Ore Genesis of the Abu Ghalaga Ferro-Ilmenite Ore Associated with Neoproterozoic Massive-Type Gabbros, South-Eastern Desert of Egypt: Evidence from Texture and Mineral Chemistry
Abstract
:1. Introduction
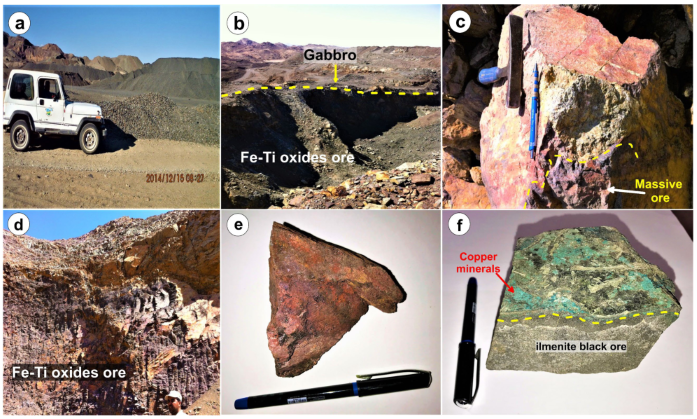
2. Geological Setting

3. Materials and Methods
4. Results and Analysis
4.1. Petrography of Gabbros
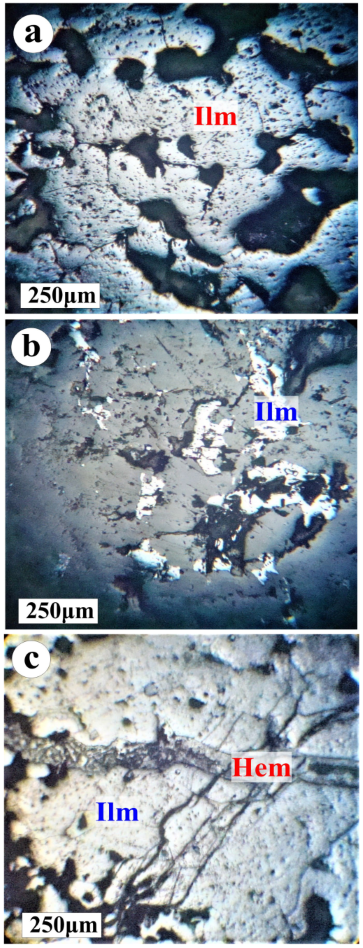
4.2. Mineralogy and Petrography of Fe-Ti Oxides
4.3. Microchemical Analysis by Scanning Electron Microscope (SEM-EDX)
4.4. X-ray Diffraction Analysis (XRD)
4.5. Chemical Composition of the Fe-Ti Oxides
5. Discussion
5.1. Formation of Fe-Ti Oxide Ore
5.2. Genesis of the Abu Ghalaga Ilmenite Ore

6. Conclusions
- XRD analyses confirmed that the SEM and reflected microscopy results were characterized by the presence of ilmenite (Ilm), hematite (Hem), and pyrite (Py).
- The area of study is affected by the Nugrus shear zone (NW–SE fault; parallel to the Red Sea direction), which represents the southern continuation of the Hafafit shear zone.
- The ilmenite grains enclose exsolution bodies of hematite lamellae that are segregated within the ilmenite host. Small grains of hematite are exsolved and segregated at the borders of ilmenite grains. Pyrite occurs as independent dissemination in ilmenite samples. The replacement of ilmenite or hematite by pyrite indicates oxidizing conditions. Barium was recorded in the SEM as barite (BaSO4) and an indicator of highly stable elements in oxidizing (sulfate-stable) environments.
- Segregation of an immiscible Fe-Ti-rich magma, fractional crystallizations with oxide settling (perhaps accompanied by plagioclase flotation), magma mixing, and solid-state crystallization are some of the major mechanisms responsible for ore formation. Exsolution of magnetite from the titano-magnetite solid solution, shaping either distinct lamellae of magnetite in titano-magnetite or the granular exsolution of ilmenite around grains of magnetite, was created by oxidation during the cooling of the intrusions. The studied samples reveal that the analyzed ilmenite plots on the ferranilmenite line formed by continuous solid solution above 800 °C; meanwhile, the analyzed magnetite and Ti–magnetite plots close to the magnetite lie at the end of the continuous solid solution above 600 °C.
