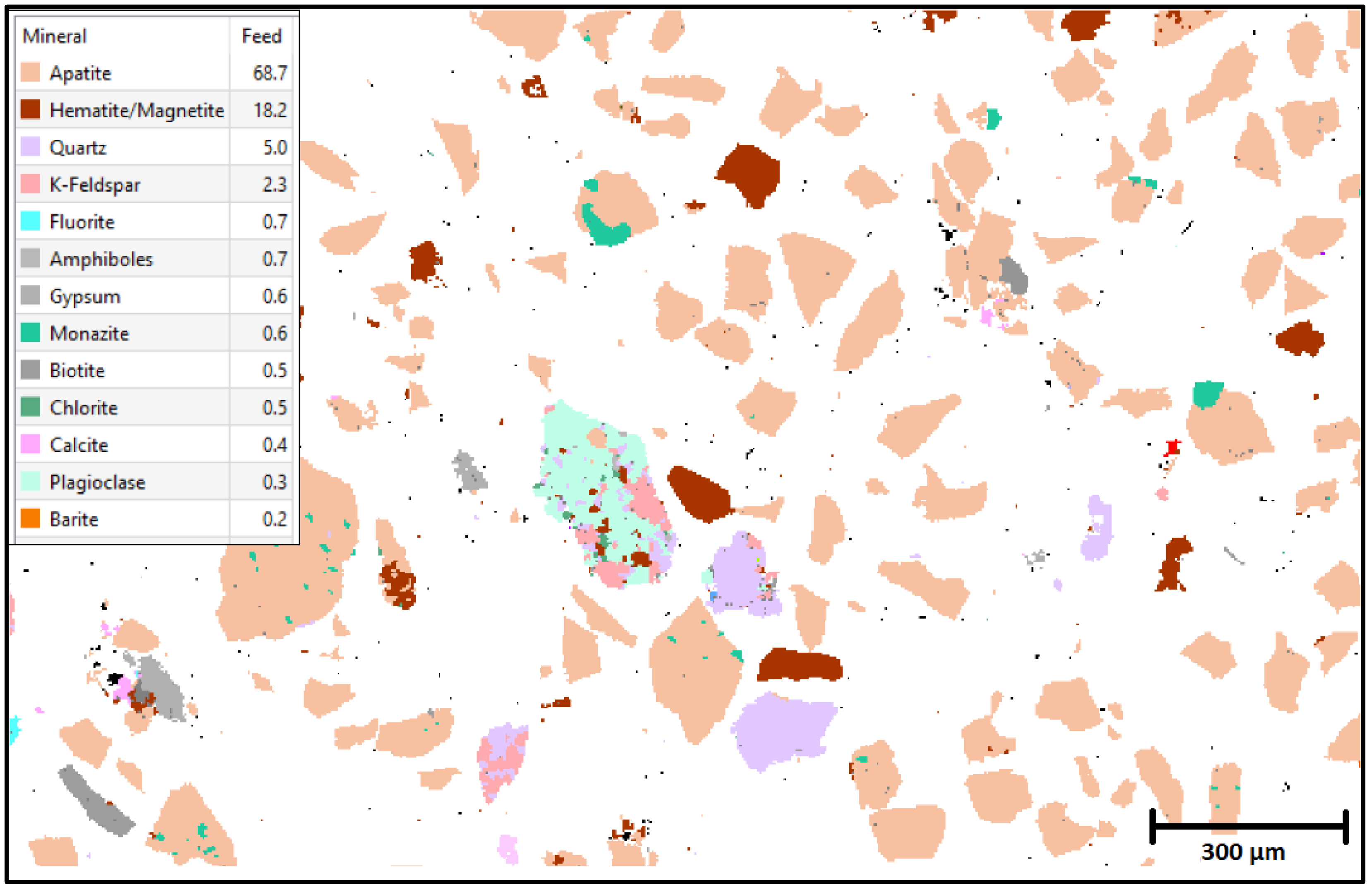
2.7. TESCAN’s Integrated Mineral Analysis Studies
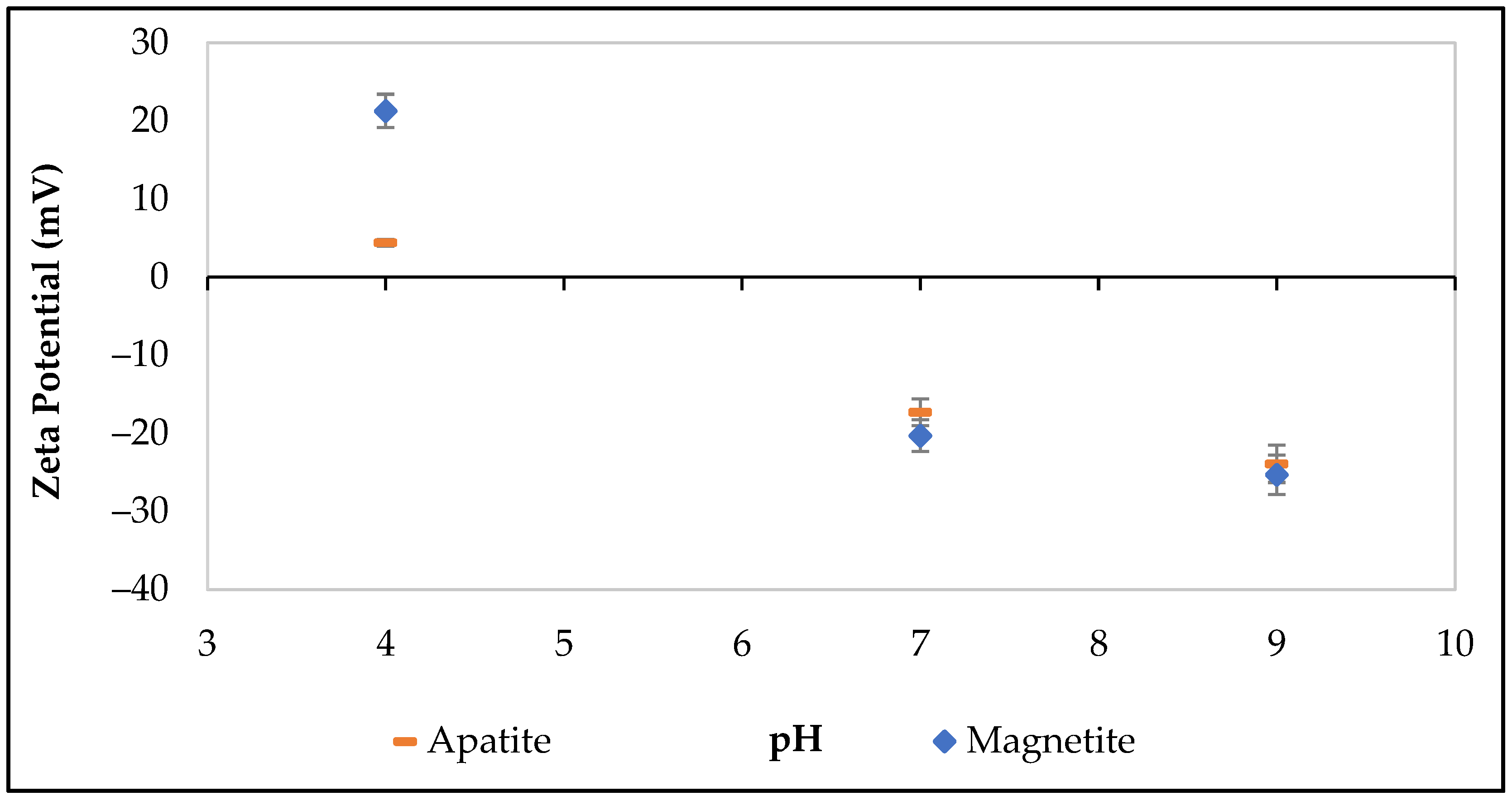
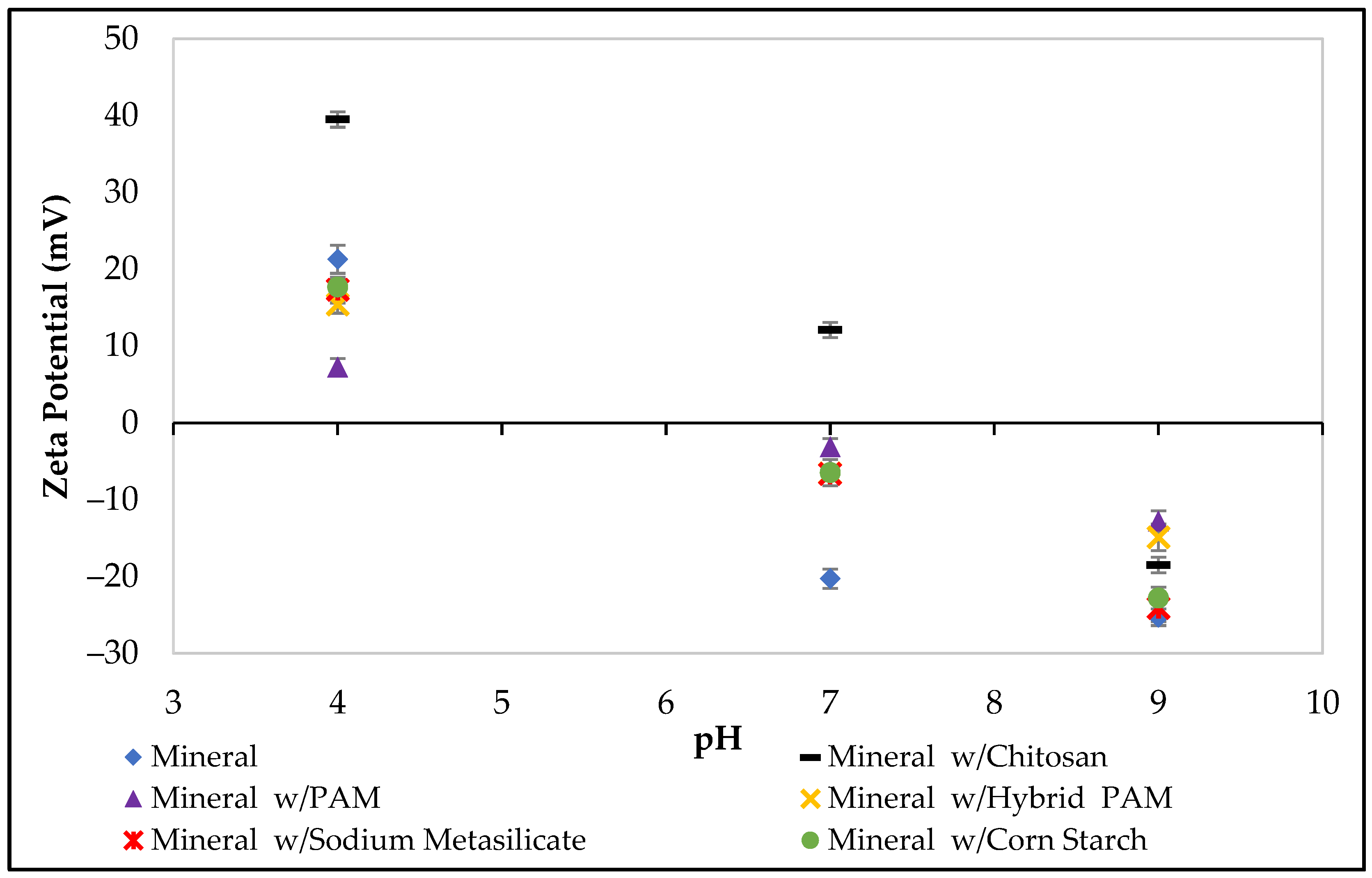
2.8. Zeta Potential Measurements
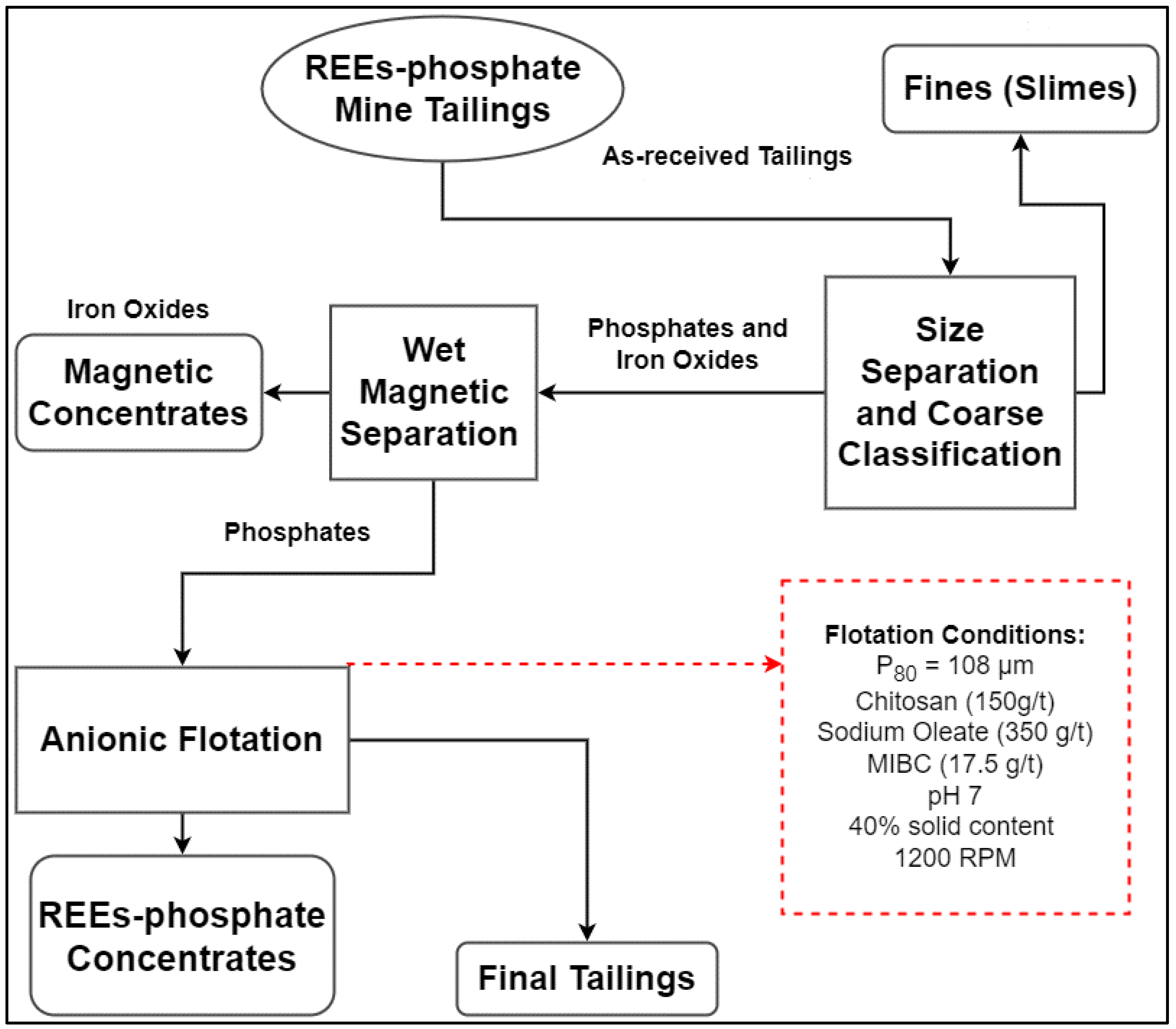
2.9. Batch Flotation Experiments
3. Results
3.1. Characterization Studies
3.2. Zeta Potential Experiments
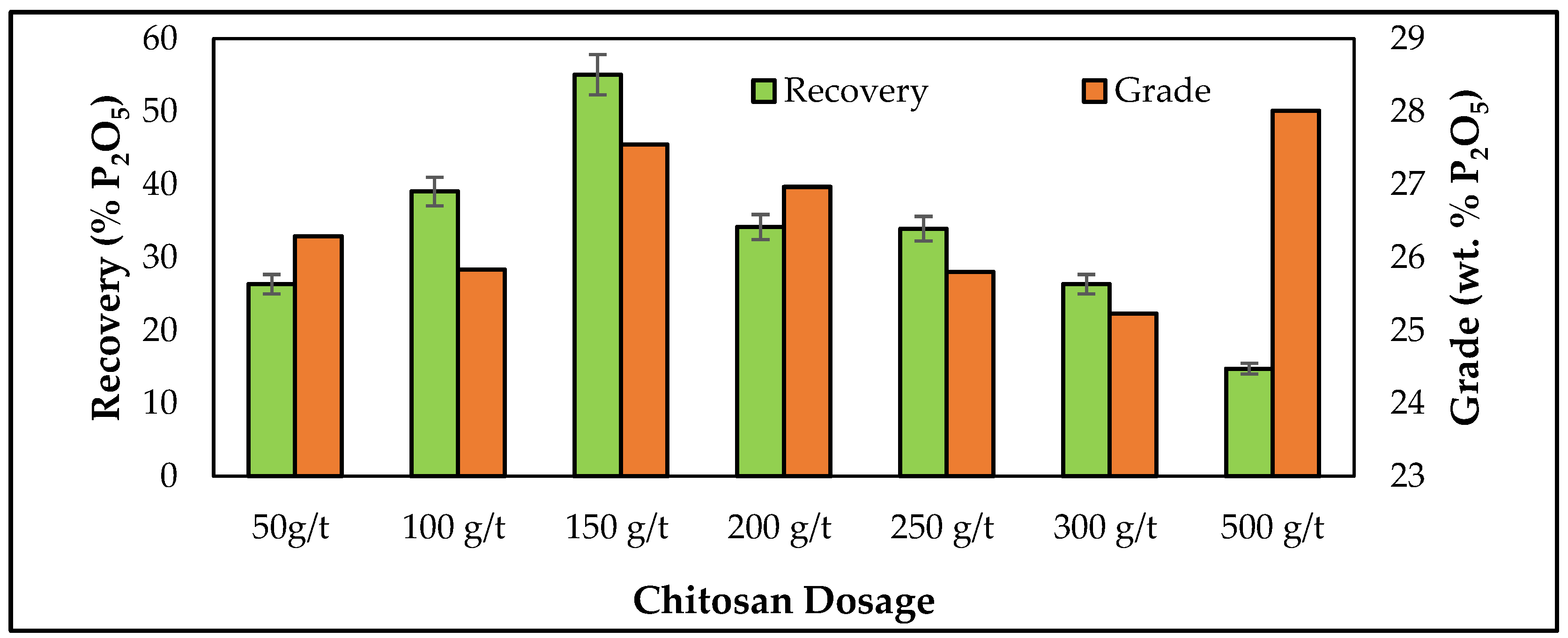
3.3. Batch Flotation Studies
3.4. Deportment Studies on REE Minerals in Flotation Products
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- DOE. Critical Materials Rare Earths Supply Chain: A Situational White Paper; DOE: Washington, DC, USA, 2020; pp. 1–21.