- According to the binary relations between FeO and MnO, P2O5, and Al2O3, the iron content was divided into three groups: low iron content (LIC), which contained FeO ranging from 0 to 11 wt.%, medium iron content (MIC) with FeO ranging from 45 to 51 wt.%, and high iron content (HIC) containing FeO ranging from 70 to 90 wt.%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lister, G.F. The composition and origin of selected iron-titanium deposits. Econ. Geol. 1966, 61, 275–310. [Google Scholar] [CrossRef]
- Shirazy, A.; Ziaii, M.; Hezarkhani, A.; Timkin, T. Geostatistical and Remote Sensing Studies to Identify High Metallogenic Potential Regions in the Kivi Area of Iran. Minerals 2020, 10, 869. [Google Scholar] [CrossRef]
- Khedr, M.Z.; Takazawa, E.; Arai, S.; Stern, R.J.; Morishita, T.; El-Awady, A. Styles of Fe–Ti–V ore deposits in the Neoproterozoic layered mafic-ultramafic intrusions, south Eastern Desert of Egypt: Evidence for fractional crystallization of V-rich melts. J. Afr. Earth Sci. 2022, 194, 104620. [Google Scholar] [CrossRef]
- Gross, G.A. Algoma-type iron-formation. Sel. Br. Columbia Miner. Depos. Profiles 1996, 2, 1–13. [Google Scholar]
- Shirazy, A.; Hezarkhani, A.; Timkin, T.; Shirazi, A. Investigation of Magneto-/Radio-Metric Behavior in Order to Identify an Estimator Model Using K-Means Clustering and Artificial Neural Network (ANN) (Iron Ore Deposit, Yazd, IRAN). Minerals 2021, 11, 1304. [Google Scholar] [CrossRef]
- Charlier, B.; Skar, O.; Korneliussen, A.; Duchesne, J.C.; Vander Auwera, J. Ilmenite composition in the Tellnes Fe-Ti deposit, SW Norway: Fractional crystallization, post cumulus evolution and ilmenite-zircon relation. Contrib. Mineral. Petrol. 2007, 154, 119–134. [Google Scholar] [CrossRef]
- El Khalloufi, M.; Drevelle, O.; Soucy, G. Titanium: An overview of resources and production methods. Minerals 2021, 11, 1425. [Google Scholar] [CrossRef]
- Towner, R.R.; Gray, J.M.; Porter, L.M. International Strategic Minerals Inventory Summary Report, Titanium; US Geological Survey: Reston, VA, USA, 1988. [Google Scholar]
- Force, E.R. Titanium content and titanium partitioning in rocks. In Geology and Resources of Titanium; U.S. Government Printing Office: Washington, DC, USA, 1976; pp. A2–A3. [Google Scholar]
- Abu El-Rus, M.A. Geological studies on Abu Ghalaga area, Eastern Desert, Egypt. Master’s Thesis, Assiut University, Assiut, Eqypt, 1991; 255p. [Google Scholar]
- Greiling, R.O.; Abdeen, M.M.; Dardir, A.A.; El-Akhal, H.; El-Ramly, M.F.; Kamal El-Din, G.M.; Osman, A.F.; Rashwan, A.A.; Rice, A.H.; Sadek, M.F. A structural synthesis of the Proterozoic Arabian-Nubian Shield in Egypt. Geol. Rundsch. 1994, 83, 484–501. [Google Scholar] [CrossRef]
- Hassan, S.M.; El-Kazzaz, Y.A.; Taha, M.N.; Mohammad, A.T. Late Neoproterozoic basement rocks of Meatiq area, Central Eastern Desert, Egypt: Petrography and remote sensing characterizations. J. Afr. Earth Sci. 2017, 131, 14–31. [Google Scholar] [CrossRef]