- DOE. Critical Materials Strategy; DOE: Washington, DC, USA, 2010; pp. 1–166.
- Peck, D. A Historical Perspective of Critical Materials, 1939 to 2006. In Critical Materials: Underlying Causes and Sustainable Mitigation Strategies; Erik Offerman, S., Ed.; World Scientific Publishing Co.: Hackensack, NJ, USA, 2019; Volume 5, pp. 85–101. [Google Scholar]
- Bobba, S.; Carrrara, S.; Mathieux, F.; Pavel, C. Critical Raw Materials for Strategic Technologies and Sectors in the EU: A Foresight Study; EU Publications: Luxembourg, 2020; pp. 1–100. [Google Scholar] [CrossRef]
- Agency for Natural Resources and Energy. Japan’s New International Resource Strategy to Secure Rare Metals/Special Contents—Energy Japan. Available online: https://www.enecho.meti.go.jp/en/category/special/article/detail_158.html (accessed on 21 September 2021).
- Geoscience Australia. Australia Critical Minerals. Available online: https://www.ga.gov.au/about/projects/resources/critical-minerals (accessed on 21 September 2021).
- Australia Government|Department of Industry, Science, Energy and Resources. The Opportunity for the Critical Minerals Sector. Available online: https://www.industry.gov.au/data-and-publications/australias-critical-minerals-strategy/the-opportunity-for-the-critical-minerals-sector (accessed on 21 September 2021).
- Seal, R.R.; Piatak, N.M. Environmental Attributes and Resource Potential of Mill Tailings from Diverse Mineral Deposit Types. In Proceedings of the Mine Water and Circular Economy; Wolkersdorfer, C., Sartz, L., Sillanpää MHäkkinen, A., Eds.; IMWA: Lappeenranta, Finland, 2017; pp. 603–609. [Google Scholar]
- USGS. USGS Rare Earths—Mineral Commodity Summaries 2020; USGS: Reston, VA, USA, 2020.