- El-Kalioubi, B.; Fowler, A.; Abdelmalik, K. Chapter 6: The Metamorphism and Deformation of the Basement Complex in Egypt. In Geology of Egypt; Springer: Berlin/Heidelberg, Germany, 2020; pp. 191–252. [Google Scholar] [CrossRef]
- El-Desoky, H.M.; Tende, A.W.; Abdel-Rahman, A.M.; Ene, A.; Awad, H.A.; Fahmy, W.; El-Awny, H.; Zakaly, H.M. Hydrothermal alteration mapping using landsat 8 and ASTER data and geochemical characteristics of Precambrian rocks in the Egyptian shield: A Case Study from Abu Ghalaga, Southeastern Desert, Egypt. Remote Sens. 2022, 14, 3456. [Google Scholar] [CrossRef]
- Nasr, B.B.; Sadek, M.F.; Masoud, M.S. Some new occurrences of layered titanomagnetite. Eastern Desert, Egypt. Ann. Geol. Surv. Egypt 2008, 23, 679–690. [Google Scholar]
- Makhlouf, A.; Beniamin, N.Y.; Mansour, M.M.; Mansour, S.A.; El-Shrbeni, H. Mafic-ultramafic intrusion of South Korab Kansi area with emphasis on titanomagnetite ores, southern Eastern Desert, Egypt. Ann. Geol. Surv. Egypt 2008, 3, 1–20. [Google Scholar]
- Saleh, G.M.; Khaleal, F.M.; Lasheen, E.S.R. Petrogenesis of ilmenite-bearing mafic intrusions: A case study of Abu Ghalaga area, South Eastern Desert, Egypt. Arab. J. Geosci. 2022, 15, 1508. [Google Scholar] [CrossRef]
- Mikulski, S.Z.; Sadłowska, K.; Wiszniewska, J.; Małek, R. Vanadium and Cobalt Occurrence in the Fe-Ti-V Oxide Deposits Related to Mesoproterozoic AMCG Complex in NE Poland. Appl. Sci. 2022, 12, 6277. [Google Scholar] [CrossRef]
- Fritz, H.; Wallbrecher, E.; Khudeir, A.A.; Abu-El Ela, F.; Dallmeyer, D.R. Formation of Neoproterozoic core complexes during oblique convergence (Eastern Desert, Egypt). J. Afr. Earth Sci. 1996, 23, 311–329. [Google Scholar] [CrossRef]
- Abd El-Wahed, M.A. Oppositely dipping thrusts and transpressional imbricate zone in the Central Eastern Desert of Egypt. J. Afr. Earth Sci. 2014, 100, 42–59. [Google Scholar] [CrossRef]
- Abdel Aziz, Y.M. Manganoan Ilmenite from the gabbroic rocks of Abu Ghalaga area, Eastern Desert, Egypt. In Proceedings of the 2nd International Conference on the Geology of Africa, Assiut, Egypt, October; 2001; Volume 1, pp. 101–121. [Google Scholar]
- Maurice, A.E.; Basta, F.F.; Khiamy, A.A. Neoproterozoic nascent island arc volcanism from the Nubian Shield of Egypt: Magma genesis and generation of continental crust in intra-oceanic arcs. Lithos 2012, 132–133, 1–20. [Google Scholar] [CrossRef]
- Amin, M.S. The ilmenite deposit of Abu Ghalaga. Econ. Geol. 1954, 49, 77–87. [Google Scholar] [CrossRef]
- Abdel-Karim, A.A.M.; Elwan, W.I.; Helmy, H.; El-Shafey, S.A. Spinels, Fe–Ti oxide minerals, apatites, and carbonates hosted in the ophiolites of Eastern Desert of Egypt: Mineralogy and chemical aspects. Arab. J. Geosci. 2014, 7, 693–709. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock Forming Minerals; Longman: London, UK, 1992; p. 696. [Google Scholar]
- Mehdilo, A.; Irannajad, M. Effects of mineralogical and textural characteristics of ilmenite; concentrate on synthetic rutile production. Arab. J. Geosci. 2013, 6, 3865–3876. [Google Scholar] [CrossRef]