- Fuerstenau, M.C.; Han, K.N. Principles of Mineral Processing; Society for Mining, Metallurgy, and Exploration: Littleton, CO, USA, 2003; ISBN 0873351673. [Google Scholar]
- Abaka-Wood, G.B.; Zanin, M.; Addai-Mensah, J.; Skinner, W. Recovery of Rare Earth Elements Minerals from Iron Oxide–Silicate Rich Tailings—Part 1: Magnetic Separation. Miner. Eng. 2019, 136, 50–61. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Zanin, M.; Addai-Mensah, J.; Skinner, W. Recovery of Rare Earth Elements Minerals from Iron Oxide–Silicate Rich Tailings—Part 2: Froth Flotation Separation. Miner. Eng. 2019, 142, 105888. [Google Scholar] [CrossRef]
- Monyake, K.C.; Alagha, L. Enhanced Separation of Base Metal Sulfides in Flotation Systems Using Chitosan-Grafted-Polyacrylamides. Sep. Purif. Technol. 2021, 281, 119818. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. The Flotation Process Can Go Green. Processes 2019, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Wei, Q.; Jiao, F.; Qin, W. Utilization of Polyepoxysuccinic Acid as the Green Selective Depressant for the Clean Flotation of Phosphate Ores. J. Clean. Prod. 2021, 282, 124532. [Google Scholar] [CrossRef]
- Leonida, C. Froth Flotation for the 21st Century: Engineering, Geology, Mineralogy, Metallurgy, Chemistry, Etc. Eng. Min. J. 2019, 220, 58–64. [Google Scholar]
- Satur, J.V.; Calabia, B.P.; Hoshino, M.; Morita, S.; Seo, Y.; Kon, Y.; Takagi, T.; Watanabe, Y.; Mutele, L.; Foya, S. Flotation of Rare Earth Minerals from Silicate-Hematite Ore Using Tall Oil Fatty Acid Collector. Miner. Eng. 2016, 89, 52–62. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. Review of Flotation and Physical Separation of Rare Earth Element Minerals. In Proceedings of the 4th UMaT Biennial International Mining and Mineral Conference, Tarkwa, Ghana, 4 August 2016; Volume 4, pp. 55–62. [Google Scholar]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A Review of the Beneficiation of Rare Earth Element Bearing Minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Andrews, W.H.; Collins, D.N.; Hollick, C.T. The Flotation of Rare Earths-A Contribution to Industrial Hygiene. In The Mineral Industry in New Zealand: Proceedings of the AusIMM Annual Conference; AusIMM: Rotorua, New Zealand, 1990. [Google Scholar]
- Pradip. The Surface Properties and Flotation of Rare-Earth Minerals. Ph.D. Thesis, University of California, Berkeley, CA, USA, 1981. [Google Scholar]
- Anderson, C.D.; Taylor, P.R.; Anderson, C.G. Rare Earth Flotation Fundamentals: A Review. In IMPC 2016: XXVIII International Mineral Processing Congress Proceedings; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2016; pp. 1–15. [Google Scholar]
- Zhang, B.; Liu, C.; Li, C.; Jiang, M. A Novel Approach for Recovery of Rare Earths and Niobium from Bayan Obo Tailings. Miner. Eng. 2014, 65, 17–23. [Google Scholar] [CrossRef]
- Jordens, A.; Sheridan, R.S.; Rowson, N.A.; Waters, K.E. Processing a Rare Earth Mineral Deposit Using Gravity and Magnetic Separation. Miner. Eng. 2014, 62, 9–18. [Google Scholar] [CrossRef]
- Pavez, O.; Peres, A.E.C. Effect of Sodium Metasilicate and Sodium Sulphide on the Floatability of Monazite-Zircon-Rutile with Oleate and Hydroxamates. Miner. Eng. 1993, 6, 69–78. [Google Scholar] [CrossRef]
- Cheng, T.W.; Holtham, P.N.; Tran, T. Froth Flotation of Monazite and Xenotime. Miner. Eng. 1993, 6, 341–351. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, L.; Cao, M.; Liu, Q. Undefined Slime Coatings in Froth Flotation: A Review. Miner. Eng. 2017, 114, 26–36. [Google Scholar] [CrossRef]
- Alagha, L.; Wang, S.; Xu, Z.; Masliyah, J. Adsorption Kinetics of a Novel Organic-Inorganic Hybrid Polymer on Silica and Alumina Studied by Quartz Crystal Microbalance. J. Phys. Chem. C 2011, 115, 15390–15402. [Google Scholar] [CrossRef]