- Du, Y.; Meng, Q.; Yuan, Z.; Ma, L.; Zhao, X.; Xu, Y. Study on the flotation behavior and mechanism of ilmenite and titanaugite with sodium oleate. Miner. Eng. 2020, 152, 106366. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Haggerty, S.E. Opaque Mineral Oxides in Terrestrial Igneous Rocks. In Oxide Minerals; Reviews in Mineralogy and Geochemistry; Rumble, D., Ed.; Mineralogical Society of America: Chantilly, VA, USA, 1976; Volume 3, pp. Hg101–Hg300. [Google Scholar]
- Dunlop, D.; Ozdemir, O. Fundamental Frontiers; Cambridge Studies in Magnetism; Cambridge University Press: New York, NY, YSA, 1997; 573p. [Google Scholar]
- Bai, Z.J.; Zhong, H.; Naldrett, A.J.; Zhu, W.G.; Xu, G.W. Whole-rock and mineral composition of constraints on the genesis of the giant Hongge Fe–Ti–V oxide deposit in the Emeishan LIP, southwest China. Econ. Geol. 2012, 107, 481–506. [Google Scholar] [CrossRef]
- Pang, K.N.; Zhow, M.F.; Lindsley, D.; Zhao, D.; Malpas, J. Origin of Fe–Ti oxide ores in mafic intrusions: Evidence from the Panzhihua intrusion, SW China. J. Petrol. 2008, 49, 295–313. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.F.; Chen, W.T.; Wang, C.Y.; Prevec, S.A.; Liu, P.P.; Howarth, G. Two stages of immiscible liquid separation in the formation of Panzhihua-type of Fe–Ti–V oxide deposits, SW China. Geosci. Front. 2013, 4, 481–502. [Google Scholar] [CrossRef]
- Charlier, B.; Grove, T.L. Experiments on liquid immiscibility along tholeiitic liquid lines of descent. Contrib. Mineral. Petrol. 2012, 164, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Withers, P.J.; Ulen, B.; Stamm, C.; Bechmann, M. Incidental phosphorus losses—Are they significant and can they predicted. J. Plant Nutr. Soil Sci. 2003, 166, 459–468. [Google Scholar] [CrossRef]
- Broska, I.; Uher, P.; Ondrejka, M. Geochemical and Mineralogical Characterization of the Fe-Ti Oxide Paragenesis in the Magmatic and Hydrothermal Systems of the Western Carpathians; Slovak Academy of Sciences: Bratislava, Slovakia, 2003; Available online: http://www.geol.sav.sk/broska/projects.html (accessed on 23 August 2022).
- Buddington, A.F.; Lindsley, D.H. Iron- titanium oxide minerals and synthetic equivalent. J. Petrol. 1964, 5, 310–357. [Google Scholar] [CrossRef]
- Al-Mohandis, A.A. The opaque minerals of Jabal Sha layered intrusion. Saudi Arabia. J. Coll. Sci. Riyadh 1980, 11, 171–188. [Google Scholar]
- Wenk, H.R.; Chen, K.; Smith, R. Morphology and microstructure of magnetite and ilmenite inclusions in plagioclase from Adirondack anorthositic gneiss. Am. Mineral. 2011, 96, 1316–1324. [Google Scholar] [CrossRef]
- Hall, A.L. The Bushweld Igneous complex of the Central Transvaal; Memoir No. 28; Geological Survey of the Union of South Africa: Pretoria, South Africa, 1932. [Google Scholar]
- Bateman, A.M. The formation of late magmatic oxide ores. Econ. Geol. 1951, 46, 404–426. [Google Scholar] [CrossRef]
- Kolker, A. Mineralogy and geochemistry of Fe-Ti oxide and apatite (nelsonite) deposits and evaluation of liquid immiscibility hypothesis, Econ. Geol. 1982, 77, 1146–1158. [Google Scholar]
- Philpotts, A.R. Origin of certain iron-titanium oxide and apatite rocks. Econ. Geol. 1967, 62, 303–315. [Google Scholar] [CrossRef]
- Reynolds, I.M. The nature and origin of titaniferous magnetite-rich layers on the upper zone of the Bushveld complex: A review and synthesis. Econ. Geol. 1985, 80, 1089–1108. [Google Scholar] [CrossRef]
- Charlier, B.; Duchesne, J.C.; Vander Auwera, J. Magma chamber processes in the Tellnes ilmenite deposit (Rogaland Anorthosite Province, SW Norway) and the formation of Fe-Ti ores in massif-type anorthos. Chem. Geol. 2006, 234, 264–290. [Google Scholar] [CrossRef]
- Woodruff, L.G.; Nicholson, S.W.; Fey, D.L. A deposit model for magmatic iron-titanium oxide deposits related to Proterozoic massif anorthosite plutonic suites. U.S. Geol. Surv. Sci. Investig. Rep. 2013, 5091, 47. [Google Scholar]
- Zhou, M.F.; Robinson, P.T.; Lesher, C.M.; Keays, R.R.; Zhang, C.J.; Malpas, J. Geochemistry, petrogenesis and metallogenesis of the Panzhihua gabbroic layered intrusion and associated Fe-Ti-V oxide deposits, Sichuan Province, SW China. J. Petrol. 2005, 46, 22–53. [Google Scholar] [CrossRef] [Green Version]
- Hou, T.; Zheng, Z.; Encarnacion, J.; Santosh, M. Petrogenesis and metallogenesis of the Taihe gabbroic intrusion associated with Fe-Ti oxide ores in the Panxi district, Emeishan large igneous province. Ore Geol. Rev. 2012, 49, 109–127. [Google Scholar] [CrossRef]
- El-Metwally, A.A. Petrogenesis of gabbroic rock intrusions from south-central Sinai massif: A transition from arc to intraplate magmatism. In Proceedings of the Third International Conference on Geochemistry, Alexandria, Egypt, 5–6 June 2015; pp. 49–66. [Google Scholar]
- Attia, M.I. The Geology of the Iron-Ore Deposits; Geological Survey: Cairo, Egypt, 1950. [Google Scholar]
- El-Shazly, E.M. The Ilmenite Ore at Abu Ghalaga, Eastern Desert, Egypt; Report 3759; Geological Survey: Cairo, Egypt, 1959. [Google Scholar]
- Cao, J.; Wang, X.; Tao, J. Petrogenesis of the Piqiang mafic-ultramafic layered intrusion and associated Fe-Ti-V oxide deposit in Tarim Large Igneous Province, NW China. Int. Geol. Rev. 2019, 61, 2249–2275. [Google Scholar] [CrossRef]
- Basta, E.Z.; Takla, M.A. Petrological Studies on Abu Ghalaga Ilmenite Occurrence, Eastern Desert. Egypt J. Geol. 1968, 12, 43–71. [Google Scholar]
- Hussein, A.A. Mineral deposits. In The Geology of Egypt; Said, R., Ed.; Routledge: Abingdon, UK, 2001; pp. 537–539. [Google Scholar]
- Shirazi, A.; Hezarkhani, A.; Beiranvand Pour, A.; Shirazy, A.; Hashim, M. Neuro-Fuzzy-AHP (NFAHP) Technique for Copper Exploration Using Advanced Spaceborne Thermal Emission and Reflection Radiometer (ASTER) and Geological Datasets in the Sahlabad Mining Area, East Iran. Remote Sens. 2022, 14, 5562. [Google Scholar] [CrossRef]
- Hawa, Y.M.A. Mineral chemistry of extraordinary ilmenite from the Gabbroic rocks of Abu Ghalaga area, Eastern Desert, Egypt: Evidence to metamorphic modification. In Proceedings of the 6th International Conference on Geological Sciences and Engineering (ICGSE), Paris, France, 28–29 August 2014; Volume 1, p. 68, (Abstract). Available online: www.waset.org/abstracts/7253 (accessed on 6 August 2020).