- Nanda, D.; Mandre, N.R. Mechanism of Polymeric Adsorption in Selective Flocculation of Low-Grade Iron Ore. Sep. Sci. Technol. 2021, 56, 68–77. [Google Scholar] [CrossRef]
- Molatlhegi, O.; Alagha, L. Ash Depression in Fine Coal Flotation Using a Novel Polymer Aid. Int. J. Clean Coal Energy 2016, 05, 65–85. [Google Scholar] [CrossRef] [Green Version]
- Khodakarami, M.; Alagha, L. High-Performance Polymers for Separation and Purification Processes: An Overview. Polym.-Plast. Technol. Eng. 2017, 56, 2019–2042. [Google Scholar] [CrossRef]
- Wang, L. The Use of Polyacrylamide as a Selective Depressant in the Separation of Chalcopyrite and Galena. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2013. [Google Scholar]
- Alsafasfeh, A.; Alagha, L. Recovery of Phosphate Minerals from Plant Tailings Using Direct Froth Flotation. Minerals 2017, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Alsafasfeh, A.; Khodakarami, M.; Alagha, L.; Moats, M.; Molatlhegi, O. Selective Depression of Silicates in Phosphate Flotation Using Polyacrylamide-Grafted Nanoparticles. Miner. Eng. 2018, 127, 198–207. [Google Scholar] [CrossRef]
- Lü, T.; Zhang, S.; Qi, D.; Zhang, D.; Zhao, H. Enhanced Demulsification from Aqueous Media by Using Magnetic Chitosan-Based Flocculant. J. Colloid Interface Sci. 2018, 518, 76–83. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of Wastewater Treatment Methods with Special Focus on Biopolymer Chitin-Chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.B.; Alagha, L.; Sannan, S.M. Flotation Behavior of Complex Sulfide Ores in the Presence of Biodegradable Polymeric Depressants. Int. J. Polym. Sci. 2017, 2017, 4835842. [Google Scholar] [CrossRef]
- Monyake, K.; Alagha, L. Depression of Pyrite in Polymetallic Sulfide Flotation Using Chitosan-Grafted-Polyacrylamide. In Proceedings of the MineXchange 2020 SME Annual Conference and Expo, Phoenix, AZ, USA, 23–26 February 2020. [Google Scholar]
- Alsafasfeh, A.; Alagha, L.; Alzidaneen, A.; Nadendla, V.S.S. Optimization of Flotation Efficiency of Phosphate Minerals in Mine Tailings Using Polymeric Depressants: Experiments and Machine Learning. Physicochem. Probl. Miner. Process. 2022, 54, 150477. [Google Scholar] [CrossRef]
- Ovanic, J. Mining Operations at Pea Ridge Iron Ore Company—A Case Study. In Underground Mining Methods; Hustrulid, W.A., Bullock, R.L., Eds.; SME, Inc.: Littleton, CO, USA, 2001; pp. 229–234. [Google Scholar]
- Yang, W.Y.; Qian, J.W.; Shen, Z.Q. A Novel Flocculant of Al(OH)3-Polyacrylamide Ionic Hybrid. J. Colloid Interface Sci. 2004, 273, 400–4005. [Google Scholar] [CrossRef]
- E276-13; Standard Test Method for Particle Size or Screen Analysis at No. 4 (4.75-Mm) Sieve and Finer for Metal-Bearing Ores and Related Materials. ASTM International: West Conshohocken, PA, USA, 2013.
- C136/C136M-19; Standard Test Method for Sieve Analysis of Fine and Coarse Aggregate. ASTM International: West Conshohocken, PA, USA, 2006.
- Geo Labs. 2022 Geoscience Laboratories Schedule of Fees and Services. Geosci. Lab. 2022, 13, 12–13. [Google Scholar]
- Actlabs. Geochemistry Schedule of Services & Fees 2023 International. Actlabs 2023, 8, 9–11. Available online: https://actlabs.com/geochemistry/ores-concentrates-and-commodities/commodities/ (accessed on 21 September 2021).
- Qi, G.W.; Parentich, A.; Little, L.; Warren, L. Selective Flotation of Apatite from Iron Oxides. Int. J. Miner. Process. 1992, 34, 83–102. [Google Scholar] [CrossRef]
- Alsafasfeh, A. Recovery of Phosphate Minerals from Plant Tailings Using Direct Froth Flotation Process. Master’s Thesis, Missouri University of Science and Technology, Rolla, MO, USA, 2017. [Google Scholar]