| Type of Samples | Number of Samples | Methods of Analysis |
|---|---|---|
| Hand specimens of gabbro and Fe-Ti oxides | 52 | |
| Thin section of gabbro (petrography) | 12 | Polarizing microscope. |
| Thin polished sections (mineralogy) | 11 | Reflected light microscope. |
| Fe-Ti ore samples | 11 | X-ray diffractometer (XRD) Scanning electron microscope (SEM-EDX) |
| Mineral chemistry analyses of Fe-Ti ore | 38 | Electron microprobe analysis (EPMA) |
| Mineral and Abbreviation | Formula | Relative Content | |
|---|---|---|---|
| 1 | Ilmenite (Ilm) | FeTiO3 | 20–85% |
| 2 | Hematite (Hem) | Fe2O3 | 13–77% |
| 3 | Chlorite (Chl) | Fe, Mg, Al)6(Si, Al)4O10(OH)8 | 4–14% |
| 4 | Anorthite (An) | CaAl2Si2O8 | 6% |
| 5 | Chlinochlore | Mg5Al(AlSi3O10)(OH)8 | 5–20% |
| 6 | Actinolite (Act) | Ca(Mg4.5-2.5Fe0.5-2.5)Si8O22OH2 | 2–21% |
| 7 | Calcite (Cal) | CaCO3 | 1% |
| 8 | Dolomite (Dol) | CaMg(CO3)2 | 3% |
| 9 | Kaolinite (Kln) | Al2Si2O5(OH)4 | 2–10% |
| 10 | Hornblende (Hbl) | (Ca,Na)2(Mg,Fe,Al)5(Al,Si)8O22 (OH)2 | 15% |
| 11 | Anthophyllite (Ath) | (Mg, Fe2+)7Si8O22(OH)2 | 3% |
| Sample (%) | Un 99 6 ROI1 | Un 100 6 ROI2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 829 | 830 | 831 | 832 | 833 | 838 | 841 | 842 | 843 | 844 | 845 | 850 | 851 | |
| SiO2 | 0.008 | 0.026 | 0.018 | ||||||||||
| TiO2 | 47.97 | 48.81 | 48.23 | 48.8 | 48.4 | 48.64 | 48.46 | 48.45 | 47.95 | 47.94 | 48.98 | 45.4 | 44.51 |
| Al2O3 | 0.02 | 0.02 | 0.01 | 0.05 | 0.01 | 0.26 | |||||||
| FeOt | 48.94 | 48.85 | 49.15 | 48.77 | 48.91 | 49.32 | 49.65 | 49.89 | 50.28 | 50.59 | 49.72 | 51.76 | 52.43 |
| MgO | 1.642 | 1.707 | 1.628 | 1.678 | 1.823 | 1.67 | 0.93 | 0.919 | 0.884 | 0.873 | 0.781 | 1.055 | 1.007 |
| Cr2O3 | 0.02 | 0.06 | 0.03 | 0.01 | 0.02 | 0.03 | 0 | 0.02 | 0.01 | 0.05 | 0.03 | 0.06 | 0.05 |
| MnO | 0.29 | 0.3 | 0.26 | 0.34 | 0.29 | 0.34 | 0.32 | 0.29 | 0.34 | 0.28 | 0.32 | 0.33 | 0.22 |
| NiO | 0.021 | ||||||||||||
| ZnO | 0.04 | 0.32 | 0.008 | 0.23 | 0.04 | 0.22 | 0.31 | 0.008 | |||||
| Total | 98.92 | 99.77 | 99.63 | 99.65 | 99.46 | 100 | 99.6 | 99.6 | 99.6 | 100 | 100.2 | 98.7 | 98.5 |
| Structural formula is based on 6 oxygen atoms | |||||||||||||
| Si | 0.28 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 | 0.28 | 0.28 | 0.29 | 0.28 | 0.28 |
| Ti | 1.67 | 1.69 | 1.68 | 1.69 | 1.68 | 1.68 | 1.69 | 1.69 | 1.67 | 1.67 | 1.7 | 1.59 | 1.56 |
| Al | 0.001 | 0.001 | 0.001 | 0.003 | 0.001 | 0.01 | |||||||
| Fe2+ | 1.9 | 1.88 | 1.89 | 1.88 | 1.88 | 1.89 | 1.93 | 1.93 | 1.95 | 1.96 | 1.92 | 2.02 | 2.05 |
| Mg | 0.11 | 0.11 | 0.11 | 0.11 | 0.12 | 0.11 | 0.06 | 0.06 | 0.06 | 0.06 | 0.05 | 0.07 | 0.07 |
| Cr | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | 0.002 | 0.002 | |||
| Mn | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.009 |
| Ni | 0.29 | 0.28 | 0.28 | 0.29 | |||||||||
| Zn | 0.005 | 0.004 | 1.69 | 1.67 | 1.67 | 1.7 | |||||||
| Sample | Un 100 6 ROI2 | Un 99 6 ROI3 | |||||||||||
| (%) | 852 | 853 | 856 | 857 | 858 | 859 | 864 | 866 | 868 | 869 | 870 | 871 | |
| SiO2 | |||||||||||||
| TiO2 | 49.07 | 46.77 | 48.9 | 48.3 | 48.8 | 47.9 | 47.6 | 48.7 | 48.2 | 48.1 | 49 | 48.9 | |
| Al2O3 | 0 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| FeOt | 48.99 | 51.1 | 48.8 | 49.4 | 49.1 | 49.8 | 48.9 | 49 | 48.8 | 49.2 | 48 | 48.5 | |
| MgO | 1.01 | 0.95 | 1.82 | 1.83 | 1.86 | 1.76 | 1.8 | 1.88 | 1.79 | 1.76 | 1.9 | 1.78 | |
| CaO | 0.05 | 0.01 | 0.01 | 0.01 | 0 | 0 | 0 | 0.02 | |||||
| Na2O | 0.01 | 0.02 | 0.01 | 0.03 | 0.03 | 0.01 | |||||||
| K2O | 0.01 | ||||||||||||
| Cr2O3 | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 | 0.02 | 0.05 | 0.01 | 0.04 | 0.04 | 0.01 | 0.01 | |
| MnO | 0.29 | 0.31 | 0.25 | 0.31 | 0.26 | 0.26 | 0.32 | 0.25 | 0.32 | 0.28 | 0.29 | 0.3 | |
| NiO | |||||||||||||
| ZnO | 0.04 | 0.3 | 0.16 | 0.44 | 0.2 | 0.02 | |||||||
| Total | 99.5 | 99.5 | 100 | 99.9 | 100.6 | 99.8 | 99 | 99.8 | 99.2 | 99.4 | 99.2 | 99.6 | |
| Cation ratios based on O = 6 | |||||||||||||
| Si | 0.29 | 0.28 | 0.29 | 0.28 | 0.29 | 0.28 | 0.28 | 0.29 | 0.29 | 0.28 | 0.29 | 0.29 | |
| Ti | 1.71 | 1.64 | 1.69 | 1.67 | 1.68 | 1.66 | 1.66 | 1.68 | 1.68 | 1.67 | 1.7 | 1.69 | |
| Al | 0.001 | 0.001 | 0.001 | ||||||||||
| Fe2+ | 1.9 | 1.99 | 1.877 | 1.9 | 1.88 | 1.91 | 1.9 | 1.88 | 1.89 | 1.9 | 1.85 | 1.87 | |
| Mg | 0.07 | 0.06 | 0.125 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.13 | 0.12 | |
| Cr | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.002 | 0 | 0.001 | |||||
| Mn | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.013 | 0.01 | 0.01 | 0.01 | 0.01 | |||
| Zn | 0.003 | 0.007 | 0.003 | ||||||||||
| Samples(%) | Titano-Magnetite | Ti-Poor Magnetite | Titano-Magnetite | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 834 | 835 | 836 | 837 | 846 | 847 | 848 | 849 | 861 | 862 | 863 | 865 | 867 | |
| SiO2 | 0.472 | 0.354 | 0.48 | 0.78 | |||||||||
| TiO2 | 11.57 | 12.45 | 12.56 | 18.52 | 1.38 | 1.96 | 1.57 | 1.66 | 11.3 | 12.5 | 11.4 | 7.5 | 11.1 |
| Al2O3 | 0.04 | 0.06 | 0.01 | 0.06 | 0.17 | 0.20 | 0.14 | 0.35 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 |
| FeO | 80.95 | 80.41 | 80.74 | 76.38 | 88.13 | 88.57 | 88.59 | 86.99 | 80.6 | 79.6 | 81.2 | 85.6 | 81.3 |
| Fe2O3 | 6.86 | 6.49 | 6.06 | 4.46 | 9.43 | 8.41 | 8.86 | 10.26 | 7.48 | 6.95 | 6.67 | 6.36 | 6.96 |
| MgO | 0.15 | 0.22 | 0.24 | 0.18 | 0.02 | 0.008 | 0.02 | 0.06 | 0.17 | 0.27 | 0.18 | 0.07 | 0.15 |
| CaO | 0.01 | 0.01 | 0.01 | 0.04 | 0.03 | 0.04 | 0.06 | ||||||
| Na2O | 0.05 | 0.01 | 0.05 | 0.02 | 0.03 | 0.03 | 0.01 | ||||||