- Corchado, J. Characterization and liberation analysis of rare earth elements inclusions within the apatite-rich tailing from the pea ridge iron mine. In Proceedings of the GSA Connects 2021; The Geological Society of America: Portland, OR, USA, 2021. [Google Scholar]
- Corchado, J.; Alagha, L. Enrichment of Rare Earth Bearing Phosphates in Mine Tailings by Froth Flotation Process. In Proceedings of the 17th International Conference on Mineral Processing and Geometallurgy, Virtual, 21 October 2021; Velásquez, C., Gutiérrez, L., Eds.; Gecamin: Santiago, Chile, 2021; pp. 1–10. [Google Scholar]
- Corchado-Albelo, J.L.; Alagha, L. Assaying rare earth elements within the apatite-rich tailing from the pea ridge iron mine for froth flotation processing. In Proceedings of the SME Annual Meeting, Salt Lake City, UT, USA, 2 March 2022; SME, Inc.: Salt Lake City, UT, USA, 2022; pp. 1–8. [Google Scholar]
- Hrstka, T.; Gottlieb, P.; Skála, R.; Breiter, K.; Motl, D. Automated Mineralogy and Petrology—Applications of TESCAN Integrated Mineral Analyzer (TIMA). J. Geosci. 2018, 63, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Somasundaran, P. Zeta Potential of Apatite in Aqueous Solutions and Its Change during Equilibration. J. Colloid Interface Sci. 1968, 27, 659–666. [Google Scholar] [CrossRef]
- Sun, Z.-X.; Su, F.-W.; Forsling, W.; Samskog, P.-O. Surface Characteristics of Magnetite in Aqueous Suspension. J. Colloid Interface Sci. 1998, 197, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Liu, Q.; Liu, X.; Li, W.; Feng, J.; Chi, R.A. Surface Electrical Behaviors of Apatite, Dolomite, Quartz, and Phosphate Ore. Front. Mater. 2020, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Meng, Q.; Yuan, Z.; Zhang, Y.; Li, L. Effect of Sodium Silicate on the Magnetic Separation of Ilmenite from Titanaugite by Magnetite Selective Coating. Powder Technol. 2019, 344, 233–241. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Yu, Z.; Jia, D. In Situ Mineralization of Magnetite Nanoparticles in Chitosan Hydrogel. Nanoscale Res. Lett. 2009, 4, 1041–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, E. Chitosan: A Versatile Biomaterial; Hasirci, N., Hasirci, V., Eds.; Springer: Boston, MA, USA, 2004; ISBN 978-0-306-48584-8. [Google Scholar]
- Chen, S.C.; Wu, Y.C.; Mi, F.L.; Lin, Y.H.; Yu, L.C.; Sung, H.W. A Novel PH-Sensitive Hydrogel Composed of N,O-Carboxymethyl Chitosan and Alginate Cross-Linked by Genipin for Protein Drug Delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Bratlie, K.M. PH Sensitive Methacrylated Chitosan Hydrogels with Tunable Physical and Chemical Properties. Biochem. Eng. J. 2018, 132, 38–46. [Google Scholar] [CrossRef]
- Peng, Y.; Xiao, J.; Deng, B.; Wang, Z.; Liu, N.; Yang, D.; Ding, W.; Chen, T.; Wu, Q. Study on Separation of Fine-Particle Ilmenite and Mechanism Using Flocculation Flotation with Sodium Oleate and Polyacrylamide. Physicochem. Probl. Miner. Process. 2020, 56, 161–172. [Google Scholar] [CrossRef]
- Sun, W.; Long, J.; Xu, Z.; Masliyah, J.H. Study of Al(OH)3-Polyacrylamide-Induced Pelleting Flocculation by Single Molecule Force Spectroscopy. Langmuir 2008, 24, 14015–14021. [Google Scholar] [CrossRef]
- Filho, E.; Brito, E.; Silva, R.; Streck, L.; Bohn, F.; Fonseca, J. Superparamagnetic Polyacrylamide/Magnetite Composite Gels. J. Dispers. Sci. Technol. 2021, 42, 1504–1512. [Google Scholar] [CrossRef]
- Araujo, A.C.; Viana, P.R.M.; Peres, A.E.C. Reagents in Iron Ores Flotation. Miner. Eng. 2005, 18, 219–224. [Google Scholar] [CrossRef]
- Guimarães, R.C.; Araujo, A.C.; Peres, A.E.C. Reagents in Igneous Phosphate Ores Flotation. Miner. Eng. 2005, 18, 199–204. [Google Scholar] [CrossRef]