| K2O | 0.002 | 0.003 | |||||||||||
| Cr2O3 | 0.33 | 0.33 | 0.33 | 0.30 | 0.67 | 0.54 | 0.53 | 0.58 | 0.35 | 0.36 | 0.41 | 0.32 | 0.37 |
| MnO | 0.03 | 0.01 | 0.04 | 0.04 | 0.02 | 0.01 | 0.03 | 0.04 | 0.02 | ||||
| NiO | 0.01 | ||||||||||||
| ZnO | 0.14 | 0.25 | 0.18 | 0.11 | |||||||||
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Cation ratios based on O = 6 | |||||||||||||
| Si | 0.22 | 0.22 | 0.22 | 0.23 | 0.21 | 0.21 | 0.20 | 0.21 | 0.24 | 0.22 | 0.22 | 0.21 | 0.22 |
| Ti | 0.42 | 0.45 | 0.45 | 0.66 | 0.05 | 0.07 | 0.01 | 0.06 | 0.72 | 0.41 | 0.46 | 0.27 | 0.41 |
| Al | 0.003 | 0.003 | 0.001 | 0.003 | 0.01 | 0.01 | 0.02 | 0.02 | 0.002 | 0.005 | 0.01 | 0.003 | 0.005 |
| Fe2+ | 3.31 | 3.28 | 3.28 | 3.06 | 3.69 | 3.67 | 3.71 | 3.66 | 2.96 | 3.32 | 3.26 | 3.48 | 3.33 |
| Mg | 0.01 | 0.01 | 0.01 | 0.01 | 0.002 | 0.001 | 0.002 | 0.005 | 0.04 | 0.01 | 0.02 | 0.005 | 0.01 |
| Cr | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | |||||
| Mn | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | 11.3 | 12.5 | 11.4 | 7.5 | 11.1 | |||
| Ni | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | ||||||||
| Zn | 0.003 | 0.005 | 80.6 | 79.6 | 81.2 | 85.6 | 81.3 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Desoky, H.M.; Abdel-Rahman, A.M.; Fahmy, W.; Khalifa, I.; Mohamed, S.A.; Shirazi, A.; Hezarkhani, A.; Shirazy, A.; Pour, A.B. Ore Genesis of the Abu Ghalaga Ferro-Ilmenite Ore Associated with Neoproterozoic Massive-Type Gabbros, South-Eastern Desert of Egypt: Evidence from Texture and Mineral Chemistry. Minerals 2023, 13, 307. https://doi.org/10.3390/min13030307
El-Desoky HM, Abdel-Rahman AM, Fahmy W, Khalifa I, Mohamed SA, Shirazi A, Hezarkhani A, Shirazy A, Pour AB. Ore Genesis of the Abu Ghalaga Ferro-Ilmenite Ore Associated with Neoproterozoic Massive-Type Gabbros, South-Eastern Desert of Egypt: Evidence from Texture and Mineral Chemistry. Minerals. 2023; 13(3):307. https://doi.org/10.3390/min13030307
Chicago/Turabian StyleEl-Desoky, Hatem M., Ahmed M. Abdel-Rahman, Wael Fahmy, Ibrahim Khalifa, Salah A. Mohamed, Aref Shirazi, Ardeshir Hezarkhani, Adel Shirazy, and Amin Beiranvand Pour. 2023. "Ore Genesis of the Abu Ghalaga Ferro-Ilmenite Ore Associated with Neoproterozoic Massive-Type Gabbros, South-Eastern Desert of Egypt: Evidence from Texture and Mineral Chemistry" Minerals 13, no. 3: 307. https://doi.org/10.3390/min13030307
APA StyleEl-Desoky, H. M., Abdel-Rahman, A. M., Fahmy, W., Khalifa, I., Mohamed, S. A., Shirazi, A., Hezarkhani, A., Shirazy, A., & Pour, A. B. (2023). Ore Genesis of the Abu Ghalaga Ferro-Ilmenite Ore Associated with Neoproterozoic Massive-Type Gabbros, South-Eastern Desert of Egypt: Evidence from Texture and Mineral Chemistry. Minerals, 13(3), 307. https://doi.org/10.3390/min13030307