- Rao, K.H.; Dwari, R.K.; Lu, S.; Vilinska, A.; Somasundaran, P. Mixed Anionic/Non-Ionic Collectors in Phosphate Gangue Flotation from Magnetite Fines. Open Miner. Process. J. 2010, 4, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Molatlhegi, O.K. Studies on the Role of Organic-Inorganic Hybrid Polyacrylamides in Fine Coal Flotation Fine Coal Flotation. Master’s Thesis, Missouri University of Science and Technology, Rolla, MO, USA, 2015. [Google Scholar]
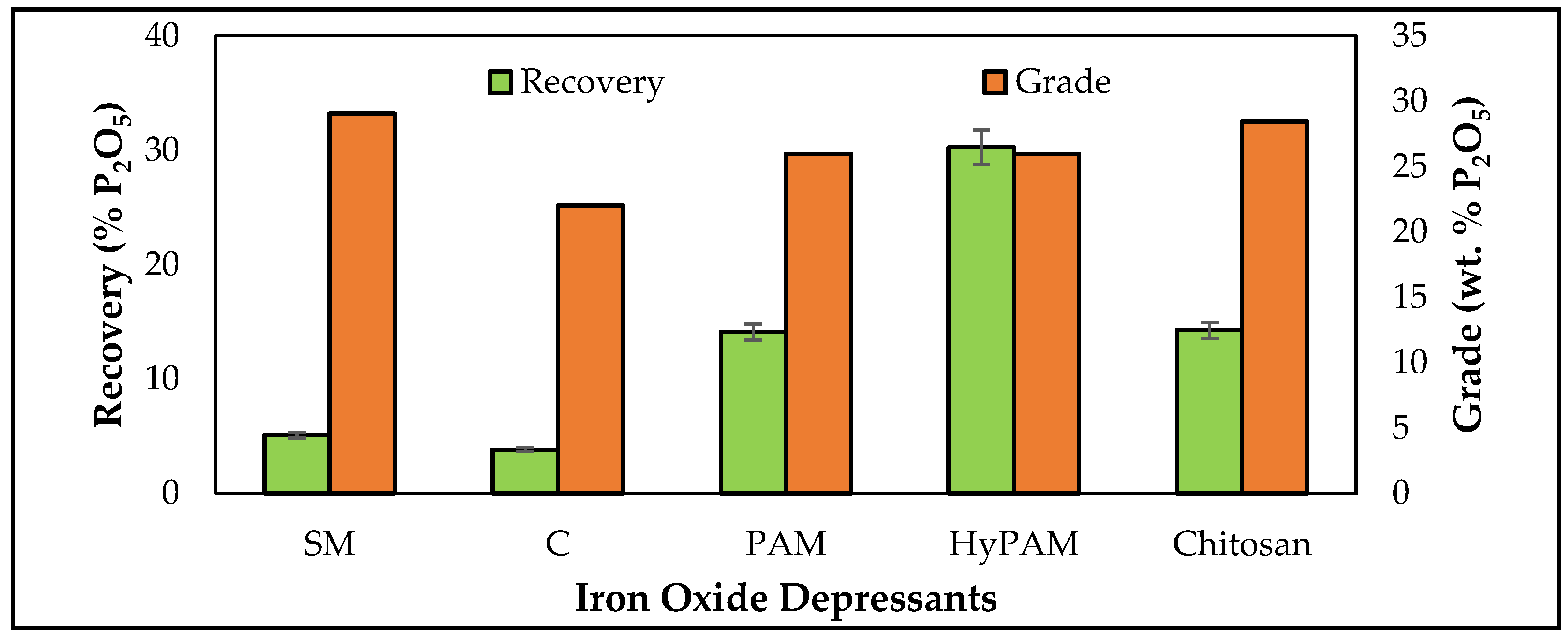
| Mineral | Cerium (%) | Lanthanum (%) | Neodymium (%) |
|---|---|---|---|
| Monazite | 96.9 | 96.7 | 97.3 |
| Synchysite | 1.7 | 1.8 | 1.7 |
| Parisite | 0.9 | 0.9 | 1 |
| Allanite | 0.5 | 0.6 | T 1 |
| Total | 100 | 100 | 100 |
| Mineral | Feed (%) |
|---|---|
| Apatite | 69.7 |
| Hematite/Magnetite | 3.1 |
| Quartz | 1.9 |
| Gypsum | 1.2 |
| Calcite | 0.5 |
| K-Feldspar | 0.3 |
| Chlorite | 0.3 |
| Free Surface | 22.4 |
| Mineral | Monazite (%) | Synchysite (%) | Parisite (%) | Allanite (%) | Xenotime (%) |
|---|---|---|---|---|---|
| Apatite | 77.5 | 4.7 | 3.3 | 0.2 | 77.9 |
| Hematite/Magnetite | 5.7 | 65.7 | 82.4 | 0.1 | 12.4 |
| Quartz | 3.3 | 8.4 | 1.6 | 60.7 | 3.2 |
| Chlorite | 1.7 | 0 | 0 | 17.6 | 0.6 |
| Monazite | 5 | 5.9 | 0.1 | 2.1 | |
| Amphiboles | 0 | 0 | 0 | 5.2 | 0.1 |
| K-Feldspar | 0.1 | 0 | 0 | 4.4 | 0.3 |
| Free particles | 7.5 | 14.8 | 6.6 | 11.7 | 3.1 |
| Mineral | Tailing | Concentrate |
|---|---|---|
| Apatite | 71.8 | 59.8 |
| Hematite/Magnetite | 1.3 | 4.9 |
| Quartz | 2.4 | 2.7 |
| Gypsum | 0.8 | 0.9 |
| K-Feldspar | 0.1 | 0.8 |
| Calcite | 0.3 | 0.3 |
| Fluorite | 0 | 0.5 |
| Free surface | 22.9 | 29.4 |
| Mineral (%) | Monazite | Synchysite | Parisite | Allanite | Xenotime |
|---|---|---|---|---|---|
| Monazite | 1.3 | 75 | 0 | 2.6 | |
| Synchysite | 1.2 | 0 | 0 | 0 | |
| Parisite | 0.2 | 0 | 0 | 0 | |
| Allanite | 0 | 0 | 0 | 0 | |
| Xenotime | 0 | 0 | 0 | ||
| Apatite | 67.3 | 0 | 25 | 20.2 | 80 |
| Hematite/Magnetite | 11.8 | 0 | 0 | 0 | 5.6 |
| Quartz | 4.7 | 0 | 0 | 0 | 3.5 |
| Gypsum | 2.4 | 0 | 0 | 0 | 0 |
| Calcite | 1 | 0 | 0 | 4.9 | 0 |
| Chlorite | 0 | 68 | 0 | 0 | 0 |
| Garnet | 0 | 30.7 | 0 | 0 | 0.7 |
| Free particles | 10.6 | 0 | 0 | 73.4 | 7.6 |
| Mineral (%) | Monazite | Synchysite | Parisite | Allanite | Xenotime |
|---|---|---|---|---|---|
| Monazite | 0 | 1.6 | 0 | 0.06 | |
| Synchysite | 0 | 0 | 0 | 0 | |
| Parisite | 0 | 0 | 0 | 0 | |
| Allanite | 0 | 0 | 0 | 0 | |
| Xenotime | 0 | 0 | 0 | 0 | |
| Apatite | 78.8 | 0 | 3.1 | 0.6 | 40.8 |
| Quartz | 4.3 | 0 | 0 | 0 | 3.8 |
| Gypsum | 2.2 | 0 | 0 | 0 | 0 |
| Hematite/Magnetite | 1.3 | 0 | 0.3 | 0 | 53 |
| Amphiboles | 0 | 0 | 0 | 9.9 | 0 |
| Chlorite | 0 | 0 | 1.8 | 6 | 0 |
| Garnet | 0 | 0 | 0 | 77.4 | 0 |
| Free Particles | 12.8 | 100 | 93.3 | 4.5 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corchado-Albelo, J.L.; Alagha, L. Studies on the Enrichment Feasibility of Rare Earth-Bearing Minerals in Mine Tailings. Minerals 2023, 13, 301. https://doi.org/10.3390/min13030301
Corchado-Albelo JL, Alagha L. Studies on the Enrichment Feasibility of Rare Earth-Bearing Minerals in Mine Tailings. Minerals. 2023; 13(3):301. https://doi.org/10.3390/min13030301
Chicago/Turabian StyleCorchado-Albelo, Jose L., and Lana Alagha. 2023. "Studies on the Enrichment Feasibility of Rare Earth-Bearing Minerals in Mine Tailings" Minerals 13, no. 3: 301. https://doi.org/10.3390/min13030301
APA StyleCorchado-Albelo, J. L., & Alagha, L. (2023). Studies on the Enrichment Feasibility of Rare Earth-Bearing Minerals in Mine Tailings. Minerals, 13(3), 301. https://doi.org/10.3390/min13030301








